Abstract
Background:
Changes in lipoproteins levels in sickle cell disease (SCD) patients are well-known, but the physiological ramifications of the low levels observed have not been entirely resolved.
Aim:
The aim of this study is to evaluate the impact of decreased levels of high density lipoprotein cholesterol (HDL-c) on hematological indices in steady state SCD patients.
Subjects and Methods:
The study was conducted on 84 SCD patients on steady clinical state, 36 males and 48 females with a mean age 21 (6) years. All those who have had blood transfusion within 4 months, infection, chronic kidney disease, and inflammatory episodes were excluded. Full blood count, total cholesterol, HDL-c, low density lipoprotein cholesterol, very low density lipoprotein cholesterol, and triglyceride were assayed. Hematological indices of SCD patients with decreased levels of HDL-c were compared with those with normal HDL-c levels.
Results:
The SCD patients with decreased levels of HDL-c presented with lower levels of hemoglobin (P < 0.01), hematocrit (P < 0.001), total leukocyte count (P = 0.02), red blood cell count (P < 0.01), absolute neutrophil count (P = 0.04), absolute monocyte count (P < 0.01), and triglyceride (P = 0.02). Of the 47 SCD with decreased levels of HDL-c, 82.9% (39/47) have had blood transfusion while 48.6% (18/37) out of 37 SCD with normal HDL-c have had blood transfusion.
Conclusion:
SCD patients with decreased levels of HDL-c had more severe anemia, higher leukocyte and platelet counts than those with normal HDL-c levels. The low HDL-c marker may assist in the prediction of adverse clinical events in these patients.
Keywords: Blood transfusion, Hematological indices, High density lipoprotein cholesterol, Sickle cell disease
Introduction
Sickle cell disease (SCD) is an inherited hemolytic anemia with a variable course and severity. The knowledge of prognosis biomarkers may help in the establishment of therapeutic intervention, management, and follow-up of patients. Studies have shown associations between some laboratory parameters and the clinical events predicting prognosis of the SCD.[1,2] Some of the well-known biomarkers that predict SCD clinical prognosis includes fetal hemoglobin, leukocyte counts, and serum Lactate dehydrogenase.[2,3,4] Other factors that may predict the risk of complications and death in SCD patients are vaso-occlusive episodes resulting from a complex interaction of events with participation of stressed reticulocytes, sickled red blood cells, leukocytes, platelets, and endothelial activation.[5,6] Bacterial infection associated with leukocytosis, malaria, dehydration, extremes of temperatures, stress, and low oxygen tension have all been shown to precipitate sickle cell crises and consequent adverse clinical outcome.
Even though, decreased levels of cholesterol and lipoprotein in SCD patients are well-documented, the pathophysiological implications of these changes have not been completely resolved.[1] In our previous report, it was observed that the levels of high density lipoprotein cholesterol (HDL-c) in SCD patients were lower (≤0.8 mmol/L) than normal and that some of the values were within normal limits (≥0.8 mmol/L)[3] probably related to the severity of anemia. Furthermore, lipid profile of SCD patients was different from that of subjects with normal hemoglobin and Nigerian SCD patients were reported to have lower lipid profile levels compared to their counterparts in America and Middle East.[3,4] The variations have been attributed to differences in age, diet, weight, smoking, disease severity, and treatment regimen.[5,7]
In this study, we evaluated the impact of decreased levels of HDL-c on hematological indices, which has been postulated to predispose SCD patients to severe hemolysis and hence anemia and its association with other hematological parameters in steady state patients.
Subjects and Methods
This was a prospective hospital based cross-sectional study in which 84 consecutive SCD patients aged 15 years and above attending the adult hematology clinic of Aminu Kano Teaching Hospital, Kano was recruited from January 2007 to December 2007. SCD patients with chronic kidney disease and other chronic complications were excluded from the study. Approval for the study was obtained from the ethical review board of the hospital, and written informed consent was signed by the patients or their parents. Clinical and demographic data were documented and only those patients with steady clinical state of the disease were included. Steady clinical state was defined as a period without any acute events and no blood transfusion for about 4 months prior to blood sample collection. A total of 56 subjects who have had blood transfusion within 4 months, infection and inflammatory episodes were excluded.
A total of 5 mL of fasting blood samples were collected with 2 mL each dispensed into the tube containing ethylene diamine tetra acetic acid anticoagulant for full blood count and the remaining 3 mL was emptied into plain container. This was allowed to clot at room temperature and then centrifuged at 3000 rpm for 10 min to obtain serum. The full blood count was carried out using electronic cell counter, Coulter CELL DYE 3700 auto-analyzer (Coulter Corporation, USA). The total cholesterol and triglyceride were determined using the enzyme catalyzed colorimetric technique by Randox Laboratories, UK while HDL-c was assayed using the supernatant after precipitation with magnesium chloride-phosphotungstic acid solution. The low density lipoprotein cholesterol (LDL-c) was calculated using the Friedewald formula.[8]
Statistical analysis
A two sample unpaired t-test was used to determine the statistical significance of the means between groups. A P ≤ 0.05 was considered statistically significant. Bivariate correlation analyses were carried out to determine the correlation between HDL-c and other measured variables using Pearson's correlation (r). Data analyses were performed using SPSS version 16.0 (Chicago, IL, USA).
Results
The results are as shown in Tables 1-3. Table 1 shows the association of HDL-c with the measured parameters. HDL-c correlated positively with the red blood cell count (P < 0.01), absolute lymphocyte count (P < 0.001) and negatively with LDL-c (P < 0.001). While the other parameters showed no significant association with HDL-c.
Table 1.
Association of HDL-cholesterol with hematological indices and lipoproteins levels in SCD patients
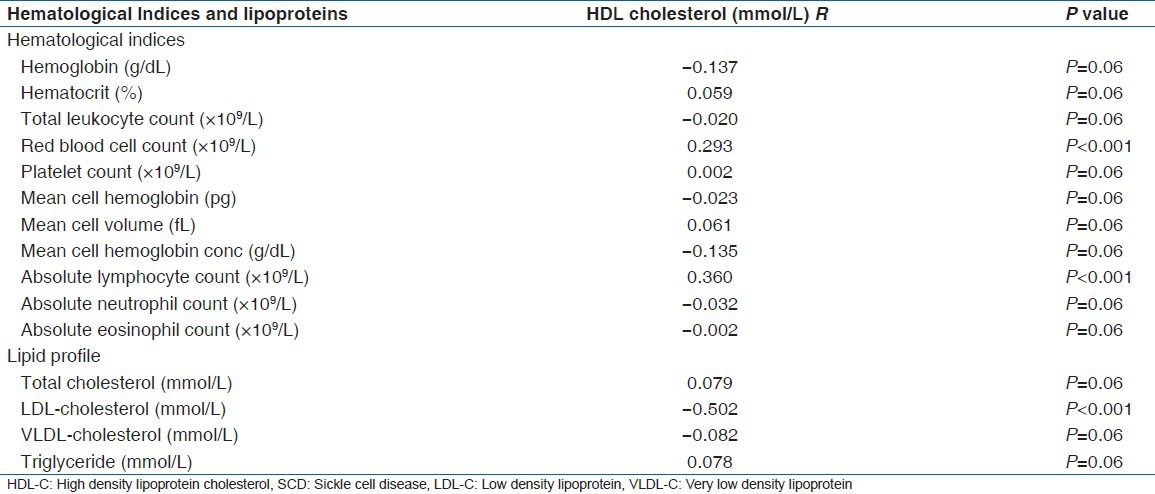
Table 3.
HDL-cholesterol levels based on SCD severity
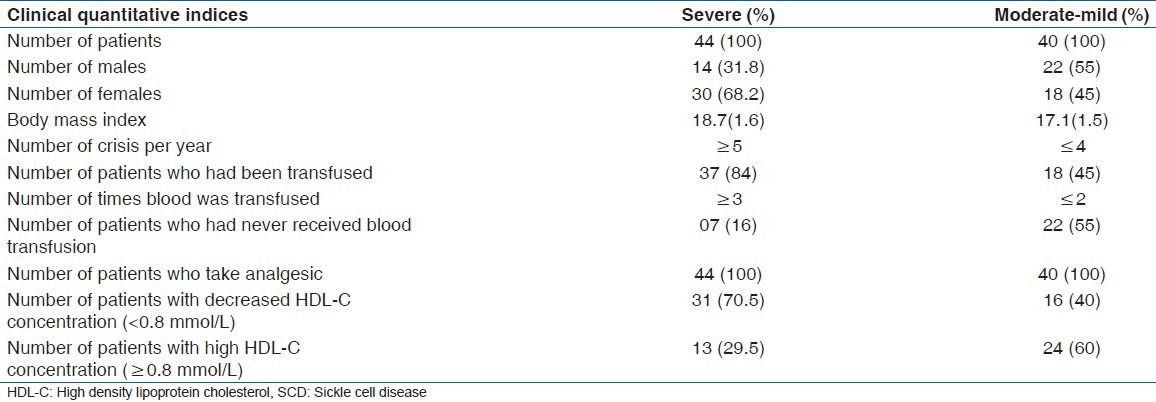
Table 2 indicates the comparison of measured parameters in SCD patients with HDL-c levels (decrease (<0.8 mmol/L) and normal HDL-c (≥0.8 mmol/L). There were 47 patients with HDL-c < 0.8 mmol/L and 37 with HDL-c ≥ 0.8 mmol/L. Those with decreased levels of HDL-c presented with lower levels of hemoglobin (P < 0.01), hematocrit (P < 0.001), total leukocyte count (P = 0.02), red blood cell count (P < 0.01), absolute neutrophil count (P = 0.04), absolute monocyte count (P < 0.01), and triglyceride (P = 0.02). In addition, out of the 47 SCD patients with decreased HDL-c levels, 82.9% (39/47) had been transfused with blood while 48.6% (18/37) of the SCD patients with normal HDL-c had received blood transfusion in the past. There was however no record of the number of units of blood received by the patients.
Table 2.
Comparison of hematological indices and lipid profile between SCD patients with low and normal serum HDL-C concentrations (mean [SEM])
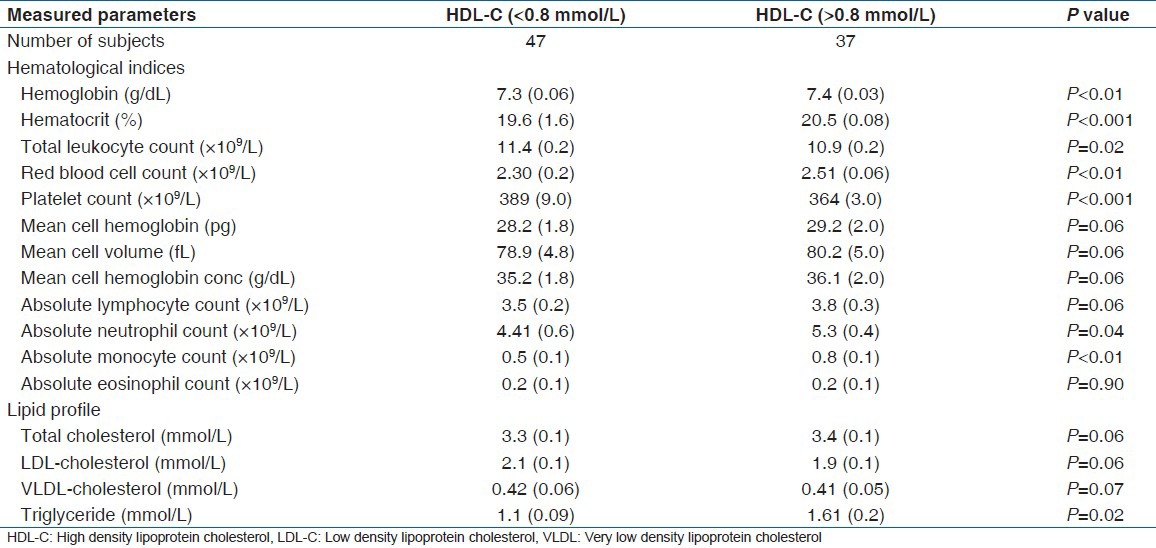
Table 3 shows HDL-c levels based on SCD severity of the study group. patients were grouped into severe and moderate-mild disease based on markers of disease severity, which include age of patients at diagnosis, numbers of hospitalization, numbers and types of crisis, presence or absence of Pneumococcal infection, major organs involvement, and number of times blood transfusion was received. From the data, all the SCD patients were diagnosed at childhood and none had symptoms of organ involvement or Pneumococcal infection. A total of 44 SCD patients were classified as severe while 40 were grouped under moderate-mild disease severity. The severe group had a vaso-occlusive crisis ≥ 5 times/year, 84% (37/44) of the patients had received blood transfusion at least 3 times in their life time while 16% (07/44) had never received blood transfusion. Among the severe group, 70.5% (31/44) of the patients had decreased (<0.8 mmol/L) HDL-c while 29.5% (13/44) had normal (≥0.8 mmol/L) HDL-c levels. On the other hand, the moderate-mild group had a vaso-occlusive crisis less than 4 times in a year and 45% (18/40) of them had received blood transfusion at least 2 times in their life time. Twenty two (55%) had never received blood transfusion, 40% (16/40) had decreased (<0.8 mmol/L) HDL-c and 60% (24/40) had normal (>0.8 mmol/L) HDL-c levels. The record of a number of hospitalizations was not available.
Discussion
The data presented indicated that SCD patients with decreased HDL-c (<0.08 mmol/L) had lower levels of markers of hemolysis (anemia), absolute neutrophil, monocyte count, total cholesterol, and triglyceride while platelet count was significantly higher than those with normal HDL-c levels (≥0.8 mmol/L) as was previously reported.[1,6,9] SCD patients with decreased HDL-c levels presented with higher risk of hemolysis and anemia,[10] which may be related to high consumption of cholesterol due to increased erythropoietic activities of the bone marrow cells during hemolytic crisis. It is proposed that therapeutic intervention aimed at increasing the levels of HDL-c in these patients may improve patients’ outcome.
About 84% (37/44) of our study patients with decreased HDL-c had received a blood transfusion as against 45% (18/40) of those with normal HDL-c levels. This observation agreed with Ohene-Frempong and Steinberg[11] who reported that SCD patients with decreased HDL-c levels are likely to have more blood transfusion than those with normal HDL-c levels. This may be an indication of poor prognosis though, transfusion is a therapeutic regimen used to prevent several clinical symptoms in these patients, repeated transfusions have been shown to increase other long-term complications such as alloimmunization, iron overload, graft versus host disease, infections to mention a few.
Our data indicated that markers of anemia and total cholesterol were significantly lower in SCD patients with decreased HDL-c levels compared to those with normal HDL-c levels. This is also consistent with that of Zorcas et al.[1] who reported that low lipoprotein levels in SCD were associated with severity of anemia while increased triglyceride level was associated with hemolysis, vascular dysfunction, and increased prevalence of pulmonary hypertension.
The decreased levels of HDL-c in SCD may reduce its anti-inflammatory and anti-oxidative functions.[6] The function of HDL-c does not only includes reverse-cholesterol transport, but modulation of inflammation. In healthy individuals, in the absence of systemic oxidative stress and inflammation, HDL is anti-inflammatory.[12,13,14] HDL-c is capable of preventing LDL-c oxidation and inflammatory response induced by LDL-c deposition in the endothelial space where the oxidized LDL-c caused the cells to synthesize and secrete monocyte chemoattractant protein-1; which is a potent inflammatory agent as seen in atherosclerosis.[15] The HDL consists of several particles of different compositions and functions.[12,13] One of such components of HDL is the Lecithin: Cholesterol Acyltransferase; an important enzyme involved in the esterification of cholesterol which had earlier been reported to be low in SCD patients.[14]
The hematological indices in SCD patients with decreased HDL-c are different from those with normal HDL-c levels. In this study, more of the SCD patients with decreased HDL-c had a higher frequency of vaso-occlusive crisis compared to those with normal HDL-c levels. Increase in leukocyte count was also observed in SCD patients with decreased HDL-c compared to those with normal HDL-c, which may be associated with bacterial infection since bacterial infection in SCD patients is a predisposing factor to crisis. Many other complications of SCD such as pain, silent infarction, stroke, and acute chest syndrome are associated with leukocytosis.[16]
The increased platelet counts observed in patients with decreased HDL-c may be due to factors such as auto-splenectomy resulting from recurrent splenic vessels occlusion, which may be predominant in this group compared to those with normal HDL-c levels. In addition, the increased platelet counts observed in these patients with decreased HDL-c may lead to platelet aggregation in areas of stasis during the vaso-occlusive crisis. The Spleen in normal subjects has been shown to retain newly formed platelets for up to 2 days before release. These young and more biologically active platelets show greater responsiveness in platelet function tests. Thus, the increased platelet counts in SCD patients in steady state may reflects the absence of splenic pooling of young active platelets rather than chronic intravascular activation of platelets in the micro-circulation.[15,16] Others also attributed the increase to a negative feedback effect on erythropoietin production in SCD patients due to anemia because erythropoietin has a structural homology with thrombopoietin, even though thrombopoietin has an identity with or similarity to erythropoietin at the N-terminal region.[17]
Conclusion
Decreased HDL-c levels in SCD patients appears to portend severe anemia, higher leukocyte and platelet counts and may predict adverse clinical course and thus, this group of patients may likely benefit from more frequent evaluation. More studies are however, required to ascertain whether this indicates the use chronic transfusion therapy and/or hydroxyurea in order to prevent or delay development of complications.
Footnotes
Source of Support: Nil
Conflict of Interest: None declared.
References
- 1.Zorca S, Freeman L, Hildesheim M, Allen D, Remaley AT, Taylor JG, 6th, et al. Lipid levels in sickle-cell disease associated with haemolytic severity, vascular dysfunction and pulmonary hypertension. Br J Haematol. 2010;149:436–45. doi: 10.1111/j.1365-2141.2010.08109.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Seixas MO, Rocha LC, Carvalho MB, Menezes JF, Lyra IM, Nascimento VM, et al. Levels of high-density lipoprotein cholesterol (HDL-C) among children with steady-state sickle cell disease. Lipids Health Dis. 2010;9:91. doi: 10.1186/1476-511X-9-91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Emokpae MA, Abdu A, Uadia PO, Borodo MM. Lipid profile in sickle cell disease patients with chronic kidney disease. Sahel Med J. 2010;13:20–3. [Google Scholar]
- 4.Emokpae MA, Uadia PO. Association of oxidative stress markers with atherogenic index of plasma in adult sickle cell nephropathy. Anemia 2012. 2012 doi: 10.1155/2012/767501. 767501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Marzouki ZM, Khoja SM. Plasma and red blood cells membrane lipid concentration of sickle cell disease patients. Saudi Med J. 2003;24:376–9. [PubMed] [Google Scholar]
- 6.Choy E, Sattar N. Interpreting lipid levels in the context of high-grade inflammatory states with a focus on rheumatoid arthritis: A challenge to conventional cardiovascular risk actions. Ann Rheum Dis. 2009;68:460–9. doi: 10.1136/ard.2008.101964. [DOI] [PubMed] [Google Scholar]
- 7.Emokpae MA, Uadia PO. Sickle cell disease and renal disease. In: Manisha S, editor. Disease of Renal Parenchyma. Rijeka, Croatia: InTech Publisher; 2012. pp. 113–34. [Google Scholar]
- 8.Friedewald WT, Levy RI, Fredrickson DS. Estimation of the concentration of low-density lipoprotein cholesterol in plasma, without use of the preparative ultracentrifuge. Clin Chem. 1972;18:499–502. [PubMed] [Google Scholar]
- 9.Johnson C, Telen MJ. Adhesion molecules and hydroxyurea in the pathophysiology of sickle cell disease. Haematologica. 2008;93:481–5. doi: 10.3324/haematol.12734. [DOI] [PubMed] [Google Scholar]
- 10.Shores J, Peterson J, VanderJagt D, Glew RH. Reduced cholesterol levels in African-American adults with sickle cell disease. J Natl Med Assoc. 2003;95:813–7. [PMC free article] [PubMed] [Google Scholar]
- 11.Ohene-Frempong K, Steinberg MH. Clinical aspects of sickle cell anaemia in adults and children. In: Steinberg MH, Forget BG, Higgs DR, Nagel RL, editors. Disorders of Hemoglobin: Genetics, in Pathophysiology and Clinical Management. New York: Cambridge University Press; 2001. pp. 611–70. [Google Scholar]
- 12.Miyazaki O, Fukamachi I, Mori A, Hashimoto H, Kawashiri MA, Nohara A, et al. Formation of prebeta1-HDL during lipolysis of triglyceride-rich lipoprotein. Biochem Biophys Res Commun. 2009;379:55–9. doi: 10.1016/j.bbrc.2008.11.146. [DOI] [PubMed] [Google Scholar]
- 13.Emokpae MA, Osadolor HB, Uwumarongie HO. Sex differences in oxidative stress markers and association with proteinuria in sickle cell anaemia patients with proteinuria. J Med Biomed Res. 2011;10:17–22. [Google Scholar]
- 14.Emokpae MA, Uwumarongie OH, Osadolor HB. Sex dimorphism in serum lecithin: Cholesterol acyltransferase and lipoprotein lipase activities in adult sickle cell anaemia patients with proteinuria. Indian J Clin Biochem. 2011;26:57–61. doi: 10.1007/s12291-010-0096-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Navab M, Anantharamaiah GM, Reddy ST, Van Lenten BJ, Fogelman AM. HDL as a biomarker, potential therapeutic target, and therapy. Diabetes. 2009;58:2711–7. doi: 10.2337/db09-0538. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Omoti CE. Haematological values in sickle cell anaemia in steady state and during vaso-occlussive crisis in Benin City, Nigeria. Ann Afr Med. 2005;4:62–7. [Google Scholar]
- 17.Akinbami A, Dosunmu A, Adediran A, Oshinaike O, Adebola P, Arogundade O. Haematological values in homozygous sickle cell disease in steady state and haemoglobin phenotypes AA controls in Lagos, Nigeria. BMC Res Notes. 2012;5:396. doi: 10.1186/1756-0500-5-396. [DOI] [PMC free article] [PubMed] [Google Scholar]


