Abstract
Homeostasis of aqueous humor (AH) outflow and intraocular pressure (IOP) is essential for normal vision. Impaired AH outflow through the trabecular meshwork (TM) and a resultant elevation in IOP are common changes in primary open-angle glaucoma (POAG), which is the most prevalent form of glaucoma. Although elevated IOP has been recognized as a definitive risk factor for POAG and lowering elevated IOP remains a mainstay for glaucoma treatment, little is known about the molecular mechanisms, especially external cues and intracellular pathways, involved in the regulation of AH outflow in both normal and glaucomatous eyes. In addition, despite the recognition that increased resistance to AH outflow via the conventional pathway consisting of TM and Schlemm's canal is the main cause for elevated IOP, there are no clinically approved drugs that target the conventional pathway to lower IOP in glaucoma patients. The aim of this article is to briefly review published work on the importance of bioactive lysophospholipids (eg, lysophosphatidic acid and sphingosine-1-phosphate), their receptors, metabolism, signaling, and role in the regulation of AH outflow via the TM and IOP, and to discuss pharmacological targeting of key proteins in the lysophospholipid signaling pathways to lower IOP in glaucoma patients.
Introduction
Aqueous humor outflow and intraocular pressure in normal and glaucomatous eyes
Glaucoma is the second leading cause of blindness globally, and more than 2.5 million people are affected by glaucoma in the United States alone.1 Glaucoma, if untreated, can lead to irreversible blindness due to optic nerve degeneration and loss of retinal ganglion cells.1,2 Although genetic, age, metabolic, environmental, and ethnic factors are recognized to influence the incidence and onset of glaucoma, a broader and clearer understanding of glaucoma pathobiology has remained elusive.3–5 Primary open-angle glaucoma (POAG), the most prevalent form of glaucoma in the United States, is associated with elevated intraocular pressure (IOP), which is considered a definitive risk factor for POAG.2,6 Importantly, lowering IOP has been shown to delay vision loss in glaucoma patients, and lowering IOP has remained a primary treatment option for glaucoma.2,6–9 Although several different drugs are currently available for lowering IOP, the efficacy of available drugs is not adequate to control elevated IOP to the desired levels in different glaucoma patients.10–12 Therefore, there is an immediate unmet need for novel and targeted therapy to effectively manage elevated IOP and prevent loss of vision in glaucoma patients. To develop novel IOP-lowering treatments, however, it is imperative that we identify the external cues and unravel different intracellular pathways which regulate IOP and understand the molecular basis of increased IOP.
IOP is maintained primarily by a balance between the amounts of aqueous humor (AH) secreted by the ciliary epithelium into the eye anterior chamber (inflow) and its outflow via the pressure-dependent conventional route and non-pressure-dependent uveoscleral route.2,13 It is commonly believed that elevated IOP derives primarily from the increased resistance to AH outflow through the conventional or trabecular pathway consisting of trabecular meshwork (TM), Schlemm's canal (SC), and the juxtacanalicular connective tissue (JCT).13–15 The TM is a unique structure consisting of highly porous beams of collagen covered by endothelial-like cells with extracellular material occupying the spaces between the beams. The JCT region between the TM and SC comprises cells that are embedded in extracellular matrix (ECM). The SC is a continuous endothelial lined canal that drains AH into the collecting channels and aqueous veins.13 Structurally, the conventional AH outflow pathway is considered as having developed to support the maintenance of optimal IOP by regulating resistance to AH outflow, which is required for normal eye shape and vision. Although the causes underlying the development of increased resistance to AH outflow are not completely clear, glaucomatous eyes have been found to exhibit fewer cells in the TM, alterations in ECM organization, and turnover in the JCT region, and accumulate sheath-like plaque material in the outflow pathway.13–18 It is also widely believed that changes such as tissue stiffness due to altered cellular contraction, oxidative damage, and altered metabolic activity of TM tissue are associated with increased resistance to AH outflow and elevated IOP.13,19–23 Little is known, however, about the cellular and molecular mechanisms that drive the increase in resistance to AH outflow and trigger the associated changes in glaucomatous eyes.
In addition to the changes described earlier, it is becoming increasingly evident that AH derived from the glaucoma patients contains elevated levels of transforming growth factor-beta (TGF-β), endothelin-1, connective tissue growth factor (CTGF), myocilin, and several other cytokines.23–28 A great deal of effort has gone into exploring how these different bioactive agents influence TM tissue properties and cell biology in the context of AH outflow in both in vitro and in vivo studies.15,16,24,29–36 These efforts are beginning to unravel the participation of several different intracellular signaling mechanisms, including Rho GTPase, Wnt, ECM/mechanotransduction, integrins, nitric oxide, PKC, BMPs/SMADs, MAP kinases, and others, in regulating contractile properties of TM cells, ECM turnover, adhesive interactions, biomechanical properties, permeability, and survival of outflow pathway tissues and cells.14,24,37–40 These different observations offer significant insights into the regulation of AH outflow and suggest several novel avenues to target selected signaling pathways and other molecular targets for increasing AH outflow through the conventional pathway, and for the development of new and mechanism-based IOP lowering drugs.10,13,40,41 Importantly, since the conventional AH outflow pathway is not only recognized to be the main site for increased resistance to AH drainage but also account for more than 80% of total AH drainage, it is ideal to have drugs targeted to this pathway to lower IOP in glaucoma patients and none are currently available. Although the prostaglandin F2α receptor agonists are widely used to lower elevated IOP and considered the first line of treatment for glaucoma, their efficacy was found to be only moderate with their long-term use.10,11 Therefore, there is an increasing interest and need in developing the TM-targeted and efficacious IOP-lowering drugs. This effort is beginning to show some promising progress, and a number of new and TM-targeted drugs are in human clinical trials, including the Rho kinase inhibitors, nitric oxide modulators, and adenosine agonists.10
As a part of recent efforts to explore and unravel additional cellular mechanisms regulating AH outflow via the TM and SC, we and others have identified that certain bioactive lipids, especially lysophospholipids, influence the TM cell contractile and cell adhesive properties, AH outflow, and IOP, highlighting a significant role for these molecules in both normal and aberrant regulation of outflow dynamics.42–44 In the rest of this review, I will be focusing my discussion primarily on the role and mechanism of action of lysophospholipids [eg, lysophosphatidic acid (LPA) and sphingosine-1-phosphate (S1P)] in AH outflow and IOP, lysophospholipid metabolism, and receptors, and will be addressing the significance of the targeting of certain key molecules in the lysophospholipid signaling pathways to lower IOP and treatment of glaucoma. Importantly, dysregulation of these signaling pathways is implicated in various disease processes and is considered a viable therapeutic target for the treatment of several diseases.45–47 Therefore, I will start with a brief background on lysophospholipids and their physiological and pathological roles in a broader context.
Lysophospholipids: role in physiological and pathological contexts
Lysophospholipids are membrane-derived lysolipids with a broad range of biological activities, and they participate in a myriad of physiological and pathological processes, including development, immunity, smooth muscle contraction, neurite extension, cancer, inflammation, fibrosis, obesity, angiogenesis, and atherosclerosis.45,46,48–60 Lysophospholipids refer to any phospholipids that are missing one of their 2 O-acyl chains. They are divided into 2 major groups: lysoglycerophospholipids and lysosphingolipids with LPA and S1P being prominent representatives of the 2 classes, respectively. LPA and S1P act at the extracellular and intracellular levels, mediating their biological effects via different G-protein-coupled receptors (GPCRs), which are linked to the subunits of Gα12/13, Gq/11 and Gi heterotrimeric G-proteins.45,48,51,60 There are several well-characterized and cognate subtypes of GPCRs for S1P and LPA, including S1P1–5 and LPA1–6, respectively.45,51,57 These different GPCRs belong to either EDG or P2Y receptor clusters and are expressed widely in different tissues and cell types. In addition to these well-characterized GPCRs, there are some additional putative receptors that have been shown to participate in LPA-mediated responses.57 These bioactive lysolipids, which act in an autocrine and paracrine fashion, are generated primarily by erythrocytes and platelets with circulating plasma levels in the nanomolar to micromolar range.45,46,51 The GPCRs for both LPA and S1P have high binding affinity for their respective ligands, typically in the low nanomolar range. Agonist stimulation of these receptors activates and regulates several different intracellular signaling pathways.45,54,60 In addition to the different species of LPA and S1P lysolipids, there are other lysolipids, including lysophosphatidylcholine (LPC), lysophosphatidylserine, lysophosphatidylinositol, lysophospholipidthreonine, lysophosphatidylethanolamine, and sphingosyl phosphorylcholine. The relative roles of these latter lysolipids in intracellular signaling are not as well understood as those of LPA and S1P. In addition, unlike S1P, which is a single molecular type, LPA is a diverse group of molecules consisting of either a saturated or unsaturated fatty acid chain esterified at sn-1 or sn-2 positions of the glycerol moiety.60,61
LPA, a mono-acylglycerol-3-phosphate, is produced largely from lysophosphatidyl choline by autotaxin (ATX)/lysophospholipase D (LysoPLD), a secretory ectonucleotide pyrophosphatase/phosphodiesterase (ENPP2) enzyme.62,63 While LysoPLD-catalyzed production of LPA is predominantly extracellular, it is also produced intracellularly through different mechanisms.46 Phospholipase A1/Phospholipase A2 (PLA2) and phospholipase-D (PLD) enzymes produce LPA by PLD-mediated conversion of phosphatidylcholine to phosphatidic acid and subsequent conversion of phosphatidic acid to LPA by PLA1/2.46,56,62 In addition, Acyl glycerol kinase also produces LPA from monoacyl glycerol.64 LPA can be metabolized rapidly by lipid phosphate phosphatases (LPP) through dephosphorylation. There are 3 integral membrane LPPs that act as ecto-enzymes to dephosphorylate extracellular LPA.65,66 In plasma and serum, LPA exists predominantly in a bound form with albumin and gelsolin.60 Bioactive LPA regulates a wide variety of cellular processes, including cell proliferation, differentiation, migration, transcription, and survival, by activating different signaling pathways that are mediated by Ras, Rho, and Rac, adenylyl cyclase, PLC, PLD, AKT, and PI3 kinases.48,52,56–58,60 By activating these different intracellular molecules via their cognate GPCRs, lysophospholipids regulate multiple and redundant cellular processes.60 LPA has been also shown to mediate its intracellular effects by regulating calcium influx and peroxisome proliferator-activated receptor-gamma nuclear receptor activity.67–69 Dysregulated signaling mediated by LPA, LPA receptors, and ATX has been shown to be involved in the pathobiology of various diseases, including cancer, neurological diseases, fibrosis, inflammation, and atherosclerosis.52,67,70 The definitive roles for LPA, LPA receptors, and ATX have been confirmed by the phenotypes associated with mutations in the respective genes in humans, gene knockout models, and by the use of specific pharmacological inhibitors.46,52,71,72 Moreover, various antagonists and agonists of LPA receptors and ATX inhibitors are being developed to explore their therapeutic potential in targeted treatments for different diseases.52,73,74
Similar to LPA, S1P is also a pleiotropic bioactive lipid mediator that acts extracellularly via specific GPCRs on the cell surface and intracellularly via distinct target sites.45,51 S1P is produced from sphingosine by sphingosine kinase (SK). Sphingosine, in turn, is generated from ceramide degradation by ceramidase. SKs (SK1 and SK2), which phosphorylate sphingosine, are activated by various external factors, including growth factors, cytokines, and agonists.45,51 S1P can be reversibly dephosphorylated by the action of 2 specific sphingosine-1 phosphate phosphatases50,66 and LPP.65,66 In addition, S1P can be hydrolyzed irreversibly by S1P lyase. S1P generated intracellularly can be secreted into the extracellular spaces by the Spinster 2 transporter and ATP-binding cassette transporters.45,51,54,75 S1P acts extracellularly through 5 specific cell surface GPCRs-S1P1–5.45,50,54 Engagement of these receptors is linked to the activation of various intracellular signaling pathways that are mediated by Rho, Rac, Ras, ERK, Wnt, PLC, adenylyl cyclase, AKT, and PI3 kinase.45,50,51,54 In addition, various growth factors activate SKs, and there appears to be some cross-talk and feed-forward relationship between S1P and TGF-β signaling.67 S1P in plasma is bound to high-density lipoprotein (HDL) and low-density lipoprotein (LDL) proteins, and some of the recognized effects of HDL and LDL are actually attributed to the bound S1P.45,54 Many lines of evidence also point to an intracellular or second messenger role for S1P, involving direct interaction with and activation of intracellular targets, including histone deacetylase, tumor necrosis factor receptor-associated factor 2, PKCδ, and amyloid precursor protein cleaving enzyme-1.45,51,54 S1P plays a vital role in various cellular processes, including proliferation, differentiation, migration and survival, cell adhesive interactions, and permeability.45,50,51,54 Dysregulation of S1P synthesis and its signaling mechanisms has been shown to be associated with various diseases, including cancer, inflammation, angiogenesis, immune cell trafficking, cardiac, and pulmonary complications.45,50,54 Interestingly, a sphingolipid receptor modulator (FTY720; Fingolimod/Gilenya) was recently approved for human use as a treatment for relapsed Multiple Sclerosis, representing an exciting advancement that has catalyzed the development and screening for inhibitors and activators of LPA and S1P receptors, and analogs and inhibitors of LPA and S1P for therapeutic use.47,54,76–78 Similar to LPA, the definitive roles of S1P in various physiological and disease processes were derived from gene-targeted mouse models and mutations in humans.45,54 Taken together, lysophospholipids are potent bioactive mediators, and this is an exciting research area for both basic and translational work. Importantly, this area is primed for drug development and for exploring the role(s) of lysophospholipids in the etiology of different diseases.45,51,79
In the context of AH outflow and IOP, both LPA and S1P have been demonstrated to activate Rho and Rac GTPase signaling and to regulate actin cytoskeletal organization, cell adhesive interactions, cell–cell junctions, permeability, and contraction in the cells of AH outflow pathway, including the TM and SC.42,44,80 In addition, enzymes generating these lysolipids were found to influence AH outflow and IOP.43,81 There is also some evidence of dysregulation of LPA production in the AH and optic nerve head of glaucoma patients.43,82,83 In addition, these bioactive lipids have been shown to play a critical role in the expression of ECM and α-smooth muscle actin.84 Therefore, the rest of this discussion will focus on the biological effects of LPA, S1P, ATX, and PLA2, and other lipids in the regulation of AH outflow and IOP in both normal and glaucoma subjects.
Lysophospholipids in regulation of AH outflow and IOP
AH outflow through the TM or conventional route is a predominant drainage pathway, and it is becoming increasingly evident that AH drainage is regulated by various external factors, including growth factors, steroids, and force/pressure.40 Therefore, there is a great deal of interest in identifying and characterizing various external cues involved in modulating AH outflow through the TM. For TM and SC cells, changes in cell contractile properties, mechanotransduction, cell shape, phagocytosis, stiffness, and cell adhesive interactions are some of the characteristics recognized to participate and influence AH outflow.13,40,41 Based on these cellular characteristics, a considerable amount of effort has been invested into identifying the bioactive agents that regulate TM and SC cell contractile properties, actomyosin organization, and cell–ECM and cell–cell interactions in the context of homeostasis of AH outflow and IOP.40,41 In addition to growth factors, steroids, and ECM molecules, various fatty acids, including eicosanoids, prostaglandins, and other lipids such as lysophospholipids, are recognized to influence cell contractile and cell adhesive properties of TM and SC cells.42,44 From this point onward, the discussion will be focused on the known effects of lysophospholipids in TM and SC cells and in AH outflow and IOP.
Lysophosphatidic acid
The presence of LPA in various biological fluids, including AH, has been well documented,85–87 and confirmed by both enzyme-based and high pressure liquid chromatography in conjunction with mass spectrometry.85 In addition to LPA, the presence of its major precursor, LPC has been confirmed in the AH of rabbits.85 Although LPA is expected to be present in human AH, there is little to no information currently on the levels of LPA in human AH.87 There is, however, overwhelming evidence that LPA influences cell adhesive, actin cytoskeletal organization, and contractile properties of TM cells derived from different species, including human and porcine.42,80,84,88 Moreover, both TM cells and TM tissue derived from human eyes have been shown to express various LPA-specific GPCR receptors that are specific to LPA.42 In serum-starved TM and SC cells, LPA has been shown to induce a robust increase in actin stress fibers, focal adhesions (vinculin and paxillin based), and contraction in association with increased myosin light chain (MLC) phosphorylation.42 These effects were found to be mediated predominantly by LPA-induced activation of Rho GTPase signaling.42,84 LPA was one of the earliest biological agents recognized to be a potent activator of Rho GTPase signaling in various endothelial and epithelial cell types.89 In TM cells, LPA also has been shown to increase the expression of various ECM proteins and CTGF.84,90 In addition, LPA has been reported to increase SC cell stiffness and intracellular calcium levels.19,42 Interestingly, myocilin-induced anti or de-adhesive interactions in TM cells have been found to be suppressed in the presence of LPA.91,92 LPA has been demonstrated to increase SC cell permeability barrier activity by following the diffusion of horse radish peroxidase using the transwell cell culture system.80 In human TM cells, expression of one of the GPCR receptors of LPA, LPAR1 has been shown to be regulated by miRNA200c.88 Most significantly, LPA perfusion has been demonstrated to increase resistance to AH outflow in enucleated porcine eyes, confirming a direct influence of LPA on AH outflow, which is expected to influence IOP.42 Based on these consistent observations, it is deemed important to evaluate the in vivo effects of LPA and its receptor activation on IOP in live animals. Most importantly, it is necessary to determine whether deregulation of LPA production or expression of its cell surface receptors is associated with elevated IOP in glaucoma patients. In addition, to gain further insights into the role of LPA in AH outflow, we need to evaluate the effects of antagonists of LPA or direct targeting of LPA receptors on AH outflow and IOP. Several antagonists of LPA and specific inhibitors and antibodies of LPA receptors are being explored for therapeutic importance in the treatment of different medical conditions, including cancer and fibrosis.93 Extracellular LPA is produced predominantly by ATX, a secretory LysoPLD that is ubiquitously expressed.52
Autotaxin/lysophospholipase D
ATX was originally discovered as an autocrine motility factor comprising ∼900 amino acids released by human melanoma cells.94 This functional property was the basis for its name ATX. It is also known as ENPP2. To date, at least 3 splice variants/isoforms (α, β, and γ) of ATX have been identified in both human and mouse.95 ATX is expressed in a number of different tissues, and high levels of ATX protein have been observed in various biological fluids.52 ATX is a secreted glycoprotein with LysoPLD activity that hydrolyzes LPC to produce equimolar amounts of LPA and choline.62,63,96 ATX has also been reported to generate S1P but is not the main source for S1P.96 Aberrant expression and activity of ATX has been shown to be associated with various pathological conditions, including cancer, neuropathic pain, and fibrosis.52,97 It is a multidomain protein consisting of 2 N-terminal somatomedin B-like domains, a central catalytic phosphodiesterase domain, and a C-terminal nuclease-like domain.79,97,98 ATX binds to β1 and β3 integrins, and this binding facilitates the generation of LPA on demand in the proximity of signaling receptors on the cell surface.99–102 A functionally active, non-catalytic domain of ATX mediates the matricellular and anti-adhesive role of this glycoprotein, which possesses heparin-binding properties.103 The crystal structure of ATX has been elucidated, and its catalytic activity domain is well characterized.79,98,100,104 Based on the elevated levels of ATX in various pathological conditions, this protein has been considered a promising therapeutic target for inflammation, fibrosis, and cancer.52,73,97 Importantly, both pharmacological inhibition and gene targeting of ATX have been found to reduce the levels of LPA in plasma and different tissues.62,105,106 Therefore, the development of ATX inhibitors has engendered a great deal of interest from pharmaceutical companies.74,98
Human TM cells express all 3 isoforms of ATX.43 In human AH, ATX was identified as one of the abundant proteins based on quantitative proteomics.43 ATX is also present in AH of different species, including rabbits, rodents, and porcine.43 Importantly, LysoPLD activity of ATX was found to be significantly elevated in the AH of POAG patients.43 This recent observation motivated us to explore the influence of ATX inhibition on IOP and the inhibition of ATX activity using a small molecular inhibitor (S32826), which significantly reduces IOP in a live animal model when presented via either the topical or the intracameral route of delivery.43 Moreover, the suppression of ATX expression in human TM cells by siRNA revealed decreases in actin stress fibers and MLC phosphorylation.43 Collectively, these promising preliminary observations warrant additional and detailed studies on the levels of LPA and LPC, ATX, and its LysoPLD activity in the AH of glaucoma patients in comparison with age-matched controls to explore the possible involvement of dysregulated ATX activity in the pathobiology of increased IOP and glaucoma. Further support for the involvement of ATX in the pathobiology of glaucoma was derived via the noted 10-fold increase in ATX expression in astrocytes derived from the optic nerve head of POAG patients as compared with normal subjects.82 Development and characterization of ATX-specific inhibitors is being actively explored for the treatment of different diseases.74 Therefore, there is an opportunity to test the translational benefit of several of these small molecular inhibitors in lowering IOP and treatment of glaucoma. For proof of concept, in addition to testing the effects of ATX inhibitors on IOP, it is necessary to target the expression of ATX in the AH outflow pathway or TM tissue using ATX siRNA and determining their specific effects on AH outflow.
Phospholipase A2
Phospholipids, which are essential components of cellular membranes, are the major source for free fatty acids such as arachidonic acid and lysophospholipids. Both these products, in turn, influence various cellular processes by acting as second messengers and engaging GPCRs.51,59,60 Phospholipids are characterized by a glycerol backbone to which a polar phosphodiester group is linked at the sn-3 carbon, and 2 fatty acid-derived acyl residues are linked at the sn-1 and sn-2 carbons.61 Lysophospholipids are glycerophospholipids in which one acyl chain is lacking.107,108 The superfamily of PLA2 enzymes is responsible for the hydrolysis of the sn-2 position fatty acid of membrane phosphatidylcholine, resulting in the generation of free fatty acid such as arachidonic acid and LPC.61,108,109 Arachidonic acid generated by PLA2s can be further metabolized by cyclooxygenases, lypooxygenases, and cytochrome 450 enzymes and generate various eicosanoids, which can, in turn, influence various biological processes.108 Similarly, LPC can be converted to LPA by ATX/LysoPLD. Therefore, the PLA2s are expected to play a vital role in regulating various cellular processes and pathological conditions.109 PLA2s are also known to influence membrane homeostasis. Similar to LPA and S1P, inhibitors of PLA2, knockout mouse models, and human gene mutations have demonstrated a definitive role for the lysophospholipids in various physiological and pathological processes.61,108,109
More than 30 different PLA2s have been found to be expressed in mammalian tissues and classified into different subtypes based on their size, distribution, substrate specificity, and calcium requirement, including secretory PLA2, cytosolic PLA2s, and calcium-independent PLA2s.109 The tissues of AH outflow pathway of humans, including the TM, have been shown to express all 3 subtypes of PLA2s based on immunohistochemistry and immunoblot analysis.83 In addition, glaucomatous TM tissue derived from POAG patients has been reported to contain elevated levels of PLA2s, indicating the potential role of PLA2s in TM biology and AH outflow in both normal and glaucomatous eyes.83 Lysosomal PLA2 has been also reported to be present in AH of humans.110 Currently, however, only little is known about the role played by PLA2s in AH outflow and IOP despite the known critical and predominant role of this class of enzymes in producing the eicosanoids and lysophospholipids that are confirmed to modulate AH outflow and IOP.111
Very recently, we not only confirmed the distribution pattern of 2 isoforms of calcium-independent phospholipaseA2 (iPLA2β and iPLA2γ) in human TM and cultured TM cells, but also established their role in regulation of AH outflow facility.81 Using isoform-specific small molecular inhibitors of iPLA2 (S-Bromoenol lactone) and (R-Bromoenol lactone), it has been demonstrated that iPLA2γ plays a significant role in the regulation of TM cell contractile properties, cell shape, and cell adhesive interactions by controlling MLC phosphorylation.81 These changes appear to be mediated by the signaling activities of Rho GTPase, PKC, and arachidonic acid in TM cells.81 Importantly, the inhibition of iPLA2γ but not iPLA2β appears to suppress Rho GTPase activation and decrease the levels of total arachidonic acid in TM cells.81 Significantly, the inhibition of iPLA2γ in perfused enucleated porcine eyes led to an increased AH outflow facility in association with increased TM relaxation.81 These observations confirm the importance of iPLA2γ in homeostasis of AH outflow. Therefore, further mechanistic studies exploring whether iPLA2γ-mediated effects on AH outflow and contractile properties are predominantly controlled by changes in arachidonic acid or levels of LPA or both would be insightful for understanding the importance of iPLA2 in homeostasis of IOP. In addition, whether changes in iPLA2 expression in the outflow pathway are associated with elevated IOP are necessary to glean information regarding a potential role for iPLA2 in the pathobiology of elevated IOP and glaucoma. Of course, further studies targeting these and other subtypes of PLA2s and exploring their therapeutic potential in lowering IOP might also offer novel insights into new treatment options for elevated IOP in glaucoma patients.
Sphingosine-1-phosphate
S1P is a potent bioactive lipid that is known to regulate various cellular processes, including proliferation, differentiation, survival, and migration. It has been shown to play a vital role in vascular development, permeability, angiogenesis, and immune cell trafficking and is involved in cancer, autoimmune and vascular problems.45,50,51 Significantly, dysregulation of S1P levels is associated with various diseases.45,51 Both TM and SC cells have been shown to express different S1P-specific receptors.42,112 TM and SC cells treated with S1P have been demonstrated to undergo changes in actin cytoskeletal organization, stimulation of Rho and Rac GTPase activation, increase permeability barrier, cell adhesive interactions (both focal adhesions and adherens junctions), and MLC phosphorylation.42,113 Importantly, S1P has been shown to influence AH outflow facility, and perfusion with S1P has been shown to decrease outflow facility in different species.42,112,114 Interestingly, an antagonist of S1P2 receptor but not S1P1 or S1P3 receptors was reported to suppress S1P-induced decrease in AH outflow facility in human eyes.44 These different observations offer definitive evidence for the importance of S1P in the regulation of AH outflow and IOP. However, little is known about S1P levels, expression profiles of S1P kinases, S1P phosphatases, and receptors in the glaucomatous human eye. Most significantly, pharmacological manipulation of S1P signaling has been shown to have therapeutic importance in the treatment of multiple sclerosis, offering possibilities for their utility in the treatment of other diseases, including glaucoma.47,51
Lipidomics
Based on existing data on lysophospholipids and their role in AH outflow facility and IOP, it is reasonable to speculate that alterations in lysophospholipid biology in both AH and the tissues of outflow pathway might be linked to altered IOP in glaucoma.43 At present, we have very limited information about the different species of phospholipids and lysophospholipids present in AH and TM tissue and their alteration in glaucoma. Only, very recently, lipidomic analyses have been undertaken to examine the distribution profile of different phospholipids, ceramides, and glycosphingolipids in AH and TM tissue from both normal and glaucomatous eyes by mass spectrometric methods.115–117 Importantly, these emerging analyses have started to unravel the presence of several distinct and common phospholipids, ceramides, and glycosphingolipids in normal and glaucoma specimens.115–117 In addition, the levels of both phospholipids and glycosphingolipids were reported to be decreased in the glaucomatous AH and TM, indicating their potential role in the pathophysiology of glaucoma and elevated IOP.115,116 Therefore, an additional and comprehensive lipidomics analysis of various bioactive lipids in glaucoma and normal human subjects of both AH and TM tissue might provide significant insights into their role in elevated IOP and glaucoma pathobiology.
Concluding Remarks
Different species of LPA and S1P and other lysophospholipids such as LPC are considered important bioactive factors that are involved in the regulation of various cellular activities. In addition, there is overwhelming evidence in support of their role in several physiological and pathological processes, and the value of key proteins involved in lysophospholipid metabolism and signal transduction as drug targets is being actively explored for the treatment of different diseases. We have strong experimental evidence in support of the involvement of both LPA and S1P, their receptors, and metabolic enzymes in regulating AH outflow via the conventional pathway and in modulation of IOP (Fig. 1). However, we have very limited information on the regulation status of lysophospholipid production and metabolism in TM and SC cells and in the AH outflow pathway in the context of homeostasis of AH outflow resistance and IOP. Therefore, further studies that explore and understand the potential role of LPA and S1P, their receptors, their production and metabolism, and their regulation in glaucomatous specimens in comparison to age-matched human subjects are expected to unravel novel insights into their involvement in both homeostasis of IOP and pathobiology of glaucoma. This knowledge is also expected to offer novel insights into drug targeting of the LPA and S1P biology to lower IOP and treatment of glaucoma. Specific antagonists and analogs of LPA and S1P, receptor antagonists, and inhibitors of enzymes (ATX, SK and PLA2s) that produce these lipid growth factors are being developed and screened currently by various pharmaceutical companies to explore their therapeutic significance for the treatment of different diseases, including cancer, inflammation, autoimmune diseases, and fibrosis. Encouragingly, this is an area of promise as evidenced by the recent approval of drugs (Fingolimod, an analog of S1P) targeting the S1P receptor for the clinical treatment of Multiple Sclerosis. A number of new drug compounds, targeting the LPA receptors and ATX, are also in clinical trials for the treatment of cancer and fibrosis. Therefore, additional studies focused on testing the drug targets and inhibitors of LPA, S1P, ATX, and PLA2, along with lipidomics of TM tissue and AH derived from both normal and glaucomatous eyes, are expected to uncover new targets and drugs for the treatment of glaucoma and elevated IOP, and to identify changes in lipid metabolites and understand their role in increased IOP and glaucoma pathobiology. Overall, this emerging area of bioactive lipid growth factors appears to be worthy of further exploratory efforts toward understanding the role of lysosphingolipids in the homeostasis of IOP, AH outflow resistance, and elevated IOP in glaucoma and serves as fertile ground for the discovery of novel drug targets to lower IOP in glaucoma patients.
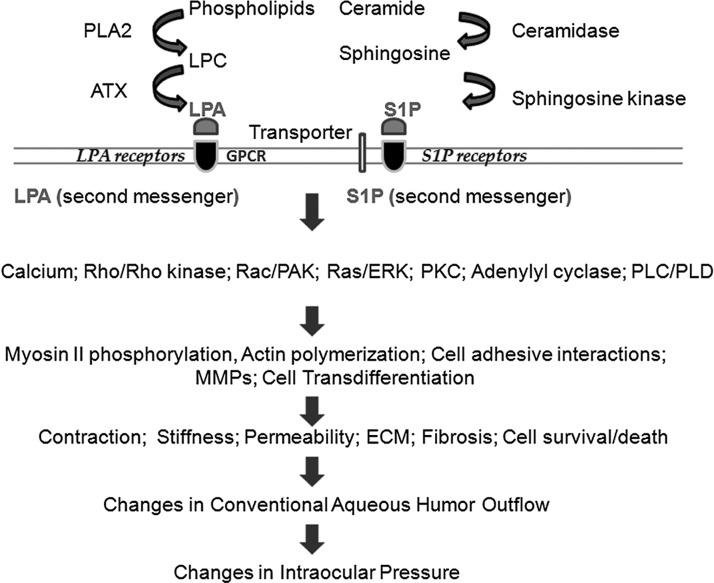
FIG. 1.
Schematic illustration of generation of lysophosphatidic acid (LPA) and sphingosine-1-phosphate (S1P) and the role of their signaling pathways in the regulation of aqueous humor (AH) outflow and intraocular pressure (IOP). LPA is generated largely extracellularly by autotaxin (ATX) by converting lysophosphatidylcholine (LPC) to LPA generated by different phospholipase A2s (PLA2s). In contrast to LPA, S1P is generated predominantly inside the cell by the conversion of sphingosine to S1P by sphingosine kinase. Sphingosine is generated from ceramide by ceramidase. S1P can be transported to extracellular spaces through the ABC transporters and spinster 2. Both LPA and S1P act extracellularly through specific cell surface G-protein coupled receptors (GPCRs) and activate a wide variety of intracellular signaling pathways, including Rho/Rho kinase, Rac/p21-activated kinase (PAK), Ras/extracellular-receptor kinase (ERK), protein kinase (PKC), phospholipase C (PLC), and phospholipase D (PLD). LPA can be also produced intracellularly, and inside the cell, both LPA and S1P are known to serve as second messengers acting through different targets. The GPCR-stimulated signaling events, in turn, influence different cellular processes such as contraction, migration, fibrogenic activity, permeability, cell adhesion, transdifferentiation, and others. These processes in trabecular meshwork and Schlemm's canal cells, in turn, appear to modulate AH outflow and subsequently, IOP.
Acknowledgments
The authors thank all the individuals from their laboratory who contributed to this area of research. Their research on lipid growth factors of trabecular meshwork and outflow pathway was supported by the NIH funding (R01EY018590).
Author Disclosure Statement
No competing financial interests exist.
References
- 1.Quigley H.A., and Broman A.T.The number of people with glaucoma worldwide in 2010 and 2020. Br. J. Ophthalmol. 90:262–267, 2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Weinreb R.N., and Khaw P.T.Primary open-angle glaucoma. Lancet 363:1711–1720, 2004 [DOI] [PubMed] [Google Scholar]
- 3.Leske M.C.Open-angle glaucoma—an epidemiologic overview. Ophthalmic Epidemiol. 14:166–172, 2007 [DOI] [PubMed] [Google Scholar]
- 4.Leske M.C., Wu S.Y., Hennis A., Honkanen R., and Nemesure B.Risk factors for incident open-angle glaucoma: the Barbados Eye Studies. Ophthalmology 115:85–93, 2008 [DOI] [PubMed] [Google Scholar]
- 5.Coleman A.L., and Miglior S.Risk factors for glaucoma onset and progression. Surv. Ophthalmol. 53Suppl 1:S3–S10, 2008 [DOI] [PubMed] [Google Scholar]
- 6.Kwon Y.H., Fingert J.H., Kuehn M.H., and Alward W.L.Primary open-angle glaucoma. N. Engl. J. Med. 360:1113–1124, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Aptel F., Chiquet C., and Romanet J.P.Intraocular pressure-lowering combination therapies with prostaglandin analogues. Drugs 72:1355–1371, 2012 [DOI] [PubMed] [Google Scholar]
- 8.De Moraes C.G., Demirel S., Gardiner S.K., et al. . Effect of treatment on the rate of visual field change in the ocular hypertension treatment study observation group. Invest. Ophthalmol. Vis. Sci. 53:1704–1709, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Higginbotham E.J., Gordon M.O., Beiser J.A., et al. . The Ocular Hypertension Treatment Study: topical medication delays or prevents primary open-angle glaucoma in African American individuals. Arch. Ophthalmol. 122:813–820, 2004 [DOI] [PubMed] [Google Scholar]
- 10.Lee A.J., and Goldberg I.Emerging drugs for ocular hypertension. Expert Opin. Emerg. Drugs 16:137–161, 2011 [DOI] [PubMed] [Google Scholar]
- 11.Toris C.B.Pharmacotherapies for glaucoma. Curr. Mol. Med. 10:824–840, 2010 [DOI] [PubMed] [Google Scholar]
- 12.Bagnis A., Papadia M., Scotto R., and Traverso C.E.Current and emerging medical therapies in the treatment of glaucoma. Expert Opin. Emerg. Drugs 16:293–307, 2011 [DOI] [PubMed] [Google Scholar]
- 13.Gabelt B.T., and Kaufman P.L.Changes in aqueous humor dynamics with age and glaucoma. Prog. Retin. Eye Res. 24:612–637, 2005 [DOI] [PubMed] [Google Scholar]
- 14.Keller K.E., Aga M., Bradley J.M., Kelley M.J., and Acott T.S.Extracellular matrix turnover and outflow resistance. Exp. Eye Res. 88:676–682, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Tamm E.R., and Fuchshofer R.What increases outflow resistance in primary open-angle glaucoma? Surv. Ophthalmol. 52Suppl 2:S101–S104, 2007 [DOI] [PubMed] [Google Scholar]
- 16.Lutjen-Drecoll E.Morphological changes in glaucomatous eyes and the role of TGFbeta2 for the pathogenesis of the disease. Exp. Eye Res. 81:1–4, 2005 [DOI] [PubMed] [Google Scholar]
- 17.Alvarado J., Murphy C., and Juster R.Trabecular meshwork cellularity in primary open-angle glaucoma and nonglaucomatous normals. Ophthalmology 91:564–579, 1984 [DOI] [PubMed] [Google Scholar]
- 18.Tektas O.Y., and Lutjen-Drecoll E.Structural changes of the trabecular meshwork in different kinds of glaucoma. Exp. Eye Res. 88:769–775, 2009 [DOI] [PubMed] [Google Scholar]
- 19.Zhou E.H., Krishnan R., Stamer W.D., et al. . Mechanical responsiveness of the endothelial cell of Schlemm's canal: scope, variability and its potential role in controlling aqueous humour outflow. J. R. Soc. Interface 9:1144–1155, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Russell P., and Johnson M.Elastic modulus determination of normal and glaucomatous human trabecular meshwork. Invest. Ophthalmol. Vis. Sci. 53:117, 2012 [DOI] [PubMed] [Google Scholar]
- 21.Liton P.B., Gonzalez P., and Epstein D.L.The role of proteolytic cellular systems in trabecular meshwork homeostasis. Exp. Eye Res. 88:724–728, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Izzotti A., Longobardi M., Cartiglia C., and Sacca S.C.Mitochondrial damage in the trabecular meshwork occurs only in primary open-angle glaucoma and in pseudoexfoliative glaucoma. PLoS One 6:e14567, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Wang N., Chintala S.K., Fini M.E., and Schuman J.S.Activation of a tissue-specific stress response in the aqueous outflow pathway of the eye defines the glaucoma disease phenotype. Nat. Med. 7:304–309, 2001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Fuchshofer R., and Tamm E.R.The role of TGF-beta in the pathogenesis of primary open-angle glaucoma. Cell Tissue Res. 347:279–290, 2012 [DOI] [PubMed] [Google Scholar]
- 25.Tripathi R.C., Li J., Chan W.F., and Tripathi B.J.Aqueous humor in glaucomatous eyes contains an increased level of TGF-beta 2. Exp. Eye Res. 59:723–727, 1994 [DOI] [PubMed] [Google Scholar]
- 26.Browne J.G., Ho S.L., Kane R., et al. . Connective tissue growth factor is increased in pseudoexfoliation glaucoma. Invest. Ophthalmol. Vis. Sci. 52:3660–3666, 2011 [DOI] [PubMed] [Google Scholar]
- 27.Noske W., Hensen J., and Wiederholt M.Endothelin-like immunoreactivity in aqueous humor of patients with primary open-angle glaucoma and cataract. Graefes Arch. Clin. Exp. Ophthalmol. 235:551–552, 1997 [DOI] [PubMed] [Google Scholar]
- 28.Howell K.G., Vrabel A.M., Chowdhury U.R., Stamer W.D., and Fautsch M.P.Myocilin levels in primary open-angle glaucoma and pseudoexfoliation glaucoma human aqueous humor. J. Glaucoma 19:569–575, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Iyer P., Maddala R., Pattabiraman P.P., and Rao P.V.Connective tissue growth factor-mediated upregulation of neuromedin U expression in trabecular meshwork cells and its role in homeostasis of aqueous humor outflow. Invest. Ophthalmol. Vis. Sci. 53:4952–4962, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Junglas B., Kuespert S., Seleem A.A., et al. . Connective tissue growth factor causes glaucoma by modifying the actin cytoskeleton of the trabecular meshwork. Am. J. Pathol. 180:2386–2403, 2012 [DOI] [PubMed] [Google Scholar]
- 31.Sethi A., Jain A., Zode G.S., Wordinger R.J., and Clark A.F.Role of TGFbeta/Smad signaling in gremlin induction of human trabecular meshwork extracellular matrix proteins. Invest. Ophthalmol. Vis. Sci. 52:5251–5259, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Fleenor D.L., Shepard A.R., Hellberg P.E., Jacobson N., Pang I.H., and Clark A.F.TGFbeta2-induced changes in human trabecular meshwork: implications for intraocular pressure. Invest. Ophthalmol. Vis. Sci. 47:226–234, 2006 [DOI] [PubMed] [Google Scholar]
- 33.Fitzgerald A.M., Benz C., Clark A.F., and Wordinger R.J.The effects of transforming growth factor-beta2 on the expression of follistatin and activin A in normal and glaucomatous human trabecular meshwork cells and tissues. Invest. Ophthalmol. Vis. Sci. 53:7358–7369, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Rao P.V., Deng P., Sasaki Y., and Epstein D.L.Regulation of myosin light chain phosphorylation in the trabecular meshwork: role in aqueous humour outflow facility. Exp. Eye Res. 80:197–206, 2005 [DOI] [PubMed] [Google Scholar]
- 35.Renieri G., Choritz L., Rosenthal R., Meissner S., Pfeiffer N., and Thieme H.Effects of endothelin-1 on calcium-independent contraction of bovine trabecular meshwork. Graefes Arch. Clin. Exp. Ophthalmol. 246:1107–1115, 2008 [DOI] [PubMed] [Google Scholar]
- 36.Zhang X., Clark A.F., and Yorio T.Interactions of endothelin-1 with dexamethasone in primary cultured human trabecular meshwork cells. Invest. Ophthalmol. Vis. Sci. 44:5301–5308, 2003 [DOI] [PubMed] [Google Scholar]
- 37.Rao P.V., Deng P.F., Kumar J., and Epstein D.L.Modulation of aqueous humor outflow facility by the Rho kinase-specific inhibitor Y-27632. Invest. Ophthalmol. Vis. Sci. 42:1029–1037, 2001 [PubMed] [Google Scholar]
- 38.Wang W.H., McNatt L.G., Pang I.H., et al. . Increased expression of the WNT antagonist sFRP-1 in glaucoma elevates intraocular pressure. J. Clin. Invest. 118:1056–1064, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Zhang M., Maddala R., and Rao P.V.Novel molecular insights into RhoA GTPase-induced resistance to aqueous humor outflow through the trabecular meshwork. Am. J. Physiol. Cell Physiol. 295:C1057–C1070, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Stamer W.D., and Acott T.S.Current understanding of conventional outflow dysfunction in glaucoma. Curr. Opin. Ophthalmol. 23:135–143, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Rao V.P., and Epstein D.L.Rho GTPase/Rho kinase inhibition as a novel target for the treatment of glaucoma. BioDrugs 21:167–177, 2007 [DOI] [PubMed] [Google Scholar]
- 42.Mettu P.S., Deng P.F., Misra U.K., Gawdi G., Epstein D.L., and Rao P.V.Role of lysophospholipid growth factors in the modulation of aqueous humor outflow facility. Invest. Ophthalmol. Vis. Sci. 45:2263–2271, 2004 [DOI] [PubMed] [Google Scholar]
- 43.Iyer P., Lalane R., 3rd, Morris C., Challa P., Vann R., and Rao P.V.Autotaxin-lysophosphatidic acid axis is a novel molecular target for lowering intraocular pressure. PLoS One 7:e42627, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Sumida G.M., and Stamer W.D.S1P(2) receptor regulation of sphingosine-1-phosphate effects on conventional outflow physiology. Am. J. Physiol. Cell Physiol. 300:C1164–C1171, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Hla T., and Dannenberg A.J.Sphingolipid signaling in metabolic disorders. Cell Metab. 16:420–434, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.van Meeteren L.A., and Moolenaar W.H.Regulation and biological activities of the autotaxin-LPA axis. Prog. Lipid Res. 46:145–160, 2007 [DOI] [PubMed] [Google Scholar]
- 47.Pyne S., and Pyne N.J.Translational aspects of sphingosine 1-phosphate biology. Trends Mol. Med. 17:463–472, 2011 [DOI] [PubMed] [Google Scholar]
- 48.Xiang S.Y., Dusaban S.S., and Brown J.H.Lysophospholipid receptor activation of RhoA and lipid signaling pathways. Biochim. Biophys. Acta 1831:213–222, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Takuwa Y., Okamoto Y., Yoshioka K., and Takuwa N.Sphingosine-1-phosphate signaling in physiology and diseases. Biofactors 38:329–337, 2012 [DOI] [PubMed] [Google Scholar]
- 50.Pyne N.J., Tonelli F., Lim K.G., Long J.S., Edwards J., and Pyne S.Sphingosine 1-phosphate signalling in cancer. Biochem. Soc. Trans. 40:94–100, 2012 [DOI] [PubMed] [Google Scholar]
- 51.Maceyka M., Harikumar K.B., Milstien S., and Spiegel S.Sphingosine-1-phosphate signaling and its role in disease. Trends Cell Biol. 22:50–60, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Houben A.J., and Moolenaar W.H.Autotaxin and LPA receptor signaling in cancer. Cancer Metastasis Rev. 30:557–565, 2011 [DOI] [PubMed] [Google Scholar]
- 53.Murakami M., Taketomi Y., Sato H., and Yamamoto K.Secreted phospholipase A2 revisited. J. Biochem. 150:233–255, 2011 [DOI] [PubMed] [Google Scholar]
- 54.Spiegel S., and Milstien S.The outs and the ins of sphingosine-1-phosphate in immunity. Nat. Rev. Immunol. 11:403–415, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Chi H.Sphingosine-1-phosphate and immune regulation: trafficking and beyond. Trends Pharmacol. Sci. 32:16–24, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Okudaira S., Yukiura H., and Aoki J.Biological roles of lysophosphatidic acid signaling through its production by autotaxin. Biochimie 92:698–706, 2010 [DOI] [PubMed] [Google Scholar]
- 57.Choi J.W., Herr D.R., Noguchi K., et al. . LPA receptors: subtypes and biological actions. Annu. Rev. Pharmacol. Toxicol. 50:157–186, 2010 [DOI] [PubMed] [Google Scholar]
- 58.Smyth S.S., Cheng H.Y., Miriyala S., Panchatcharam M., and Morris A.J.Roles of lysophosphatidic acid in cardiovascular physiology and disease. Biochim. Biophys. Acta 1781:563–570, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Pyne N.J., and Pyne S.Sphingosine 1-phosphate, lysophosphatidic acid and growth factor signaling and termination. Biochim. Biophys. Acta 1781:467–476, 2008 [DOI] [PubMed] [Google Scholar]
- 60.Moolenaar W.H., van Meeteren L.A., and Giepmans B.N.The ins and outs of lysophosphatidic acid signaling. Bioessays 26:870–881, 2004 [DOI] [PubMed] [Google Scholar]
- 61.Murakami M., and Lambeau G.Emerging roles of secreted phospholipase A(2) enzymes: an update. Biochimie 95:43–50, 2013 [DOI] [PubMed] [Google Scholar]
- 62.Umezu-Goto M., Kishi Y., Taira A., et al. . Autotaxin has lysophospholipase D activity leading to tumor cell growth and motility by lysophosphatidic acid production. J. Cell Biol. 158:227–233, 2002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Tokumura A., Majima E., Kariya Y., et al. . Identification of human plasma lysophospholipase D, a lysophosphatidic acid-producing enzyme, as autotaxin, a multifunctional phosphodiesterase. J. Biol. Chem. 277:39436–39442, 2002 [DOI] [PubMed] [Google Scholar]
- 64.Bektas M., Payne S.G., Liu H., Goparaju S., Milstien S., and Spiegel S.A novel acylglycerol kinase that produces lysophosphatidic acid modulates cross talk with EGFR in prostate cancer cells. J. Cell Biol. 169:801–811, 2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Pyne S., Long J.S., Ktistakis N.T., and Pyne N.J.Lipid phosphate phosphatases and lipid phosphate signalling. Biochem. Soc. Trans. 33:1370–1374, 2005 [DOI] [PubMed] [Google Scholar]
- 66.Sciorra V.A., and Morris A.J.Roles for lipid phosphate phosphatases in regulation of cellular signaling. Biochim. Biophys. Acta 1582:45–51, 2002 [DOI] [PubMed] [Google Scholar]
- 67.Watterson K.R., Lanning D.A., Diegelmann R.F., and Spiegel S.Regulation of fibroblast functions by lysophospholipid mediators: potential roles in wound healing. Wound Repair Regen. 15:607–616, 2007 [DOI] [PubMed] [Google Scholar]
- 68.Zhang C., Baker D.L., Yasuda S., et al. . Lysophosphatidic acid induces neointima formation through PPARgamma activation. J. Exp. Med. 199:763–774, 2004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.McIntyre T.M., Pontsler A.V., Silva A.R., et al. . Identification of an intracellular receptor for lysophosphatidic acid (LPA): LPA is a transcellular PPARgamma agonist. Proc. Natl. Acad. Sci. U. S. A. 100:131–136, 2003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Shea B.S., and Tager A.M.Role of the lysophospholipid mediators lysophosphatidic acid and sphingosine 1-phosphate in lung fibrosis. Proc. Am. Thorac. Soc. 9:102–110, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Okabe K., Hayashi M., Fujii M., et al. . Mutations of lysophosphatidic acid receptor genes in human osteosarcoma cells. Pathobiology 77:278–282, 2010 [DOI] [PubMed] [Google Scholar]
- 72.Pasternack S.M., von Kugelgen I., Al Aboud K., et al. . G protein-coupled receptor P2Y5 and its ligand LPA are involved in maintenance of human hair growth. Nat. Genet. 40:329–334, 2008 [DOI] [PubMed] [Google Scholar]
- 73.Tager A.M.Autotaxin emerges as a therapeutic target for idiopathic pulmonary fibrosis: limiting fibrosis by limiting lysophosphatidic acid synthesis. Am. J. Respir. Cell Mol. Biol. 47:563–565, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Albers H.M., and Ovaa H.Chemical evolution of autotaxin inhibitors. Chem. Rev. 112:2593–2603, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Nishi T., Kobayashi N., Hisano Y., Kawahara A., and Yamaguchi A.Molecular and physiological functions of sphingosine 1-phosphate transporters. Biochim. Biophys. Acta pii: , 2013 [DOI] [PubMed] [Google Scholar]
- 76.Orr Gandy K.A., and Obeid L.M.Targeting the sphingosine kinase/sphingosine 1-phosphate pathway in disease: review of sphingosine kinase inhibitors. Biochim. Biophys. Acta 1831:157–166, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Brinkmann V.FTY720 (fingolimod) in multiple sclerosis: therapeutic effects in the immune and the central nervous system. Br. J. Pharmacol. 158:1173–1182, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Edmonds Y., Milstien S., and Spiegel S.Development of small-molecule inhibitors of sphingosine-1-phosphate signaling. Pharmacol. Ther. 132:352–360, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Hausmann J., Perrakis A., and Moolenaar W.H.Structure-function relationships of autotaxin, a secreted lysophospholipase D. Adv. Biol. Regul. 53:112–117, 2013 [DOI] [PubMed] [Google Scholar]
- 80.Kumar J., and Epstein D.L.Rho GTPase-mediated cytoskeletal organization in Schlemm's canal cells play a critical role in the regulation of aqueous humor outflow facility. J. Cell Biochem. 112:600–606, 2011 [DOI] [PubMed] [Google Scholar]
- 81.Pattabiraman P.P., Lih F.B., Tomer K.B., and Rao P.V.The role of calcium-independent phospholipase A2gamma in modulation of aqueous humor drainage and Ca2+ sensitization of trabecular meshwork contraction. Am. J. Physiol. Cell. Physiol. 302:C979–C991, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Hernandez M.R., Agapova O.A., Yang P., Salvador-Silva M., Ricard C.S., and Aoi S.Differential gene expression in astrocytes from human normal and glaucomatous optic nerve head analyzed by cDNA microarray. Glia 38:45–64, 2002 [DOI] [PubMed] [Google Scholar]
- 83.Ronkko S., Rekonen P., Kaarniranta K., Puustjarvi T., Terasvirta M., and Uusitalo H.Phospholipase A2 in chamber angle of normal eyes and patients with primary open angle glaucoma and exfoliation glaucoma. Mol. Vis. 13:408–417, 2007 [PMC free article] [PubMed] [Google Scholar]
- 84.Pattabiraman P.P., and Rao P.V.Mechanistic basis of Rho GTPase-induced extracellular matrix synthesis in trabecular meshwork cells. Am. J. Physiol. Cell Physiol. 298:C749–C763, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Tokumura A., Taira S., Kikuchi M., Tsutsumi T., Shimizu Y., and Watsky M.A.Lysophospholipids and lysophospholipase D in rabbit aqueous humor following corneal injury. Prostaglandins Other Lipid Mediat. 97:83–89, 2012 [DOI] [PubMed] [Google Scholar]
- 86.Liliom K., Guan Z., Tseng J.L., Desiderio D.M., Tigyi G., and Watsky M.A.Growth factor-like phospholipids generated after corneal injury. Am. J. Physiol. 274:C1065–C1074, 1998 [DOI] [PubMed] [Google Scholar]
- 87.Pages C., Simon M., Valet P., and Saulnier-Blache J.S.Lysophosphatidic acid synthesis and release(1). Prostaglandins 64:1–10, 2001 [DOI] [PubMed] [Google Scholar]
- 88.Luna C., Li G., Huang J., et al. . Regulation of trabecular meshwork cell contraction and intraocular pressure by miR-200c. PLoS One 7:e51688, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Ridley A.J., and Hall A.The small GTP-binding protein rho regulates the assembly of focal adhesions and actin stress fibers in response to growth factors. Cell 70:389–399, 1992 [DOI] [PubMed] [Google Scholar]
- 90.Chudgar S.M., Deng P., Maddala R., Epstein D.L., and Rao P.V.Regulation of connective tissue growth factor expression in the aqueous humor outflow pathway. Mol. Vis. 12:1117–1126, 2006 [PubMed] [Google Scholar]
- 91.Peters D.M., Herbert K., Biddick B., and Peterson J.A.Myocilin binding to Hep II domain of fibronectin inhibits cell spreading and incorporation of paxillin into focal adhesions. Exp. Cell Res. 303:218–228, 2005 [DOI] [PubMed] [Google Scholar]
- 92.Shen X., Koga T., Park B.C., SundarRaj N., and Yue B.Y.Rho GTPase and cAMP/protein kinase A signaling mediates myocilin-induced alterations in cultured human trabecular meshwork cells. J. Biol. Chem. 283:603–612, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Pyne N.J., Dubois G., and Pyne S.Role of sphingosine 1-phosphate and lysophosphatidic acid in fibrosis. Biochim. Biophys. Acta 1831:228–238, 2013 [DOI] [PubMed] [Google Scholar]
- 94.Stracke M.L., Krutzsch H.C., Unsworth E.J., et al. . Identification, purification, and partial sequence analysis of autotaxin, a novel motility-stimulating protein. J. Biol. Chem. 267:2524–2529, 1992 [PubMed] [Google Scholar]
- 95.Giganti A., Rodriguez M., Fould B., et al. . Murine and human autotaxin alpha, beta, and gamma isoforms: gene organization, tissue distribution, and biochemical characterization. J. Biol. Chem. 283:7776–7789, 2008 [DOI] [PubMed] [Google Scholar]
- 96.Clair T., Aoki J., Koh E., et al. . Autotaxin hydrolyzes sphingosylphosphorylcholine to produce the regulator of migration, sphingosine-1-phosphate. Cancer Res. 63:5446–5453, 2003 [PubMed] [Google Scholar]
- 97.Oikonomou N., Mouratis M.A., Tzouvelekis A., et al. . Pulmonary autotaxin expression contributes to the pathogenesis of pulmonary fibrosis. Am. J. Respir. Cell Mol. Biol. 47:566–574, 2012 [DOI] [PubMed] [Google Scholar]
- 98.Nishimasu H., Ishitani R., Aoki J., and Nureki O.A 3D view of autotaxin. Trends Pharmacol. Sci. 33:138–145, 2012 [DOI] [PubMed] [Google Scholar]
- 99.Moolenaar W.H., and Perrakis A.Insights into autotaxin: how to produce and present a lipid mediator. Nat. Rev. Mol. Cell Biol. 12:674–679, 2011 [DOI] [PubMed] [Google Scholar]
- 100.Hausmann J., Kamtekar S., Christodoulou E., et al. . Structural basis of substrate discrimination and integrin binding by autotaxin. Nat. Struct. Mol. Biol. 18:198–204, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Tabchy A., Tigyi G., and Mills G.B.Location, location, location: a crystal-clear view of autotaxin saturating LPA receptors. Nat. Struct. Mol. Biol. 18:117–118, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Fulkerson Z., Wu T., Sunkara M., Kooi C.V., Morris A.J., and Smyth S.S.Binding of autotaxin to integrins localizes lysophosphatidic acid production to platelets and mammalian cells. J. Biol. Chem. 286:34654–34663, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Yuelling L.M., and Fuss B.Autotaxin (ATX): a multi-functional and multi-modular protein possessing enzymatic lysoPLD activity and matricellular properties. Biochim. Biophys. Acta 1781:525–530, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Nishimasu H., Okudaira S., Hama K., et al. . Crystal structure of autotaxin and insight into GPCR activation by lipid mediators. Nat. Struct. Mol. Biol. 18:205–212, 2011 [DOI] [PubMed] [Google Scholar]
- 105.Albers H.M., Dong A., van Meeteren L.A., et al. . Boronic acid-based inhibitor of autotaxin reveals rapid turnover of LPA in the circulation. Proc. Natl. Acad. Sci. U. S. A. 107:7257–7262, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Tanaka M., Okudaira S., Kishi Y., et al. . Autotaxin stabilizes blood vessels and is required for embryonic vasculature by producing lysophosphatidic acid. J. Biol. Chem. 281:25822–25830, 2006 [DOI] [PubMed] [Google Scholar]
- 107.Sevastou I., Kaffe E., Mouratis M.A., and Aidinis V.Lysoglycerophospholipids in chronic inflammatory disorders: the PLA(2)/LPC and ATX/LPA axes. Biochim. Biophys. Acta 1831:42–60, 2013 [DOI] [PubMed] [Google Scholar]
- 108.Cedars A., Jenkins C.M., Mancuso D.J., and Gross R.W.Calcium-independent phospholipases in the heart: mediators of cellular signaling, bioenergetics, and ischemia-induced electrophysiologic dysfunction. J. Cardiovasc. Pharmacol. 53:277–289, 2009 [PMC free article] [PubMed] [Google Scholar]
- 109.Burke J.E., and Dennis E.A.Phospholipase A2 structure/function, mechanism, and signaling. J. Lipid Res. 50Suppl:S237–S242, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Abe A., Hiraoka M., Inatomi S., Ohguro I., and Ohguro H.Lysosomal phospholipase A2 activity in pig aqueous humor. Invest. Ophthalmol. Vis. Sci. 53:152–156, 2012 [DOI] [PubMed] [Google Scholar]
- 111.Wan Z., Woodward D.F., and Stamer W.D.Endogenous bioactive lipids and the regulation of conventional outflow facility. Expert Rev. Ophthalmol. 3:457–470, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Stamer W.D., Read A.T., Sumida G.M., and Ethier C.R.Sphingosine-1-phosphate effects on the inner wall of Schlemm's canal and outflow facility in perfused human eyes. Exp. Eye Res. 89:980–988, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Sumida G.M., and Stamer W.D.Sphingosine-1-phosphate enhancement of cortical actomyosin organization in cultured human Schlemm's canal endothelial cell monolayers. Invest. Ophthalmol. Vis. Sci. 51:6633–6638, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Boussommier-Calleja A., Bertrand J., Woodward D.F., Ethier C.R., Stamer W.D., and Overby D.R.Pharmacologic manipulation of conventional outflow facility in ex vivo mouse eyes. Invest. Ophthalmol. Vis. Sci. 53:5838–5845, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Aribindi K., Guerra Y., Lee R.K., and Bhattacharya S.K.Comparative phospholipid profiles of control and glaucomatous human trabecular meshwork. Invest. Ophthalmol. Vis. Sci. 54:3037–3044, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Aribindi K., Guerra Y., Piqueras Mdel C., Banta J.T., Lee R.K., and Bhattacharya S.K.Cholesterol and glycosphingolipids of human trabecular meshwork and aqueous humor: comparative profiles from control and glaucomatous donors. Curr. Eye Res. 38:1017–1026, 2013 [DOI] [PubMed] [Google Scholar]
- 117.Aljohani A.J., Munguba G.C., Guerra Y., Lee R.K., and Bhattacharya S.K.Sphingolipids and ceramides in human aqueous humor. Mol. Vis. 19:1966–1984, 2013 [PMC free article] [PubMed] [Google Scholar]