Abstract
Purpose: To support the growing promise of regenerative medicine in glaucoma, we characterized the similarities and differences between human trabecular meshwork (HTM) cells and human mesenchymal stem cells (hMSCs).
Methods: HTM cells and hMSCs were phenotypically characterized by flow cytometry. Using quantitative polymerase chain reaction, the expression of myoc, angptl7, sox2, pou5f1, and notch1 was determined in both cell types with and without dexamethasone (Dex). Immunosuppressive behavior of HTM cells and hMSCs was determined using T cells activated with phytohemagglutinin. T-cell proliferation was determined using BrdU incorporation and flow cytometry. Multipotency of HTM cells and hMSCs was determined using adipogenic and osteogenic differentiation media as well as aqueous humor (AH). Alpha-smooth muscle actin (αSMA) expression was determined in HTM cells, hMSCs, and HTM tissue.
Results: Phenotypically, HTM and hMSCs expressed CD73, CD90, CD105, and CD146 but not CD31, CD34, and CD45 and similar sox2, pou5f1, and notch1 expression. Both cell types suppressed T-cell proliferation. However, HTM cells, but not hMSCs, upregulated myoc and angptl7 in response to Dex. Additionally, HTM cells did not differentiate into adipocytes or osteocytes. Culture of hMSCs in 20%, but not 100%, AH potently induced alkaline phosphatase activity. HTM cells in culture possessed uniformly strong expression of αSMA, which contrasted with the limited expression in hMSCs and spatially discrete expression in HTM tissue.
Conclusions: HTM cells possess a number of important similarities with hMSCs but lack multipotency, one of the defining characteristics of stem cells. Further work is needed to explore the molecular mechanisms and functional implications underlying the phenotypic similarities.
Introduction
A key contributor to the progression of primary open-angle glaucoma is the reduction in outflow facility through the human trabecular meshwork (HTM). HTM cellularity is reported to decrease steadily throughout life, and glaucoma is correlated with a more rapid decline.1–4 Taken together, these data have led to speculation that healthy cell populations may be needed to maintain HTM function and outflow facility. The progressive loss of HTM cells in vivo is puzzling considering the presence of dividing cells in the HTM and animal TMs in response to injury,5–9 especially in the nonfiltering anterior region of the meshwork.5 Several researchers have speculated that this region, the so-called insert region located near Schwalbe's line, may contain a progenitor cell population, which could be induced to differentiate and repopulate the filtering HTM.10–14 Indeed, in the spontaneous glaucoma beagle model, there is a marked decrease of cells near Schwalbe's line.15 These data point to renewing the HTM cell population as a potential therapeutic target for the treatment of glaucoma.
A knowledge gap exists, however, in our understanding of the HTM cell progenitor pool and what distinguishes progenitors from the mature HTM population. The root of this problem rests in the poor classification of HTM cells. While the HTM is known to express numerous genes, such as myocilin,16–19 angiopoietin-related protein 7,20–23 α-smooth muscle actin (αSMA),24–26 chitinase-3-like-1,27–29 and aquaporin 1,30 none of these biomarkers are specific to the HTM. In place of a unique gene expression signature, the identity of HTM cells is frequently verified through their responsiveness to glucocorticoids, such as dexamethasone (Dex). In a behavior that is thought to be a unique attribute of the HTM, Dex treatment induces the upregulation of myocilin (myoc) and angiopoietin-related protein 7 (angptl7) expression.31,32 Thorough characterization of HTM cells is the starting point for understanding, and exploiting, the distinction between mature and progenitor populations.
The ability of TM cell populations to regenerate in vivo was first observed over 2 decades ago in a feline model after TM cells were exposed to an inflammatory challenge via zymosan injections.9 In this study, cellularity was acutely decreased but ultimately recovered. Later work identified cell proliferation, localized primarily in the anterior meshwork, after laser trabeculoplasty (LTP) in ex vivo human models.5 Indeed, proliferation can lead to the failure of LTP with some cases exhibiting the overgrowth of “cell sheets” into the intertrabecular spaces.7 Despite the knowledge of the existence of a replicating population, research has yet to uncover a method for utilizing this in the treatment of glaucoma.
There is some evidence that these cells, or another progenitor pool, have successfully been cultured. Gonzalez et al. isolated “free-floating spheres” from HTM primary cultures.11 Similar spheres have exhibited characteristics of multipotent progenitors in other tissue culture systems,33–35 and the HTM free-floating spheres exhibited gene expression profiles similar to both cultured HTM cells and progenitor cells. More recently, Du et al. isolated a side population of primary HTM cells and characterized them as lacking typical HTM markers and possessing multipotency.36 Importantly, these cells could be differentiated into phagocytically active HTM cells through exposure to aqueous humor (AH) or serum. As a demonstration of the therapeutic potential of these cells, they were safely injected in a mouse eye and localized to the TM, whereas similarly injected fibroblasts were distributed throughout the eye.37 Although such results are very promising and offer direct evidence of an adult stem cell pool within the TM, regenerative medicine in the HTM remains in its infancy. Fortunately, there is a large and still growing body of research on adult stem cells from which we can draw.
Adult stem cells are known to be expressed in numerous tissues where they are thought to maintain a stable population of cells and replenish the population after injury or insult. A subset of adult stem cells, human mesenchymal stem cells (hMSCs), are an attractive option for regenerative medicine as they can be isolated relatively easily, can be expanded readily in vitro, and possess multipotency. Additionally, a growing body of clinical and animal studies provides evidence of their safety and efficacy in vivo.38 There is agreement that hMSCs are CD73, CD90, CD105, and CD146 positive, and CD31, CD34, and CD45 negative, have trilineage potential (adipo-, osteo-, and chondro-genic), and can suppress the proliferative response of T cells.39–43 Similar to HTM cells, Dex is known to have potent effects on hMSCs. A recent report by Kwon et al. revealed that hMSCs also exhibit myoc upregulation in response to Dex and that myocilin induces osteogenesis.44 This work adds potential insight to the long practice of using Dex to induce in vitro differentiation of hMSCs.45,46
Tay et al. have recently reported the isolation of hMSCs subsequent to collagenase digestion of TM. The isolated cells possessed both the proper surface markers and trilineage potential characteristic of hMSCs.47 We have similarly observed that HTM explant cultures exhibit several characteristics similar to cultures of adipose-derived hMSCs. There is a long incubation phase before cells migrate from the tissue, and once they have exited the tissue, HTM cells have a large expansion capacity, are plastic adherent, and fibroblastic in appearance. All of these are characteristics of hMSCs. The study reported herein was undertaken to critically compare and contrast HTM cells with adipose-derived MSCs in culture.
Methods
Isolation and culture of cells
All work involving human tissue was performed in a manner consistent with the Declaration of Helsinki. Primary cultures of TM cells (HTM) were isolated from donor human corneoscleral rims. Briefly, the iris and ciliary body were removed from the corneoscleral rim, revealing the meshwork. The meshwork was then carefully dissected out of the tissue in 10- to 20-mm segments and placed with 0.2% Cytodex beads (Sigma) in Dulbecco's modified Eagle medium/Nutrient Mixture F-12 (50:50; DMEM/F12) supplemented with 10% fetal bovine serum (FBS) and 1% penicillin/streptomycin (P/S)/fungizone (Life Technologies). Cells that migrated out of the tissue were maintained in supplemented DMEM/F12. Cultures were used up to passage 7. All cultures used were confirmed as HTM by myoc upregulation in response to 100 nM Dex. Equivolume treatments of ethanol were used as a vehicle control.
Primary cultures of MSCs (hMSCs) were isolated and cultured from donor adipose tissue as previously described.48–50 Briefly, 10–13 g of fat was minced and rocked at 37°C for 2 h in 50 mL of phosphate-buffered saline (PBS; Invitrogen) with 0.1% collagenase/1% bovine serum albumin (Worthington) followed by centrifugation to remove the lipid layer and repeated washes with PBS. Cell pellets were resuspended with culture media DMEM (low glucose) supplemented with 10% FBS and 1% P/S (Life Technologies), plated, and incubated at 37°C, 5% CO2. Cells were passaged at 70% confluence and maintained in supplemented DMEM (low glucose).
Immortalized corneal fibroblasts [human corneal fibroblast (HCFs)] were kindly gifted by Dr. Jamie Jester.51 They were maintained in supplemented DMEM (high glucose). To induce differentiation into myofibroblasts, HCFs were treated with 2 ng/mL transforming growth factor-β1 (TGF-β1) (Sigma) for 48 h, similar to previous reports.51
Flow cytometry for surface antigens
Flow cytometric analysis of surface markers, CD31 (Clone: WM59; BD Pharmingen), CD34 (Clone: 581; BD Pharmingen), CD45 (Clone: HI30; BD Pharmingen), CD73 (Clone: AD2; BD Pharmingen), CD90 (Clone: 5E10; BD Pharmingen), CD105 (Clone: 266; BD Pharmingen), CD146 (Clone: 541-10B2; Milentyi Biotec), were performed as previously described.52
Isolation of mRNA and quantitative polymerase chain reaction
Cells were washed with PBS, and mRNA was extracted using RNeasy minikits (Qiagen) according to the manufacturer protocols. Concentration of mRNA was determined using a NanoDrop (ThermoFisher). Quantitative polymerase chain reaction (qPCR) was performed using the SensiFAST Hi-ROX One-Step mastermix (BioLine). All reactions were performed in triplicate. All assays were performed with commercially available aptamers (Life Technologies) for myocilin (myoc, Hs00165345_m1), angiopoietin-related protein 7 (angptl7, Hs00221727_m1), SRY (sex-determining region Y)-box 2 (sox2, Hs01053049_s1), octamer-binding transcription factor 4 (pou5f1, Hs00999632_g1), notch 1 (notch1, Hs01062014_m1), αSMA (acta2, Hs00426835_g1), and normalized to the expression of rRNA 18S (Hs99999901_s1). The qPCR reactions were performed in a StepOne qPCR machine (Life Technologies) with the following parameters: 30 min at 50°C followed by 10 min at 95°C and 40 cycles of 60°C for 1 min and 95°C for 15 s.
Isolation of protein and western blotting
HTM cells were washed with PBS and lysed using RIPA buffer (ThermoScientific) containing the HALT protease/phosphatase inhibitor cocktail (ThermoScientific) on ice. The lysate was homogenized and centrifuged for 1 min to remove cellular debris. Protein was quantified using a modified Lowry assay (DC protein assay; Bio-Rad) with bovine serum albumin as the standard. Protein was denatured in NuPAGE sample buffer (Life Technologies) by heating to 85°C. Approximately 40 μg of protein was loaded into wells of TGX 10% polyacrylamide precast gel (Bio-Rad) for separation before transfer to polyvinylidene difluoride membranes. The membranes were blocked using milk diluent (KPL) for 1 h at 37°C. As a positive control for Oct-3/4 expression, lysate from the F9 embryonal carcinoma cell line was loaded at 5 μg of protein. Immunoblotting was performed with anti-human Oct-3/4 (C-10; Santa Cruz Biotechnologies) for 2 h at 37°C. Rabbit anti-human heat shock protein-90 (HSP90; Cell Signaling) was used as a loading control. Following primary antibody incubation and washing, secondary antibodies conjugated with horseradish peroxidase (HRP) were added for 1 h at 37°C. Bands were detected using the WesternBright Quantum HRP substrate (Advansta) and imaged using an ImageQuant 350 system (GE Healthcare Life Sciences).
Differentiation assays
Cells (HTM and hMSC) were plated at 25,000 and 125,000 cells/well in 24- and 6-well plates, respectively, and allowed to grow to ∼80% confluence (∼1–3 days). The cells were then switched to either induction or control media and maintained for the specified time with media changes every 3–4 days. For adipogenesis, control media was DMEM (high glucose) supplemented with 10% FBS and 1% P/S. Adipogenic media was control media further supplemented with 500 μM 3-isobutyl-1-methyl-xanthine (Sigma), 1 μM Dex (Sigma), 10 μg/mL insulin (Sigma), and 10 μg/mL indomethacin (Sigma). For osteogenesis, control media was DMEM (low glucose) supplemented with 10% FBS and 1% P/S, and osteogenic media was the Osteocyte Differentiation Tool (ATCC). For AH differentiation experiments, the control media was DMEM (high glucose) supplemented with 10% FBS and 1% P/S. The experimental media were the control medium with 20% sterile-filtered bovine AH, 100% bovine AH, or the osteogenic medium. AH was removed using a 25-gauge needle from enucleated bovine eyes shipped overnight on ice (Pel-freez).
Adipogenesis was assayed using Oil Red O stain for lipids. Briefly, the cells were washed with PBS and fixed in 4% formaldehyde in PBS for 20 min and washed again. The cells were rinsed with 60% isopropanol (Fisher Scientific) and then stained with 0.3% Oil Red O (Sigma) in 60% isopropanol for 10 min. The cells were rinsed with 60% isopropanol and PBS and then imaged.
Osteogenic potential was assayed using an alkaline phosphatase activity stain. Briefly, the cells were washed with PBS and fixed in 4% formaldehyde in PBS for 5 min and washed again. The cells were then stained with 0.1% naphthol AS-MX phosphate (Sigma) and 0.1% fast red violet LB (Sigma) dissolved in 56 mM 2-amino-2-methyl-1,3-propanediol (pH 9.9; Sigma) for 15 min.
Differentiation into TM cells was assayed using a 3-day Dex treatment followed by the measurement of the expression of myoc and angptl7 message expression as described above.
Peripheral blood mononuclear cell proliferation assay
Peripheral blood from a human donor was collected into tubes containing acid-citrate dextrose (BD Biosciences). Peripheral blood mononuclear cells (PBMCs) were obtained by mixing 20 mL blood with 15 mL of PBS (Hyclone) and subsequently underlaying with 10 mL Ficoll-Paque Plus (GE Healthcare). The blood was centrifuged (500g, 20 min, no brake), and PBMCs were isolated, washed with PBS (400g, 10 min), and resuspended in supplemented DMEM (low glucose). The T-cell population of the PBMCs was subsequently enriched in nylon wool.53–55 Nylon wool (0.5 g; Polysciences, Inc., Warrington, PA) was loaded into a 12-mL syringe, autoclaved, and then incubated in media for 1 h before the addition of PBMCs. PBMCs were layered over the nylon wool in 2 mL of media and incubated at 37°C for 1 h. The nylon wool was then washed with media. The flowthrough was centrifuged (400g, 10 min). T-cell-enriched PBMCs were resuspended in 5 mL media and kept on ice until plating.
To prevent the proliferation of HTM and hMSCs, cells were detached and 15 mL suspensions were placed in T75 flasks (Corning) and irradiated (10 Gy, Varian 2100C linear accelerator, Varian Medical Systems, Inc.). After irradiation, HTM and hMSCs were spun down (300g, 10 min) and resuspended in supplemented DMEM (high glucose) and kept on ice until plating.
Enriched T cells, HTM cells, and hMSCs were plated in 24-well plates (Falcon) at a ratio of 1:5 HTM/hMSC: enriched T-cells and were incubated with supplemented media at 37°C, 5% CO2 for 4 days. To induce T-cell proliferation, the cultures were treated with 5 μg/mL phytohemagglutinin (PHA; Sigma). T cells in monoculture with PHA were considered to have the maximal rate of proliferation. In the final 24 h of coculture, cells were treated with BrdU (1 mM) (BD Biosciences). Cells were collected and processed as per the manufacturer directions (FITC BrdU Flow Kit; BD Biosciences) and analyzed on a flow cytometer (Cytomics FC500; Beckman Coulter). As it has been shown that the nylon wool method of enrichment can increase the basal level of T-cell activation,53 T cells in monoculture without PHA were used as a basal control.
Immunohistochemistry and immunocytochemistry
For in vitro αSMA staining, cells were cultured on 13-mm glass coverslips for 3 days. Cells were fixed in 4% formaldehyde in PBS (pH 7.0) for 20 min and permeabilized in 0.1% Triton X-100 for 5 min. Endogenous peroxidase in cells was quenched by incubation with ice cold 0.3% H2O2 in PBS for 30 min. Blocking was performed in PBS containing 10% superblock, 10% FBS, and 0.2% fish gelatin for 1 h at 37°C. Cells were then incubated with primary antibody (mouse anti-αSMA, 1:250 dilution; Sigma-Aldrich) for 1 h at room temperature. They were rinsed 3 times in PBS and incubated with secondary antibody (goat anti-mouse DyLight 488 nm, 1:500 dilution; Fisher Scientific) for 20 min at room temperature. All antibodies were prepared in blocking buffer. F-actin in cells was stained by incubation with AlexaFluor 568-conjugated phallodin for 15 min at room temperature, and nuclei were counterstained with 4′,6-diamidino-2-phenylindole (DAPI) (5 min). Coverslips were briefly rinsed in deionized water and then mounted onto glass slides using Mowiol with DABCO as antifade.
For tissue staining, paraffin-embedded sections of corneoscleral rim from a 51-year-old donor were incubated twice in xylene for 6 min each. Deparaffinized sections were saturated 3 times in 100% ethanol (1 min each) and successively rehydrated in 95% ethanol (1 min) and 70% ethanol (1 min) in deionized water. Endogenous peroxidase was quenched with ice cold 0.3% H2O2 in methanol for 30 min and washed in PBS. Antigen retrieval was performed at 95–100°C in a steamer by incubation in 1×saline–sodium citrate (pH 6.0) buffer for 20 min. Sections were then cooled to room temperature and rinsed in PBS containing 0.1% Tween-20 (PBS-T). Blocking was performed in PBS containing 10% superblock, 10% FBS, 0.2% fish gelatin, and 0.02% sodium-azide. Tissue sections were incubated in primary antibody (mouse anti-αSMA, 1:200 dilution) or PBS (negative control) overnight at 4°C. Sections were washed twice in PBS-T and once in PBS and incubated with secondary antibody (goat anti-mouse DyLight 488 nm, 1:500 dilution) for 45 min at room temperature. All antibodies were prepared in blocking buffer. Sections were washed twice in PBS-T and once in PBS, and nuclei were counterstained with DAPI for 3 min at room temperature, rinsed in deionized water, and mounted using glass coverslips with Mowiol with DABCO.
For both cultures and tissue sections, images were obtained using a Zeiss Axiovert 200M inverted epifluorescent microscope (Zeiss AG). Exposure times were consistent for all samples.
Statistics
Pairwise testing between samples was performed using Student's t-test in SigmaPlot 11.0 (Systat). Pairwise significance is denoted with *, **, *** (P<0.05; P<0.01; P<0.001). In the case of myoc and angptl7 expression data, ### indicates the difference from the HTM control to the P<0.001 significance level. In the AH-treated sox2 and pou5f1 expression data, ### indicates the difference from the hMSC control to the P<0.001 significance level. For the immunosuppression data, pairwise comparisons were also performed using Student's t-test, and results are indicated as mean±standard error in the text.
Results
Characterization of HTM cell surface markers
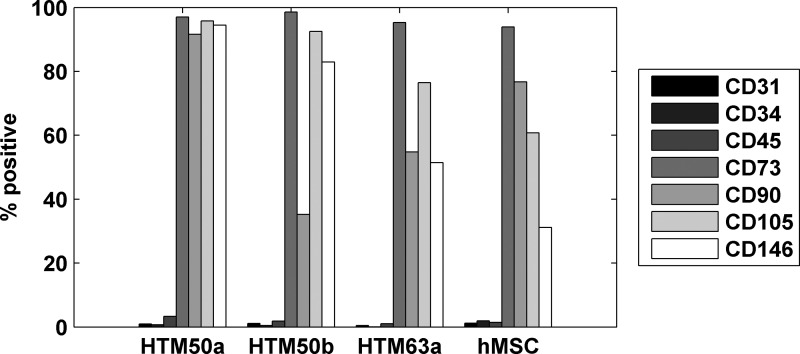
We examined the HTM cells for the expression of surfaces markers that are phenotypically characteristic of MSCs to compare the 2 cell populations. We chose 3 negative markers (CD31, CD34, and CD45) and 4 positive markers (CD73, CD90, CD105, and CD146) of hMSCs and used flow cytometry to determine the percent of HTM cells that expressed the markers. Surprisingly, HTM cells exhibited surface marker expression remarkably consistent with the hMSCs run as a positive control. As shown in Fig. 1, 3 separate HTM isolations (50A, 50B, and 63A) uniformly lacked the negative markers while expressing high levels of the positive markers when compared to adipose-derived hMSCs.
FIG. 1.
Human trabecular meshwork (HTM) cells express surface markers consistent with human mesenchymal stem cells (hMSCs). Donor cells extracted from 3 meshworks (HTM 50a, 50b, and 63a; n=3) all expressed positive markers of hMSCs (CD73, CD90, CD105, CD146) and did not express negative markers (CD31, CD34, CD45). This was consistent with the positive control of an adipose-derived hMSC (hMSC; n=1).
Expression of stem cell signaling molecules
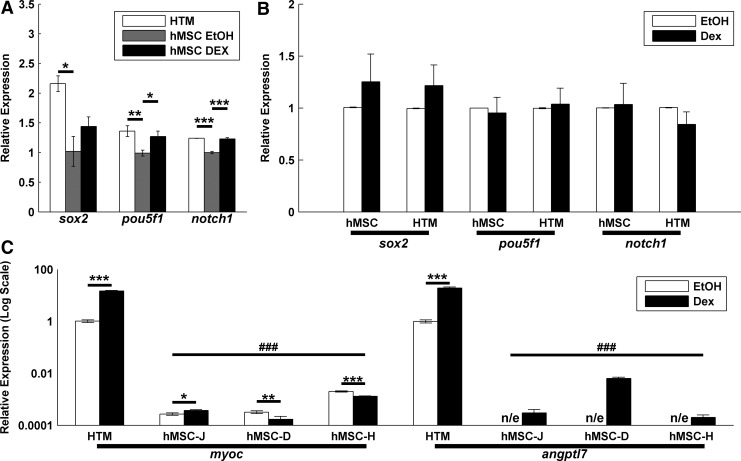
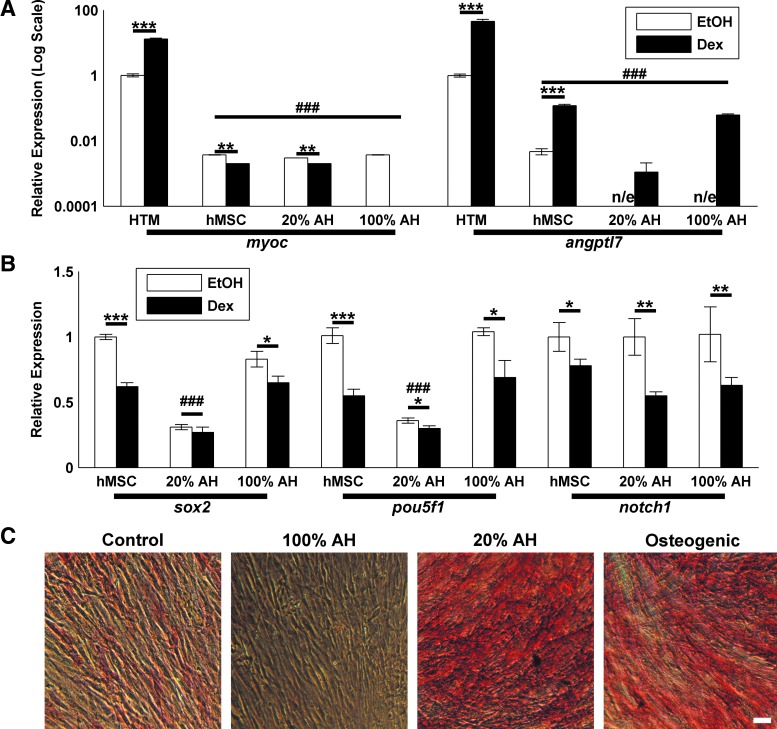
Given the similarity between HTM and hMSC surface protein expression, we then determined if they also expressed stem cell-related genes. We chose 2 known transcription factors important in maintaining pluripotency, sox2 and pou5f1.56,57 We also chose notch1 as an important receptor in differentiation and lineage commitment.58–60 Again, we used hMSCs as a positive control. As we wished to compare gene expression during typical culture, we cultured HTM cells in DMEM/F12 and hMSCs in DMEM (low glucose), as is routine practice in our laboratory. We observed equivalent or higher expression at the message level in HTM cells compared to hMSC cells (n=6 and n=3, respectively), representative data shown in Fig. 2A. Additionally, as Dex is used to induce differentiation in hMSCs,45,46 we treated cells with 10−7 M Dex or vehicle control (EtOH) for 3 days. Although we observed small, but significant, increases in pou5f1 and sox2 with Dex in a single hMSC line (Fig. 2A), we did not observe a consistent effect of Dex in 3 hMSC lines and 6 HTM lines (Fig. 2B). Dex is also known to induce the expression of myoc and angptl7 in HTM cells31,32 and has recently been reported to upregulate myoc in hMSCs.44 As shown in Fig. 2C, HTM cells respond with a dramatic upregulation of both genes while hMSCs have lower basal levels of expression. All hMSCs exhibited an increase in angptl7; however, only 1 of the 3 hMSC lines increased myoc expression.
FIG. 2.
HTMs and hMSCs have similar expression of self-renewal transcription factors but differ in Dex responsiveness. (A) When compared to hMSCs, HTMs express equivalent or higher levels of the self-renewal/pluripotency genes sox2 and pouf5f1. They additionally express similar levels of notch1, a transmembrane receptor known to be expressed by hMSCs and important in differentiation (n=3; representative results shown). (B) The expression of these proteins is not influenced by 10−7 M dexamethasone (Dex) treatment when compared to the vehicle control (EtOH). Data mean±standard error of the mean of cells from 3 (hMSC) and 6 (HTM) donors. (C) hMSCs (donors J, D, and H) myoc and angptl7 expression is far lower than HTM cells and lacks the consistent and robust HTM response to Dex. Significance between EtOH/Dex indicated with *, **, ***. Significance between HTM/hMSC indicated with ###. “n/e” indicates no reliable detectable expression.
Expression of OCT4A protein in HTM cells
As pou5f1 is known to produce 2 gene products that can be difficult to differentiate at the message level, commonly referred to as OCT4A and OCT4B,61 we wished to determine if HTM cells expressed the pluripotency-associated isoform, OCT4A. We used an antibody for OCT4A that is nonreactive with OCT4B, and as a positive control we used F9 embryonal carcinoma cell lysate. We observed positive OCT4A immunostaining at the appropriate molecular weight in 4 HTM cultures, although weaker than the F9 lysate control. A representative western blot including the loading control HSP90 is shown in Fig. 3.
FIG. 3.
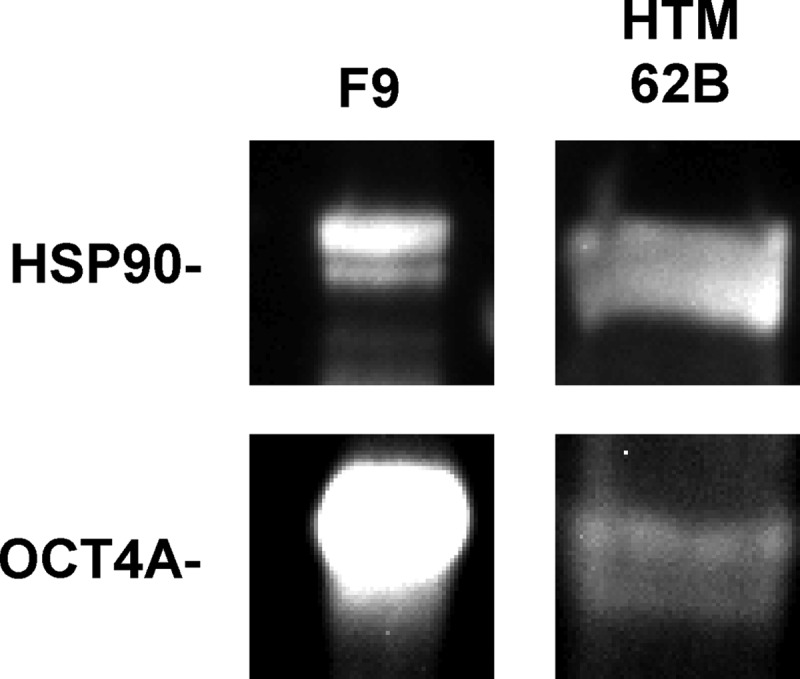
HTMs express low levels of the OCT4A isoform. When compared to the highly expressing F9 embryonal carcinoma cell line, HTM cells exhibit distinct, albeit limited, immunostaining at the appropriate molecular weight. For clarity of the HTM band, the F9 control was overexposed. Heat shock protein-90 (HSP90) used as a loading control. Blot representative of 4 HTM cultures.
Immunomodulatory characteristics of HTM cells
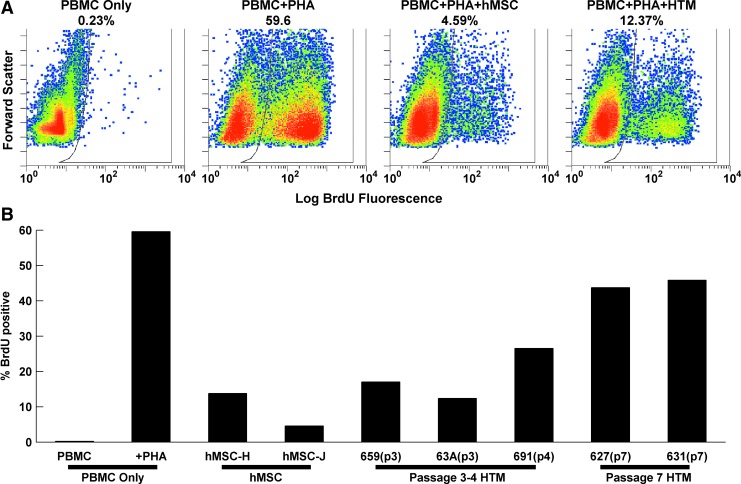
hMSCs are well recognized for possessing immunomodulatory properties.43 To test the extent with which HTM cells also possessed this property, we cocultured HTM cells with human enriched T cells activated with PHA. In T-cell-only controls, PHA resulted in a dramatic increase in BrdU-positive cells (0.23–59.6%; Fig. 4), which was effectively repressed by coculture with 2 separate hMSC lines (9.2±4.6%). HTM cells revealed similar immunomodulatory behaviors to the hMSCs, although it was highly passage dependent. Low-passage (passage 3–4) HTM cultures suppressed T-cell proliferation (18.7±4.2%) to a similar extent as hMSCs (P=0.23). However, later passage HTM cultures (passage 7) inhibited T-cell proliferation (44.8±1.0%) to a much lower degree compared to earlier passages (P=0.0172).
FIG. 4.
HTM cells have a passage-dependent immunomodulatory effect. (A) Log BrdU fluorescence (horizontal axis) and forward scatter (vertical axis) flow cytometry data. T cells in monoculture have limited proliferation without phytohemagglutinin (PHA) and substantial proliferation with PHA. The effect of PHA is inhibited by coculture with hMSCs or HTM cells. (B) Quantification of BrdU-positive cells for the different experimental conditions. HTM cells exhibited a passage-dependent immunomodulatory effect. Color images available online at www.liebertpub.com/jop
Differentiation assays
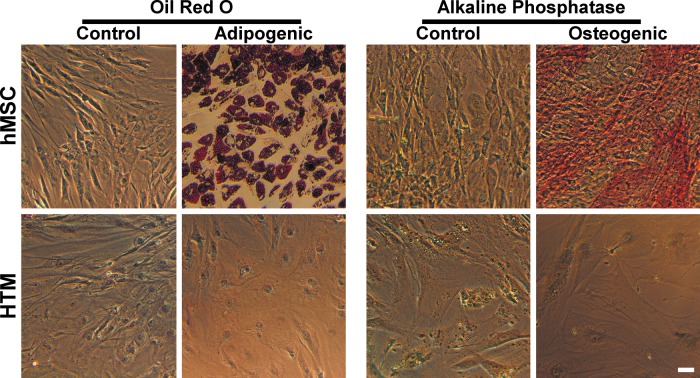
One of the most important characteristics of hMSCs is their multipotency,42,43 or ability to differentiate into distinct cell types, including adipocytes and osteocytes. We wished to determine if explant HTM cultures could likewise differentiate, similar to a previous report.47 To this end, we maintained HTM and hMSC cells in adipogenic or osteogenic media for 19 days. The cells were then fixed and stained for lipid content or alkaline phosphatase activity, respectively. hMSCs (n=3) demonstrated altered morphology and positive staining consistent with differentiation into adipocytes and osteocytes (Fig. 5). HTM cells (n=3) exhibited minor morphological changes in the differentiation media compared to control media but were negative for lipid or alkaline phosphatase activity. This demonstrates that they were either already committed to the HTM lineage or that their potential differentiation fates do not include adipose and bone.
FIG. 5.
HTM cells do not differentiate into adipocytes or osteocytes. HTM cells and hMSCs were exposed to adipogenic and osteogenic media for 19 days. Adipogenic and osteogenic differentiation of the hMSCs was indicated by the presence of lipids and alkaline phosphatase, respectively. HTMs did not stain for lipids or alkaline phosphatase with or without differentiation stimulus. Scale bar is 50 μm. Color images available online at www.liebertpub.com/jop
To test the capacity of hMSCs to differentiate into HTM cells, we cultured hMSCs in AH. AH has previously been shown to induce myoc expression and secretion62,63 and to differentiate TM-derived stem cells (TMSC) into HTM cells.36 We used a 3-day Dex induction of myoc and angptl7 expression as markers for successful HTM differentiation, as these are known characteristics of HTM cells, and hMSCs do not have this response in our experiments (Fig. 2). At 14 days, with Dex treatment for the final 3 days, both 20% and 100% AH treatments did not increase the basal level of myoc or angptl7 expression to HTM levels (Fig. 6A). There was no detectable basal angptl7 expression in the AH cultured samples. Likewise, there was no increase of myoc after Dex treatment. Expression of angptl7 did increase after Dex treatment; however, this behavior was not influenced by AH culture.
FIG. 6.
Aqueous humor does not differentiate hMSCs into HTM cells. (A) Expression of myoc and angptl7 mRNA remained lower than control HTM cells. Additionally, there was a limited and inconsistent response to 100 nM Dex. (B) Pluripotency factors sox2 and pou5f1 were significantly decreased by culture in 20% bovine aqueous humor (AH) but not 100% AH. Expression of notch1 was unaffected by either media. In all cases, 100 nM Dex treatment reduced the expression of sox2 in control media and 100% AH, and notch1 and pou5f1. Significance between EtOH/Dex indicated with *, **, ***. Significant difference from HTM control (for myoc/angptl7) or from control hMSC (for sox2/pou5f1) indicated with ###. “n/e” indicates no detectable expression. (C) Alkaline phosphatase staining of hMSCs. Minimal staining is observed in control or 100% AH cultured cells, but prominent staining is observed in 20% AH and osteogenic cultures. Scale bar is 50 μm. Color images available online at www.liebertpub.com/jop
We did observe altered morphology of the AH-treated hMSCs, suggesting that they may have differentiated. We assayed for the expression of pluripotency markers pou5f1 and sox2 and found them to be significantly decreased after culture in 20% AH, regardless of Dex treatment (Fig. 6B). 100% AH treatment did not alter the expression of either gene. We also observed a Dex-dependent decrease of pou5f1 and sox2 and notch1 in most cases. As sox2 is known to negatively regulate osteogenic differentiation of MSCs64–66 and is decreased during osteogenesis,66 we wished to test whether AH had increased the osteogenic potential. We again cultured hMSCs (n=3) in AH for 11 days before assaying for alkaline phosphatase activity. As a positive control, we additionally cultured cells in osteogenic media. 20% AH, but not 100% AH, resulted in a dramatic increase in alkaline phosphatase activity, comparable with the osteogenic media (Fig. 6C). Similarly, cultured HTM cells (n=2) did not exhibit alkaline phosphatase activity and remained Dex responsive (not shown).
Expression and localization of SMA
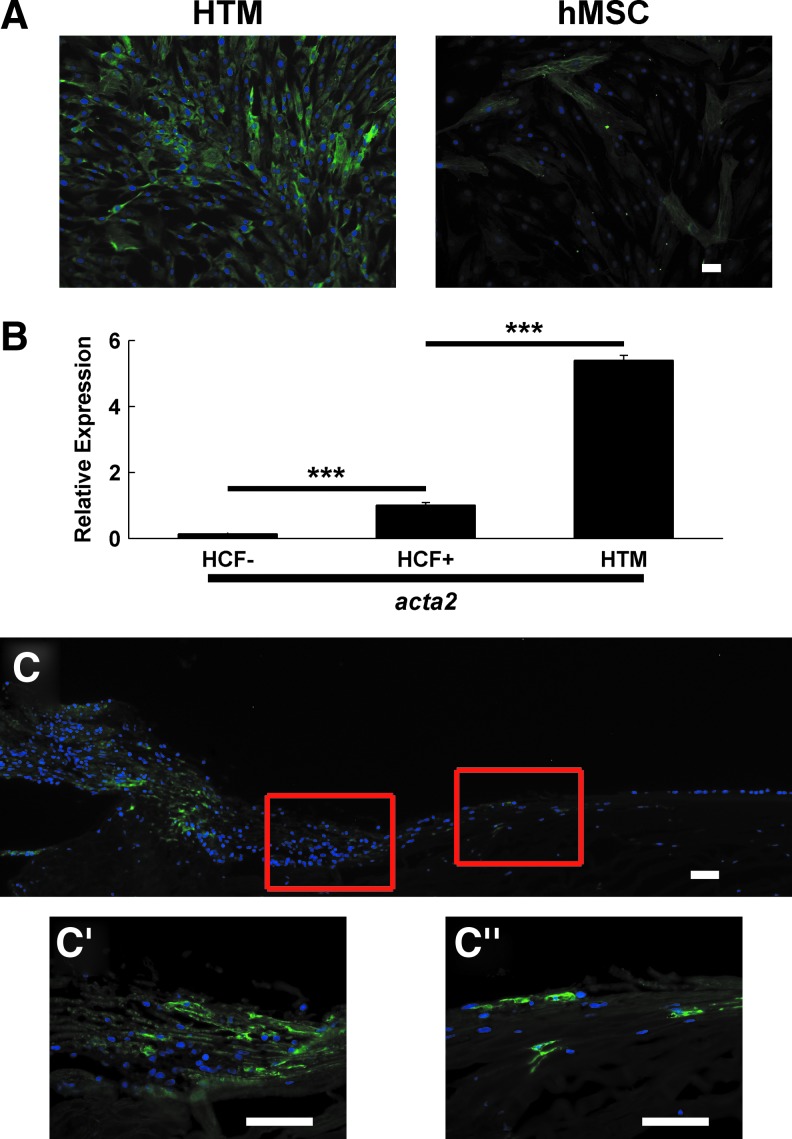
As αSMA is considered a marker for HTM24–26 but is not broadly expressed in undifferentiated adipose-derived hMSCs,67–70 we then determined the expression of αSMA in these cells. Cultured HTM cells were strongly positive for αSMA, whereas only a subpopulation of hMSCs were positive (n=3; representative results shown in Fig. 7A). To further characterize the expression level of αSMA in HTM explant cultures, we compared acta2 (αSMA) mRNA expression in HTM cells to a known strongly expressing cell type, immortalized corneal fibroblasts (HCFs) treated with 2 ng/mL TGFβ for 48 h.51 HTM cells displayed comparable expression to the myofibroblasts, further demonstrating their robust expression (n=2; representative results shown in Fig. 7B). As previous reports have identified a heterogeneous distribution of αSMA-positive cells in the TM,25,26 we wished to compare our in vitro αSMA staining with cells in HTM tissue using immunohistochemistry. We stained a donor corneoscleral rim for αSMA (n=1; Fig. 7C). Consistent with previous results, there was prominent immunoreactivity in the ciliary body and posterior meshwork, with weak staining throughout the remainder of the TM. Staining increased in the anterior meshwork (Fig. 7C′), and isolated cells in the insert region were brightly labeled (Fig. 7C′′).
FIG. 7.
HTM cells express α-smooth muscle actin (αSMA). (A) Fresh HTM explant cultures stain strongly positive for αSMA, whereas hMSCs have sparse immunoreactivity. (B) HTM explant cultures have higher mRNA expression than transforming growth factor-beta-treated human corneal fibroblasts (HCF+). Untreated human corneal fibroblasts are used as a negative control (HCF−). Significance between groups indicated with ***. (C) The HTM stains weakly positive for αSMA overall. Ciliary muscle exhibits strong immunoreactivity, which is diminished in the posterior meshwork, but the anterior meshwork (C′) and the insert region (C′′) display individually strongly immunoreactive cells. Scale bars are 50 μm. Color images available online at www.liebertpub.com/jop
Discussion
Results from the studies performed are consistent with previous reports identifying stem-like properties among cells resident in the HTM.11,36,37,47 These properties include a surface antigen signature consistent with hMSCs and high expression of the pluripotency and self-renewal markers pou5f1 and sox2. Importantly, and similar to hMSCs, HTM cells also display potent and passage-dependent immunosuppressive behavior. Although sharing these important attributes with hMSCs, HTM cells failed to differentiate along known hMSC lineages when given the appropriate culture environment. In aggregate, these findings suggest either terminal differentiation of the HTM cells or that HTM cells possess distinct potential lineages from hMSCs. To further explore the cell fate relationships between hMSCs and HTM cells, we attempted to differentiate hMSCs using AH and found that AH promoted osteogenic, but not trabecular, potential. As an additional characterization of HTM cells, we found them to strongly express αSMA. This further distinguishes them from hMSCs and is suggestive of derivation from the anterior nonfiltering meshwork and insert region near Schwalbe's line, thought to harbor the HTM progenitor populations.
A primary objective of this study was to broaden the known phenotype of HTM cells to better enable their isolation and identification. Cells isolated from the HTM possess a similar surface antigen signature to hMSCs (Fig. 1), consistent with a previous report.47 We note that our study used a different HTM isolation and culture method yet yielded similar results. An important caveat is that this surface antigen signature, while meeting the current consensus on hMSC markers,42,43 is not unique.40 The verification of the hMSC identity requires this surface antigen signature combined with a trilineage potential when provided the appropriate culture environment, a condition that HTM cells did not meet. To further compare these 2 cell types, we used a known behavior of HTM cells, that is, the upregulation of myoc and angptl7 in response to Dex. HTM cells had high basal expression of myoc and angptl7 and additionally exhibited strong upregulation of these proteins by Dex treatment, whereas hMSCs had a significantly lower basal expression of these genes and inconsistent Dex-responsiveness (Fig. 2C). These results provide both a surface antigen signature of HTM cells and a crucial distinction between the phenotypically similar HTM cells and adipose-derived hMSCs. We note, however, a recent publication reports myoc Dex-responsiveness in hMSCs,44 a finding not congruous with our studies. The discrepancy in results may be due to the length of Dex exposure (we used 3-day exposure, whereas Kwon et al.44 observed a Dex response at 4 days, but no response at 2 days, and tests at day 3 were not reported), differences between message and protein (we assayed mRNA, whereas Kwon et al. used western blotting), or the source of hMSCs (adipose vs. bone marrow derived).
In addition to the similarity in surface antigens between hMSCs and HTM cells, there are other indications of potential stemness of HTM cells. They also expressed pou5f1, sox2, and notch1 at similar levels to hMSCs (Fig. 2A). It is important to note that we were attempting to assay the gene expression under standard culture conditions, and thus, the hMSCs and HTM cells were cultured in DMEM (low glucose) and DMEM/F12, respectively. The different formulations (especially glucose levels) could certainly influence gene expression, but the data are representative of the gene expression during routine culture. These data are suggestive of the self-renewal capability and pluripotency,71,72 but there are several important caveats. There are 2 gene products of pou5f1 and only one has been linked to pluripotency, and the expression of pou5f1 in hMSCs is currently under debate.61 While we observed the expression of the pluripotency-linked pou5f1 gene product OCT4A at the protein level, the expression was far lower than a known positive cell line (Fig. 3). This could be explained by low expression or expression limited to a subpopulation of cells similar to those observed by Du et al.36 Expression of sox2 is considered a key factor in self-renewal and pluripotency in embryonic stem cells,71,72 the maintenance of adult stem cell/progenitor populations73,74 and inducing pluripotency in induced pluripotent stem cells.75,76 However, sox2 has been implicated in additional roles in differentiation with a recent report identifying the importance of sox2 expression in hMSC osteo/adipo lineage selection.66 That study found that sox2 inhibited osteogenesis through Wnt antagonism and that both differentiated adipocytes and adipose tissue maintained high levels of sox2 expression. Taken together, these data document roles beyond self-renewal, and further studies will be required to detail the specific role of sox2 in the HTM.
Immune modulation is another important characteristic of MSCs, although it is shared by other cell types, including fibroblasts.77 To the best of our knowledge, our results are the first to document HTM cells to have immunosuppressive attributes. We found early passage HTM cells inhibit lymphocyte proliferation comparable to hMSCs (Fig. 4). The functional ramifications of this in vivo remain to be explored, but this finding does fit into the larger picture of immune function of the eye. Rat TM has been reported to host dendritic cells and potential antigen-presenting cells.78 HTM cells have previously been reported to express major histocompatibility complex (MHC) proteins79,80 and have been speculated to play a role in anterior segment-associated immune deviation,80,81 an intricate system where antigens presented in the eye result in systemic suppression of immune response to that antigen.81,82 Additionally, the expression of MHC proteins in equine TM cells is increased in equine recurrent uveitis,83 suggesting that the TM is responsive, if not contributory, to the immune status of eye. Especially relevant to the current study, AH has been identified as a key component of the immunosuppressive environment of the eye and specifically has been found to limit immune cell proliferation and activity.84–90 Taken together with the results of this study, these data suggest that HTM cells may secrete soluble factors that contribute to the immunosuppressive nature of AH and to the immune privilege of the eye as a whole.
We additionally observed decreased immune suppression in 2 extended passage HTM cultures, which is consistent with a previous report concerning hMSCs.91 In the study by Liu and colleagues, changing cytokine activity in the media accounted for the difference. It is possible that HTM cells likewise secrete a changing profile of cytokines with increased passage. As there is also a loss of differentiation capacity with extended culture,92 it is possible that loss of immune suppression is linked to a loss of stemness. As hMSCs in that report tolerated several more passages than the HTM cells in this study, this may suggest that HTM cells are isolated in a partially or totally differentiated state, although other variables, such as donor age, could also explain the differences.
Our results also differ from previous literature concerning stem-like cells in the HTM, leading to a complex intersection of stem-like and HTM-like behaviors. Multipotency is one of the most important hMSC characteristics and has been reported in both the TMSCs of Du et al.36,37 and the HTM-MSCs of Tay et al.47 Our isolates, however, lacked adipogenic and osteogenic capacity (Fig. 5), confirming that they are distinct from hMSCs. Both TMSCs and HTM-MSCs possess multipotency, whereas the cells described herein did not. Conversely, these cells and HTM-MSCs possess HTM markers (eg, myoc, angptl7, chi3l1), whereas TMSCs do not.
Although HTM cells were clearly not multipotent hMSCs, we were struck by the numerous similarities described above and hypothesized that hMSCs may have a potential HTM differentiation pathway. As Du et al.36 has shown that TMSCs differentiate to TM cells in AH, we attempted a similar experiment with hMSCs. Our preliminary assay for HTM, myoc/angptl7 upregulation in response to Dex revealed that the hMSCs had not differentiated into HTM (Fig. 6). However, AH did potently increase alkaline phosphatase activity in hMSCs, but only when combined with supplemented media (Fig. 6). Although we observed robust cell growth in pure AH, aqueous is nutrient poor and unbuffered compared to supplemented media. A likely possibility is that culturing in pure aqueous prevents differentiation through starvation, pH imbalance, or the lack of key growth factors provided by serum. Although further work is needed to fully characterize the components of aqueous responsible for differentiation, one likely candidate is myocilin, which is a component of aqueous93,94 and has been recently identified as a stimulant of osteogenesis of hMSCs.44
As a final characterization of the HTM explant cultures, we stained passage 0 HTM cells and hMSCs for αSMA, finding uniformly strong immunolabeling in the HTM cells but only scattered reactivity in hMSCs (Fig. 7A). This is consistent with previous reports for both the HTM24–26 and hMSCs67–70 and serves as a further cellular attribute for differentiation between the cell types. Strikingly, HTM cells also expressed several times more acta2 than myofibroblasts, known to be strongly positive for αSMA.51 These data point to strong uniform expression in HTM cultures, but these results conflict with the focal immunoreactivity reported in tissue sections,25,26 which we also observed in a representative donor corneoscleral rim (Fig. 7C). While broader expression may be found in younger individuals,26 reports conflict on this,25 and our typical HTM cultures tend to be isolated from older donors. Taken together, these data point to 1 of the 2 possibilities. One, the expression of αSMA is induced during in vitro HTM culture, or two, explant culture selectively isolates the αSMA-positive cells. As αSMA-positive cells can be enriched in anterior meshwork26 (Fig. 7C′) and insert region (Fig. 7C′′), regions thought to contain a progenitor population capable of pou5f1 and sox2 expression,10–14 it may be possible that αSMA expression correlates with progenitor status in HTM cells. Of course, without better characterization of both progenitor and mature HTM populations, this is currently just a hypothesis.
In conclusion, the current study provides additional cellular attributes enabling the characterization of isolated TM cells through cell surface markers, cytoskeletal constituents, and transcription factor expression. We additionally reported for the first time potent immunomodulatory effects of HTM cells and the increase of alkaline phosphatase in hMSCs following AH treatment. These exciting findings open up new avenues of research in immune function and regenerative medicine of the eye.
Acknowledgments
This research was funded by grants from the National Institutes of Health (R01EY019475 and R01EY019970) and unrestricted funds from Research to Prevent Blindness.
Author Disclosure Statement
No competing financial interests exist.
References
- 1.Grierson I., and Howes R.C.Age-related depletion of the cell population in the human trabecular meshwork. Eye (Lond). 1 (Pt 2):204–210, 1987 [DOI] [PubMed] [Google Scholar]
- 2.Liton P.B., Challa P., Stinnett S., Luna C., Epstein D.L., and Gonzalez P.Cellular senescence in the glaucomatous outflow pathway. Exp. Gerontol. 40:745–748, 2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Alvarado J., Murphy C., and Juster R.Trabecular meshwork cellularity in primary open-angle glaucoma and nonglaucomatous normals. Ophthalmology 91:564–579, 1984 [DOI] [PubMed] [Google Scholar]
- 4.Alvarado J., Murphy C., Polansky J., and Juster R.Age-related changes in trabecular meshwork cellularity. Invest. Ophthalmol. Vis. Sci. 21:714–727, 1981 [PubMed] [Google Scholar]
- 5.Acott T.S., Samples J.R., Bradley J.M., Bacon D.R., Bylsma S.S., and Van Buskirk E.M.Trabecular repopulation by anterior trabecular meshwork cells after laser trabeculoplasty. Am. J. Ophthalmol. 107:1–6, 1989 [DOI] [PubMed] [Google Scholar]
- 6.Bylsma S.S., Samples J.R., Acott T.S., and Van Buskirk E.M.Trabecular cell division after argon laser trabeculoplasty. Arch. Ophthalmol. 106:544–547, 1988 [DOI] [PubMed] [Google Scholar]
- 7.Alexander R.A., and Grierson I.Morphological effects of argon laser trabeculoplasty upon the glaucomatous human meshwork. Eye (Lond). 3 (Pt 6):719–726, 1989 [DOI] [PubMed] [Google Scholar]
- 8.Dueker D.K., Norberg M., Johnson D.H., Tschumper R.C., and Feeney-Burns L.Stimulation of cell division by argon and Nd:YAG laser trabeculoplasty in cynomolgus monkeys. Invest. Ophthalmol. Vis. Sci. 31:115–124, 1990 [PubMed] [Google Scholar]
- 9.Johnson D.H., Richardson T.M., and Epstein D.L.Trabecular meshwork recovery after phagocytic challenge. Curr. Eye Res. 8:1121–1130, 1989 [DOI] [PubMed] [Google Scholar]
- 10.Kelley M.J., Rose A.Y., Keller K.E., Hessle H., Samples J.R., and Acott T.S.Stem cells in the trabecular meshwork: present and future promises. Exp. Eye Res. 88:747–751, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Gonzalez P., Epstein D.L., Luna C., and Liton P.B.Characterization of free-floating spheres from human trabecular meshwork (HTM) cell culture in vitro. Exp. Eye Res. 82:959–967, 2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Whikehart D.R., Parikh C.H., Vaughn A.V., Mishler K., and Edelhauser H.F.Evidence suggesting the existence of stem cells for the human corneal endothelium. Mol. Vis. 11:816–824, 2005 [PubMed] [Google Scholar]
- 13.McGowan S.L., Edelhauser H.F., Pfister R.R., and Whikehart D.R.Stem cell markers in the human posterior limbus and corneal endothelium of unwounded and wounded corneas. Mol. Vis. 13:1984–2000, 2007 [PubMed] [Google Scholar]
- 14.Yu W.Y., Sheridan C., Grierson I., Mason S., Kearns V., Lo A.C., and Wong D.Progenitors for the corneal endothelium and trabecular meshwork: a potential source for personalized stem cell therapy in corneal endothelial diseases and glaucoma. J. Biomed. Biotechnol. 2011:412743, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Samuelson D., Plummer C., Lewis P., and Gelatt K.Schwalbe line's cell in the normal and glaucomatous dog. Vet. Ophthalmol. 4:47–53, 2001 [DOI] [PubMed] [Google Scholar]
- 16.Polansky J.R., Fauss D.J., Chen P., Chen H., Lutjen-Drecoll E., Johnson D., Kurtz R.M., Ma Z.D., Bloom E., and Nguyen T.D.Cellular pharmacology and molecular biology of the trabecular meshwork inducible glucocorticoid response gene product. Ophthalmologica 211:126–139, 1997 [DOI] [PubMed] [Google Scholar]
- 17.Polansky J.R., Fauss D.J., and Zimmerman C.C.Regulation of TIGR/MYOC gene expression in human trabecular meshwork cells. Eye (Lond). 14 (Pt 3B):503–514, 2000 [DOI] [PubMed] [Google Scholar]
- 18.Tamm E.R., and Polansky J.R.The TIGR/MYOC gene and glaucoma: opportunities for new understandings. J. Glaucoma 10:S9–S12, 2001 [DOI] [PubMed] [Google Scholar]
- 19.Fingert J.H., Ying L., Swiderski R.E., Nystuen A.M., Arbour N.C., Alward W.L., Sheffield V.C., and Stone E.M.Characterization and comparison of the human and mouse GLC1A glaucoma genes. Genome Res. 8:377–384, 1998 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Kuchtey J., Kallberg M.E., Gelatt K.N., Rinkoski T., Komaromy A.M., and Kuchtey R.W.Angiopoietin-like 7 secretion is induced by glaucoma stimuli and its concentration is elevated in glaucomatous aqueous humor. Invest. Ophthalmol. Vis. Sci. 49:3438–3448, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Comes N., Buie L.K., and Borras T.Evidence for a role of angiopoietin-like 7 (ANGPTL7) in extracellular matrix formation of the human trabecular meshwork: implications for glaucoma. Genes Cells 16:243–259, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Raghunathan V.K., Morgan J.T., Dreier B., Reilly C.M., Thomasy S.M., Wood J.A., Ly I., Tuyen B.C., Hughbanks M., Murphy C.J., and Russell P.Role of substratum stiffness in modulating genes associated with extracellular matrix and mechanotransducers YAP and TAZ. Invest. Ophthalmol. Vis. Sci. 54:378–386, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Thomasy S.M., Wood J.A., Kass P.H., Murphy C.J., and Russell P.Substratum stiffness and latrunculin B regulate matrix gene and protein expression in human trabecular meshwork cells. Invest. Ophthalmol. Vis. Sci. 53:952–958, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Clark A.F., Wilson K., McCartney M.D., Miggans S.T., Kunkle M., and Howe W.Glucocorticoid-induced formation of cross-linked actin networks in cultured human trabecular meshwork cells. Invest. Ophthalmol. Vis. Sci. 35:281–294, 1994 [PubMed] [Google Scholar]
- 25.de Kater A.W., Shahsafaei A., and Epstein D.L.Localization of smooth muscle and nonmuscle actin isoforms in the human aqueous outflow pathway. Invest. Ophthalmol. Vis. Sci. 33:424–429, 1992 [PubMed] [Google Scholar]
- 26.Flügel C., Tamm E., Lütjen-Drecoll E., and Stefani F.H.Age-related loss of [alpha]-smooth muscle actin in normal and glaucomatous human trabecular mesh work of different age groups. J. Glaucoma 1:165–173, 1992 [Google Scholar]
- 27.Paylakhi S.H., Yazdani S., April C., Fan J.B., Moazzeni H., Ronaghi M., and Elahi E.Non-housekeeping genes expressed in human trabecular meshwork cell cultures. Mol. Vis. 18:241–254, 2012 [PMC free article] [PubMed] [Google Scholar]
- 28.Liton P.B., Luna C., Challa P., Epstein D.L., and Gonzalez P.Genome-wide expression profile of human trabecular meshwork cultured cells, nonglaucomatous and primary open angle glaucoma tissue. Mol. Vis. 12:774–790, 2006 [PMC free article] [PubMed] [Google Scholar]
- 29.Liton P.B., Liu X., Stamer W.D., Challa P., Epstein D.L., and Gonzalez P.Specific targeting of gene expression to a subset of human trabecular meshwork cells using the chitinase 3-like 1 promoter. Invest. Ophthalmol. Vis. Sci. 46:183–190, 2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Stamer W.D., Seftor R.E., Snyder R.W., and Regan J.W.Cultured human trabecular meshwork cells express aquaporin-1 water channels. Curr. Eye Res. 14:1095–1100, 1995 [DOI] [PubMed] [Google Scholar]
- 31.Rozsa F.W., Reed D.M., Scott K.M., Pawar H., Moroi S.E., Kijek T.G., Krafchak C.M., Othman M.I., Vollrath D., Elner V.M., and Richards J.E.Gene expression profile of human trabecular meshwork cells in response to long-term dexamethasone exposure. Mol. Vis. 12:125–141, 2006 [PubMed] [Google Scholar]
- 32.Nehme A., Lobenhofer E.K., Stamer W.D., and Edelman J.L.Glucocorticoids with different chemical structures but similar glucocorticoid receptor potency regulate subsets of common and unique genes in human trabecular meshwork cells. BMC Med. Genomics. 2:58, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Jensen J.B., and Parmar M.Strengths and limitations of the neurosphere culture system. Mol. Neurobiol. 34:153–161, 2006 [DOI] [PubMed] [Google Scholar]
- 34.Amano S., Yamagami S., Mimura T., Uchida S., and Yokoo S.Corneal stromal and endothelial cell precursors. Cornea 25:S73–S77, 2006 [DOI] [PubMed] [Google Scholar]
- 35.Shi X., Gipp J., and Bushman W.Anchorage-independent culture maintains prostate stem cells. Dev. Biol. 312:396–406, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Du Y., Roh D.S., Mann M.M., Funderburgh M.L., Funderburgh J.L., and Schuman J.S.Multipotent stem cells from trabecular meshwork become phagocytic TM cells. Invest. Ophthalmol. Vis. Sci. 53:1566–1575, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Du Y., Yun H., Yang E., and Schuman J.S.Stem cells from trabecular meshwork home to TM tissue in vivo. Invest. Ophthalmol. Vis. Sci. 54:1450–1459, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Voswinkel J., Francois S., Simon J.M., Benderitter M., Gorin N.C., Mohty M., Fouillard L., and Chapel A.Use of mesenchymal stem cells (MSC) in chronic inflammatory fistulizing and fibrotic diseases: a comprehensive review. Clin. Rev. Allergy Immunol. 45:180–192, 2013 [DOI] [PubMed] [Google Scholar]
- 39.Blasi A., Martino C., Balducci L., Saldarelli M., Soleti A., Navone S.E., Canzi L., Cristini S., Invernici G., Parati E.A., and Alessandri G.Dermal fibroblasts display similar phenotypic and differentiation capacity to fat-derived mesenchymal stem cells, but differ in anti-inflammatory and angiogenic potential. Vasc. Cell 3:5, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Kundrotas G.Surface markers distinguishing mesenchymal stem cells from fibroblasts. Acta Medica Lituanica 19:75–79, 2012 [Google Scholar]
- 41.Halfon S., Abramov N., Grinblat B., and Ginis I.Markers distinguishing mesenchymal stem cells from fibroblasts are downregulated with passaging. Stem Cells Dev. 20:53–66, 2011 [DOI] [PubMed] [Google Scholar]
- 42.Horwitz E.M., Le Blanc K., Dominici M., Mueller I., Slaper-Cortenbach I., Marini F.C., Deans R.J., Krause D.S., and Keating A.Clarification of the nomenclature for MSC: The International Society for Cellular Therapy position statement. Cytotherapy 7:393–395, 2005 [DOI] [PubMed] [Google Scholar]
- 43.Chamberlain G., Fox J., Ashton B., and Middleton J.Concise review: mesenchymal stem cells: their phenotype, differentiation capacity, immunological features, and potential for homing. Stem Cells 25:2739–2749, 2007 [DOI] [PubMed] [Google Scholar]
- 44.Kwon H.S., Johnson T.V., and Tomarev S.I.Myocilin stimulates osteogenic differentiation of mesenchymal stem cells through mitogen-activated protein kinase signaling. J. Biol. Chem. 288:16882–16894, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Guo L., Kawazoe N., Hoshiba T., Tateishi T., Chen G., and Zhang X.Osteogenic differentiation of human mesenchymal stem cells on chargeable polymer-modified surfaces. J. Biomed. Mater. Res. A 87:903–912, 2008 [DOI] [PubMed] [Google Scholar]
- 46.Herbertson A., and Aubin J.E.Dexamethasone alters the subpopulation make-up of rat bone marrow stromal cell cultures. J. Bone Miner. Res. 10:285–294, 1995 [DOI] [PubMed] [Google Scholar]
- 47.Tay C.Y., Sathiyanathan P., Chu S.W., Stanton L.W., and Wong T.T.Identification and characterization of mesenchymal stem cells derived from the trabecular meshwork of the human eye. Stem Cells Dev. 21:1381–1390, 2012 [DOI] [PubMed] [Google Scholar]
- 48.Toupadakis C.A., Wong A., Genetos D.C., Cheung W.K., Borjesson D.L., Ferraro G.L., Galuppo L.D., Leach J.K., Owens S.D., and Yellowley C.E.Comparison of the osteogenic potential of equine mesenchymal stem cells from bone marrow, adipose tissue, umbilical cord blood, and umbilical cord tissue. Am. J. Vet. Res. 71:1237–1245, 2010 [DOI] [PubMed] [Google Scholar]
- 49.Chung D.J., Hayashi K., Toupadakis C.A., Wong A., and Yellowley C.E.Osteogenic proliferation and differentiation of canine bone marrow and adipose tissue derived mesenchymal stromal cells and the influence of hypoxia. Res. Vet. Sci. 92:66–75, 2012 [DOI] [PubMed] [Google Scholar]
- 50.Wood J.A., Chung D.J., Park S.A., Zwingenberger A.L., Reilly C.M., Ly I., Walker N.J., Vernau W., Hayashi K., Wisner E.R., Cannon M.S., Kass P.H., Cherry S.R., Borjesson D.L., Russell P., and Murphy C.J.Periocular and intra-articular injection of canine adipose-derived mesenchymal stem cells: an in vivo imaging and migration study. J. Ocul. Pharmacol. Ther. 28:307–317, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Jester J.V., Huang J., Fisher S., Spiekerman J., Chang J.H., Wright W.E., and Shay J.W.Myofibroblast differentiation of normal human keratocytes and hTERT, extended-life human corneal fibroblasts. Invest. Ophthalmol. Vis. Sci. 44:1850–1858, 2003 [DOI] [PubMed] [Google Scholar]
- 52.Carrade D.D., Owens S.D., Galuppo L.D., Vidal M.A., Ferraro G.L., Librach F., Buerchler S., Friedman M.S., Walker N.J., and Borjesson D.L.Clinicopathologic findings following intra-articular injection of autologous and allogeneic placentally derived equine mesenchymal stem cells in horses. Cytotherapy 13:419–430, 2011 [DOI] [PubMed] [Google Scholar]
- 53.Wohler J.E., and Barnum S.R.Nylon wool purification alters the activation of T cells. Mol. Immunol. 46:1007–1010, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Julius M.H., Simpson E., and Herzenberg L.A.A rapid method for the isolation of functional thymus-derived murine lymphocytes. Eur. J. Immunol. 3:645–649, 1973 [DOI] [PubMed] [Google Scholar]
- 55.Eisen S.A., Wedner H.J., and Parker C.W.Isolation of pure human peripheral blood T-lymphocytes using nylon wool columns. Immunol. Commun. 1:571–577, 1972 [DOI] [PubMed] [Google Scholar]
- 56.Tai M.H., Chang C.C., Kiupel M., Webster J.D., Olson L.K., and Trosko J.E.Oct4 expression in adult human stem cells: evidence in support of the stem cell theory of carcinogenesis. Carcinogenesis 26:495–502, 2005 [DOI] [PubMed] [Google Scholar]
- 57.Wei X., and Shen C.Y.Transcriptional regulation of oct4 in human bone marrow mesenchymal stem cells. Stem Cells Dev. 20:441–449, 2011 [DOI] [PubMed] [Google Scholar]
- 58.Li H., Yu B., Zhang Y., Pan Z., and Xu W.Jagged1 protein enhances the differentiation of mesenchymal stem cells into cardiomyocytes. Biochem. Biophys. Res. Commun. 341:320–325, 2006 [DOI] [PubMed] [Google Scholar]
- 59.Xu N., Liu H., Qu F., Fan J., Mao K., Yin Y., Liu J., Geng Z., and Wang Y.Hypoxia inhibits the differentiation of mesenchymal stem cells into osteoblasts by activation of Notch signaling. Exp. Mol. Pathol. 94:33–39, 2013 [DOI] [PubMed] [Google Scholar]
- 60.Yamamizu K., Matsunaga T., Uosaki H., Fukushima H., Katayama S., Hiraoka-Kanie M., Mitani K., and Yamashita J.K.Convergence of Notch and beta-catenin signaling induces arterial fate in vascular progenitors. J. Cell Biol. 189:325–338, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Mueller T., Luetzkendorf J., Nerger K., Schmoll H.J., and Mueller L.P.Analysis of OCT4 expression in an extended panel of human tumor cell lines from multiple entities and in human mesenchymal stem cells. Cell Mol. Life Sci. 66:495–503, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Resch Z.T., Hann C.R., Cook K.A., and Fautsch M.P.Aqueous humor rapidly stimulates myocilin secretion from human trabecular meshwork cells. Exp. Eye Res. 91:901–908, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Fautsch M.P., Howell K.G., Vrabel A.M., Charlesworth M.C., Muddiman D.C., and Johnson D.H.Primary trabecular meshwork cells incubated in human aqueous humor differ from cells incubated in serum supplements. Invest. Ophthalmol. Vis. Sci. 46:2848–2856, 2005 [DOI] [PubMed] [Google Scholar]
- 64.Basu-Roy U., Ambrosetti D., Favaro R., Nicolis S.K., Mansukhani A., and Basilico C.The transcription factor Sox2 is required for osteoblast self-renewal. Cell Death Differ. 17:1345–1353, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Seo E., Basu-Roy U., Zavadil J., Basilico C., and Mansukhani A.Distinct functions of Sox2 control self-renewal and differentiation in the osteoblast lineage. Mol. Cell Biol. 31:4593–4608, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Seo E., Basu-Roy U., Gunaratne P.H., Coarfa C., Lim D.S., Basilico C., and Mansukhani A.SOX2 regulates YAP1 to maintain stemness and determine cell fate in the osteo-adipo lineage. Cell Rep. 3:2075–2087, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Yun D.H., Song H.Y., Lee M.J., Kim M.R., Kim M.Y., Lee J.S., and Kim J.H.Thromboxane A(2) modulates migration, proliferation, and differentiation of adipose tissue-derived mesenchymal stem cells. Exp. Mol Med. 41:17–24, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Jeon E.S., Park W.S., Lee M.J., Kim Y.M., Han J., and Kim J.H.A Rho kinase/myocardin-related transcription factor-A-dependent mechanism underlies the sphingosylphosphorylcholine-induced differentiation of mesenchymal stem cells into contractile smooth muscle cells. Circ. Res. 103:635–642, 2008 [DOI] [PubMed] [Google Scholar]
- 69.Jeon E.S., Moon H.J., Lee M.J., Song H.Y., Kim Y.M., Bae Y.C., Jung J.S., and Kim J.H.Sphingosylphosphorylcholine induces differentiation of human mesenchymal stem cells into smooth-muscle-like cells through a TGF-beta-dependent mechanism. J. Cell Sci. 119:4994–5005, 2006 [DOI] [PubMed] [Google Scholar]
- 70.Park W.S., Heo S.C., Jeon E.S., Hong D.H., Son Y.K., Ko J.H., Kim H.K., Lee S.Y., Kim J.H., and Han J.Functional expression of smooth muscle-specific ion channels in TGF-beta1-treated human adipose-derived mesenchymal stem cells. Am. J. Physiol. Cell Physiol. 305:C377–391, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Niwa H.How is pluripotency determined and maintained? Development 134:635–646, 2007 [DOI] [PubMed] [Google Scholar]
- 72.Masui S., Nakatake Y., Toyooka Y., Shimosato D., Yagi R., Takahashi K., Okochi H., Okuda A., Matoba R., Sharov A.A., Ko M.S., and Niwa H.Pluripotency governed by Sox2 via regulation of Oct3/4 expression in mouse embryonic stem cells. Nat. Cell Biol. 9:625–635, 2007 [DOI] [PubMed] [Google Scholar]
- 73.Pevny L.H., and Nicolis S.K.Sox2 roles in neural stem cells. Int. J. Biochem. Cell Biol. 42:421–424, 2010 [DOI] [PubMed] [Google Scholar]
- 74.Arnold K., Sarkar A., Yram M.A., Polo J.M., Bronson R., Sengupta S., Seandel M., Geijsen N., and Hochedlinger K.Sox2(+) adult stem and progenitor cells are important for tissue regeneration and survival of mice. Cell Stem Cell 9:317–329, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Takahashi K., and Yamanaka S.Induction of pluripotent stem cells from mouse embryonic and adult fibroblast cultures by defined factors. Cell 126:663–676, 2006 [DOI] [PubMed] [Google Scholar]
- 76.Yoshioka N., Gros E., Li H.R., Kumar S., Deacon D.C., Maron C., Muotri A.R., Chi N.C., Fu X.D., Yu B.D., and Dowdy S.F.Efficient generation of human iPSCs by a synthetic self-replicative RNA. Cell Stem Cell 13:246–254, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Haniffa M.A., Collin M.P., Buckley C.D., and Dazzi F.Mesenchymal stem cells: the fibroblasts' new clothes? Haematologica 94:258–263, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.McMenamin P.G., and Holthouse I.Immunohistochemical characterization of dendritic cells and macrophages in the aqueous outflow pathways of the rat eye. Exp. Eye Res. 55:315–324, 1992 [DOI] [PubMed] [Google Scholar]
- 79.Tripathi B.J., Tripathi R.C., Wong P., and Raja S.Expression of HLA by the human trabecular meshwork and corneal endothelium. Exp. Eye Res. 51:269–276, 1990 [DOI] [PubMed] [Google Scholar]
- 80.Latina M., Flotte T., Crean E., Sherwood M.E., and Granstein R.D.Immunohistochemical staining of the human anterior segment. Evidence that resident cells play a role in immunologic responses. Arch. Ophthalmol. 106:95–99, 1988 [DOI] [PubMed] [Google Scholar]
- 81.Streilein J.W.Ocular immune privilege: therapeutic opportunities from an experiment of nature. Nat. Rev. Immunol. 3:879–889, 2003 [DOI] [PubMed] [Google Scholar]
- 82.Biros D.Anterior chamber-associated immune deviation. Vet. Clin. North Am Small Anim. Pract. 38:309–321, vi–vii, 2008 [DOI] [PubMed] [Google Scholar]
- 83.Romeike A., Brugmann M., and Drommer W.Immunohistochemical studies in equine recurrent uveitis (ERU). Vet. Pathol. 35:515–526, 1998 [DOI] [PubMed] [Google Scholar]
- 84.Taylor A.W., Streilein J.W., and Cousins S.W.Immunoreactive vasoactive intestinal peptide contributes to the immunosuppressive activity of normal aqueous humor. J. Immunol. 153:1080–1086, 1994 [PubMed] [Google Scholar]
- 85.Kaiser C.J., Ksander B.R., and Streilein J.W.Inhibition of lymphocyte proliferation by aqueous humor. Reg. Immunol. 2:42–49, 1989 [PubMed] [Google Scholar]
- 86.Taylor A.W., and Yee D.G.Somatostatin is an immunosuppressive factor in aqueous humor. Invest. Ophthalmol. Vis. Sci. 44:2644–2649, 2003 [DOI] [PubMed] [Google Scholar]
- 87.Taylor A.W., Yee D.G., and Streilein J.W.Suppression of nitric oxide generated by inflammatory macrophages by calcitonin gene-related peptide in aqueous humor. Invest. Ophthalmol. Vis. Sci. 39:1372–1378, 1998 [PubMed] [Google Scholar]
- 88.Apte R.S., Sinha D., Mayhew E., Wistow G.J., and Niederkorn J.Y.Cutting edge: role of macrophage migration inhibitory factor in inhibiting NK cell activity and preserving immune privilege. J. Immunol. 160:5693–5696, 1998 [PubMed] [Google Scholar]
- 89.Namba K., Kitaichi N., Nishida T., and Taylor A.W.Induction of regulatory T cells by the immunomodulating cytokines alpha-melanocyte-stimulating hormone and transforming growth factor-beta2. J. Leukoc. Biol. 72:946–952, 2002 [PubMed] [Google Scholar]
- 90.Taylor A.W., Streilein J.W., and Cousins S.W.Identification of alpha-melanocyte stimulating hormone as a potential immunosuppressive factor in aqueous humor. Curr. Eye Res. 11:1199–1206, 1992 [DOI] [PubMed] [Google Scholar]
- 91.Liu H., Lu K., MacAry P.A., Wong K.L., Heng A., Cao T., and Kemeny D.M.Soluble molecules are key in maintaining the immunomodulatory activity of murine mesenchymal stromal cells. J. Cell Sci. 125:200–208, 2012 [DOI] [PubMed] [Google Scholar]
- 92.Kretlow J.D., Jin Y.Q., Liu W., Zhang W.J., Hong T.H., Zhou G., Baggett L.S., Mikos A.G., and Cao Y.Donor age and cell passage affects differentiation potential of murine bone marrow-derived stem cells. BMC Cell Biol. 9:60, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Rao P.V., Allingham R.R., and Epstein D.L.TIGR/myocilin in human aqueous humor. Exp. Eye Res. 71:637–641, 2000 [DOI] [PubMed] [Google Scholar]
- 94.Russell P., Tamm E.R., Grehn F.J., Picht G., and Johnson M.The presence and properties of myocilin in the aqueous humor. Invest. Ophthalmol. Vis. Sci. 42:983–986, 2001 [PubMed] [Google Scholar]