Abstract
Rho-kinase inhibitors affect actomyosin cytoskeletal networks and have been shown to significantly increase outflow facility and lower intraocular pressure in various animal models and human eyes. This article summarizes common morphological changes in the trabecular meshwork induced by Rho-kinase inhibitors and specifically compares the morphological and hydrodynamic correlations with increased outflow facility by Rho-kinase inhibitor, Y-27632, in bovine, monkey, and human eyes under similar experimental conditions. Interspecies comparison has shown that morphological changes in the juxtacanalicular connective tissue (JCT) of these 3 species were different. However, these different morphological changes in the JCT, no matter if it's separation between the JCT and inner wall in bovine eyes, or separation between the JCT cells or between the JCT cells and their matrix in monkey eyes, or even no separation between the inner wall and the JCT but a more subtle expansion of the JCT in human eyes, appear to correlate with the increased percent change of outflow facility. More importantly, these different morphological changes all resulted in an increase in effective filtration area, which was positively correlated with increased outflow facility in all 3 species. These results suggest a link among changes in outflow facility, tissue architecture, and aqueous outflow pattern. Y-27632 increases outflow facility by redistributing aqueous outflow through a looser and larger area in the JCT.
Introduction
Primary open-angle glaucoma (POAG) is a leading cause of blindness that affects 60.5 million people worldwide.1 Elevated intraocular pressure (IOP) is a major risk factor for the development and progression of POAG, and currently, lowering IOP is the only effective way of treating glaucoma.2–7 IOP is maintained within a normal range from a dynamic balance between continuous production of aqueous humor by the ciliary epithelium and drainage through the trabecular and uveoscleral outflow pathways.8 The trabecular outflow pathway, consisting of the trabecular meshwork (TM), Schlemm's canal (SC), collector channels (CCs), and episcleral veins, is the major aqueous drainage pathway where ∼70–90% of aqueous humor exits.9,10 Although the mechanism behind increased outflow resistance in POAG remains unclear, the consensus is that the majority of outflow resistance resides in the TM outflow pathway proximal to upstream of SC, consisting of the inner wall endothelium and its underlying juxtacanalicular connective tissue (JCT).11,12 Current glaucoma drugs lower IOP by decreasing aqueous production (beta-blockers, carbonic anhydrase inhibitors, alpha-2 agonists, and epinephrine and analogs), increasing uveoscleral outflow (prostaglandins and alpha-2 agonists), or increasing trabecular outflow through ciliary muscle contraction (cholinergic agents).13 However, none of these drugs directly target the trabecular outflow pathway, the considered site of the initial problem. The lack of drugs specifically targeting the trabecular outflow pathway may explain that even with the availability of multiple drug classes, many patients still fail to adequately control IOP, resulting in disease progression and further invasive surgeries to control IOP.14 Thus, there is a need to develop the next generation of glaucoma drugs to directly target the TM outflow pathway to control IOP.
The Rho and Rho-associated coiled coil-forming protein kinase (ROCK) pathway has been studied extensively for the past decade as a potential target for the treatment of glaucoma. More recently, several glaucoma drug candidates that target the Rho/ROCK pathway are undergoing phase I and phase II clinical trials,15–18 which underscores the importance on understanding the underlying mechanism behind Rho-kinase inhibitors that lower IOP. In the past several years, Y-27632, a Rho-kinase inhibitor, has been studied extensively in both animal and human models in an attempt to understand its mechanisms of increasing outflow facility. The purpose of this review was to summarize common morphological changes in the TM, induced by Rho-kinase inhibitors, and specifically compare the morphological and hydrodynamic correlations with increased outflow facility by Rho-kinase inhibitor, Y-27632, in bovine, monkey, and human eyes under similar experimental conditions.
Effect on Aqueous Outflow Facility and IOP
An overview of the Rho/ROCK pathway reveals that the activation of the Rho/ROCK pathways results in increased outflow resistance, thereby decreasing outflow facility and elevating IOP. Agonists of the Rho/ROCK pathway, such as endothelin-1,19 transforming growth factor-beta,20 lysophospholipids (lysophosphatidic acid and sphingosine-1-phosphate),21 and expression of RhoAV14,22 have been shown to decrease aqueous outflow and/or increase IOP. In contrast, inhibition of the Rho/ROCK pathways results in decreased outflow resistance, thereby increasing outflow facility and lowering IOP. Antagonists of the Rho/ROCK pathway, such as ROCK inhibitors (Y-27632, Y-39983, HA-1077, H-1152),23–37 myosin light-chain kinase inhibitor (ML-9),38 and Lim kinase-2 inhibitor,39 and silencing RhoA expression,40 have all shown to increase aqueous outflow and/or decrease IOP in various animal models as well as in human eyes. A summary of the effect of the Rho-kinase inhibitors on aqueous outflow facility and IOP is shown in Table 1.
Table 1.
Effect of Rho-Kinase Inhibitors on Aqueous Outflow Facility and Intraocular Pressure in Various Animal Models and Human Eyes
| Rho-kinase inhibitors | Outflow facility (C)/IOP | Species | Method | References | |
|---|---|---|---|---|---|
| Y-27632 | ↑ | C | Bovine | Ex vivo whole globe perfusion | 23 |
| ↑ | C | Monkey | Ex vivo whole globe perfusion | 24,42 | |
| ↑ | C | Human | Ex vivo whole globe perfusion | 25 | |
| ↑ | C | Porcine | Ex vivo whole globe perfusion | 41 | |
| ↑ | C | Monkey | In vivo: intracameral | 34 | |
| ↑ | C | Rabbit | In vivo: topical | 29 | |
| ↓ | IOP | Rabbit | In vivo: topical, intracameral, intravitreal | 29,35 | |
| ↓ | IOP | Mouse | In vivo: topical | 27 | |
| Y-39983 | ↑ | C | Rabbit | In vivo: topical | 26 |
| ↓ | IOP | Rabbit | In vivo: topical | 26 | |
| ↓ | IOP | Monkey | In vivo: topical | 26 | |
| ↓ | IOP | Mouse | In vivo: topical | 27 | |
| Y-39983 (SNJ-1656)a | ↓ | IOP | Human | Phase I clinical, In vivo: topical | 28 |
| H-1152P | ↑ | C | Porcine | Ex vivo whole globe perfusion | 32 |
| ↓ | IOP | Rabbit | In vivo: topical | 31 | |
| H-1152 | ↓ | IOP | Rat | In vivo: topical | 33 |
| Fasudil (HA-1077) | ↑ | C | Rabbit | In vivo: topical | 36 |
| ↓ | IOP | Rabbit | In vivo: topical, intracameral, intravitreal | 30,36 | |
| K-115a | ↓ | IOP | Human | Phase 1/2 clinical, In vivo: topical | 16,17 |
| AR-12286a | ↓ | IOP | Human | Phase 2A clinical, In vivo: topical | 18 |
| SR-3677 | ↑ | C | Porcine | Ex vivo whole globe perfusion | 37 |
| Dominant-negative RhoA | ↑ | C | Human | Anterior segment perfusion | 40 |
All Rho-kinase inhibitors increase outflow facility (C) and decrease intraocular pressure (IOP).
Clinical trial.
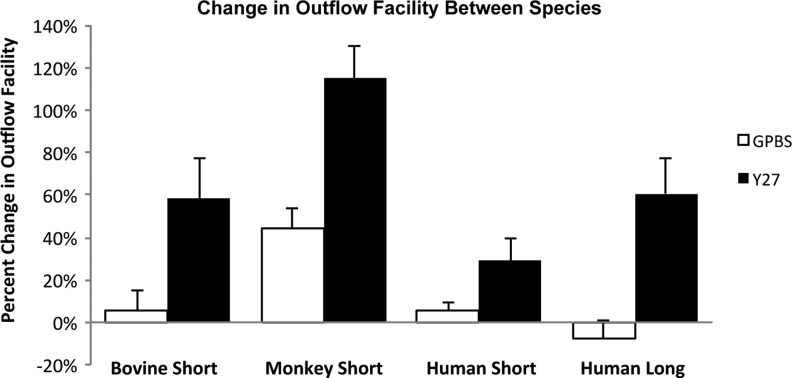
A popular Rho-kinase inhibitor used in studies of the trabecular outflow pathway has been Y-27632. Other groups have demonstrated that perfusion with 50 μM of Y-27632 for a minimum of 60 and 170 min in enucleated porcine41 and monkey42 eyes significantly increased the outflow facility, respectively. However, their results are difficult to compare because of the different perfusion pressures as well as possible different perfusion methods. On the other hand, Y-27632 in different species under similar experimental conditions (same concentration and perfusion time), studied by our group, has shown to have a greater increase in outflow facility in bovine and monkey eyes compared to human eyes.23–25 Human eyes required a longer perfusion time to achieve the similar percent increase in outflow facility as observed in nonhuman eyes (Fig. 1).
FIG. 1.
Comparison of outflow facility in bovine, monkey, and human eyes. Percent change in outflow facility in bovine, monkey, and human eyes after Y-27632 treatment (50 μM). Human eyes required a longer perfusion time (3 h) with Y-27632 to achieve a similar percent increase in outflow facility as found in bovine eyes when perfused for a short duration (30 min), whereas this increase was still less than the level achieved in monkey eyes when perfused for the same duration (30 min) with Y-27632 (from Yang et al.25).
Morphological Changes Following Y-27632 Treatment
Morphological changes at the cellular level under cell culture conditions
TM cells
Studies have shown that TM cells possess smooth muscle-like properties, which can enable them to relax and contract and may play a role in regulating the aqueous outflow.41,43,44 In particular, one study has shown that Y-27632 can cause TM cell relaxation, disassembly of actin stress fibers, and focal adhesions,41 which could potentially lower the aqueous outflow resistance by increasing paracellular fluid flow or alteration of the pathway through the JCT.
Inner wall endothelial cells of SC
In addition to TM cells, studies have shown that SC cells treated with Rho-kinase inhibitors or other cytoskeletal altering agents have a significant influence on the cell actin cytoskeleton,41 cortex,45 stiffness, and contractility.46 Specifically, Y-27632 resulted in a decrease in junctional resistance of inner wall endothelial cells of SC from both human41 and monkey42 eyes, presumably due to the loss of actin stress fibers and a decrease in cell stiffness/contractility. Although the mechanism of how cell stiffness and contractility leads to an increase in outflow facility is still unclear, it has been hypothesized that decreasing cell stiffness and contractility can decrease outflow resistance by increasing the ability of SC cells to form giant vacuoles (GVs) and pores.47 However, further studies are warranted to prove this hypothesis.
Morphological changes in ex vivo whole globe perfusion model
General morphological changes
Aqueous outflow tissue that has been treated with ROCK inhibitors often share common morphological features associated with increased outflow facility. Such features include the expansion of the JCT (human25), SC inner wall endothelium and JCT separation (bovine23 and monkey24), and distension of the SC inner wall endothelium (porcine41). These morphological observations are likely due to cell relaxation and disassembly of both actin stress fibers and focal adhesions.41
Comparison of the morphological changes associated with increased outflow facility with Y-27632 treatment in bovine, monkey and human eyes
Our group has studied the morphological changes associated with increased outflow facility after Y-27632 treatment in bovine, monkey, and human eyes under similar experimental conditions.23–25 We found that, in both bovine and monkey eyes, light microscopy reveals a similar separation between the inner wall and JCT, and this separation showed a positive correlation with increased outflow facility23,24 (Fig. 2). However, upon closer inspection at the electron microscopic level, bovine eyes displayed a separation between the basal lamina of the inner wall endothelial cells and the extracellular matrix of the JCT (matrix–matrix separation), whereas monkey eyes revealed a separation between the JCT cells (cell–cell separation) or between the JCT cells and their matrix (cell–matrix separation) (Fig. 3). These similar morphological differences between bovine (matrix–matrix separation)48,49 and monkey (cell–cell and cell–matrix separations)24 eyes were also reported following the washout effect (a perfusion volume-dependent phenomenon whereby outflow facility progressively increases during prolonged perfusion in nonhuman eyes12), and similar cell–cell separation in the JCT was also observed in monkey eyes after treatment with latrunculin-B, which disrupts microfilament organization of the cells.50 These results suggest that monkey eyes may have a stronger connection between the basal lamina of the inner wall cells and the JCT than that between the JCT cells and between the JCT cells and their matrix.
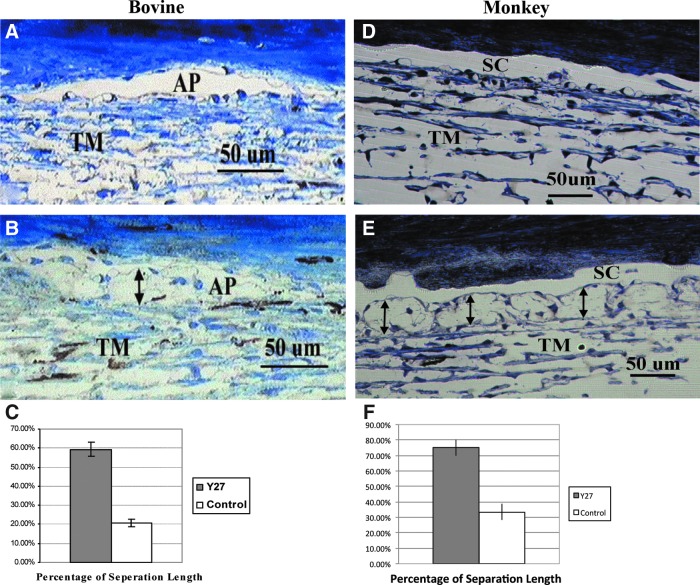
FIG. 2.
Light microscopic analysis in bovine and monkey eyes. (A) An image from a control bovine eye, no separation was found between the inner wall of the aqueous plexus (AP) and juxtacanalicular connective tissue (JCT). (B) An image from a Y-27632-treated bovine eye, separation was found between the inner wall of the AP and JCT (double headed arrows). (C) Percent separation length (PSL) in bovine eyes was significantly increased in Y-27632- treated eyes compared to control eyes. (D) An image from a control monkey eye, the inner wall (IW) of Schlemm's canal (SC) appeared in contact with the underlying JCT matrix. (E) An image from a Y-27632-treated monkey eye, separation was found between the inner wall of SC and JCT (double headed arrows). (F) PSL in the JCT region was significantly increased in Y-27632-treated eyes compared to control eyes. TM, trabecular meshwork. (A–C) from Lu et al.23; (D–F) from Lu et al.24
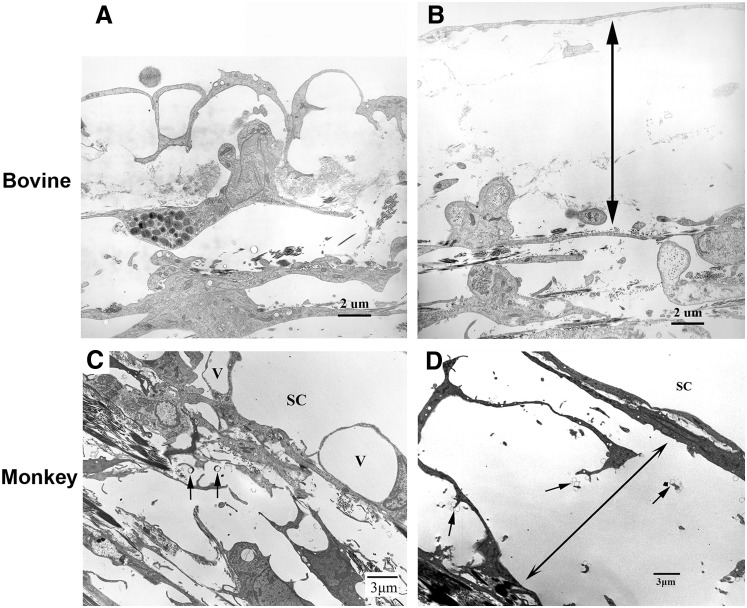
FIG. 3.
Electron microscopic analysis in bovine and monkey eyes. (A) An image from the inner wall and JCT region of a control bovine eye, the connection between the inner wall endothelial cells and underlying matrix of the JCT was maintained. (B) An image from the inner wall and JCT region of a Y-27632-treated bovine eye, separation between the basal lamina of the inner wall endothelium and underlying extracellular matrix of JCT was seen (double headed arrows). (C) An image from the inner wall and JCT region of a control monkey eye, the inner wall of SC appeared attached to the underlying JCT structures in most regions. Giant vacuoles (V) were seen along the inner wall of SC. Microspheres (arrows) were usually seen in the open spaces of the JCT region. (D) An image from the inner wall and JCT region of a Y-27632-treated monkey eye, the inner wall appeared distended to SC. The JCT appeared loose and disorganized. Significant separations between the inner wall and JCT were found (double headed arrows). The separation was mainly between JCT cells and between the JCT cells and their extracellular matrix. In separated areas, the connections between JCT cells were lost and less extracellular matrix was seen. (A, B) from Lu et al.23; (C, D) from Lu et al.24
Unlike the findings in bovine and monkey eyes, perfusion of human eyes with Y-27632 for the same time (30 min) or longer (3 h) showed no obvious separation between the inner wall and the JCT under light microscopy (Fig. 4). Interestingly, using a detailed imaging analysis, a more subtle expansion of the JCT region (2 μm increase in JCT thickness) was observed in human eyes25 (Figs. 5 and 6). Despite this difference in morphological alterations, a positive correlation was found between the JCT thickness and increased outflow facility in human eyes, which was similar to the positive correlation between percent separation length and increased outflow facility in bovine and monkey eyes (Fig. 7). These results suggested that anatomical differences among species may affect the extent of changes in morphology and outflow facility, which is consistent with previous reports that human eyes do not exhibit the washout effect49,51 or inner wall/JCT separation following prolonged perfusion.49 The morphological findings associated with an increase in outflow facility after Y-27632 treatment support the “funneling” model.11 This hypothesis states that aqueous humor flowing through the JCT must cross through the pores of the inner wall endothelium of SC, where aqueous outflow resistance decreases with an increase in available area for flow and that the bulk of resistance is generated within the inner wall endothelium of the SC and JCT region. Therefore, Y-27632 could greatly attenuate the outflow resistance in the inner wall and JCT region by affecting the JCT/SC connectivity and increasing the thickness of the JCT through relaxing the inner wall and JCT cells as discussed above. However, the mechanism behind the changes in the outflow resistance as a result of Rho-kinase inhibitor treatment is likely not only due to JCT/SC connectivity and JCT geometrical changes, but the inner wall pore density/size may also play a role according to the funneling model, which is discussed further in the next section.
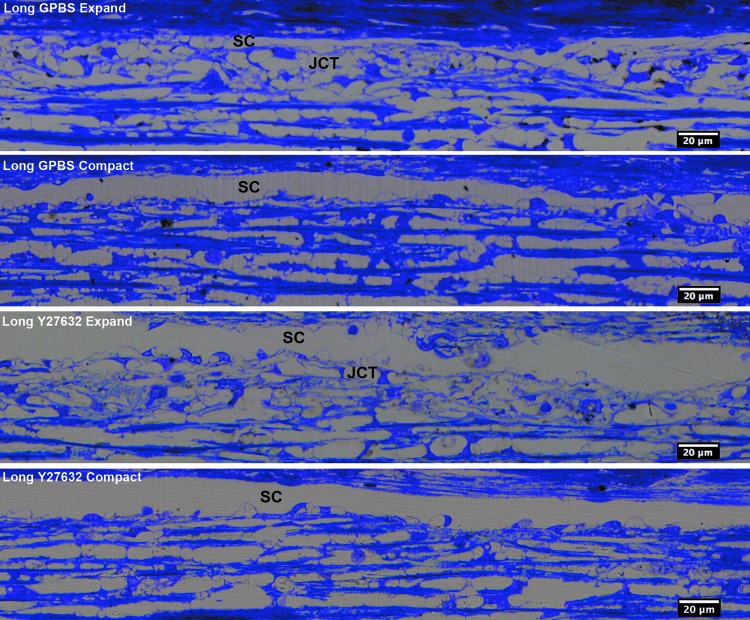
FIG. 4.
Light microscopic analysis in human eyes. JCT expansion and compact regions were observed in both long-duration Y-27632-treated eyes and its control groups. SC, Schlemm's canal (from Yang et al.25).
FIG. 5.
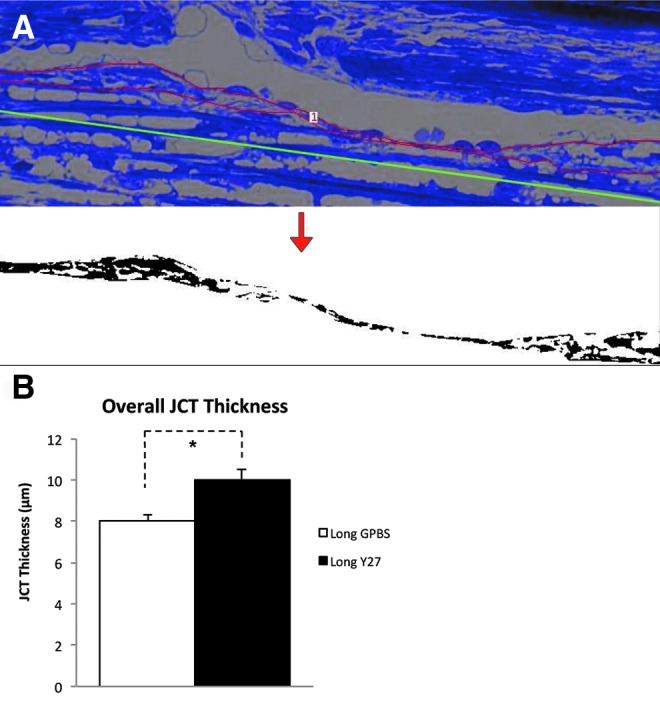
Morphological analysis of JCT thickness in human eyes. (A) Morphological analysis of JCT. Red line represents the JCT area, and the green line represents the length of JCT. The JCT area was converted to binary image (below) to obtain the percent JCT empty space. The average JCT thickness (ΣJCT area/ΣJCT length) and the percent JCT optically empty space (ΣJCT optically empty space/ΣJCT area) of each eye were calculated. (B) JCT thickness. A significant increase in average JCT thickness was found in long-duration Y-27632 group compared to its control (*P<0.01) (from Yang et al.25).
FIG. 6.
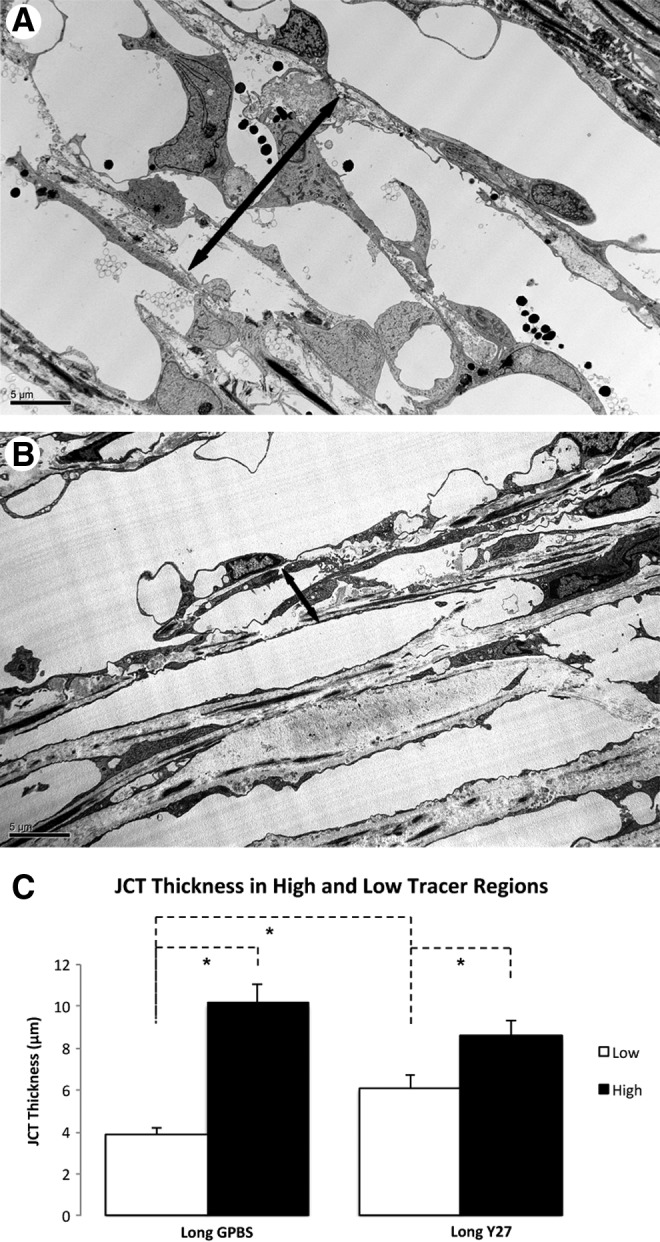
JCT thickness in human eyes. (A–C) An increased JCT thickness (double headed arrows) was found in the high-tracer regions (A) compared to the low-tracer regions (B) in both Y-27632-treated and control human eyes (*P<0.05) (C) (from Yang et al.25).
FIG. 7.
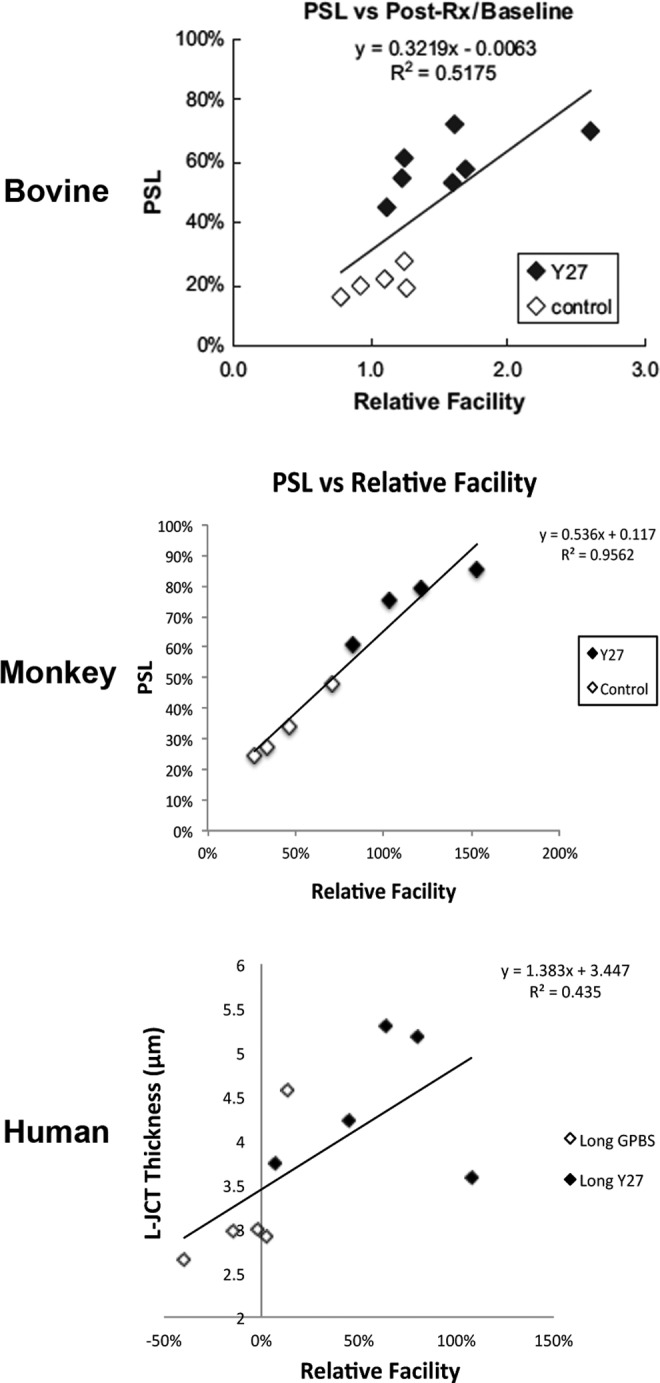
Correlation between changes in the JCT and outflow facility. In both bovine and monkey eyes, a significant positive correlation was found between percent separation length (PSL) and change in outflow facility (with respect to baseline). In human eyes, a significant positive correlation was found between loose JCT (L-JCT) thickness and change in outflow facility (with respect to baseline). (Bovine data from Lu et al.23; monkey data from Lu et al.24; and human data from Yang et al.25)
Effect on pores and GVs
After traversing through the TM, the aqueous humor encounters the inner wall endothelium of SC, a confluent layer of cells lying upon a discontinuous basement membrane. The endothelium of SC forms 2 types of pores: intracellular pores and paracellular pores.52 Aqueous humor is presumed to enter SC through these pores. To date, only one study has demonstrated an increase in number and size of paracellular pores after perfusing enucleated human eyes with a cytoskeletal disrupting agent, latrunculin-B.53 Based on this study, one could infer that an increase in number and size of paracellular pores may also play a role in increased outflow facility by Rho-kinase inhibitors. On the other hand, the same group did not observe differences in paracellular pore density after perfusion with sphingosine-1-phosphate (S1P), an agonist of the Rho/ROCK pathway.54 Therefore, whether drugs that target the Rho/ROCK pathway affect pore formation remains unclear.
Another feature of the endothelial lining cells of SC is the formation of GVs. GVs are pressure-sensitive structures caused by the pressure drop across the inner wall endothelium of SC.55 When cells are attenuated and the cytoplasm becomes thin, intracellular pores are more likely to form and are often associated with GVs. An increase in GV density was reported near the CC ostia, where preferential flow exists.56 Conflicting reports regarding increased GV formations after Rho-kinase inhibitors have made it difficult to associate these changes with higher aqueous outflow facility. There has been evidence that there is an increase in GV number compared to controls in Y-27632-treated enucleated porcine,41 bovine,23 and monkey24,42 eyes; however, there is also evidence that no increase in GV numbers exist in human eyes after Y-27632 treatment.25 Conversely, agonists of Rho/ROCK pathway, lysophosphatidic acid or S1P, perfused in enucleated porcine eyes showed an increase in GV numbers,21 whereas human eyes perfused with S1P showed no difference compared to its control.54
Two potential mechanisms can be used to explain the conflicting findings regarding GVs: (1) modulation of the aqueous outflow resistance at the JCT/SC region affects the pressure drop across SC; (2) direct attenuation of SC endothelial cell stiffness and contractility. The first mechanism pertains to the modulation of morphology of the TM by expanding the JCT/TM, causing a more uniform flow and lower outflow resistance, according to the “funneling” model,11,48 and resulting in more GV collapse and therefore more difficulty detecting them.53 The second possible mechanism relates to the direct attenuation of the SC cell stiffness and contractility and potentially increase GV formation.47 However, whether Y-27632 has effects on the inner wall GV formation and pore density/size remains to be determined.
Effect on Effective Filtration Area
Aqueous outflow is segmental in normal eyes
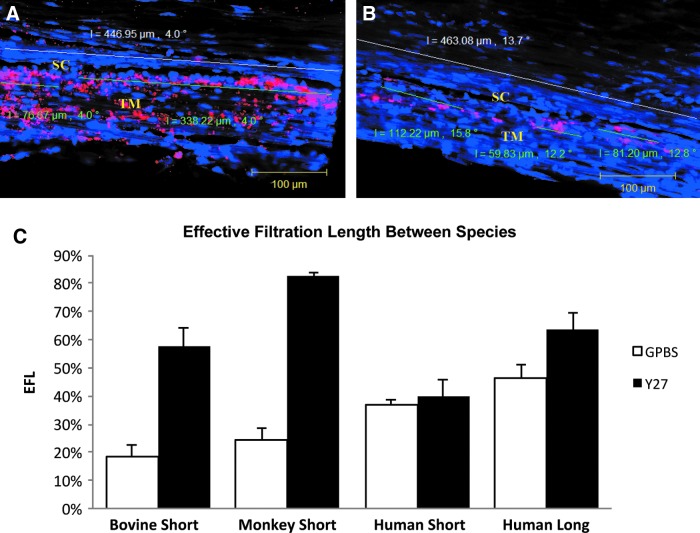
Aqueous humor outflow through the trabecular outflow pathway has been reported to be segmental or circumferentially nonuniform through studies observing the distribution of pigment in the TM57 and tracer perfused into the anterior chamber.23,24,57–65 These results suggest that at any given time, only a fraction of the outflow pathways are actively involved in aqueous humor drainage. This active area is termed the effective filtration area (EFA), which has been quantified by our group through the percent effective filtration length [PEFL=(length of the inner wall exhibiting tracer labeling/total length of inner wall)×100%]. This can serve as a one-dimensional measurement of the EFA where the fluorescent tracers reached the inner wall of the SC (Fig. 8). A greater concentration of tracer was observed in the TM adjacent to CC ostia, but not all the CC ostia are active at a given time.25 Additionally, segmental outflow has also been reported in mouse,66 porcine,67 bovine,23,59 monkey,24 and human eyes.57,67,68 The implication of segmental flow is significant because it confers the existence of circumferential variations of the TM structure, meaning that cells/tissues may respond to drugs at varying magnitudes, depending on the amount of flow (and therefore, amount of the drug) through that region of the TM. This effect raises some doubt about the precision of unbiased random sampling of the TM to study morphological changes.
FIG. 8.
Confocal microscopy and percent effective filtration length (PEFL). (A, B) PEFL=FL/TL. Green line represents tracer-decorated filtration length (FL) and white line represents total length (TL) of SC. The tracer distribution was shown by the pink color. After Y-27632 treatment, tracer distribution was more uniform throughout the TM with extensive labeling along the IW of SC in human long-term groups compared to the controls. (C) Percent EFL in bovine,23 monkey,24 and human25 eyes after Y-27632 treatment. No change in EFL was found between short-duration Y-27632 treatment and its controls in human eyes. An increase with respect to its controls in EFA after long duration of Y-27632 perfusion in human eyes is smaller compared to short-duration Y-27632 treatment in both bovine and monkey eyes. (A–C) from Yang et al.25
EFA decreases with increasing IOP and in POAG
EFA was found to reduce with acute elevation of IOP in bovine eyes. Outflow patterns in the JCT and inner wall transitioned from less segmental (more uniform) patterns at normal IOP (7 mmHg in enucleated eyes) to an increased segmental pattern at higher IOP (15–45 mmHg).59 This decrease in EFA is associated with decreased outflow facility and is reversible when pressure is reduced from a high level to a normal level.69 A decrease in EFA was also reported in chronic elevation of IOP in the laser-induced monkey glaucoma model,70 with a lesser amount or no tracer was found in the areas of the TM where they had received laser injury, including the CC ostia region. Moreover, active outflow was found to be shifted away from areas with laser injury to areas without it. Significant reduction of EFA was also reported in POAG eyes compared to normal eyes in a tracer study (Cha ED, et al: Annual meeting of Association for Research in Vision and Ophthalmology (ARVO), Invest. Ophthalmol. Vis. Sci., 2013; 54:E-Abstract 2291). In addition, an inverse correlation between EFA and IOP was recently documented in an ocular hypotensive mouse model.66 Collectively, these results suggest that the EFA is a valuable method of measuring outflow resistance and the effects of changes in IOP in humans and a number of different species.
EFA increases with Y-27632 treatment in bovine, monkey, and human eyes
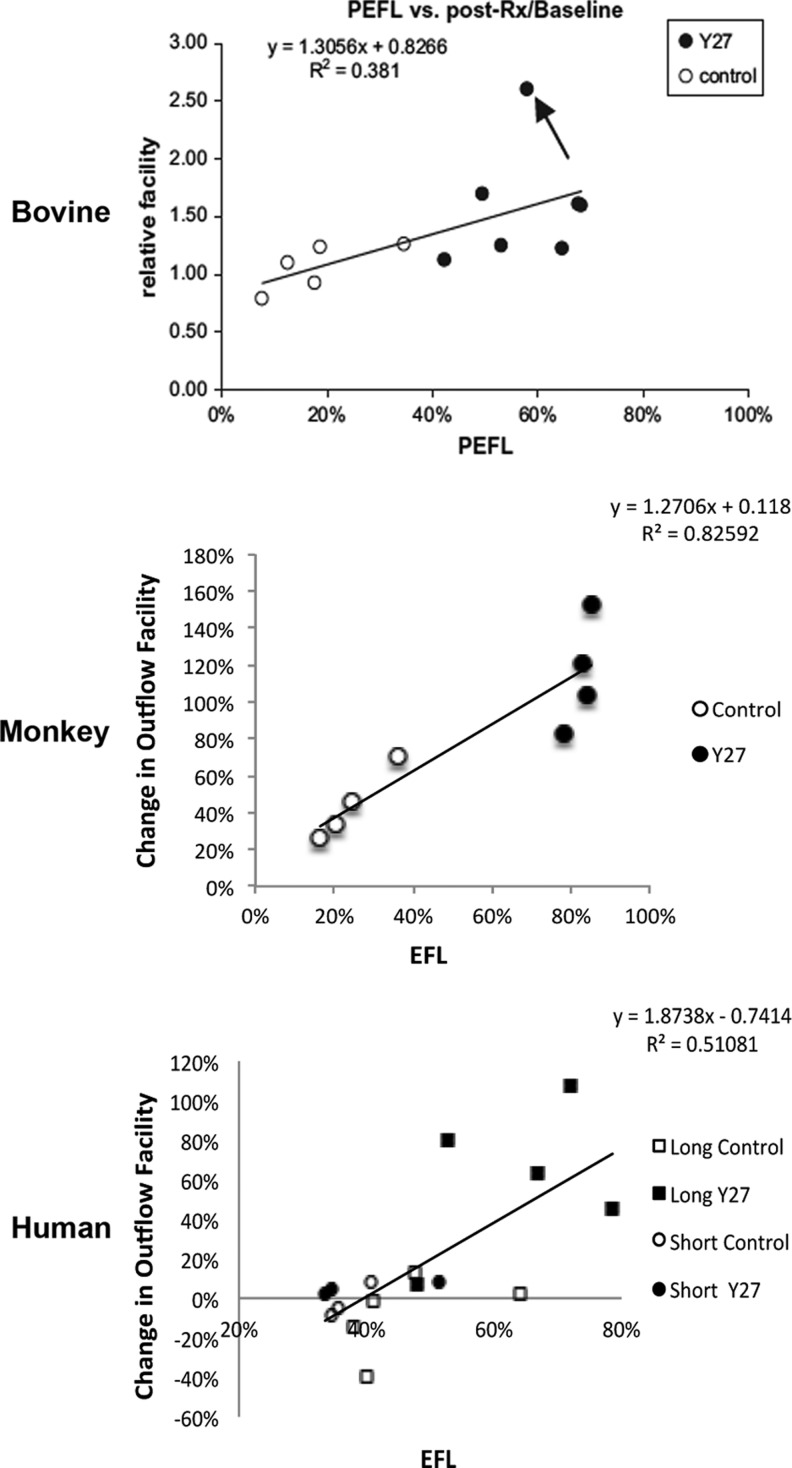
In addition to the different morphological changes that were found associated with increased outflow facility in bovine,23,24 monkey,24 and human eyes25 as described above, an increase in EFA was also found in all these 3 species following the Y-27632 treatment compared to their normal controls (Fig. 8). A positive correlation was found between outflow facility and EFA in all 3 species (Fig. 9). Similar to outflow facility, the magnitude of the increase in EFA varies among the species, where human eyes take a significantly longer time to observe a similar magnitude of change in outflow facility and EFA compared to bovine and monkey eyes (Fig. 8), presumably due to anatomical differences between species. It is also important to note that in these studies, an increase in outflow facility was shown to strongly correlate with an increase in EFA, regardless of the species or drug perfusion time (Fig. 9). Additionally, high tracer regions were also found to associate with an increase in TM thickness (innermost uveoscleral beam to the inner wall endothelium) in human eyes25 (Fig. 10). These results suggest that active flow area can be used as a guiding tool to accurately assess morphological changes in the TM and their correlation with the changes in outflow facility and may also be used to study the effect of other drugs on the aqueous outflow pathway. One caveat of the studies involving the Rho/ROCK pathway is that none of its activators have studied whether EFA decreases with increased outflow resistance, presenting a void that needs to be filled in the future.
FIG. 9.
Correlation between the PEFL and outflow facility. A significant positive correlation was found between PEFL and change in outflow facility (with respect to baseline) in bovine, monkey, and human eyes with and without Y-27632 treatment. (Bovine data from Lu et al.23; monkey data from Lu et al.24; and human data from Yang et al.25)
FIG. 10.
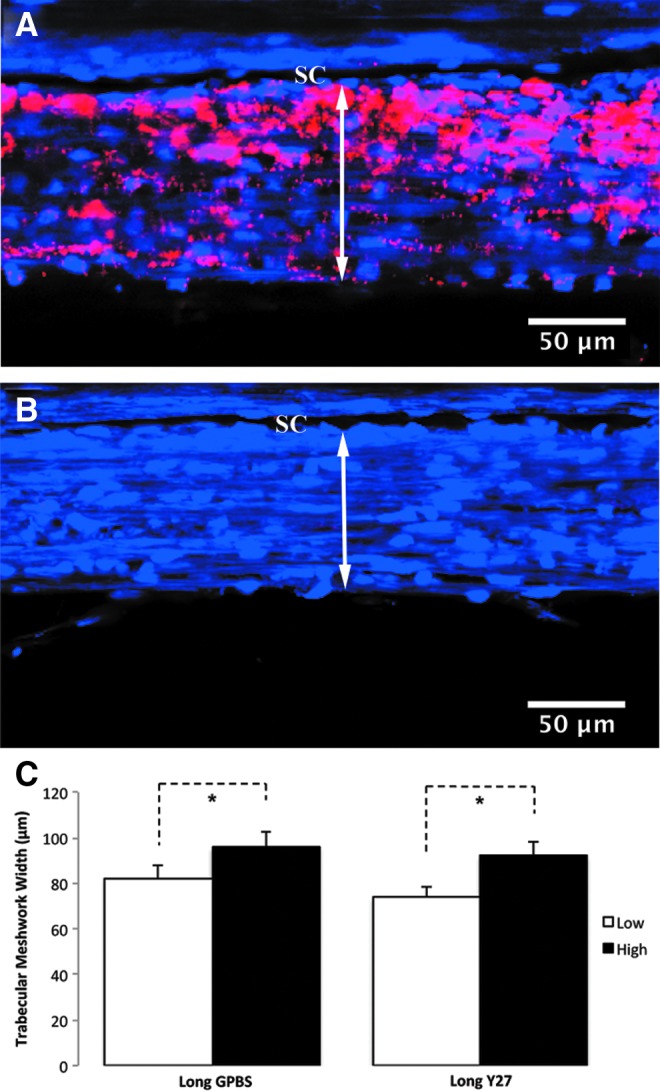
TM thickness in high- and low-tracer regions of human eyes. (A–C) An increased TM thickness (double headed arrows) was found in the high-tracer regions (A) compared to the low-tracer regions (B) in both Y-27632-treated and control eyes (C). (A–C) from Yang et al.25
Summary
In summary, Rho-kinase inhibitors significantly increase outflow facility and IOP in various animal models and human eyes. The morphological changes in the TM associated with increased outflow facility include separation between the JCT and inner wall found in bovine eyes, separation between the JCT cells and between the JCT cells and their matrix found in monkey eyes, and an increased expansion in the TM and JCT in human eyes. Despite the different morphological changes in the JCT across these species, they all appear to correlate with percent changes of increased outflow facility. More importantly, these different morphological changes all resulted in an increase in EFA, which was positively correlated with an increase in outflow facility in all 3 species. These results suggest a link among changes in outflow facility, tissue architecture, and aqueous outflow pattern. With all these aqueous outflow-related changes considered, it is likely that Rho-kinase inhibitor Y-27632 increases outflow facility and thus lowers the IOP by redistributing aqueous outflow through a looser and larger area in the JCT. Whether the changes in the pore density/size of the inner wall also play a role in the increased outflow facility and EFA by Y-27632 remains to be determined. To date, all studies of the effect of Y-27632 on outflow facility and IOP have been performed in normal animal models and human eyes, and how its effect on altered structure of the trabecular outflow pathway in POAG eyes is warranted to be explored.
Acknowledgments
Original work was supported by BrightFocus Foundation (formerly American Health Assistance Foundation), NIH Grant EY018712, EY022634, the Boston University School of Medicine Wing Tat Lee Fund, and the Massachusetts Lions Eye Research Fund.
Author Disclosure Statement
The authors have no commercial relationships.
References
- 1.Quigley H.A., and Broman A.T.The number of people with glaucoma worldwide in 2010 and 2020. Br. J. Ophthalmol. 90:262–267, 2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.The Advanced Glaucoma Intervention Study (AGIS). 7. The relationship between control of intraocular pressure and visual field deterioration. The AGIS Investigators. Am. J. Ophthalmol. 130:429–440, 2000 [DOI] [PubMed] [Google Scholar]
- 3.Bergeå B., Bodin L., and Svedbergh B.Impact of intraocular pressure regulation on visual fields in open-angle glaucoma. Ophthalmology 106:997–1004, 1999; discussion 1004–1005. [DOI] [PubMed] [Google Scholar]
- 4.Leske M.C., Hyman L., Hussein M., Heijl A., and Bengtsson B.Comparison of glaucomatous progression between untreated patients with normal-tension glaucoma and patients with therapeutically reduced intraocular pressures. The effectiveness of intraocular pressure reduction in the treatment of normal-tension glaucoma. Am. J. Ophthalmol. 127:625–626, 1999 [PubMed] [Google Scholar]
- 5.Mao L.K., Stewart W.C., and Shields M.B.Correlation between intraocular pressure control and progressive glaucomatous damage in primary open-angle glaucoma. Am. J. Ophthalmol. 111:51–55, 1991 [DOI] [PubMed] [Google Scholar]
- 6.Heijl A., Leske M.C., Bengtsson B., Hyman L., Hussein M., and EMGT Group. Reduction of intraocular pressure and glaucoma progression: results from the Early Manifest Glaucoma Trial. Arch. Ophthalmol. 120:1268–1279, 2002 [DOI] [PubMed] [Google Scholar]
- 7.Kass M.A., Gordon M.O., Hoff M.R., et al. Topical timolol administration reduces the incidence of glaucomatous damage in ocular hypertensive individuals. A randomized, double-masked, long-term clinical trial. Arch. Ophthalmol. 107:1590–1598, 1989 [DOI] [PubMed] [Google Scholar]
- 8.Brubaker R.Clinical measurements of aqueous dynamics: implications for addressing glaucoma. In: Civan M., ed. The Eye's Aqueous Humor from Secretion to Glaucoma. San Diego, CA: Academic Press, 1998, pp. 233–284 [Google Scholar]
- 9.Townsend D.J., and Brubaker R.F.Immediate effect of epinephrine on aqueous formation in the normal human eye as measured by fluorophotometry. Invest. Ophthalmol. Vis. Sci. 19:256–266, 1980 [PubMed] [Google Scholar]
- 10.Toris C.B., Yablonski M.E., Wang Y.L., and Camras C.B.Aqueous humor dynamics in the aging human eye. Am. J. Ophthalmol. 127:407–412, 1999 [DOI] [PubMed] [Google Scholar]
- 11.Overby D.R., Stamer W.D., and Johnson M.The changing paradigm of outflow resistance generation: towards synergistic models of the JCT and inner wall endothelium. Exp. Eye Res. 88:656–670, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Gong H., and Freddo T.F.The washout phenomenon in aqueous outflow—why does it matter? Exp. Eye Res. 88:729–737, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Schacknow P.N., and Samples J.R.Medications used to treat glaucoma. The Glaucoma Book. Medford, MA: Springer, 2010, pp. 583–628 [Google Scholar]
- 14.Schwartz K., and Budenz D.Current management of glaucoma. Curr. Opin. Ophthalmol. 15:119–126, 2004 [DOI] [PubMed] [Google Scholar]
- 15.Chen J., Runyan S.A., and Robinson M.R.Novel ocular antihypertensive compounds in clinical trials. Clin. Ophthalmol. 5:667–677, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Tanihara H., Inoue T., Yamamoto T., et al. Phase 1 clinical trials of a selective Rho kinase inhibitor, K-115. JAMA Ophthalmol. 131:1288–1295, 2013 [DOI] [PubMed] [Google Scholar]
- 17.Tanihara H., Inoue T., Yamamoto T., et al. Phase 2 randomized clinical study of a Rho kinase inhibitor, K-115, in primary open-angle glaucoma and ocular hypertension. Am. J. Ophthalmol. 156:731–736, 2013 [DOI] [PubMed] [Google Scholar]
- 18.Williams R.D., Novack G.D., van Haarlem T., Kopczynski C., and Group A-PAS.Ocular hypotensive effect of the Rho kinase inhibitor AR-12286 in patients with glaucoma and ocular hypertension. Am. J. Ophthalmol. 152:834–841.e831,2011 [DOI] [PubMed] [Google Scholar]
- 19.Wiederholt M., Bielka S., Schweig F., Lütjen-Drecoll E., and Lepple-Wienhues A.Regulation of outflow rate and resistance in the perfused anterior segment of the bovine eye. Exp. Eye Res. 61:223–234, 1995 [DOI] [PubMed] [Google Scholar]
- 20.Gottanka J., Chan D., Eichhorn M., Lütjen-Drecoll E., and Ethier C.R.Effects of TGF-beta2 in perfused human eyes. Invest. Ophthalmol. Vis. Sci. 45:153–158, 2004 [DOI] [PubMed] [Google Scholar]
- 21.Mettu P.S., Deng P.F., Misra U.K., Gawdi G., Epstein D.L., and Rao P.V.Role of lysophospholipid growth factors in the modulation of aqueous humor outflow facility. Invest. Ophthalmol. Vis. Sci. 45:2263–2271, 2004 [DOI] [PubMed] [Google Scholar]
- 22.Zhang M., Maddala R., and Rao P.V.Novel molecular insights into RhoA GTPase-induced resistance to aqueous humor outflow through the trabecular meshwork. Am. J. Physiol. Cell Physiol. 295:C1057–C1070, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Lu Z., Overby D.R., Scott P.A., Freddo T.F., and Gong H.The mechanism of increasing outflow facility by rho-kinase inhibition with Y-27632 in bovine eyes. Exp. Eye Res. 86:271–281, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Lu Z., Zhang Y., Freddo T.F., and Gong H.Similar hydrodynamic and morphological changes in the aqueous humor outflow pathway after washout and Y27632 treatment in monkey eyes. Exp. Eye Res. 93:397–404, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Yang C.Y., Liu Y., Lu Z., Ren R., and Gong H.Effects of y27632 on aqueous humor outflow facility with changes in hydrodynamic pattern and morphology in human eyes. Invest. Ophthalmol. Vis. Sci. 54:5859–5870, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Tokushige H., Inatani M., Nemoto S., et al. Effects of topical administration of y-39983, a selective rho-associated protein kinase inhibitor, on ocular tissues in rabbits and monkeys. Invest. Ophthalmol. Vis. Sci. 48:3216–3222, 2007 [DOI] [PubMed] [Google Scholar]
- 27.Whitlock N.A., Harrison B., Mixon T., et al. Decreased intraocular pressure in mice following either pharmacological or genetic inhibition of ROCK. J. Ocul. Pharmacol. Ther. 25:187–194, 2009 [DOI] [PubMed] [Google Scholar]
- 28.Tanihara H., Inatani M., Honjo M., Tokushige H., Azuma J., and Araie M.Intraocular pressure-lowering effects and safety of topical administration of a selective ROCK inhibitor, SNJ-1656, in healthy volunteers. Arch. Ophthalmol. 126:309–315, 2008 [DOI] [PubMed] [Google Scholar]
- 29.Honjo M., Tanihara H., Inatani M., et al. Effects of rho-associated protein kinase inhibitor Y-27632 on intraocular pressure and outflow facility. Invest. Ophthalmol. Vis. Sci. 42:137–144, 2001 [PubMed] [Google Scholar]
- 30.Fukunaga T., Ikesugi K., Nishio M., et al. The effect of the Rho-associated protein kinase inhibitor, HA-1077, in the rabbit ocular hypertension model induced by water loading. Curr. Eye Res. 34:42–47, 2009 [DOI] [PubMed] [Google Scholar]
- 31.Nishio M., Fukunaga T., Sugimoto M., et al. The effect of the H-1152P, a potent Rho-associated coiled coil-formed protein kinase inhibitor, in rabbit normal and ocular hypertensive eyes. Curr. Eye Res. 34:282–286, 2009 [DOI] [PubMed] [Google Scholar]
- 32.Rao P.V., Deng P., Sasaki Y., and Epstein D.L.Regulation of myosin light chain phosphorylation in the trabecular meshwork: role in aqueous humour outflow facility. Exp. Eye Res. 80:197–206, 2005 [DOI] [PubMed] [Google Scholar]
- 33.Yu M., Chen X., Wang N., et al. H-1152 effects on intraocular pressure and trabecular meshwork morphology of rat eyes. J. Ocul. Pharmacol. Ther. 24:373–379, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Tian B., and Kaufman P.L.Effects of the Rho kinase inhibitor Y-27632 and the phosphatase inhibitor calyculin A on outflow facility in monkeys. Exp. Eye Res. 80:215–225, 2005 [DOI] [PubMed] [Google Scholar]
- 35.Waki M., Yoshida Y., Oka T., and Azuma M.Reduction of intraocular pressure by topical administration of an inhibitor of the Rho-associated protein kinase. Curr. Eye Res. 22:470–474, 2001 [DOI] [PubMed] [Google Scholar]
- 36.Honjo M., Inatani M., Kido N., et al. Effects of protein kinase inhibitor, HA1077, on intraocular pressure and outflow facility in rabbit eyes. Arch. Ophthalmol. 119:1171–1178, 2001 [DOI] [PubMed] [Google Scholar]
- 37.Feng Y., Yin Y., Weiser A., et al. Discovery of substituted 4-(pyrazol-4-yl)-phenylbenzodioxane-2-carboxamides as potent and highly selective Rho kinase (ROCK-II) inhibitors. J. Med. Chem. 51:6642–6645, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Honjo M., Inatani M., Kido N., et al. A myosin light chain kinase inhibitor, ML-9, lowers the intraocular pressure in rabbit eyes. Exp. Eye Res. 75:135–142, 2002 [DOI] [PubMed] [Google Scholar]
- 39.Harrison B.A., Whitlock N.A., Voronkov M.V., et al. Novel class of LIM-kinase 2 inhibitors for the treatment of ocular hypertension and associated glaucoma. J. Med. Chem. 52:6515–6518, 2009 [DOI] [PubMed] [Google Scholar]
- 40.Vittitow J.L., Garg R., Rowlette L.L., Epstein D.L., O'Brien E.T., and Borrás T.Gene transfer of dominant-negative RhoA increases outflow facility in perfused human anterior segment cultures. Mol. Vis. 8:32–44, 2002 [PubMed] [Google Scholar]
- 41.Rao P.V., Deng P.F., Kumar J., and Epstein D.L.Modulation of aqueous humor outflow facility by the Rho kinase-specific inhibitor Y-27632. Invest. Ophthalmol. Vis. Sci. 42:1029–1037, 2001 [PubMed] [Google Scholar]
- 42.Kameda T., Inoue T., Inatani M., et al. The effect of Rho-associated protein kinase inhibitor on monkey Schlemm's canal endothelial cells. Invest. Ophthalmol. Vis. Sci. 53:3092–3103, 2012 [DOI] [PubMed] [Google Scholar]
- 43.Rosenthal R., Choritz L., Schlott S., et al. Effects of ML-7 and Y-27632 on carbachol- and endothelin-1-induced contraction of bovine trabecular meshwork. Exp. Eye Res. 80:837–845, 2005 [DOI] [PubMed] [Google Scholar]
- 44.McKee C.T., Wood J.A., Shah N.M., et al. The effect of biophysical attributes of the ocular trabecular meshwork associated with glaucoma on the cell response to therapeutic agents. Biomaterials 32:2417–2423, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Vargas-Pinto R., Gong H., Vahabikashi A., and Johnson M.The effect of the endothelial cell cortex on atomic force microscopy measurements. Biophys. J. 105:300–309, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Zhou E.H., Krishnan R., Stamer W.D., et al. Mechanical responsiveness of the endothelial cell of Schlemm's canal: scope, variability and its potential role in controlling aqueous humour outflow. J. R. Soc. Interface 9:1144–1155, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Overby D.R.The mechanobiology of aqueous humor transport across Schlemm's canal endothelium. In: Jiro N., ed., Mechanobiology Handbook. Boca Raton, FL: CRC Press, 2011, pp. 367–390 [Google Scholar]
- 48.Overby D., Gong H., Qiu G., Freddo T.F., and Johnson M.The mechanism of increasing outflow facility during washout in the bovine eye. Invest. Ophthalmol. Vis. Sci. 43:3455–3464, 2002 [PubMed] [Google Scholar]
- 49.Scott P.A., Overby D.R., Freddo T.F., and Gong H.Comparative studies between species that do and do not exhibit the washout effect. Exp. Eye Res. 84:435–443, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Sabanay I., Tian B., Gabelt B.T., Geiger B., and Kaufman P.L.Latrunculin B effects on trabecular meshwork and corneal endothelial morphology in monkeys. Exp. Eye Res. 82:236–246, 2006 [DOI] [PubMed] [Google Scholar]
- 51.Erickson-Lamy K., Schroeder A.M., Bassett-Chu S., and Epstein D.L.Absence of time-dependent facility increase (“washout”) in the perfused enucleated human eye. Invest. Ophthalmol. Vis. Sci. 31:2384–2388, 1990 [PubMed] [Google Scholar]
- 52.Ethier C.R., Coloma F.M., Sit A.J., and Johnson M.Two pore types in the inner-wall endothelium of Schlemm's canal. Invest. Ophthalmol. Vis. Sci. 39:2041–2048, 1998 [PubMed] [Google Scholar]
- 53.Ethier C.R., Read A.T., and Chan D.W.Effects of latrunculin-B on outflow facility and trabecular meshwork structure in human eyes. Invest. Ophthalmol. Vis. Sci. 47:1991–1998, 2006 [DOI] [PubMed] [Google Scholar]
- 54.Stamer W.D., Read A.T., Sumida G.M., and Ethier C.R.Sphingosine-1-phosphate effects on the inner wall of Schlemm's canal and outflow facility in perfused human eyes. Exp. Eye Res. 89:980–988, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Inomata H., Bill A., and Smelser G.K.Aqueous humor pathways through the trabecular meshwork and into Schlemm's canal in the cynomolgus monkey (Macaca irus). An electron microscopic study. Am. J. Ophthalmol. 73:760–789, 1972 [DOI] [PubMed] [Google Scholar]
- 56.Parc C.E., Johnson D.H., and Brilakis H.S.Giant vacuoles are found preferentially near collector channels. Invest. Ophthalmol. Vis Sci. 41:2984–2990, 2000 [PubMed] [Google Scholar]
- 57.Hann C.R., and Fautsch M.P.Preferential fluid flow in the human trabecular meshwork near collector channels. Invest. Ophthalmol. Vis. Sci. 50:1692–1697, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Camp J.J., Hann C.R., Johnson D.H., Tarara J.E., and Robb R.A.Three-dimensional reconstruction of aqueous channels in human trabecular meshwork using light microscopy and confocal microscopy. Scanning 19:258–263, 1997 [DOI] [PubMed] [Google Scholar]
- 59.Battista S.A., Lu Z., Hofmann S., Freddo T., Overby D.R., and Gong H.Reduction of the available area for aqueous humor outflow and increase in meshwork herniations into collector channels following acute IOP elevation in bovine eyes. Invest. Ophthalmol. Vis. Sci. 49:5346–5352, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Hann C.R., Bahler C.K., and Johnson D.H.Cationic ferritin and segmental flow through the trabecular meshwork. Invest. Ophthalmol. Vis. Sci. 46:1–7, 2005 [DOI] [PubMed] [Google Scholar]
- 61.Ethier C.R., and Chan D.W.Cationic ferritin changes outflow facility in human eyes whereas anionic ferritin does not. Invest. Ophthalmol. Vis. Sci. 42:1795–1802, 2001 [PubMed] [Google Scholar]
- 62.de Kater A.W., Melamed S., and Epstein D.L.Patterns of aqueous humor outflow in glaucomatous and nonglaucomatous human eyes. A tracer study using cationized ferritin. Arch. Ophthalmol. 107:572–576, 1989 [DOI] [PubMed] [Google Scholar]
- 63.Epstein D.L., and Rohen J.W.Morphology of the trabecular meshwork and inner-wall endothelium after cationized ferritin perfusion in the monkey eye. Invest. Ophthalmol. Vis. Sci. 32:160–171, 1991 [PubMed] [Google Scholar]
- 64.Melamed S., and Epstein D.L.Alterations of aqueous humour outflow following argon laser trabeculoplasty in monkeys. Br. J. Ophthalmol. 71:776–781, 1987 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Karg S.J., Garron L.K., Feeney M.L., and Mcewen W.K.Perfusion of human eyes with latex microspheres. AMA Arch. Ophthalmol. 61:68–71, 1959 [DOI] [PubMed] [Google Scholar]
- 66.Swaminathan S.S., Oh D.J., Kang M.H., et al. Secreted protein acidic and rich in cysteine (SPARC)-null mice exhibit more uniform outflow. Invest. Ophthalmol. Vis. Sci. 54:2035–2047, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Keller K.E., Bradley J.M., Vranka J.A., and Acott T.S.Segmental versican expression in the trabecular meshwork and involvement in outflow facility. Invest. Ophthalmol. Vis. Sci. 52:5049–5057, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Yang C.-Y., Liu Y., and Gong H.Effects of Y27632 on aqueous humor outflow facility with changes in hydrodynamic pattern and morphology in human eyes. Invest. Ophthalmol. Vis. Sci. 54:5859–5970, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Zhu J.Y., Ye W., Wang T., and Gong H.Y.Reversible changes in aqueous outflow facility, hydrodynamics, and morphology following acute intraocular pressure variation in bovine eyes. Chin. Med. J. (Engl). 126:1451–1457, 2013 [PMC free article] [PubMed] [Google Scholar]
- 70.Zhang Y., Toris C.B., Liu Y., Ye W., and Gong H.Morphological and hydrodynamic correlates in monkey eyes with laser induced glaucoma. Exp. Eye Res. 89:748–756, 2009 [DOI] [PubMed] [Google Scholar]