Abstract
Background: As the role of palliative care (PC) has yet to be clearly defined in patients with heart failure (HF), such patients may face barriers regarding PC referral. In order to maximally meet the needs of HF patients, it is necessary to understand how they compare to the classic PC population: patients with cancer.
Objective: To characterize the unresolved symptom and treatment needs with which patients with HF and those with cancer present to PC.
Methods: We used data from the Palliative Care Research Registry (PCRR), a repository of quality improvement data from three community-based PC organizations. We abstracted first PC visit data from the PCRR for patients with primary diagnoses of HF or cancer seen between 2008 and 2012. We assessed the association of primary diagnosis (i.e., HF or cancer) on three outcomes: unresolved symptoms, treatment gaps, and a composite indicator of symptom control and quality of life. Analyses included descriptive statistics and multivariate Poisson regression.
Results: Our analytic sample comprised 334 patients with HF and 697 patients with cancer, the majority of whom were white and male. Compared to patients with cancer, patients with HF presented with fewer unresolved symptoms, both overall and at moderate/severe distress levels. Patients with HF more commonly reported moderately/severely distressful dyspnea (25% versus 18%, p=0.02), and more commonly experienced dyspnea-related treatment gaps (17% versus 8%, p<0.001).
Conclusions: Patients with HF possess care needs that are squarely within the purview of PC. Future work is needed to delineate how PC referral policies should be refined to optimize PC access for patients with HF.
Introduction
Historically, palliative care (PC) in the United States has largely served patients with cancer.1 Nevertheless, patients with other serious illnesses, including heart failure (HF), may benefit from PC. HF is a progressive condition that affects more than 5,000,000 Americans,2 and is associated with significant physical and psychosocial distress for patients and caregivers.3–7 Given its prevalence and improved survival resulting from life-prolonging therapies, the burden of chronic HF is greater than ever and is expected to grow.8
Internationally, numerous consensus statements support PC for patients with HF, ranging from specialist PC consultation to comanagement models.7,9–11 In the United States, the American College of Cardiology recommends PC for patients with advanced disease,9 however, patients with HF access palliative services far less often than patients with cancer.1 Although patients with HF and those with cancer exhibit similar disease-related burdens,12 patients with HF with worse health status experience greater physical and psychological symptom burdens.6 Patients with HF and those with cancer (and their caregivers) may differ in other ways. First, patients with HF may be less enagaged in health care decision-making and have less information regarding their illness and treatment options.13,14 Second, patients with HF experience poorer care coordination.13,15,16 Third, the pattern of decline in physical and psychological well-being vastly differs between patients with HF and patients with cancer.13 Last, the unpredictable trajectory of HF may impede PC referral.15–18 Moreover, because provider-related factors may delay PC referral among patients with HF,16 they may present with more advanced symptoms than patients with cancer.
Community-based PC is expanding in the United States,19,20 and holds promise to increase PC access for patients with HF. As such, it is important for community-based PC providers to understand the unmet palliative needs of patients with HF at first consultation, and how they differ from those of patients with cancer. Our goals are to: (1) describe patients with HF and patients with cancer receiving community-based PC; (2) determine the impact of having HF versus cancer on unresolved symptoms and treatment gaps; and (3) assess associations between primary diagnosis and outcomes. We seek to provide potential priorities for initial community-based PC consultations of patients with HF and those with cancer.
Methods
Data
We analyzed data from the Palliative Care Research Registry (PCRR), the repository of retrospective quality improvement data for the Carolinas Palliative Care Consortium. Established in 2008, the Consortium is a community-academic partnership between three North Carolina PC organizations and Duke University.21,22 We extracted data from patients' initial PC visits between June 1, 2008 and January 1, 2012.
At each PC visit, Consortium providers collected patient- or proxy-reported data on the distress of 11 symptoms: agitation, anorexia, anxiety, constipation, depression, diarrhea, dyspnea, fatigue, insomnia, nausea, and pain using the McCorkle Symptom Distress Scale.23 Symptom tolerability was also asssessed.
Measures
Our dependent variables were: (1) number of unresolved symptoms; (2) number of treatment care gaps; and, (3) palliative care patient health status (PC-PHS), a composite indicator of adequate symptom control and QOL. Unresolved symptoms are those rated as causing moderate or severe distress for each of the 11 symptoms in the PCRR (range, 0–11). Thus, “unresolved” signifies symptom distress persisting at the initial PC consultation—likely reflecting care received prior to PC referral. Somatic symptom burden (i.e., frequency and severity) may be a resonable indicator of latent emotional distress and poor quality of life in cardiovascular disease.24,25 A treatment gap was defined as the lack of a documented pharmacologic or nonpharmacologic intervention for a symptom whose distress was rated as moderate or severe. As interventions were documented for pain, dyspnea, constipation, and depression, treatment gap counts ranged between 0–4. Similar to unresolved symptoms, our measure reflects care received prior to PC referral.
Finally, PC-PHS is a composite indicator of symptom control and quality of life. We focused on pain and dyspnea, which are relevant to patients with HF and those with cancer26,27 and are often targets of PC interventions. Providers asked patients about both current and maximum tolerable levels of each symptom. Patients were considered to have adequate control if current symptoms were less than the maximum tolerable level, or the patient reported no current symptom. Although this may not constitute the ideal goal for symptom resolution, it represents a minimum standard of control. Furthermore, it is a patient-centered approach, assessing treatment effectiveness based on a specific patients' symptom experience. Quality of life was assessed with a single item (poor, fair, good).28 Given that only 5% of patients reported good quality of life, we created a binary measure (poor versus fair/good QOL). The resulting PC-PHS variable is a binary indicator with 1 (better health status) indicating all 3 criteria were met (i.e., controlled pain, controlled dyspnea, and fair/good quality of life), and 0 otherwise.
Our independent variable was primary diagnosis (i.e., HF or cancer). Diagnostic codes used to identify cohorts are provided in Table 1.
Table 1.
Diagnostic Codes Used to Identify Patient Cohorts
| Cohort | ICD-9-CM Diagnostic Codes |
|---|---|
| Heart failure29 | 428.xx (heart failure); 429.3 (cardiomegaly); 402.01 (malignant hypertensive heart disease with HF); 402.11 (benign hypertensive heart disease with HF); 402.91 (unspecified hypertensive heart disease with HF); and, 425.xx (cardiomyopathy). |
| Cancer | 140-239.9 |
Control variables included: patient age, gender, race, care setting, and respondent (i.e., patient-reported, proxy-reported). Performance status was assessed using the Palliative Performance Scale (PPS).30,31 We transformed PPS into a three-category variable for clinical relevance (i.e., 0–30, low; 40–60, medium; 70–100, high).32,33
Statistical analysis
All analyses were cross-sectional, patient-level, and assessed at the time of first PC visit. First, we used Pearson's χ2 tests, Student's t tests, and Wilcoxon-Mann-Whitney tests to examine bivariate differences by primary diagnosis. Next, we estimated risk ratios (RRs) to assess the effect of primary diagnosis on the probability of the PC-PHS outcome. We calculated RRs using modified Poisson regression with robust errors.34,35 Statistical tests were two-tailed with a critical α-level of 0.05. We conducted numerous sensitivity analyses, as well as assessments of model fit (e.g., Akaike's Information Criterion [AIC], deviance). Analyses were conducted using Stata/IC, version 12 (StataCorp, College Station, TX).36 This study was approved by the Institutional Review Boards of Duke University and the University of North Carolina.
Results
Of 1031 patients meeting study criteria, 334 (32%) had a primary diagnosis of HF; their characteristics are presented in Table 2. Most patients had a do-not-resuscitate order. The median PPS score for all patients was 40%, indicating predominantly bedridden patients with extensive evidence of disease. Approximately half of each disease group reported fair/good quality of life. Compared to patients with cancer, patients with HF were significantly older (84 versus 71 years, p<0.001), had one or more prior hospitalization in the preceding 6 months (81% versus 63%, p<0.001), and likelier to reside in a nursing home (18% versus 4%, p<0.001).
Table 2.
Demographic and Disease Characteristics of Study Cohort
| Characteristic | Patients with heart failure n (%) | Patients with cancer n (%) | p value |
|---|---|---|---|
| n | 334 | 697 | |
| Age in years, median [range] | 84 [33–102] | 71 [12–101] | <0.001 |
| Male gender | 138 (41) | 327 (47) | 0.09 |
| Race | 0.001 | ||
| White | 302 (90) | 602 (86) | |
| Black | 16 (5) | 78 (11) | |
| Other or unknown | 16 (5) | 17 (2) | |
| Advance care planning activities completed prior to or during initial palliative care visit | |||
| Do-not-resuscitate status declaration | 217 (65) | 472 (68) | 0.64 |
| Living will completed | 136 (41) | 249 (36) | 0.003 |
| MOST form completed | 16 (5) | 24 (3) | 0.30 |
| Designation of healthcare surrogate | 191 (57) | 472 (68) | <0.001 |
| Number of hospitalizations within 6 months before first palliative care visit | <0.001 | ||
| 0 | 64 (19) | 256 (37) | |
| 1 | 90 (27) | 183 (26) | |
| 2 | 53 (16) | 97 (14) | |
| 3 | 28 (8) | 38 (5) | |
| <3 | 17 (5) | 15 (2) | |
| Unknown | 82 (25) | 108 (15) | |
| Care setting at time of first palliative care visit | <0.001 | ||
| Hospital inpatient | 230 (69) | 544 (78) | |
| Nursing home or assisted living facility | 61 (18) | 30 (4) | |
| Patient home | 25 (8) | 83 (12) | |
| Outpatient clinic | 1 (0.3) | 6 (0.7) | |
| Respondent | 0.03 | ||
| Patient | 222 (66) | 507 (73) | |
| Caregiver or provider | 110 (33) | 183 (26) | |
| Palliative Performance Scale, median [range] | 40 [10–80] | 40 [10–90] | 0.27 |
| Low (10%–30%) | 92 (28) | 236 (34) | |
| Medium (40%–60%) | 154 (46) | 296 (42) | |
| High (70%–100%) | 13 (4) | 74 (11) | |
| General quality of life rating | 0.99 | ||
| Poor | 134 (40) | 301 (43) | |
| Fair/good | 153 (46) | 344 (49) | |
Values may not sum to 100 due to rounding and/or missing data. The Palliative Performance Scale rates functional status across five domains from 0%–100% in 10-percentage point increments, with greater scores indicating higher performance. χ2 tests of independence were calculated for categorical variables, Student's t tests were used for normally distributed continuous variables, and Wilcoxon-Mann-Whitney tests calculated for interval or non-normally distributed continuous outcomes.
MOST, Medical Orders for Scope of Treatment.
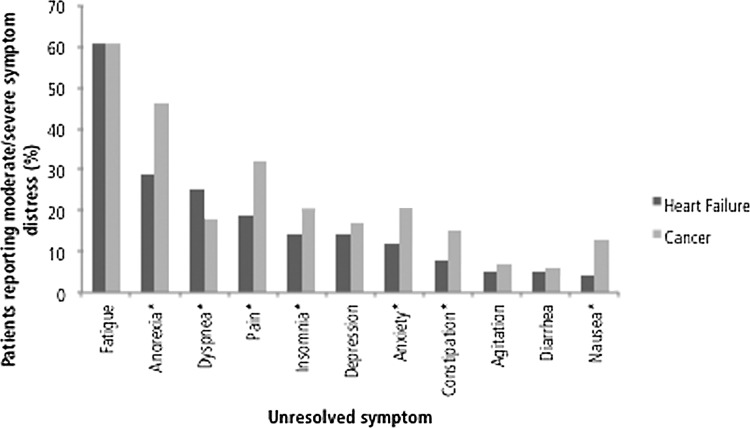
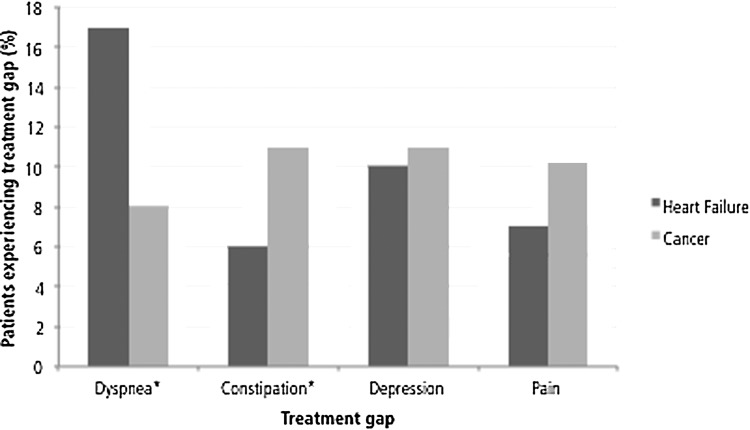
Patients with HF presented with fewer moderately and severely distressful unresolved symptoms than did patients with cancer (1.77 versus 2.24, p=0.0001) and overall (3.36 versus 3.87, p=0.0002), patients with HF, relative to patients with cancer, less frequently rated the following symptoms as moderately/severely distressful: anorexia (29% versus 46%, p<0.001), pain (19% versus 32%, p<0.001), insomnia (14% versus 20%, p=0.02), anxiety (12% versus 20%, p=0.002), constipation (8% versus 15%, p=0.004), and nausea (4% versus 13%, p<0.001). Only dyspnea was more commonly reported by patients with HF than patients with cancer as moderately/severely distressful (25% versus 18%, p=0.02; Fig. 1). Patients with HF experienced more dyspnea treatment gaps (17% versus 8%, p<0.001; Fig. 2); cancer patients had more constipation-related treatment gaps (11% versus 6%, p=0.008).
FIG. 1.
Prevalence of unresolved symptoms at first palliative care consultation. A symptom was defined as unresolved when the distress that it caused was rated as either moderate or severe. *Pearson's χ2 test indicates significant difference in unresolved symptom prevalence between heart failure patients and patients with cancer at the α=0.05 level.
FIG. 2.
Prevalence of treatment gaps at first palliative care consultation. We defined a treatment gap as the lack of a documented intervention for a symptom whose distress was rated as moderate or severe. *Pearson's χ2 test indicates significant difference in treatment gap prevalence between heart failure patients and patients with cancer at the α=0.05 level.
In bivariate analysis, diagnosis was not associated with PC-PHS (unadjusted RR: 1.20, 95% confidence interval [CI]: 0.96, 1.50, Table 3). However, after adjusting for clinical and demographic factors, patients with HF had lower probability of positive health status (adjusted RR: 0.70, 95% CI: 0.55, 0.90).
Table 3.
Associations between Various Demographic and Clinical Variables and Palliative Care Patient Health Status at First Palliative Care Consultation
| Unadjusted RR | p value | Adjusted RR | p value | |
|---|---|---|---|---|
| Heart failure (vs. cancer) | 1.20 (0.96–1.50) | 0.11 | 0.70 (0.55–0.90)a | 0.01 |
| Age, in years | 1.01 (1.00–1.02)a | 0.01 | 1.02 (1.01–1.03)a | <0.001 |
| Male gender | 0.75 (0.62–0.92)a | 0.01 | 1.34 (1.10–1.62)a | 0.003 |
| Non-white race | 1.24 (0.88–1.73) | 0.22 | 0.86 (0.62–1.20) | 0.37 |
| Proxy-reported symptom assessment (vs. patient-reported) | 1.69 (1.30–2.20)a | <0.001 | 0.58 (0.45–0.76)a | <0.001 |
| Outpatient (i.e., not hospitalized) at time of visit | 0.75 (0.61–0.93)a | 0.01 | 1.27 (1.04–1.57)b | 0.02 |
Significant at the 1% level.
Significant at the 5% level.
The outcome variable (PC-PHS, palliative care patient health status) was a binary indicator defined as positive if a patient reported all three of the following criteria: fair/good quality of life; adequately controlled pain; and, adequately controlled dyspnea.
Akaike's Information Criterion (AIC) of adjusted model: 1.24.
RR, risk ratio; CI, confidence interval.
Discussion
Patients with HF possess care needs that are clearly within the purview of PC. Prior work comparing the physical and psychosocial needs of patients with HF and those with cancer found the groups to be indistinguishable vis-à-vis symptom burden, despite differences regarding specific symptom prevalence.6,37 Our conclusions generally support these findings. We found that patients with cancer reported more unresolved moderately/severely distressful symptoms (2.24 versus 1.77). Whether this difference is sufficient to affect clinical decisionmaking is unknown; our experience suggests that symptom prioritization becomes paramount when people suffer from multiple problems simultaneously. Nevertheless, the question remains whether cumulative symptom burden or distress from a specific symptom prompts provider intervention. Echoing previous work,37,38 we found greater dyspnea in patients with HF than in patients with cancer; dyspnea, like pain, is a high-priority symptom that must be addressed, otherwise quality of life degrades and caregiver burden escalates.38–42 Last, having HF was associated with poorer health, even after controlling for the advanced age of our patients with HF. Our findings suggest that patients with HF and patients with cancer are appropriate for PC, however, the pattern of burden appears to differ between the two illnesses. Therefore, the portfolio of palliative interventions must be appropriately tailored.
Several limitations merit comment. First, PC-PHS is an exploratory composite measure. Although it has not yet been formally validated, it has face validity based on clinical and intuitive logic. Second, our cross-sectional study means that conclusions be judiciously interpreted. Third, data come from a community-based PC consortium in North Carolina and our patients were referred for PC; these factors may limit generalizability.
Ours is the first HF-focused analysis of community-based PC in the United States. We hope that it will serve to further describe a model of care through which we may expect a growing number of patients with HF to receive supportive services.7,19 With an estimated additional 3,000,000 Americans to be diagnosed with HF by 2030,43 our work can spark discussion regarding PC workforce planning, ensuring that PC teams have the skills and resources necessary to care for people with HF.
Acknowledgments
The first author sincerely thanks Joseph Kelly, William Downey, MSW, Gregory Samsa, PhD, Janet Bull, MD, Jeanne Bailey, C. Steve Stinson, MD, and Melanie Kelly, MSN, for their assistance in the conduct of this study. This material is the result of work supported in part (Dr. Dev) with resources and the use of facilities at the Phoenix VA Health Care System. The contents do not represent the views of the Department of Veterans Affairs or the United States Government.
Dr. Kavalieratos was supported by a Ruth L. Kirschstein National Research Service Award Pre-doctoral Traineeship from the Agency for Healthcare Research and Quality, sponsored by the Cecil G. Sheps Center for Health Services Research, University of North Carolina at Chapel Hill, Grant No. T32-HS-000032-19. Dr. Kavalieratos is currently supported by a National Research Service Award Post-doctoral Traineeship from the Agency for Healthcare Research and Quality, sponsored by the Division of General Internal Medicine at the University of Pittsburgh, Grant No. T32-HS-017587-05.
This study was presented, in part, at the 2012 American Public Health Association Annual Meeting; October 29, 2012; San Francisco, California.
Author Disclosure Statement
Dr. Abernethy has research funding from the National Institute of Nursing Research, National Cancer Institute, Agency for Healthcare Research and Quality, Robert Wood Johnson Foundation, Biovex, DARA, Helsinn, MiCo, Dendreon and Pfizer; these funds are all distributed to Duke University Medical Center to support research including salary support for Dr. Abernethy. Pending industry funded projects include: Bristol Myers Squibb and Genentech. In the last 2 years she has had nominal consulting agreements with or received honoraria from (less than $10,000 annually) Novartis, Bristol Myers Squibb and Pfizer. Further consulting with Bristol Meyers Squibb is pending in 2013, for role as Co-Chair of a Scientific Advisory Committee. Dr. Abernethy has a paid leadership role with American Academy of Hospice and Palliative Medicine (President). She has corporate leadership responsibility in Advoset (an education company that has a contract with Novartis) and Orange Leaf Associates LLC (an IT development company). Dr. Biddle receives industry funding from Bristol Myers Squibb to oversee a fellowship program. Dr. Carey receives funding from NIH, AHRQ and PCORI. Dr. Weinberger receives research funding from NIH, AHRQ, and the Department of Veterans Affairs. All other authors have no disclosures.
References
- 1.Andersen J: NHPCO Facts and Figures: Hospice Care in America. 2009 ed. Alexandria, VA: NHPCO, 2009, pp. 1–15 [Google Scholar]
- 2.Lloyd-Jones D, Adams RJ, Brown TM, Carnethon M, Dai S, De Simone G, Ferguson TB, Ford E, Furie K, Gillespie C, Go A, Greenlund K, Haase N, Hailpern S, Ho PM, Howard V, Kissela B, Kittner S, Lackland D, Lisabeth L, Marelli A, McDermott MM, Meigs J, Mozaffarian D, Mussolino M, Nichol G, Roger VL, Rosamond W, Sacco R, Sorlie P, Roger VL, Thom T, Wasserthiel-Smoller S, Wong ND, Wylie-Rosett J; American Heart Association Statistics Committee and Stroke Statistics Subcommittee: Heart disease and stroke statistics—2010 update: A report from the American Heart Association. Circulation 2010;121:e46–e215 [DOI] [PubMed] [Google Scholar]
- 3.Blinderman CD, Homel P, Billings JA, Portenoy RK, Tennstedt Symptom distress and quality of life in patients with advanced congestive heart failure. J Pain Symptom Manage 2008;35:594–603 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Walke LM, Byers AL, Tinetti ME, Dubin JA, McCorkle R, Fried TR: Range and severity of symptoms over time among older adults with chronic obstructive pulmonary disease and heart failure. Arch Intern Med 2007;167:2503–2508 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Bekelman DB, Havranek EP, Becker DM, Kutner JS, Peterson PN, Wittstein IS, Gottlieb SH, Yamashita TE, Fairclough DL, Dy SM: Symptoms, depression, and quality of life in patients with heart failure. J Card Fail 2007;13:643–648 [DOI] [PubMed] [Google Scholar]
- 6.Bekelman DB, Rumsfeld JS, Havranek EP, Yamashita TE, Hutt E, Gottlieb SH, Dy SM, Kutner JS: Symptom burden, depression, and spiritual well-being: A comparison of heart failure and advanced cancer patients. J Gen Intern Med 2009;24:592–598 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Goodlin S, Hauptman P, Arnold R, Grady K, Hershberger RE, Kutner J, Masoudi F, Spertus J, Dracup K, Cleary JF, Medak R, Crispell K, Piña I, Stuart B, Whitney C, Rector T, Teno J, Renlund DG: Consensus statement: Palliative and supportive care in advanced heart failure. J Card Fail 2004;10:200–209 [DOI] [PubMed] [Google Scholar]
- 8.Goodlin SJ: Palliative care in congestive heart failure. J Am Coll Cardiol. 2009;54:386–396 [DOI] [PubMed] [Google Scholar]
- 9.Yancy CW, Jessup M, Bozkurt B, Butler J, Casey DE, Jr, Drazner MH, Fonarow GC, Geraci SA, Horwich T, Januzzi JL, Johnson MR, Kasper EK, Levy WC, Masoudi FA, McBride PE, McMurray JJ, Mitchell JE, Peterson PN, Riegel B, Sam F, Stevenson LW, Tang WH, Tsai EJ, Wilkoff BL; American College of Cardiology Foundation/American Heart Association Task Force on Practice Guidelines: 2013 ACCF/AHA Guideline for the Management of Heart Failure: A Report of the American College of Cardiology Foundation/American Heart Association Task Force on Practice Guidelines. Circulation 2013;128:e240–327 [DOI] [PubMed] [Google Scholar]
- 10.Jaarsma T, Beattie JM, Ryder M, Rutten FH, McDonagh T, Mohacsi P, Murray SA, Grodzicki T, Bergh I, Metra M, Ekman I, Angermann C, Leventhal M, Pitsis A, Anker SD, Gavazzi A, Ponikowski P, Dickstein K, Delacretaz E, Blue L, Strasser F, McMurray J; Advanced Heart Failure Study Group of the HFA of the ESC: Palliative care in heart failure: a position statement from the palliative care workshop of the Heart Failure Association of the European Society of Cardiology. Eur J Heart Fail 2009;11:433–443 [DOI] [PubMed] [Google Scholar]
- 11.McKelvie RS, Moe GW, Cheung A, Costigan J, Ducharme A, Estrella-Holder E, Ezekowitz JA, Floras J, Giannetti N, Grzeslo A, Harkness K, Heckman GA, Howlett JG, Kouz S, Leblanc K, Mann E, O'Meara E, Rajda M, Rao V, Simon J, Swiggum E, Zieroth S, Arnold JM, Ashton T, D'Astous M, Dorian P, Haddad H, Isaac DL, Leblanc MH, Liu P, Sussex B, Ross HJ: The 2011 Canadian Cardiovascular Society heart failure management guidelines update: Focus on sleep apnea, renal dysfunction, mechanical circulatory support, and palliative care. Can J Cardiol 2011;27:319–338 [DOI] [PubMed] [Google Scholar]
- 12.Steinhauser KE, Arnold RM, Olsen MK, Lindquist J, Hays J, Wood LL, Burton AM, Tulsky JA: Comparing three life-limiting diseases: Does diagnosis matter or is sick, sick? J Pain Symptom Manage 2011;42:331–341 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Murray SA, Boyd K, Kendall M, Worth A, Benton TF, Clausen H: Dying of lung cancer or cardiac failure: Prospective qualitative interview study of patients and their carers in the community. BMJ 2002;325:929. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Rogers AE, Addington-Hall JM, Abery AJ, McCoy AS, Bulpitt C, Coats AJ, Gibbs JS: Knowledge and communication difficulties for patients with chronic heart failure: Qualitative study. BMJ 2000;321:605–607 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Hanratty B, Hibbert D, Mair F, May C, Ward C, Capewell S, Litva A, Corcoran G: Doctors' perceptions of palliative care for heart failure: Focus group study. BMJ 2002;325:581–585 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Kavalieratos D, Mitchell EM, Carey TS, Dev S, Biddle AK, Reeve BB, Abernethy AP, Weinberger M: Not the ‘grim reaper service’: An assessment of provider knowledge, attitudes, and perceptions regarding palliative care referral barriers in heart failure. J Amer Heart Assn 2014;3:e544. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Wotton K, Borbasi S, Redden M: When all else has failed: Nurses' perception of factors influencing palliative care for patients with end-stage heart failure. J Cardiovasc Nurs 2005;20:18–25 [DOI] [PubMed] [Google Scholar]
- 18.Green E, Gardiner C, Gott M, Ingleton C: Exploring the extent of communication surrounding transitions to palliative care in heart failure: The perspectives of health care professionals. J Palliat Care 2011;27:107–116 [PubMed] [Google Scholar]
- 19.Meier DE, Beresford L: Outpatient clinics are a new frontier for palliative care. J Palliat Med 2008;11:823–828 [DOI] [PubMed] [Google Scholar]
- 20.Muir JC, Daly F, Davis MS, Weinberg R, Heintz JS, Paivanas TA, Beveridge R: Integrating palliative care into the outpatient, private practice oncology setting. J Pain Symptom Manage 2010;40:126–135 [DOI] [PubMed] [Google Scholar]
- 21.Bull J, Zafar SY, Wheeler JL, Harker M, Gblokpor A, Hanson L, Hulihan D, Nugent R, Morris J, Abernethy AP: Establishing a regional, multisite database for quality improvement and service planning in community-based palliative care and hospice. J Palliat Med 2010;13:1013–1020 [DOI] [PubMed] [Google Scholar]
- 22.Abernethy AP, Wheeler JL, Bull J: Development of a health information technology-based data system in community-based hospice and palliative care. Am J Prev Med 2011;40(5 Suppl 2):S217–224 [DOI] [PubMed] [Google Scholar]
- 23.McCorkle R, Young K: Development of a symptom distress scale. Cancer Nurs 1978;1:373–378 [PubMed] [Google Scholar]
- 24.Kohlmann S, Gierk B, Hümmelgen M, Blankenberg S, Löwe B: Somatic symptoms in patients with coronary heart disease: Prevalence, risk factors, and quality of life. JAMA Intern Med 2013;173:1469–1471 [DOI] [PubMed] [Google Scholar]
- 25.O'Malley PG: Symptom number and severity as a sign of emotional distress in patients with cardiovascular disease. JAMA Intern Med 2013;173:1471–147123939518 [Google Scholar]
- 26.Solano J, Gomes B, Higginson I: A comparison of symptom prevalence in far advanced cancer, AIDS, heart disease, chronic obstructive pulmonary disease and renal disease. J Pain Symptom Manage 2006;31:58–69 [DOI] [PubMed] [Google Scholar]
- 27.Steinhauser KE, Christakis NA, Clipp EC, McNeilly M, McIntyre L, Tulsky JA: Factors considered important at the end of life by patients, family, physicians, and other care providers. JAMA 2000;284:2476–2482 [DOI] [PubMed] [Google Scholar]
- 28.Cohen SR, Mount BM, Strobel MG, Bui F: The McGill Quality of Life Questionnaire: A measure of quality of life appropriate for people with advanced disease. A preliminary study of validity and acceptability. Palliat Med 1995;9:207–219 [DOI] [PubMed] [Google Scholar]
- 29.Heidenreich PA, Sahay A, Kapoor JR, Pham MX, Massie B: Divergent trends in survival and readmission following a hospitalization for heart failure in the Veterans Affairs health care system 2002 to 2006. J Am Coll Cardiol 2010;56:362–368 [DOI] [PubMed] [Google Scholar]
- 30.Anderson F, Downing GM, Hill J, Casorso L, Lerch N: Palliative performance scale (PPS): A new tool. J Palliat Care 1996;12:5–11 [PubMed] [Google Scholar]
- 31.Mor V, Laliberte L, Morris JN, Wiemann M: The Karnofsky Performance Status Scale. An examination of its reliability and validity in a research setting. Cancer 1984;53:2002–2007 [DOI] [PubMed] [Google Scholar]
- 32.Ezekowitz JA, Thai V, Hodnefield TS, Sanderson L, Cujec B: The correlation of standard heart failure assessment and palliative care questionnaires in a multidisciplinary heart failure clinic. J Pain Symptom Manage 2011;42:379–387 [DOI] [PubMed] [Google Scholar]
- 33.Kamal AH, Bull J, Kavalieratos D, Taylor DH, Jr., Downey W, Abernethy AP: Palliative care needs of patients with cancer living in the community. J Oncol Pract 2011;7:382–388 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Zou G: A modified poisson regression approach to prospective studies with binary data. Am J Epidemiol 2004;159:702–706 [DOI] [PubMed] [Google Scholar]
- 35.McNutt L-A, Wu C, Xue X, Hafner JP: Estimating the relative risk in cohort studies and clinical trials of common outcomes. Am J Epidemiol 2003;157:940–943 [DOI] [PubMed] [Google Scholar]
- 36.Case RB, Moss AJ, Case N, McDermott M, Eberly S: Living alone after myocardial infarction: Impact on prognosis. JAMA 1992;267:515–519 [PubMed] [Google Scholar]
- 37.O'Leary N, Murphy NF, O'Loughlin C, Tiernan E, McDonald K: A comparative study of the palliative care needs of heart failure and cancer patients. Eur J Heart Fail 2009;11:406–412 [DOI] [PubMed] [Google Scholar]
- 38.Currow DC, Smith J, Davidson PM, Newton PJ, Agar MR, Abernethy AP: Do the trajectories of dyspnea differ in prevalence and intensity by diagnosis at the end of life? A consecutive cohort study. J Pain Symptom Manage 2010;39:680–690 [DOI] [PubMed] [Google Scholar]
- 39.Feinstein AR, Fisher MB, Pigeon JG: Changes in dyspnea-fatigue ratings as indicators of quality of life in the treatment of congestive heart failure. Am J Cardiol 1989;64:50–55 [DOI] [PubMed] [Google Scholar]
- 40.Moody LE, McMillan S: Dyspnea and quality of life indicators in hospice patients and their caregivers. Health Qual Life Outcomes 2003;1:9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Abernethy AP, Wheeler JL: Total dyspnoea. Curr Opin Support Palliat 2008;2:110–113 [DOI] [PubMed] [Google Scholar]
- 42.Reddy SK, Parsons HA, Elsayem A, Palmer JL, Bruera E: Characteristics and correlates of dyspnea in patients with advanced cancer. J Palliat Med 2009;12:29–36 [DOI] [PubMed] [Google Scholar]
- 43.Heidenreich PA, Trogdon JG, Khavjou OA, Butler J, Dracup K, Ezekowitz MD, Finkelstein EA, Hong Y, Johnston SC, Khera A, Lloyd-Jones DM, Nelson SA, Nichol G, Orenstein D, Wilson PW, Woo YJ; American Heart Association Advocacy Coordinating Committee; Stroke Council; Council on Cardiovascular Radiology and Intervention; Council on Clinical Cardiology; Council on Epidemiology and Prevention; Council on Arteriosclerosis; Thrombosis and Vascular Biology; Council on Cardiopulmonary; Critical Care; Perioperative and Resuscitation; Council on Cardiovascular Nursing; Council on the Kidney in Cardiovascular Disease; Council on Cardiovascular Surgery and Anesthesia, and Interdisciplinary Council on Quality of Care and Outcomes Research: Forecasting the future of cardiovascular disease in the United States: A policy statement from the American Heart Association. Circulation 2011;123:933–944 [DOI] [PubMed] [Google Scholar]