Abstract
Integrins are a family of heterodimeric transmembrane receptors that mediate adhesion to the extracellular matrix (ECM). However, integrins are not just adhesion receptors. They can act as “bidirectional signal transducers” that coordinate a large number of cellular activities in response to the extracellular environment and intracellular signaling events. Among the activities regulated by integrins are cell adhesion, assembly of the ECM, growth factor signaling, apoptosis, organization of the cytoskeleton, and cytoskeleton-mediated processes such as contraction, endocytosis, and phagocytosis. Integrins regulate these activities through a complex network of intracellular signaling kinases and adaptor proteins that associate with the transmembrane and cytoplasmic domains of the integrin subunits. In this review, we will discuss how some of the known integrin-mediated activities can control the function of the trabecular meshwork. We will also discuss how integrin activity is a tightly regulated process that involves conformation changes within the heterodimer which are mediated by specific integrin-binding proteins.
Introduction
The function of the trabecular meshwork (TM) is to regulate the outflow of aqueous humor (AH) from the anterior chamber in order to maintain intraocular pressure (IOP). It is generally believed that the major components responsible for the movement of AH are the contractile forces of the TM and the composition of extracellular matrix (ECM) of the juxtacanalicular canal (JCT). The ECM in the JCT is a highly dynamic structure that can influence the outflow resistance of the TM through interactions with cell surface receptors which bind the components of the ECM.
One major family of receptors that interact with ECM proteins is the integrins. Integrins are a family of heterodimeric transmembrane receptors that are composed of α- and β-subunits. These integrins are distributed throughout the TM with the heaviest localization observed along cells on the beams.1–3 There are 11 different integrins in the TM and all of them are known to promote adhesion to the ECM. Integrins found in the TM and their ligands are listed in Table 1. In general, integrins are divided into subgroups depending on the binding motif that they recognize in the ligands.4 The largest subgroup is the integrins that bind an RGD sequence found in many proteins. These integrins are the most promiscuous members of the family, because they bind a large number of ECM proteins as well as growth factors such as CTGF and VEGF and the latent forms of TGFβ1 and β3. The latent form of TGFβ2 does not contain an RGD motif. The other subgroup of integrins interacts with proteins through a functionally related LDV (L/I-D/E-V/S/T-P/S) motif. In the TM, this includes α4β1, α4β7, and α9β1 integrins. The third subgroup of integrins in the TM contains an αI-domain, and their ligand binding is often coordinated by a cation-binding domain. These integrins interact with collagen or laminins. The motif that collagen-binding integrins recognize is GFOGER. A consensus sequence motif has not been identified for laminin-binding integrins.
Table 1.
Integrins and Their Ligands
| Integrin | Ligands | Consensus motif |
|---|---|---|
| α1β1a | Collagens (I, IV, IX), laminin, galectin-8 | GFOGER in collagen |
| α2β1a | Collagens (I, IV, IX), laminin, E-cadherin, TSP-1, tenascin-C, | GFOGER in collagen |
| α3β1a | Laminin, TSP-1, galectin-8 | Not identified |
| α4β1 | Fibronectin, VCAM-1, ICAM-4, TSP-1, osteopontin, ADAM2 | LI/-D/E-V/S/T-P/S (LDV) |
| α5β1 | Fibronectin, fibrillin, osteopontin, TSP-1, galectin-8, ADAM9, 12, 15, 23 | RGD |
| α6β1a | Laminin, TSP, ADAM9, 12, 15, 23 | Not identified |
| α7β1 | Laminin | Not identified |
| α8β1 | Fibronectin, vitronectin, osteopontin, LAP-TGF-β | RGD |
| α9β1 | Tenascin-C, VEGF-C, VEGF-D, VCAM-1, osteopontin, Adam2, 9, 12, 15, 23 | LI/-D/E-V/S/T-P/S (LDV) |
| α10β1 | Collagens (I, IV, VI, IX), laminin | GFOGER in collagen |
| α11β1 | Collagens (I, IV, IX) | GFOGER in collagen |
| αvβ1 | Fibronectin, vitronectin, LAP-TGF-β, osteopontin, ADAM9, 12, 15, 23, galectin-8 | RGD |
| αvβ3 | Fibronectin, vitronectin, TSP-1, 2 and collagen IV, CTGF, MMP-2, LAP-TGF-β, osteopontin | RGD |
| αvβ5 | Fibronectin, vitronectin, LAP-TGF-β, osteopontin, ADAM9, 12, 15, 23 | RGD |
| αvβ6 | Fibronectin, LAP-TGF-β, osteopontin | RGD |
| αvβ8 | Fibronectin, LAP-TGF-β | RGD |
| α6β4a | Laminin | Not identified |
| α4β7 | Fibronectin, VCAM-1, osteopontin | LI/-D/E-V/S/T-P/S (LDV) |
Integrins are not just adhesion receptors, but they also act as conduits to convey information about the extracellular environment into the cell and cellular changes to the extracellular environment. The signals generated by integrins regulate a large number of biological processes that are relevant to TM function, including growth factor signaling, cell death, senescence, autophagy, phagocytosis, and the assembly and turnover of extracellular matrices. These signaling events also activate members of the Rho GTPase family that control contractility of the actomyosin cytoskeleton and play a critical role in regulating AH outflow. Integrins are, therefore, likely to play a role in regulating outflow facility.
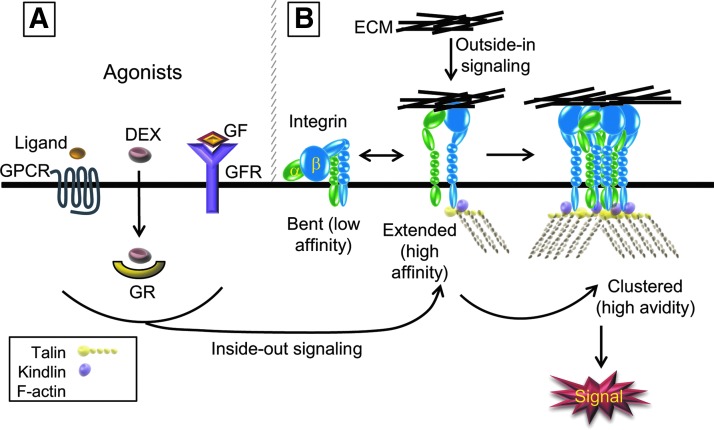
The conformation of the integrin plays a critical role in integrin signaling (Fig 1). A large number of studies have now established that the physiological low-affinity resting state of an unoccupied integrin on the cell surface is a bent conformation of the extracellular domain with the cytoplasmic tails of the α and β subunits bound together with a salt bridge.5 On exposure to an ECM ligand, conformational changes are transmitted to the transmembrane and cytoplasmic domains of the α- and β-integrin subunits that result in a high affinity state. This is called outside-in signaling and it regulates cell shape, migration, growth, and survival. Critical for integrin signaling is the formation of a signaling complex. Integrins do not have any intrinsic enzymatic activity in their cytoplasmic tails, so outside-in signaling events are mediated by interactions between adaptor, or scaffold proteins to link the integrins to kinases such as focal adhesion kinase (FAK) and Src.6 These cytoplasmic interactions are mediated by the conformational changes that are triggered by the ligand binding to the extracellular ligand domain. The signaling kinases are then activated by a series of auto- and trans-phosphorylation events that are triggered by the physical clustering of the integrins induced by the multi-valency of their ECM ligands. Once the integrins cluster, integrin binding to their ligand occurs with a higher avidity (Fig. 1).
FIG. 1.
Schematic of integrin signaling. (A) Integrins can be activated by inside-out signaling events that are mediated by G-protein-coupled receptor (GPCR) or growth factor receptor (GFR) signaling pathways. These intracellular signaling pathways cause a conformational change in integrins into the extended, high-affinity conformation. Ligands (yellow oval) that activate integrins via GPCRs are chemokines involved in lymphocyte trafficking,100 whereas growth factors which activate integrins include FGF, HGF, and VEGF.101 In trabecular meshwork (TM) cells: dexamethasone (DEX) also appears to act as an agonist to trigger the active conformation11,16 by signaling through the glucocorticoid receptor (GR). (B) Integrins exist in a bent conformation when they are unoccupied. In this conformation, the transmembrane and cytoplasmic domains are closely associated. During activation, the transmembrane and cytoplasmic domains are separated and cytoplasmic proteins such as talin or kindlin can bind and stabilize the extended conformation. Integrins can also undergo a conformational change to the high-affinity state through outside-in signaling, by binding to specific domains of extracellular matrix (ECM) ligands. Clustering of the integrin induced by the multi-valency of the ECM proteins enhances the signal.
It should be noted that unoccupied integrins have been reported to exist on the cell surface,7,8 so changes in the expression of the ECM could theoretically trigger a new signaling event without necessarily disrupting cell adhesion. For instance, one of the changes in the ECM seen in glaucoma is an increased deposition of fibronectin. Fibronectin contains 7 possible integrin binding sites, and it can bind 7 of the 11 integrins found in the TM. Thus, an increase in fibronectin expression could contribute to the pathogenesis of glaucoma by altering outside-in integrin signaling, leading to changes in the actin cytoskeleton.
In addition to outside-in signaling, integrins are unique because they can be activated to bind their ligand in a process called inside-out signaling, which shifts the integrin from the low-affinity (or resting) state to a high-affinity (active) state (Fig. 1). Inside-out signaling is usually the result of an agonist signal triggering an intracellular event that induces a conformational change in the integrin which stabilizes the extended conformation and exposes the external ligand binding site.9 This change is triggered through many signaling intermediaries that cause the cytoplasmic tails of the integrins to interact with a number of cytoplasmic proteins. The best-studied integrin regulators are talin and kindlin, but other molecules can also regulate integrin activation such as integrin linked kinase and filamin.10 These intracellular signals can be the result of external cues such as stretch, injury, or inflammation. Within the TM, recent studies suggest that treatment with dexamethasone may activate the high-affinity state of αvβ3 integrin, possibly through an inside-out signaling event.11
Control of Integrin Expression
Integrin signaling is responsive to stretch, steroids, CTGF, and TGF-β, all of which are factors that affect IOP. In the TM, stretch up-regulates the expression of αv, α5, and β1 integrins12, and steroids up-regulate the expression of α2, α5, αv, and the β3 integrin subunits.11–16 In contrast, the α3 and α4 integrin subunits are not affected by dexamethasone.13 Integrin expression in the TM is also affected by CTGF, which up-regulates the expression of the αv and β1 integrin subunits.17 Surprisingly, integrin expression in the TM does not appear to be significantly affected by TGFβ1 or TGFβ2, although in other cells types, both growth factors clearly affect the expression of αvβ3 and αvβ5 integrins18,19 and several β1 integrins, especially α5β1 integrin.20,21 TGFβ2 caused only a small increase in the expression of the β1 and α2 integrin subunits in the TM,22 while neither TGFβ1 nor TGFβ2 seemed to affect the expression of αvβ3 integrin (D.M. Peters, unpublished data). Finally, expression of the β1 integrin subunit in the TM can be regulated by miR-18323 and miR-204.24
In some instances, these factors may not change integrin expression levels, but instead affect integrin activity. For example, α4β1, α5β1, α2β1, and αvβ3 integrins can switch from the inactive (low affinity) state to an active (high affinity) state in response to stretch25 or external stimuli such as shear force or cytokines.26 In the TM, one such external stimulus could be dexamethasone, which can activate αvβ3 integrin.11 The exact manner in which dexamethasone activates αvβ3 integrin is unknown, but most likely, it involves activation of cytoplasmic proteins, leading to inside-out activation of the integrin. Recent proteomic studies support this idea and show that a large number of cytoplasmic proteins which are capable of activating αvβ3 integrin are up-regulated by dexamethasone.15 The dexamethasone-induced increase in αvβ3 integrin is the result of an increase in the transcription of β3 integrin mRNA through a secondary glucocorticoid response mechanism,27 similar to what has been shown for myocilin.28,29 This increase was dependent on calcineurin, a phosphatase that activates the NFAT family of transcription factors.27 Treatment with dexamethasone not only increased both expression and activation of αvβ3 integrin at the cell surface, but this effect also persisted even after the removal of dexamethasone. This suggests that long-term treatment with dexamethasone could result in a dysregulation of the αvβ3 integrin signaling pathway. The induction of an αvβ3 integrin signaling pathway(s) by dexamethasone can be significant. It can lead to the inhibition of phagocytosis,16 and αvβ3 integrin signaling can lead to cytoskeleton changes such as the development of cross-linked actin networks (CLANs) in TM cells.11,30 CLANs are thought to play a role in altering the contractile properties of the TM in glaucoma.31,32 Thus, the different mechanisms of integrin activation can affect the manner in which TM cells adapt to changes in IOP in order to regulate outflow facility.
Biological Processes Controlled by Integrin Signaling
Although integrins are best known for their role in mediating cell adhesion, individual integrins can perform specialized functions. For instance, only certain integrins (α4β1, α5β1) regulate matrix deposition while other integrins (αvβ5, α2β1) regulate phagocytosis. Some integrins are capable of regulating multiple cellular functions such as the ability of αvβ3 integrin to regulate both matrix deposition and phagocytosis. Thus, the repertoire of integrins can impart unique functions to the TM and changes in the repertoire, or activity of integrins, is, therefore, likely to affect outflow facility by altering the integrin-mediated signaling events that regulate matrix deposition and remodeling, phagocytosis, and/or contractility. These changes in integrin signaling can be triggered by changes in the types of ECM proteins deposited in the TM, such as those induced by TGFβ or dexamethasone. They can also be affected by the expression or phosphorylation of intracellular proteins that modulate integrin activity via an inside-out signaling event (Fig. 1). In the next sections, we will discuss how specific integrins regulate these biological processes.
Role of Integrins in Matrix Assembly
An important role for integrins is their ability to control the deposition of the ECM. The assembly of both fibronectin and collagen fibrils is a highly controlled, cell-mediated process involving specific integrins. For instance, in vascular smooth muscle cells, both collagen and fibronectin fibrillogenesis can be inhibited using anti-α2β1 or α5β1 integrin antibodies, respectively.33,34 Interestingly, if the assembly of fibronectin matrices is impaired, ECM deposition overall may be impaired because the incorporation of a number of ECM molecules into the matrix is dependent on the assembly of fibronectin fibrils.35,36 ECM proteins that use fibronectin as a scaffold for their deposition into the ECM include collagens I and III, fibrillin, tenascin-C, and LAP-TGFβ. Thus, when fibronectin fibril formation is disrupted, a decrease in microfibril formation, collagen fibril formation, and LAP-TGFβ incorporation into the ECM is also observed. Interestingly, lysyl oxidase (LOX), the enzyme found to be altered in pseudoexfoliation glaucoma,37 also interacts with fibronectin in the matrix and this interaction stimulates LOX activity. Finally, integrins may also control the turnover of matrix. Recent studies have shown that the β1 integrin is involved in the endocytosis of soluble fibronectin38 and indirectly influences the endocytosis of type I collagen matrix.39 Together, these observations suggest that integrin-mediated control of fibronectin matrices plays a critical role in both ECM deposition and turnover in the TM and that a disruption in integrin-mediated matrix assembly could have far-reaching consequences outside the assembly of collagen and fibronectin fibrils.
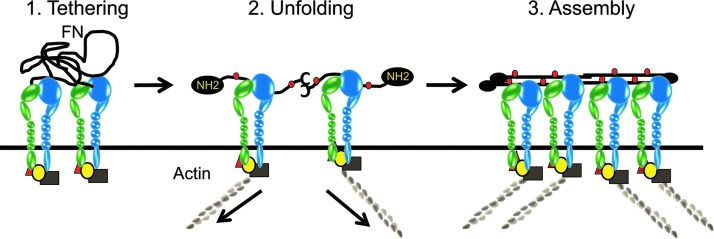
Mechanistically, integrins control matrix assembly by tethering fibronectin to specific cell surface sites and triggering a conformation change in fibronectin that exposes fibronectin–fibronectin binding sites which are responsible for the polymerization of fibronectin into fibrils40 (Fig. 2). The primary integrin known to regulate matrix assembly is the α5β1 integrin, which binds the RGD site in fibronectin. Although the interaction with integrins is usually mediated via the RGD site in fibronectin, not all RGD-binding integrins are capable of mediating the assembly of fibronectin into fibrils.35 Other integrins reported to regulate fibronectin fibrillogenesis are α4β1,41 αvβ1,42 and αvβ343 integrins. The αIIbβ3 integrin is also known to control fibril formation,44 but since αIIbβ3 is only found on platelets, it does not play a role in assembling the ECM in the TM. The reason that only certain integrins are capable of promoting matrix deposition is unclear. One explanation may be the avidity of the interaction between the integrin and its ligand. Although α5β1 integrin binding to fibronectin occurs primarily at the RGD site in fibronectin, the bond strength between α5β1 integrin and fibronectin is further enhanced via interactions between α5β1 integrin and fibronectin's synergy site (PHRSN). In the case of α4β1, αvβ1, αvβ3, and αIIbβ3 integrins, the necessary bond strength for matrix assembly may be acquired by activating the high-affinity state of these integrins. Thus, when the αvβ3 integrin is activated in the TM, this enhances fibronectin matrix deposition, especially in the presence of TGF-β2 (D.M. Peters, unpublished data).
FIG. 2.
Role of integrins in matrix assembly. The first step is the tethering of fibronectin (FN) to the cell surface via specific integrins. Soluble fibronectin is a heterodimer that is secreted as a compact globular protein. The second step is the unfolding of the globular domain to expose the amino termini and multiple other FN-FN binding sites (red dots) that are involved in fibril formation. This conformational change is triggered by contractile forces (arrows) that are generated by integrin-actomyosin linkages. The third step is the polymerization of fibronectin into fibrils. As integrins cluster, the multiple FN-FN binding sites mediate lateral and linear association of fibronectin heterodimers into fibrils.
Factors that are likely to affect matrix assembly in the TM are contractility, activation of Rho kinase, and changes in the affinity of the integrin.35 A critical feature of the integrin-mediated assembly of fibronectin fibrils is the cytoskeletal connections with the integrins' cytoplasmic tails. These connections provide the necessary contractile forces that induce the conformational change in fibronectin from a compact globular molecule to a more extended, open conformation (Fig. 2). These contractile forces are mediated by Rho kinase through the formation of actomyosin networks40 and enhanced Rho kinase activity via treatment with either lysophosphatidic acid45 or sphingosine-1-phosphate46 will facilitate matrix deposition. In the absence of contractile forces, the assembly of fibronectin fibrils is impaired. Thus, one reason that only certain integrins may participate in fibril formation is the need for sufficient bond strength with the integrin to transmit the necessary contractile forces to change the conformation of fibronectin.
Integrin-mediated matrix assembly is also facilitated by rigid substrates, as these provide a counter-force for the cell to pull on and alter the conformation of fibronectin. Thus, cells form a matrix on tensioned collagen gels or other rigid substrates, while assembly is hampered on soft substrates or floating collagen gels.47,48 Therefore, the increased stiffness of the TM in glaucomatous tissue reported by Last et al.49 could enhance integrin-mediated matrix deposition.
Integrins and Phagocytosis
Phagocytosis is the receptor-mediated engulfment of large particles (≥0.5 μm), and it is a normal TM cellular function that helps in maintaining cell and tissue homeostasis. In healthy eyes, TM cells are thought to be highly phagocytic and capable of efficiently clearing away debris to facilitate outflow of AH.50–54 In vivo, TM cells are capable of ingesting pigment granules, erythrocytes, and pseudoexfoliative material,53–56 and their phagocytic activity modulates expression of ECM remodeling genes.57
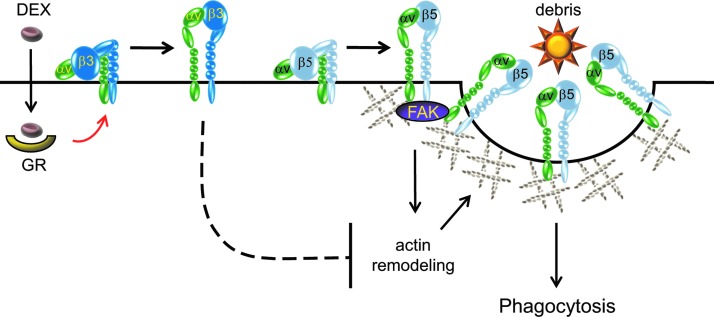
In TM cells, this process appears to involve an αvβ5 integrin-FAK signaling pathway, as there is a significant reduction in phagocytic activity when β5 integrin expression is knocked down or when FAK activity is inhibited.16 Thus, phagocytosis in TM cells is similar to that observed in other highly phagocytic, nonimmune cells, such as retinal pigmented epithelial cells, which also utilize the αvβ5 integrin-FAK signaling pathway to phagocytose rod outer segments.58,59 During phagocytosis, the role of the αvβ5 integrin-mediated signaling pathway in the TM may not be to directly bind the ingested material but rather to locally direct cytoskeletal remodeling in order to facilitate the formation of the F-actin-rich phagocytic cup in conjunction with FAK and co-receptors such as MerTK58,60,61 (Fig. 3).
FIG. 3.
Model of integrin-mediated phagocytosis in TM cells. Phagocytosis in TM cells involves an αvβ5 integrin–focal adhesion kinase (FAK) signaling pathway that can regulate actin cytoskeleton remodeling to form the phagocytic cup in TM cells. Activation of the αvβ3 integrin via dexamethasone (DEX) hinders αvβ5 integrin-FAK-mediated phagocytosis, possibly by preventing actin remodeling.
Multiple studies have shown that treatment with steroids such as dexamethasone significantly impairs phagocytosis in TM cells.51,54 Recent studies in cultured TM cells suggest that the dexamethasone-induced decrease in the phagocytic function of TM cells is due to the activation of αvβ3 integrin.16 Thus, in TM cells, the αvβ5 integrin-FAK signaling pathway that mediates phagocytosis is modulated by the activation state of the αvβ3 integrin. Interestingly, activation of the αvβ3 integrin has previously been implicated in the formation of CLANs in TM cells.11,30,62 CLAN formation utilizes an Rac1/Trio signaling pathway to reorganize the actin cytoskeleton into rigid geodesic dome-like structures. Hence, the Rac1/Trio signaling through αvβ3 integrin activation could prevent the utilization of Rac1 by αvβ5 integrin61 during the formation of the phagocytic cup (Fig. 3).
Growth Factor Signaling
One area of integrin research that has not been explored in the TM, but has been extensively studied in other cells and tissues, is the cooperation between integrin and growth factor receptor (GFR) signaling.63 This is a dynamic and reciprocal process. For example, in the TM, CTGF up-regulates the expression of the αv and β1 integrin subunits,17 and there are numerous reports of TGFβ-1 and -2 up-regulating the expression of integrin-binding ECM molecules such as fibronectin, collagen, thrombospondin-1, and SPARC.22,64–67 Integrins, on the other hand, can also control the expression of TGF-β receptors. In fibroblasts, expression of the α5 integrin subunit induces expression of TGF-βRII, which, in turn, renders the cells more responsive to TGF-β signaling.68 In the absence of the β3 integrin subunit, the expression of both TGF-βRI and TGF-βRII is enhanced, suggesting that the αvβ3 integrin represses the expression of these receptors in fibroblasts.69 Both integrins and growth factors cooperate with each other by orchestrating the endocytosis and recycling of the GFRs and integrins. In endothelial cells, VEGF-A can induce the recycling of αvβ3 integrin,70 and inhibition of αvβ3 integrin induces recycling of VEGFR2 back to the cell surface.71
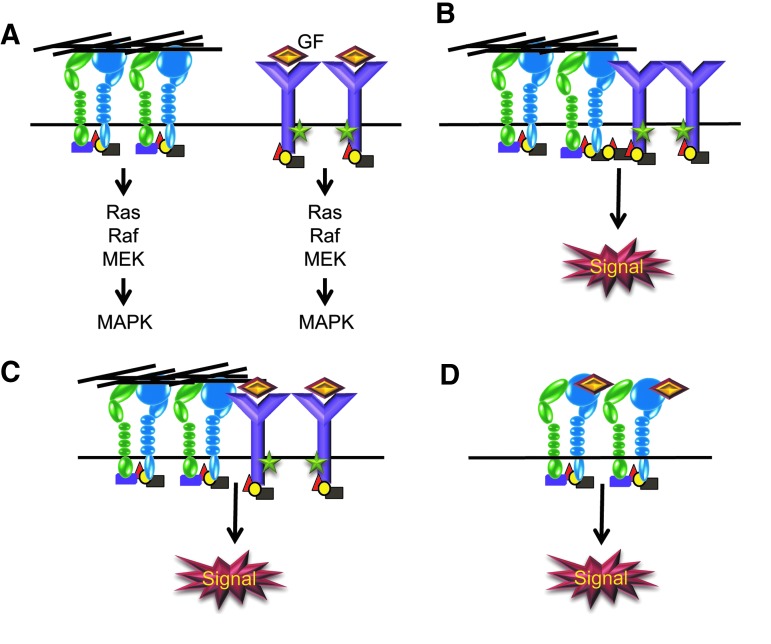
Integrins can also regulate growth factor signaling pathways. It is well established that integrins can transmit information from the ECM into the cell by activating the same signal transduction pathways which are regulated by GFRs.63,72 Some of the pathways shared by integrins and GFRs include the MAPK, PI-3Kinase, AKT, and Rho GTPase-mediated pathways (Fig. 4). The independent activation of these pathways by integrins, in essence, helps amplify the signals generated from the GFR-mediated pathway and is thought to provide a “maintenance” signal for cell survival.
FIG. 4.
Collaboration between integrins, growth factors, and GFRs. (A) Integrins and GFRs can activate the same signaling pathways independent of each other. The Ras-MAPK pathway is illustrated as an example of a GFR pathway that can be amplified by independent integrin signaling. (B) Integrins and unoccupied GFRs can physically interact with each other via bridges formed by cytoplasmic adaptor proteins that cluster the 2 types of receptors together. The clustering of GFRs leads to receptor transactivation and phosphorylation (green stars) of key residues in the GFR cytoplasmic domains followed by induction of the GF signaling pathway. Examples of this scenario include EGFR, PDGFR, VEGFR2, or IGFR interacting with αvβ3 integrins. (C) Soluble GFs, for example, FGF-1 or IGF-1, can bind both integrins and their cognate receptors, thus coupling the 2 receptor types. As discussed above, this clustering leads to transactivation and induction of GF signaling pathways. (D) Certain GFs, such as VEGF-A and angiopoietins 1 and 2, are able to bind integrins independent of their cognate receptors and activate specific signaling pathways.
In addition to independently activating these pathways, integrins also collaborate with GFRs to regulate these signaling events. This collaborative interaction between integrins and GFRs has been described for many GFRs, including the receptors for IGF, VEGF, TGF-β, HGF, PDGF, and EGF.63,72 However, the integrin-mediated activation of specific growth factor signaling pathways is dependent on the expression of specific integrin subunits. For example, the VEGFR and TGF-βRII are only found in complexes with αvβ3 integrin, whereas the EGFR interacts with several β1, β3, and β4 integrins.72–75 Since the association with GFRs appears to only occur with specific integrins and cells express specific profiles of integrin subunits, this suggests that integrins act as “environmental sensors” which determine the outcome of the growth factor signaling by creating or localizing the GFR to the correct spatial location. Any changes in the integrin profile or ECM environment are, therefore, likely to affect growth factor-mediated cell behavior.
There are at least 2 different mechanisms by which integrins and GFRs collaborate.63,73 First, integrins can directly induce the GFR pathway in the absence of the soluble growth factor via a physical interaction between the unoccupied GFR and the associated integrin (Fig. 4). For example, αvβ3 integrins have been shown to physically associate with a number of GFRs, including EGFR, PDGFR, VEGFR2, and IGFR-1.63,73 The association of these receptors with αvβ3 integrin triggers a transactivation event that leads to induction of the GF signaling pathway in the absence of the soluble growth factor.
The second mechanism involves the integrin assisting the GFR signaling pathway by mediating the organization of an integrin-GFR signaling platform. This can be accomplished in a number of ways. First, integrins can recruit and control GFR signaling complexes through the recruitment of specific adaptors to the plasma membrane. For instance, both the EGFR and the PDGFR have been shown to associate with integrins via the FAK/Src complex on the cytoplasmic tails of the integrins.76 Second, as shown in Table 1, a number of integrins can bind growth factors. Thus, integrins can act as bio-reservoirs for growth factors. Binding of the growth factors to integrins helps bring them to the cell surface and introduce them to the GFR. For instance, binding of FGF-1 or IGF-1 to αvβ3 integrin is known to promote signaling by the FGFR or IGFR-1, respectively.63 Another salient feature of this model is that it helps localize the growth factor and GFR to specific regions of the cells. This is especially critical during cell migration to promote directional migration.74 Alternatively, some integrin-mediated signaling pathways, in particular α5β1 and αvβ5 integrins, can be activated by direct binding of certain growth factors to the integrins in the absence of their own cognate receptors. For example, angiopoietins 1 and 2 can mediate integrin-dependent adhesion and spreading of both HUVECs and fibroblasts.77 Angiopoietin-1 also promotes integrin-mediated migration of HUVECs, while angiopoietin-2 does not.
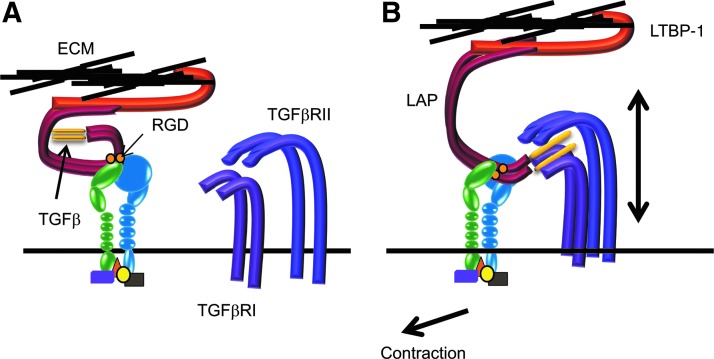
Perhaps the best known example of an integrin-growth factor-GFR complex is the interaction of αv integrins with the large latent TGF-β complex consisting of LAP-TGF-β-LTPB-1. The propeptide of LAP-TGF-β contains an RGD motif to which all the αv integrins (αvβ1, αvβ3, αvβ5, αvβ6, and αvβ8) as well as α8β1 integrin can bind.78 Although all these integrins are capable of binding the RGD sequence in the LAP fragment of TGFβ1 and TGFβ3, αvβ6 and αvβ8 integrins appear to be the predominant integrins that bind and activate these TGF-βs (Fig. 5). The αvβ6 integrin activates the LAP-TGF-β complex containing the latent TGF-β-binding protein-1 (LTBP-1) by triggering an allosteric change in the conformation of the LAP-TGF-β complex which unveils the TGF-β in such a manner that it can bind to the TGFβRs on the same cell or on neighboring cells. In this instance, the contractile forces generated by the cell stretch the αvβ6 integrin-bound LAP-TGF-β complex, which is also bound to the ECM via LTBP-1, and unveils the active TGF-β. The αvβ8 integrin, on the other hand, introduces LAP-TGF-β to matrix metalloproteinase-14, thereby causing the proteolytic release of TGF-β.78
FIG. 5.
Integrins are involved in activation of TGFβ. (A) The LAP-TGF-β complex is a dimeric, inactive protein consisting of the active C-terminal growth factor that is noncovalently linked to a pro-peptide termed the latency-associated peptide (LAP, in red). It is bound to certain integrins on the cell surface via an RGD sequence within the LAP peptide. It is also tethered to the ECM via the latent TGF-β-binding protein-1 (LTBP-1, in orange), which itself binds to ECM proteins in the absence of any contraction. The LAP masks the active growth factor and prevents its interactions with its cognate receptors (TGFβRI, TGFβRII). (B) On contraction of the actomyosin network, the cell pulls away from the ECM, thus stretching the inactive TGFβ propeptide such that the active growth factor is now able to recognize and bind to its cognate receptors.
Of course, not all integrin-GFR interactions lead to activation of the growth factor signaling pathway. These interactions can also lead to the suppression of the signal. For example, the α1β1 collagen-binding integrin can bind T-cell protein tyrosine phosphatase. This interaction serves to activate the phosphatase and recruit it to the EGFR where the ligand-induced EGFR signal is inhibited.79
Growth Arrest and Apoptosis
The collaboration between integrins and growth factor signaling for cell survival is largely believed to be the reason that nonadherent normal cells die. It is widely accepted that a cell's responsiveness to growth factors depends on integrin-mediated adhesion events. Any disruption in cell attachment results in anoikis (detachment-induced apoptosis) or growth arrest.80 In certain instances, the mis-expression of a certain integrin or absence of its ligand can lead to cell death. For example, up-regulation of the β4 integrin subunit can mediate cell death, senescence, or autophagy in response to cell stress,81 while expression of the αvβ3 integrin in the absence of a ligand triggers apoptosis in endothelial cells.7 At the present time, it is not known whether changes in integrin expression are observed in glaucomatous TM tissue, but it is tempting to speculate that the ECM changes observed in glaucomatous tissue could be triggering the wrong ECM-integrin signals needed for cell survival.
Control of Cytoskeleton
It is well established that integrins can activate members of the Rho GTPase family and, therefore, mediate contractility.82–85 The best studied example of this, of course, is the integrin-mediated formation of the actomyosin network known as stress fibers via RhoA in focal adhesions. The organization of the actomyosin network has been shown to play a critical role in regulating outflow facility86,87 and will be covered in another review in this special issue.
Integrins, however, are not always found in focal adhesions nor do they always trigger stress fiber formation. One such exception is the α4β1 integrin, which is expressed predominantly at the leading edge of highly migratory cells such as circulating leukocytes, rather than at focal adhesions.88 In fact, the α4 integrin subunit is unique, because not only is its expression limited to a number of cell types, but it also inhibits focal adhesion formation and decreases contractility. This is consistent with studies which found that activation of the α4β1 integrin by the HepII domain of fibronectin decreased contractility and stress fiber formation in cultured TM cells89–91 and caused an increase in outflow facility in both human and monkey organ cultured anterior segments.89,92 As discussed next, in order to decrease contractility in TM cells, the α4β1 integrin requires co-signaling with a collagen-binding integrin.
Cross-Talk Between Integrins
Integrin signaling in the TM, as in all cells, is complex and controlled, in part, by the presence of other integrins and matrix proteins that co-direct signaling events between integrins. Thus, changes in the expression of an integrin, its activation state, or ECM ligand can alter the signaling events mediated by another integrin. A number of instances of integrin cross-talk have been observed in the TM that reflects how changes in the composition of the ECM, or integrin expression, can alter the actomyosin network. For example, the formation of CLANs in TM cells involves co-operative signaling between a β1 integrin subunit and an αvβ3 integrin.30 For maximal CLAN formation mediated by the activation of αvβ3 integrin, the β1 integrin subunit may be paired with either the α5 or α4 subunit, both of which bind fibronectin, or with the α2 subunit, which binds collagen. Activation of αvβ3 integrin alone, either with a β3 integrin activating antibody or by engaging an αvβ3 integrin with a ligand such as vitronectin, does not appear to be sufficient to induce significant CLAN formation,11,30,62 supporting the concept of integrin cross-talk in CLAN formation. Since αvβ3 integrin can be activated by a fragment of thrombospondin-1,11,30,62 this suggests that up-regulation of thrombospondin-1 by either TGFβ2 or dexamethasone65 or the activation of αvβ3 integrin by dexamethasone11,16 would enhance the probability of CLAN formation occurring in the TM, and this could explain why formation is enhanced under these circumstances.31,32,93,94
Co-signaling between integrins also regulates the formation of stress fibers in TM cells. In both subconfluent95 and confluent cultures of TM cells,91 activation of α4β1 and α5β1 integrin signaling pathways enhances stress fiber formation. In contrast, co-signaling between the α2β1 collagen-binding integrin and α4β1 integrin appears to trigger the disassembly of actin filaments in TM cultures and to reduce the contractile properties of TM cells.91 This suggests that Rho signaling is down-regulated by α2β1 and α4β1 integrin co-signaling and offers a possible explanation as to how the α4β1 integrin-binding HepII domain of fibronectin96 was able to increase outflow facility in both human and porcine anterior segment organ cultures.89,92 This type of cross-talk is antagonistic and is often referred to as transdominant inhibition.97 Another example of transdominant inhibition would be activated αvβ3 integrin inhibiting αvβ5 integrin-mediated phagocytosis in TM cells.16 Together, these studies suggest that co-signaling between different integrins is likely to play an important role in the TM and once again illustrates how changes in the composition of the ECM could alter TM cell function.
Several mechanisms have been reported to control integrin cross-talk. The most frequently observed mechanism generally involves the binding of cytoskeleton and signaling adaptor molecules to the cytoplasmic tails of the integrin subunits during inside-out signaling as discussed earlier (Fig. 1). The best-characterized examples are the interaction between talin 1 or kindlin 1 with the β3 integrin subunit and paxillin with the α4 integrin subunit. Integrin cross-talk, however, may also occur through regulation of integrin mRNA stability.98 For example, in GD25 cells, expression of the β1 subunit caused a down-regulation of αvβ3 integrin by decreasing the stability of the β3 integrin mRNA. Integrin cross-talk can also be affected by the endocytosis and cell surface expression of an integrin heterodimer.99 For example, recycling of the αvβ3 integrin to the cell surface is controlled by the Rab-coupling protein (RAB11FIP1), which associates directly with the β3 integrin subunit. If αvβ3 integrin function is impaired, RAB11FIP1 dissociates from αvβ3 integrin and binds to the β1 integrin subunit, thereby bringing α5β1 integrin to the cell surface.
Therapeutic Applications
Currently, integrin therapies are not being used to treat glaucoma. However, integrin antagonists to αIIbβ3 integrin have been used for more than 15 years to treat acute coronary syndromes,100 and antagonists to α4β1 and α4β7 integrins are being used to treat inflammatory diseases. Antagonists to αvβ3 integrin are also currently being tested as treatment for wet AMD and diabetic retinopathy.101 To date, antagonists have been developed that could target integrins found in the TM, including α1β1, α4β1, α4β7, αvβ3, and αvβ5 integrins. These inhibitors of integrin function include blocking monoclonal antibodies (ie, vitaxin,102 natalizumab103), peptide antagonists (ie, cilengitide104), and small molecules such as disintegrins.101,105
The key to using anti-integrin therapies will be to (i) know which integrin to target, (ii) design a specific probe based on the sequence surrounding the integrin-binding motif, and (iii) determine the correct conformation of the peptide.101 Generic RGD peptides target all RGD-binding integrins and, when perfused into cultured bovine anterior segments106 or human anterior segments,107 have no effect on outflow facility. However, a loss of cell attachment, especially of Schlemm's canal cells, was observed. In contrast, when a fragment from the HepII domain of fibronectin that lacks an RGD sequence and should only bind α4β1 integrin was used,92 an increase in outflow facility was observed.92 At the higher concentration used in cultured human anterior segments, this fragment also caused the detachment of Schlemm's canal cells. However, when an 8-fold lower concentration was perfused into monkey anterior segments, outflow facility was still increased without the loss of cell attachment.89
Another important feature to be kept in mind when using integrin therapies is the ability of integrins to adopt different affinities. Thus, an integrin-binding peptide or antibody could act as either an agonist or as an antagonist depending on how it affects the integrin conformation.108 Experimental support for this comes from the finding that RGD peptides can facilitate the assembly of focal adhesions and block production of PDGF.109 To further complicate matters, some RGD peptides have been found to have a biphasic effect. At high concentrations, they act as antagonists, but at low concentrations, they are agonists.110
In addition to their use as antagonists, RGD-containing peptides and proteins have been successfully used to control drug bio-distribution and to act as agents to target nanoparticles (liposomes or polymers) to cells. Although this approach has been primarily investigated as a mechanism to deliver anti-cancer drugs, cyclic RGD peptides conjugated to silver nanoparticles are also being investigated as a way to deliver a sustained controlled substance to prevent retinal angiogenesis.111 Whether such an approach could be beneficial for the delivery of drugs or viral vectors to the TM remains to be determined.
Conclusions
In addition to mediating cell adhesion, the different integrins in the TM are likely to have unique and specialized functions, such as modulating matrix assembly, cell contractility, and phagocytosis. Undoubtedly, with so many integrin-assisted processes involved in the regulation of AH outflow, having a better understanding of how integrins function in the TM in vivo is warranted.
Acknowledgment
The authors acknowledge support from the NIH-NEI grants EY017006, EY002490, R21 EY023343, and P30 EY 016665.
Author Disclosure Statement
No competing financial interests exist for any author.
References
- 1.Tervo K., Paallysaho T., Virtanen I., and Tervo T.Integrins in human anterior chamber angle. Graefes Arch. Clin. Exp. Ophthal. 233:291–295, 1995 [DOI] [PubMed] [Google Scholar]
- 2.Zhou L., Zhang S.R., and Yue B.Y.Adhesion of human trabecular meshwork cells to extracellular matrix proteins. Roles and distribution of integrin receptors. Invest. Ophthal. Vis. Sci. 37:104–113, 1996 [PubMed] [Google Scholar]
- 3.Zhou L., Maruyama I., Li Y., Cheng E.L., and Yue B.Y.Expression of integrin receptors in the human trabecular meshwork. Curr. Eye Res. 19:395–402, 1999 [DOI] [PubMed] [Google Scholar]
- 4.Humphries J.D., Byron A., and Humphries M.J.Integrin ligands at a glance. J. Cell Sci. 119:3901–3903, 2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Kim C., Ye F., and Ginsberg M.H.Regulation of integrin activation. Annu. Rev. Cell Dev. Biol. 27:321–345, 2011 [DOI] [PubMed] [Google Scholar]
- 6.Giancotti F.G., and Ruoslahti E.Integrin signaling. Science. 285:1028–1032, 1999 [DOI] [PubMed] [Google Scholar]
- 7.Stupack D.G., Puente X.S., Boutsaboualoy S., Storgard C.M., and Cheresh D.A.Apoptosis of adherent cells by recruitment of caspase-8 to unligated integrins. J. Cell Biol. 155:4459–4470, 2001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Zhao H., Ross F.P., and Teitelbaum S.L.Unoccupied alpha(v)beta3 integrin regulates osteoclast apoptosis by transmitting a positive death signal. Mol. Endocrinol. 19:771–780, 2005 [DOI] [PubMed] [Google Scholar]
- 9.Shattil S.J., Kim C., and Ginsberg M.H.The final steps of integrin activation: the end game. Nat. Rev. Mol. Cell Biol. 11:288–300, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Legate K.R., and Fassler R.Mechanisms that regulate adaptor binding to β-integrin cytoplasmic tails. J. Cell Sci. 122:187–198, 2009 [DOI] [PubMed] [Google Scholar]
- 11.Filla M., Schwinn M.K., Nosie A.K., Clark R.W., and Peters D.M.Dexamethasone-associated cross-linked actin network (CLAN) formation in human trabecular meshwork (HTM) cells involves β3 integrin signaling. Invest. Ophthal. Vis. Sci. 52:2952–2959, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Rose A.Y., et al. . Association for research in vision and ophthalmology. Invest. Ophthalmol. Vis. Sci. 2002, 43:E-Abstract 1045. [Google Scholar]
- 13.Dickerson J.E., Jr., Steely H.T., Jr., English-Wright S.L., and Clark A.F.The effect of dexamethasone on integrin and laminin expression in cultured human trabecular meshwork cells. Exp. Eye Res. 66:731–738, 1998 [DOI] [PubMed] [Google Scholar]
- 14.Rozsa F.W., Reed D.M., Scott K.M., et al. . Gene expression profile of human trabecular meshwork cells in response to long-term dexamethasone exposure. Mol. Vis. 12:125–141, 2006 [PubMed] [Google Scholar]
- 15.Clark R., Nosie A., Walker T.M., et al. . Comparative genomic and proteomic analysis of cytoskeletal changes in dexamethasone-treated trabecular meshwork cells. Mol. Cell Proteomics. 12:194–206, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Gagen D., Filla M., Clark R., Litton P., and Peters D.M.Integrins modulate phagocytosis in human trabecular meshwork (TM) cells. Invest. Ophthal. Vis. Sci. 54:5000–5011, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Junglass B., Yu A.H.L., Welge-Lussen U., Tamm E., and Fuchshofer R.Connective tissue growth factor induces extracellular matrix deposition in human trabecular meshwork cells. Exp. Eye Res. 88:1065–1075, 2009 [DOI] [PubMed] [Google Scholar]
- 18.Lai C.-F., Feng X., Nishimura R., et al. . Transforming Growth Factor-β up-regulates the β5 integrin subunit expression via Sp1 and Smad signaling. J. Biol. Chem. 275:36400–36406, 2000 [DOI] [PubMed] [Google Scholar]
- 19.Pechkovsky D.V., Scaffidi A.K., Hackett T.L., et al. . Transforming growth factor beta1 induces alphavbeta3 integrin expression in human lung fibroblasts via a beta3 integrin-, c-Src-, and p38 MAPK-dependent pathway. J. Biol. Chem. 283:12898–12908, 2008 [DOI] [PubMed] [Google Scholar]
- 20.Roberts C.J., Birkenmeier T.M., McQuillan J.J., Akiyama S.K., Yamada S.S., Chen W T., Yamada K.M., and McDonald J.A.Transforming growth factor β stimulates the expression of fibronectin and of both subunits of the human fibronectin receptor by cultured human lung fibroblasts. J. Biol. Chem. 263:4586–4592, 1988 [PubMed] [Google Scholar]
- 21.Heino J., Ignotz R.A., Hemler M.E., Crouse C., and Massagué J.Regulation of cell adhesion receptors by transforming growth factor-beta. Concomitant regulation of integrins that share a common beta 1 subunit. J. Biol. Chem. 264:380–388, 1989 [PubMed] [Google Scholar]
- 22.Bollinger K.E., Crabb J.S., Yuan X., et al. . Quantitative proteomics: TGFβ2 signaling in trabecular meshwork cells. Invest. Ophthal. Vis. Sci. 52:8287–8294, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Li G., Luna C., Qiu J., Epstein D.L., and Gonzalez P.Targeting of integrin beta1 and kinesin 2 alpha by microRNA 183. J. Biol. Chem. 285:5461–5471, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Paylakhi S.H., Moazzeni H., Yazdani S., et al. . FOXC1 in human trabecular meshwork cells is involved in regulatory pathway that includes miR-204, MEIS2, and ITGβ1. Exp. Eye Res. 111:112–121, 2013 [DOI] [PubMed] [Google Scholar]
- 25.Tzima E., Angel del Pozo M., Shattil S.J., Chien S., and Schwartz M.A.Activation of integrins in endothelial cells by fluid shear stress mediates Rho-dependent cytoskeletal alignment. EMBO J. 20:4639–4647, 2001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Alon R., and Dustin M.L.Force as a facilitator of integrin conformational changes during leukocyte arrest on blood vessels and antigen-presenting cells. Immunity. 26:17–27, 2007 [DOI] [PubMed] [Google Scholar]
- 27.Faralli J.A., Gagen D., Filla M.S., Crotti T.N., and Peters D.M.Dexamethasone increases αvβ3 integrin expression and affinity through a calcineurin/NFAT pathway. Biochim. Biophys. Acta. 2013. DOI: 10.1016/j.bbamcr.2013.09.020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Joe M.K., Sohn S., Kim T.E., et al. . Analysis of glucocorticoid-induced MYOC expression in human trabecular meshwork cells. Vis. Res. 51:1033–1038, 2011 [DOI] [PubMed] [Google Scholar]
- 29.Shepard A.R., Jacobson N., Fingert J.H., et al. . Delayed secondary glucocorticoid responsiveness of MYCO in human trabecular meshwork cells. Invest. Ophthal. Vis. Sci. 42:3173–3181, 2001 [PubMed] [Google Scholar]
- 30.Filla M., Woods A., Kaufman P.L., and Peters D.M.P.β1 and β3 integrins cooperate to induce syndecan-4 containing cross-linked actin networks (CLANs) in human trabecular meshwork (HTM) cells Invest. Ophthal. Vis. Sci.; 47:1956–1967, 2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Clark A.F., Wilson K., McCartney M.D., et al. . Glucocorticoid-induced formation of cross-linked actin networks in cultured human trabecular meshwork cells. Invest. Ophthal. Vis. Sci. 35:281–294, 1994 [PubMed] [Google Scholar]
- 32.Clark A.F., Brotchie D., Read A.T., et al. . Dexamethasone alters F-actin architecture and promotes cross-linked actin network formation in human trabecular meshwork tissue. Cell Motil. Cytoskeleton. 60:83–95, 2005 [DOI] [PubMed] [Google Scholar]
- 33.Fogerty F.J., Akiyama S.K., Yamada K.M., and Mosher D.F.Inhibition of binding of fibronectin to matrix assembly sites by anti-integrin (α5β1) antibodies. J Cell Biol. 111:699–708, 1990 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Li S., Van Den Diepstraten C., D'Souza S.J., Chan B.M.C., and Pickering J.G.Vascular smooth muscle cells orchestrate the assembly of type I collagen via alpha2beta1 integrin, RhoA and fibronectin polymerization. Am. J. Pathol. 163:1045–1056, 2003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Singh P., Carraher C., and Schwarzbauer J.E.Assembly of fibronectin extracellular matrix. Annu. Rev. Cell Dev. Biol. 26:397–419, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Dallas S.L., Chen Q., and Sivakumar P.Dynamics of assembly and reorganization of extracellular matrix proteins. Curr. Top. Dev. Biol. 75:1–24, 2006 [DOI] [PubMed] [Google Scholar]
- 37.Schlotzer-Schrehardt U.Molecular pathology of pseudoexfoliation syndrome/glaucoma—New insights from LOX1 gene associations. Exp. Eye Res. 88:776–785, 2009 [DOI] [PubMed] [Google Scholar]
- 38.Shi F., and Sottile J.Caveolin-1-dependent beta1 integrin endocytosis is a critical regulator of fibronectin turnover. J. Cell Sci. 121:2360–2371, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Shi F., Harman J., Fujiwara K., and Sottile J.Collagen I matrix turnover is regulated by fibronectin polymerization. Am. J. Physiol. Cell Physiol. 298:C1265–C1275, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Zhong C., Chrzanowska Wodnicka M., Brown J., et al. . Rho-mediated contractility exposes a cryptic site in fibronectin and induces fibronectin matrix assembly. J. Cell Biol. 141:539–551, 1998 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Sechler J.L., Cuminsky A.M., Gazzola D.M., and Scwarzbauer J.E.A novel RGD-independant fibronectin assembly pathway initiated by alpha4beta1 integrin binding to the alternatively spliced V region. J. Cell Sci. 113(Pt 8):1491–1498, 2000 [DOI] [PubMed] [Google Scholar]
- 42.Zhang Z., Morla A.O., Vuoori K., et al. . The αvβ1 integrin functions as a fibronectin receptor but does not support fibronectin matrix assembly and cell migration on fibronectin. J. Cell Biol. 122:235–242, 1993 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Wu C., Hughes P.E., Ginsberg M.H., and McDonald J.A.Identification of a new biological function for the integrin αvβ3: initiation of fibronectin matrix assembly. Cell Adhes. Commun. 4:149–158, 1996 [DOI] [PubMed] [Google Scholar]
- 44.Olorundare O.E., Peyruchaud O., Albrecht R.M., and Mosher D.F.Assembly of a fibronectin matrix by adherent platelets stimulated by lysophospahtidic and other agonists. Blood. 98:117–124, 2001 [DOI] [PubMed] [Google Scholar]
- 45.Zhang Q., Checovich W.J., Peters D.M., Albrecht R.M., and Mosher D.F.Modulation of cell surface fibronectin assembly sites by lysophosphatidic acid. J. Cell Biol. 127:1447–1459, 1994 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Zhang Q., Peyruchaud O., French K.J., Magnusson M.K., and Mosher D.F.Sphingosine 1-phosphate stimulates fibronectin matrix assembly through a Rho-dependent signal pathway. Blood. 93:2984–2990, 1999 [PubMed] [Google Scholar]
- 47.Halliday N.L., and Tomasek J.J.Mechanical properties of the extracellular matrix influence fibronectin fibril assembly in vitro. Exp. Cell Res. 217:109–117, 1995 [DOI] [PubMed] [Google Scholar]
- 48.Carraher C.L., and Schwarzbauer J.Regulation of matrix assembly through rigidity-dependent fibronectin conformational changes. J. Biol. Chem. 288:14805–14814, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Last J.A., Pan T., Ding Y., et al. . Elastic modulus determination of normal and glaucomatous human trabecular meshwork. Invest. Ophthal. Vis. Sci. 52:2147–2152, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Johnson D.H., Richardson T.M., and Epstein E.Trabecular meshwork recovery after phagocytic challenge. Curr. Eye Res. 8:1121–1130, 1989 [DOI] [PubMed] [Google Scholar]
- 51.Matsumoto Y., and Johnson D.H.Dexamethasone decreases phagocytosis by human trabecular meshwork cells in situ. Invest. Ophthal. Vis. Sci. 38:1902–1907, 1997 [PubMed] [Google Scholar]
- 52.Matsumoto Y., and Johnson D.H.Trabecular meshwork phagocytosis in glaucomatous eyes. Ophthalmologica. 211:147–152, 1997 [DOI] [PubMed] [Google Scholar]
- 53.Rohen J.W., and van der Zypen E.The phagocytic activity of the trabecularmeshwork endothelium. An electron-microscopic study of the vervet (Cercopithecus aethiops). Albrecht Von Graefes Arch. Klin. Exp. Ophthalmol. 175:143–160, 1968 [DOI] [PubMed] [Google Scholar]
- 54.Zhang X., Ognibene C.M., Clark A.F., and Yorio T.Dexamethasone inhibition of trabecular meshwork cell phagocytosis and its modulation by glucocorticoid receptor β. Exp. Eye Res. 84:275–284, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Grierson I., and Lee W.R.Erythrocyte phagocytosis in the human trabecular meshwork. Br. J. Ophthalmol. 57:400–415, 1973 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Grierson I., Day J., Unger W.G., and Ahmed A.Phagocytosis of latex microspheres by bovine meshwork cells in culture. Graefes Arch. Clin. Exp. Ophthalmol. 224:536–544, 1986 [DOI] [PubMed] [Google Scholar]
- 57.Porter K.M., Epstein D.L., and Liton P.B.Up-regulated expression of extracellular matrix remodeling genes in phagocytically challenged trabecular meshwork cells. PLoS One. 7:e34792, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Finnemann S.C.Focal adhesion kinase signaling promotes phagocytosis of integrin-bound photoreceptors. EMBO J. 22:4143–4154, 2003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Finnemann S.C., Bonilha V.L., Marmorstein A.D., and Rodriguez-Boulan E.Phagocytosis of rod outer segments by retinal pigment epithelial cells requires alpha(v)beta5 integrin for binding but not for internalization. Proc. Natl. Acad. Sci. USA. 94:12932–12937, 1997 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Finnemann S.C., and Nandrot E.F.MerTK activation during RPE phagocytosis in vivo requires alphaVbeta5 integrin. Adv. Exp. Med. Biol. 572:499–503, 2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Dupuy A.G., and Caron E.Integrin-dependent phagocytosis: spreading from microadhesion to new concepts. J. Cell Biol. 121:1773–1783, 2008 [DOI] [PubMed] [Google Scholar]
- 62.Filla M., Schwinn M.K., Sheibani N., Kaufman P.L., and Peters D.M.Regulation of cross-linked actin network (CLAN) formation in human trabecular meshwork (HTM) cells by convergence of distinct β1 and β3 integrin pathways Invest. Ophthal. Vis. Sci. 50:5723–5731, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Ivaska J., and Heino J.Cooperation between integrins and growth factor receptors in signaling and endocytosis. Annu. Rev. Cell Dev. Biol. 27:291–320, 2011 [DOI] [PubMed] [Google Scholar]
- 64.Zhao X., Ramsey K.I., Stephan D.A., and Russell P.Gene and protein expression changes in human trabecular meshwork cells treated with transforming growth-β. Invest. Ophthal. Vis. Sci. 45:4023–4034, 2004 [DOI] [PubMed] [Google Scholar]
- 65.Flugel-Koch C., Ohlmann A., Fuchshofer R., Welge-Lussen U., and Tamm E.R.Thrombospondin-1 in the trabecular meshwork: localization in normal and glaucomatous eyes, and induction by TGF-beta1 and dexamethasone. Exp. Eye Res. 79:649–663, 2004 [DOI] [PubMed] [Google Scholar]
- 66.Fleenor D.L., Shepard A.R., Hellberg P.E., et al. . TGFbeta2-induced changes in human trabecular meshwork: implications for intraocular pressure. Invest. Ophthal. Vis. Sci. 47:226–234, 2006 [DOI] [PubMed] [Google Scholar]
- 67.Kang M.H., Oh D.J., Kang J.H., and Rhee D.J.Regulation of SPARC by transforming growth factor β2 in human trabecular meshwork. Invest. Ophthal. Vis. Sci. 54:2523–2532, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Wang D., Sun L., Zborowska E., et al. . Control of type II transforming growth factor-β receptor expression by integrin ligation. J. Biol. Chem. 274:12840–12847, 1999 [DOI] [PubMed] [Google Scholar]
- 69.Reynolds L.E., Conti F.J., Lucas M., et al. . Accelerated re-epithelialization in 3-integrin-deficient- mice is associated with enhanced TGF-1 signaling. Nat. Med. 11:167–174, 2005 [DOI] [PubMed] [Google Scholar]
- 70.di Blasio L., Droetto S., Norman J., Bussolino F., and Primo L.Protein kinase D1 regulates VEGF-A-induced αvβ3 integrin trafficking and endothelial cell migration. Traffic. 11:1107–1018, 2010 [DOI] [PubMed] [Google Scholar]
- 71.Reynolds A.R., Hart I.R., Watson A.R., et al. . Stimulation of tumor growth and angiogenesis by low concentrations of RGD-mimetic integrin inhibitors. Nat. Med. 15:392–400, 2009 [DOI] [PubMed] [Google Scholar]
- 72.Streuli C.H., and Akhtar N.Signal co-operation between integrins and other receptor systems. Biochem. J. 418:491–506, 2009 [DOI] [PubMed] [Google Scholar]
- 73.Alam N., Goel H.L., Zarif M.J., et al. . The integrin-growth factor receptor duet. J. Cell Physiol. 213:649–653, 2007 [DOI] [PubMed] [Google Scholar]
- 74.Scaffidi A.K., Petrovic N., Moodley Y.P., et al. . αvβ3 integrin interacts with the transforming growth factor β (TGFβ) type II receptor to potentiate the proliferative effects of TGFβ1 in living human lung fibroblasts. J. Biol. Chem. 279:37726–37733, 2004 [DOI] [PubMed] [Google Scholar]
- 75.Galliher A.J., and Schiemann W.P.β3 Integrin and Src facilitate transforming growth factor-β mediated induction of epithelial-mesenchymal transition in mammary epithelial cells. Breast Cancer Res. 8:R42, 2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Sieg D.J., Hauck C.R., Llic D., et al. . FAK integrates growth-factor and integrin signals to promote cell migration. Nat. Cell Biol. 2:249–256, 2000 [DOI] [PubMed] [Google Scholar]
- 77.Carlson T.R., Feng Y., Maisonpierre P.C., Mrksich M., and Morla A.O.Direct cell adhesion to the angiopoietins mediated by integrins. J. Biol. Chem. 276:26516–26525, 2001 [DOI] [PubMed] [Google Scholar]
- 78.Munger J.S., and Sheppard D.Cross talk among TGF-β signaling pathways, integrins, and the extracellular matrix. Cold Spring Harb. Perspect. Biol. 3:a005017, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Mattila E., Pellinen T., BNevo J., et al. . Negative regulation of EGFR signaling through integrin α1β1-mediated actiavtion of protein tyrosine phosphasts TCPTP. Nat. Cell Biol. 7:78–85, 2005 [DOI] [PubMed] [Google Scholar]
- 80.Schwartz M.A., and Assoian R.K.Integrins and cell proliferation: regulation of cyclin-dependent kinases via cytoplasmic signaling pathways. J. Cell Sci. 114:2553–2560, 2001 [DOI] [PubMed] [Google Scholar]
- 81.Wang L., Dong Z., Zhang Y., and Miao J.The roles of integrin β4 in vascular endothelial cells. J. Cell Physiol. 227:474–478, 2012 [DOI] [PubMed] [Google Scholar]
- 82.Burridge K., and Chrzanowska-Wodnicka M.Focal adhesions, contractility, and signaling. Annu. Rev. Cell Dev. Biol. 12:463–518, 1996 [DOI] [PubMed] [Google Scholar]
- 83.Schoenwaelder S.M., and Burridge K.Bidirectional signaling between the cytoskeleton and integrins. Curr. Opin. Cell Biol. 11:274–286, 1999 [DOI] [PubMed] [Google Scholar]
- 84.Arthur W.T., Noren N.K., and Burridge K.Regulation of Rho family GTPases by cell-cell and cell-matrix adhesion. Biol. Res. 35:239–246, 2002 [DOI] [PubMed] [Google Scholar]
- 85.Ridley A.J.Rho GTPases and cell migration. J. Cell Sci. 114:2713–2722, 2001 [DOI] [PubMed] [Google Scholar]
- 86.Tian B., Geiger B., Epstein D.L., and Kaufman P.L.Cytoskeletal involvement in the regulation of aqueous humor outflow. Invest. Ophthal. Vis. Sci. 41:619–623, 2000 [PubMed] [Google Scholar]
- 87.Rao P.V., Peterson Y.K., Inoue T., and Casey P.J.Effects of pharmacologic inhibition of protein geranylgeranyltransferase type I on aqueous humor outflow through the trabecular meshwork. Invest. Ophthal. Vis. Sci. 49:2464–2471, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Liu S., Rose D.M., Han J., and Ginsberg M.H.α4 integrins in cardiovascular development and diseases. Trends Cardiovasc. Med. 10:253–257, 2000 [DOI] [PubMed] [Google Scholar]
- 89.Gonzalez J.M.J., Hu Y., Gabelt B., Kaufman P.L., and Peters D.M.Identification of the active site in the Heparin II domain of fibronectin that increases outflow facility in cultured monkey anterior segments. Invest. Ophthal. Vis. Sci. 50:235–241, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Gonzalez J.M.J., Peterson J.A., Peters J.M., Newman J., and Peters D.M.Effect of Heparin II domain of fibronectin on actin cytoskeleton and adherens junctions in human trabecular meshwork cultures. Invest. Ophthal. Vis. Sci. 47:2924–2931, 2006 [DOI] [PubMed] [Google Scholar]
- 91.Schwinn M.K., Gonzalez J.M.J., Gabelt B., et al. . Heparin II domain of fibronectin mediates contractility through an α4β1 integrin co-signaling pathway. Exp. Eye Res. 316:1500–1512, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Santas A.J., Bahler C., Peterson J.A., et al. . Effect of Heparin II domain of fibronectin on aqueous outflow in cultured anterior segments of human eyes. Invest. Ophthal. Vis. Sci. 44:4796–4801, 2003 [DOI] [PubMed] [Google Scholar]
- 93.Clark A.F., Miggans S.T., Wilson K., Browder S., and McCartney M.D.Cytoskeletal changes in cultured human glaucoma trabecular meshwork cells. J. Glaucoma. 4:183–188, 1995 [PubMed] [Google Scholar]
- 94.O'Reilly S., Pollock N., Currie L., et al. . Inducers of cross-linked actin networks in trabecular meshwork cells. Invest. Ophthal. Vis. Sci. 52:7316–7324, 2011 [DOI] [PubMed] [Google Scholar]
- 95.Peterson J.A., Sheibani N., David G., Garcia-Pardo A., and Peters D.M.Heparin II domain of fibronectin uses α4β1 integrin to control focal adhesion and stress fiber formation, independent of syndecan-4. J. Biol. Chem. 280:6915–6922, 2004 [DOI] [PubMed] [Google Scholar]
- 96.Sharma A., Askari J.A., Humphries M.J., Jones E.Y., and Stuart D.I.Crystal structure of a heparin- and integrin-binding segment of human fibronectin. EMBO J. 18:1468–1479, 1999 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Gonzalez A.M., Bhattacharya R., deHart G.W., and Jones J.C.Transdominant regulation of integrin function: mechanisms of crosstalk. Cell Signal. 22:578–583, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Retta S.F., Cassara G., D'Amato M., et al. . Cross talk betweem β1 and αv integrins: β1 affects β3 mRNA stability. Mol. Biol. Cell. 12:3126–3138, 2001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Caswell P.T., Vadrevu S., and Norman J.C.Integrins:masters and slaves of endocytic transport. Nat. Rev. Mol. Cell Biol. 10:843–853, 2009 [DOI] [PubMed] [Google Scholar]
- 100.Bledzka K., Smyth S.S., and Plow E.F.Integrin αIIbβ3: From discovery to efficacious therapeutic target. Circ. Res. 112:1189–1200, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Mousa S.A.αv Vitronectin receptors in vascular-mediated disorders. Med. Res. Rev. 23:190–199, 2003 [DOI] [PubMed] [Google Scholar]
- 102.Gutheil J.C., Campbell T.N., Pierce P.R., et al. . Targeted antiangiogenic therapy for cancer using Vitaxin: a humanized monoclonal antibody to the integrin αvβ3. Clin. Cancer Res. 6:3056–3061, 2000 [PubMed] [Google Scholar]
- 103.Kummer C., and Ginsberg M.H.New approaches to blockade of α4-integrins, proven therapeutic targets in chronic inflammation. Biochem. Pharmacol. 72:1460–1468, 2006 [DOI] [PubMed] [Google Scholar]
- 104.Alghisi G.C., Ponsonnet L., and Ruegg C.The integrin antagonist cilengitide activates αvβ3, disrupts VE-cadherin localization at ell junctions and enhnaces permeability in endothelial cells. PLoS One. 4:e4449, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Marcinkiewicz C., Weinreb P.H., Calvete J.J., et al. . Obtustatin: a potent slecive inhibitor of α1β1 integrin in vitro and angiogenesis in vivo. Cancer Res. 63:2020–2023, 2003 [PubMed] [Google Scholar]
- 106.Overby D., Gong H., Qiu G., Freddo T., and Johnson M.F.The mechanism of increasing outflow facility during washout in the bovine eye. Invest. Ophthal. Vis. Sci. 43:3455–3464, 2002 [PubMed] [Google Scholar]
- 107.Bahler C.K., Hann C.R., Fautsch M.P., and Johnson D.H.Pharmacologic Disruption of Schlemm's Canal Cells and Outflow Facility in Anterior Segments of Human Eyes Invest. Ophthal. Vis. Sci. 45:2246–2254, 2004 [DOI] [PubMed] [Google Scholar]
- 108.Shimaoka M., and Springer T.A.Therapeutic antagonists and conformational regulation of integrin function. Nat. Rev. Drug Discov. 2:703–716, 2003 [DOI] [PubMed] [Google Scholar]
- 109.Mogford J.E., DAvis G.E., Platts S.H., and Meininger G.A.Vascular smooth muscle αvβ3 integrin mediates arteriolar vasodilaton in response to RGD peptides. Circ. Res. 79:821–826, 1996 [DOI] [PubMed] [Google Scholar]
- 110.Legler D.F., Wiedle G., Ross F.P., and Imhof B.A.Superactivation of integrin αvβ3 by low antagonist concentrations. J. Cell Sci. 114:1545–1553, 2001 [DOI] [PubMed] [Google Scholar]
- 111.Kalishwaralal K., BarathManiKanth S., Pandian S.R.K., Deepak V., and Gurunathan S.Silver nano—A trove for retinal therapies. J. Control Release. 145:76–90, 2010 [DOI] [PubMed] [Google Scholar]