Abstract
Intraocular pressure (IOP) is the only treatable risk factor in glaucoma, one of the world's leading causes of blindness. Mechanisms that maintain IOP within a normal range have been poorly understood in contrast to intrinsic mechanisms that regulate systemic blood pressure. Vessel walls experience continuous pulse-induced cyclic pressure and flow. Pressure-dependent wall stress and flow-dependent shear stress provide sensory signals that initiate mechanotransduction responses. The responses optimize vessel wall elasticity, compliance and lumen size, providing a feedback loop to maintain intrinsic pressure homeostasis.
Aqueous humor is part of a vascular circulatory loop, being secreted into the anterior chamber of the eye from the vasculature, then returning to the vasculature by passing through the trabecular meshwork (TM), a uniquely modified vessel wall interposed between the anterior chamber and a vascular sinus called Schlemm's canal (SC). Since pressure in circulatory loops elsewhere is modulated by cyclic stresses, one might predict similar pressure modulation in the aqueous outflow system.
Recent laboratory evidence in fact demonstrates that cyclic IOP changes alter aqueous outflow while increasing cellularity and contractility of TM cells. Cyclic changes also lead to alterations in gene expression, changes in cytoskeletal networks and modulation of signal transduction. A new technology, phase-based optical coherence tomography, demonstrates in vivo pulse-dependent TM motion like that elsewhere in the vasculature. Recognition of pulse-dependent TM motion provides a linkage to well-characterized mechanisms that provide pressure homeostasis in the systemic vasculature. The linkage may permit unifying concepts of pressure control and provide new insights into IOP homeostatic mechanisms.
Introduction
Glaucoma is one of the world's leading causes of irreversible blindness with the only treatable risk factor being intraocular pressure (IOP).1 Control of IOP resides in the flow control pathways of the aqueous outflow system of the eye, yet the nature of control mechanisms remains uncertain.2 An understanding of normal flow control mechanisms is essential before abnormalities in the mechanisms can be accurately identified and modified in glaucoma patients.
Recent laboratory studies provide evidence that pulse-dependent mechanisms alter outflow facility,3 while at the same time increasing trabecular meshwork (TM) cellularity and contractility.4 Such findings are consistent with the concept that cyclic mechanical stresses function to confer a physiologic benefit important to IOP homeostasis. These studies point to the need to improve our understanding of the linkage between cyclic stresses, TM motion, and IOP regulatory homeostasis.
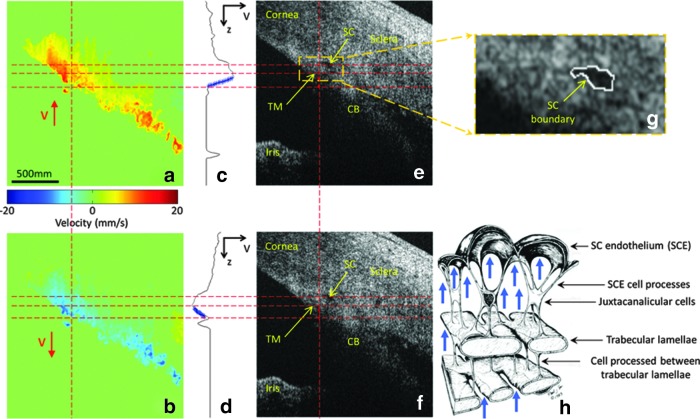
Pulse-dependent TM motion has recently been documented with phase-based optical coherence tomography (PhS-OCT)5,6 (Fig. 1) and is a functional property that provides a bridge between ocular and vascular physiology.7 Aqueous circulates within one of the vascular circulatory loops, which return fluids to the heart and the TM, as the wall of a vascular sinus exhibits pulse-dependent responses like those of the veins and lymphatics. The bridging concept permits vascular and ocular cyclic pressure control mechanisms to be reconciled at the tissue and cellular level8 as well as integrating the mechanisms into the larger framework of cellular physiology.
FIG. 1.
Ex vivo pulse-induced trabecular meshwork (TM) movement and Schlemm's canal (SC) deformation in nonhuman primate eye at 8 mmHg mean intraocular pressure (IOP). Representative cross-sectional images of tissue velocity in the corneoscleral limbus: (a) red corresponds to TM movement into the SC during systole; (b) blue corresponds to TM movement away from the SC during diastole; (c) and (d) depth-dependent velocity profiles along the vertical dashed lines in (a) and (b), respectively; (e) and (f ) corresponding optical coherence tomography microstructural images from (a) and (b). (g) Enlarged view of the area marked by the dashed yellow square in (e). The closed white curve in (g) depicts the boundary of SC. (h) Schematic of the SC endothelial attachment to the underlying trabecular lamellae. The bold arrows in (h) indicate tissue responses to deforming forces induced by IOP transients. The horizontal lines are used to mark approximately the position of TM, facilitating comparison between figures. Reproduced with permission from Li et al.5
Evidence indicates that tissue homeostasis is governed by means of a hierarchy of tissue and cellular level tensional integration, which interconnects cells, extracellular matrix (ECM), integrins, cadherins, stress-sensitive ion channels, focal adhesions, cytoskeletal filaments, cytoskeletal-linked signaling pathways, and the nuclear envelope.9 Tensionally integrated behavior additionally induces movement of nuclear filament networks providing a means of direct mechanical control of nuclear activities and functions.9,10 These tensionally integrated connections permit instantaneous tissue-wide recognition of, and contemporaneous rather than linear, responses to tension changes.
A central feature of tissue homeostasis is thought to be cellular differentiation to establish an intrinsic cellular tension.11,12 This tensional set point orchestrates evolutionarily optimized genomic relationships13 that determine sensor signal acquisition and effector signaling responses, maintaining orientation in three-dimensional space and tissue-wide coordination of motion-dependent responses.9 The set points can be tuned to match substrates.14
Application of changing tension in the environment surrounding the cell activates Rho, stimulating actin polymerization and myosin-based tension, which in turn modulates focal adhesion and stress fiber assembly permitting iterative tension optimization.9,12 In the vasculature, shear stress signal acquisition is dependent on cellular topology, a cellular configuration determined by the interplay between cytoskeletal tension and cell turgor. Shear stress in turn orchestrates a myriad of signaling responses, including optimization of cytoskeletal tension; a tightly linked contemporaneous optimization of cell turgor, cellular tension, topology, and shear stress is thus an inherent requirement for cellular homeostasis.11,12
At the cellular level, coordination of a multitude of well-studied cellular signaling pathways of the endothelial cells of the vascular system is governed by the sensory input provided by wall and shear stresses11,12 that undergo continuous cyclic pulse transients. These mechanotransduction-dependent signaling pathways optimize both cellular and extracellular wall tension9 to provide intrinsic pressure homeostasis throughout the vasculature. Evidence of pulse-dependent TM motion suggests that the same principles of cellular biomechanics so well characterized in the systemic vasculature12 will be able to provide guidance for and be reconciled with aqueous outflow system principles and pathways.
Cyclic IOP Changes Affect Signaling Pathways of the Outflow System
The TM is interposed between the anterior chamber and Schlemm's canal (SC), a vascular sinus that communicates with the venous system. Vascular endothelia elsewhere sense shear and wall stresses, mechanotransducing these stresses into biochemical signals that induce not only endothelial cytoskeletal changes, but also changes in the composition of the extracellular components of the vessel walls.11,12
The cellular components of the TM, particularly the SC endothelium and the juxtacanalicular cells, undergo marked deformation in response to IOP changes within the physiologic range.15–17 Pressure-dependent, tensionally integrated cellular deformation confers the ability to sense environmental stimuli such as IOP fluctuations and cyclic IOP changes.9
In the aqueous outflow system, cyclic changes in IOP alter conventional aqueous outflow in an ex vivo model3; a synchronous increase in cellularity has lead to the proposal that cyclic changes act to alter cellular contractile mechanisms.4 In addition, mechanical stresses lead to alterations in gene expression18–21 as well as changes in cytoskeletal networks22,23 and modulation of signal transduction.24
The composition of the ECM in the trabecular beams and juxtacanalicular space is subject to modulation by mechanical stretching.25,26 The ECM composition, in turn, controls TM tissue elasticity and compliance, properties important to the maintenance of normal function. Modulation of ECM composition becomes abnormal in glaucoma.2,27 Studies implicate changes in cell and ECM stiffness as probable factors in the glaucoma process.8,28,29 Recent elastic modulus determination in normal and glaucoma conditions30–32 has indicated a relationship between increased TM tissue stiffness and open angle glaucoma, a change also identified in clinical studies.7,33,34
Recognition of the Importance of Trabecular Tissue Motion
Morton Grant can be credited with initiating the first studies to characterize TM motion. Early studies by Grant in ex vivo eyes indicated that 75% of the normal outflow resistance35 and the abnormal resistance in glaucoma36 were in the TM leading to the concept that the control of flow was dependent on a passive filter-like mechanism.
It was the same Morton Grant who suspected a more complex explanation because of the lack of circumferential flow in the SC.35,36 The absence of circumferential flow requires much of SC lumen to be little more than a potential space, suggesting that trabecular tissues must maintain a finely tuned relationship with the SC external wall to permit aqueous access to collector channels, yet at the same time, prevent circumferential flow.
Studies by Grant and colleagues demonstrated in ex vivo eyes that outflow resistance increases markedly with increases in IOP,37 a phenomenon much more striking in 6 glaucomatous eyes.34 In a geometrically stable structure, resistance would not change as IOP increases.37 Furthermore, by using a perfusion technique that deepened the anterior chamber, known to create tension on scleral spur and dilate the SC, the increasing resistance with increasing pressure was completely eliminated.37 They concluded that the resistance increase was a result of TM motion causing progressive SC wall apposition with a simultaneous increase in resistance; the finding is especially significant because of its much greater impact in glaucomatous eyes.
To further explore the question of TM motion, Ellingsen and Grant removed the external wall of SC in a series of eyes.38 In the course of external wall removal, they directly observed TM movement outward toward the region of the now absent external wall of SC. The reduction in resistance with SC external wall removal was essentially the same as with TM removal. If removal of either of the walls of SC leaves only ∼25% of the resistance, they concluded that the relationship between the SC walls must account for much of the experimentally demonstrated changes in resistance.38
To test the hypothesis that the TM tissues were indeed in motion, Grant and I initiated a study that involved fixation of enucleated human and live primate eyes at a systematically controlled series of IOPs. Remarkable changes in tissue configuration at both the tissue15 and cellular16 levels were observed. The TM distended progressively into the SC and at near physiologic pressures, developed progressive apposition to SC external wall as predicted by Grant's earlier studies.
The above studies indicated that the TM is tensionally integrated and prestressed, criteria necessary for mechanotransduction mechanisms. Later, Van Buskirk, with lens depression studies, histologically demonstrated progressive SC wall separation with a corresponding highly linear improvement in aqueous outflow facility.39 The group of studies points to a need to maintain tightly controlled elasticity and compliance of the TM tissues to optimize SC wall relationships.
Pulsatile Aqueous Outflow: A Role in IOP Homeostasis
Aqueous outflow patterns can be studied in the eye by a means unique in physiology, that is, direct observation of aqueous flow as it passes from the SC into the aqueous and episcleral veins on the surface of the eye.7 Direct observation of flow provides assumption-free evidence of outflow mechanisms. The strength of various theories of outflow can be assessed by their ability to explain and predict the in vivo findings.
Above the heart, pulsatile mechanisms involving Starling resistor-like oscillatory flow through collapsible tubes and chokepoints have long been recognized as the regulatory framework for maintaining optimized flow and controlled pressure drops in vessels.40 Directly observable oscillatory collapse of the central retinal vein (CRV) has been considered a classic illustration of such a control mechanism.
In humans, mean IOP in the population is about 16 mm Hg, while mean episcleral venous pressure is about 8 mm Hg resulting in a pressure drop across the aqueous outflow system of about 8 mm Hg.41 Retinal vein pressures are linked to IOP since venous pressures must be maintained at a level slightly higher than IOP, otherwise, intraocular veins would collapse. Episcleral veins, normally at a pressure of about 8 mm Hg, drain to the ophthalmic vein, which must then have a pressure slightly lower than 8 mm Hg. Since the CRV also drains to the ophthalmic vein, a very similar pressure drop must be present between the aqueous and venous systems. One might reasonably expect similar oscillatory Starling resistor-like mechanisms of flow and pressure control in the 2 linked systems.
The pulse dependence of aqueous return to the episcleral vascular system has been recognized as a salient behavior from the first recognition that aqueous flows.42,43 Experimentally controlled increases in IOP induced by pressure on the side of the eye,44–46 ophthalmodynamometry,47,48 or water drinking7 result in enhanced pulsatile aqueous outflow. When IOP is reduced below its physiologic set point, pulsatile flow stops, but begins again when IOP rises to the homeostatic set point.7,49 When flow in a downstream recipient episcleral vein is temporarily blocked, pulsatile aqueous flow increases in the upstream aqueous vein, causing aqueous to enter regional tributary veins previously carrying aqueous.47,50 The aqueous influx response is indicative of an intrinsic outflow system mechanism capable of altering pulsatile flow in response to changing pressure relationships.49
Synchrony of Cardiac-Induced Pulse Waves and TM Motion Revealed by Phase-OCT
A new technology, PhS-OCT has nanometer sensitivity to tissue motion. Ex vivo studies using PhS-OCT in primate eyes have demonstrated cyclic pulse-dependent TM motion.5 Consideration of known normal volumes of aqueous flow and SC lumen geometry indicates that pulse-dependent TM excursions can carry enough volume per pulse wave to account for all of aqueous outflow. As IOP increases, a situation in which the lumen of SC begins to close,51 the response of the TM to a stable induced pulse wave amplitude decreases.5
A recent study of 20 eyes of 10 human subjects finds a highly significant relationship between TM motion peaks and cardiac pulse peaks.6 Motion of the TM in response to the ocular pulse reflects the elasticity and compliance of the trabecular tissues, a measure of their functional properties that are involved in maintaining IOP homeostasis. Knowledge of such TM properties in an individual patient may help predict who will have progressive difficulty with maintaining IOP homeostasis and benefit from an earlier initiation or escalation of therapy.
Glaucoma management decisions are inherently suboptimal because we use IOP measurements to assess adequacy of pressure control. Such measurements are typically random, infrequent (3–4/year), and only provide a few seconds of information (∼3 s/exam) within each year's time frame. PhS-OCT is noncontact, noninvasive, and measures properties that maintain homeostasis, suggesting it could be a useful clinical tool to assess the properties that normally maintain IOP within a narrow range.
PhS-OCT is a nascent technology recently developed in the laboratory and relies on principles that are fundamentally different from commercially available OCT devices. As with any new technology of this complexity, translation to a useful clinical tool may be expected to require time and considerable resources, requirements that may best be met with industry involvement. The ability to characterize tissue properties that maintain IOP homeostasis should provide a compelling argument for such a commitment.
Glaucoma Medication Management Dilemmas and Possible PhS-OCT Solutions
The first studies recognizing aqueous flow and its pulsatile character as well as multiple later studies documented that medications such as miotics42,45,47,50,52–54 and adrenergics34,43,47,50,55 as well as prostaglandins7 markedly increase pulsatile aqueous outflow; the increase in pulsatile flow precedes a reduction in IOP. As the IOP drops to a new homeostatic set point, pulsatility slows. Beyond the duration of medication action, pulsatile flow stops accompanied by an increase in IOP.
A significant number of patients also have a poor or absent medication response. The unknown response in individual patients imposes a requirement that all patients return soon after starting a medication to assess its effectiveness. Assessment of effectiveness is complicated by many factors such as a tendency to initiate medical therapy on a day when pressure is elevated resulting in the likelihood of a regression to the mean. The resulting pressure reduction may lead to the inappropriate conclusion of effectiveness following which, patients may be kept on an ineffective medication for many months to years.
Poor compliance, an extremely common problem,56 when occurring before a visit may lead to a conclusion that medication is ineffective, causing discontinuance of useful medication. Diurnal changes57 and spontaneous day-to-day variations58,59 are further confounding factors in evaluating medication effectiveness, factors not eliminated by monocular trials, which have been found as an unreliable guide for medication decisions.59
A marked increase in pulsatile aqueous flow occurs within ∼5 min with adrenergics, 5–20 min with miotics, and 20–30 min with prostaglandins.7 Pulsatile aqueous outflow results from TM motion. Accordingly, the ability to monitor TM movement offers the possibility of first measuring baseline motion with OCT, then instilling drops, and within less than an hour assessing whether a change in motion has occurred. The ability to do a same day office assessment of medication effectiveness would reduce medication response uncertainties, reducing the number of patients either taking ineffective medications or not taking medications because they were unrecognized as effective.
Summary
Morton Grant's early insight into the role of TM motion in aqueous flow control provided a new vantage point, an organizational framework that frees us mentally, allowing us to explore outflow mechanisms from a new perspective. Evidence of in vivo cyclic motion of the TM provides a means to explain pulsatile aqueous outflow from the SC. Structure and function can be integrated; all the trabecular tissues can be envisioned to be participating in regulated flow of aqueous from the anterior chamber to the venous circulation.
Evidence suggests that cyclic mechanical stresses may confer a physiologic benefit important to IOP homeostasis. Alterations in pulsatile flow may provide a means to maintain short-term IOP control. Long-term homeostasis may be maintained by mechanotransduction mechanisms that regulate TM tissue composition to maintain optimized TM cellular contractility, as well as elasticity and compliance of extracellular elements. PhS-OCT may provide a noninvasive in vivo means of using motion to assess and monitor the properties of the TM tissues, properties essential to homeostasis. PhS-OCT may also provide a clinical means of improving glaucoma management decisions.
Author Disclosure Statement
No competing financial interests exist.
References
- 1.Quigley H.A., and Broman A.T.The Number of People With Glaucoma Worldwide in 2010 and 2020. Br. J. Ophthalmol. 90:262–267, 2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Stamer W.D., and Acott T.S.Current understanding of conventional outflow dysfunction in glaucoma. Curr. Opin. Ophthalmol. 23:135–143, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Ramos R.F., and Stamer W.D.Effects of cyclic intraocular pressure on conventional outflow facility. Invest. Ophthalmol. Vis. Sci. 49:275–281, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Ramos R.F., Sumida G.M, and Stamer W.D.Cyclic mechanical stress and trabecular meshwork cell contractility. Invest. Ophthalmol. Vis. Sci. 50:3826–3832, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Li P., et al. . Phase-sensitive optical coherence tomography characterization of pulse-induced trabecular meshwork displacement in ex vivo nonhuman primate eyes. J. Biomed. Opt. 17:076026, 2012 [DOI] [PubMed] [Google Scholar]
- 6.Li P., et al. . Pulsatile motion of the trabecular meshwork in healthy human subjects quantified by phase-sensitive optical coherence tomography. Biomed. Optics Express 4:251–265, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Johnstone M., Martin E., and Jamil A.Pulsatile flow into the aqueous veins: manifestations in normal and glaucomatous eyes. Exp. Eye Res. 92:318–327, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Johnstone M.A.The aqueous outflow system as a mechanical pump: evidence from examination of tissue and aqueous movement in human and non-human primates. J. Glaucoma. 13:421–438, 2004 [DOI] [PubMed] [Google Scholar]
- 9.Ingber D.E.Tensegrity-based mechanosensing from macro to micro. Prog. Biophys. Mol. Biol. 97:163–179, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Hu S., et al. . Prestress mediates force propagation into the nucleus. Biochem. Biophys. Res. Commun. 329:423–428, 2005 [DOI] [PubMed] [Google Scholar]
- 11.Ingber D.E.Mechanical signaling and the cellular response to extracellular matrix in angiogenesis and cardiovascular physiology. Circ. Res. 91:877–887, 2002 [DOI] [PubMed] [Google Scholar]
- 12.Humphrey J.D.Vascular adaptation and mechanical homeostasis at tissue, cellular, and sub-cellular levels. Cell Biochem. Biophys. 50:53–78, 2008 [DOI] [PubMed] [Google Scholar]
- 13.Kaufmann S.A.The Origins of Order, Self-Organization, and Selection in Evolution. New York, NY: Oxford University Press; 1993 [Google Scholar]
- 14.Solon J., et al. . Fibroblast adaptation and stiffness matching to soft elastic substrates. Biophys. J. 93:4453–4461, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Johnstone M.A., and Grant W.G.Pressure-dependent changes in structures of the aqueous outflow system of human and monkey eyes. Am. J. Ophthalmol. 75:365–383, 1973 [DOI] [PubMed] [Google Scholar]
- 16.Johnstone M.A.Pressure-dependent changes in nuclei and the process origins of the endothelial cells lining Schlemm's canal. Invest. Ophthalmol. Vis. Sci. 18:44–51, 1979 [PubMed] [Google Scholar]
- 17.Grierson I., and Lee W.R.Pressure-induced changes in the ultrastructure of the endothelium lining Schlemm's canal. Am. J. Ophthalmol. 80:863–884, 1975 [DOI] [PubMed] [Google Scholar]
- 18.Tumminia S.J., et al. . Mechanical stretch alters the actin cytoskeletal network and signal transduction in human trabecular meshwork cells. Invest. Ophthalmol. Vis. Sci. 39:1361–1371, 1998 [PubMed] [Google Scholar]
- 19.Luna C., et al. . Alterations in gene expression induced by cyclic mechanical stress in trabecular meshwork cells. Mol. Vis. 15:534–544, 2009 [PMC free article] [PubMed] [Google Scholar]
- 20.Liton P.B., et al. . Induction of Il-6 expression by mechanical stress in the trabecular meshwork. Biochem. Biophys. Res. Commun. 337:1229–1236, 2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Tamm E.R., et al. . Modulation of myocilin/tigr expression in human trabecular meshwork. Invest. Ophthalmol. Vis. Sci. 40:2577–2582, 1999 [PubMed] [Google Scholar]
- 22.Mitton K.P., et al. . Transient loss of alphab-crystallin: an early cellular response to mechanical stretch. Biochem. Biophys. Res. Commun. 235:69–73, 1997 [DOI] [PubMed] [Google Scholar]
- 23.Junglas B., et al. . Connective tissue growth factor causes glaucoma by modifying the actin cytoskeleton of the trabecular meshwork. Am. J. Pathol. 180:2386–2403, 2012 [DOI] [PubMed] [Google Scholar]
- 24.Luna C., et al. . Extracellular release of Atp mediated by cyclic mechanical stress leads to mobilization of Aa in trabecular meshwork cells. Invest. Ophthalmol. Vis. Sci. 50:5805–5810, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Bradley J.M., et al. . Signaling pathways used in trabecular matrix metalloproteinase response to mechanical stretch. Invest. Ophthalmol. Vis. Sci. 44:5174–5181, 2003 [DOI] [PubMed] [Google Scholar]
- 26.Vittal V., et al. . Changes in gene expression by trabecular meshwork cells in response to mechanical stretching. Invest. Ophthalmol. Vis. Sci. 46:2857–2868, 2005 [DOI] [PubMed] [Google Scholar]
- 27.Keller K.E., et al. . Extracellular matrix turnover and outflow resistance. Exp. Eye Res. 88:676–682, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Clark R., et al. . Comparative genomic and proteomic analysis of cytoskeletal changes in dexamethasone-treated trabecular meshwork cells. Mol. Cell Proteomics. 12:194–206, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Filla M.S., et al. . Dexamethasone-associated cross-linked actin network formation in human trabecular meshwork cells involves Beta3 integrin signaling. Invest. Ophthalmol. Vis. Sci. 52:2952–2959, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Last J.A., et al. . Elastic modulus determination of normal and glaucomatous human trabecular meshwork. Invest. Ophthalmol. Vis. Sci. 52:2147–2152, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Russell P., and Johnson M.Elastic modulus determination of normal and glaucomatous human trabecular meshwork. Invest. Ophthalmol. Vis. Sci. 53:117, 2012 [DOI] [PubMed] [Google Scholar]
- 32.Thomasy S.M., et al. . Substratum stiffness and latrunculin B modulate the gene expression of the mechanotransducers Yap and Taz in human trabecular meshwork cells. Exp. Eye Res. 113:66–73, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Suson E.B., and Schultz R.O.Blood in Schlemm's canal in glaucoma suspects. A Study of the Relationship Between Blood-filling Pattern and Outflow Facility in Ocular Hypertension. Arch. Ophthalmol. 81:808–812, 1969 [DOI] [PubMed] [Google Scholar]
- 34.Johnstone M.A.A new model describes an aqueous outflow pump and explores causes of pump failure in glaucoma. In: Grehn H., and Stamper R., eds. Essentials in Ophthalmology: Glaucoma II. Heidelberg: Springer; 2006 [Google Scholar]
- 35.Grant W.M.Further studies on facility of flow through the trabecular meshwork. Arch. Ophthal. 60:523–533, 1958 [DOI] [PubMed] [Google Scholar]
- 36.Grant W.M.Experimental aqueous perfusion in enucleated human eyes. Arch. Ophthal. 69:783–801, 1963 [DOI] [PubMed] [Google Scholar]
- 37.Ellingsen B.A., and Grant W.M.The relationship of pressure and aqueous outflow in enucleated human eyes. Invest. Ophthalmol. 10:430–437, 1971 [PubMed] [Google Scholar]
- 38.Ellingsen B.A., and Grant W.M.Trabeculotomy and sinusotomy in enucleated human eyes. Invest. Ophthalmol. 11:21–28, 1972 [PubMed] [Google Scholar]
- 39.Van Buskirk E.M.Anatomic correlates of changing aqueous outflow facility in excised human eyes. Invest. Ophthalmol. Vis. Sci. 22:625–632, 1982 [PubMed] [Google Scholar]
- 40.Shapiro A.H.Physiological and Medical Aspects of Flow in Collapsible Tubes. Proc. Can. Congr. Appl. Mech., 6th. 883–906, 1977 [Google Scholar]
- 41.Kaufman P., and Gabelt B.Production and flow of aqueous humor. Times cited: 263. Kaufman P.L., et al., eds. Adler's Physiology of the Eye. New York: Elsevier Health Sciences; 2011; p. 274–307 [Google Scholar]
- 42.Ascher K.W.Glaucoma and the aqueous veins. Am. J. Ophth. 25:1309–1315, 1942 [Google Scholar]
- 43.Goldmann H.Abfluss des kammerwassers beim menschen. Ophthalmologica. 111:146–152, 1946 [DOI] [PubMed] [Google Scholar]
- 44.Goldmann H.Weitere mitteilung über den abfluss des kammerwassers beim menschen. Ophthalmologica. 112:344–346, 1946 [DOI] [PubMed] [Google Scholar]
- 45.Thomassen T.L.On aqueous veins. Acta Ophth. 25:369–378, 1947 [Google Scholar]
- 46.Thomassen T.L., Perkins E.S., and Dobree J.H.Aqueous veins in glaucomatous eyes. Br. J. Ophth. 34:221, 1950 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.De Vries S.De Zichtbare Afvoer Van Het Kamerwater. Amsterdam: Drukkerij Kinsbergen; 1947 [Google Scholar]
- 48.Kleinert H.The Compensation maximum: a new glaucoma sign in aqueous veins. Arch. Ophth. 46:618–624, 1951 [PubMed] [Google Scholar]
- 49.Ascher K.W.The Aqueous Veins: Biomicroscopic Study of Aqueous Humor Elimination. Springfield, IL: Charles C Thomas; 1961 [Google Scholar]
- 50.Ascher K.W.Physiologic importance of the visible elimination of intraocular fluid. Am. J. Ophth. 25:1174–1209, 1942 [DOI] [PubMed] [Google Scholar]
- 51.Johnstone M.A., and Grant W.M.Microsurgery of Schlemm's Canal and the human aqueous outflow system. Am. J. Ophthalmol. 76:906–917, 1973 [DOI] [PubMed] [Google Scholar]
- 52.Ascher K.W.Aqueous veins. Am. J. Ophth. 25:31–38, 1942 [Google Scholar]
- 53.Thomassen T.L.The venous tension of eyes suffering from simple glaucoma. Acta Ophth. 25:221, 1947 [Google Scholar]
- 54.Cambiaggi A.Effeto della jaluronidasi sulla pressone intraocular E Sull'asetto della vene Dell'accqueo. Boll. Soc. di Biologia Sperimentale. 34:1–7, 1958 [PubMed] [Google Scholar]
- 55.Ascher K.W.Local pharmacologic effects on aqueous veins. Am. J. Ophth. 25:1301, 1942 [Google Scholar]
- 56.Friedman D.S., et al. . Doctor–patient communication, health-related beliefs, and adherence in glaucoma: results from the glaucoma adherence and persistency study. Ophthalmology. 115:1320–1327, 2008 [DOI] [PubMed] [Google Scholar]
- 57.Liu J.H., et al. . Laboratory assessment of diurnal and nocturnal ocular perfusion pressures in humans. J. Ocul. Pharmacol. Ther. 19:291–297, 2003 [DOI] [PubMed] [Google Scholar]
- 58.Realini T., Weinreb R.N, and Wisniewski S.R.Diurnal intraocular pressure patterns are not repeatable in the short term in healthy individuals. Ophthalmology. 117:1700–1704, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Liu J.H., Realini T., and Weinreb R.N.Asymmetry of 24-hour Intraocular pressure reduction by topical ocular hypotensive medications in fellow eyes. Ophthalmology. 118:1995–2000, 2011 [DOI] [PubMed] [Google Scholar]