Abstract
Elevated intraocular pressure (IOP) is the most prevalent risk factor for glaucoma. All treatments, whether surgical or pharmaceutical, are aimed at lowering IOP. Prostaglandin analogues are a first line therapy for glaucoma due to their ability to reduce IOP, once-daily dosing, efficacy, and minimal side-effect profile. Whereas prostaglandin analogues have been known to alter aqueous humor outflow through the unconventional (uveoscleral) pathway, more recent evidence suggests their action also occurs through the conventional (trabecular) pathway. Understanding how prostaglandin analogues successfully lower IOP is important, as this information may lead to the discovery of new molecular targets for future therapeutic intervention. This review explores the current understanding of prostaglandin analogue biology as it pertains to IOP reduction and improved aqueous humor outflow facility.
Introduction
Glaucoma is a neurodegenerative disease that damages the optic nerve. There are several risk factors for the disease (age, race, family history, diabetes, and reduced cerebrospinal fluid pressure). However, elevated intraocular pressure (IOP) remains the most prevalent risk and causal factor for glaucoma. If left untreated, elevated IOP will lead to loss of retinal ganglion cells resulting in optic nerve head cupping and irreversible vision loss. At present, all treatments for glaucoma are geared toward reducing elevated IOP to slow progression of the disease.
In the normal eye, IOP is maintained through a balance between the amount of aqueous humor produced in the ciliary body epithelium and the amount drained from the anterior chamber. Whereas secretion of aqueous humor remains normal in glaucoma, the main cause of elevated IOP is the reduced ability to drain aqueous humor from the anterior chamber. In humans, between 60% and 80% of aqueous humor is drained through the conventional (trabecular) outflow pathway, which consists of the trabecular meshwork, a multilayer tissue that filters aqueous humor as it flows from the anterior chamber, and Schlemm's canal, a tubule that drains the trabecular meshwork and moves the aqueous humor through collector channels into aqueous veins and into the episcleral venous system. A portion of aqueous humor (20%–40%) is also removed through the unconventional (uveoscleral) outflow pathway by diffusion through the interstitial spaces of the ciliary muscle into the suprachoroidal space. Fluid removal through the unconventional pathway decreases with age resulting in the conventional outflow pathway becoming the primary route of aqueous humor drainage in the elderly.1 In the glaucomatous eye, impaired drainage of aqueous humor is caused by an increased resistance to fluid drainage at the interface between the trabecular meshwork and the inner wall of Schlemm's canal within the conventional outflow pathway.2 This leads to reduced outflow facility, large diurnal IOP fluctuations, and elevated IOP, which eventually will lead to glaucoma.3–5 At present, treatment aimed at lowering IOP is the only method to slow glaucoma progression and prevent irreversible blindness.
Identifying Prostaglandins as Ocular Hypotensive Agents
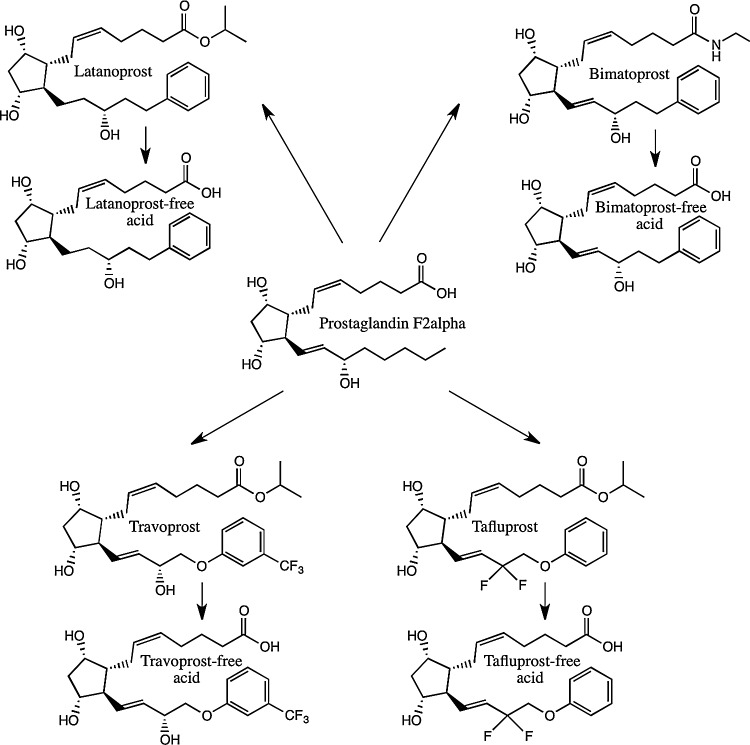
Prostaglandins elicit many effects throughout the body, including constriction and relaxation of smooth muscles and regulation of the immune response.6,7 Early studies analyzing the effects of prostaglandins in the eye revealed significant irritation and initial IOP elevation. Whereas the prevailing thought in the early 1970s was that prostaglandins elevate IOP, it was not until 1977 when Drs. Camras, Bito, and Eakins published the seminal article showing that administration of prostaglandin F2α (PGF2α) and E2 (PGE2) to uncannulated rabbit eyes lowered IOP in a dose-dependent manner following an early ocular hypertensive effect.8 This groundbreaking research led to the FDA approval in 1996 of latanoprost, an isopropyl analogue of PGF2α (Fig. 1). In 2001, bimatoprost and travoprost, followed by tafluprost in 2012, joined latanoprost as commercially available prostaglandin analogues in the United States for the treatment of glaucoma. Latanoprost, travoprost, and tafluprost are prostanoids, while bimatoprost is classified as a prostamide. All these agents are prodrugs of PGF2α.
FIG. 1.
Molecular structure of prostaglandin analogues used to treat ocular hypertension. Latanoprost, bimatoprost, travoprost, and tafluprost are prostanoids, while bimatoprost is a prostamide. Associated physiologically active free acid forms are also shown.
The popularity of prostaglandin analogues as first line pharmaceuticals for glaucoma treatment stems from their ability to effectively reduce IOP by increasing outflow facility, once-daily dosing, and minimal side effects.9 However, the responsiveness of individuals to prostaglandin analogues in the clinical setting can be highly variable.10,11 Some patients respond well to treatment using only a single prostaglandin analogue. Others may not observe a clinically significant decrease in IOP. These patients may require cotreatment with another family of drugs (β-adrenergic receptor inhibitor, timolol maleate, carbonic anhydrase inhibitor, or dorzolamide hydrochloride) to elicit pressure reduction to the normal IOP range of 12–15 mmHg.11–15 There is also a small subset of patients who are refractory to one prostaglandin analogue, but responsive to another.16–18 For example, bimatoprost has been found to be effective for patients who do not respond to latanoprost treatment.17 Clinically, bimatoprost appears slightly more effective in lowering pressure than latanoprost and travoprost, but has a more significant side-effect profile, causing greater hyperemia and eyelash growth than latanoprost, but less iridial hyperpigmentation.9,11,19–24
Prostaglandin Receptors
Prostaglandin analogues elicit their effect by binding to specific receptors localized in the cell membrane and nuclear envelope.25 There are 9 prostaglandin receptors: PGE receptor 1–4 (EP1–4), PGD receptor 1–2 (DP1–2), PGIP receptor, PGFP receptor, and thromboxane A2 receptor (TP), their designation based mainly on the prostaglandin for which binding is most specific.7,26,27 PGF2α binds the FP, EP1, and EP3 receptors with significant affinity, while travoprost binds the FP receptor with highest affinity among the prostaglandin analogues, with minimal affinity for DP, EP1, EP3, EP4, and TP receptors.26,28 Pharmacologic and pharmacokinetic data suggest the existence of a unique bimatoprost receptor, distinct from the known FP receptors; however, this receptor is yet to be cloned and bimatoprost has not been shown to work independent of FP receptor activation.29–31 In the eye, FP and all 4 EP receptors (1–4) have been identified in several ocular tissues, including the trabecular meshwork and the cells of Schlemm's canal.25,32–38 Investigation with mice lacking the FP receptor gene suggests that the FP receptor is critical for IOP reduction caused by latanoprost, bimatoprost, and travoprost, as all 3 treatments had no effect in knockout mice.39 The importance of the FP receptor was further confirmed in human nonpigmented ciliary epithelial and trabecular meshwork cells, where use of the FP receptor antagonist AL-8810 inhibited phosphoinositide turnover.40,41 The absence of EP3 receptors in mice reduced the IOP lowering effect of latanoprost, bimatoprost, and travoprost, but mice lacking EP1 and EP2 receptors showed inconclusive results.42 These studies in mice suggest that FP and EP3 are the primary receptors that trigger downstream signaling pathways and the eventual physiologic response following treatment with latanoprost, bimatoprost, and travoprost. However, in primates, the story is slightly different. EP2 receptor stimulation has been shown to increase uveoscleral outflow,43 and EP4 receptor activation reduces IOP by increasing outflow facility without effecting uveoscleral outflow.44 These results in mice and primates suggest that species-specific mechanisms may exist.
Mode of Action: Unconventional Pathway
As mentioned previously, IOP is regulated by the balance between production and removal of aqueous humor from the anterior chamber through the conventional and unconventional pathways. Several studies suggest that prostaglandins do not reduce the amount of aqueous humor production in humans,45–50 but instead have a minimal increase in aqueous humor production, although the amount is not clinically significant.51 At present, all evidence supports the role of prostaglandin analogues increasing aqueous humor outflow. In the unconventional pathway, PGF2α and prostaglandin analogues bind to EP and FP receptors in the ciliary muscle, resulting in ciliary muscle relaxation and increased aqueous humor outflow.43,52–55 Binding of prostaglandins and prostaglandin analogues to ciliary muscle FP receptors also disrupts extracellular matrix turnover.56–60 The rate of turnover is dependent on the balance between the molecules that degrade and remodel the extracellular matrix [matrix metalloproteinases (MMPs)] and their inhibitors [tissue inhibitor of metalloproteinase (TIMPs)]. MMPs degrade and remodel the extracellular matrix in the ciliary muscle, iris root, and sclera, reducing outflow resistance to fluid flow.59,61 Treatment with bimatoprost and latanoprost induced MMP-1, -3, and -9 expression in ciliary smooth muscle cells.62 MMP-1 breaks down interstitial collagens, most notably types I, II, and III, while MMP-9 is most associated with the breakdown of collagens IV and V.63–66 MMP-3 has a broad substrate specificity that includes degradation of fibronectin, laminin, elastin, proteoglycans, and several collagens, while also having the regulatory function of activating MMP-1 and MMP-9, making MMP-3 a critical molecule in connective tissue remodeling.63,65 Only TIMP-3, which is associated with heparin sulfate and chondroitin sulfate containing proteoglycans in the extracellular matrix, was induced by bimatoprost and latanoprost.62,67–69 Additionally, cynomolgus monkey ciliary muscle treated with PGF2α, latanoprost, or bimatoprost for up to 8 days showed increased space between muscle bundles indicating changes in extracellular matrix synthesis and turnover presumably increasing aqueous humor outflow.70 Overall, it appears that treatment with PGF2α and prostaglandin analogues increases the amount of MMPs, while maintaining TIMP expression. This shifts the balance in favor of degradation and remodeling of the extracellular matrix to enhance outflow facility.
Mode of Action: Conventional Pathway
Whereas increased aqueous humor outflow through the unconventional pathway has been the accepted mode of action after prostaglandin analogue treatment, studies have shown that prostaglandin analogues also play a significant role in modulating outflow facility through the conventional outflow pathway. Treatment with bimatoprost or latanoprost increased outflow facility in cultured human anterior segments, a model that contains only the conventional outflow pathway.71,72 Histological analysis of latanoprost-treated anterior segments showed focal loss of Schlemm's canal endothelial cells, separation of inner wall cells from the basal lamina, and focal loss of extracellular matrix in the juxtacanalicular region.71 Previous studies have confirmed that loss of extracellular matrix from the juxtacanalicular region and cell disconnection from the extracellular matrix increase conventional outflow.73,74 Bimatoprost treatment of cynomolgus monkeys for 1 year showed disruption of the endothelial cell monolayer of Schlemm's canal, expansion of the juxtacanalicular region of the trabecular meshwork, loss of extracellular matrix, and in some samples, widening of intertrabecular spaces in the corneoscleral region of the trabecular meshwork.75 Both collagen and the electron dense core of elastic-like fibers in the inner portion of the trabecular meshwork were lost, as well as connective tissue from the beams as a result of treatment. These changes appear to aid in increasing outflow facility through ways that are still not well understood. Prostaglandin treatment also increased outflow facility and hydraulic conductivity across human primary trabecular meshwork cell cultures.72 More recent studies on EP receptor stimulation has shown that EP2 and EP4 activation results in increased cell contractility of the trabecular meshwork, and decreased cell contractility of the inner wall of Schlemm's canal, mediating IOP through the conventional pathway.35
Prostaglandins also alter the production of MMPs in human primary trabecular meshwork cells. Latanoprost increased expression of MMP-1, -3, -17, and -24.68 Several MMPs and MMP inhibitors have been shown to increase outflow facility.76–78 MMP-17 activates aggrecanase-1 (ADAMTS4), potentially causing degradation of glycosaminoglycans and extracellular matrix.79,80 In monkeys treated unilaterally with latanoprost or bimatoprost for 12 months, significant IOP reduction was obtained. Histologically, treated eyes from these animals showed enlarged tissue space within the ciliary muscle and trabecular meshwork, confirming extracellular matrix remodeling within the conventional and unconventional outflow pathways as an effect of prostaglandin treatment.75
Molecular Targets of Prostaglandin Analogue Treatment
There is enough evidence to suggest that prostaglandin analogues lower IOP through tissue impedance changes and long-term remodeling of the extracellular matrix within the conventional and unconventional outflow pathways. However, this does not explain the early effects of prostaglandin analogue treatment in cell culture models.35 IOP was found to be lowered within 2 h of treatment in mice81 and human anterior segment culture.71 Additionally, several prospective human studies have confirmed an ocular hypotensive effect within a few hours of prostaglandin analogue treatment.45,82–85 These early effects are most likely due to rapid changes in cell signaling.
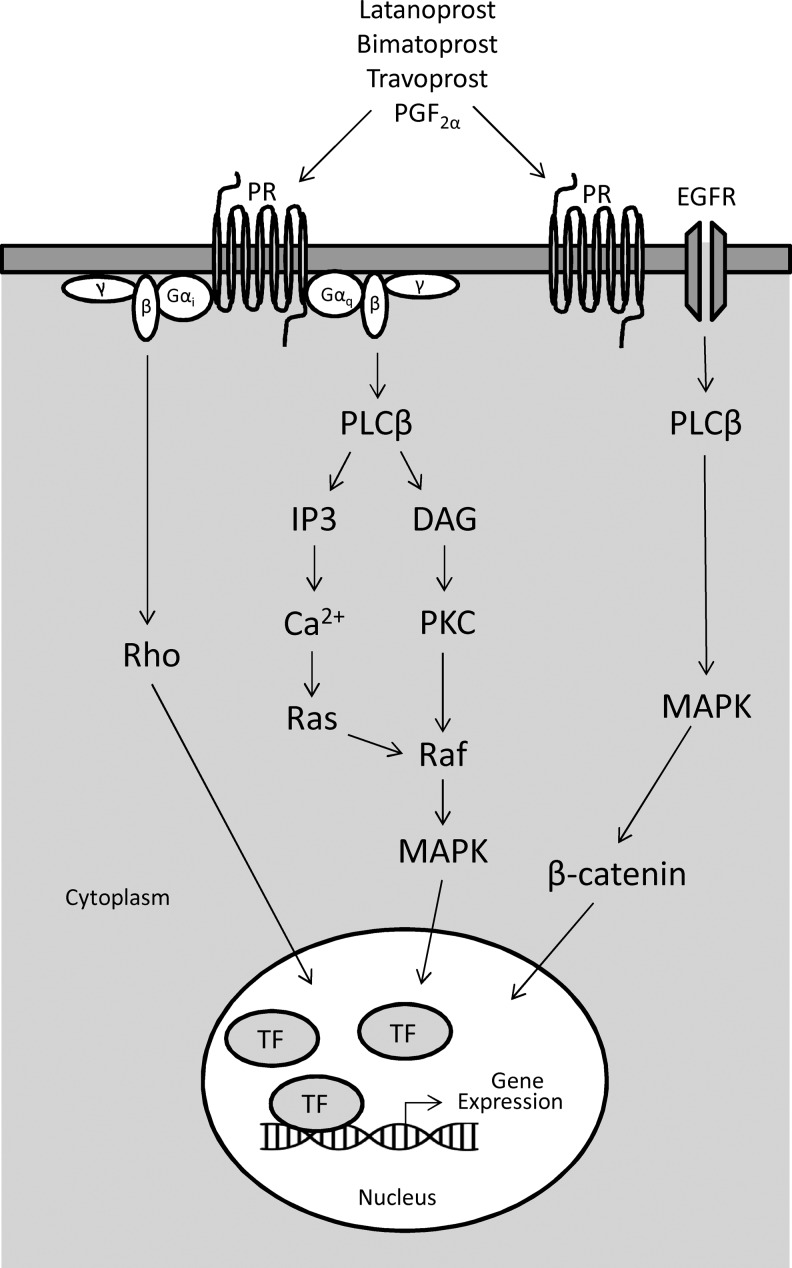
Prostaglandin FP receptors activate phosphatidylinositol metabolism through Gαq (G-coupled protein), leading to the formation of inositol triphosphate (IP3) with mobilization of intracellular free calcium (Fig. 2).32,54,86–88 Elevated intracellular calcium levels have been reported in primary open angle glaucoma trabecular meshwork cells as well as in a subset of trabecular meshwork cells after application of hydraulic pressure.89,90 Additionally, calcium-regulated potassium channels, BK(Ca), affect trabecular meshwork and Schlemm's canal cell volume, further indicating a role of calcium signaling in IOP homeostasis.91,92 Gαq is also linked to mitogen-activated protein kinase (MAPK) through calcium release and phospholipase Cβ (PLCβ)-induced activation of protein kinase C (PKC).93 PGF2α also activates Gαq-independent (Gαi) signaling leading to Rho-mediated cytoskeletal rearrangement and actin stress fiber formation.94
FIG. 2.
Supported cell signaling cascades for activation of immediate early genes following prostaglandin analogue treatment. Ligand binding to prostaglandin receptor (PR) activates G-protein-dependent (Gαq) and -independent (Gαi) pathways. Activation of Gαq-dependent pathway results in increased intracellular calcium stores and protein kinase C (PKC) induction leading to activation of the mitogen-activated protein kinase (MAPK) pathway. The Gαi-independent pathway leads to activation of Rho signaling. PR activation can also lead to phospholipase Cβ (PLCβ)-dependent transactivation of the epidermal growth factor receptor (EGFR) followed by MAPK and β-catenin activation. MAPK, Rho, and translocation of β-catenin to the nucleus induce gene expression of immediate early genes (eg, early growth response-1) and molecules involved in cell migration and cytoskeleton remodeling. TF, transcription factor; DAG, diacylglycerol; IP3, inositol triphosphate.
In addition to Gαq-dependent and -independent signaling, the wingless/integrase 1 (Wnt) signaling pathway has been linked to FP receptor activation (Fig. 2). Activated β-catenin, produced through PLCβ-dependent epidermal growth factor receptor (EGFR) and extracellular signal-regulated kinase (ERK) phosphorylation, can translocate to the nucleus and act as a transcriptional activator.95,96 In human trabecular meshwork cells, functional canonical and noncanonical Wnt signaling pathways are present.97,98 Treatment of human trabecular meshwork cells with travoprost reduced the formation of dexamethasone-induced crosslinked actin networks through a β-catenin-dependent pathway.99 Myocilin, a glaucoma-associated protein, increased cell migration through activation of focal adhesion kinase (FAK) and protein kinase B serine threonine kinase pathway (PKB/AKT).100 Together, these fast responsive intracellular signaling mechanisms (MAPK, Rho, and β-catenin) may explain some of the early effects of prostaglandin analogue treatment (Fig. 2).
In addition to signaling mechanisms, changes in transcription following prostaglandin analogue treatment have been identified in ciliary smooth muscle and trabecular meshwork cells. PGF2α upregulates several early response genes, including early growth response-1 (EGR-1), connective tissue growth factor (CTGF), hypoxia-inducible factor-1a (Hif-1a), c-fos, and Cyr61.59,101,102 All of these genes are involved in extracellular matrix remodeling through regulation of cell adhesion, migration, and survival. Many of these gene inductions appear to be working through the activation of MAPKs, specifically ERK1/2 (Fig. 2).101 Other transcriptional changes have also been reported, but with longer prostaglandin analogue treatment. Upregulation of insulin growth factor 1 (IGF1), fibroleukin, TNFSF10 (TRAIL), and promelanosome-concentrating hormone was identified in primary human trabecular meshwork cells treated with latanoprost for 9 days.103 IGF1 has been linked to changes in metabolic activity and activation of the MMPs, stromelysin and gelatinase. Fibroleukin acts as a protease, and may digest extracellular matrix components. However, glaucoma-associated proteins myocilin, optineurin, and αβ-crystallin showed no change in expression indicating that these genes may not be involved in long-term prostaglandin analogue-mediated cell signaling. Detailed genome-wide in vitro studies analyzing short- and long-term effects of prostaglandin analogue treatment will be important to further identify candidate genes involved in prostaglandin analogue-mediated IOP reduction.
Conclusion
The identification of prostaglandin analogues as ocular hypotensive agents has had an enormous positive impact on the treatment of glaucoma. Each mmHg drop in mean IOP decreases the risk of glaucoma progression by 10%.104 However, some patients do not respond to treatment with one prostaglandin analogue, but find success with another.16–18 Understanding how prostaglandin analogues can selectively lower IOP provides an avenue to understand how IOP is regulated in normal and glaucomatous conditions. Whereas prostaglandins have historically been thought of as unconventional outflow pathway modifiers, significant evidence supports their role in modifying the conventional outflow pathway, which is the site of increased outflow resistance in glaucoma. Understanding the physiologic responses of outflow tissues to prostaglandin analogue treatment will be an important area of research in the future. New analogues are being developed that show specificity for FP and EP receptors, providing new opportunities to improve IOP reduction and minimize side effects. Characterizing the molecular fingerprint of prostaglandin treatment will elevate our understanding of the signaling mechanisms leading to identification of the physiological responses associated with IOP reduction in the conventional outflow pathway. This will also lead to identification of better and more specific therapeutic targets for the treatment of glaucoma.
Acknowledgments
The authors would like to thank Dr. Uttio Roy Chowdhury for his helpful suggestions and assistance in reviewing the contents of the article. Supported, in part, by the National Institutes of Health research grants EY 07065, EY 21727; the Mayo Foundation, Rochester, MN; and Research to Prevent Blindness, New York, NY (M.P.F. is a recipient of a Lew R. Wasserman Merit Award and the Department of Ophthalmology, Mayo Clinic is the recipient of an unrestricted grant).
Author Disclosure Statement
No competing financial interests exist.
References
- 1.Toris C.B., Yablonski M.E., Wang Y.-L., and Camras C.B.Aqueous humor dynamics in the aging human eye. Am. J. Ophthal. 127:407–412, 1999 [DOI] [PubMed] [Google Scholar]
- 2.Overby D.R., Stamer W.D., and Johnson M.The changing paradigm of outflow resistance generation: towards synergistic models of the JCT and inner wall endothelium. Exp. Eye Res. 88:656–670, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Brubaker R.F.Targeting outflow facility in glaucoma management. Surv. Ophthalmol. 48Suppl 1:S17–S20, 2003 [DOI] [PubMed] [Google Scholar]
- 4.Liu J.H., Zhang X., Kripke D.F., and Weinreb R.N.Twenty-four-hour intraocular pressure pattern associated with early glaucomatous changes. Invest. Ophthalmol. Vis. Sci. 44:1586–1590, 2003 [DOI] [PubMed] [Google Scholar]
- 5.Stamer W.D., and Acott T.S.Current understanding of conventional outflow dysfunction in glaucoma. Curr. Opin. Ophthalmol. 23:135–143, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Colina-Chourio J.A., Godoy-Godoy N., and Avila-Hernandez R.M.Role of prostaglandins in hypertension. J. Hum. Hypertens. 14Suppl 1:S16–S19, 2000 [DOI] [PubMed] [Google Scholar]
- 7.Hata A.N., and Breyer R.M.Pharmacology and signaling of prostaglandin receptors: multiple roles in inflammation and immune modulation. Pharmacol. Ther. 103:147–166, 2004 [DOI] [PubMed] [Google Scholar]
- 8.Camras C.B., Bito L.Z., and Eakins K.E.Reduction of intraocular pressure by prostaglandins applied topically to the eyes of conscious rabbits. Invest. Ophthalmol. Vis. Sci. 16:1125–1134, 1977 [PubMed] [Google Scholar]
- 9.Aptel F., Cucherat M., and Denis P.Efficacy and tolerability of prostaglandin analogs: a meta-analysis of randomized controlled clinical trials. J. Glaucoma. 17:667–673, 2008 [DOI] [PubMed] [Google Scholar]
- 10.Choplin N., Bernstein P., Batoosingh A.L., and Whitcup S.M.A randomized, investigator-masked comparison of diurnal responder rates with bimatoprost and latanoprost in the lowering of intraocular pressure. Surv. Ophthalmol. 49Suppl 1:S19–S25, 2004 [DOI] [PubMed] [Google Scholar]
- 11.Costagliola C., Del Prete A., Verolino M., Antinozzi P., Fusco R., Parmeggiani F., and Mastropasqua L.Effect of 0.005% latanoprost once daily on intraocular pressure in glaucomatous patients not adequately controlled by beta-blockers twice daily: a 3-year follow-up. Experience and incidence of side effects in a prospective study on 76 patients. Graefes Arch. Clin. Exp. Ophthalmol. 240:379–386, 2002 [DOI] [PubMed] [Google Scholar]
- 12.Brubaker R.F.Mechanism of action of bimatoprost (Lumigan). Surv. Ophthalmol. 45Suppl 4:S347–S351, 2001 [DOI] [PubMed] [Google Scholar]
- 13.Cheng J.W., Li Y., and Wei R.L.Systematic review of intraocular pressure-lowering effects of adjunctive medications added to latanoprost. Ophthalmic Res. 42:99–105, 2009 [DOI] [PubMed] [Google Scholar]
- 14.Tabet R., Stewart W.C., Feldman R., and Konstas A.G.A review of additivity to prostaglandin analogs: fixed and unfixed combinations. Surv. Ophthalmol. 53Suppl 1:S85–S92, 2008 [DOI] [PubMed] [Google Scholar]
- 15.Woodward D.F., and Chen J.Fixed-combination and emerging glaucoma therapies. Expert Opin. Emerg. Drugs. 12:313–327, 2007 [DOI] [PubMed] [Google Scholar]
- 16.Eisenberg D.Latanoprost versus bimatoprost. Ophthalmology. 110:1861–1862, 2003; author reply 1862. [DOI] [PubMed] [Google Scholar]
- 17.Gandolfi S.A., and Cimino L.Effect of bimatoprost on patients with primary open-angle glaucoma or ocular hypertension who are nonresponders to latanoprost. Ophthalmology. 110:609–614, 2003 [DOI] [PubMed] [Google Scholar]
- 18.Kammer J.A., Katzman B., Ackerman S.L., and Hollander D.A.Efficacy and tolerability of bimatoprost versus travoprost in patients previously on latanoprost: a 3-month, randomised, masked-evaluator, multicentre study. Br. J. Ophthalmol. 94:74–79, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Cheng J.W., and Wei R.L.Meta-analysis of 13 randomized controlled trials comparing bimatoprost with latanoprost in patients with elevated intraocular pressure. Clin. Ther. 30:622–632, 2008 [DOI] [PubMed] [Google Scholar]
- 20.Denis P., Lafuma A., Khoshnood B., Mimaud V., and Berdeaux G.A meta-analysis of topical prostaglandin analogues intra-ocular pressure lowering in glaucoma therapy. Curr. Med. Res. Opin. 23:601–608, 2007 [DOI] [PubMed] [Google Scholar]
- 21.Gandolfi S., Simmons S.T., Sturm R., Chen K., and VanDenburgh A.M.Three-month comparison of bimatoprost and latanoprost in patients with glaucoma and ocular hypertension. Adv. Ther. 18:110–121, 2001 [DOI] [PubMed] [Google Scholar]
- 22.Holmstrom S., Buchholz P., Walt J., Wickstrom J., and Aagren M.Analytic review of bimatoprost, latanoprost and travoprost in primary open angle glaucoma. Curr. Med. Res. Opin. 21:1875–1883, 2005 [DOI] [PubMed] [Google Scholar]
- 23.Simmons S.T., Dirks M.S., and Noecker R.J.Bimatoprost versus latanoprost in lowering intraocular pressure in glaucoma and ocular hypertension: results from parallel-group comparison trials. Adv. Ther. 21:247–262, 2004 [DOI] [PubMed] [Google Scholar]
- 24.Woodward D.F., Carling R.W., Cornell C.L., Fliri H.G., Martos J.L., Pettit S.N., Liang Y., and Wang J.W.The pharmacology and therapeutic relevance of endocannabinoid derived cyclo-oxygenase (COX)-2 products. Pharmacol. Ther. 120:71–80, 2008 [DOI] [PubMed] [Google Scholar]
- 25.Schlotzer-Schrehardt U., Zenkel M., and Nusing R.M.Expression and localization of FP and EP prostanoid receptor subtypes in human ocular tissues. Invest. Ophthalmol. Vis Sci. 43:1475–1487, 2002 [PubMed] [Google Scholar]
- 26.Sharif N.A., Kelly C.R., Crider J.Y., Williams G.W., and Xu S.X.Ocular hypotensive FP prostaglandin (PG) analogs: PG receptor subtype binding affinities and selectivities, and agonist potencies at FP and other PG receptors in cultured cells. J. Ocul. Pharmacol. Ther. 19:501–515, 2003 [DOI] [PubMed] [Google Scholar]
- 27.Swymer C., and Neville M.W.Tafluprost: the first preservative-free prostaglandin to treat open-angle glaucoma and ocular hypertension. Ann. Pharmacother. 46:1506–1510, 2012 [DOI] [PubMed] [Google Scholar]
- 28.Abramovitz M., Adam M., Boie Y., Carriere M., Denis D., Godbout C., Lamontagne S., Rochette C., Sawyer N., Tremblay N.M., Belley M., Gallant M., Dufresne C., Gareau Y., Ruel R., Juteau H., Labelle M., Ouimet N., and Metters K.M.The utilization of recombinant prostanoid receptors to determine the affinities and selectivities of prostaglandins and related analogs. Biochim. Biophys. Acta. 1483:285–293, 2000 [DOI] [PubMed] [Google Scholar]
- 29.Liang Y., Woodward D.F., Guzman V.M., Li C., Scott D.F., Wang J.W., Wheeler L.A., Garst M.E., Landsverk K., Sachs G., Krauss A.H., Cornell C., Martos J., Pettit S., and Fliri H.Identification and pharmacological characterization of the prostaglandin FP receptor and FP receptor variant complexes. Br. J. Pharmacol. 154:1079–1093, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Sharif N.A., and Klimko P.Update and commentary on the pro-drug bimatoprost and a putative ‘prostamide receptor’. Expert Rev. Ophthalmol. 4:477–489, 2009 [Google Scholar]
- 31.Patil A.J., Vajaranant T.S., and Edward D.P.Bimatoprost—a review. Expert Opin. Pharmacother. 10:2759–2768, 2009 [DOI] [PubMed] [Google Scholar]
- 32.Anthony T.L., Pierce K.L., Stamer W.D., and Regan J.W.Prostaglandin F2 alpha receptors in the human trabecular meshwork. Invest. Ophthalmol. Vis. Sci. 39:315–321, 1998 [PubMed] [Google Scholar]
- 33.Kamphuis W., Schneemann A., van Beek L.M., Smit A.B., Hoyng P.F., and Koya E.Prostanoid receptor gene expression profile in human trabecular meshwork: a quantitative real-time PCR approach. Invest. Ophthalmol. Vis. Sci. 42:3209–3215, 2001 [PubMed] [Google Scholar]
- 34.Thieme H., Schimmat C., Munzer G., Boxberger M., Fromm M., Pfeiffer N., and Rosenthal R.Endothelin antagonism: effects of FP receptor agonists prostaglandin F2alpha and fluprostenol on trabecular meshwork contractility. Invest. Ophthalmol. Vis. Sci. 47:938–945, 2006 [DOI] [PubMed] [Google Scholar]
- 35.Wang J.W., Woodward D.F., and Stamer W.D.Differential effects of prostaglandin E2-sensitive receptors on contractility of human ocular cells that regulate conventional outflow. Invest. Ophthalmol. Vis. Sci. 54:4782–4790, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Davis T.L., and Sharif N.A.Quantitative autoradiographic visualization and pharmacology of FP-prostaglandin receptors in human eyes using the novel phosphor-imaging technology. J. Ocul. Pharmacol. Ther. 15:323–336, 1999 [DOI] [PubMed] [Google Scholar]
- 37.Sharif N.A., Davis T.L., and Williams G.W.[3H]AL-5848 ([3H]9beta-(+)-Fluprostenol). Carboxylic acid of travoprost (AL-6221), a novel FP prostaglandin to study the pharmacology and autoradiographic localization of the FP receptor. J. Pharm. Pharmacol. 51:685–694, 1999 [DOI] [PubMed] [Google Scholar]
- 38.Sharif N.A., Williams G.W., and Davis T.L.Pharmacology and autoradiography of human DP prostanoid receptors using [(3)H]-BWA868C, a DP receptor-selective antagonist radioligand. Br. J. Pharmacol. 131:1025–1038, 2000 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Ota T., Aihara M., Narumiya S., and Araie M.The effects of prostaglandin analogues on IOP in prostanoid FP-receptor-deficient mice. Invest. Ophthalmol. Vis. Sci. 46:4159–4163, 2005 [DOI] [PubMed] [Google Scholar]
- 40.Griffin B.W., Klimko P., Crider J.Y., and Sharif N.A.AL-8810: a novel prostaglandin F2 alpha analog with selective antagonist effects at the prostaglandin F2 alpha (FP) receptor. J. Pharmacol. Exp. Ther. 290:1278–1284, 1999 [PubMed] [Google Scholar]
- 41.Sharif N.A., Kelly C.R., and Crider J.Y.Human trabecular meshwork cell responses induced by bimatoprost, travoprost, unoprostone, and other FP prostaglandin receptor agonist analogues. Invest. Ophthalmol. Vis. Sci. 44:715–721, 2003 [DOI] [PubMed] [Google Scholar]
- 42.Ota T., Aihara M., Saeki T., Narumiya S., and Araie M.The effects of prostaglandin analogues on prostanoid EP1, EP2, and EP3 receptor-deficient mice. Invest. Ophthalmol. Vis. Sci. 47:3395–3399, 2006 [DOI] [PubMed] [Google Scholar]
- 43.Nilsson S.F., Drecoll E., Lutjen-Drecoll E., Toris C.B., Krauss A.H., Kharlamb A., Nieves A., Guerra T., and Woodward D.F.The prostanoid EP2 receptor agonist butaprost increases uveoscleral outflow in the cynomolgus monkey. Invest. Ophthalmol. Vis. Sci. 47:4042–4049, 2006 [DOI] [PubMed] [Google Scholar]
- 44.Woodward D.F., Nilsson S.F., Toris C.B., Kharlamb A.B., Nieves A.L., and Krauss A.H.Prostanoid EP4 receptor stimulation produces ocular hypotension by a mechanism that does not appear to involve uveoscleral outflow. Invest. Ophthalmol. Vis. Sci. 50:3320–3328, 2009 [DOI] [PubMed] [Google Scholar]
- 45.Alm A., and Villumsen J.PhXA34, a new potent ocular hypotensive drug. A study on dose-response relationship and on aqueous humor dynamics in healthy volunteers. Arch. Ophthalmol. 109:1564–1568, 1991 [DOI] [PubMed] [Google Scholar]
- 46.Kerstetter J.R., Brubaker R.F., Wilson S.E., and Kullerstrand L.J.Prostaglandin F2 alpha-1-isopropylester lowers intraocular pressure without decreasing aqueous humor flow. Am. J. Ophthalmol. 105:30–34, 1988 [DOI] [PubMed] [Google Scholar]
- 47.Lim K.S., Nau C.B., O'Byrne M.M., Hodge D.O., Toris C.B., McLaren J.W., and Johnson D.H.Mechanism of action of bimatoprost, latanoprost, and travoprost in healthy subjects. A crossover study. Ophthalmology. 115:790–795e794, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Linden C., and Alm A.Prostaglandin analogues in the treatment of glaucoma. Drugs Aging. 14:387–398, 1999 [DOI] [PubMed] [Google Scholar]
- 49.Toris C.B., Camras C.B., and Yablonski M.E.Effects of PhXA41, a new prostaglandin F2 alpha analog, on aqueous humor dynamics in human eyes. Ophthalmology. 100:1297–1304, 1993 [DOI] [PubMed] [Google Scholar]
- 50.Ziai N., Dolan J.W., Kacere R.D., and Brubaker R.F.The effects on aqueous dynamics of PhXA41, a new prostaglandin F2 alpha analogue, after topical application in normal and ocular hypertensive human eyes. Arch. Ophthalmol. 111:1351–1358, 1993 [DOI] [PubMed] [Google Scholar]
- 51.Toris C.B., Gabelt B.T., and Kaufman P.L.Update on the mechanism of action of topical prostaglandins for intraocular pressure reduction. Surv. Ophthalmol. 53Suppl 1:S107–S120, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Alm A., and Nilsson S.F.Uveoscleral outflow—a review. Exp. Eye Res. 88:760–768 2009 [DOI] [PubMed] [Google Scholar]
- 53.Poyer J.F., Millar C., and Kaufman P.L.Prostaglandin F2 alpha effects on isolated rhesus monkey ciliary muscle. Invest. Ophthalmol. Vis. Sci. 36:2461–2465, 1995 [PubMed] [Google Scholar]
- 54.Stamer W.D., Piwnica D., Jolas T., Carling R.W., Cornell C.L., Fliri H., Martos J., Pettit S.N., Wang J.W., and Woodward D.F.Cellular basis for bimatoprost effects on human conventional outflow. Invest. Ophthalmol. Vis. Sci. 51:5176–5181, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Schachtschabel U., Lindsey J.D., and Weinreb R.N.The mechanism of action of prostaglandins on uveoscleral outflow. Curr. Opin. Ophthalmol. 11:112–115, 2000 [DOI] [PubMed] [Google Scholar]
- 56.Lindsey J.D., Kashiwagi K., Boyle D., Kashiwagi F., Firestein G.S., and Weinreb R.N.Prostaglandins increase proMMP-1 and proMMP-3 secretion by human ciliary smooth muscle cells. Curr. Eye Res. 15:869–875, 1996 [DOI] [PubMed] [Google Scholar]
- 57.Lindsey J.D., Kashiwagi K., Kashiwagi F., and Weinreb R.N.Prostaglandin action on ciliary smooth muscle extracellular matrix metabolism: implications for uveoscleral outflow. Surv. Ophthalmol. 41Suppl 2:S53–S59, 1997 [DOI] [PubMed] [Google Scholar]
- 58.Lindsey J.D., Kashiwagi K., Kashiwagi F., and Weinreb R.N.Prostaglandins alter extracellular matrix adjacent to human ciliary muscle cells in vitro. Invest. Ophthalmol. Vis. Sci. 38:2214–2223, 1997 [PubMed] [Google Scholar]
- 59.Lindsey J.D., To H.D., and Weinreb R.N.Induction of c-fos by prostaglandin F2 alpha in human ciliary smooth muscle cells. Invest. Ophthalmol. Vis. Sci. 35:242–250, 1994 [PubMed] [Google Scholar]
- 60.Sagara T., Gaton D.D., Lindsey J.D., Gabelt B.T., Kaufman P.L., and Weinreb R.N.Reduction of collagen type I in the ciliary muscle of inflamed monkey eyes. Invest. Ophthalmol. Vis. Sci. 40:2568–2576, 1999 [PubMed] [Google Scholar]
- 61.Weinreb R.N., and Lindsey J.D.Metalloproteinase gene transcription in human ciliary muscle cells with latanoprost. Invest. Ophthalmol. Vis. Sci. 43:716–722, 2002 [PubMed] [Google Scholar]
- 62.Oh D.J., Martin J.L., Williams A.J., Peck R.E., Pokorny C., Russell P., Birk D.E., and Rhee D.J.Analysis of expression of matrix metalloproteinases and tissue inhibitors of metalloproteinases in human ciliary body after latanoprost. Invest. Ophthalmol. Vis. Sci. 47:953–963, 2006 [DOI] [PubMed] [Google Scholar]
- 63.Baker A.H., Edwards D.R., and Murphy G.Metalloproteinase inhibitors: biological actions and therapeutic opportunities. J. Cell Sci. 115:3719–3727, 2002 [DOI] [PubMed] [Google Scholar]
- 64.Nagase H., Visse R., and Murphy G.Structure and function of matrix metalloproteinases and TIMPs. Cardiovasc. Res. 69:562–573, 2006 [DOI] [PubMed] [Google Scholar]
- 65.Nagase H., and Woessner J.F., Jr., Matrix metalloproteinases. J. Biol. Chem. 274:21491–21494, 1999 [DOI] [PubMed] [Google Scholar]
- 66.Van den Steen P.E., Dubois B., Nelissen I., Rudd P.M., Dwek R.A., and Opdenakker G.Biochemistry and molecular biology of gelatinase B or matrix metalloproteinase-9 (MMP-9). Crit. Rev. Biochem. Mol. Biol. 37:375–536, 2002 [DOI] [PubMed] [Google Scholar]
- 67.Hinz B., Rosch S., Ramer R., Tamm E.R., and Brune K.Latanoprost induces matrix metalloproteinase-1 expression in human nonpigmented ciliary epithelial cells through a cyclooxygenase-2-dependent mechanism. FASEB J. 19:1929–1931, 2005 [DOI] [PubMed] [Google Scholar]
- 68.Oh D.J., Martin J.L., Williams A.J., Russell P., Birk D.E., and Rhee D.J.Effect of latanoprost on the expression of matrix metalloproteinases and their tissue inhibitors in human trabecular meshwork cells. Invest. Ophthalmol. Vis. Sci. 47:3887–3895, 2006 [DOI] [PubMed] [Google Scholar]
- 69.Weinreb R.N., Kashiwagi K., Kashiwagi F., Tsukahara S., and Lindsey J.D.Prostaglandins increase matrix metalloproteinase release from human ciliary smooth muscle cells. Invest. Ophthalmol. Vis. Sci. 38:2772–2780, 1997 [PubMed] [Google Scholar]
- 70.Lutjen-Drecoll E., and Tamm E.Morphological study of the anterior segment of cynomolgus monkey eyes following treatment with prostaglandin F2 alpha. Exp. Eye Res. 47:761–769, 1988 [DOI] [PubMed] [Google Scholar]
- 71.Bahler C.K., Howell K.G., Hann C.R., Fautsch M.P., and Johnson D.H.Prostaglandins increase trabecular meshwork outflow facility in cultured human anterior segments. Am. J. Ophthalmol. 145:114–119, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Wan Z., Woodward D.F., Cornell C.L., Fliri H.G., Martos J.L., Pettit S.N., Wang J.W., Kharlamb A.B., Wheeler L.A., Garst M.E., Landsverk K.J., Struble C.S., and Stamer W.D.Bimatoprost, prostamide activity, and conventional drainage. Investig. Ophthal. Vis. Sci. 48:4107–4115, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Bill A., Lutjen-Drecoll E., and Svedbergh B.Effects of intracameral Na2EDTA and EGTA on aqueous outflow routes in the monkey eye. Invest. Ophthalmol. Vis. Sci. 19:492–504, 1980 [PubMed] [Google Scholar]
- 74.Svedbergh B., Lutjen-Drecoll E., Ober M., and Kaufman P.L.Cytochalasin B-induced structural changes in the anterior ocular segment of the cynomolgus monkey. Invest. Ophthalmol. Vis. Sci. 17:718–734, 1978 [PubMed] [Google Scholar]
- 75.Richter M., Krauss A.H., Woodward D.F., and Lutjen-Drecoll E.Morphological changes in the anterior eye segment after long-term treatment with different receptor selective prostaglandin agonists and a prostamide. Invest. Ophthalmol. Vis. Sci. 44:4419–4426, 2003 [DOI] [PubMed] [Google Scholar]
- 76.Pang I.H., Hellberg P.E., Fleenor D.L., Jacobson N., and Clark A.F.Expression of matrix metalloproteinases and their inhibitors in human trabecular meshwork cells. Investig. Ophthal. Vis. Sci. 44:3485–3493, 2003 [DOI] [PubMed] [Google Scholar]
- 77.Parshley D.E., Bradley J.M., Samples J.R., Van Buskirk E.M., and Acott T.S.Early changes in matrix metalloproteinases and inhibitors after in vitro laser treatment to the trabecular meshwork. Curr. Eye Res. 14:537–544, 1995 [DOI] [PubMed] [Google Scholar]
- 78.Bradley J.M., Vranka J., Colvis C.M., Conger D.M., Alexander J.P., Fisk A.S., Samples J.R., and Acott T.S.Effect of matrix metalloproteinases activity on outflow in perfused human organ culture. Invest. Ophthalmol. Vis. Sci. 39:2649–2658, 1998 [PubMed] [Google Scholar]
- 79.Arai M., Anderson D., Kurdi Y., Annis-Freeman B., Shields K., Collins-Racie L.A., Corcoran C., DiBlasio-Smith E., Pittman D.D., Dorner A.J., Morris E., and LaVallie E.R.Effect of adenovirus-mediated overexpression of bovine ADAMTS-4 and human ADAMTS-5 in primary bovine articular chondrocyte pellet culture system. Osteoarthritis Cartilage. 12:599–613, 2004 [DOI] [PubMed] [Google Scholar]
- 80.Ishiguro N., and Kojima T.[Role of aggrecanase and MMP in cartilage degradation]. Clin. Calcium. 14:38–44, 2004 [PubMed] [Google Scholar]
- 81.Aihara M., Lindsey J.D., and Weinreb R.N.Reduction of intraocular pressure in mouse eyes treated with latanoprost. Invest. Ophthalmol. Vis. Sci. 43:146–150, 2002 [PubMed] [Google Scholar]
- 82.Costagliola C., dell'Omo R., Romano M.R., Rinaldi M., Zeppa L., and Parmeggiani F.Pharmacotherapy of intraocular pressure—part II. Carbonic anhydrase inhibitors, prostaglandin analogues and prostamides. Expert Opin. Pharmacother. 10:2859–2870, 2009 [DOI] [PubMed] [Google Scholar]
- 83.Hotehama Y., and Mishima H.K.Clinical efficacy of PhXA34 and PhXA41, two novel prostaglandin F2 alpha-isopropyl ester analogues for glaucoma treatment. Jpn. J. Ophthalmol. 37:259–269, 1993 [PubMed] [Google Scholar]
- 84.Larsson L.I., Mishima H.K., Takamatsu M., Orzalesi N., and Rossetti L.The effect of latanoprost on circadian intraocular pressure. Surv. Ophthalmol. 47Suppl 1:S90–S96, 2002 [DOI] [PubMed] [Google Scholar]
- 85.Nguyen Q.H.The role of prostaglandin analogues in the treatment of glaucoma in the 21st century. Int. Ophthalmol. Clin. 44:15–27, 2004 [DOI] [PubMed] [Google Scholar]
- 86.Abramovitz M., Boie Y., Nguyen T., Rushmore T.H., Bayne M.A., Metters K.M., Slipetz D.M., and Grygorczyk R.Cloning and expression of a cDNA for the human prostanoid FP receptor. J. Biol. Chem. 269:2632–2636, 1994 [PubMed] [Google Scholar]
- 87.Sugimoto Y., Hasumoto K., Namba T., Irie A., Katsuyama M., Negishi M., Kakizuka A., Narumiya S., and Ichikawa A.Cloning and expression of a cDNA for mouse prostaglandin F receptor. J. Biol. Chem. 269:1356–1360, 1994 [PubMed] [Google Scholar]
- 88.Watanabe T., Nakao A., Emerling D., Hashimoto Y., Tsukamoto K., Horie Y., Kinoshita M., and Kurokawa K.Prostaglandin F2 alpha enhances tyrosine phosphorylation and DNA synthesis through phospholipase C-coupled receptor via Ca(2+)-dependent intracellular pathway in NIH-3T3 cells. J. Biol. Chem. 269:17619–17625, 1994 [PubMed] [Google Scholar]
- 89.He Y., Ge J., and Tombran-Tink J.Mitochondrial defects and dysfunction in calcium regulation in glaucomatous trabecular meshwork cells. Invest. Ophthalmol. Vis. Sci. 49:4912–4922, 2008 [DOI] [PubMed] [Google Scholar]
- 90.Matsuo T., and Matsuo N.Intracellular calcium response to hydraulic pressure in human trabecular cells. Br. J. Ophthalmol. 80:561–566, 1996 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Dismuke W.M., and Ellis D.Z.Activation of the BKCa channel increases outflow facility and decreases trabecular meshwork cell volume. J. Ocul. Pharmacol. Ther. 4:309–313, 2009 [DOI] [PubMed] [Google Scholar]
- 92.Ellis D.Z., Sharif N.A., and Dismuke W.M.Endogenous regulation of human Schlemm's canal cell volume by nitric oxide signaling. Invest. Ophthalmol. Vis. Sci. 51:5817–5824, 2010 [DOI] [PubMed] [Google Scholar]
- 93.Bos C.L., Richel D.J., Ritsema T., Peppelenbosch M.P., and Versteeg H.H.Prostanoids and prostanoid receptors in signal transduction. Int. J. Biochem. Cell Biol. 36:1187–1205, 2004 [DOI] [PubMed] [Google Scholar]
- 94.Pierce K.L., Fujino H., Srinivasan D., and Regan J.W.Activation of FP prostanoid receptor isoforms leads to Rho-mediated changes in cell morphology and in the cell cytoskeleton. J. Biol. Chem. 274:35944–35949, 1999 [DOI] [PubMed] [Google Scholar]
- 95.Baarsma H.A., Konigshoff M., and Gosens R.The WNT signaling pathway from ligand secretion to gene transcription: molecular mechanisms and pharmacological targets. Pharmacol. Ther. 138:66–83, 2013 [DOI] [PubMed] [Google Scholar]
- 96.Sales K.J., Milne S.A., Williams A.R., Anderson R.A., and Jabbour H.N.Expression, localization, and signaling of prostaglandin F2 alpha receptor in human endometrial adenocarcinoma: regulation of proliferation by activation of the epidermal growth factor receptor and mitogen-activated protein kinase signaling pathways. J. Clin. Endocrinol. Metab. 89:986–993, 2004 [DOI] [PubMed] [Google Scholar]
- 97.Mao W., Millar J.C., Wang W.H., Silverman S.M., Liu Y., Wordinger R.J., Rubin J.S., Pang I.H., and Clark A.F.Existence of the canonical Wnt signaling pathway in the human trabecular meshwork. Invest. Ophthalmol. Vis. Sci. 53:7043–7051, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Shyam R., Shen X., Yue B.Y., and Wentz-Hunter K.K.Wnt gene expression in human trabecular meshwork cells. Mol. Vis. 16:122–129, 2010 [PMC free article] [PubMed] [Google Scholar]
- 99.Peng J., Lei C.T., Hu J.B., and Fan Y.C.Effects of travoprost on actin cytoskeleton and beta-catenin in the human trabecular meshwork cells treated with dexamethasone. Zhonghua Yan Ke Za Zhi. 47:336–341, 2011 [PubMed] [Google Scholar]
- 100.Kwon H.S., and Tomarev S.I.Myocilin, a glaucoma-associated protein, promotes cell migration through activation of integrin-focal adhesion kinase-serine/threonine kinase signaling pathway. J. Cell Physiol. 226:3392–3402, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Hutchinson A.J., Coons S.C., Chou C.L., Xu W., Stamer W.D., Woodward D.F., and Regan J.W.Induction of angiogenic immediate early genes by activation of FP prostanoid receptors in cultured human ciliary smooth muscle cells. Curr. Eye Res. 35:408–418, 2010 [DOI] [PubMed] [Google Scholar]
- 102.Liang Y., Li C., Guzman V.M., Evinger A.J., 3rd, Protzman C.E., Krauss A.H., and Woodward D.F.Comparison of prostaglandin F2alpha, bimatoprost (prostamide), and butaprost (EP2 agonist) on Cyr61 and connective tissue growth factor gene expression. J. Biol. Chem. 278:27267–27277, 2003 [DOI] [PubMed] [Google Scholar]
- 103.Zhao X., Pearson K.E., Stephan D.A., and Russell P.Effects of prostaglandin analogues on human ciliary muscle and trabecular meshwork cells. Investig. Ophthalmol. Vis. Sci. 44:1945–1952, 2003 [DOI] [PubMed] [Google Scholar]
- 104.Kass M.A., Heuer D.K., Higginbotham E.J., Johnson C.A., Keltner J.L., Miller J.P., Parrish R.K., 2nd, Wilson M.R., and Gordon M.O.The Ocular Hypertension Treatment Study: a randomized trial determines that topical ocular hypotensive medication delays or prevents the onset of primary open-angle glaucoma. Arch. Ophthalmol. 120:701–713, 2002; discussion 829–830. [DOI] [PubMed] [Google Scholar]