Abstract
Microfibrils are macromolecular aggregates located in the extracellular matrix of both elastic and nonelastic tissues that have essential functions in formation of elastic fibers and control of signaling through the transforming growth factor beta (TGFβ) family of cytokines. Elevation of systemic TGFβ and chronic activation of TGFβ signal transduction are associated with diseases caused by mutations in microfibril-associated genes, including FBN1. A role for microfibrils in glaucoma is suggested by identification of risk alleles in LOXL1 for exfoliation glaucoma and mutations in LTBP2 for primary congenital glaucoma, both of which are microfibril-associated genes. Recent identification of a mutation in another microfibril-associated gene, ADAMTS10, in a dog model of primary open-angle glaucoma led us to form the microfibril hypothesis of glaucoma, which in general states that defective microfibrils may be an underlying cause of glaucoma. Microfibril defects could contribute to glaucoma through alterations in biomechanical properties of tissue and/or through effects on signaling through TGFβ, which is well established to be elevated in the aqueous humor of glaucoma patients. Recent work has shown that diseases caused by microfibril defects are associated with increased concentrations of TGFβ protein and chronic activation of TGFβ-mediated signal transduction. In analogy with other microfibril-related diseases, defective microfibrils could provide a mechanism for the elevation of TGFβ2 in glaucomatous aqueous humor. If glaucoma shares mechanisms with other diseases caused by defective microfibrils, such as Marfan syndrome, therapeutic interventions to inhibit chronic activation of TGFβ signaling used in those diseases may be applied to glaucoma.
The Microfibril Hypothesis of Glaucoma
Identification of a mutation in ADAMTS10 in the dog model of primary open-angle glaucoma led us to form the microfibril hypothesis of glaucoma, which in general states that defective microfibrils may be an underlying cause of glaucoma.1 The microfibril hypothesis is based on genetic and biological evidence and is consistent with many key features of glaucoma that have been studied extensively. Genetic support for the microfibril hypothesis includes involvement of the microfibril-associated genes, LOXL1,2 LTBP2,3,4 and ADAMTS101 in glaucoma and the high prevalence of primary open-angle glaucoma in patients with Marfan syndrome,5 which is caused by mutations in FBN1, the gene that encodes fibrillin-1, the main component of microfibrils.6 Microfibril defects could contribute to glaucoma through alterations in biomechanical properties of tissue and/or through effects on signaling through transforming growth factor beta (TGFβ). Microfibrils are the major reservoir of latent TGFβ and are central to TGFβ localization and signaling.7–11 Defective microfibrils could provide a mechanism for the elevation of TGFβ2 in the aqueous humor of glaucoma patients. If glaucoma shares mechanisms with other diseases caused by defective microfibrils, therapeutic interventions to inhibit chronic activation of TGFβ signaling used in those diseases may be applied to glaucoma.
Discovery of Microfibrils
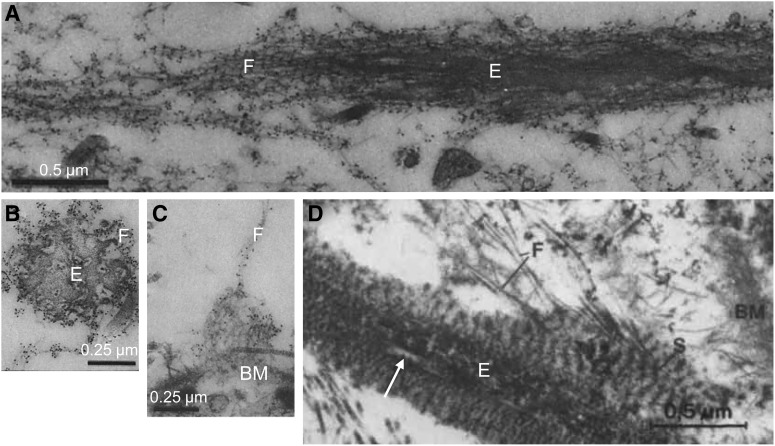
An electron microscopy study of the cornea in 1954 by Jakus12 is perhaps the earliest identification of the fine extracellular filaments in connective tissue that became known as microfibrils. In 1958, Karrer noted that these filamentous structures sometimes had a beaded appearance and were associated with elastic fibers.13 Summarizing the work of several investigators in 1962, Low described these structures as a basic element of connective tissue frequently associated with elastic fibers and basement membranes and suggested the specific name of microfibrils to describe them.14 In 1969 Ross and Bornstein more fully described microfibrils as forming sheaths surrounding elastic fibers and noted that microfibrils appeared to form an aggregate structure preceding development of elastin cores,15 an observation that has been subsequently confirmed by demonstrated requirement for formation of a microfibril scaffold on which elastin cores assemble.16 Microfibrils are now well established to be widely expressed constituents of extracellular matrix (ECM) in both elastic and nonelastic tissues. In a landmark article published in 1986, Sakai et al. identified fibrillin-1 as a major component of microfibrils.17 Microfibrils exist most often as components of elastic fibers as sheaths of fibrillar material surrounding elastin cores (Fig. 1A and B) or, alternatively, not associated with elastin, as fibers connecting to basement membrane (Fig. 1C) or performing specialized structural function such as formation of the lens zonules.
FIG. 1.
Examples of microfibril ultrastructure. Microfibrils are components of elastic fibers, forming sheaths of fibrillar material (F) surrounding elastin cores (E), as seen in a longitudinal section (A) and cross section (B) of elastic fibers in human skin that have been immunogold labeled with an antibody to fibrillin-1. Microfibrils can also form fibrous structures independent of elastin, which are sometimes seen connected to basement membrane, as shown in an anti-fibrilin-1-labeled section in which immunogold-labeled fibers appear to insert into the lamina densa portion of the basement membrane (BM) in the dermal-epidermal region of human skin (C). Elastic-like fibers in the trabecular meshwork are atypical elastic fibers, with heterogenous elastin cores displaying light patches (arrow) surrounded by a sheath containing periodic structures (S) from which fine fibrils (F) seem to derive and connect to the basement membrane in a tangential section of the trabecular meshwork (D). Images adapted from Sakai et al, © 1986 Rockefeller University Press, originally published in Journal of Cell Biology.17 (A–C) and from Fig. 3 of Rohen et al.,46 reprinted with permission from the copyright holder, the Association for Research in Vision and Ophthalmology (D).
Principal Component of Microfibrils: Fibrillin-1
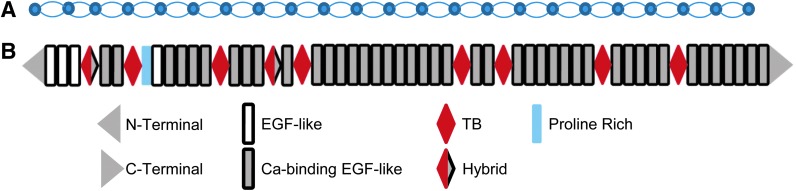
The organizing fibril structure of microfibrils is composed of polymers of fibrillin-1 assembled in a head-to-tail arrangement. Isolated microfibrils appear as 10–12 nm diameter fibers with regularly spaced bead-like structures (Fig. 2A). Though it is known that overlap of amino and carboxy termini of adjacent fibrillin-1 molecules correspond to the beaded structures, the basic orientation of fibrillin-1 within microfibrils is not established.7,10 Several competing models of the arrangement of fibrillin-1 within microfibril structures have been proposed7,10 (e.g., see Fig. 4 of Jensen et al.10).
FIG. 2.
Structure of microfibrils and fibrilin-1. Isolated microfibrils have a characteristic beads-on-a-string appearance with ∼50 nm spacing between beads as shown schematically (A). The fibrillar structure is composed of linear arrays of fibrillin-1 molecules. The exact arrangement of fibrillin-1 within microfibrils is an active area of investigation, though it is known that the bead structures correspond to overlapping N- and C- termini. Fibrillin-1 is an ∼350 kDa protein with multiple domains (B). The bulk of the structure is composed of calcium-binding epidermal growth factor (EGF)-like domains.
Fibrillin-1 is a large (∼350 kDa) multi-domain protein (Fig. 2) that serves as an extracellular docking site for multiple other proteins involved in a variety of structural and growth factor-mediated functions (Table 1).7,9,10,18 From an evolutionary perspective, the domain organization of fibrillin-1 is remarkably conserved, with the number and kinds of domains nearly identical from jellyfish to human, consistent with a central biological role for microfibrils.18,19
Table 1.
Microfibril-Associated Proteins
| Microfibril-associated protein | Microfibril-related function |
|---|---|
| Large Latency Complex | TGFβ signaling |
| (LTBP bound to latent TGFβ) | |
| GDF8, GDF5, BMP2, BMP4, BMP7, BMP10 | TGFβ superfamily signaling |
| LTBP2 | Possible structural role and/or competes for binding with other microfibril-associated proteins |
| fibulin2, fibulin4, fibulin5 | Microfibril and elastic fiber structure and assembly |
| Elastin | Elastic fiber core structure |
| ADAMTS10 | Microfibril assembly |
| ADAMTSL2 (via LTBP1), ADAMTSL4, ADAMTSL3, ADAMTSL6 | Not determined |
| MAGP1, MAGP2 | Elastic fiber assembly |
| Decorin, versican, aggrecan, perlcan | Not determined |
TGFβ, transforming growth factor beta.
The bulk of fibrillin-1 structure is composed of 47 epidermal growth factor (EGF)-like domains, 43 of which bind calcium (cb-EGF-like).7 EGF-like and cb-EGF-like domains are relatively common, occurring in many proteins other than fibrillins, and often serving as binding sites for other proteins. Coordination of calcium ions within cb-EGF-like domains and interactions with adjacent domains impose a rod-like structure to segments of fibrillin-1and is required for fibrillin-1 binding to other ECM components, including heparin, aggrecan, and versican.7,9,10
Fibrillin-1 contains 7 TGFβ binding-like (TB) domains, which are uniquely found in fibrillins and latent TGFβ-binding proteins (LTBPs).18 Despite their name, TB domains do not bind TGFβ, with the exception of TB domains found in LTBPs. The fourth TB domain of fibrillin-1 contains an RGD motif by which fibrillin-1 binds integrins.18
In humans, there are 3 fibrillins that are distinguishable by a domain located C-terminal from the first TB domain, which is proline-rich for fibrillin-1, glycine-rich for fibrillin-2 and proline, and glycine-rich for fibrillin-3.18,19 The domain structure is almost completely preserved in the 3 fibrillins, with the notable exception that fibrillin-2 and fibrillin-3 contain an additional RGD motif in addition to the one found within the fourth TB domain of fibrillin-1.18,19 Fibrillin-1 is by far the best-studied and most prevalent fibrillin, forming the backbone of mature microfibrils. Fibrillin-2 is also found in microfibrils and probably shares many of the functional capabilities of fibrillin-1 since it may be able to partially compensate for fibrillin-1 deficiency.20 Fibrillin-2 appears to play a more prominent role during development. For example, recent work has shown fibrillin-2 is the dominant fibrillin in microfibrils of the developing lens, but is replaced by fibrillin-1 in the adult eye.21
Microfibrils Control Location and Activation of TGFβ
Localization and activation of TGFβ is controlled by microfibrils, which are the major storage depot of latent TGFβ in the ECM.8,11,22,23 TGFβ is localized to extracellular microfibrils via the large latency complex (LLC), which is comprised of latent TGFβ dimers covalently attached to LTBP. TGFβ is cleaved into latency-associated peptide (LAP) and active peptide halves in the Golgi apparatus, with LAP remaining noncovalently bound, forming the small latency complex (SLC). LTBP binds the SLC forming the LLC, which is secreted into the ECM where it binds fibrillin-1 microfibrils through noncovalent interaction with LTBP. There are 3 TGFβs: TGFβ1, TGFβ2, and TGFβ3 and 4 LTBPs: LTBP1, LTBP2, LTBP3, and LTBP4. LTBP2 does not bind any of the TGFβ/LAP complexes, but it does bind microfibrils where it may play a structural role or participate in formation of microfibril-associated protein complexes by competing with other LTBPs for binding to fibrillin-1.24,25 LTBP1 and LTBP3 bind all 3 TGFβ/LAP complexes with equal affinity while LTBP4 weakly binds TGFβ1/LAP.18,25
Microfibrils regulate activation of TGFβ, which involves release of LAP to allow interaction of the active TGFβ with its receptor.11 Mechanisms of TGFβ activation include interactions with thrombospondin-1,26 protease activity, integrin interactions, and tissue elasticity.11,27 Integrins can activate TGFβ by binding an RGD motif in LAP. Mice with a targeted mutation disrupting the RGD motif in LAP of TGFβ1 develop pathology identical to TGFβ1 null mice, indicating that integrin activation of TGFβ through the RGD domain of LAP is a major mechanism of activation.28 While important, integrin activation with LAP does not apply to TGFβ2 activation because it does not have an RGD domain in its LAP, unlike the other TGFβs. A critical role for microfibrils in TGFβ activation is suggested by a number of diseases that have chronic activation of TGFβ signaling associated with mutations in fibrillin-1 or other microfibril-related diseases, including Marfan syndrome,29–32 Weill-Marchesani syndrome,33 acromicric dysplasia,34 geleophysic dysplasia,34,35 congenital scleroderma,36 and cutis laxa.37,38
Microfibril Assembly and Generation of Elastic Fibers
Assembly of fibrillin-1 into microfibrils is initiated intracellularly and continues paracellularly, though many fundamental steps are not yet fully understood. Formation of the microfibrils continues near the cell surface after fibrillin-1 secretion in a process that requires a previously formed layer of fibronectin.39,40 To form the macromolecular structures, fibrillin-1 is assembled in a head-to-tail fashion with overlapping amino and carboxy termini corresponding to the beads of the characteristic beads-on-a-string ultrastructure of microfibrils.7,9,10 Beyond the head-to tail assembly, the basic arrangement of fibrillin-1 monomers within the polymeric assembly is not precisely known, though several competing models have been proposed. These models attempt to explain the ∼50 nm periodicity of the bead structures, accommodate location of putative transglutaminase cross-links, and supply mechanisms for the elastic properties of microfibrils.7,9,10
Assembly of elastic fibers requires microfibrils, which serve as a scaffold for deposition and cross-linking of tropoelastin to form the elastin core. Disordered elastic fibers are characteristic of patients with Marfan syndrome,32,41 which is caused by mutations in FBN1, and in mice with targeted mutations of Fbn1.42,43 Several microfibril-associated proteins are necessary for proper formation of elastic fibers, including fibulins, lysyl oxidases (Lox), and lysyl oxidase-like (LoxL) proteins. Though elastin cores are always sheathed in microfibrils, microfibrils can form structures without elastin (Fig. 1C).16
Microfibrils in the Outflow Pathway
A ring of elastic fibers parallel to the internal axis of Schlemm's canal is formed by abundant elastic fibers in the trabecular meshwork (TM).44,45 The network of elastic fibers is found in the cores of trabecular beams in the corneoscleral portion of the TM,45–48 within the juxtacanalicular tissue (JCT)46,48–50 and the inner and outer walls of Schlemm's canal.51 The circumferential elastic fibers within the JCT region (also referred to as the cribriform meshwork46) are connected to the elastic fibers of ciliary muscle tendons and to the basement membranes of Schlemm's canal and trabecular endothelial cells by connecting fibrils.46,52 The interconnected circumferential elastic fiber network resembles elastic fiber structure in blood vessels53 and likely provides necessary elastic properties to the aqueous humor outflow system, which is constantly challenged with pulsatile force.
The elastic fibers in the TM have an atypical ultrastructural appearance compared with those in other tissues, as noted in early45 and subsequent46,48,49 electron microscopic studies. The TM elastic fibers display an “electron-dense core containing light strands and a surrounding sheath of periodic structure embedded in matrix,”46 rather than a homogenous core with an obvious fibrillar coat, as found in other tissues (Fig. 1D). Because of their atypical appearance, elastic fibers in the TM have been referred to as elastic-like fibers.46 Subsequent immunogold labeling for elastin demonstrated that the cores of the elastic-like fibers contain elastin,47,48,50,54 though they are resistant to digestion by elastase.49 The sheath material surrounding the cores contains fibrillin-1, similar to typical elastic fibers, however, in the JCT, fibrillin-1 is expressed in the cores as well.47,50 Additional matrix components have been identified in TM elastic fibers, including abundant expression of decorin and type VI collagen in the cores and fibronectin, vitronectin, tenascin, decorin, and hyaluronic acid in the sheaths.47,50 The distribution of the ECM components is different for elastic fibers in the corneoscleral meshwork compared with those in the JCT.47,50 The distinct structural and compositional characteristics of TM elastic fibers suggests tissue-specific specialization of function. However, elastic-like fibers in the TM can broadly be classified as elastic fibers, based on having elastin cores surrounded by microfibril sheaths containing fibrillin-1. Expression of fibrillin-1 in the TM as revealed by immunostaining and light microscopy confirm a distribution of microfibrils overlapping with TM elastic fibers, with prominent expression of fibrillin-1 adjacent to Schlemm's canal51,55,56 and within the JCT.51
Sheath-Derived Plaques
In 1971 Rohen and Witmer reported that in glaucomatous eyes, “deposits of homogeneous osmiophilic material (plaques) were found between the cell layers of the cribriform area of the TM adjacent to the inner wall of Schlemm's canal,” which “were not present to such an extent within the control specimens.”57 These structures located in the JCT and walls of Schlemm's canal are composed of fibrillar material embedded in homogenous matrix, with banded appearance in some cases, which appear to originate from the sheaths of the elastic-like fibers, as extensively characterized in electron microscopic studies by Rohen, Lutjen-Drecoll, and colleagues who referred to them as sheath-derived plaques.46,49 Fibrillin-1 was later found to be expressed in sheath-derived plaques and in the sheaths of elastic fibers in the JCT and corneoscleral meshwork in immunoelectron microscopy studies by Ueda et al.47,50 Although accumulation of these structures occurs with normal aging, qualitative and quantitative studies indicated a greater accumulation of sheath-derived plaques in TM samples from glaucoma patients, suggesting a pathogenic role.46,49,58,59
Microfibril defects result in disordered elastic fibers. Fragmented and degenerated elastic fibers and thickening of the aortic wall due to accumulation of amorphous matrix are found in Marfan syndrome patients and in mouse models of Marfan syndrome caused by mutations of Fbn1.41,42,60 Genetic variants in FBN1, or microfibril-associated genes, could contribute to the accelerated accumulation of sheath-derived plaques observed in the TM of glaucoma patients.
Pulsatile Outflow
A pumping mechanism of AH outflow has been proposed by Johnstone, in which periodic oscillation of intraocular pressure (IOP) due to cardiac pulse causes expansion and contraction of the TM, which expels aqueous humor into collector channels and into episcleral veins.61–63 The expansion and contraction of the TM, which drives the pumping action, has been shown to involve movement on the order of 1–2 μm, depending on IOP.63 In analogy with arterial mechanics,53 the elastic properties of the TM, collector channels, and episcleral veins would govern the behavior of the pump mechanism. In addition to the extensive network of elastic fibers in the TM,52 elastic fibers are found in collector channel walls51 and very likely in episcleral veins. Microfibril defects cause changes in vascular biomechanics as evidenced by the decreased arterial distensibility and increased aortic stiffness in Marfan syndrome.64 As reviewed by Johnstone et al., abnormalities of pulsatile flow in glaucomatous eyes have been demonstrated62 and could account for reduced aqueous humor outflow and increased IOP. Microfibril defects could alter elasticity of the TM, collector channels, and episcleral veins, which could impede the pumping action of the TM and the pulsatile outflow of aqueous humor in glaucoma.
Microfibril Genes Associated with Glaucoma
Since glaucoma has a significant genetic component, if microfibril defects cause glaucoma, then risk alleles in microfibril-related genes should be associated with the disease. Studies with Mendelian forms of glaucoma have identified rare mutations in 2 microfibril-related genes, LTBP23,4 and ADAMTS10.1 So far, genome-wide association studies have found only one microfibril-related gene associated with glaucoma, LOXL1.2 As with other complex diseases, there is an apparent deficit between the number of cases that could be accounted for by all identified glaucoma risk alleles and the expected heritability of glaucoma. One possible explanation for this deficit is that rare mutations difficult to detect in genome-wide association studies account for a greater proportion of heritability.65 If the microfibril hypothesis is correct, future large scale re-sequencing studies may identify additional rare variant risk alleles in microfibril-related genes.
Single nucleotide polymorphisms in LOXL1 were identified as risk factors for pseudoexfoliation glaucoma in a large-scale genome-wide association study involving more than 10,000 subjects from northern European populations.2 LOXL1 encodes LoxL protein-1, which is required for normal assembly of elastic fibers.16,66 The risk variants originally identified for LOXL1 cause amino acid substitutions in the amino-terminal pro-peptide and, as might be expected, do not affect enzymatic activity.67 During elastic fiber formation, inactive LOXL1 is tethered via its pro-peptide domain to the microfibril scaffold through binding to fibulin-5, which binds to fibrillin-1.66 Although enzyme activity is not affected, the LOXL1 variants in the pro-domain could affect localization of the protein by disrupting interactions with fibulin-5.67
The microfibril-associated protein LTBP2 is associated with glaucoma. LTBP2 mutations have been shown to cause primary congenital glaucoma3,4 and recent evidence suggests they might be risk factors for primary open angle and pseudoexfoliation glaucoma.68 A truncation of LTBP2 was also identified as the causative mutation in a cat model of primary congenital glaucoma.69 Unlike other LTBPs, LTBP2 does not bind TGFβ, but it does bind fibrilllin-1 and may perform a structural role in microfibril and elastic fiber formation70 and/or compete with other LTBPs for interaction with microfibrils.25
A mutation in ADAMTS10 has been reported as likely causative for glaucoma in a canine model of hereditary primary open-angle glaucoma.1,71 ADAMTS10 is a secreted matrix metalloproteinase that can cleave fibrillin-1.72,73 Although its exact function is not known, a role for ADAMTS10 in microfibril structure and function was first suggested by the finding that Weill-Marchesani syndrome can be caused either by recessive ADAMTS10 mutations74 or dominant mutations in FBN1.75 Clinically, the dominant and recessive forms of Weill-Marchesani are indistinguishable, suggesting common functional roles for ADAMTS10 and FBN1.76 FBN1 mutations also account for most cases of Marfan syndrome.6 Although Weill-Marchesani patients are opposite to Marfan patients in outward appearance, the syndromes share a common molecular mechanism, which is defective fibrillin-1 microfibrils.33,73,77 Further supporting a role for ADAMTS10 in microfibril function, co-localization of ADAMTS10 and fibrillin-1 has been shown by immunohistochemistry of human skin33,73 and ADAMTS10 has been shown to promote microfibril formation in cell culture.73 Specific and high-affinity binding of ADAMTS10 to fibrillin-1 has been demonstrated by affinity blotting assays, affinity pull-down assays, and surface plasmon resonance.33,73 The binding site on fibrillin-1 for ADAMTS10 overlaps with the binding site for other ADAMTS proteins,33 suggesting competition for the fibrillin-1 binding site or complex formation with other microfibril-associated proteins.33
ADAMTS10 may play a role in outflow resistance since in the canine model of primary open-angle glaucoma, affected dogs homozygous for the ADAMTS10 mutation display impaired outflow facility and increased IOP at an early age, well before evidence of optic nerve damage.78 ADAMTS10 protein is abundantly expressed in the TM, consistent with a function in regulating outflow resistance.1
Glaucoma in Marfan Patients
Marfan syndrome is well established as a disease caused by microfibril deficiency.6 An association between Marfan syndrome and glaucoma was suggested by a retrospective study of 573 Marfan syndrome patients examined by ophthalmologists, which found that primary open-angle glaucoma was the most common form of glaucoma, with a prevalence higher than in the general population.5 Since glaucoma is a late onset disease, careful study of glaucoma in Marfan syndrome patients is complicated by their shortened lifespan. Ectopia lentis is common in Marfan syndrome, which further complicates the study of glaucoma because elevated IOP can be secondary to lens displacement.6 Marfan syndrome patients have thin corneas,79,80 which is a significant risk factor for glaucoma.81 With thin corneas, measurements of IOP by tonography underestimate IOP,82,83 which may lead to an underestimation of the prevalence of glaucoma in Marfan syndrome patients. Further study is needed to establish a link between Marfan syndrome and glaucoma.
Microfibril Defects and Chronic Over-Activation of TGFβ Signaling
TGFβ may play a central role in glaucoma pathogenesis in general, and in particular may cause decreased aqueous humor outflow through the TM. Since the initial discovery in 1994 by Tripathi et al.,84 that the aqueous humor of glaucoma patients contains elevated levels of TGFβ2, evidence has accumulated supporting elevated TGFβ2 in the aqueous humor,85–91 and additionally, the ability of TGFβ2 to increase IOP, as recently reviewed by Fuchshofer and Tamm.92 Perfusion of human anterior segments with TGFβ2 results in decreased facility of outflow of AH and accumulation of matrix material in the TM.93,94 In vivo, overexpression of TGFβ in mouse eyes by transgenic or viral expression results in elevated IOP.95–97 A plausible mechanism by which increased TGFβ2 elevates IOP is by altering ECM turnover in the TM resulting in reduced aqueous humor outflow facility.92–94,98,99
Although a link between TGFβ and microfibrils in glaucoma is at present hypothetical, a complete discussion of TGFβ-mediated disease must include the topic of microfibrils. Microfibrils perform a central fundamental role in controlling the localization and activation of TGFβ signaling.8,9,11 Elevated TGFβ levels and chronic activation of TGFβ signaling coincide with highly penetrant diseases caused by mutations in FBN1 and other microfibril-associated genes (Table 2).29–38 In a mouse model of Marfan syndrome caused by a C1039G mutation of Fbn1, aortic aneurysm, mitral valve prolapsed, and myopathy were prevented by TGFβ-neutralizing antibody,31,100,101 suggesting a cause and effect relationship between TGFβ and disease phenotypes. Similarly, in another mouse model of Marfan syndrome caused by heterozygous deletion of Fbn1, development of alveolar septation was blocked by neutralization of TGFβ,30 further supporting the hypothesis that disease in microfibril deficiencies is mediated by TGFβ.102 In human Marfan patients, TGFβ signaling is hyper-activated32 and plasma TGFβ is elevated.29,32
Table 2.
Microfibril Diseases Associated with Chronic Over-Activation of Transforming Growth Factor Beta Signaling
| Disease name | Microfibril-associated genetic mutation(s) | References related to TGFβ |
|---|---|---|
| Marfan syndrome | FBN1 | Human,29,32 mouse model30,31 |
| Weill-Marchesani syndrome | FBN1, ADAMTS10, ADAMTS17 | FBN1, mouse model33 |
| Acromicric dysplasia | FBN1 | Human34 |
| Geleophysic dysplasia | FBN1, ADAMTSL2 | Human FBN1,34 ADAMTSL235 |
| Congenital scleroderma | FBN1 | Human36 |
| Cutis laxa type I | LTBP4, FBLN4, FBLN5 | LTBP4,37FBLN438 |
A large body of research strongly implicates elevated TGFβ in increased resistance to aqueous humor outflow by inducing changes in the ECM.92 Though the consequences of elevated TGFβ are well studied, the mechanisms of increased TGFβ in the aqueous humor are not known. In diseases associated with microfibril defects, such as Marfan syndrome, TGFβ signaling is hyper-activated and TGFβ concentration is elevated.29,30 Since prominent fibrillin-1 expression is found in the peripheral cornea endothelium, cilliary body, and iris,55,56,103 defective microfibrils could provide a mechanism for the well-established elevation of TGFβ concentration in the aqueous humor of glaucoma patients.84,92
Therapeutic Implications of the Microfibril Hypothesis of Glaucoma
If glaucoma is thought of as a microfibril deficiency, a rational approach to glaucoma therapy would consider treatments used for other microfibril deficiencies such as Marfan syndrome. The standard of care for Marfan patients has been to lower blood pressure with β-adrenergic receptor blockers and/or angiotensin-converting enzyme (ACE) inhibitors to slow onset of life-threatening cardiovascular events such as aortic dissection and rupture.104 More recently, losartan has emerged as a promising drug for treating Marfan syndrome, based on a series of studies100,101 using the mouse model caused by a heterozygous C1039G mutation of Fbn1.42 In these studies, many of the disease phenotypes, including thickening of the aorta wall that could be blocked by neutralization of TGFβ, could also be prevented by treatment with losartan100,101,105,106 with greater efficacy than treatment with β-adrenergic receptor blockers100 or ACE inhibitors.106
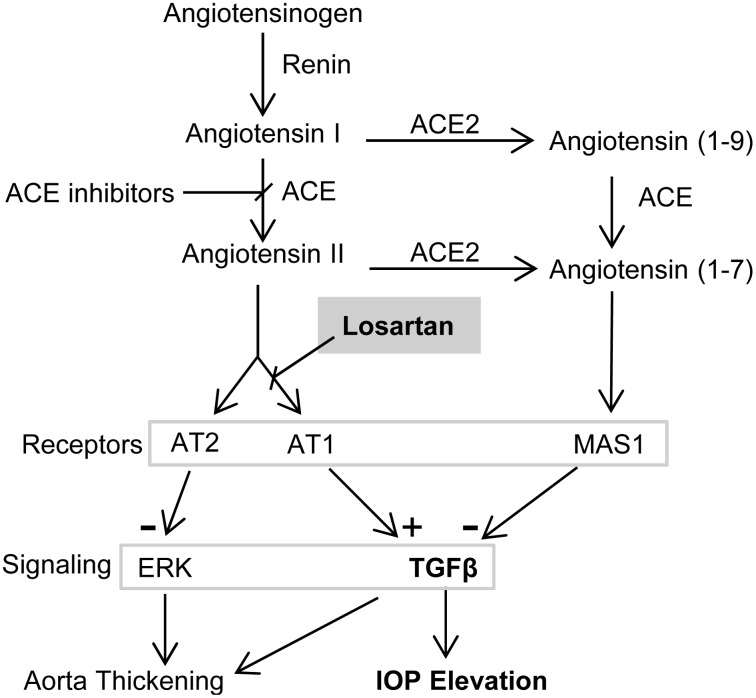
Losartan is an angiotensin II type 1 receptor (AT1) blocker in common use for the treatment of hypertension. Several AT1 blockers have been developed based on the structure of losartan, a class of drugs that can be referred to as “sartans,” including valsartan, irbesartan, azilsartan, candesartan, telmisartan, olmesartan, and eprosartan.107 The “sartans” are designed to interfere with the renin-angiotensin system (RAS), which regulates blood pressure. In the RAS, renin converts angiotensinogen to angiotensin I, which is converted to angiotensin II by ACE (Fig. 3). Angiotensin II is an 8-amino acid peptide hormone that binds AT1 and AT2 and increases blood pressure via AT1. However, efficacy of losartan in treating Marfan syndrome is due to indirect inhibition of TGFβ signaling, rather than direct effects on RAS.
FIG. 3.
Interaction of renin-angiotensin and transforming growth factor beta (TGFβ) signal transduction with intraocular pressure (IOP). Inhibition of chronic TGFβ signal transduction could reverse pathogenic remodeling of the trabecular meshwork (TM) and lower IOP. In Marfan syndrome, losartan is more effective than angiotensin-converting enzyme (ACE) inhibitors because it inhibits TGFβ signaling through angiotensin II type 1 receptor (AT1) without removing AT2-mediated inhibition of extracellular signal-regulated kinase (ERK), which is activated by noncanonical TGFβ signaling and mediates aortic wall thickening. AT1 blockers such as losartan, ACE inhibitors, or ACE2 activators may be effective at lowering IOP through reduction of TGFβ signaling.
In their original study, Habashi et al.100 chose to use losartan to treat the Marfan model mice because in addition to reducing hypertension, losartan had been reported to have inhibitory effects on TGFβ signaling. For example, losartan has been shown to prevent increases in TGFβ in the plasma and renal cortex of uremic rats108 and to block myocardial expression of TGFβ in a mouse model of hypertrophic cardiomyopathy.109 The TGFβ-blocking effect of losartan was confirmed in the Marfan mouse model.100,101,105,106 In a mouse model of autoimmune encephalitis, candesartan has been shown to reduce activation of TGFβ signaling.110 Consistent with an inhibitory effect on TGFβ, Marfan syndrome patients receiving losartan were shown to have significantly lower plasma TGFβ levels.29 Based on the evidence with the Marfan mouse model that TGFβ is causative and losartan inhibits the action of TGFβ and prevents disease phenotypes, several clinical trials are underway testing the efficacy of losartan in treating Marfan syndrome patients.104,111
Targeting the RAS for treating ocular hypertension has been extensively considered and investigated.112 AT1 blockers have been studied in animal models and human subjects before the mechanism of reducing TGFβ activity was appreciated. Losartan and olmesartan (also called CS-088) moderately reduced IOP in rabbits with ocular hypertension induced by α-chymotrypsin within 4 h after topical application.113,114 In monkeys with laser-induced ocular hypertension, 4% olmesartan eye drops were shown to mildly reduce IOP within 1 h of application.115 In a study with human subjects, an oral dose of 50 mg losartan reduced IOP within 3 h and by as much as 20%.116 In the human study, the magnitude of the reduction of IOP was greater in patients with existing ocular hypertension compared with patients with normal IOP.116 While losartan reduced blood pressure in arterial hypertensive patients, it did not affect blood pressure in subjects without arterial hypertension,116 suggesting that the mechanism of lowering IOP was not related to blood pressure effects of losartan. Tonographic measurements in the human study of losartan showed an increase in aqueous humor outflow facility coinciding with reduced IOP in patients receiving losartan,116 suggesting that losartan acts by increasing aqueous humor outflow facility. Efficacy of AT1 blockers were recently investigated when topical olmesartan was entered into clinical trials for lowering IOP by Santen Pharmaceutical Company. However, the company discontinued an early phase II clinical trial reporting only a small reduction of IOP without a clear dose–response relationship.117
In the Marfan mouse model, selective inhibition of AT1 with losartan more effectively prevented thickening of the aortic wall than did inhibition of both AT1 and AT2 with ACE inhibitors (Fig. 3), even though ACE inhibitors were more effective in reducing TGFβ signaling.106 This was unexpected because thickening of the aortic wall is mediated by TGFβ, specifically via activation of extracellular signal-regulated kinase (ERK) via noncanonical TGFβ signaling.118 Habashi et al. showed that blocking AT2 removed an inhibition of ERK activation, which counteracts the beneficial effect of inhibition of AT1106 (Fig. 3), explaining the greater benefit of losartan over ACE inhibition despite less reduction of TGFβ activation.
In the context of glaucoma, the relative importance of canonical SMAD-mediated versus noncanonical TGFβ signaling is not known. Greater reductions of TGFβ signaling through use of ACE inhibitors could prove more effective than AT1 blockade. Another approach could be through the more recently discovered component of the RAS in which angiotensin (1–7), a 7-amino acid derivative of angiotensin formed by the action of ACE2, binds to its receptor, Mas1119 (Fig. 3). Activation of Mas1 by angiotensin (1–7) has opposing effects to AT1 activation, including inhibition of TGFβ signaling.120 Recently, in a rat model of induced ocular hypertension, activation of ACE2 with diminazene aceturate was shown to prevent glaucoma and reduce IOP, possibly by increasing aqueous humor outflow.121
Although the moderate reductions of IOP seen in humans after short-term treatment with oral or topical AT1 blockers suggest limited usefulness for this class of compounds, a slow developing phase of further reduction of IOP would be predicted by the microfibril hypothesis of glaucoma. If glaucoma is a microfibril deficiency, available evidence suggests that the accompanying chronic TGFβ activation would affect IOP by remodeling ECM over an extended period of time.92,98,99 Experiments with in vitro perfusion of anterior segments with TGFβ2 have shown decreased outflow facility that develops over the course of 2–3 days.93,94 In the microfibril-deficient Marfan mouse model, aortas appear normal until about 2 months of age, after which gradual thickening develops that is dependent on TGFβ signaling.42 Reversal of chronic TGFβ-mediated alterations of ECM in the TM may require an extended treatment period. The microfibril hypothesis motivates re-evaluation of targeting the RAS with AT1 blockers, ACE inhibitors, ACE2 activators, or other means to treat ocular hypertension and suggests that after an initial small drop in IOP, sustained use of these compounds could rejuvenate the diseased outflow pathway by reducing chronic TGFβ activity and result in a prolonged second phase of more substantial reduction of IOP.
Acknowledgments
Association for Research in Vision and Ophthalmology is acknowledged as copyright holder of Fig. 1D, which was taken from Fig. 3 of Rohen et al.46 Supported by NEI grant EY020894 (R.W.K.), a Departmental Unrestricted Award from Research to Prevent Blindness, Inc., and Vanderbilt Vision Research Center (P30EY008126).
Author Disclosure Statement
No competing financial interests exist.
References
- 1.Kuchtey J., Olson L.M., Rinkoski T., et al. Mapping of the disease locus and identification of ADAMTS10 as a candidate gene in a canine model of primary open angle glaucoma. PLoS Genet. 7:e1001306, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Thorleifsson G., Magnusson K.P., Sulem P., et al. Common sequence variants in the LOXL1 gene confer susceptibility to exfoliation glaucoma. Science. 317:1397–1400, 2007 [DOI] [PubMed] [Google Scholar]
- 3.Narooie-Nejad M., Paylakhi S.H., Shojaee S., et al. Loss of function mutations in the gene encoding latent transforming growth factor beta binding protein 2, LTBP2, cause primary congenital glaucoma. Hum. Mol. Genet. 18:3969–3977, 2009 [DOI] [PubMed] [Google Scholar]
- 4.Ali M., McKibbin M., Booth A., et al. Null mutations in LTBP2 cause primary congenital glaucoma. Am. J. Hum. Genet. 84:664–671, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Izquierdo N.J., Traboulsi E.I., Enger C., and Maumenee I.H.Glaucoma in the Marfan syndrome. Trans. Am. Ophthalmol. Soc. 90:111–117; discussion 118–122, 1992 [PMC free article] [PubMed] [Google Scholar]
- 6.Faivre L., Collod-Beroud G., Loeys B.L., et al. Effect of mutation type and location on clinical outcome in 1,013 probands with Marfan syndrome or related phenotypes and FBN1 mutations: an international study. Am. J. Hum. Genet. 81:454–466, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Kielty C.M., Sherratt M.J., Marson A., and Baldock C.Fibrillin microfibrils. Adv. Protein Chem. 70:405–436, 2005 [DOI] [PubMed] [Google Scholar]
- 8.Ramirez F., and Rifkin D.B.Extracellular microfibrils: contextual platforms for TGFbeta and BMP signaling. Curr. Opin. Cell Biol. 21:616–622, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Ramirez F., and Sakai L.Y.Biogenesis and function of fibrillin assemblies. Cell Tissue Res. 339:71–82, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Jensen S.A., Robertson I.B., and Handford P.A.Dissecting the fibrillin microfibril: structural insights into organization and function. Structure. 20:215–225, 2012 [DOI] [PubMed] [Google Scholar]
- 11.Horiguchi M., Ota M., and Rifkin D.B.Matrix control of transforming growth factor-beta function. J. Biochem. 152:321–329, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Jakus M.A.Studies on the cornea. I. The fine structure of the rat cornea. Am. J. Ophthalmol. 38:40–53, 1954 [PubMed] [Google Scholar]
- 13.Karrer H.E.The fine structure of connective tissue in the tunica propria of bronchioles. J. Ultrastruct. Res. 2:96–121, 1958 [DOI] [PubMed] [Google Scholar]
- 14.Low F.N.Microfibrils: fine filamentous components of the tissue space. Anat. Rec. 142:131–137, 1962 [DOI] [PubMed] [Google Scholar]
- 15.Ross R., and Bornstein P.The elastic fiber. I. The separation and partial characterization of its macromolecular components. J. Cell Biol. 40:366–381, 1969 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Yanagisawa H., and Davis E.C.Unraveling the mechanism of elastic fiber assembly: the roles of short fibulins. Int. J. Biochem. Cell. Biol. 42:1084–1093, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Sakai L.Y., Keene D.R., and Engvall E.Fibrillin, a new 350-kD glycoprotein, is a component of extracellular microfibrils. J. Cell Biol. 103:2499–2509, 1986 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Robertson I., Jensen S., and Handford P.TB domain proteins: evolutionary insights into the multifaceted roles of fibrillins and LTBPs. Biochem. J. 433:263–276, 2011 [DOI] [PubMed] [Google Scholar]
- 19.Piha-Gossack A., Sossin W., and Reinhardt D.P.The evolution of extracellular fibrillins and their functional domains. PLoS One 7:e33560, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Carta L., Pereira L., Arteaga-Solis E., et al. Fibrillins 1 and 2 perform partially overlapping functions during aortic development. J. Biol. Chem. 281:8016–8023, 2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Shi Y., Tu Y., De Maria A., Mecham R.P., and Bassnett S.Development, composition, and structural arrangements of the ciliary zonule of the mouse. Invest. Ophthalmol. Vis. Sci. 54:2504–2515, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Chaudhry S.S., Cain S.A., Morgan A., Dallas S.L., Shuttleworth C.A., and Kielty C.M.Fibrillin-1 regulates the bioavailability of TGFbeta1. J. Cell Biol. 176:355–367, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Massam-Wu T., Chiu M., Choudhury R., et al. Assembly of fibrillin microfibrils governs extracellular deposition of latent TGF beta. J. Cell Sci. 123:3006–3018, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Hirani R., Hanssen E., and Gibson M.A.LTBP-2 specifically interacts with the amino-terminal region of fibrillin-1 and competes with LTBP-1 for binding to this microfibrillar protein. Matrix Biol. 26:213–223, 2007 [DOI] [PubMed] [Google Scholar]
- 25.Todorovic V., and Rifkin D.B.LTBPs, more than just an escort service. J. Cell Biochem. 113:410–418, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Sweetwyne M.T., and Murphy-Ullrich J.E.Thrombospondin1 in tissue repair and fibrosis: TGF-beta-dependent and independent mechanisms. Matrix Biol. 31:178–186, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Worthington J.J., Klementowicz J.E., and Travis M.A.TGFbeta: a sleeping giant awoken by integrins. Trends Biochem. Sci. 36:47–54, 2011 [DOI] [PubMed] [Google Scholar]
- 28.Yang Z., Mu Z., Dabovic B., et al. Absence of integrin-mediated TGFbeta1 activation in vivo recapitulates the phenotype of TGFbeta1-null mice. J. Cell Biol. 176:787–793, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Matt P., Schoenhoff F., Habashi J., et al. Circulating transforming growth factor-beta in Marfan syndrome. Circulation. 120:526–532, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Neptune E.R., Frischmeyer P.A., Arking D.E., et al. Dysregulation of TGF-beta activation contributes to pathogenesis in Marfan syndrome. Nat. Genet. 33:407–411, 2003 [DOI] [PubMed] [Google Scholar]
- 31.Ng C.M., Cheng A., Myers L.A., et al. TGF-beta-dependent pathogenesis of mitral valve prolapse in a mouse model of Marfan syndrome. J. Clin. Invest. 114:1586–1592, 2004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Kim K.L., Yang J.H., Song S.H., et al. Positive correlation between the dysregulation of transforming growth factor-beta1 and aneurysmal pathological changes in patients with Marfan syndrome. Circ. J. 77:952–958, 2013 [DOI] [PubMed] [Google Scholar]
- 33.Sengle G., Tsutsui K., Keene D.R., et al. Microenvironmental regulation by fibrillin-1. PLoS Genet. 8:e1002425, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Le Goff C., Mahaut C., Wang L.W., et al. Mutations in the TGFbeta binding-protein-like domain 5 of FBN1 are responsible for acromicric and geleophysic dysplasias. Am. J. Hum. Genet. 89:7–14, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Le Goff C., Morice-Picard F., Dagoneau N., et al. ADAMTSL2 mutations in geleophysic dysplasia demonstrate a role for ADAMTS-like proteins in TGF-beta bioavailability regulation. Nat. Genet. 40:1119–1123, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Loeys B.L., Gerber E.E., Riegert-Johnson D., et al. Mutations in fibrillin-1 cause congenital scleroderma: stiff skin syndrome. Sci. Transl. Med. 2:23ra20, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Callewaert B., Su C.T., Van Damme T., et al. Comprehensive clinical and molecular analysis of 12 families with type 1 recessive cutis laxa. Hum. Mutat. 34:111–121, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Renard M., Holm T., Veith R., et al. Altered TGFbeta signaling and cardiovascular manifestations in patients with autosomal recessive cutis laxa type I caused by fibulin-4 deficiency. Eur. J. Hum. Genet. 18:895–901, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Kinsey R., Williamson M.R., Chaudhry S., et al. Fibrillin-1 microfibril deposition is dependent on fibronectin assembly. J. Cell Sci. 121:2696–2704, 2008 [DOI] [PubMed] [Google Scholar]
- 40.Sabatier L., Chen D., Fagotto-Kaufmann C., et al. Fibrillin assembly requires fibronectin. Mol. Biol. Cell 20:846–858, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Abraham P.A., Perejda A.J., Carnes W.H., and Uitto J.Marfan syndrome. Demonstration of abnormal elastin in aorta. J. Clin. Invest. 70:1245–1252, 1982 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Judge D.P., Biery N.J., Keene D.R., et al. Evidence for a critical contribution of haploinsufficiency in the complex pathogenesis of Marfan syndrome. J. Clin. Invest. 114:172–181, 2004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Mariko B., Pezet M., Escoubet B., et al. Fibrillin-1 genetic deficiency leads to pathological ageing of arteries in mice. J. Pathol. 224:33–44, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Ashton N., Brini A., and Smith R.Anatomical studies of the trabecular meshwork of the normal human eye. Br. J. Ophthalmol. 40:257–282, 1956 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Iwamoto T.Light and electron microscopy of the presumed elastic components of the trabeculae and scleral spur of the human eye. Invest. Ophthalmol. 3:144–156, 1964 [PubMed] [Google Scholar]
- 46.Rohen J.W., Futa R., and Lutjen-Drecoll E.The fine structure of the cribriform meshwork in normal and glaucomatous eyes as seen in tangential sections. Invest. Ophthalmol. Vis. Sci. 21:574–585, 1981 [PubMed] [Google Scholar]
- 47.Ueda J., and Yue B.Y.Distribution of myocilin and extracellular matrix components in the corneoscleral meshwork of human eyes. Invest. Ophthalmol. Vis. Sci. 44:4772–4779, 2003 [DOI] [PubMed] [Google Scholar]
- 48.Gong H.Y., Trinkaus-Randall V., and Freddo T.F.Ultrastructural immunocytochemical localization of elastin in normal human trabecular meshwork. Curr. Eye Res. 8:1071–1082, 1989 [DOI] [PubMed] [Google Scholar]
- 49.Lutjen-Drecoll E., Futa R., and Rohen J.W.Ultrahistochemical studies on tangential sections of the trabecular meshwork in normal and glaucomatous eyes. Invest. Ophthalmol. Vis. Sci. 21:563–573, 1981 [PubMed] [Google Scholar]
- 50.Ueda J., Wentz-Hunter K., and Yue B.Y.Distribution of myocilin and extracellular matrix components in the juxtacanalicular tissue of human eyes. Invest. Ophthalmol. Vis. Sci. 43:1068–1076, 2002 [PubMed] [Google Scholar]
- 51.Hann C.R., and Fautsch M.P.The elastin fiber system between and adjacent to collector channels in the human juxtacanalicular tissue. Invest. Ophthalmol. Vis. Sci. 52:45–50, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Tektas O.Y., and Lutjen-Drecoll E.Structural changes of the trabecular meshwork in different kinds of glaucoma. Exp. Eye Res. 88:769–775, 2009 [DOI] [PubMed] [Google Scholar]
- 53.Wagenseil J.E., and Mecham R.P.Vascular extracellular matrix and arterial mechanics. Physiol. Rev. 89:957–989, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Umihira J., Nagata S., Nohara M., Hanai T., Usuda N., and Segawa K.Localization of elastin in the normal and glaucomatous human trabecular meshwork. Invest. Ophthalmol. Vis. Sci. 35:486–494, 1994 [PubMed] [Google Scholar]
- 55.Wheatley H.M., Traboulsi E.I., Flowers B.E., et al. Immunohistochemical localization of fibrillin in human ocular tissues. Relevance to the Marfan syndrome. Arch. Ophthalmol. 113:103–109, 1995 [DOI] [PubMed] [Google Scholar]
- 56.Schlotzer-Schrehardt U., von der Mark K., Sakai L.Y., and Naumann G.O.Increased extracellular deposition of fibrillin-containing fibrils in pseudoexfoliation syndrome. Invest Ophthalmol Vis Sci 38:970–984, 1997 [PubMed] [Google Scholar]
- 57.Rohen J.W., and Witmer R.Electron microscopic studies on the trabecular meshwork in glaucoma simplex. Albrecht Von Graefes Arch. Klin. Exp. Ophthalmol. 183:251–266, 1972 [DOI] [PubMed] [Google Scholar]
- 58.Lutjen-Drecoll E., Shimizu T., Rohrbach M., and Rohen J.W.Quantitative analysis of ‘plaque material’ in the inner- and outer wall of Schlemm's canal in normal- and glaucomatous eyes. Exp. Eye Res. 42:443–455, 1986 [DOI] [PubMed] [Google Scholar]
- 59.Rohen J.W., Lutjen-Drecoll E., Flugel C., Meyer M., and Grierson I.Ultrastructure of the trabecular meshwork in untreated cases of primary open-angle glaucoma (POAG). Exp. Eye. Res. 56:683–692, 1993 [DOI] [PubMed] [Google Scholar]
- 60.Chung A.W., Au Yeung K., Sandor G.G., Judge D.P., Dietz H.C., and van Breemen C.Loss of elastic fiber integrity and reduction of vascular smooth muscle contraction resulting from the upregulated activities of matrix metalloproteinase-2 and -9 in the thoracic aortic aneurysm in Marfan syndrome. Circ. Res. 101:512–522, 2007 [DOI] [PubMed] [Google Scholar]
- 61.Johnstone M.A.The aqueous outflow system as a mechanical pump: evidence from examination of tissue and aqueous movement in human and non-human primates. J. Glaucoma 13:421–438, 2004 [DOI] [PubMed] [Google Scholar]
- 62.Johnstone M., Martin E., and Jamil A.Pulsatile flow into the aqueous veins: manifestations in normal and glaucomatous eyes. Exp. Eye Res. 92:318–327, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Li P., Reif R., Zhi Z., et al. Phase-sensitive optical coherence tomography characterization of pulse-induced trabecular meshwork displacement in ex vivo nonhuman primate eyes. J. Biomed. Opt. 17:076026, 2012 [DOI] [PubMed] [Google Scholar]
- 64.Jeremy R.W., Huang H., Hwa J., McCarron H., Hughes C.F., and Richards J.G.Relation between age, arterial distensibility, and aortic dilatation in the Marfan syndrome. Am. J. Cardiol. 74:369–373, 1994 [DOI] [PubMed] [Google Scholar]
- 65.Gibson G.Rare and common variants: twenty arguments. Nat. Rev. Genet. 13:135–145, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Liu X., Zhao Y., Gao J., et al. Elastic fiber homeostasis requires lysyl oxidase-like 1 protein. Nat. Genet. 36:178–182, 2004 [DOI] [PubMed] [Google Scholar]
- 67.Kim S., and Kim Y.Variations in LOXL1 associated with exfoliation glaucoma do not affect amine oxidase activity. Mol. Vis. 18:265–270, 2012 [PMC free article] [PubMed] [Google Scholar]
- 68.Jelodari-Mamaghani S., Haji-Seyed-Javadi R., Suri F., et al. Contribution of the latent transforming growth factor-beta binding protein 2 gene to etiology of primary open angle glaucoma and pseudoexfoliation syndrome. Mol. Vis. 19:333–347, 2013 [PMC free article] [PubMed] [Google Scholar]
- 69.Kuehn M.H., McLellan G.J., Pfleging A., Snella E.M., and Ellinwood N.M.Spontaneous mutations In LTBP2 are associated with congenital glaucoma in cats. Invest. Ophthalmol. Vis. Sci. 52:2424, 2011 [Google Scholar]
- 70.Hirai M., Horiguchi M., Ohbayashi T., Kita T., Chien K.R., and Nakamura T.Latent TGF-beta-binding protein 2 binds to DANCE/fibulin-5 and regulates elastic fiber assembly. EMBO J. 26:3283–3295, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Kuchtey J., Kunkel J., Esson D., et al. Screening ADAMTS10 in dog populations supports Gly661Arg as the glaucoma-causing variant in beagles. Invest. Ophthalmol. Vis. Sci. 54:1881–1886, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Somerville R.P., Jungers K.A., and Apte S.S.Discovery and characterization of a novel, widely expressed metalloprotease, ADAMTS10, and its proteolytic activation. J. Biol. Chem. 279:51208–51217, 2004 [DOI] [PubMed] [Google Scholar]
- 73.Kutz W.E., Wang L.W., Bader H.L., et al. ADAMTS10 protein interacts with fibrillin-1 and promotes its deposition in extracellular matrix of cultured fibroblasts. J. Biol. Chem. 286:17156–17167, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Dagoneau N., Benoist-Lasselin C., Huber C., et al. ADAMTS10 mutations in autosomal recessive Weill-Marchesani syndrome. Am. J. Hum. Genet. 75:801–806, 2004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Faivre L., Gorlin R.J., Wirtz M.K., et al. In frame fibrillin-1 gene deletion in autosomal dominant Weill-Marchesani syndrome. J. Med. Genet. 40:34–36, 2003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Faivre L., Dollfus H., Lyonnet S., et al. Clinical homogeneity and genetic heterogeneity in Weill-Marchesani syndrome. Am. J. Med. Genet. A. 123A:204–207, 2003 [DOI] [PubMed] [Google Scholar]
- 77.Hollister D.W., Godfrey M., Sakai L.Y., and Pyeritz R.E.Immunohistologic abnormalities of the microfibrillar-fiber system in the Marfan syndrome. N. Engl. J. Med. 323:152–159, 1990 [DOI] [PubMed] [Google Scholar]
- 78.Gelatt K.N., Peiffer R.L., Jr., Gwin R.M., Gum G.G., and Williams L.W.Clinical manifestations of inherited glaucoma in the beagle. Invest. Ophthalmol. Vis. Sci. 16:1135–1142, 1977 [PubMed] [Google Scholar]
- 79.Heur M., Costin B., Crowe S., et al. The value of keratometry and central corneal thickness measurements in the clinical diagnosis of Marfan syndrome. Am. J. Ophthalmol. 145:997–1001, 2008 [DOI] [PubMed] [Google Scholar]
- 80.Sultan G., Baudouin C., Auzerie O., De Saint Jean M., Goldschild M., and Pisella P.J.Cornea in Marfan disease: orbscan and in vivo confocal microscopy analysis. Invest. Ophthalmol. Vis. Sci. 43:1757–1764, 2002 [PubMed] [Google Scholar]
- 81.Gordon M.O., Beiser J.A., Brandt J.D., et al. The Ocular Hypertension Treatment Study: baseline factors that predict the onset of primary open-angle glaucoma. Arch. Ophthalmol. 120:714–720; discussion 829–730, 2002 [DOI] [PubMed] [Google Scholar]
- 82.Gelaw Y., Kollmann M., Irungu N.M., and Ilako D.R.The influence of central corneal thickness on intraocular pressure measured by goldmann applanation tonometry among selected Ethiopian communities. J. Glaucoma. 19:514–518, 2010 [DOI] [PubMed] [Google Scholar]
- 83.Park S.J., Ang G.S., Nicholas S., and Wells A.P.The effect of thin, thick, and normal corneas on Goldmann intraocular pressure measurements and correction formulae in individual eyes. Ophthalmology. 119:443–449, 2011 [DOI] [PubMed] [Google Scholar]
- 84.Tripathi R.C., Li J., Chan W.F., and Tripathi B.J.Aqueous humor in glaucomatous eyes contains an increased level of TGF-beta 2. Exp. Eye Res. 59:723–727, 1994 [DOI] [PubMed] [Google Scholar]
- 85.Inatani M., Tanihara H., Katsuta H., Honjo M., Kido N., and Honda Y.Transforming growth factor-beta 2 levels in aqueous humor of glaucomatous eyes. Graefes Arch. Clin. Exp. Ophthalmol. 239:109–113, 2001 [DOI] [PubMed] [Google Scholar]
- 86.Ochiai Y., and Ochiai H.Higher concentration of transforming growth factor-beta in aqueous humor of glaucomatous eyes and diabetic eyes. Jpn. J. Ophthalmol. 46:249–253, 2002 [DOI] [PubMed] [Google Scholar]
- 87.Picht G., Welge-Luessen U., Grehn F., and Lutjen-Drecoll E.Transforming growth factor beta 2 levels in the aqueous humor in different types of glaucoma and the relation to filtering bleb development. Graefes Arch. Clin. Exp. Ophthalmol. 239:199–207, 2001 [DOI] [PubMed] [Google Scholar]
- 88.Schlotzer-Schrehardt U., Zenkel M., Kuchle M., Sakai L.Y., and Naumann G.O.Role of transforming growth factor-beta1 and its latent form binding protein in pseudoexfoliation syndrome. Exp. Eye Res. 73:765–780, 2001 [DOI] [PubMed] [Google Scholar]
- 89.Ozcan A.A., Ozdemir N., and Canataroglu A.The aqueous levels of TGF-beta2 in patients with glaucoma. Int. Ophthalmol. 25:19–22, 2004 [DOI] [PubMed] [Google Scholar]
- 90.Yamamoto N., Itonaga K., Marunouchi T., and Majima K.Concentration of transforming growth factor beta2 in aqueous humor. Ophthalmic Res. 37:29–33, 2005 [DOI] [PubMed] [Google Scholar]
- 91.Min S.H., Lee T.I., Chung Y.S., and Kim H.K.Transforming growth factor-beta levels in human aqueous humor of glaucomatous, diabetic and uveitic eyes. Korean J. Ophthalmol. 20:162–165, 2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Fuchshofer R., and Tamm E.R.The role of TGF-beta in the pathogenesis of primary open-angle glaucoma. Cell Tissue Res. 347:279–290, 2012 [DOI] [PubMed] [Google Scholar]
- 93.Gottanka J., Chan D., Eichhorn M., Lutjen-Drecoll E., and Ethier C.R.Effects of TGF-beta2 in perfused human eyes. Invest. Ophthalmol. Vis. Sci. 45:153–158, 2004 [DOI] [PubMed] [Google Scholar]
- 94.Fleenor D.L., Shepard A.R., Hellberg P.E., Jacobson N., Pang I.H., and Clark A.F.TGFbeta2-induced changes in human trabecular meshwork: implications for intraocular pressure. Invest. Ophthalmol. Vis. Sci. 47:226–234, 2006 [DOI] [PubMed] [Google Scholar]
- 95.Robertson J.V., Golesic E., Gauldie J., and West-Mays J.A.Ocular gene transfer of active TGF-beta induces changes in anterior segment morphology and elevated IOP in rats. Invest. Ophthalmol. Vis. Sci. 51:308–318, 2010 [DOI] [PubMed] [Google Scholar]
- 96.Shepard A.R., Millar J.C., Pang I.H., Jacobson N., Wang W.H., and Clark A.F.Adenoviral gene transfer of active human transforming growth factor-{beta}2 elevates intraocular pressure and reduces outflow facility in rodent eyes. Invest. Ophthalmol. Vis. Sci. 51:2067–2076, 2010 [DOI] [PubMed] [Google Scholar]
- 97.Robertson J.V., Siwakoti A., and West-Mays J.A.Altered expression of transforming growth factor beta 1 and matrix metalloproteinase-9 results in elevated intraocular pressure in mice. Mol. Vis. 19:684–695, 2013 [PMC free article] [PubMed] [Google Scholar]
- 98.Johnson M.‘What controls aqueous humour outflow resistance?’. Exp. Eye Res. 82:545–557, 2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Acott T.S., and Kelley M.J.Extracellular matrix in the trabecular meshwork. Exp. Eye Res. 86:543–561, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Habashi J.P., Judge D.P., Holm T.M., et al. Losartan, an AT1 antagonist, prevents aortic aneurysm in a mouse model of Marfan syndrome. Science. 312:117–121, 2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Cohn R.D., van Erp C., Habashi J.P., et al. Angiotensin II type 1 receptor blockade attenuates TGF-beta-induced failure of muscle regeneration in multiple myopathic states. Nat. Med. 13:204–210, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Le Goff C., and Cormier-Daire V.From tall to short: the role of TGFbeta signaling in growth and its disorders. Am. J. Med. Genet. C Semin. Med. Genet. 160C:145–153, 2012 [DOI] [PubMed] [Google Scholar]
- 103.Ljubimov A.V., Saghizadeh M., Spirin K.S., Mecham R.P., Sakai L.Y., and Kenney M.C.Increased expression of fibrillin-1 in human corneas with bullous keratopathy. Cornea. 17:309–314, 1998 [PubMed] [Google Scholar]
- 104.Lacro R.V., Dietz H.C., Wruck L.M., et al. Rationale and design of a randomized clinical trial of beta-blocker therapy (atenolol) versus angiotensin II receptor blocker therapy (losartan) in individuals with Marfan syndrome. Am. Heart J. 154:624–631, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Nistala H., Lee-Arteaga S., Carta L., et al. Differential effects of alendronate and losartan therapy on osteopenia and aortic aneurysm in mice with severe Marfan syndrome. Hum. Mol. Genet. 19:4790–4798, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Habashi J.P., Doyle J.J., Holm T.M., et al. Angiotensin II type 2 receptor signaling attenuates aortic aneurysm in mice through ERK antagonism. Science. 332:361–365, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Mavromoustakos T., Agelis G., and Durdagi S.AT1 antagonists: a patent review (2008–2012). Expert Opin. Ther. Pat. 23:1483–1494, 2013 [DOI] [PubMed] [Google Scholar]
- 108.Lavoie P., Robitaille G., Agharazii M., Ledbetter S., Lebel M., and Lariviere R.Neutralization of transforming growth factor-beta attenuates hypertension and prevents renal injury in uremic rats. J. Hypertens. 23:1895–1903, 2005 [DOI] [PubMed] [Google Scholar]
- 109.Lim D.S., Lutucuta S., Bachireddy P., et al. Angiotensin II blockade reverses myocardial fibrosis in a transgenic mouse model of human hypertrophic cardiomyopathy. Circulation. 103:789–791, 2001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Lanz T.V., Ding Z., Ho P.P., et al. Angiotensin II sustains brain inflammation in mice via TGF-beta. J. Clin. Invest. 120:2782–2794, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Radonic T., de Witte P., Baars M.J., Zwinderman A.H., Mulder B.J., and Groenink M.Losartan therapy in adults with Marfan syndrome: study protocol of the multi-center randomized controlled COMPARE trial. Trials. 11:3, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Vaajanen A., Luhtala S., Oksala O., and Vapaatalo H.Does the renin-angiotensin system also regulate intra-ocular pressure? Ann. Med. 40:418–427, 2008 [DOI] [PubMed] [Google Scholar]
- 113.Shah G.B., Sharma S., Mehta A.A., and Goyal R.K.Oculohypotensive effect of angiotensin-converting enzyme inhibitors in acute and chronic models of glaucoma. J. Cardiovasc. Pharmacol. 36:169–175, 2000 [DOI] [PubMed] [Google Scholar]
- 114.Inoue T., Yokoyoma T., Mori Y., et al. The effect of topical CS-088, an angiotensin AT1 receptor antagonist, on intraocular pressure and aqueous humor dynamics in rabbits. Curr. Eye Res. 23:133–138, 2001 [DOI] [PubMed] [Google Scholar]
- 115.Wang R.F., Podos S.M., Mittag T.W., and Yokoyoma T.Effect of CS-088, an angiotensin AT1 receptor antagonist, on intraocular pressure in glaucomatous monkey eyes. Exp. Eye Res. 80:629–632, 2005 [DOI] [PubMed] [Google Scholar]
- 116.Costagliola C., Verolino M., De Rosa M.L., Iaccarino G., Ciancaglini M., and Mastropasqua L.Effect of oral losartan potassium administration on intraocular pressure in normotensive and glaucomatous human subjects. Exp. Eye Res. 71:167–171, 2000 [DOI] [PubMed] [Google Scholar]
- 117.Chen J., Runyan S.A., and Robinson M.R.Novel ocular antihypertensive compounds in clinical trials. Clin. Ophthalmol. 5:667–677, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Holm T.M., Habashi J.P., Doyle J.J., et al. Noncanonical TGFbeta signaling contributes to aortic aneurysm progression in Marfan syndrome mice. Science. 332:358–361, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Santos R.A., Ferreira A.J., and Simoes E.S.A.C.Recent advances in the angiotensin-converting enzyme 2-angiotensin(1–7)-Mas axis. Exp. Physiol. 93:519–527, 2008 [DOI] [PubMed] [Google Scholar]
- 120.Silveira K.D., Barroso L.C., Vieira A.T., et al. Beneficial effects of the activation of the angiotensin-(1–7) MAS receptor in a murine model of adriamycin-induced nephropathy. PLoS One 8:e66082, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Foureaux G., Nogueira J.C., Nogueira B.S., et al. Antiglaucomatous effects of the activation of intrinsic angiotensin-converting enzyme 2. Invest. Ophthalmol. Vis. Sci. 54:4296–4306, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]