Abstract
Glucocorticoid (GC)-induced ocular hypertension (OHT) is a serious side effect of GC therapy in susceptible individuals. This OHT is due to increased aqueous humor (AH) outflow resistance in the trabecular meshwork (TM) caused by GC-mediated changes in TM structure and function. GCs may also play a role in the development of primary open-angle glaucoma (POAG). Elevated cortisol levels in the AH or enhanced GC sensitivity may be one of the reasons for elevated intraocular pressure in POAG patients. The GC OHT responder population is at greater risk of developing POAG compared with non-responders. We recently have gained insight into the molecular mechanisms responsible for this differential GC responsiveness, which is attributed to differences in GC receptor isoform expression in the TM. This article summarizes current knowledge on alternative GC receptor splicing to generate GC receptor alpha (GRα) and GRβ and their roles in the regulation of GC responsiveness in normal and glaucoma TM.
Introduction
The glucocorticoid receptor (GR) is a ligand-activated transcription factor that mediates numerous physiological functions and is the target for anti-inflammatory and immunosuppressive glucocorticoids (GCs). GCs are a group of natural (cortisol) or synthetic [dexamethasone (DEX), prednisolone] ligands that maintain normal metabolism, homeostasis, and immune regulation. Many of these properties of GCs have been exploited for anti-inflammatory, anti-allergic, and immunosuppressive uses. However, prolonged exposure to GCs can lead to a number of serious systemic and local adverse side effects. These adverse effects of GCs are dependent on GC potency, dosage form, pharmacokinetics, and route of administration. The systemic side effects include hyperglycemia, osteoporosis, immunodeficiency, and altered protein and lipid metabolism.
Ocular side effects of GCs include cataract and ocular hypertension (OHT) that can lead to glaucoma. Like primary open-angle glaucoma (POAG), GC-induced OHT is due to impaired aqueous humor (AH) outflow in the trabecular meshwork (TM). GCs have diverse effects on TM cells.1 Experimentally, ex vivo studies using human and bovine eye anterior segments2,3 and in vivo work in multiple species1 prove the direct role of GCs in increased AH outflow resistance that is similar to glaucoma pathology in the TM. GCs increase extracellular matrix (ECM) proteins4–7 decrease matrix metalloproteinase activity8,9 and increase expression of TIMP1,10 resulting in the deposition of ECM in the TM.11 GCs reduce phagocytic activity of TM cells12,13 that may further lead to increased outflow resistance and elevated intraocular pressure (IOP). In addition, GCs reorganize the TM cytoskeleton that affects TM cell migration, proliferation, and function,14,15 and most likely also TM cell contractibility. GCs also increase the expression of myocilin, the first identified glaucoma gene,16,17 but it is currently not known whether this plays any role in GC-induced OHT. Elevated levels of cortisol in the plasma18–20 and AH19 of POAG patients and their interactions with other pathways21–23 may worsen the outcome of their disease.
Not all individuals equally respond to GC therapy, and there are clear cases of GC resistance and enhanced GC responsiveness.24–31 There also are individual differences in GC responsiveness to the ocular side effect of GC-induced OHT. Normal individuals who are “steroid responders” develop elevated IOP (>6 mm Hg) following topical administration of GCs 3–4 times a day for 4–6 weeks.11,32 Approximately 40% of the normal population are considered to be “steroid responders.” In contrast, almost all POAG patients are steroid responders.32 Interestingly, non-glaucomatous steroid responders are at higher risk for developing POAG compared to non-glaucomatous non-responders.33,34 One main reason for differential responsiveness to GCs at the molecular level has been attributed to the relative expression levels of the 2 alternatively spliced GR isoforms GRα and GRβ.24–26,28,30,35,36
Regulation of GC Activity
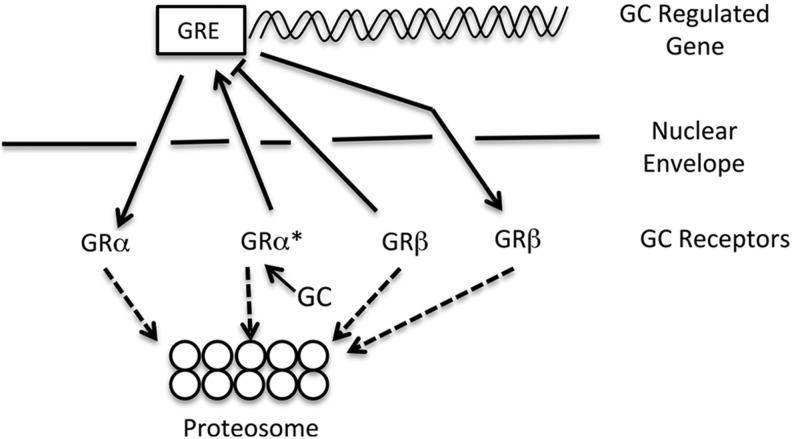
There are a number of mechanisms that regulate GC activity (Table 1). GRα is the biological receptor for GCs and acts as a ligand-activated transcription factor. GRα is a >90 kDa protein that normally resides in cytoplasm. Binding to its highly lipophilic ligand (GC) causes a conformational change and the release of accessory proteins. Ligand-bound GRα is recognized by importins and translocated to nucleus (Fig. 1). Glucocorticoid response elements (GREs) on GC regulated genes facilitate the homodimerization of the GC-bound GR complex. This interaction between ligand-bound GR dimer and GREs drives transcription of various genes (transactivation). Binding of GRα homodimers to negative GREs can suppress gene transcription. Activated GRα can also alter gene expression in a GRE-independent manner by directly binding to and inhibiting other transcription factors such as AP-1 and NFkB in a process known as transrepression.21–23 Activated GRα can also form heterodimers with other steroid receptors such as estrogen,37 mineralocorticoid,38 or androgen receptors.39 Depending on which accessory proteins (coactivator/corepressor/HDAC) become associated with this complex, there is either inhibition or activation of GRE-mediated transcription. Similarly, the receptor heterodimers can bind to other responsive sequences on DNA such as estrogen response elements and depending upon the other factors recruited, transcription is either activated (transactivation) or repressed (transrepression). It was initially proposed that the major anti-inflammatory actions of GCs were mediated via transrepression, but this hypothesis has been recently challenged.40 In addition to regulated interaction with other transcription factors, GR activity can also be regulated by transport into and out of the nucleus (Fig. 1). GCs can also trigger rapid signaling cascade independent of direct transcriptional or genomic changes.41–43 Examples include effects on mitogen-associated protein kinases44–52 and protein kinase C.53–55
Table 1.
Mechanisms Regulating Glucocorticoid Activity in Trabecular Meshwork Cells
| Mechanism | References |
|---|---|
| Potency of GC ligand | 1,11 |
| Alternative splicing regulating levels of GRα and GRβ | 78,83 |
| [Multiple translation initiation sites on GR mRNA] | 58 |
| [Factors affecting stability of GRα and GRβ mRNA] | |
| Nuclear import of GRα and/or GRβ | 62,65 |
| [Nuclear export of GRα and/or GRβ] | |
| Proteosome degradation of GRα and/or GRβ | 62 |
| [Heterodimerization with other steroid receptors] | 37–39 |
| [Presence of tissue-specific enhancers and/or repressors] | |
“[]” indicates mechanisms shown in other cell types.
GC, glucocorticoid; GR, glucocorticoid receptor.
FIG. 1.
Nuclear import and export of glucocorticoid receptor alpha (GRα) and GRβ and proteosomal degradation regulate glucocorticoid (GC) activities. Both GRα and GRβ proteins reside within the cytoplasm. Upon binding glucocorticoids (GC), GRα becomes activated (GRα*) and is translocated into the nucleus through the nuclear pore complex. GRα* then dimerizes and binds to glucocorticoid response elements (GRE) on GC-regulated genes to increase or decrease gene transcription. GRβ is translocated into the nucleus in a ligand-independent manner where it then acts as a dominant negative regulator by blocking GRα* activity. The levels of both GRα and GRβ can also be regulated by degradation of these proteins in the proteasome complex.
GC Receptor Beta
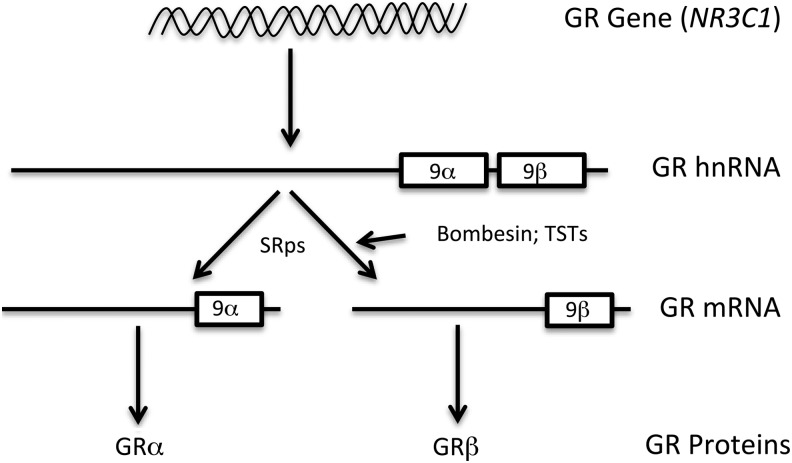
GR activity is also regulated by the different ratios of the alternatively spliced GRα and GRβ isoforms (Fig. 2). The GR gene NR3C1 pre-mRNA (hnRNA) consists of 9 exons, and the last exon consists of 2 exons, 9α and 9β, separated by a short intronic sequence. Alternative splicing generates 2 isoforms: one incorporates exon 9α to form GRα and the other instead splices in exon 9β giving rise to GRβ.56 Since the exon9 encodes the ligand-binding (LB) domain (LBD), the resultant 2 isoforms greatly differ in their LB properties. GRα is a longer isoform with 777 amino acids and a fully functional LBD, whereas GRβ is a shorter version with 742 amino acids that loses its LBD and is thus unable to bind GCs.56 The GRβ isoform is still capable of forming heterodimers with GRα, but the complex has substantially diminished transcriptional activity compared with the GRα-GRα homodimer. In this way, GRβ acts as a dominant negative inhibitor of GRα transcriptional activities and provides enhanced resistance to the biological and pharmacological effects of GCs. There is evidence that GRβ does possess transcriptional activity in stably transfected HeLa cells, but this is mainly due to interaction with other transcription factors or ligand-independent transactivation via the DNA-binding domain.57 High GRβ levels are associated with GC-resistant asthma24 and GC-resistant rheumatoid arthritis.27 Both GRα and GRβ are expressed in the TM.30,31 GRβ levels were found to be lower in TM cells isolated from glaucoma patients' eyes compared with TM cells derived from normal individuals.30 Lower GRβ expression in the TM of glaucoma patients or steroid responders makes these cells more susceptible to GCs, leading to ECM buildup and cytoskeletal changes in the TM either by endogenous cortisol (such as found to be higher in AH of glaucoma patients) or by the administration of exogenous GCs (like DEX). This may explain the elevated IOP in glaucoma or the inherited tendency to elevate IOP in steroid responders. Adding further complexity to alternative splicing of the GR, multiple additional GRα and GRβ isoforms can be formed post-transcriptionally as the result of alternate translation initiation sites that can also regulate GC activity.58
FIG. 2.
Alternative splicing of the human GR. The GR gene (NR3C1) contains terminal exons 9α and 9β that are alternatively spliced from the primary hnRNA transcript to generate GRα and/or GRβ mRNAs. SRps 20, 30, and 40 in the spliceosome complex are involved in this differential splicing. Exogeneous compounds [bombesin or thailanstatins (TSTs)] also regulate this alternative splicing. The GRα and GRβ mRNAs are translated to form the ligand (GC) binding GRα isoform and the dominant negative regulator isoform GRβ.
The GRβ isoform has been conserved evolutionarily and can be traced back to zebrafish, suggesting its importance in regulation of GC activity. Although the patterns of splicing events differ among species, the ultimate properties and functions of GRβ remain the same by inhibition of GRα activities. In zebrafish, the splicing occurs at exon 8 of the GR gene instead of exon 9 of human GR gene.59 The mouse GRβ (mGRβ) arises from alternative splicing utilizing intron 8 rather than exon 9 as in humans.60 Similar intron inclusion occurs in generation of rat GRβ.61 These GRβ proteins do not possess LB properties, show similar subcellular localization and expression levels, and act as dominant negative inhibitors of GRα activities in all these species.59,60,61 Therefore, the GRβ isoform is invaluable in the study of steroid responsiveness. We have recently reported differential DEX responsiveness in an ex vivo perfusion culture model of bovine eyes anterior segments.3 We are now in the process of determining whether altered expression of bovine GRβ is responsible for these differences in DEX-induced OHT.
Regulated Nuclear Transport
Differences in the levels of GRβ can determine the steroid responsiveness among the individuals. In addition, nuclear import and export of the 2 GRα and GRβ isoforms can also regulate the GC activity (Fig. 1). The heat-shock protein 90 (HSP90), a molecular chaperone, is involved in nuclear transport of GRα and GRβ as shown by co-immunoprecipitation and transfection experiments.62 Once in nucleus, GRβ inhibits the GRα transcriptional activity and provides GC resistance. Treatment with 17-AAG, an inhibitor of HSP90 chaperone activity, blocks the nuclear transport of GRβ and facilitates its degradation.62 Therefore, proteosomal degradation of GRα or GRβ would alter the GRα/GRβ ratio and also regulate GC responsiveness in TM cells.62 The FK-506 binding immunophilin FKBP51 has been implicated in GC resistance in primates.63,64 In cultured human TM cells, FKBP51 maintains the constitutive GC-independent transport of GRα and GRβ from cytoplasm to nucleus, whereas FKBP52 is involved in nuclear transport of the GC-bound activated GRα, but FKBP52 is not involved in GRβ translocation.65 FK506 facilitated FKBP51-mediated nuclear transport of GRβ and significantly reduced DEX-mediated GRE-luciferase activity in normal TM (NTM) cells, but had little effect on GRβ translocation in glaucoma glaucomatous TM (GTM) cells.65 Indeed, FK506 potentiated DEX-mediated GRE-luciferase activity in GTM cells. These differential responses to FK506 may be explained by differences in cellular levels and/or activities of FKBP51 and FKBP52.
Regulation of GR Splicing
Alternative mRNA splicing occurs for the majority of protein-encoding genes; more than 95% of transcriptome undergoes alternative splicing to generate diversity of mRNAs and proteins.66 This process is tightly regulated inside cells. Any disruption in either cis-acting sequences on pre-mRNA or in activities/levels of trans-acting elements can affect this process, causing diseases. Alternative splicing is carried out by a specialized assembly of proteins and RNA called the spliceosome, which consists of 5 small nuclear ribonucleoproteins and different serine-arginine (SR) proteins (SRps).67 RNA–RNA, RNA–protein, and protein–protein interactions ensure inclusion of the right number and order of exons in the final mRNA product.68 In addition to 3 core sequences (i.e., 5′ splice site, 3′ splice site, and the branch sequence), there are additional sequences located within introns or exons or intron–exon junctions that recruit SRps.69–71 Each SRp has a signature RNA recognition motif, and their levels or activities can modulate splice site recognition to promote alternative splicing. A critical balance of SRps and their antagonistic regulators is necessary for exon inclusion in mRNA transcripts.72 These interactive SRps are also involved in mRNA nuclear export and translation, further elucidating their importance inside cells.73–77
In fact, SRps are master regulators controlling alternative mRNA splicing of GR (Fig. 2) and other genes in the TM78 and other cell types.79,80 Transient transfection experiments showed that SRp20 favors more GRα splicing, whereas SRp30c and SRp40 overexpression increases GRβ levels in TM cells.78 The increased GRβ levels by overexpressing SRp30c or SRp40 are associated with decreased DEX activity in TM cells.78 SRp20 overexpression increases GC responsiveness in NTM cells (which are low GC responder cells), whereas SRp30c and SRp40 overexpression decreases GCs responsiveness in GTM cells (which have greater GC responsiveness). There are differences in predicted binding sites for SRps on exon 9 of the GR gene, with more SRp20 sites on exon 9β and more SRp40 sites on exon 9α.78 Also, SRp40 mRNA expression was lower in GTM cell strains compared with NTM cell strains, which correlated to lower GRβ expression in GTM cell strains.78 It would be interesting to determine what other TM cell genes SRp40 regulates in terms of alternative splicing and whether these genes are involved in glaucoma pathophysiology. Single-nucleotide polymorphism genotyping of DNA samples from normal controls, POAG patients, and steroid responders did not show any significant allele frequency differences for SRp20, SRp30c, or SRp40 between cohorts.81 This suggests it could be the relative levels or activities of SRps that may determine GC responsiveness in these groups.
Peptides such as bombesin78,79 and other chemical modulators of alternative splicing have further illustrated a role for SRps in determining GC responsiveness in TM cells. Thailanstatins (TSTs) are a new class of microbial-derived compounds that are very potent regulators of alternative mRNA splicing.82 Bombesin and 3 TSTs (TST-A, TST-B, and TST-C) increase GRβ levels in TM cells and decrease GC responsiveness assessed by DEX induction of ECM (fibronectin) production, myocilin secretion, and GRE-luciferase reporter activity.78,83 Currently, we are studying the TST mechanism of action and performing translational studies in ex vivo and in vivo models of steroid responsiveness to further clarify this mechanism. We will determine the effects of specific spliceosome modulators on a broad range of SRps. RNA interference-mediated knock down studies will also help identify what other SRps (in addition to SRp20, 30c, and 40) are involved in GR splicing. It is also possible that these compounds affect mRNA stability or the rate of mRNA transcription rather than direct interaction with the spliceosome.
Future Directions
The process of GR alternative splicing could possibly be exploited for therapeutic intervention. Manipulation of the alternative splicing to generate more GRβ in the TM would diminish endogenous cortisol activity in POAG patients and prevent GC-induced OHT in steroid responders. Another potential therapeutic approach to treat GC-induced OHT and glaucoma would be to use gene therapy to selectively overexpress GRβ in the TM. Several viral expression vectors, including adenovirus Ad5 and lentiviruses, have selective tropism for the TM,84–87 which would prevent GC-induced biochemical, morphological, and physiological changes in TM but still allow the use of GCs for treating inflammatory conditions in steroid responders, including POAG patients. Bombesin and spliceosome modulators like TSTs have shown great promise in vitro in increasing GRβ in TM cell strains.78,83 It would be worth testing these agents in ex vivo and in vivo models of GC-induced OHT. GC-induced OHT occurs in multiple species including non-human primates,88 rabbits,89 cats,90 cows,91 sheep,92 rats,93 and mice.94 However, the trabecular outflow pathway in mice95,96 and rats is more similar to primates, compared with these other species.
The present findings reinforce the need for appropriate steroid-induced glaucoma models. Ex vivo models of DEX-induced OHT involving perfusion of eye anterior segments provide fast and reliable tools to test these alternative splicing promoting compounds and determine their molecular mechanisms of action. We have demonstrated that DEX-induced OHT occurs in perfusion organ culture models of human eyes.2 Mao et al. recently developed an ex vivo model of steroid-induced OHT using anterior segments of bovine eyes that is fast, reliable, and cost-effective.3 GC-induced OHT in both these models occurs in ∼40% of the DEX perfused eyes, which mimics the GC response seen clinically, and will allow us to determine the potential roles of GRβ in determining this dichotomous GC response on IOP. Cloning of the endogenous bovine GRβ will strengthen the effectiveness of the bovine model. Generation of GRβ knockout and GRβ transgenic mice will also help in understanding the physiological role of GRβ in GC-induced OHT. Overexpressing hGRβ or mGRβ in the mouse TM using viral expression vectors can be used to study the local effects on topically or systemically administered GCs. These proposed studies would help us better understand the physiological role of GR splicing and GRβ in IOP regulation. This would also help in understanding the role of GRβ in glaucoma etiology to aid in the discovery of novel therapeutic targets and strategies.
Acknowledgments
This work was supported by NEI/NIH grant R01 EY016242.
Author Disclosure Statement
No competing financial interests exist.
References
- 1.Clark A.F., and Wordinger R.J.The role of steroids in outflow resistance. Exp. Eye Res. 88:752–759, 2009 [DOI] [PubMed] [Google Scholar]
- 2.Clark A.F., Wilson K., de Kater A.W., Allingham R.R., and McCartney M.D.Dexamethasone-induced ocular hypertension in perfusion-cultured human eyes. Invest. Ophthalmol. Vis. Sci. 36:478–489, 1995 [PubMed] [Google Scholar]
- 3.Mao W., Tovar-Vidales T., Yorio T., Wordinger R.J., and Clark A.F.Perfusion-cultured bovine anterior segments as an ex vivo model for studying glucocorticoid-induced ocular hypertension and glaucoma. Invest. Ophthalmol. Vis. Sci. 52:8068–8075, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Yun A.J., Murphy C.G., Polansky J.R., Newsome D.A., and Alvarado J.A.Proteins secreted by human trabecular cells. Glucocorticoid and other effects. Invest. Ophthalmol. Vis. Sci. 30:2012–2022, 1989 [PubMed] [Google Scholar]
- 5.Steely H.T., Browder S.L., Julian M.B., Miggans S.T., Wilson K.L., and Clark A.F.The effects of dexamethasone on fibronectin expression in cultured human trabecular meshwork cells. Invest. Ophthalmol. Vis. Sci. 33:2242–2250, 1992 [PubMed] [Google Scholar]
- 6.Dickerson J.E., Jr., Steely H.T., Jr., English-Wright S.L., and Clark A.F.The effect of dexamethasone on integrin and laminin expression in cultured human trabecular meshwork cells. Exp. Eye Res. 66:731–738, 1998 [DOI] [PubMed] [Google Scholar]
- 7.Zhou L., Li Y., and Yue B.Y.Glucocorticoid effects on extracellular matrix proteins and integrins in bovine trabecular meshwork cells in relation to glaucoma. Int. J. Mol. Med. 1:339–346, 1998 [PubMed] [Google Scholar]
- 8.Snyder R.W., Stamer W.D., Kramer T.R., and Seftor R.E.Corticosteroid treatment and trabecular meshwork proteases in cell and organ culture supernatants. Exp. Eye Res. 57:461–468, 1993 [DOI] [PubMed] [Google Scholar]
- 9.Seftor R.E., Stamer W.D., Seftor E.A., and Snyder R.W.Dexamethasone decreases tissue plasminogen activator activity in trabecular meshwork organ and cell cultures. J. Glaucoma 3:323–328, 1994 [PubMed] [Google Scholar]
- 10.Samples J.R., Alexander J.P., and Acott T.S.Regulation of the levels of human trabecular matrix metalloproteinases and inhibitor by interleukin-1 and dexamethasone. Invest. Ophthalmol. Vis. Sci. 34:3386–3395, 1993 [PubMed] [Google Scholar]
- 11.Wordinger R.J., and Clark A.F.Effects of glucocorticoids on the trabecular meshwork: towards a better understanding of glaucoma. Prog. Retin Eye Res. 18:629–667, 1999 [DOI] [PubMed] [Google Scholar]
- 12.Matsumoto Y., and Johnson D.H.Dexamethasone decreases phagocytosis by human trabecular meshwork cells in situ. Invest. Ophthalmol. Vis. Sci. 38:1902–1907, 1997 [PubMed] [Google Scholar]
- 13.Zhang X., Ognibene C.M., Clark A.F., and Yorio T.Dexamethasone inhibition of trabecular meshwork cell phagocytosis and its modulation by glucocorticoid receptor beta. Exp. Eye Res. 84:275–284, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Clark A.F., Wilson K., McCartney M.D., Miggans S.T., Kunkle M., and Howe W.Glucocorticoid-induced formation of cross-linked actin networks in cultured human trabecular meshwork cells. Invest. Ophthalmol. Vis. Sci. 35:281–294, 1994 [PubMed] [Google Scholar]
- 15.Clark A.F., Brotchie D., Read A.T., et al. . Dexamethasone alters F-actin architecture and promotes cross-linked actin network formation in human trabecular meshwork tissue. Cell Motil. Cytoskeleton 60:83–95, 2005 [DOI] [PubMed] [Google Scholar]
- 16.Clark A.F., Steely H.T., Dickerson J.E. Jr., et al. . Glucocorticoid induction of the glaucoma gene MYOC in human and monkey trabecular meshwork cells and tissues. Invest. Ophthalmol. Vis. Sci. 42:1769–1780, 2001 [PubMed] [Google Scholar]
- 17.Nguyen T.D., Chen P., Huang W.D., Chen H., Johnson D., and Polansky J.R.Gene structure and properties of TIGR, an olfactomedin-related glycoprotein cloned from glucocorticoid-induced trabecular meshwork cells. J. Biol. Chem. 273:6341–6350, 1998 [DOI] [PubMed] [Google Scholar]
- 18.Ray S., Mehra K.S., Misra S., and Singh R.Plasma cortisol in glaucoma. Ann. Ophthalmol. 9:1151–1154, 1977 [PubMed] [Google Scholar]
- 19.Rozsival P., Hampl R., Obenberger J., Starka L., and Rehak S.Aqueous humour and plasma cortisol levels in glaucoma and cataract patients. Curr. Eye Res. 1:391–396, 1981 [DOI] [PubMed] [Google Scholar]
- 20.Schwartz B., McCarty G., and Rosner B.Increased plasma free cortisol in ocular hypertension and open angle glaucoma. Arch. Ophthalmol. 105:1060–1065, 1987 [DOI] [PubMed] [Google Scholar]
- 21.McKay L.I., and Cidlowski J.A.Cross-talk between nuclear factor-kappa B and the steroid hormone receptors: mechanisms of mutual antagonism. Mol. Endocrinol. 12:45–56, 1998 [DOI] [PubMed] [Google Scholar]
- 22.Song C.Z., Tian X., and Gelehrter T.D.Glucocorticoid receptor inhibits transforming growth factor-beta signaling by directly targeting the transcriptional activation function of Smad3. Proc. Natl. Acad. Sci. U. S. A. 96:11776–11781, 1999 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Schule R., Rangarajan P., Kliewer S., et al. . Functional antagonism between oncoprotein c-Jun and the glucocorticoid receptor. Cell. 62:1217–1226, 1990 [DOI] [PubMed] [Google Scholar]
- 24.Hamid Q.A., Wenzel S.E., Hauk P.J., et al. . Increased glucocorticoid receptor beta in airway cells of glucocorticoid-insensitive asthma. Am. J. Respir. Crit. Care Med. 159:1600–1604, 1999 [DOI] [PubMed] [Google Scholar]
- 25.Hamilos D.L., Leung D.Y., Muro S., et al. . GRbeta expression in nasal polyp inflammatory cells and its relationship to the anti-inflammatory effects of intranasal fluticasone. J. Allergy Clin. Immunol. 108:59–68, 2001 [DOI] [PubMed] [Google Scholar]
- 26.Longui C.A., Vottero A., Adamson P.C., et al. . Low glucocorticoid receptor alpha/beta ratio in T-cell lymphoblastic leukemia. Horm. Metab. Res. 32:401–406, 2000 [DOI] [PubMed] [Google Scholar]
- 27.Derijk R.H., Schaaf M.J., Turner G., et al. . A human glucocorticoid receptor gene variant that increases the stability of the glucocorticoid receptor beta-isoform mRNA is associated with rheumatoid arthritis. J. Rheumatol. 28:2383–2388, 2001 [PubMed] [Google Scholar]
- 28.Walker B.R., Phillips D.I., Noon J.P., et al. . Increased glucocorticoid activity in men with cardiovascular risk factors. Hypertension. 31:891–895, 1998 [DOI] [PubMed] [Google Scholar]
- 29.Kino T., De Martino M.U., Charmandari E., Mirani M., and Chrousos G.P.Tissue glucocorticoid resistance/hypersensitivity syndromes. J. Steroid Biochem. Mol. Biol. 85:457–467, 2003 [DOI] [PubMed] [Google Scholar]
- 30.Zhang X., Clark A.F., and Yorio T.Regulation of glucocorticoid responsiveness in glaucomatous trabecular meshwork cells by glucocorticoid receptor-beta. Invest. Ophthalmol. Vis. Sci. 46:4607–4616, 2005 [DOI] [PubMed] [Google Scholar]
- 31.Zhang H., Zhao G.H., Zhang Q., et al. . [Relationship between glucocorticoid receptors in the peripheral blood lymphocytes and trabecular meshwork and glucocorticoid induced glaucoma]. Zhonghua Yan Ke Za Zhi. 42:431–434, 2006 [PubMed] [Google Scholar]
- 32.Armaly M.F., and Becker B.Intraocular pressure response to topical corticosteroids. Fed. Proc. 24:1274–1278, 1965 [PubMed] [Google Scholar]
- 33.Lewis J.M., Priddy T., Judd J., et al. . Intraocular pressure response to topical dexamethasone as a predictor for the development of primary open-angle glaucoma. Am. J. Ophthalmol. 106:607–612, 1988 [DOI] [PubMed] [Google Scholar]
- 34.Kitazawa Y., and Horie T.The prognosis of corticosteroid-responsive individuals. Arch. Ophthalmol. 99:819–823, 1981 [DOI] [PubMed] [Google Scholar]
- 35.Honda M., Orii F., Ayabe T., et al. . Expression of glucocorticoid receptor beta in lymphocytes of patients with glucocorticoid-resistant ulcerative colitis. Gastroenterology. 118:859–866, 2000 [DOI] [PubMed] [Google Scholar]
- 36.Hagg P.M., Hurskainen T., Palatsi R., Ilves M., and Oikarinen A.Increased expression of glucocorticoid receptor beta in lymphocytes of patients with severe atopic dermatitis unresponsive to topical corticosteroid. Br. J. Dermatol. 162:318–324, 2010 [DOI] [PubMed] [Google Scholar]
- 37.Karmakar S., Jin Y., and Nagaich A.K.Interaction of glucocorticoid receptor (GR) with estrogen receptor (ER) alpha and activator protein 1 (AP1) in dexamethasone-mediated interference of ERalpha activity. J. Biol. Chem. 288:24020–24034, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Savory J.G., Prefontaine G.G., Lamprecht C., et al. . Glucocorticoid receptor homodimers and glucocorticoid-mineralocorticoid receptor heterodimers form in the cytoplasm through alternative dimerization interfaces. Mol. Cell. Biol. 21:781–793, 2001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Chen S., Wang J., Yu G., Liu W., and Pearce D.Androgen and glucocorticoid receptor heterodimer formation. A possible mechanism for mutual inhibition of transcriptional activity. J. Biol. Chem. 272:14087–14092, 1997 [DOI] [PubMed] [Google Scholar]
- 40.Vandevyver S., Dejager L., Tuckermann J., and Libert C.New insights into the anti-inflammatory mechanisms of glucocorticoids: an emerging role for glucocorticoid-receptor-mediated transactivation. Endocrinology. 154:993–1007, 2013 [DOI] [PubMed] [Google Scholar]
- 41.Croxtall J.D., Choudhury Q., and Flower R.J.Glucocorticoids act within minutes to inhibit recruitment of signalling factors to activated EGF receptors through a receptor-dependent, transcription-independent mechanism. Br. J. Pharmacol. 130:289–298, 2000 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Hafezi-Moghadam A., Simoncini T., Yang Z., et al. . Acute cardiovascular protective effects of corticosteroids are mediated by non-transcriptional activation of endothelial nitric oxide synthase. Nat. Med. 8:473–479, 2002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Limbourg F.P., Huang Z., Plumier J.C., et al. . Rapid nontranscriptional activation of endothelial nitric oxide synthase mediates increased cerebral blood flow and stroke protection by corticosteroids. J. Clin. Invest. 110:1729–1738, 2002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Krstic M.D., Rogatsky I., Yamamoto K.R., and Garabedian M.J.Mitogen-activated and cyclin-dependent protein kinases selectively and differentially modulate transcriptional enhancement by the glucocorticoid receptor. Mol. Cell. Biol. 17:3947–3954, 1997 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Swantek J.L., Cobb M.H., and Geppert T.D.Jun N-terminal kinase/stress-activated protein kinase (JNK/SAPK) is required for lipopolysaccharide stimulation of tumor necrosis factor alpha (TNF-alpha) translation: glucocorticoids inhibit TNF-alpha translation by blocking JNK/SAPK. Mol. Cell. Biol. 17:6274–6282, 1997 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Hulley P.A., Gordon F., and Hough F.S.Inhibition of mitogen-activated protein kinase activity and proliferation of an early osteoblast cell line (MBA 15.4) by dexamethasone: role of protein phosphatases. Endocrinology. 139:2423–2431, 1998 [DOI] [PubMed] [Google Scholar]
- 47.Mitchell R.A., Metz C.N., Peng T., and Bucala R.Sustained mitogen-activated protein kinase (MAPK) and cytoplasmic phospholipase A2 activation by macrophage migration inhibitory factor (MIF). Regulatory role in cell proliferation and glucocorticoid action. J. Biol. Chem. 274:18100–18106, 1999 [DOI] [PubMed] [Google Scholar]
- 48.Bell L.M., Leong M.L., Kim B., et al. . Hyperosmotic stress stimulates promoter activity and regulates cellular utilization of the serum- and glucocorticoid-inducible protein kinase (Sgk) by a p38 MAPK-dependent pathway. J. Biol. Chem. 275:25262–25272, 2000 [DOI] [PubMed] [Google Scholar]
- 49.Lucki A., Klein E., Karry R., and Ben-Shachar D.Dexamethasone in the presence of desipramine enhances MAPK/ERK1/2 signaling possibly via its interference with beta-arrestin. J. Neural. Transm. 2013. Epub October17 PMID: [DOI] [PubMed] [Google Scholar]
- 50.Bouazza B., Debba-Pavard M., Amrani Y., et al. . Basal p38 MAPK regulates unliganded glucocorticoid receptor function in airway smooth muscle cells. Am. J. Respir. Cell Mol. Biol. 50:301–315, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Li W., Lin J.T., Sun L.C., Zhou T.L., Zhang L., and Shu J.[Mechanism of p38 mitogen-activated protein kinase inhibitor SB203580 to glucocorticoid sensitivity]. Zhonghua Yi Xue Za Zhi. 92:2570–2573, 2012 [PubMed] [Google Scholar]
- 52.Ayroldi E., Cannarile L., Migliorati G., Nocentini G., Delfino D.V., and Riccardi C.Mechanisms of the anti-inflammatory effects of glucocorticoids: genomic and nongenomic interference with MAPK signaling pathways. FASEB J. 26:4805–4820, 2012 [DOI] [PubMed] [Google Scholar]
- 53.Gadson P., McCoy J., Wikstrom A.C., and Gustafsson J.A.Suppression of protein kinase C and the stimulation of glucocorticoid receptor synthesis by dexamethasone in human fibroblasts derived from tumor tissue. J. Cell. Biochem. 43:185–198, 1990 [DOI] [PubMed] [Google Scholar]
- 54.McConkey D.J., Hartzell P., Jondal M., and Orrenius S.Inhibition of DNA fragmentation in thymocytes and isolated thymocyte nuclei by agents that stimulate protein kinase C. J. Biol. Chem. 264:13399–13402, 1989 [PubMed] [Google Scholar]
- 55.Migliorati G., Pagliacci M.C., D'Adamio F., Crocicchio F., Nicoletti I., and Riccardi C.Glucocorticoid-induced DNA fragmentation: role of protein-kinase-C activity. Pharmacol. Res. 26Suppl 2:5–9, 1992 [DOI] [PubMed] [Google Scholar]
- 56.Oakley R.H., Sar M., and Cidlowski J.A.The human glucocorticoid receptor beta isoform. Expression, biochemical properties, and putative function. J. Biol. Chem. 271:9550–9559, 1996 [DOI] [PubMed] [Google Scholar]
- 57.Kino T., Manoli I., Kelkar S., Wang Y., Su Y.A., and Chrousos G.P.Glucocorticoid receptor (GR) beta has intrinsic, GRalpha-independent transcriptional activity. Biochem. Biophys. Res. Commun. 381:671–675, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Lu N.Z., and Cidlowski J.A.Translational regulatory mechanisms generate N-terminal glucocorticoid receptor isoforms with unique transcriptional target genes. Mol. Cell 18:331–342, 2005 [DOI] [PubMed] [Google Scholar]
- 59.Schaaf M.J., Champagne D., van Laanen I.H., et al. . Discovery of a functional glucocorticoid receptor beta-isoform in zebrafish. Endocrinology. 149:1591–1599, 2008 [DOI] [PubMed] [Google Scholar]
- 60.Hinds T.D., Jr., Ramakrishnan S., Cash H.A., et al. . Discovery of glucocorticoid receptor-beta in mice with a role in metabolism. Mol. Endocrinol. 24:1715–1727, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.DuBois D.C., Sukumaran S., Jusko W.J., and Almon R.R.Evidence for a glucocorticoid receptor beta splice variant in the rat and its physiological regulation in liver. Steroids. 78:312–320, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Zhang X., Clark A.F., and Yorio T.Heat shock protein 90 is an essential molecular chaperone for nuclear transport of glucocorticoid receptor beta. Invest. Ophthalmol. Vis. Sci. 47:700–708, 2006 [DOI] [PubMed] [Google Scholar]
- 63.Reynolds P.D., Ruan Y., Smith D.F., and Scammell J.G.Glucocorticoid resistance in the squirrel monkey is associated with overexpression of the immunophilin FKBP51. J. Clin. Endocrinol. Metab. 84:663–669, 1999 [DOI] [PubMed] [Google Scholar]
- 64.Scammell J.G., Denny W.B., Valentine D.L., and Smith D.F.Overexpression of the FK506-binding immunophilin FKBP51 is the common cause of glucocorticoid resistance in three New World primates. Gen. Comp. Endocrinol. 124:152–165, 2001 [DOI] [PubMed] [Google Scholar]
- 65.Zhang X., Clark A.F., and Yorio T.FK506-binding protein 51 regulates nuclear transport of the glucocorticoid receptor beta and glucocorticoid responsiveness. Invest. Ophthalmol. Vis. Sci. 49:1037–1047, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Pan Q., Shai O., Lee L.J., Frey B.J., and Blencowe B.J.Deep surveying of alternative splicing complexity in the human transcriptome by high-throughput sequencing. Nat. Genet. 40:1413–1415, 2008 [DOI] [PubMed] [Google Scholar]
- 67.Will C.L., and Luhrmann R.Spliceosome structure and function. Cold Spring Harb. Perspect. Biol. 3(7), 2011. PMID: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Kohtz J.D., Jamison S.F., Will C.L., et al. . Protein-protein interactions and 5'-splice-site recognition in mammalian mRNA precursors. Nature. 368:119–124, 1994 [DOI] [PubMed] [Google Scholar]
- 69.Wu J.Y., and Maniatis T.Specific interactions between proteins implicated in splice site selection and regulated alternative splicing. Cell. 75:1061–1070, 1993 [DOI] [PubMed] [Google Scholar]
- 70.Blencowe B.J.Exonic splicing enhancers: mechanism of action, diversity and role in human genetic diseases. Trends Biochem. Sci. 25:106–110, 2000 [DOI] [PubMed] [Google Scholar]
- 71.Black D.L.Mechanisms of alternative pre-messenger RNA splicing. Annu. Rev. Biochem. 72:291–336, 2003 [DOI] [PubMed] [Google Scholar]
- 72.Martinez-Contreras R., Cloutier P., Shkreta L., Fisette J.F., Revil T., and Chabot B.hnRNP proteins and splicing control. Adv. Exp. Med. Biol. 623:123–147, 2007 [DOI] [PubMed] [Google Scholar]
- 73.Caceres J.F., Screaton G.R., and Krainer A.R.A specific subset of SR proteins shuttles continuously between the nucleus and the cytoplasm. Genes Dev. 12:55–66, 1998 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Huang Y., and Steitz J.A.Splicing factors SRp20 and 9G8 promote the nucleocytoplasmic export of mRNA. Mol. Cell 7:899–905, 2001 [DOI] [PubMed] [Google Scholar]
- 75.Huang Y., Gattoni R., Stevenin J., and Steitz J.A.SR splicing factors serve as adapter proteins for TAP-dependent mRNA export. Mol. Cell 11:837–843, 2003 [DOI] [PubMed] [Google Scholar]
- 76.Sanford J.R., Gray N.K., Beckmann K., and Caceres J.F.A novel role for shuttling SR proteins in mRNA translation. Genes Dev. 18:755–768, 2004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Michlewski G., Sanford J.R., and Caceres J.F.The splicing factor SF2/ASF regulates translation initiation by enhancing phosphorylation of 4E-BP1. Mol. Cell 30:179–189, 2008 [DOI] [PubMed] [Google Scholar]
- 78.Jain A., Wordinger R.J., Yorio T., and Clark A.F.Spliceosome protein (SRp) regulation of glucocorticoid receptor isoforms and glucocorticoid response in human trabecular meshwork cells. Invest. Ophthalmol. Vis. Sci. 53:857–866, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Zhu J., Gong J.Y., Goodman O.B., Jr., Cartegni L., Nanus D.M., and Shen R.Bombesin attenuates pre-mRNA splicing of glucocorticoid receptor by regulating the expression of serine-arginine protein p30c (SRp30c) in prostate cancer cells. Biochim. Biophys. Acta 1773:1087–1094, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Yan X.B., Tang C.H., Huang Y., et al. . Alternative splicing in exon 9 of glucocorticoid receptor pre-mRNA is regulated by SRp40. Mol. Biol. Rep. 37:1427–1433, 2010 [DOI] [PubMed] [Google Scholar]
- 81.Fingert J.H., Alward W.L., Wang K., Yorio T., and Clark A.F.Assessment of SNPs associated with the human glucocorticoid receptor in primary open-angle glaucoma and steroid responders. Mol. Vis. 16:596–601, 2010 [PMC free article] [PubMed] [Google Scholar]
- 82.Liu X., Biswas S., Berg M.G., et al. . Genomics-guided discovery of thailanstatins A, B, and C As pre-mRNA splicing inhibitors and antiproliferative agents from Burkholderia thailandensis MSMB43. J. Nat. Prod. 76:685–693, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Jain A., Liu X., Wordinger R.J., Yorio T., Cheng Y.Q., and Clark A.F.Effects of thailanstatins on glucocorticoid response in trabecular meshwork and steroid-induced glaucoma. Invest. Ophthalmol. Vis. Sci. 54:3137–3142, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Borras T., Matsumoto Y., Epstein D.L., and Johnson D.H.Gene transfer to the human trabecular meshwork by anterior segment perfusion. Invest. Ophthalmol. Vis. Sci. 39:1503–1507, 1998 [PubMed] [Google Scholar]
- 85.Borras T., Rowlette L.L., Erzurum S.C., and Epstein D.L.Adenoviral reporter gene transfer to the human trabecular meshwork does not alter aqueous humor outflow. Relevance for potential gene therapy of glaucoma. Gene Ther. 6:515–524, 1999 [DOI] [PubMed] [Google Scholar]
- 86.Loewen N., Fautsch M.P., Teo W.L., Bahler C.K., Johnson D.H., and Poeschla E.M.Long-term, targeted genetic modification of the aqueous humor outflow tract coupled with noninvasive imaging of gene expression in vivo. Invest. Ophthalmol. Vis. Sci. 45:3091–3098, 2004 [DOI] [PubMed] [Google Scholar]
- 87.Loewen N., Bahler C., Teo W.L., et al. . Preservation of aqueous outflow facility after second-generation FIV vector-mediated expression of marker genes in anterior segments of human eyes. Invest. Ophthalmol. Vis. Sci. 43:3686–3690, 2002 [PubMed] [Google Scholar]
- 88.Fingert J.H., Clark A.F., Craig J.E., et al. . Evaluation of the myocilin (MYOC) glaucoma gene in monkey and human steroid-induced ocular hypertension. Invest. Ophthalmol. Vis. Sci. 42:145–152, 2001 [PubMed] [Google Scholar]
- 89.Lorenzetti O.J.Effects of corticosteroids on ocular dynamics in rabbits. J. Pharmacol. Exp. Ther. 175:763–772, 1970 [PubMed] [Google Scholar]
- 90.Zhan G.L., Miranda O.C., and Bito L.Z.Steroid glaucoma; corticosteroid-induced ocular hypertension in cats. Exp. Eye Res. 54:211–218, 1992 [DOI] [PubMed] [Google Scholar]
- 91.Gerometta R., Podos S.M., Candia O.A., et al. . Steroid-induced ocular hypertension in normal cattle. Arch. Ophthalmol. 122:1492–1497, 2004 [DOI] [PubMed] [Google Scholar]
- 92.Gerometta R., Podos S.M., Danias J., et al. . Steroid-induced ocular hypertension in normal sheep. Invest. Ophthalmol. Vis. Sci. 50:669–673, 2009 [DOI] [PubMed] [Google Scholar]
- 93.Sawaguchi K., Nakamura Y., Nakamura Y., et al. . Myocilin gene expression in the trabecular meshwork of rats in a steroid-induced ocular hypertension model. Ophthalmic Res. 37:235–242, 2005 [DOI] [PubMed] [Google Scholar]
- 94.Whitlock N.A., McKnight B., Corcoran K.N., et al. . Increased intraocular pressure in mice treated with dexamethasone. Invest. Ophthalmol. Vis. Sci. 51:6496–6503, 2010 [DOI] [PubMed] [Google Scholar]
- 95.Smith R.S., Zabeleta A., Savinova O.V., et al. . The mouse anterior chamber angle and trabecular meshwork develop without cell death. BMC Dev. Biol. 1:3, 2001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Ko M.K., and Tan J.C.Contractile markers distinguish structures of the mouse aqueous drainage tract. Mol. Vis. 19:2561–2570, 2013 [PMC free article] [PubMed] [Google Scholar]