Abstract
Myocilin is a secreted glaucoma-associated protein, specifically induced by dexamethasone in human trabecular meshwork cells, where it was discovered. Myocilin is expressed in several tissues of the body, but it causes disease only in the eye. The protein contains two domains: an N-terminal region with significant homologies to nonmuscle myosin, and a C-terminal region, which is similar to the olfactomedin proteins. Forty percent of myocilin undergoes an intracellular endoproteolytic cleavage by calpain II, a calcium-dependent cysteine protease, which releases the 2 domains. The protein is known to interact with intracellular and extracellular matrix proteins, and some is released into the extracellular space associated with exosomes. Myocilin mutations are linked to glaucoma and induce elevated intraocular pressure. Most of the glaucoma-causative mutations map to the olfactomedin domain, which appears to be a critical domain for the function of the protein. Myocilin mutants are misfolded, aggregate in the endoplasmic reticulum, and are not secreted. Overexpression of myocilin and of its mutants in primary human trabecular meshwork cells triggers changes in the expression of numerous genes, many of which have been known to be involved in mechanisms important for the physiology and pathology of the tissue. Here we review recent studies from our laboratory and those of others that deal with trabecular meshwork genes, which are altered by the overexpression of wild-type and glaucoma-causative mutant myocilin genes.
Introduction
Ever since the discovery of the trabecular meshwork–specific induction of myocilin by dexamethasone (DEX) (originally termed “trabecular meshwork glucocorticoid-inducible protein,” TIGR),1,2 and of the consequent association of the gene encoding the protein to glaucoma,3 there has been enormous interest in identifying the mechanisms by which the protein causes the disease. Interestingly, the initial discovery of an increased expression of the wild-type form of the protein under known glaucomatous conditions appeared contradictory to subsequent findings involving the discovery of numerous mutations in the gene that were linked to the disease worldwide. Mutations in the myocilin gene amount to 3% of the global primary open-angle glaucoma. To date, over 250 different variants have been reported (http://www.myocilin.com/, last updated March 17, 2013) and from those, about 40% are disease causing. The majority of the myocilin-causing mutations (90%) map to exon 3 of the gene, which encodes for the olfactomedin domain of the protein (residues 244–504). Most mutations are missense, and each of their phenotypes varies considerably, showing glaucomas of different severities, different age of onset, different characteristics of the amino acid mutated, environmental factors, etc. (review in4). Recently, some myocilin mutations have shown association with pigment dispersion syndrome and pigmentary glaucoma.5 Studies addressing the biology of the protein resulted in the identification of numerous associated mechanisms such as, among others, endoplasmic reticulum stress, cell adhesion, intercellular communication through exosomes, involvement of the Wnt and RhoA pathways, regulated processing of the protein etc. It would seem quite likely that myocilin uses more than one mechanism to induce trabecular meshwork resistance and elevated pressure. It is well accepted that myocilin mutations are caused by a gain of function.
An approach toward the identification of glaucoma-causative mechanisms of myocilin has been the characterization of those trabecular meshwork genes that are modified by either overexpression of the wild-type or the mutated forms of the protein. Although full concordance does not occur among the different studies, there appears to be a common trend and, importantly, all of them reveal a group of genes with functions that could have an impact in the regulation of aqueous humor outflow facility. In this short review, the major alterations triggered by wild-type and mutated myocilin in different species and under different conditions will be compared. A schematic summary of the selected relevant genes and functions reviewed here is shown in Tables 1A and 1B.
Table 1A.
Selected Trabecular Meshwork Relevant Genes Altered by Wild-Type and Mutant Myocilin
| Gene name | Symbol | FC | RNA/protein origin | Overexpress model | Species | Analysis | Function | Citation |
|---|---|---|---|---|---|---|---|---|
| Wild-type Myocilin | ||||||||
| Podoplanin | PDPN | 4.7 | HTM cells* | Adh.myocWT | Human | AffyU133P2.0 | Lymph.marker/ Rho activ. | Kennedy 2012 |
| Prostaglandin D2 synthase | PTGDS | 3.4 | HTM cells* | Adh.myocWT | Human | AffyU133P2.0 | Neuromodulator | Kennedy 2012 |
| Chitinase 3-like 1 | CHI3L1 | 3.0 | HTM cells* | Adh.myocWT | Human | AffyU133P2.0 | Cartilage ECM | Kennedy 2012 |
| αB-Crystallin | CRYAB | 1.8 | HTM cells* | Adh.myocWT | Human | AffyU133P2.0 | Chaperone | Kennedy 2012 |
| Matrix metallopeptidase 1 | MMP1 | 26.0 | HTM cells** | AdhTIG3.WT | Human | AffyU133 A | ECM remodeling | Borrás 2006 |
| Thrombomodulin | THBD | 20.0 | HTM cells** | AdhTIG3.WT | Human | AffyU133 A | Binds thrombin/clotting | Borrás 2006 |
| Angiopoietin 2 | ANGPT2 | 39.0 | HTM cells** | AdhTIG3.WT | Human | AffyU133 A | Pro-angiogenic | Borrás 2006 |
| Swiss cheese | sws | 34.0 | Transgenic* | UAS-WT | Drosophila | AffyDros 2.0 | Neurodegenerative prt. | Borrás 2003 |
| Aqp1049 | CG4019 | −1.8 | Transgenic* | UAS-WT | Drosophila | AffyDros 2.0 | Water chanel activity | Borrás 2003 |
| Cytochrome P450 | CYP315A1 | −2.6 | Transgenic* | UAS-WT | Drosophila | AffyDros 2.0 | Detoxification | Borrás 2003 |
| Conexin46 | Gja3 | 5.0 | Transgenic** | βB1-Cry-Myoc | Mouse | Affy 430.2.0A | Gap junct/cell-cell sign. | Paper 2008 |
| αB-Crystallin | Cryab | 1.9 | Transgenic** | βB1-Cry-Myoc | Mouse | Affy 430.2.0A | Response to heat | Paper 2008 |
| Spondin | Spon2 | 1.6 | Transgenic** | βB1-Cry-Myoc | Mouse | Affy 430.2.0A | Cell adhesion | Paper 2008 |
| Homeobox protein Six1 | Six1 | −1.8 | Transgenic** | βB1-Cry-Myoc | Mouse | Affy 430.2.0A | Transcription factor | Paper 2008 |
| Neural Wiskott-Aldrich prt. | Wasl | −1.8 | Transgenic** | βB1-Cry-Myoc | Mouse | Affy 430.2.0A | Cytoskeleton signaling | Paper 2008 |
| Carcinoembryonic antigen1 | Ceacam1 | −1.7 | Transgenic** | βB1-Cry-Myoc | Mouse | Affy 430.2.0A | Cell adhesion | Paper 2008 |
| Myocilin mutants | ||||||||
| Q368X | ||||||||
| Prot disulfide isomerase A4 | PDIA4 | 2.1 | HTM cells* | Adh.Q368X | Human | AffyU133P2.0 | Chaperone | Kennedy 2012 |
| Secret frizz-related prot 1 | SFRP1 | −2.5 | HTM cells* | Adh.Q368X | Human | AffyU133P2.0 | Wnt signaling | Kennedy 2012 |
| Matrix Gla protein | MGP | −2.0 | HTM cells* | Adh.Q368X | Human | AffyU133P2.0 | Inhibitor of calcification | Kennedy 2012 |
| Lysyl oxidase-like 1 | LOXL1 | −2.0 | HTM cells* | Adh.Q368X | Human | AffyU133P2.0 | Elastin network | Kennedy 2012 |
| Heat shock prot 90B1 (GRP94) | HSP90B1 | 3.3 | HTM cells* | Adh.Q368X | Human | AffyU133P2.0 | Unfo Prot Respon (UPR) | Kennedy 2012 |
| Calreticulin | CALR | 2.3 | HTM cells* | Adh.Q368X | Human | AffyU133P2.0 | Unfo Prot Respon (UPR) | Kennedy 2012 |
| Glycoprotein 93 | Gp93 | up | Transgenic* | UAS-Q368X | Drosophila | AffyDros 2.0 | Unfo Prot Respon (UPR) | Carbone 2009 |
| Calreticulin | Crc | up | Transgenic* | UAS-Q368X | Drosophila | AffyDros 2.0 | Unfo Prot Respon (UPR) | Carbone 2009 |
| R342K | ||||||||
| Ras-related protein 39B | RAB39B | 3.2 | HTM cells* | Adh.R342K | Human | AffyU133P2.0 | Vesicular trafficking | Kennedy 2012 |
| D380N | ||||||||
| Matrix metallopeptidase 1 | MMP1 | −5.5 | HTM cells* | Adh.D380N | Human | AffyU133P2.0 | ECM remodeling | Kennedy 2012 |
| Fibronectin 1 | FN1 | 1.8 | HTM cells* | Adh.D380N | Human | AffyU133P2.0 | Cell adhesion | Kennedy 2012 |
| Synuclein alpha | SNCA | 2.5 | HTM cells* | Adh.D380N | Human | AffyU133P2.0 | Presynaptic signal | Kennedy 2012 |
| K423E | ||||||||
| Insulin-like growth factor 1 | IGF1 | 3.9 | HTM cells* | Adh.K423E | Human | AffyU133P2.0 | Signaling hormone | Kennedy 2012 |
| Cytochrome P450, 1B1 | CYP1B1 | −2.7 | HTM cells* | Adh.K423E | Human | AffyU133P2.0 | Detoxification | Kennedy 2012 |
| Q368X, R342K,D380N,K423E (of 73 shared) | ||||||||
| Chemokine (C-X-C motif)12 | CXCL12 | −3.0 | HTM cells* | Adh.Q368X | Human | AffyU133P2.0 | Signaling cytokine | Kennedy 2012 |
| Cystatin A (Stefin A) | CSTA | 3.4 | HTM cells* | Adh.R342K | Human | AffyU133P2.0 | Protein processing | Kennedy 2012 |
| Endothelin 1 | EDN1 | −1.6 | HTM cells* | Adh.D380N | Human | AffyU133P2.0 | Vasoactive peptides | Kennedy 2012 |
| Stanniocalcin 1 | STC1 | −1.8 | HTM cells* | Adh.K423E | Human | AffyU133P2.0 | Calcium homeostasis | Kennedy 2012 |
| P370L | ||||||||
| Glucose-reg Prot78 (BIP) | GRP78 | −1.5 | HTM cells* | pcDNA3.P370L | Human | WB | Unfo Prot Respon (UPR) | Wang 2007 |
| Y437H | ||||||||
| Glucose-reg Prot78 (BIP) | GRP78 | up | HEK 293*. | Y437H | Human | WB | Unfo Prot Respon (UPR) | Joe 2010 |
| Protein disulfide isomerase | PDI | up | HEK 293* | Y437H | Human | WB | Chaperone | Joe 2010 |
| I477N | ||||||||
| Glucose-reg Prot94 (HSP90B1) | GRP94 | up | HEK 293**. | I477N | Human | co-IP | Unfo Prot Respon (UPR) | Suntharalingam 2012 |
| Protein disulfide isomerase | PDI | up | HEK 293** | I477N | Human | WB | Chaperone | Joe 2010 |
| Glucose-reg Prot78 (BIP) | GRP78 | up | HEK 293** | I477N | Human | WB | Unfo Prot Respon (UPR) | Joe 2010 |
 ECM
ECM  Protein synthesis/modification
Protein synthesis/modification  Stress / defense
Stress / defense  Cytoskeleton/structural
Cytoskeleton/structural  Signaling/cytokines
Signaling/cytokines  Calcification
Calcification
HTM cells*, primary human trabecular meshwork cells derived directly from TM explants; HTM cells**, primary human trabecular meshwork cells derived from collagenase treatment of TMs.
Transgenic*, Heads from transgenic flies.
Transgenic**, Mouse eyes (minus lens) from transgenic mice.
HEK 293*, HEK 293 Tet-On stably transfected with pTRE-Y437H MYOC.
HEK 293**, HEK 293 Tet-On stably transformed with pTRE-I477N MYOC.
HTMs, human trabecular meshworks; WB, Western blot; ECM, extracellular matrix.
Table 1B.
Selected Trabecular Meshwork Functions Altered by Wild-Type and Mutant Myocilin
| Function | Model | Method | Vector | Species | Citation |
|---|---|---|---|---|---|
| Wild-type myocilin | |||||
| Loss of stress fibers | Transi.transf.HTM/ HVJ liposome/ 48h | Immunofluoresc.Phalloidin | pcDNA3.1.myoc | Human | Wentz-Hunter 2004 |
| Stably transf.HTM cells/ FuGene | Immunofluoresc.Phalloidin | Human | |||
| Induction stress fibers | Exogen myoc on HTM / 3h | Immunofluoresc.Phalloidin | purified myocilin | Human | Kwon 2009 |
| from transfec 293 media | |||||
| β-Catenin translocation | Exogenous myoc on NIH3T3 cells | Immunofluoresc.βcatenin | from transfec 293 media | Mouse | Kwon 2009 |
| Wnt pathway activation | Tcf/Lef binding site/ luciferase reporter | Chemiluminescence | Myoc+pTOP-Flash | Human | Shen 2012 |
| Transf.Colorectal adenocarcinoma cell | |||||
| Reduce RhoA activation | Transi.transf.HTM/ FuGene&Lipof.LTX | Pull-down assay | pTarget.myocilin | Human | Shen 2008 |
| Reduce fibronectin deposition | Transi.transf.HTM | Fluores. Histocytochem. | pTarget.myocilin | Human | Shen 2008 |
| Higher sensitivity to apoptosis | Transi.transf.HTM/ HVJ liposome/ 48h | Anti-Fas apoptosis inducti | pcDNA3.1.myoc | Human | Wentz-Hunter 2004 |
| Conversion to osteoblasts | Mesenchimal stem.cells (MSC) | AlizaRed stain./Alkal.Phos. | purified myocilin | Human | Kwon 2013 |
| 3μg myoc for 6d | from transfec 293 media | ||||
| Inhibits cell spreading | Hskin fibroblast on coated myoc well | Count spread/round cells | Hmyoc fr transf insectcells | Human | Peters 2005 |
| Inhibits incorp Paxillin in FA | Hskin fibrobroblast+soluble myocilin | Immunofluoresc. Vin&paxil | Hmyoc fr transf insectcells | Human | Peters 2005 |
| Mutant myocilin | |||||
| Disturbs ER stress response | Transi.transf.HTM/ Lipofectmine | pcr.GRP78 & P.eIF2a | pCDNA3.P370L | Human | Wang 2007 |
| Disturbs Mitchon membr potential | Transi.transf.HTM/ Lipofectmine | Flow cytometry. JC1probe | pCDNA3.P370L | Human | Wang 2007 |
| Loss of stress fibers | Transi.transf.HTM/ FuGene&Lipof.LTX | FuGene & Lipofecta.LTX | pMyoc.P370L-EGFP | Human | Shen 2012 |
| Increase prot kinA (PKA) activitity | Transi.transf.HTM/ FuGene&Lipof.LTX | Peptag L-R-R-S-L-G | pMyoc.P370L-EGFP | Human | Shen 2012 |
| Reduce RhoA activation | Transi.transf.HTM/ FuGene&Lipof.LTX | Pull-down assay | pMyoc.P370L-EGFP | Human | Shen 2012 |
| Incre sensitivity to oxidative stress | HEK 293*/ 100μM H2O2 | Apoptosis/ TUNEL | pTRE-Y437H MYOC | Human | Joe 2010 |
| Mild glaucoma | Transg.mice/ hmyoc | IOP/ RGC&Axon count | BAC RP11-23L17.Y437H | Mouse | Zhou 2008 |
| Glaucoma | Transg.mice/ CMV.cDNAhmyoc | IOP/ RGC&Axon count | pCS2.Y437H | Mouse | Zode 2011 |
| Inhibition of myoc processing | Transi.transf. HEK293, COS, hCMsv | WB | pCDNA3.D380A | H &Monk | Aroca-Aguilar 2005 |
HEK 293*, HEK 293 Tet-On stably transfected.
HTM, primary human trabecular meshwork cells; ICC, immunocytochemistry; FA, focal adhesion; ER, endoplasmic reticulum; IOP, intraocular pressure.
Genes Affected by Overexpression of Wild-Type Myocilin
Microarrays
Overexpression of recombinant wild-type myocilin in primary human trabecular meshwork (HTM) cells induces changes in the expression of numerous genes of the cell's transcriptome. The latest microarray study used an adenoviral vector carrying the full coding myocilin gene under the control of a ubiquitous cytomegalovirus (CMV) promoter versus an empty vector with the same background.6 The number of nonredundant genes altered ≥1.5-fold in the myocilin-infected cells was 4337 out of 54,678 spots in the Affymetrix Human Genome U133 Plus 2.0 chip, or approximately 8%. Of these altered genes, 2,008 were upregulated and 2,329 downregulated, or approximately 50%. These arrays were conducted with RNA from primary HTM cells generated by the explant method from corneal rims at passage 4, infected with a newly constructed adenoviral vector (Adh.myocWT) and harvested at 72 h post-infection.6 An example of trabecular meshwork relevant (TMR) genes from this wild-type myocilin list included: podoplanin (PDPN, 4.7-fold), prostaglandin D2 synthase (PGD2, 3.4-fold), chitinase-3-like 1 (CHI3L1, 3.0-fold) and αB-crystallin (CRYAB, 1.8-fold). Some of the genes altered by the wild-type had also been altered by some mutants.6 PDPN is a gene that was shown to be induced by elevated pressure in perfused human anterior segments7 and a mediator in the activation of the RhoA pathway in lymphatic cells.8 PGD2 catalyzes the formation of prostaglandin D2, a molecule with a long history of reducing intraocular pressure (IOP).9 CH13L1 is a trabecular meshwork marker10 and CRYAB, expressed in the juxtacanalicular region of the trabecular meshwork, is significantly regulated by elevated pressure.7
Earlier microarrays11 conducted with RNA from primary HTM cells generated by the collagenase method,12 infected with a different myocilin adenoviral vector construct,13 hybridized with the older generation Human Genome U133 A chips, and compared against vehicle-treated cells, gave a slightly different profile. For instance, among the top TMR changers there was an increased MMP1 (26-fold in the array, 5.8-fold taqman PCR), thrombomodulin (THBD, 20-fold), and angiopoietin 2 (ANGPT2, 39-fold).11 These 3 genes also appeared altered in the more recent myocilin chips, albeit some of them downregulated. MMP1 and THBD were downregulated while ANGPT2 was increased by the myocilin wild-type.6 The functions of these three genes are known to influence the trabecular meshwork. MMP1 is an interstitial collagenase known to increase outflow facility and lower IOP.14,15 THBD is a vascular endothelial cell receptor involved in blood clotting, induced by elevated IOP, and believed to influence the fluidity of the aqueous humor,16,17 while ANGPT2, a pro-angiogenic protein that acts by loosening up cell–matrix interactions, could be a facilitator of outflow facility.
Experiments using myocilin overexpression in other species and analyzed on their corresponding arrays yielded additional genes. Crosses between a Drosophila melanogaster transgenic line carrying the human myocilin cDNA under the control of the UAS promoter, with the gmr-GAL4 line, carrying the transposon P(GAL4-ninaE.GMR), drove myocilin expression to the eye of the fly. RNAs extracted from the fly heads whose eyes overexpressed human myocilin were hybridized to high-density arrays and compared to the parental lines. Analysis of these arrays yielded 50 transcripts with very significant P-values. The highest overexpressed gene, the swiss cheese gene, was upregulated 34-fold.18 Interestingly, the swiss cheese human ortholog, neuropathy target esterase (NTE), was upregulated in the trabecular meshwork of perfused human anterior segment cultures in response to adenovirus-mediated overexpression of wild-type myocilin.18 Among the Drosophila down-regulated genes were a water transporter-like protein (CG4019) and a Cytochrome P-450 isoenzyme (CYP315A1), whose human homologs Aquaporin4 and Cytochrome P-450 (CYP315A1) have been identified as associated with aqueous humor dynamics and congenital glaucoma.19,20
Subjecting the mouse eye to elevated levels of myocilin protein in the aqueous humor also caused changes in the entire eye gene expression.21 Transgenic mice carrying myocilin cDNA under the control of the βB1-crystallin promoter expressed myocilin in the lens and secreted the protein into the aqueous humor. Transcriptome of the whole mouse eye (minus the lens) was compared to that of the wild-type littermate. Hybridization to Affymetrix mouse 430 2.0 arrays yielded 7 differentially expressed genes with fold change values≥1.5 and significant P-values lower than 0.05. Of the top changers, connexin 46 (Gja3), αB-crystallin (Cryab), and spondin 2 (Spon2) were increased 5.0-, 1.9- and 1.6-fold, respectively. The other 4 genes, homeobox protein SIX1 (Six1), cyclin-dependent kinase 14 (Pftk1), neural Wiskott-Aldrich syndrome protein (Wasl) and carcinoembryonic antigen1 (Ceacam1) were down-regulated between 1.8- and 1.7-fold. In a similar manner to what it occurred in the fruit fly, the expression change of the correspondent human genes was reproduced in primary HTM cells. For that, RNA extracted from HTM cells exposed to different concentrations of recombinant myocilin (isolated from supernatants of HEK293 cells transfected with a recombinant myocilin plasmid) showed concordant down-regulation of SIX1, PFTK1, WASL, and CEACAM1 and reversed (down- rather than up) regulation of SPON2.21 SIX1 is a transcription factor, PFTK1 is a cycle-dependent kinase, WASL is a cytoskeletal signaling protein, and CEACAM1 and SPON2 are involved in cell adhesion. The functions of the proteins encoded by these genes would be relevant to regulation of outflow facility by the trabecular meshwork. The overlapping up-regulation of CRYAB in human cells and transgenic mice gives this gene a stronger myocilin mediator role. A list of selected relevant genes from this section is shown in Table 1A.
Single-function studies: stress fibers, RhoA, Wnt pathway, and osteogenesis
Several studies on the overexpression of myocilin in the trabecular meshwork have focused on the effect of high levels of the protein on a given cell function. Yue's team constructed a pcDNA3.1 plasmid containing the coding myocilin cDNA, the CMV promoter, and the V5 and 6XHis tags at its 3’-end. The authors overexpressed the gene by transfecting primary HTM cells, either by the use of hemagglutinating virus of Japan (HVJ)-liposome system22 with harvesting at 48 h, or by generating stably transfected cells using the FuGene kit (La Roche) and G418 selection. In both transfection methods, an increase in myocilin levels by 3- to 4- fold caused a significant loss of actin fibers and focal adhesions.23 In contrast, studies from a different group24 reported that treatment of primary HTM cells with exogenous purified myocilin induced the formation, not the loss of stress fibers. Interestingly, both groups showed that either the loss, or the induction of stress fibers induced by myocilin was reverted by treatment of the cultures with secreted frizzled-related protein 1 (SFRP1), an inhibitor of the Wnt pathway.24,25 This would indicate that the actions of wild-type myocilin in the cells were mediated by the Wnt pathway. In addition to the Wnt pathway interfering with cell characteristics induced by myocilin, it was also proposed that, reversely, myocilin could itself be a modulator of the Wnt pathway.24 Immunoprecipitation experiments have demonstrated the interaction of myocilin with SFRP1, SFRP3, and several frizzled receptors, and treatment of murine NIH3T3 cells with recombinant purified human myocilin protein induced cellular translocation of β-catenin, albeit not to the nucleus as occurs in the canonical Wnt pathway.24 The activation of the Wnt pathway by (increased?) myocilin was also shown by a direct assay using TOP-Flash/FOP-Flash luciferase reporter plasmid.25 This reporter plasmid contains 2 repeats of three copies of the Tcf/Lef binding site in front of a basic promoter driving the luciferase gene, and it is activated by β-catenin. Co-transfection of the myocilin and pTOP-Flash plasmids into human colorectal carcinoma cells induced a 2.7-fold activation of luciferase (Wnt pathway) over controls. However, when the co-transfection was performed into primary HTM cells, the increase of luciferase and thus of the Wnt pathway was marginal.25 It is likely that in this experiment, as the authors interpret, the very low Wnt induction in cells of the outflow facility was due to low transfection efficiencies. Although the presence of the Wnt pathway in the trabecular meshwork has been demonstrated,26,27 newer designs overcoming transfection barriers are needed to ascertain the correlation of myocilin with this pathway in the trabecular meshwork.
In addition to the loss of stress fibers, adhesion assays have shown that increased myocilin tends to diminish adhesion of the cells to the extracellular matrix (ECM), making it logical to speculate the existence of a correlation of these events with a beneficial outcome for regulation of outflow facility.28 Supporting these effects are experiments where coating cell culture dishes with myocilin isolated from transfected Sf9 insect cells inhibited cell spreading of human neonatal skin fibroblasts, and treating these same cells with the soluble protein inhibited incorporation of paxillin into focal adhesions.29 At the same time, though, it was observed that cells containing high levels of myocilin exhibited an increased sensitivity to apoptosis, which would indicate that cells overexpressing the wild-type are sicker and more subjective to pathological events.23 Based on such overexpression experiments, it would seem that myocilin might be involved in a balancing act between an association of the disruption of cytoskeleton/adhesion with increased outflow facility and a weaker cell, which would compromise coping with oxidative stress and/or other insults.
Myocilin also reduced the deposition of fibronectin and the activity of RhoA as measured by the pull-down assay.30 Furthermore, the loss of actin stress fibers in cells overexpressing myocilin was prevented by co-transfection with a constitutive active RhoA plasmid.30 Altogether, those findings suggested that at least some overexpressed myocilin effects might be mediated through the regulation of the RhoA pathway.
An intriguing recent finding on myocilin effects is that of its role in the differentiation of mesenchymal stem cells (MSC) into osteoblasts, and in osteogenesis in vivo. Myocilin was detected in human bone-marrow-derived MSC, and treatment of the cultures with purified myocilin enhanced osteogenesis as measured by alizarin red staining.31 Evidence of the existence of a calcification process in the trabecular meshwork was first introduced in 2006 by Xue et al.,32 followed by a second report on the increased concentration of the calcification marker alkaline phosphatase in the trabecular meshwork of postmortem glaucomatous specimens.33 It was hypothesized then that this process could involve the conversion of HTM cells into osteoblasts.33 Whether myocilin is mediating this process is not yet known. It is well established that induction of the Wnt pathway promotes calcification and osteogenic differentiation.34,35 Perhaps then, the induction of osteogenesis by myocilin is mediated by its activation of the Wnt pathway. This newly described property of myocilin could implicate overexpressed myocilin as having a detrimental role in outflow facility and inducing elevated IOP. A list of selected relevant functions from this section is shown in Table 1B.
Genes Affected by Overexpression of Glaucoma-Causative Myocilin Mutants
Mutants in myocilin have been undoubtedly linked to glaucoma. Moreover, there is no direct evidence in the global population that overexpression of wild-type myocilin is linked to the disease or to glaucomatous conditions in humans. The paradox exists though, in the cases of medical treatment with corticosteroids. Topical ocular treatment with corticosteroids induces elevated IOP in 30%–40% of the population, and these responders are more likely to develop primary open-angle glaucoma than the nonresponders. It is quite intriguing, then, that treatment of trabecular meshwork tissue and cells with DEX specifically induces myocilin.2,36 Morphological characteristics observed on the outflow pathway of human specimens with steroid-induced glaucoma indicate that the cause of elevated pressure is an increased ECM deposition. It is well known that corticosteroids elicit alteration of expression in a multitude of genes, many of them associated with remodeling of the ECM.37 Thus, whether other DEX-induced genes are involved, or whether myocilin overexpression is the central mediator of glucocorticoid-associated glaucoma is not known, but it would seem less likely. On the contrary, numerous myocilin mutations have been strongly linked to families and individuals with glaucoma (http://www.myocilin.com/). A number of studies have searched for the genes affected by overexpression of different myocilin mutation mutants.
Microarrays
Our laboratory conducted a thorough comparative study using 4 selected glaucoma-causative myocilin mutations (Q368X, R342K, D380N, and K423E), primary HTM cells from the same individual and passage number, and Affymetrix U133 Plus 2.0 GeneChips.6 Each mutant cDNA was carried on an adenoviral vector and compared to an adeno-null control; the cutoff for differential expression was 1.5-fold. The 4 representative mutants were selected based on different clinical outcomes, population, or relevance of the mutated codon. The Q368X variant is the most common (31.3% of the disease-causing variants, http://www.myocilin.com/), and results in a mild phenotype (mean IOP 21 mmHg); mutations R342K and D380N comprise 0.7% of the causing variants each, and are very severe, with a mean maximum IOP of 54 and 39 mmHg, respectively.38 The amino acid #380, aspartic acid, highly conserved in all vertebrates, has produced 4 disease-causing variants,4 an extremely rare occurrence in genetics. The fourth selected mutant, K423E (mean IOP 31 mmHg), was selected because it occurred in two unrelated Caucasian populations, has a severe clinical outcome and presents the interesting feature that homozygous patients do not develop the disease.39
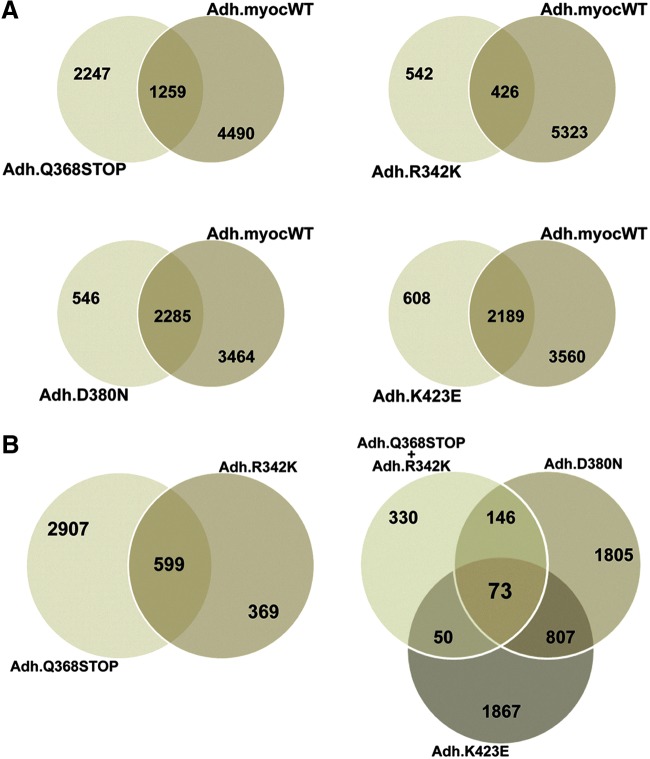
Gene lists generated from each of the human myocilin mutants shared different percentages of altered genes with the myocilin wild-type (Fig. 1). Thus, 64% of genes altered by the stop mutation Q368X did not overlap with genes altered by the wild-type, the highest nonoverlapping proportion of the 4 mutants. Mutant R342K, which had the lowest absolute number of altered genes, still contained a high 56% nonoverlap. The other 2 mutants, D380N and K423E, had a lower number of nonoverlapping genes (24% and 22%, respectively), sharing more of the changes with the wild-type (Fig. 1A). Regarding affecting the trabecular meshwork transcriptome, these 4 mutations appear to fall into 2 sets: Q368X/R342K (set 1) and D380N/K423E (set 2), at both the overall and individual affected gene level. This categorization does not correlate with their IOP outcome. All 4 mutants shared just 73 altered genes among themselves. Among these 73 genes were the signaling cytokine CXCL12, the vasoconstrictor endothelin 1 (EDN1), the ECM protein ECM2, the cysteine protease Cystatin A (CSTA), the calcium homeostasis proteins stanniocalcin 1 and 2 (STC1, STC2), and the growth factors insulin-like growth factor 1 (IGF1) and fibroblast growth factor 1 (FGF1).6 Together these genes encode proteins with numerous diverse functions, from signaling, to matrix components, to calcium regulation. It is likely that a combination of their encoded functions would be responsible for the glaucoma caused by myocilin. Furthermore, of the 73 shared altered genes, 10 were not altered in the wild-type. One of them (CSTA, a cysteine protease inhibitor) is described below.
FIG. 1.
Venn maps of genes of the trabecular meshwork altered by overexpression of wild-type and 4 glaucoma-causative myocilin mutants. Hybridization was conducted on Affymetrix GeneChips (n=17) and analyzed with GeneSpring 10 software. Nonredundant gene lists had cutoff fold-change values of≥and ≤1.5. (A) Number of genes altered in each of the mutants that overlap with genes altered by the wild-type. (B) Number of genes altered in all mutants.6
Among the individually affected candidate genes (i.e., genes that were top-changers in just 1 of the 4 mutants), there were many that had been historically connected to trabecular meshwork physiology and pathology. Some of them had known myocilin associations such as MMP1, calreticulin (CALR), protein disulfide isomerase 4 (PDIA4), secreted frizzled-related protein 1 (SFRP1), and fibronectin 1 (FN1) while others did not, such as EDN1, Matrix Gla (MGP), and IGF1. Some of the genes were top-changers only in 1 of the mutants such as lysyl oxidase-like 1 (LOXL1) or Cytochrome P450 family 1B1 (CYP1B1) (in mutations Q368X and K423E, respectively), pointing to an individual response to a particular mutant. Some of the found genes were also new to the field, such as synuclein alpha (SNCA), STC1, CXCL12, and CSTA.6 In each case, however, it is not difficult to speculate that their specific encoded functions could be potential modulators of outflow facility, giving myocilin a wide range of possibilities by which it could cause glaucoma.
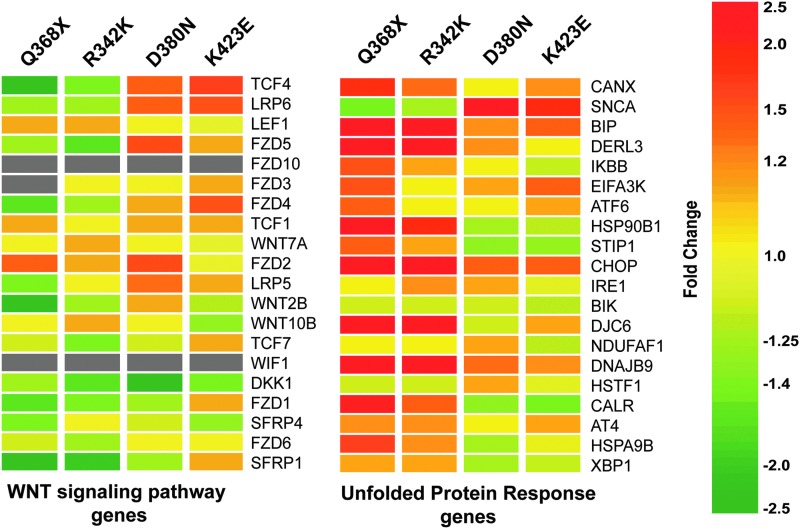
In this human study,6 lists of genes representing 4 relevant trabecular meshwork functions [those of stress and unfolded protein response (UPR), Wnt pathway, collagen-elastin crosslinking and calcification genes] were custom generated by literature review and analyzed in the 4 mutants.6 Two of the 4, the WNT pathway and the UPR, are shown in Fig. 2. These expression heat maps have revealed that the gene list comprising the endoplasmic reticulum (ER) stress/UPR were the most affected and up-regulated, in particular by Q368X and R342K mutants. Curiously, the same mutants caused the most down-regulation for the set of genes of the Wnt pathway (Fig. 2). A Drosophila study using the gmr-Gal4/UAS system described above used the same set of myocilin mutants and analyzed the RNA of 2–3-day-old whole fly heads in their corresponding arrays.40 Interestingly, the UPR response genes were also altered in the myocilin mutant flies and as in the human, the Q368X mutant was the one having a major effect. Moreover, to confirm the UPR response elicited by the myocilin mutants, these authors generated myocilin mutant flies carrying an additional green fluorescent protein (GFP) reporter gene under the control of an ER response element.41 Although a considerable amount of autofluorescence was present, a punctate GFP expression was clearly observed in the mutant flies, especially in the Q368X mutant.40 This correlation of elicited functions known to be induced by ER stress between such diverse species as human and insects gives a greater strength to validity of the UPR response, and at the same time validates the fly system as a good genetic model of glaucoma. A list of selected relevant genes from this section is shown in Table 1A.
FIG. 2.
Heat maps depicting changes induced by myocilin mutants on the WNT pathway and Unfolded Protein Response trabecular meshwork genes. Gene lists for each pathway were custom-generated by literature review. Each row represents the fold change (Adeno.myocilin.mutant over Adeno.Null) for a single gene in each of the 4 mutants. Each column represents the fold changes for all genes of the category in one myocilin mutant. The fold change for each gene is visually represented by a color, which is given by the scale bar on the right. Gray cells indicate that the expression of the given gene was below the signal intensity cutoff value and was considered absent. Modified from reference 6 with permission.
Focused myocilin mutant studies
Two other myocilin mutations associated with early-onset, or juvenile open-angle glaucoma, Y437H and P370L, have been the subject of several studies. The Y437H mutation was first described in 1998,42 amounts to 7.3% of the myocilin disease-causing variants, and has a mean IOP of 43.7 mmHg (mean age of diagnosis, 19.1 years). The P370L is 3.9% of the variants, has a mean IOP of 29.8 mmHg, and a mean age of diagnosis of 13 years.
Overexpression of the P370L in primary HTM cells for 40 h was found to down-regulate the ER stress response and to damage the mitochondrial membrane potential.43 In that study, ER stress was induced with 2.5 μg/mL of tucanamycin for 6 h and evaluated by the expression levels of glucose-regulated protein GRP78 (also known as BIP). Mitochondrial membrane potential was measured by exposing the cells to the JC-1 fluorescence probe for 15 min, harvesting the cells, and analyzing them immediately by flow cytometry. In a different laboratory, and also using HTM cells, the same P370L mutation caused loss of actin stress fibers, an increase in protein kinase A (PKA) activity, and Rho inactivation.25 The PKA increase was reverted by treatment with SFRP1, an inhibitor of the Wnt pathway.
The human myocilin gene, containing the Y437H mutation with its own promoter and regulatory regions, was used to generate transgenic mice. The human gene was expressed in the mouse eye and produced changes that, although moderate, were characteristic of glaucoma.44 HEK293 cells stably transformed with the same myocilin mutant were more sensitive to oxidative stress and up-regulated the chaperone ER stress marker GRP78.45 Importantly, a second transgenic mouse generated in a different laboratory and carrying the human myocilin Y437H cDNA driven by a CMV promoter developed a more robust glaucomatous phenotype and induced ER stress.46 This transgenic mouse showed elevated IOP plus retinal ganglion cells and axon degeneration at 3–4 months of age.46 Moreover, topical administration 2× daily with a 0.2% solution of sodium 4-phenylbutyrate (PBA) significantly reduced the elevated IOP in these mice.47 PBA is a clinically approved chemical chaperone, which has been shown to reduce ER stress in several diseases.47 Together, all these findings corroborate the relevance of the ER response in the myocilin mutant trigger of glaucoma.
One of the hallmarks of myocilin mutant effects is that of being sequestered inside the cell. A study comprising 26 glaucoma-linked variants showed that 20 of them were retained intracellularly and 6 were secreted (80% secreted).48 When the temperature of the incubator was lowered from 37°C to 30°C, a process known to facilitate protein folding, 11 of the 20 mutants originally retained were now secreted. Lowering the temperature also increased the originally lower secretion level of variant T377M to that of the other secreted variants.48 The variant T377M comprising 14.8% of the glaucoma-linked mutations exhibits a moderate IOP (16.7 mmHg) with an age of onset of 21.4 years of age. The sequestration of the myocilin mutants is not specific to glaucoma-causative mutations but is most likely due to localization of the mutation to the olfactomedin domain of the protein. This sequestration was first reported in a laboratory-created arbitrary mutant in the olfactomedin domain.49 All data together strongly indicates that myocilin mutants are misfolded. Proteins that fail to fold correctly are sequestered at the ER and induce ER stress.50 Hetero-oligomers of myocilin wild-type/mutants have also been shown to be sequestered in the ER51 and to activate the same UPR response genes that are seen after overexpression of myocilin variants.6,40 The overwhelming majority of myocilin-causative glaucoma occurs in heterozygous individuals, where the formation of wild-type/mutant hetero-oligomers leads to the formation of insoluble aggregates.49,51 Four cases of myocilin mutation homozygosity have been reported.39,52–54 The T377M homozygous (2 subjects) presents a glaucoma that is more severe than its more frequent heterozygous state,50 the variants K423G (4 siblings)39 and G368X (1 subject)53 present normal ocular exams, and the fourth variant reported, R46X, appears to be a neutral mutation.52
Recently, it has been discovered that the aspartic acid amino acid 380, which as described above, leads to the unusual event of 4 different glaucoma-causative mutants (D380N, D380A, D380G, and D380H),55 is an integral part of a calcium-binding site buried in the olfactomedin domain.56 The glaucoma phenotypes corresponding to the 4 residue substitutions of aspartic acid 380 present a clinical onset that is intermediate between juvenile and adult-onset glaucoma. Their IOPs are also intermediate between the severe ones of P370L and the milder one of the most common Q368X. The aspartic acid 380 residue appears to be important for myocilin function.55 The variant D380A significantly inhibits the endoproteolytic processing of myocilin and its triton solubility,57 and the variant D380N specifically elevates Cystatin A, which also inhibits the myocilin processing.6 The newly found role of D380 in the calcium-binding domain of the myocilin protein adds to its importance and brings up the relevance of calcium on the olfactomedin domain stability.56 It also points to an involvement of myocilin in the trabecular meshwork tissue calcification, which has been documented to be associated with glaucoma postmortem specimens.4 These data would be in good agreement with 1 of the functions of the calcium binding sites, which is conferring thermal stability and proteolytic protection to extracellular proteins.58
Another gene, HSP90B1, is upregulated by myocilin mutants,6 especially by the Q368X mutation, which triggers the highest UPR response.6 This gene is most recently known as GRP94 and encodes a chaperone, ER resident protein, which has been shown to have unique characteristics and be of special importance in the ER. GRP94 is induced upon glucose deprivation, and its up-regulation is used as a hallmark of responses of the ER to stress.59 Recent studies have shown that myocilin is a client of GRP94 and that this chaperone specifically recognizes the mutant myocilin and targets it for degradation via the ubiquitin-dependent/proteasome ER degradation (ERAD).60 Using HEK293 cells, the authors demonstrated that silencing GRP94 with siRNA diverts the mutated protein for degradation via the more robust autophagy pathway. Although the results need to be validated in HTM cells, where mutant myocilin directly affects the outcome of IOP function, they nevertheless bring an interesting rational mechanism that could explain why myocilin mutants cause glaucoma. Still, there is a lack of correlation between up-regulation of HSP90B1, encoding GRP94, and the severity of the IOP associated with the mutants. The stop mutation Q368X associated with moderate IOP (mean IOP 21 mmHg) induces high up-regulation of the HSP90B1 gene, while mutations D380N and K423E with means IOP of 39 mmHg and 28 mmHg barely show up-regulation of the same chaperone (Fig. 2).6 A list of selected relevant functions from this section is shown in Table 1B.
One Potential Common Gene Link for Myocilin Disease-Causing Mutants: Cystatin A
It is unlikely that one single mechanism would govern the glaucoma-causative effect of all myocilin mutants. Myocilin wild-type per se exerts and affects numerous cell processes.22–24,30,31 However, because most myocilin disease variants fall in the olfactomedin domain, it would make sense to assume that a potential common mechanism would be one that implicates this region of the protein. In normal conditions, 40% of wild-type myocilin gets cleaved in the ER by the cysteine protease calpain II between amino acids arginine 226 and Ieucine 227, which are located in the central linker between the 2 protein domains. This processing releases a 35-kDa fragment containing the olfactomedin domain, which is co-secreted with the wild-type.57 Although an olfactomedin domain fraction purified from the medium of 293 EBNA cells transfected with a human myocilin cDNA plasmid did not affect outflow facility in perfused human and porcine eyes,61 the full consequences of this domain and of this processing are not yet known. It has been shown that myocilin mutants inhibit the proteolytic process, and that the extent of the inhibition correlates with the severity of the glaucoma phenotypes. It is also known that this proteolytic process modulates the molecular interactions of myocilin and reduces the formation of myocilin homo-aggregates.62
As mentioned above, the search for specific genes that would be involved in a common myocilin mutant mechanism led to the finding that 73 genes had been altered after overexpression of all 4 mutants of the study (Q368X, R342K, D380N, and K423E). From those 73 genes, only 10 were altered specifically in the mutants but were not shared with the altered genes in the wild-type.6 Analysis of the functions of the 10 genes revealed the presence of a cysteine protease inhibitor, Cystatin A (CSTA), which showed a no-change 1.0-fold in the myocilin wild-type and an increase of 3.4, 3.2, 1.5, and 1.9-fold in Q368X, R342K, D380N, and K423E, respectively.6 Functional assay of CSTA in primary HTM cells and transformed H293K cells showed that indeed this protein inhibited the proteolytic cleavage of wild-type myocilin. Because the protein involved in the processing of myocilin is a cysteine protease and because myocilin cleavage is inhibited by myocilin mutants, it is logical to assume that CSTA plays a role in the inhibition process.6,57 It is not yet known whether the elevation of expression of CSTA in the myocilin mutants is directly inhibiting calpain II. The cysteine protease CSTA inhibits the activity of the papain-type proteases cathepsin B, L, and H. However, CSTA was not known to inhibit calpains. However, a recent study investigating cell death in macrophages has shown that calpain activation occurs downstream of cathepsin B, therefore concluding that cathepsin B activates calpain.63 Since CSTA inhibits cathepsin B, CSTA will in turn prevent the activation of calpain. It is possible then, that increased levels of CSTA seen in myocilin mutant cells would result in the inactivation of calpain by inhibiting cathepsin B. All together these gene and protein findings from 2 unrelated laboratories do reinforce the mechanism of myocilin processing as a potential common link of myocilin mutants.
Conclusions
Because of the continuous global finding of genetic linkages of myocilin mutants with glaucoma (http://www.myocilin.com/), the search for the function(s) of this protein is of great relevance. Understanding its biology and mechanisms leading to the disease could lead to the design of new rational drugs. The approach of elucidating myocilin function through the identification of genes altered by overexpression and underexpression of wild-type and mutants in the relevant cell type provides the first insight into those mechanisms. Studies of the last decade have demonstrated that most likely there is not a single central mechanism associated with myocilin-causative glaucoma. Analyses of gene profiles, as well as of individual functions associated with them, have revealed the involvement of numerous distinct mechanisms. Most of these, when analyzed individually, do make good physiological sense as would-be contributors to the maintenance of aqueous humor resistance. This is in part due to the universal role of trabecular meshwork tissue of maintaining a physiological IOP. Given the nature of the tissue, it is not difficult to visualize that such function could be achieved through many different pathways, from the reorganization of the ECM and contractility status of its cells, to the inhibition of calcification and stiffness, to its processing and/or intercellular signaling. The trabecular meshwork may use them separately or together to exert its function. Many myocilin-causative mechanisms remain to be learned, but many have been found. The relatively minor discrepancies seen among different laboratories will need to be resolved, but they may also be an indication that the properties of this gene/protein are very much in tune with different experimental conditions. Thus, different individual cell lines, passages, and cell culture conditions could cause an apparently different result. Overall, identification of consequent altered gene expression remains one of the best tools to further explore the myocilin contribution to glaucoma. Nature usually does not waste resources, and cells would not be using them unless the genes expressed would be needed for something. Keeping an open mind and observing these genes' changes can take us to the design of numerous targeted drugs for controlling elevated IOP and as a consequence to ameliorate glaucoma.
Acknowledgments
The author thanks laboratory members La Kisha K. Buie and Brandon Lane for critical reading of the manuscript and helpful comments. This study was supported by National Institutes of Health Grants EY11906 (TB) and EY13126 (TB) and by an unrestricted grant from Research to Prevent Blindness to the Department of Ophthalmology at the University of North Carolina at Chapel Hill.
Author Disclosure Statement
No competing financial interests exist.
References
- 1.Polansky J.R., Kurtz R.M., Fauss D.J., Kim R.Y., and Bloom E.In vitro correlates of glucocorticoid effects on intraocular pressure. In: Krieglstein G.K., ed. Glaucoma Update IV. Berlin, Heidelberg: Springer-Verlag; 1991; p. 20–29 [Google Scholar]
- 2.Nguyen T.D., Chen P., and Huang W.D., et al. . Gene structure and properties of TIGR, an olfactomedin-related glycoprotein cloned from glucocorticoid-induced trabecular meshwork cells. J. Biol. Chem. 273:6341–6350, 1998 [DOI] [PubMed] [Google Scholar]
- 3.Stone E.M., Fingert J.H., Alward W.L., et al. . Identification of a gene that causes primary open angle glaucoma. Science. 275:668–670, 1997 [DOI] [PubMed] [Google Scholar]
- 4.Gong G., Kosoko-Lasaki O., Haynatzki G.R., and Wilson M.R.Genetic dissection of myocilin glaucoma. Hum. Mol. Genet. 13(Spec No 1):R91–R102, 2004 [DOI] [PubMed] [Google Scholar]
- 5.Lascaratos G., Shah A., and Garway-Heath D.F.The genetics of pigment dispersion syndrome and pigmentary glaucoma. Surv. Ophthalmol. 58:164–175, 2013 [DOI] [PubMed] [Google Scholar]
- 6.Kennedy K.D., AnithaChristy S.A., Buie L.K., and Borrás T.Cystatin a, a potential common link for mutant myocilin causative glaucoma. PLoS ONE. 7:e36301, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Comes N., and Borrás T.Individual molecular response to elevated intraocular pressure in perfused postmortem human eyes. Physiol. Genomics. 38:205–225, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Navarro A., Perez R.E., Rezaiekhaligh M., Mabry S.M., and Ekekezie I.I.T1alpha/podoplanin is essential for capillary morphogenesis in lymphatic endothelial cells. Am. J. Physiol. Lung Cell. Mol. Physiol. 295:L543-L551, 2008 [DOI] [PubMed] [Google Scholar]
- 9.Goh Y., Nakajima M., Azuma I., and Hayaishi O.Prostaglandin D2 reduces intraocular pressure. Br. J. Ophthalmol. 72:461–464, 1988 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Gonzalez P., Epstein D.L., and Borrás T.Characterization of gene expression in human trabecular meshwork using single-pass sequencing of 1060 clones. Invest. Ophthalmol. Vis Sci. 41:3678–3693, 2000 [PubMed] [Google Scholar]
- 11.Borrás T., Bryant P.A., and Chisolm S.S.First look at the effect of overexpression of TIGR/MYOC on the transcriptome of the human trabecular meshwork. Exp. Eye Res. 82:1002–1010, 2006 [DOI] [PubMed] [Google Scholar]
- 12.Stamer W.D., Seftor R.E., Williams S.K., Samaha H.A., and Snyder R.W.Isolation and culture of human trabecular meshwork cells by extracellular matrix digestion. Curr. Eye Res. 14:611–617, 1995 [DOI] [PubMed] [Google Scholar]
- 13.Caballero M., and Borrás T.Inefficient processing of an olfactomedin-deficient myocilin mutant: potential physiological relevance to glaucoma. Biochem. Biophys. Res. Commun. 282:662–670, 2001 [DOI] [PubMed] [Google Scholar]
- 14.Bradley J.M., Vranka J., Colvis C.M., et al. . Effect of matrix metalloproteinases activity on outflow in perfused human organ culture. Invest. Ophthalmol. Vis. Sci. 39:2649–2658, 1998 [PubMed] [Google Scholar]
- 15.Gerometta R., Spiga M.G., Borrás T., and Candia O.A.Treatment of sheep steroid-induced ocular hypertension with a glucocorticoid-inducible MMP1 gene therapy virus. Invest. Ophthalmol. Vis. Sci. 51:3042–3048, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Borrás T.What is functional genomics teaching us about intraocular pressure regulation and glaucoma. In: Civan M.M., ed. The Eye's Aqueous Humor, Second Edition. San Diego: Elsevier; 2008; p. 323–377 [Google Scholar]
- 17.Ikeda T., Ishii H., Higuchi T., et al. . Localization of thrombomodulin in the anterior segment of the human eye. Invest. Ophthalmol. Vis. Sci. 41:3383–3390, 2000 [PubMed] [Google Scholar]
- 18.Borrás T., Morozova T.V., Heinsohn S.L., et al. . Transcription profiling in Drosophila eyes that overexpress the human glaucoma-associated trabecular meshwork-inducible glucocorticoid response protein/myocilin (TIGR/MYOC). Genetics. 163:637–645, 2003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Han Z., Wax M.B., and Patil R.V.Potential role of aquaporins and atrial natriuretic peptides in the aqueous humor dynamics. Exp. Eye. Res. 67:251–253, 1998 [DOI] [PubMed] [Google Scholar]
- 20.Stoilov I., Akarsu A.N., and Sarfarazi M.Identification of three different truncating mutations in cytochrome P4501B1 (CYP1B1) as the principal cause of primary congenital glaucoma (Buphthalmos) in families linked to the GLC3A locus on chromosome 2p21. Hum. Mol. Genet. 6:641–647, 1997 [DOI] [PubMed] [Google Scholar]
- 21.Paper W., Kroeber M., Heersink S., et al. . Elevated amounts of myocilin in the aqueous humor of transgenic mice cause significant changes in ocular gene expression. Exp. Eye Res. 87:257–267, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Kaneda Y., Uchida T., Kim J., Ishiura M., and Okada Y.The improved efficient method for introducing macromolecules into cells using HVJ (Sendai virus) liposomes with gangliosides. Exp Cell Res. 173:56–69, 1987 [DOI] [PubMed] [Google Scholar]
- 23.Wentz-Hunter K., Shen X., Okazaki K., Tanihara H., and Yue B.Y.Overexpression of myocilin in cultured human trabecular meshwork cells. Exp. Cell Res. 297:39–48, 2004 [DOI] [PubMed] [Google Scholar]
- 24.Kwon H.S., Lee H.S., Ji Y., Rubin J.S., and Tomarev S.I.Myocilin is a modulator of Wnt signaling. Mol. Cell. Biol. 29:2139–2154, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Shen X., Ying H., and Yue B.Y.Wnt activation by wild type and mutant myocilin in cultured human trabecular meshwork cells. PLoS ONE. 7:e44902, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Wang W.H., McNatt L.G., Pang I.H., et al. . Increased expression of the WNT antagonist sFRP-1 in glaucoma elevates intraocular pressure. J Clin. Invest. 118:1056–1064, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Mao W., Millar J.C., Wang W.H., et al. . Existence of the canonical Wnt signaling pathway in the human trabecular meshwork. Invest. Ophthalmol. Vis. Sci. 53:7043–7051, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Tian B., Geiger B., Epstein D.L., and Kaufman P.L.Cytoskeletal involvement in the regulation of aqueous humor outflow. Invest. Ophthalmol. Vis. Sci. 41:619–623, 2000 [PubMed] [Google Scholar]
- 29.Peters D.M., Herbert K., Biddick B., and Peterson J.A.Myocilin binding to Hep II domain of fibronectin inhibits cell spreading and incorporation of paxillin into focal adhesions. Exp. Cell. Res. 303:218–228, 2005 [DOI] [PubMed] [Google Scholar]
- 30.Shen X., Koga T., Park B.C., SundarRaj N., and Yue B.Y.Rho GTPase and cAMP/protein kinase A signaling mediates myocilin-induced alterations in cultured human trabecular meshwork cells. J. Biol. Chem. 283:603–612, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Kwon H.S., Johnson T.V., and Tomarev S.I.Myocilin stimulates osteogenic differentiation of mesenchymal stem cells through mitogen-activated protein kinase signaling. J. Biol. Chem. 288:16882–16894, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Xue W., Wallin R., Olmsted-Davis E.A., and Borráas T.Matrix GLA protein function in human trabecular meshwork cells: inhibition of BMP2-induced calcification process. Invest. Ophthalmol. Vis. Sci. 47:997–1007, 2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Xue W., Comes N., and Borráas T.Presence of an established calcification marker in trabecular meshwork tissue of glaucoma donors. Invest. Ophthalmol. Vis. Sci. 48:3184–3194, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Faverman L., Mikhaylova L., Malmquist J., and Nurminskaya M.Extracellular transglutaminase 2 activates beta-catenin signaling in calcifying vascular smooth muscle cells. FEBS Lett. 582:1552–1557, 2008 [DOI] [PubMed] [Google Scholar]
- 35.Gaur T., Lengner C.J., Hovhannisyan H., et al. . Canonical WNT signaling promotes osteogenesis by directly stimulating Runx2 gene expression. J Biol Chem. 280:33132–33140, 2005 [DOI] [PubMed] [Google Scholar]
- 36.Lo W.R., Rowlette L.L., Caballero M., et al. . Tissue differential microarray analysis of dexamethasone induction reveals potential mechanisms of steroid glaucoma. Invest. Ophthalmol. Vis. Sci. 44:473–485, 2003 [DOI] [PubMed] [Google Scholar]
- 37.Steely H.T., Browder S.L., Julian M.B., et al. . The effects of dexamethasone on fibronectin expression in cultured human trabecular meshwork cells. Invest. Ophthalmol. Vis. Sci. 33:2242–2250, 1992 [PubMed] [Google Scholar]
- 38.Challa P., Herndon L.W., Hauser M.A., et al. . Prevalence of myocilin mutations in adults with primary open-angle glaucoma in Ghana, West Africa. J. Glaucoma. 11:416–420, 2002 [DOI] [PubMed] [Google Scholar]
- 39.Morissette J., Clepet C., Moisan S., et al. . Homozygotes carrying an autosomal dominant TIGR mutation do not manifest glaucoma. Nat. Genet. 19:319–321, 1998 [DOI] [PubMed] [Google Scholar]
- 40.Carbone M.A., Ayroles J.F., Yamamoto A., et al. . Overexpression of myocilin in the Drosophila eye activates the unfolded protein response: implications for glaucoma. PLoS ONE. 4:e4216, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Ryoo H.D., Domingos P.M., Kang M.J., and Steller H.Unfolded protein response in a Drosophila model for retinal degeneration. EMBO J. 26:242–252, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Wiggs J.L., Allingham R.R., Vollrath D., et al. . Prevalence of mutations in TIGR/myocilin in patients with adult and juvenile primary open-angle glaucoma. Am. J. Hum. Genet. 63:1549–1552, 1998 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Wang L., Zhuo Y., Liu B., et al. . Pro370Leu mutant myocilin disturbs the endoplasm reticulum stress response and mitochondrial membrane potential in human trabecular meshwork cells. Mol. Vis. 13:618–625, 2007 [PMC free article] [PubMed] [Google Scholar]
- 44.Zhou Y., Grinchuk O., and Tomarev S.I.Transgenic mice expressing the Tyr437His mutant of human myocilin protein develop glaucoma. Invest. Ophthalmol. Vis. Sci. 49:1932–1939, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Joe M.K., and Tomarev S.I.Expression of myocilin mutants sensitizes cells to oxidative stress-induced apoptosis: implication for glaucoma pathogenesis. Am. J. Pathol. 176:2880–2890, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Zode G.S., Kuehn M.H., Nishimura D.Y., et al. . Reduction of ER stress via a chemical chaperone prevents disease phenotypes in a mouse model of primary open angle glaucoma. J. Clin. Invest. 121:3542–3553, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Zode G.S., Bugge K.E., Mohan K., et al. . Topical ocular sodium 4-phenylbutyrate rescues glaucoma in a myocilin mouse model of primary open-angle glaucoma. Invest. Ophthalmol. Vis. Sci. 53:1557–1565, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Gobeil S., Letartre L., and Raymond V.Functional analysis of the glaucoma-causing TIGR/myocilin protein: integrity of amino-terminal coiled-coil regions and olfactomedin homology domain is essential for extracellular adhesion and secretion. Exp. Eye Res. 82:1017–1029, 2006 [DOI] [PubMed] [Google Scholar]
- 49.Caballero M., Rowlette L.L., and Borrás T.Altered secretion of a TIGR/MYOC mutant lacking the olfactomedin domain. Biochim. Biophys. Acta. 1502:447–460, 2000 [DOI] [PubMed] [Google Scholar]
- 50.Sidrauski C., Chapman R., and Walter P.The unfolded protein response: an intracellular signalling pathway with many surprising features. Trends Cell. Biol. 8:245–249, 1998 [DOI] [PubMed] [Google Scholar]
- 51.Gobeil S., Rodrigue M.A., Moisan S., et al. . Intracellular sequestration of hetero–oligomers formed by wild-type and glaucoma-causing myocilin mutants. Invest. Ophthalmol. Vis. Sci. 45:3560–3567, 2004 [DOI] [PubMed] [Google Scholar]
- 52.Wirtz M.K., Konstas A.G., Samples J.R., et al. . Myocilin variations and familial glaucoma in Taxiarchis, a small Greek village. Mol. Vis. 14:774–781, 2008 [PMC free article] [PubMed] [Google Scholar]
- 53.Hewitt A.W., Bennett S.L., Dimasi D.P., Craig J.E., and Mackey D.A.A myocilin Gln368STOP homozygote does not exhibit a more severe glaucoma phenotype than heterozygous cases. Am J Ophthalmol. 141:402–403, 2006 [DOI] [PubMed] [Google Scholar]
- 54.Yoon S.J., Kim H.S., Moon J.I., Lim J.M., and Joo C.K.Mutations of the TIGR/MYOC gene in primary open-angle glaucoma in Korea. Am. J. Hum. Genet. 64:1775–1778, 1999 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Wirtz M.K., Samples J.R., Choi D., Gaudette N.D.Clinical features associated with an Asp380His myocilin mutation in a US family with primary open-angle glaucoma. Am. J. Ophthalmol. 144:75–80, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Donegan R.K., Hill S.E., Turnage K.C., Orwig S.D., and Lieberman R.L.The glaucoma-associated olfactomedin domain of myocilin is a novel calcium binding protein. J. Biol. Chem. 287:43370–43377, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Aroca-Aguilar J.D., Sanchez-Sanchez F., Ghosh S., Coca-Prados M., and Escribano J.Myocilin mutations causing glaucoma inhibit the intracellular endoproteolytic cleavage of myocilin between amino acids Arg226 and Ile227. J. Biol. Chem. 280:21043–21051, 2005 [DOI] [PubMed] [Google Scholar]
- 58.Maurer P., Hohenester E., and Engel J.Extracellular calcium-binding proteins. Curr. Opin. Cell. Biol. 8:609–617, 1996 [DOI] [PubMed] [Google Scholar]
- 59.Eletto D., Dersh D., and Argon Y.GRP94 in ER quality control and stress responses. Semin. Cell. Dev Biol. 21:479–485, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Suntharalingam A., Abisambra J.F., O'Leary J.C. III, et al. . Glucose-regulated protein 94 triage of mutant myocilin through endoplasmic reticulum-associated degradation subverts a more efficient autophagic clearance mechanism. J. Biol. Chem. 287:40661–40669, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Goldwich A., Ethier C.R., Chan D.W., and Tamm E.R.Perfusion with the olfactomedin domain of myocilin does not affect outflow facility. Invest. Ophthalmol. Vis. Sci. 44:1953–1961, 2003 [DOI] [PubMed] [Google Scholar]
- 62.Aroca-Aguilar J.D., Martinez-Redondo F., Sanchez-Sanchez F., Coca-Prados M., and Escribano J.Functional role of proteolytic processing of recombinant myocilin in self-aggregation. Invest. Ophthalmol. Vis. Sci. 51:72–78, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Hentze H., Lin X.Y., Choi M.S., and Porter A.G.Critical role for cathepsin B in mediating caspase-1-dependent interleukin-18 maturation and caspase-1-independent necrosis triggered by the microbial toxin nigericin. Cell Death Differ. 10:956–968, 2003 [DOI] [PubMed] [Google Scholar]