Abstract
Glaucoma patients routinely take multiple medications, with multiple daily doses, for years or even decades. Benzalkonium chloride (BAK) is the most common preservative in glaucoma medications. BAK has been detected in the trabecular meshwork (TM), corneal endothelium, lens, and retina after topical drop installation and may accumulate in those tissues. There is evidence that BAK causes corneal and conjunctival toxicity, including cell loss, disruption of tight junctions, apoptosis and preapoptosis, cytoskeleton changes, and immunoinflammatory reactions. These same effects have been reported in cultured human TM cells exposed to concentrations of BAK found in common glaucoma drugs and in the TM of primary open-angle glaucoma donor eyes. It is possible that a relationship exists between chronic exposure to BAK and glaucoma. The hypothesis that BAK causes/worsens glaucoma is being tested experimentally in an animal model that closely reflects human physiology.
Introduction
Glaucoma refers to a group of progressive optic neuropathies that are the leading cause of irreversible blindness in the world.1 Open-angle and angle-closure glaucoma, together, are the second leading cause of world blindness after cataracts.2 Glaucoma is often undiagnosed until vision loss has occurred. It is estimated that worldwide 90% of affected people are undiagnosed, while in developed countries, the estimate drops to 50%.3 As of 2010, there were 44.7 million people in the world with open-angle glaucoma and the prevalence is projected to increase to 58.6 million globally by 2020.3
Several large clinical trials have shown that lowering intraocular pressure slows glaucoma progression, preserving visual function.4–7 Lowering IOP remains the mainstay of glaucoma treatment. Once diagnosed, patients generally begin treatment with eye drops.8 The most commonly prescribed drugs act on either the ciliary muscle cells to increase the uveoscleral outflow (prostaglandin analogs that bind to and activate prostaglandin FP receptors) or the ciliary process epithelia to reduce aqueous humor production (eg, beta-adrenergic receptor antagonists).9 Most patients will be treated with multiple classes of eye drops, in various combinations, with multiple daily doses, over their lifetime.
Medical therapy for chronic ocular diseases such as glaucoma can lead to ocular surface disease (OSD),10–14 which is a constellation of disorders affecting the eyelids, conjunctiva, and/or the multilayered corneal surface. Symptoms include burning, redness, irritation, fatigue, fluctuating visual acuity, infection, and potential loss of vision. Although OSD is seen in approximately 15% of the general elderly population,15 as many as 59% of patients with medically treated glaucoma or ocular hypertension report symptoms of OSD in at least 1 eye.10 Symptom severity is correlated with the number of IOP-lowering medications used and is often attributed to preservatives in the formulation, most commonly benzalkonium chloride (BAK).11–13 It is likely that some patients are more sensitive to BAK than others. Recognizing these BAK-sensitive individuals may be important when considering treatment options, weighing the costs and benefits of using preservative-free solutions.14
Preservative use is associated with a number of adverse effects. It can increase the number of inflammatory cells in the conjunctiva,16 which is a risk factor for failed trabeculectomy.17–19 Filtration surgery failure can also result from subconjunctival fibrosis, which can be caused by fibroblast proliferation, collagen synthesis, and glycosaminoglycan deposition. A recent study showed a dose–response curve for the amount of preoperative BAK exposure and trabeculectomy failure; an increased amount of preserved drops used per day increased the risk for surgical failure. This study identified BAK as the most likely etiologic agent.20
Preservatives are required by regulatory agency pharmacopeia guidelines to prevent bacterial and fungal contamination in multidose eye drop containers,21,22 and BAK is the most common preservative in ocular formulations. It is used in approximately 70% of preserved ophthalmic solutions, while only 10% use other preservatives.14 The typical BAK concentration in commercial glaucoma medications is 0.005% to 0.02%. BAK is a detergent polyquaternary ammonium compound, which lyses cell membranes.23 It has excellent efficacy as a preservative and is familiar across industry and regulatory agencies. Its ability to break cell–cell junctions in the corneal epithelium facilitates the penetration of the topically applied medication into the anterior chamber.23,24
There are global regulatory differences (between the FDA, EMEA, Japanese and Australian agencies) in requirements for preservatives used in multiple-dose vials. This can make formulation decisions difficult for companies developing ophthalmic drop products. It is more cost effective to get regulatory approval and manufacture a single formulation for global use and it is more cost effective to make multiple-dose vials than unit dose packaging, of particular consideration in emerging economies. Nevertheless, the accumulated evidence of deleterious effects of BAK has spurred the development of different classes of preservatives with a goal of improved side effect profiles.25,26 These include Polyquaternium-1 (Polyquad), a detergent-type preservative related to BAK, initially developed for contact lens solutions. Bacterial cells tend to attract it, yet human corneal epithelial cells may repel the compound.14 Another class is oxidizing preservatives. Stabilized oxychloro complex (SOC) is marketed under the trade name Purite (Allergan, Irvine, CA). SOC appears to be well tolerated by the ocular surface and has broad antimicrobial activity, even at very low concentrations (0.005%).14,27 Sodium perborate, also known as GenAqua, was one of the first of the oxidative-type preservatives and is used in lubricants such as Genteal eye drops (Novartis Ophthalmics, East Hanover, NJ).14,28 A fourth class is ionic-buffered preservatives such as sofZia, found in travoprost Z (Alcon, Fort Worth, TX). In vitro and in vivo studies have shown a reduced toxicity to corneal and conjunctival epithelial cells using these alternatives, compared with BAK-containing glaucoma medications.29,30
Multiple studies have found the IOP lowering efficacy of glaucoma drugs using alternative preservatives, such as SofZia and polyquad, as well as preservative-free formulations, to be equivalent (or non-inferior) to BAK-containing formulations.31–34 Results are mixed regarding the reduction in OSD symptoms for patients using alternative preservatives or preservative-free formulations. One review article stated that the ill effects reported for BAK are often not relevant to clinical practice because they are based on cell and animal studies.35 A 2012 study with 353 patients on either BAK-preserved travoprost or Polyquad- preserved travoprost found no differences in subjective symptoms, SPK, or conjunctival hyperemia.33 On the other hand, many crossover studies have shown clear improvement in OSD symptoms.36–39 Part of this discrepancy may be the timing/duration of treatment issue. Patients treated for 2 weeks32 to 3 months40 showed no significant differences in ocular tolerability between BAK and SofZia or preservative-free compounds. A longer term study found that patients switching from latanoprost to BAK-free travoprost showed no decrease in hyperemia after 1 month, but showed significant decreases at 3 and 12 months compared with baseline (P<0.05).41
That reductions in symptoms can take 12 or more weeks may be the reason that shorter-term studies have not shown a difference between preservative and preservative-free groups. Another study comparing BAK-preserved latanoprost to BAK-free travoprost12 found that in the overall cohort of patients, mean OSD scores at the 12-week time point were not statistically different, but significant improvement was seen in the subsets of patients with mild OSD scores at baseline and in patients who had long-term exposure to BAK-preserved latanoprost 0.005% (more than 24 months) before entry into the study. The latter finding suggests a cumulative, long-term adverse effect of BAK, consistent with its persistence in ocular tissues.42 (Fig. 1) OSD effects may also depend somewhat on the active ingredient BAK is combined with. In several in vitro and ex vivo studies, BAK alone in commercial formulation concentrations and BAK with timolol produced more deleterious effects in conjunctival cells than BAK with prostaglandin analogues.43–46
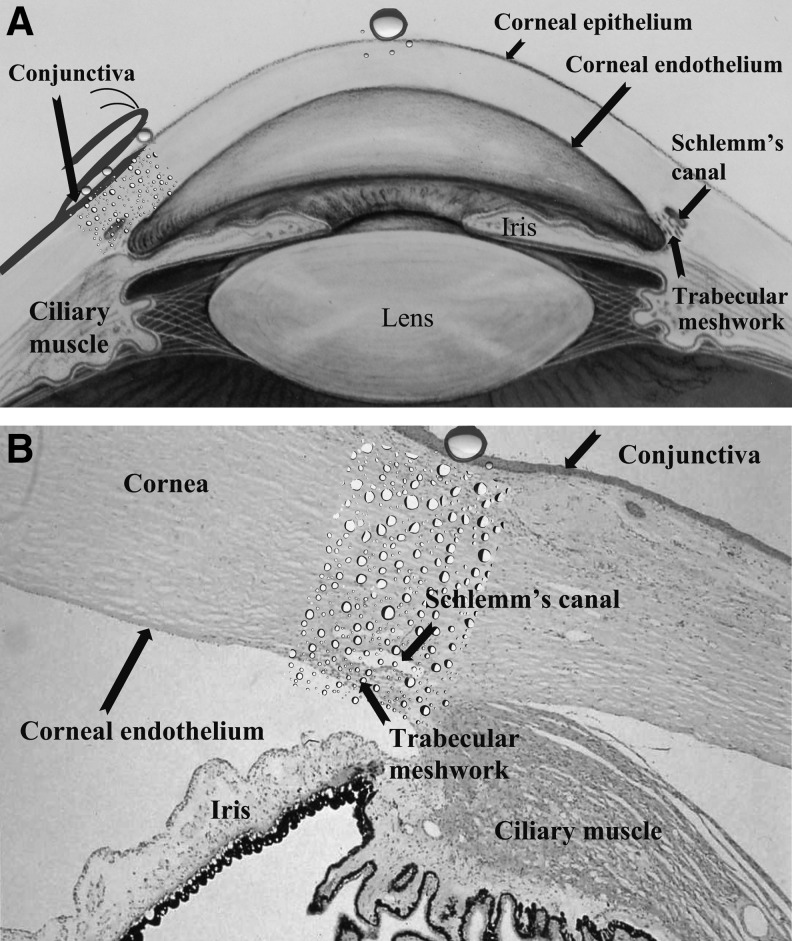
FIG. 1.
(A) BAK is reported to affect/accumulate in the cornea, lens, conjunctiva, iris, ciliary muscle epithelium, and trabecular meshwork. Adapted from the National Eye Institute, National Institutes of Health Ref#: NEA04. (B) The trabecular meshwork and Schlemm's canal are less than 1 mm away from the corneal and conjunctival surfaces, where drops are applied. Image courtesy of Dr. Morton Smith. BAK, benzalkonium chloride.
BAK Effects in Cells and the Trabecular Meshwork Mimic Glaucoma
The trabecular meshwork (TM) of glaucoma patients is characterized by loss of TM cells, accumulation of extracellular matrix (ECM), changes in the cytoskeleton, and accelerated senescence.47,48 Also implicated in glaucoma pathogenesis are increased apoptosis, reactive oxygen species (ROS), and inflammation.49,50 These findings are also reported in studies of multiple cell types treated with BAK. Human conjunctival and corneal epithelial cells treated with BAK alone and glaucoma drugs containing BAK showed reduced cell viability as well as increased apoptosis and oxidative stress markers compared with cells treated with PBS or alternative preservatives.25,29,30,44,45
Studies using human trabecular meshwork (HTM) cells show similar effects. Damage to TM cells by glaucoma drugs such as epinephrine and pilocarpine, with and without BAK, have been documented by several groups.51–53 Effects include reduced cell numbers, inhibition of cell growth, and altered cell morphology, for example, disassembly of stress fibers and filaments. Several of these studies included experiments testing BAK alone. In human TM cells, BAK concentrations as low as 0.00002% induced a significant reduction in cell density and cell growth,51 whereas in bovine TM cells, the BAK induced cell loss, vacuole formation, and growth inhibition were not seen until concentrations of 0.001%.52 The discrepancy in results was thought to be due to species differences or a difference in the state of the cells. HTM cell cultures treated with concentrations of BAK, found in common glaucoma medications, showed a dose-dependent, significant decrease in the percent of live cells. BAK treatment also caused elevated levels of MMP-9, a matrix metalloproteinase possibly implicated in the pathogenesis of glaucoma.54 In another study using HTM cells, travoprost+BAK treatment left statistically fewer live cells than both travoprost+sofZia and travoprost+PQ. A BAK dose-dependent reduction in cell viability was seen in both HTM and nonpigmented ciliary epithelial (NPCE) cells, a cell type relevant to aqueous inflow. After exposure to a 1:10 dilution of 0.02% BAK (ie, 0.002% BAK), there were significantly more living NPCE cells than HTM cells, indicating that NPCE cells may be less affected by BAK.55 By extension, BAK might thus have less impact on aqueous humor formation than on outflow through the TM, further promoting an increased IOP.
Comparing proapoptotic activity on cultured HTM cells treated with glaucoma medications, with and without preservatives, it was determined that beta-blockers alone did not exhibit proapoptotic activity. BAK alone significantly increased 3 apoptotic markers, while BAK containing beta-blockers and prostaglandin analogues produced mild expression of 1 out of 3 apoptotic markers. Similar to studies noted above using conjunctival cells, PGs and timolol may be somewhat cytoprotective against the proapoptotic effects of BAK in TM cells.56 In HTM cells exposed to the range of concentrations of BAK common to glaucoma drugs, BAK caused immediate necrosis at high concentrations, but apoptosis after treatment with low concentration.54
In a study investigating oxidative stress in TM cells, treatment with BAK concentrations found in commercial glaucoma drugs induced increased fibronectin mRNA expression, decreased MMP-2 mRNA, increased senescence biomarker SA-B-Gal activity, and cell death in nonstressed TM cells.46 ROS production, measured with the total ROS measurement probe H2DCF-DA, was significantly increased in HTM3 cells treated with BAK in concentrations ranging from 0.005% to 0.02%.57 These findings indicate that BAK may play a role in the production of ROS, which can lead to stress-induced premature senescence, another characteristic TM finding in primary open-angle glaucoma (POAG).48,58 It is interesting to note that BAK effects were consistently more pronounced in H2O2-stressed TM cells than in cells treated with BAK alone, suggesting that the stressed TM of glaucoma patients might also be more susceptible to the deleterious effects of BAK.
Immunohistochemical analyses of TM biopsy samples collected from patients undergoing trabeculectomy revealed a higher rate of inflammatory cell infiltrates and fibroblasts in patients who were treated with preserved glaucoma medications compared with untreated participants (who underwent primary surgery). Patients who received 2 or more drugs for at least 1 year had greater expression of inflammatory markers compared with those treated with just a beta-blocker for 1 year.59 In another study investigating TM specimens from glaucoma patients, inflammatory cells were found together with a dramatic decrease in TM cells.60 BAK has also been associated with subclinical anterior chamber inflammation/blood–aqueous barrier impairment. Specifically, in a randomized prospective, single-masked clinical trial, in which previously untreated ocular hypertension patients received timolol, preservative free or with BAK, a significant increase in anterior chamber aqueous flare (measured with a flare meter) was found in the BAK-treated eyes after 1 month.61
Criticisms of BAK toxicity studies point to their use of doses higher than those found in commercial drops, the difficulty of translating data from cell monolayers and in vivo rabbit studies to the clinical setting, and exposure times that exceed what would occur postdrop installation in a human eye with a normal tear film that dilutes BAK quickly.35 Glaucoma patients, however, have an abnormally high rate of OSD, including compromised tear film layers. Although tear washout can reduce the exposure time/concentration of BAK on the cornea, the preservative has a long half-life (20 h in the corneal and conjunctival epithelium and 11 h in deeper conjunctival structures).42 BAK was detected in the conjunctiva 168 h after a single 30 mL drop of 0.01% BAK in rabbits.42 Accumulation of BAK was found in the TM, iris, and lens samples obtained during cataract and/or glaucoma surgery in patients who had been treated for glaucoma for at least 10 years, including at least 5 years with 2 or more BAK-containing medications62(Fig. 1).
BAK Effects on the TM in Animals
Recently, an in vivo BAK localization and quantification study was done in rabbits.63 Topical drops of 0.01% BAK were administered twice a day for 5 months to 1 group, 0.2% BAK 1 drop a day for 1 month to another group, and a control group received no drops. BAK was detected in multiple deeper ocular structures: the sclera, the TM, near the optic nerve, and in the choroid.63 Additionally, inflammatory markers CD45-, RLA-DR-, and vimentin-positive cells were increased in these tissues in all BAK-treated eyes compared with controls (some positive cells are normally found in the conjunctiva and the limbus), and were elevated more in low-dose/long-term treated eyes than high-dose/short-term treated eyes, highlighting the duration of exposure as a key element in BAK toxicity as well as its ability to accumulate in deeper ocular structures.
Little in vivo data are available showing effects of BAK on IOP or outflow. In a rat model, after subconjunctival injections of 0.01% BAK, the IOP rose significantly at day 7 in BAK-injected eyes compared with vehicle-injected eyes. Inflammation was seen initially, but subsided after several days. A second injection was given on day 7 and IOP remained elevated for 6 more days, at which point, outflow facility measurements were performed. Outflow was significantly reduced in BAK-treated eyes compared with control eyes. Histological analysis by TUNEL labeling showed an increased density of apoptotic cells in the TM and iris root in BAK-injected eyes.57
Does BAK Worsen Glaucoma?
BAK can accumulate in deeper ocular structures, including the TM, and has the potential to cause changes similar to those found in the outflow pathways of glaucoma patients (cell senescence, apoptosis, cell loss, ECM accumulation, increased ROS, decreased MMPs, increased fibronectin, and an increase in inflammatory biomarkers. (Table 1) Several groups have hypothesized that long-term exposure to BAK could contribute to glaucoma worsening. Samples et al. noted that BAK-induced damage to TM cells occurred at very low concentrations and that chronic exposure to BAK containing drugs could impair the normal function of the TM, decrease outflow, and mimic the disease.51 Baudouin et al. hypothesized that BAK could accumulate in the TM, adding to the trabecular pathology characteristic of POAG by inducing chronic inflammation, inflammatory cytokine release, immune cell infiltration, and TM cell apoptosis, and that a possible mechanism for the apparent progressive loss of efficacy of topical IOP-lowering drugs could be the toxicity of the preservative exerting a significant pro-oxidative and/or proinflammatory effect on the TM.57 Brignole-Baudouin et al. suggested that BAK side effects might be indistinguishable from the disease outcome, as the treatment would be protective, but at the same time, although to a lesser extent, deleterious. They hypothesized that BAK effects could account for the percentage of glaucomatous patients whose disease progresses even with good IOP control.63 Ammar and Kahook pointed out that given the BAK ability to be absorbed and accumulate over time, the concentration of BAK in the aqueous humor may be much higher than in the active ingredient. Since brief treatment of cultured TM cells with BAK increased apoptotic cell markers and significantly decreased cell growth at levels 1/100th of that used as a preservative, the potentially harmful effect of BAK on TM cells may be an underappreciated concern.54 Hopes and Broadway concluded that since even low concentrations of BAK cause HTM cell toxic changes and apoptosis, long-term use of BAK-preserved topical drops could contribute to impaired trabecular function and potentially worsen any glaucomatous process within the TM.64
Table 1.
BAK, TGFβ2, and POAG Induce Similar Changes in TM Tissue/HTM Cells
| BAK exposure-HTM cells and animal models | TGFβ2 exposure-HTM cells and animal models | TM of POAG patients |
|---|---|---|
| Loss of cells | Loss of cells | Loss of cells |
| Increased: accumulation of ECM, apoptosis, ROS, inflammatory markers, fibronectin | Increased: accumulation of ECM, apoptosis, ROS, inflammatory markers, fibronectin | Increased: accumulation of ECM, apoptosis, ROS, inflammatory markers, fibronectin |
| Changes in the cytoskeleton | Changes in the cytoskeleton | Changes in the cytoskeleton |
| Cellular senescence-associated changes | Cellular senescence-associated changes | Cellular senescence-associated changes |
| Decreased outflow | Decreased outflow | Decreased outflow |
| ?????? | Presence of CLANS | Presence of CLANS |
BAK might play a role in the pathogenesis of POAG.
BAK, benzalkonium chloride; TGFβ2, transforming growth factor beta 2; POAG, primary open-angle glaucoma; TM, trabecular meshwork; HTM, human trabecular meshwork; ECM, extracellular matrix; ROS, reactive oxygen species.
??????, no data currently available.
Strategy for Future Studies
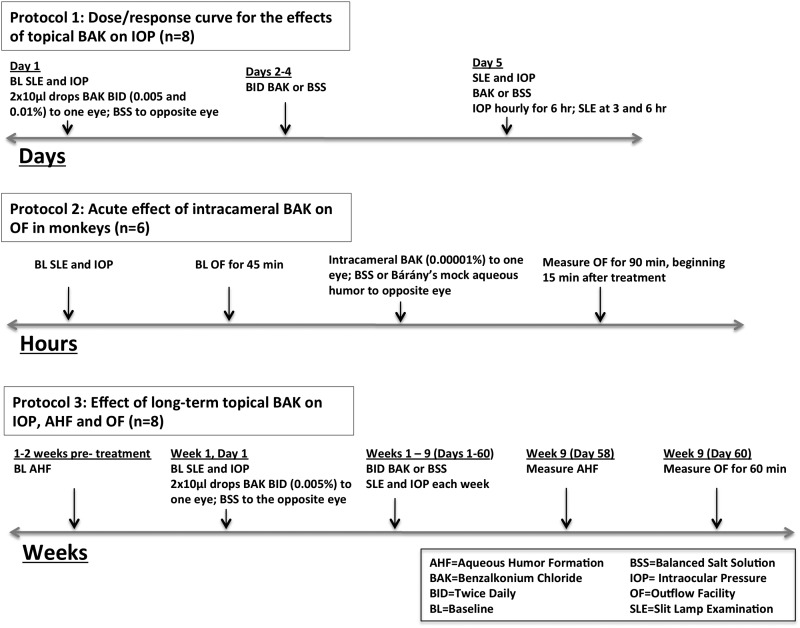
Studies in nonhuman primates, whose aqueous humor formation and drainage system most closely resemble that of humans, are the logical next step. We have begun in vivo IOP, aqueous formation, and outflow facility studies, in cynomolgus monkeys (Macaca fascicularis) to help elucidate the effects of BAK on aqueous humor dynamics. All experiments adhere to the Association for Research in Vision and Ophthalmology (ARVO) statement for the Use of Animals in Ophthalmic and Vision Research. The hypothesis is that chronic medical therapy with drugs containing BAK makes glaucomatous outflow tract pathology worse and itself damages the TM, decreasing outflow facility and possibly contributing to elevated IOP. The central question to answer in this series of nonhuman primate experiments is, does BAK given topically or intracamerally raise IOP in ocular normotensive monkeys and decrease outflow facility or the facility responses to other compounds known to increase facility directly (epinephrine, Rho kinase/myosin light-chain kinase inhibitors, latrunculins) or indirectly (pilocarpine)? The design for these studies includes experiments to determine the ocular tolerability and effects of different clinically relevant concentrations of BAK (0.005, 0.01, 0.05%) on IOP, aqueous humor formation, and total aqueous outflow facility65 (Fig. 2).
FIG. 2.
Proposed experimental protocols for studies on the effects of BAK on aqueous humor dynamics in cynomolgus monkeys. IOP, as measured by Goldmann applanation tonometry; AHF, as measured by scanning ocular fluorophotometry; outflow facility, as measured by the 2-level constant pressure perfusion of the anterior chamber technique. All monkeys determined to be ocularly normal by slit-lamp biomicroscopy before each experiment.
In summary, there is ample evidence that exposure to BAK is deleterious to cells and some ocular structures. It can accumulate over time in outflow tract tissues, including the TM, and has the potential to damage its structure/function in a variety of ways. These could include damaging mechanosensitivity, flexibility, signaling pathways, cytoskeleton, cell–cell and cell–ECM adhesion, and cellular and overall tissue contraction/relaxation properties and their regulation. Studies in a species whose anatomy, physiology, and pharmacological responses closely resemble humans are needed, with clinically relevant concentrations and dosing regimens of BAK. Understanding whether and how BAK affects aqueous humor dynamics will help inform clinical decision making and antiglaucoma drug development.
Acknowledgments
Supported by grants from the National Institutes of Health/National Eye Institute (University of Wisconsin-Madison Core Grant for Vision Research (P30 EY016665) and P51 RR000167); Merck, Inc.; Research to Prevent Blindness, Inc., New York, NY, unrestricted departmental and Physician-Scientist awards; Ocular Physiology Research and Education Foundation; and Walter Helmerich Chair from the Retina Research Foundation.
Author Disclosure Statement
C.A. Rasmussen: No competing financial interests exist.
P.L. Kaufman: AGTC (C, R), Lens AR, Inc. (F), WARF (F, P), Z Lens, LLC (F), Alcon (C, R), Allergan (C, R), Altheos, Inc. (C, R), Bausch & Lomb (C, R), Amakem Therapeutics (C, R), Johnson & Johnson (C, R), Merck (C, R, F), Pfizer (C, R), Santen (F, C, R), Refocus (C, R). C, consultant; R, honoraria, F, financial support; P, patent.
J.A. Kiland: No competing financial interests exist.
References
- 1.Coleman A.L., and Brigatti L.The glaucomas. Minerva Med. 92:365–379, 2001 [PubMed] [Google Scholar]
- 2.Resnikoff S., Pascolini D., Etya'ale D., et al. . Global data on visual impairment in 2002. Bull. World Health Organ. 82:844–845, 2004 [PMC free article] [PubMed] [Google Scholar]
- 3.Quigley H.A., and Broman A.The number of persons with glaucoma worldwide in 2010 and 2020. Br. J. Ophthalmol. 90:151–156, 2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bengtsson B., Leske M.C., Hyman L., and Heijl A.Early Manifest Glaucoma Trial Group. Fluctuation of intraocular pressure and glaucoma progression in the early manifest glaucoma trial. Ophthalmology. 114:205–209, 2007 [DOI] [PubMed] [Google Scholar]
- 5.Collaborative Normal-Tension Glaucoma Study Group. Comparison of glaucomatous progression between untreated patients with normal tension glaucoma and patients with therapeutically reduced intraocular pressure. Am. J. Ophthalmol. 126:487–497, 1998 [DOI] [PubMed] [Google Scholar]
- 6.Kass M.A., Heuer D.K., Higginbotham E.J., et al. . The ocular hypertension treatment study. Arch. Ophthalmol. 120:701–713, 2002 [DOI] [PubMed] [Google Scholar]
- 7.Chandrasekaran S., Cumming R.G., Rochtchina E., and Mitchell P.Associations between elevated intraocular pressure and glaucoma, use of glaucoma medications, and 5-year incident cataract: the Blue Mountains Eye Study. Ophthalmology. 113:417–424, 2006 [DOI] [PubMed] [Google Scholar]
- 8.Quigley H.A.Glaucoma. Lancet. 377:1367–1377, 2011 [DOI] [PubMed] [Google Scholar]
- 9.Medeiros F.A., and Weinreb R.N.Medical backgrounders: glaucoma. Drugs Today. 38:563, 2002 [DOI] [PubMed] [Google Scholar]
- 10.Leung E.W., Medeiros F.A., and Weinreb R.N.Prevalence of ocular surface disease in glaucoma patients. J. Glaucoma. 17:350–355, 2008 [DOI] [PubMed] [Google Scholar]
- 11.Fechtner R.D., Godfrey D.G., et al. . Prevalence of ocular surface complaints in patients with glaucoma using topical intraocular pressure-lowering medications. Cornea. 29:618–621, 2010 [DOI] [PubMed] [Google Scholar]
- 12.Katz G., Springs C.L., Craven E.R., and Montecchi-Palmer M.Ocular surface disease in patients with glaucoma or ocular hypertension treated with either BAK-preserved latanoprost or BAK-free travoprost. Clin. Ophthalmol. 4:1253–1261, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Rossi G.C., Pasinetti G.M., Scudeller L., Raimondi M., Lanteri S., and Bianchi P.E.Risk factors to develop ocular surface disease in treated glaucoma or ocular hypertension patients. Eur. J. Ophthalmol. 23:296–302, 2013 [DOI] [PubMed] [Google Scholar]
- 14.Freeman P.D., and Kahook M.Y.Preservatives in topical ophthalmic medications: historical and clinical perspectives. Expert Rev. Ophthalmol. 4:59–64, 2009 [Google Scholar]
- 15.Schein O.D., Munoz B., Tielsch J.M., Bandeen-Roche K., and West S.Prevalence of dry eye among the elderly. Am. J. Ophthalmol. 124:723–728, 1997 [DOI] [PubMed] [Google Scholar]
- 16.Malvitte L., Montange T., Vejux A., et al. . Measurement of inflammatory cytokines by multicytokine assay in tears of patients with glaucoma topically treated with chronic drugs. Br. J. Ophthalmol. 91:29–32, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Sherwood M.B., Grierson I., Millar L., and Hitchings R.A.Long-term morphologic effects of antiglaucoma drugs on the conjunctiva and Tenon's capsule in glaucomatous patients. Ophthalmology. 96:327–335, 1989 [DOI] [PubMed] [Google Scholar]
- 18.Lavin M.J., Wormald RPL, Migdal C.S., and Hitchings R.A.The influence of prior therapy on the success of trabeculectomy. Arch. Ophthalmol. 108:1543–1548, 1990 [DOI] [PubMed] [Google Scholar]
- 19.Broadway D.C., Grierson I., O'Brien C., et al. . Adverse effect of antiglaucoma medication: II. The outcome of filtration surgery. Arch. Ophthalmol. 112:1446–1454, 1994 [DOI] [PubMed] [Google Scholar]
- 20.Boimer C., and Birt C.M.Preservative exposure and surgical outcomes in glaucoma patients: the PESO Study. J. Glaucoma. 2013. DOI: 10.1097/IJG.0b013e31825af67d [DOI] [PubMed] [Google Scholar]
- 21.The United State Pharmacopoeia 24th revision. The National Formulary, 19th edition. Rockville, MD: the United States Pharmacopeial Convention, Inc.; 2000 [Google Scholar]
- 22.European Pharmacopoeia, 7th edition Council of Europe, Strasbourg; 2010 [Google Scholar]
- 23.Tripathi B.J., Tripathi R.C., and Killi S.P.Cytotoxicity of ophthalmic preservatives on human corneal epithelium. Lens Eye Toxic Res. 9:361–337, 1992 [PubMed] [Google Scholar]
- 24.Yee R.W.The effect of drop vehicle on the efficacy and side effects of topical glaucoma therapy: a review. Curr. Opin. Ophthalmol. 18:134–139, 2007 [DOI] [PubMed] [Google Scholar]
- 25.Brignole-Baudouin F., Riancho L., Liang H., Nakib Z., and Baudouin C.In vitro comparative toxicology of polyquad-preserved and benzalkonium chloride-preserved travoprost/timolol fixed combination and latanoprost/timolol fixed combination. J. Ocul. Pharmacol. Ther. 27:273–280, 2011 [DOI] [PubMed] [Google Scholar]
- 26.Baudouin C., Labbé A, Liang H., Pauly A., and Brignole-Baudouin F.Preservatives in eyedrops: the good, the bad and the ugly. Prog. Retin. Eye Res. 29:312–334, 2010 [DOI] [PubMed] [Google Scholar]
- 27.Noecker R.J., Herrygers L.A., and Anwaruddin R.Corneal and conjunctival changes caused by commonly used glaucoma medications. Cornea. 23:490–496, 2004 [DOI] [PubMed] [Google Scholar]
- 28.Epstein S.P., Ahdoot M., Marcus E., and Asbell P.A.Comparative toxicity of preservatives on immortalized corneal and conjunctival epithelial cells. J. Ocul. Pharmacol. Ther. 25:113–119, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Aihara M., Oshima H., Araie M; EXTraKT study group. Effects of SofZia-preserved travoprost and benzalkonium chloride-preserved latanoprost on the ocular surface—a multicentre randomized single-masked study. Acta Ophthalmol. 91:e7–e14, 2013 [DOI] [PubMed] [Google Scholar]
- 30.Ammar D.A., Noecker R.J., and Kahook M.Y.Effects of benzalkonium chloride-preserved, polyquad-preserved, and sofZia-preserved topical glaucoma medications on human ocular epithelial cells. Adv. Ther. 27:837–845, 2010 [DOI] [PubMed] [Google Scholar]
- 31.Rouland J.F., Traverso C.E., Stalmans I., et al. . Efficacy and safety of preservative-free latanoprost eyedrops, compared with BAK-preserved latanoprost in patients with ocular hypertension or glaucoma. Br. J. Ophthalmol. 97:196–200, 2013 [DOI] [PubMed] [Google Scholar]
- 32.Gross R.L., Peace J.H., Smith S.E., et al. . Duration of IOP reduction with travoprost BAK-free solution. J. Glaucoma. 17:217–222, 2008 [DOI] [PubMed] [Google Scholar]
- 33.Gandolfi S., Paredes T., Goldberg I., et al. . Comparison of a travoprost BAK-free &&formulation preserved with polyquaternium-1 with BAK-preserved travoprost in ocular hypertension or open-angle glaucoma. Eur. J. Ophthalmol. 22:34–44, 2012 [DOI] [PubMed] [Google Scholar]
- 34.Renieri G., Fuhrer K., Scheithe K., et al. . Efficacy and tolerability of preservative- free eye drops containing a fixed combination of dorzolamide and timolol in glaucoma patients. J. Ocul. Pharmacol. Ther. 26:597–603, 2010 [DOI] [PubMed] [Google Scholar]
- 35.Tressler C.S., Beatty R., and Lemp M.A.Preservative use in topical glaucoma medications. Ocul. Surf. 9:140–158, 2011 [DOI] [PubMed] [Google Scholar]
- 36.Januleviciene I., Derkac I., Grybauskiene L., et al. . Effects of preservative-free tafluprost on tear film osmolarity, tolerability, and intraocular pressure in previously treated patients with open-angle glaucoma. Clin. Ophthalmol. 6:103–109, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Jaenen N., Baudouin C., Pouliquen P., et al. . Ocular symptoms and signs with preserved and preservative-free glaucoma medications. Eur. J. Ophthalmol. 17:341–349, 2007 [DOI] [PubMed] [Google Scholar]
- 38.Uusitalo H., Chen E., Pfeiffer N., et al. . Switching from a preserved to a preservative-free prostaglandin preparation in topical glaucoma medication. Acta Ophthalmol. 88:329–336, 2010 [DOI] [PubMed] [Google Scholar]
- 39.Renieri G., Fuhrer K., Scheithe K., et al. . Efficacy and tolerability of preservative- free eye drops containing a fixed combination of dorzolamide and timolol in glaucoma patients. J. Ocul. Pharmacol. Ther. 26:597–603, 2010 [DOI] [PubMed] [Google Scholar]
- 40.Whitson J.T., Trattler W.B., Matossian C., Williams J., and Hollander D.A.Ocular surface tolerability of prostaglandin analogs in patients with glaucoma or ocular hypertension. J. Ocul. Pharmacol. Ther. 26:287–292, 2010 [DOI] [PubMed] [Google Scholar]
- 41.Aihara M., Otani S.I., Kozaki J., et al. . Long-term effect of BAK-free travoprost on ocular surface and intraocular pressure in glaucoma patients after transition from latanoprost. J. Glaucoma. 21:60–64, 2012 [DOI] [PubMed] [Google Scholar]
- 42.Champeau E., and Edelhauser H.Effect of ophthalmic preservatives on the ocular surface: conjunctival and corneal uptake and distribution of benzalkonium chloride and chlorhexidine digluconate. In: Holly F., Lamberts D., MacKeen D., eds. The Preocular Tear Film in Health, Disease, and Contact Lens Wear. Lubbock, TX: Dry Eye Institute, Inc.; 292–302, 1986 [Google Scholar]
- 43.Guenoun J.M., Baudouin C., Rat P., et al. . In vitro study of inflammatory potential and toxicity profile between latanoprost, travoprost, and bimatoprost on conjunctiva-derived epithelial cells. Invest. Ophthalmol. Vis. Sci. 46:2444–2450, 2005 [DOI] [PubMed] [Google Scholar]
- 44.Pisella P.J., Debbasch C., Hamard P., et al. . Conjunctival proinflammatory and proapoptotic effects of latanoprost and preserved and unpreserved timolol: an ex vivo and in vitro study. Invest. Ophthalmol. Vis. Sci. 45:1360–1368, 2004 [DOI] [PubMed] [Google Scholar]
- 45.Guenoun J.M., Baudouin C., Rat P., et al. . In vitro comparison of cytoprotective and antioxidative effects of latanoprost, travoprost, and bimatoprost on conjunctiva-derived epithelial cells. Invest. Ophthalmol. Vis. Sci. 2005;40:4594–4599 [DOI] [PubMed] [Google Scholar]
- 46.Yu A.L., Fuchshofer R., Kampik A., and Welge-Lüssen U.Effects of oxidative stress in trabecular meshwork cells are reduced by prostaglandin analogues. Invest. Ophthalmol. Vis. Sci. 49:4872–4880, 2008 [DOI] [PubMed] [Google Scholar]
- 47.Lütjen-Drecoll E.Morphological changes in glaucomatous eyes and the role of TGFbeta2 for the pathogenesis of the disease. Exp. Eye. Res. 81:1–4, 2005 [DOI] [PubMed] [Google Scholar]
- 48.Liton P.B., Challa P., Stinnett S., et al. . Cellular senescence in the glaucomatous outflow pathway. Exp. Gerontol. 40:745–748, 2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Izzotti A., Bagnis A., and Sacca S.C.The role of oxidative stress in glaucoma. Mutat. Res. 612:105–114, 2006 [DOI] [PubMed] [Google Scholar]
- 50.Baleriola J., García-Feijoo J., Martínez-de-la-Casa J.M., et al. . Apoptosis in the trabecular meshwork of glaucomatous patients. Mol. Vis. 14:1513–1516, 2008 [PMC free article] [PubMed] [Google Scholar]
- 51.Samples J.R., Binder P.S., and Nayak S.The effect of epinephrine and benzalkonium chloride on cultured corneal endothelial and trabecular meshwork cells. Exp. Eye Res. 49:1–12, 1989 [DOI] [PubMed] [Google Scholar]
- 52.Kawa J.E., Higginbotham E.J., Chang I.L., and Yue B.Y.Effects of antiglaucoma medications on bovine trabecular meshwork cells in vitro. Exp. Eye Res. 57:557–565, 1993 [DOI] [PubMed] [Google Scholar]
- 53.Tripathi B.J., Tripathi R.C., and Millard C.B.Epinephrine-induced toxicity of human trabecular cells in vitro. Lens Eye Toxic. Res. 6:141–156, 1989 [PubMed] [Google Scholar]
- 54.Ammar D.A., and Kahook M.Y.Effects of benzalkonium chloride- or polyquad-preserved fixed combination glaucoma medications on human trabecular meshwork cells. Mol. Vis. 17:1806–1813, 2011 [PMC free article] [PubMed] [Google Scholar]
- 55.Ammar D.A., and Kahook M.Y.Effects of glaucoma medications and preservatives on cultured human trabecular meshwork and non-pigmented ciliary epithelial cell lines. Br. J. Ophthalmol. 95:1466–1469, 2011 [DOI] [PubMed] [Google Scholar]
- 56.Hamard P., Blondin C., Debbasch C., et al. . In vitro effects of preserved and unpreserved antiglaucoma drugs on apoptotic marker expression by human trabecular cells. Graefes Arch. Clin. Exp. Ophthalmol. 241:1037–1043, 2003 [DOI] [PubMed] [Google Scholar]
- 57.Baudouin C., Denoyer A., Desbenoit N., Hamm G., and Grise A.In vitro and in vivo experimental studies on trabecular meshwork degeneration induced by benzalkonium chloride (An American Ophthalmological Society Thesis). Trans. Am. Ophthalmol. Soc. 110:40–63, 2012 [PMC free article] [PubMed] [Google Scholar]
- 58.Toussaint O., Medrano E.E., and von Zglinicki T.Cellular and molecular mechanisms of stress-induced premature senescence (SIPS) of human diploid fibroblasts and melanocytes. Exp. Gerontol. 35:927–945, 2000 [DOI] [PubMed] [Google Scholar]
- 59.Baudouin C., Pisella P.J., Fillacier K., et al. . Ocular surface inflammatory changes induced by topical antiglaucoma drugs: human and animal studies. Ophthalmology. 106:556–563, 1999 [DOI] [PubMed] [Google Scholar]
- 60.Hamard P., Valtot F., Sourdille P., Bourles-Dagonet F., and Baudouin C.Confocal microscopic examination of trabecular meshwork removed during ab externo trabeculectomy. Br. J. Ophthalmol. 86:1046–1052, 2002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Stevens A.M., Kestelyn P.A., De Bacquer D., and Kestelyn P.G.Benzalkonium chloride induces anterior chamber inflammation in previously untreated patients with ocular hypertension as measured by flare meter: a randomized clinical trial. Acta Ophthalmol. 90:e221–e224, 2012 [DOI] [PubMed] [Google Scholar]
- 62.Desbenoit N., Schmitz-Afonso I., Baudouin C., et al. . Localisation and quantification of benzalkonium chloride in eye tissue by TOF-SIMS imaging and liquid chromatography mass spectrometry. Anal. Bioanal. Chem. 405:4039–4049, 2013 [DOI] [PubMed] [Google Scholar]
- 63.Brignole-Baudouin F., Desbenoit N., Hamm G., et al. . A new safety concern for glaucoma treatment demonstrated by mass spectrometry imaging of benzalkonium chloride distribution in the eye, an experimental study in rabbits. PLoS One. 7:e50180, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Hopes M., and Broadway D.Preservative-free treatment in glaucoma is a sensible and realistic aim for the future. Eur. Ophthalmic Rev. 4:23–28, 2010 [Google Scholar]
- 65.Bárány E.H.Simultaneous measurement of changing intraocular pressure and outflow facility in the vervet monkey by constant pressure infusion. Invest. Ophthalmol. 3:135–143, 1964 [PubMed] [Google Scholar]