Abstract
The zebrafish larva is a promising whole-animal model for safety pharmacology, environmental risk assessment, and developmental toxicity. This model has been used for the high-throughput toxicity screening of various compounds. Our aim here is to identify possible phenotypic markers of teratogenicity in zebrafish embryos that could be used for the assaying compounds for reproductive toxicity. We have screened a panel of 60 water-soluble toxicants to examine their effects on zebrafish development. A total of 22,080 wild-type zebrafish larvae were raised in 250 μL defined buffer in 96-well plates at a plating density of one embryo per well. They were exposed for a 96-h period starting at 24 h post-fertilization. A logarithmic concentration series was used for range-finding, followed by a narrower geometric series for developmental toxicity assessment. A total of 9017 survivors were analyzed at 5 days post-fertilization for nine phenotypes, namely, (1) normal, (2) pericardial oedema, (3) yolk sac oedema, (4) melanophores dispersed, (5) bent tail tip, (6) bent body axis, (7) abnormal Meckel's cartilage, (8) abnormal branchial arches, and (9) uninflated swim bladder. For each toxicant, the EC50 (concentration required to produce one or more of these abnormalities in 50% of embryos) was also calculated. For the majority of toxicants (55/60) there was, at the population level, a statistically significant, concentration-dependent increase in the incidence of abnormal phenotypes among survivors. The commonest abnormalities were pericardial oedema, yolk sac oedema, dispersed melanophores, and uninflated swim bladder. It is possible therefore that these could prove to be general indicators of reproductive toxicity in the zebrafish embryo assay.
Introduction
The zebrafish embryo is a promising alternative model in some fields of biomedical research, such as drug screening, safety pharmacology, and developmental toxicity assessment.1–9 This whole-animal model may be useful as a rapid, high-throughput, low-cost assay in the early stages of the drug-development pipeline.8 Recent studies reported that the zebrafish embryo model has good predictivity for the toxicity and teratogenicity of compounds in rodents.4,10–17
The zebrafish has been extensively used in toxicological studies to screen either single compounds or small panels of compounds (reviewed by refs.18–22). Examples include the use of adult zebrafish for the testing of lead and uranium,23 malathion,24 colchicine,25 anilines,26 and metronidazole,27 and the use of juveniles and embryos for testing agricultural biocides.28,29 Not only adult zebrafish, but also zebrafish embryos or larvae are used in toxicity studies (reviewed by Truong et al.30). Examples of this application include the toxicity testing of nanoparticles31–34 and chemical compounds from different pharmacological classes,11 and developmental toxicity testing of ethanol35–37 and other compounds.13,14,38–47
There are several advantages of using zebrafish embryos in biomedical research (reviewed in Ali et al.20). These include the small size of the embryo, the small volume of test compound required for testing, and the relatively rapid development of the embryo. Many major organ systems are partially developed at 5 days post-fertilization (dpf).5,48 Further, cellular and molecular pathways implicated in the response to chemicals or stress, as well as many developmental pathways, show evolutionary conservation between the zebrafish and mammals.48–51 Broad homologies of zebrafish to other vertebrate species (including rodents and humans) include similarities in their genome, brain patterning, and the structure and function of several neural and physiological systems, including the stress-regulating axis.3,52–64
Although there are some essential similarities between zebrafish and mammals, there are also some important differences. The zebrafish is ectothermic, lacks cardiac septa, synovial joints, lungs, and other structures.65–67 The last common ancestor of zebrafish and mammals probably lived around 445 million years ago.68 For these and other reasons, some phenotypic effects produced by toxicants in humans are difficult or impossible to model in the zebrafish. Another negative issue concerning the use of the early zebrafish embryo in compound screening is that it remains inside the chorion (an extraembryonic membrane) until at least 48 hours post-fertilization (hpf).48 This is significant because the chorion may constitute a barrier to compound diffusion into the embryo.69,70
Given that the use of zebrafish embryos in biomedical research is increasing, there is a need for further research into the model.7 Our aim here is to examine the teratogenicity to zebrafish embryos of a panel of 60 toxicants. Because we add the compounds to the water in which the embryos develop, we focus on water-soluble compounds to avoid any confusing effects of carrier solvents. The compounds were selected (in a previous study11) to represent a range of chemical classes and toxicological mechanisms without a priori knowledge of their effects on the zebrafish embryo. Survivors were analyzed at 5 dpf for the presence of eight abnormal phenotypes (the remainder being classified as normal). We did not aim to make a comprehensive survey of organ morphology since this would be impracticable given the large scale of this study and the number of compounds and replicates used. Instead, we selected readouts for their ease of screening in a medium-throughput context. Note that the present study is part of a large-scale toxicity study in this lab, and the LC50 data (96-h duration of exposure) of this same panel of 60 water-soluble toxicants have been already published by us.11 The same set of embryos used in that study are analyzed here. In addition, we have previously tested the effects of these 60 compounds on zebrafish embryo behavioral responses.71
Materials and Methods
The embryos analyzed in this study are the same specimens for which mortality rates (and behavioral responses to toxicants) have already been reported.11,71 For the sake of completeness, we give the materials and methods used in that study11 in the following sections, together with additional information on the EC50 calculations and malformations.
Ethics statement
All animal experimental procedures were conducted in accordance with local and international regulations. The local regulation is the Wet op de dierproeven (Article 9) of Dutch Law (National) and the same law administered by the Bureau of Animal Experiment Licensing, Leiden University (Local). This local regulation serves as the implementation of Guidelines on the protection of experimental animals by the Council of Europe, Directive 86/609/EEC, which allows zebrafish embryos to be used up to the moment of free living (∼5–7 days after fertilization). Because embryos used here were no more than 5 days old, no license is required by Council of Europe (1986), Directive 86/609/EEC, or the Leiden University ethics committee.
Animals
Male and female adult zebrafish (Danio rerio) of AB wild-type were purchased from Selecta Aquarium Speciaalzaak who obtains stock from Europet Bernina International BV. The AB strain is a wild-type strain (see www.zfin.org) and shows high genetic diversity, increasing the likelihood that we will detect idiosyncratic responses to the toxicants. Fish were kept at a maximum density of 100 individuals in glass recirculation aquaria (L=80 cm, H=50 cm, and W=46 cm) on a 14-h light:10-h dark cycle (lights on at 08.00). Water and air were temperature controlled (26±0.5°C and 23°C, respectively). The fish were fed twice daily with “Sprirulina” brand flake food (O.S.L. Marine Lab., Inc.) and twice a week with frozen Artemia (Dutch Select Food; Aquadistri BV).
Defined embryo buffer
To produce a defined and standardized vehicle (control) for these experiments, we used 10% Hanks' balanced salt solution (made from cell-culture-tested, powdered Hanks' salts, without sodium bicarbonate, Cat. No. H6136-10X1L; Sigma-Aldrich) at a concentration of 0.98 g/L in Milli-Q water (resistivity=18.2 MΩ·cm), with the addition of sodium bicarbonate at 0.035 g/L (cell culture tested; Sigma Cat. No. S5761), and adjusted to pH 7.46. A similar medium has been used previously as a zebrafish embryo buffer.11,35,72
Embryo care
Eggs were obtained by random pairwise mating of zebrafish. Three adult males and four females were placed together in small breeding tanks (Ehret GmbH) the evening before eggs were required. The breeding tanks (L=26 cm, H=12.5 cm, and W=20 cm) had mesh egg traps to prevent the eggs from being eaten. The eggs were harvested the following morning and transferred into 92-mm plastic Petri dishes (50 eggs per dish) containing 40 mL fresh embryo buffer. Eggs were washed four times to remove debris. Further, unfertilized, unhealthy, and dead embryos were identified under a dissecting microscope and removed by selective aspiration with a pipette. At 3.5 hpf, embryos were again screened and any further dead and unhealthy embryos were removed. Throughout all procedures, the embryos and the solutions were kept at 28±0.5°C, either in the incubator or a climatized room under a light cycle of 14 h light:10 h dark (lights on at 08.00). All pipetting was done manually, with an eight-channel pipetter.
Test compounds
We used water-soluble toxic compounds representing a range of different chemical classes and biochemical activities (Supplementary Table S1; Supplementary Data are available online at www.liebertpub.com/zeb). These compounds have been screened by us for embryo lethality in two previous studies.11,71 The required dilution was always freshly prepared in buffer just prior to the assay on zebrafish embryos.
Range finding
To determine a suitable range of concentrations for testing, we performed range finding using a logarithmic series (0, 1, 10, 100, and 1000 mg/L) as recommended in standard protocols.73 Zebrafish embryos of 24 hpf were gently transferred from the Petri dish using a sterile plastic pipette into 96-well sterile microtitre plates (Costar 3599; Corning, Inc.). A single embryo was plated per well, so that dead embryos would not affect others, and also to allow individual embryos to be tracked for the whole duration of the experiment. A static nonreplacement regime was used (thus, there was no replacement or refreshment of buffer after the addition of compound). Each well contained 250 μL of either freshly prepared test compound, or vehicle only (buffer) as control. All pipetting of compounds and vehicle was done manually, with an eight-channel pipetter. We used 16 embryos for each concentration and 16 embryos as controls for each compound. The embryos for controls and treatment groups for each compound were plated in the same 96-well microtitre plates in each independent experiment.
Mortality scoring
Mortality rates at 48, 72, 96, and 120 hpf in both logarithmic series and geometric series were determined using a dissecting stereomicroscope as previously described.11
Geometric series
After the range-finding experiments, a series of concentrations lying in the range between 0% and 100% mortality were selected. The actual concentrations used are shown in Supplementary Table S2. The concentrations were in a geometric series in which each concentration was double the next lowest value.73 Each geometric series of concentrations of each compound was repeated three times (in total 48 embryos per concentration and 48 embryos for vehicle for each compound). The embryos for controls and treatment groups for each compound were plated in the same 96-well microtitre plates in each independent experiment.
Morphological assessment of larval phenotypes in the survivor population
Larvae at 5 days were fixed in 4% paraformaldehyde in phosphate-buffered saline at pH 7.2 at 4°C overnight. They were then rinsed five times in distilled water and dehydrated in a graded ethanol series (25%, 50%, and 70%) for 5 min each. Larvae were then rinsed in acid alcohol (1% concentrated hydrochloric acid in 70% ethanol) for 10 min. They were then placed in filtered Alcian blue solution (0.03% Alcian blue in acid alcohol) overnight. Larvae were subsequently differentiated in acid alcohol for 1 h and washed 2×30 min in distilled water. Finally, they were cleared and stored in glycerol. All larvae remained in their original multiwell plates, so that each individual could be tracked throughout the entire experimental and analysis procedure. Analysis of larval morphology was carried out using a dissecting stereomicroscope. The phenotypes were scored according to the criteria listed in Table 1.
Table 1.
Phenotype Analysis
| Larval phenotype | Criteria |
|---|---|
| 1. Normal | Absence of any of the phenotypes listed below |
| 2. Pericardial oedema | Pericardium abnormally swollen by the accumulation of pellucid fluid |
| 3. Yolk sac oedema | Yolk sac swollen with accumulated pellucid fluid |
| 4. Melanophores (pigment cells) dispersed | Melanophores overlying ventral half of yolk sac region are relatively pale in color, with dispersed melanosomes (in contrast to the normal phenotype in which the melanophores are dark in color, punctuate, and have aggregated melanosomes). Note: we did not score iridophores or xanthophores |
| 5. Tail bent | Tail at the axial level of the caudal fin is abnormally flexed dorsoventrally or laterally |
| 6. Body axis bent | Primary axis (excluding the “tail” as defined above) is abnormally flexed dorsoventrally or laterally |
| 7. Meckel's cartilage abnormal | Meckel's cartilage grossly hypoplastic, missing or unfused in midline. These effects may be unilateral or bilateral |
| 8. Branchial arches abnormal | One or more cartilages of the branchial skeleton hypoplastic or missing |
| 9. Swim bladder uninflated | The swim bladder is unexpanded (in contrast to the normal phenotype at this stage in which there is a prominent, dilated lumen) |
Description of the nine categories used to score larval phenotypes at 5 dpf. See Figure 2 for selected illustrations of these phenotypes.
We take the presence of any one or more of the seven phenotypic abnormalities listed in this table to classify a compound as a teratogen for the purposes of this study.
dpf, days post-fertilization.
Statistical analysis and EC50 determination
Statistical analyses were performed using GraphPad Prism for Windows (version 5.03). To see the impact of compounds on zebrafish larvae development, we used one-way analysis of variance and Dunnett's multiple-comparison test with a probability level of 5% as the minimal criterion of significance. EC50 (expressed in mg compound/L of buffer) was determined based on morphological assessment of three independent experiments from geometric series using Regression Probit Analysis with SPSS Statistics for Windows version 17.0 (SPSS, Inc.). The EC50 in mg/L was converted into EC50 mM using the molecular weights shown in Supplementary Table S1.
Results and Discussion
We have studied the effects of differing concentrations of a panel of 60 toxicants on zebrafish development. Embryos were exposed continuously from 24 to 120 hpf. They were then assessed for malformations at 5 dpf. An overview of the data is given in Table 2 and Supplementary Table S3. Figures 1 and 2 show examples of abnormal phenotypes and the profile of teratogenic effects is summarized in Figure 3.
Table 2.
Summary of Outcomes of Compound Treatment Tested in Zebrafish Larvae Assay
| Compounds | Significant phenotypic abnormalities observeda | NOAEC (mg/L) | |
|---|---|---|---|
| 1 | Aconitine | None | 50 |
| 2 | Atropine | Pericardial oedema, yolk sac oedema, dispersed pigment cells, body axis bent, Meckel's cartilage hypoplasia, swim bladder uninflated | 400 |
| 3 | Berberine chloride | Pericardial oedema, yolk sac oedema, swim bladder uninflated | 50 |
| 4 | Colchicine | Pericardial oedema, yolk sac oedema, pigment cells dispersed | 20 |
| 5 | Coniine | Yolk sac oedema | 20 |
| 6 | α-Lobeline hydrochloride | Pericardial oedema, yolk sac oedema, dispersed pigment cells, body axis bent | 10 |
| 7 | Morphine hydrochloride | Yolk sac oedema, dispersed pigment cells | 2000 |
| 8 | Nicotine | Yolk sac oedema, dispersed pigment cells, tail bent, body axis bent, swim bladder uninflated | 0 |
| 9 | Quinine sulfate | Dispersed pigment cells, tail bent, swim bladder uninflated | 240 |
| 10 | (−)-Scopolamine hydrobromide trihydrate | Pericardial oedema, yolk sac oedema, dispersed pigment cells, tail bent, Meckel's cartilage hypoplasia, swim bladder uninflated | 2000 |
| 11 | Strychnine hydrochloride | Pericardial oedema, yolk sac oedema, body axis bent, swim bladder uninflated | 0 |
| 12 | Theobromine | Pericardial oedema, yolk sac oedema, dispersed pigment cells, body axis bent, swim bladder uninflated | 30 |
| 13 | (+)-Tubocurarine chloride hydrate | Yolk sac oedema | 200 |
| 14 | Yohimbine hydrochloride | Pericardial oedema, yolk sac oedema, dispersed pigment cells, swim bladder uninflated | 10 |
| 15 | Amygdalin | Yolk sac oedema, dispersed pigment cells, body axis bent, swim bladder uninflated | 10 |
| 16 | Arbutin | Dispersed pigment cells | 80 |
| 17 | Convallatoxin | None | 0 |
| 18 | Coumarin | Pericardial oedema, yolk sac oedema, dispersed pigment cells, body axis bent, Meckel's cartilage hypoplasia, brachial arch hypoplasia, swim bladder uninflated | 70 |
| 19 | Digitoxin | Dispersed pigment cells | 0 |
| 20 | Gentamycin sulfate | Dispersed pigment cells, swim bladder uninflated | 200 |
| 21 | Glycyrrhizin | Pericardial oedema, yolk sac oedema, dispersed pigment cells, Meckel's cartilage hypoplasia, brachial arch hypoplasia, swim bladder uninflated | 20 |
| 22 | Hesperidin | Yolk sac oedema, dispersed pigment cells, Meckel's cartilage hypoplasia | 40 |
| 23 | Kanamycin monosulfate | Yolk sac oedema, dispersed pigment cells, Meckel's cartilage hypoplasia, swim bladder uninflated | 250 |
| 24 | Naringin | Tail bent, Meckel's cartilage hypoplasia, swim bladder uninflated | 400 |
| 25 | Neohesperidin | Yolk sac oedema, dispersed pigment cells, body axis bent, swim bladder uninflated | 20 |
| 26 | Ouabain octahydrate | Body axis bent, swim bladder uninflated | 100 |
| 27 | Phloridzin dihydrate | Pericardial oedema, yolk sac oedema, dispersed pigment cells, tail bent, Meckel's cartilage hypoplasia, brachial arch hypoplasia, swim bladder uninflated | 280 |
| 28 | Rutin hydrate | Pericardial oedema, yolk sac oedema, dispersed pigment cells, body axis bent, Meckel's cartilage hypoplasia, brachial arch hypoplasia, swim bladder uninflated | 2000 |
| 29 | Streptomycin sulfate | Yolk sac oedema, Meckel's cartilage hypoplasia, swim bladder uninflated | 1000 |
| 30 | Cadmium(II) chloride | Pericardial oedema, yolk sac oedema, dispersed pigment cells, tail bent, Meckel's cartilage hypoplasia, brachial arch hypoplasia, swim bladder uninflated | 0 |
| 31 | Copper(II) nitrate trihydrate | Pericardial oedema, yolk sac oedema, dispersed pigment cells, body axis bent, swim bladder uninflated | 0 |
| 32 | Lead acetate trihydrate | None | 40 |
| 33 | Lithium chloride | Pericardial oedema, yolk sac oedema, dispersed pigment cells, Meckel's cartilage hypoplasia, brachial arch hypoplasia, swim bladder uninflated | 1000 |
| 34 | Chloramphenicol | Pericardial oedema, yolk sac oedema, dispersed pigment cells, Meckel's cartilage hypoplasia, brachial arch hypoplasia, swim bladder uninflated | 200 |
| 35 | Ethanol | Pericardial oedema, dispersed pigment cells | 4000 |
| 36 | Glycerol | Body axis bent | 8000 |
| 37 | Tween 80 | Dispersed pigment cells, body axis bent, branchial arch hypoplasia | 100 |
| 38 | Acetic acid | Pericardial oedema, yolk sac oedema, dispersed pigment cells, Meckel's cartilage hypoplasia, brachial arch hypoplasia, swim bladder uninflated | 50 |
| 39 | Salicylic acid | Yolk sac oedema | 15 |
| 40 | Sodium oxalate | Pericardial oedema, dispersed pigment cells, Meckel's cartilage hypoplasia, brachial arch hypoplasia, swim bladder uninflated | 100 |
| 41 | Trichloroacetic acid | Pericardial oedema, swim bladder uninflated | 40 |
| 42 | Ampicillin sodium | None | 4000 |
| 43 | Cyclophosphamide monohydrate | Pericardial oedema, yolk sac oedema, dispersed pigment cells, swim bladder uninflated | 1000 |
| 44 | Paracetamol | None | 400 |
| 45 | Phenacetin | Pericardial oedema, yolk sac oedema, Meckel's cartilage hypoplasia, swim bladder uninflated | 100 |
| 46 | Benserazide hydrochloride | Pericardial oedema, yolk sac oedema, swim bladder uninflated | 0 |
| 47 | Chlorpromazine hydrochloride | Pericardial oedema, yolk sac oedema, dispersed pigment cells, Meckel's cartilage hypoplasia, swim bladder uninflated | 1 |
| 48 | Isoniazid | Pericardial oedema, yolk sac oedema, dispersed pigment cells, Meckel's cartilage hypoplasia, swim bladder uninflated | 200 |
| 49 | Phenelzine sulfate | Pericardial oedema, yolk sac oedema, Meckel's cartilage hypoplasia | 5 |
| 50 | Ethambutol dihydrochloride | Pericardial oedema, yolk sac oedema, tail bent, body axis bent, branchial arch hypoplasia, swim bladder uninflated | 4000 |
| 51 | Verapamil hydrochloride | Pericardial oedema, dispersed pigment cells, Meckel's cartilage hypoplasia, swim bladder uninflated | 20 |
| 52 | Phenol | Pericardial oedema, yolk sac oedema, dispersed pigment cells, body axis bent, Meckel's cartilage hypoplasia, swim bladder uninflated | 20 |
| 53 | Sodium azide | Pericardial oedema, yolk sac oedema, dispersed pigment cells, branchial arch hypoplasia, swim bladder uninflated | 0.5 |
| 54 | Dimethyl sulfoxide | Pericardial oedema, swim bladder uninflated | 8000 |
| 55 | Formaldehyde | Yolk sac oedema, dispersed pigment cells, swim bladder uninflated | 8 |
| 56 | Phenformin hydrochloride | Yolk sac oedema, swim bladder uninflated | 200 |
| 57 | Ropinirole hydrochloride | Pericardial oedema, yolk sac oedema, dispersed pigment cells, swim bladder uninflated | 100 |
| 58 | Amitriptyline hydrochloride | Pericardial oedema, yolk sac oedema, dispersed pigment cells, Meckel's cartilage hypoplasia, swim bladder uninflated | 0 |
| 59 | Sodium dodecyl sulfate | Yolk sac oedema, dispersed pigment cells, swim bladder uninflated | 2 |
| 60 | Barbital sodium | Pericardial oedema, yolk sac oedema, dispersed pigment cells, body axis bent, Meckel's cartilage hypoplasia, branchial arch hypoplasia, swim bladder uninflated | 500 |
For the full range of concentrations used, see Supplementary Tables S2 and S3.
The presence of teratogenic phenotypes (i.e., any of the abnormal phenotypes 2–9 in Table 1) was scored in this column only if the incidence was significantly higher after exposure to compound as compared with vehicle.
NOAEC, no observed adverse effects (malformations) concentration.
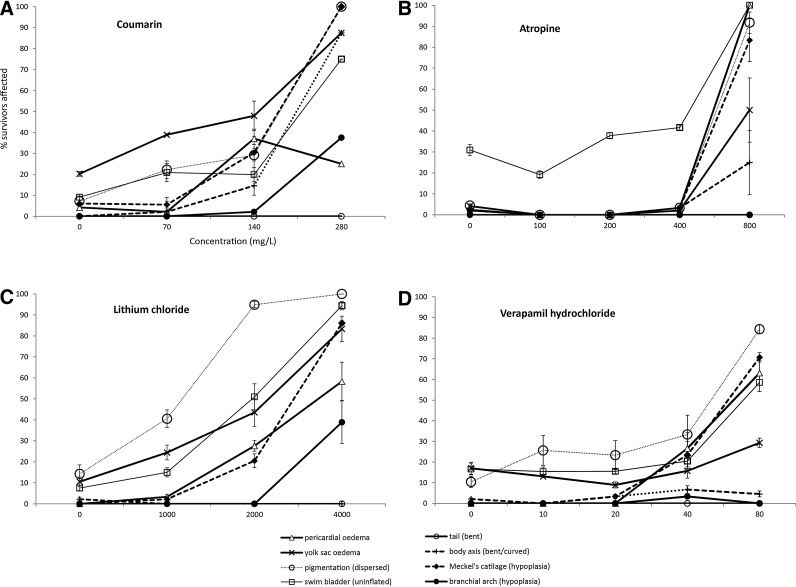
FIG. 1.
Concentration-dependent phenotypic abnormalities in zebrafish larvae (survivors) produced by selected toxicants [(A), coumarin, (B) atropine, (C) lithium chloride, and (D) verapamil hydrochloride]. In each case, the ordinate (y-axis) indicates the percentage of survivors showing a particular phenotype, and the abscissa (x-axis) indicates the concentration of compound tested in mg/mL. Error bars±standard error of the mean of N=48 larvae for vehicle (0 mg/L) and surviving larvae for each concentration for each compound from three independent experiments. Note that for coumarin at 200 mg/mL the error was nil.
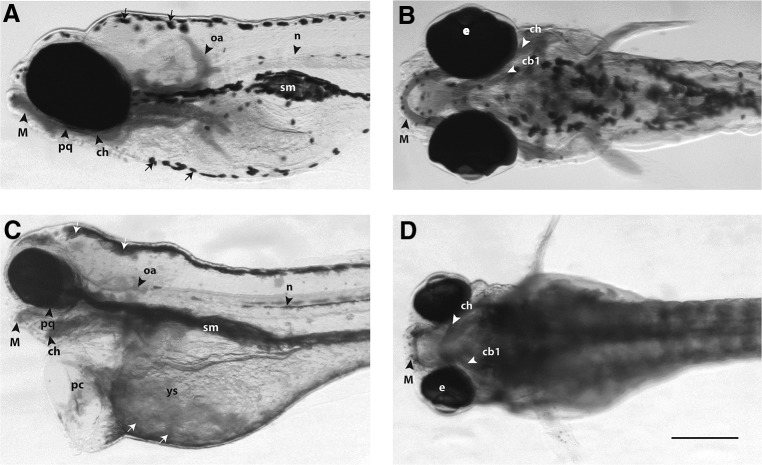
FIG. 2.
Morphological analysis of zebrafish larvae at 5 days post-fertilization. The larvae were fixed, stained with Alcian blue, and cleared in glycerol to show cartilage and other structures in the head and branchial region. The aim of this figure is to show examples of the range of malformations obtained. (A, C) Left lateral views; (B, D) ventral views. In all figures, rostral is to the left. All embryos are shown to the same scale, indicated by the scale bar (250 μm) in (D). (A, B) Vehicle only, normal phenotype. (C, D) Scopolamine hydrobromide trihydrate treated (4 g/L), having pericardial and yolk sac oedema, grossly hypoplastic Meckel's and branchial cartilages, dispersed phenotype of melanocytes on the dorsal surface of head and yolk sac area [white arrows in (C)], and also an uninflated swim bladder [sm in (C)]. The eyes in the scopolamine-treated larva (C, D) also appear smaller, although this was not quantified. cb1, First ceratobranchial cartilage; ch, ceratohyal cartilage; e, eye; M, Meckel's cartilage; n, notochord; oa, occipital arches; pc, pericardium and heart oedema; pq, palatoquadrate; ys, yolk sac oedema; sm, swim bladder.
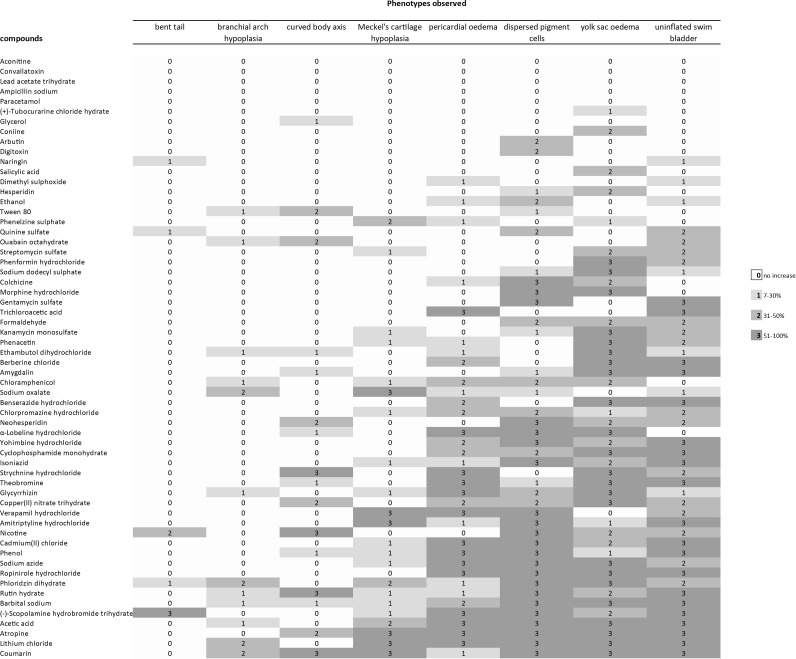
FIG. 3.
Profile of developmental effects in zebrafish larvae after exposure to different compounds. Phenotypic effects of compounds were compared to embryos exposed to vehicle only. The percentage increase of malformations at any given compound concentration is indicated by the color of the cell (see legend). The excess incidence of phenotypic defects at each concentration of each compound was calculated from three independent experiments. Only increases that were statistically significant are included; thus, in the range 7–30% increase, the values for 1–6% increase are omitted because they were not statistically significant. For full dataset, see Supplementary Table S3. As can be seen, from top to bottom, there is a general increase in the severity of effects; thus, the compounds at the top of that were not associated with a statistically significant increase in any abnormal phenotypes in survivors; by contrast, those at the bottom caused multiple abnormal phenotypes. By reading the figure form left to right, the frequency of different abnormal phenotypes is seen, with the least common on the left and the most common on the right. For the five remaining toxicants (aconitine, convallotoxin, lead acetate trihydrate, ampicillin sodium, and paracetomol) we could not find a statistically significant increase in the survivor population. This is either because the compounds are not teratogenic, or because they are so toxic that the number of survivors was too small to detect a statistically significant increase in abnormal phenotypes.
Discussion and criticism of the methodology used
Recently, in a study using the same culture protocols (and the same embryos that we have reanalyzed here), we found that, in controls (embryo buffer only), 5% of zebrafish eggs were unfertilized, and a further 9% represented embryos that died spontaneously in the first 24 hpf.11 Spontaneous mortality of 5–25% was also reported for zebrafish development.74 To avoid this early mortality, we began our assays at 24 hpf. This also makes our study consistent with a previous one, in which the zebrafish was exposed to various compounds from 24 hpf to find the predictivity of zebrafish assays for the toxicity of compounds in rodents.4 Therefore, we must assume that compounds that induce phenotype abnormalities only following early exposure will not be detected in our assays. The results are summarized in Tables 2 and 3. In previous studies, similar morphological parameters have been used to examine zebrafish embryos/larvae.13,31,75–78
Table 3.
Classification of Compounds Tested in Zebrafish Larvae Assay
| Compound class | Teratogenic in zebrafish assay (this study) | Non-teratogenic in zebrafish assay (this study) |
|---|---|---|
| Alkaloids | Atropine, berberine chloride, colchicine, coniine, α-lobeline hydrochloride, morphine hydrochloride, nicotine, quinine sulfate, (−)-scopolamine hydrobromide trihydrate, strychnine hydrochloride, theobromine, tubocurarine chloride hydrate yohimbine hydrochloride | Aconitine |
| Glycosides | Amygdalin, arbutin, coumarin, digitoxin, hesperidin, gentamycin sulfate, glycyrrhizin, naringin, rutin hydrate, kanamycin monosulfate, neohesperidin, ouabain octahydrate, phloridzin dihydrate, streptomycin sulfate | — |
| Carboxylic acids | Acetic acid, sodium oxalate, salicylic acid, trichloroacetic acid | |
| Alcohols | Tween 80, chloramphenicol, ethanol, glycerol | |
| Amides | Cyclophosphamide monohydrate, phenacetin | Ampicillin sodium, paracetamol |
| Others | Amitriptyline hydrochloride, barbital sodium, benserazide hydrochloride, cadmium(II) chloride, chlorpromazine hydrochloride, copper(ii)nitrate trihydrate, dimethyl sulfoxide, ethambutol dihydrochloride, formaldehyde, isoniazid, lithium chloride, phenelzine sulfate, phenformin hydrochloride, phenol, ropinirole hydrochloride, sodium azide, sodium dodecyl sulfate, verapamil hydrochloride | Lead acetate trihydrate |
Note that for aconitine, convallotoxin, lead acetate, ampicillin sodium, and paracetomol, we could not find a statistically significant increase in phenotypic abnormalities in the survivor population. This is either because the compounds are not teratogenic, or because they are so toxic that the number of survivors was too small to detect a statistically significant increase in abnormal phenotypes. Producing a statistically significant increase in any one or more of the abnormal phenotypes in Table 1.
General findings
Of the 60 toxicants tested, 55 produced one or more phenotypic effects at the range of concentrations used (Fig. 3 and Table 2; Supplementary Table S3). For the five remaining toxicants (aconitine, convallotoxin, lead acetate trihydrate, ampicillin sodium, and paracetomol) we could not find a statistically significant increase in the survivor population. This could be because these compounds are not teratogenic according to the criteria used here. An alternative explanation is that these five compounds are so toxic that there were too few survivors to yield a statistically significant change. EC50 values of compounds were estimated and shown in Table 4.
Table 4.
Zebrafish Embryo EC50 Values
| Compounds | Zebrafish embryo EC50 (mg/L±SEM) | Zebrafish embryo EC50 (mM±SEM) | |
|---|---|---|---|
| 1 | Aconitine | n/a | n/a |
| 2 | Atropine | 559.1±22.39 | 1.93206±0.0774 |
| 3 | Berberine chloride | 90.6±15.01 | 0.24367±0.0404 |
| 4 | Colchicine | 26.4±1.53 | 0.06601±0.0038 |
| 5 | Coniine | 75.0±11.58 | 0.58922±0.0910 |
| 6 | α-Lobeline hydrochloride | 11.5±0.97 | 0.03067±0.0026 |
| 7 | Morphine hydrochloride | 1335.6±58.41 | 3.15082±0.1378 |
| 8 | Nicotine | 9.2±0.03 | 0.05690±0.0002 |
| 9 | Quinine sulfate | 198.6±5.20 | 0.50732±0.0133 |
| 10 | (−)-Scopolamine hydrobromidetrihydrate | 1833.3±190.13 | 4.18258±0.4338 |
| 11 | Strychnine hydrochloride | 8.2±1.04 | 0.02211±0.0028 |
| 12 | Theobromine | 108.3±19.58 | 0.60132±0.1087 |
| 13 | (+)-Tubocurarine chloride hydrate | 285.5±12.07 | 0.41889±0.0177 |
| 14 | Yohimbine hydrochloride | 14.5±3.25 | 0.03718±0.0083 |
| 15 | Amygdalin | 36.5±13.43 | 0.07987±0.0294 |
| 16 | Arbutin | 81.5±6.23 | 0.29923±0.0229 |
| 17 | Convallatoxin | n/a | n/a |
| 18 | Coumarin | 97.0±14.82 | 0.00025±0.0000 |
| 19 | Digitoxin | n/a | n/a |
| 20 | Gentamycin sulfate | 115.9±12.67 | 0.20139±0.0220 |
| 21 | Glycyrrhizin | 16.2±1.24 | 0.01933±0.0015 |
| 22 | Hesperidin | 3.1±0.20 | 0.00513±0.0003 |
| 23 | Kanamycin monosulfate | 399.3±21.55 | 0.68538±0.0370 |
| 24 | Naringin | 159.4±27.93 | 0.27463±0.0481 |
| 25 | Neohesperidin | 27.9±0.87 | 0.04575±0.0014 |
| 26 | Ouabainoctahydrate | 54.8±9.19 | 0.07520±0.0126 |
| 27 | Phloridzindihydrate | 157.2±33.10 | 0.33281±0.0701 |
| 28 | Rutin hydrate | 5769.4±127.15 | 9.45003±0.2083 |
| 29 | Streptomycin sulfate | 1110.4±11.57 | 0.76194±0.0079 |
| 30 | Cadmium(II) chloride | 5.2±0.80 | 0.01091±0.0017 |
| 31 | Copper(II) nitrate trihydrate | 6.2±0.13 | 0.02580±0.0006 |
| 32 | Lead acetate trihydrate | 30.1±0.37 | 0.07944±0.0010 |
| 33 | Lithium chloride | 296.4±37.40 | 6.99222±0.8823 |
| 34 | Chloramphenicol | 319.0±25.82 | 0.98726±0.0799 |
| 35 | Ethanol | 11693.3±887.59 | 253.81666±19.2661 |
| 36 | Glycerol | 10250.7±374.87 | 111.28723±4.0698 |
| 37 | Tween 80 | 117.4±6.00 | 0.08962±0.0046 |
| 38 | Acetic acid | 56.4±1.57 | 0.93866±0.0261 |
| 39 | Salicylic acid | 27.3±2.28 | 0.19790±0.0165 |
| 40 | Sodium oxalate | 72.0±10.19 | 0.53731±0.0760 |
| 41 | Trichloroacetic acid | 40.8±7.24 | 0.24952±0.0443 |
| 42 | Ampicillin sodium | 347.6±22.97 | 0.93601±0.0618 |
| 43 | Cyclophosphamide monohydrate | 1185.1±99.27 | 4.24603±0.3557 |
| 44 | Paracetamol | 282.0±10.03 | 1.86567±0.0664 |
| 45 | Phenacetin | 151.4±8.78 | 0.84459±0.0490 |
| 46 | Benserazide hydrochloride | 790.1±74.00 | 2.69016±0.2520 |
| 47 | Chlorpromazine hydrochloride | 1.9±0.26 | 0.00535±0.0007 |
| 48 | Isoniazid | 485.8±64.93 | 3.54237±0.4735 |
| 49 | Phenelzine sulfate | 7.8±0.45 | 0.03315±0.0019 |
| 50 | Ethambutol dihydrochloride | 1946.0±208.68 | 7.02008±0.7528 |
| 51 | Verapamil hydrochloride | 24.2±2.71 | 0.04935±0.0055 |
| 52 | Phenol | 19.0±2.53 | 0.20154±0.0269 |
| 53 | Sodium azide | 0.7±0.23 | 0.01128±0.0036 |
| 54 | Dimethyl sulfoxide | 9136.4±479.56 | 78.54047±6.1380 |
| 55 | Formaldehyde | 6.7±0.30 | 0.22311±0.0100 |
| 56 | Phenformin hydrochloride | 201.5±21.84 | 0.83361±0.0904 |
| 57 | Ropinirole hydrochloride | 123.8±14.66 | 0.41695±0.0494 |
| 58 | Amitriptyline hydrochloride | 2.0±0.03 | 0.00648±0.0001 |
| 59 | Sodium dodecyl sulfate | 1.4±0.35 | 0.00484±0.0012 |
| 60 | Barbital sodium | 1065.5±18.76 | 5.16731±0.0910 |
We could not estimate EC50 because only few survivors were obtained on only lowest concentration of these compounds.
n/a, not applicable; SEM, standard error of the mean.
Relationship between malformations and concentration of compound
For most of compounds, the incidence of malformations was concentration dependent. To give one example, the incidence of yolk sac oedema for coumarin was 38.9% at 70 mg/L, 47.9% (140 mg/L), and 87.5% (240 mg/L). The data in the same case for dispersed pigment phenotype were 22.2% (70 mg/L), 29.2% (140 mg/L), and 100% (240 mg/L) (see Fig. 1A). Further, selected examples are shown in Figure 1B–D and the full dataset in Supplementary Table S3. These data are consistent with previous studies14,38,76,77 in which incidences of malformations were concentration dependent.
The results of morphological analyses of larvae are summarized in Table 2. The wide range of phenotypic effects that can be seen in one treatment group are illustrated in Figure 2, which compares an untreated larva (Fig. 2A, B) with larva exposed to scopolamine hydrobromide trihydrate with 4 g/L (Fig. 2C, D). The larva in Figure 2C and D shows a range of phenotypic abnormalities, including Meckel's cartilage hypoplasia, branchial arch hypoplasia, pericardial oedema, yolk sac oedema, “dispersed” morphology of melanocytes, and uninflated swim bladder.
Compound specificity of malformations
A range of phenotypic effects were recorded in zebrafish larvae after exposure (Fig. 3 and Table 2; Supplementary Table S3). Uninflated swim bladder, yolk sac oedema, and dispersed pigment cells were the phenotypes most frequently observed. Bent tail, brachial arch hypoplasia, and bent body axis were the least recorded phenotypes (Fig. 3). Five compounds (Fig. 3) did not produce any significant effects. Five produced only one effect (Fig. 3) while the majority of compounds (50) produced multiple phenotypic effects (Fig. 3).
That types of malformation that we report here after exposure to cadmium(II) chloride, chloramphenicol, lithium chloride, chlorpromazine hydrochloride, kanamycin, and isoniazid (Fig. 3) are consistent with previous studies using zebrafish embryos.14,38,77,79 Pericardial oedema induced by glycyrrhizin has also been reported in a human adult.80 Other literatures are consistent with the compound-specific malformation reported here (Fig. 3): the report of craniofacial malformations in mouse embryos exposure to lithium,81 craniofacial defects in monkeys exposed to Isoniazid, and craniofacial defects in rats exposed to ethambutol (reviewed in Holdiness82). The teratogenicity of colchicine is known from one case study of a human fetus that had heart malformations83; however, another larger scale study found no evidence of teratogenicity of colchicine in humans.84
It could be argued that, by beginning exposure at 24 h, we are missing out on early developmental toxicity effects, such as the action of compounds on gastrula stages. However, this is likely to be a general phenomenon because other compounds mainly cause embryo death at these early stages. For example, we showed35 that exposure of zebrafish embryos at early stages (dome to 26-somite) to ethanol resulted in high mortality, while exposure at later stages (prim-6 and prim-16) led to a high incidence of malformations. Other compounds, such as copper and cadmium, also show a development window of sensitivity and are more toxic to larval stages than to embryonic and adult stages of freshwater fish species.85,86
These previous results are consistent with a time window of sensitivity within the range of stages exposed here. It should also be remembered that early embryos may be shielded from teratogen actions because of the presence of the chorion at early stages. This membrane acts as a possible barrier to diffusion of some compounds.35,69,70
Teratogenicity per compound class in zebrafish larvae
To see whether the variation in developmental toxicity of compounds screened in the zebrafish assay was due to compound class, we sorted the compounds by chemical class according to Ali et al.11 The classes were alcohols, alkaloids, amides, carboxylic acids, glycosides, and the remaining compounds (others). The break-down by compound class shows that teratogens were detected in all compound classes (Table 3).
Conclusions
Our findings show that teratogenicity assessment on zebrafish larvae can provide a sensitive evaluation of the teratogenicity of a wide range of toxicants. Thus, it could, in principle, also provide a useful tool in the screening of new drugs for treating human diseases. The zebrafish larval assay is compatible with high-throughput screening and can be implemented early in the drug-discovery pipeline for early assessment of drug safety. However, future work in the validation of the zebrafish larval assay must include a wider range of compounds, including those that are known teratogens in humans.
Supplementary Material
Acknowledgments
The authors thank Merijn A.G. de Bakker and Peter J. Steenbergen for expert technical assistance and zebrafish breeding.
This work was supported by the Smart Mix Programme of the Netherlands Ministry of Economic Affairs and the Netherlands Ministry of Education, Culture and Science (M.K.R.) under grant number SSM06010 and University of Azad Jammu and Kashmir, Pakistan (S.A.) under project (no. F-3/PD/Main and Mirpur/369/2007). Note that the funding bodies had no role in designing, conducting the study, analyzing data, and writing article.
Authors' Contributions
S.A. and M.K.R. designed the experiments; S.A. conducted the experiments; M.K.R. provided the materials and facilities; J.A. scored the zebrafish larvae for morphological malformations (and S.A. rechecked a sample); S.A., J.A., and M.K.R. analyzed the data; and S.A. and M.K.R. wrote the article.
Disclosure Statement
The authors have no competing interests to declare.
References
- 1.Barros TP, Alderton WK, Reynolds HM, Roach AG, Berghmans S. Zebrafish: an emerging technology for in vivo pharmacological assessment to identify potential safety liabilities in early drug discovery. Br J Pharmacol 2008;154:1400–1413 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Spitsbergen JM, Kent ML. The state of the art of the zebrafish model for toxicology and toxicologic pathology research—advantages and current limitations. Toxicol Pathol 2003;31Suppl:62–87 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Lieschke GJ, Currie PD. Animal models of human disease: zebrafish swim into view. Nat Rev Genet 2007;8:353–367 [DOI] [PubMed] [Google Scholar]
- 4.Parng C, Seng WL, Semino C, McGrath P. Zebrafish: a preclinical model for drug screening. Assay Drug Dev Technol 2002;1:41–48 [DOI] [PubMed] [Google Scholar]
- 5.Rubinstein AL. Zebrafish: from disease modeling to drug discovery. Curr Opin Drug Discov Dev 2003;6:218–223 [PubMed] [Google Scholar]
- 6.Berghmans S, Butler P, Goldsmith P, Waldron G, Gardner I, Golder Z, et al. Zebrafish based assays for the assessment of cardiac, visual and gut function—potential safety screens for early drug discovery. J Pharmacol Toxicol Methods 2008;58:59–68 [DOI] [PubMed] [Google Scholar]
- 7.McGrath P, Li CQ. Zebrafish: a predictive model for assessing drug-induced toxicity. Drug Discov Today 2008;13:394–401 [DOI] [PubMed] [Google Scholar]
- 8.Redfern WS, Waldron G, Winter MJ, Butler P, Holbrook M, Wallis R, et al. Zebrafish assays as early safety pharmacology screens: paradigm shift or red herring? J Pharmacol Toxicol Methods 2008;58:110–117 [DOI] [PubMed] [Google Scholar]
- 9.Pardo-Martin C, Chang TY, Koo BK, Gilleland CL, Wasserman SC, Yanik MF. High-throughput in vivo vertebrate screening. Nat Methods 2010;7:634–636 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Milan DJ, Peterson TA, Ruskin JN, Peterson RT, Macrae CA. Drugs that induce repolarization abnormalities cause bradycardia in zebrafish. Circulation 2003;107:1355–1358 [DOI] [PubMed] [Google Scholar]
- 11.Ali S, van Mil HG, Richardson MK. Large-scale assessment of the zebrafish embryo as a possible predictive model in toxicity testing. PLoS ONE 2011;6:e21076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Rihel J, Prober DA, Arvanites A, Lam K, Zimmerman S, Jang S, et al. Zebrafish behavioral profiling links drugs to biological targets and rest/wake regulation. Science 2010;327:348–351 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Brannen KC, Panzica-Kelly JM, Danberry TL, Augustine-Rauch KA. Development of a zebrafish embryo teratogenicity assay and quantitative prediction model. Birth Defects Res B Dev Reprod Toxicol 2010;89:66–77 [DOI] [PubMed] [Google Scholar]
- 14.Selderslaghs IW, Blust R, Witters HE. Feasibility study of the zebrafish assay as an alternative method to screen for developmental toxicity and embryotoxicity using a training set of 27 compounds. Reprod Toxicol 2012;33:142–154 [DOI] [PubMed] [Google Scholar]
- 15.Sipes NS, Padilla S, Knudsen TB. Zebrafish: as an integrative model for twenty-first century toxicity testing. Birth Defects Res C Embryo Today 2011;93:256–267 [DOI] [PubMed] [Google Scholar]
- 16.Knudsen T, Martin M, Chandler K, Kleinstreuer N, Judson R, Sipes N. Predictive models and computational toxicology. Methods Mol Biol 2013;947:343–374 [DOI] [PubMed] [Google Scholar]
- 17.Hermsen SA, Pronk TE, van den Brandhof EJ, van der Ven LT, Piersma AH. Concentration-response analysis of differential gene expression in the zebrafish embryotoxicity test following flusilazole exposure. Toxicol Sci 2012;127:303–312 [DOI] [PubMed] [Google Scholar]
- 18.Hill AJ, Teraoka H, Heideman W, Peterson RE. Zebrafish as a model vertebrate for investigating chemical toxicity. Toxicol Sci 2005;86:6–19 [DOI] [PubMed] [Google Scholar]
- 19.Teraoka H, Dong W, Hiraga T. Zebrafish as a novel experimental model for developmental toxicology. Congenit Anom (Kyoto) 2003;43:123–132 [DOI] [PubMed] [Google Scholar]
- 20.Ali S, Champagne DL, Spaink HP, Richardson MK. Zebrafish embryos and larvae: a new generation of disease models and drug screens. Birth Defects Res C Embryo Today 2011;93:115–133 [DOI] [PubMed] [Google Scholar]
- 21.Brittijn SA, Duivesteijn SJ, Belmamoune M, Bertens LF, Bitter W, de Bruijn JD, et al. Zebrafish development and regeneration: new tools for biomedical research. Int J Dev Biol 2009;53:835–850 [DOI] [PubMed] [Google Scholar]
- 22.McCollum CW, Ducharme NA, Bondesson M, Gustafsson JA. Developmental toxicity screening in zebrafish. Birth Defects Res C Embryo Today 2011;93:67–114 [DOI] [PubMed] [Google Scholar]
- 23.Labrot F, Narbonne JF, Ville P, Saint DM, Ribera D. Acute toxicity, toxicokinetics, and tissue target of lead and uranium in the clam Corbicula fluminea and the worm Eisenia fetida: comparison with the fish Brachydanio rerio. Arch Environ Contam Toxicol 1999;36:167–178 [DOI] [PubMed] [Google Scholar]
- 24.Kumar K, Ansari BA. Malathion toxicity: effect on the liver of the fish Brachydanio rerio (Cyprinidae). Ecotoxicol Environ Saf 1986;12:199–205 [DOI] [PubMed] [Google Scholar]
- 25.Roche H, Boge G, Peres G. Acute and chronic toxicities of colchicine in Brachydanio rerio. Bull Environ Contam Toxicol 1994;52:69–73 [DOI] [PubMed] [Google Scholar]
- 26.Zok S, Gorge G, Kalsch W, Nagel R. Bioconcentration, metabolism and toxicity of substituted anilines in the zebrafish (Brachydanio rerio). Sci Total Environ 1991;109–110:411–421 [DOI] [PubMed] [Google Scholar]
- 27.Lanzky PF, Halling-Sorensen B. The toxic effect of the antibiotic metronidazole on aquatic organisms. Chemosphere 1997;35:2553–2561 [DOI] [PubMed] [Google Scholar]
- 28.Gorge G, Nagel R. Toxicity of lindane, atrazine, and deltamethrin to early life stages of zebrafish (Brachydanio rerio). Ecotoxicol Environ Saf 1990;20:246–255 [DOI] [PubMed] [Google Scholar]
- 29.El-Amrani S, Pena-Abaurrea M, Sanz-Landaluze J, Ramos L, Guinea J, Cámara C. Bioconcentration of pesticides in zebrafish eleutheroembryos (Danio rerio). Sci Total Environ 2012;425:184–190 [DOI] [PubMed] [Google Scholar]
- 30.Truong L, Harper SL, Tanguay RL. Evaluation of embryotoxicity using the zebrafish model. Methods Mol Biol 2011;691:271–279 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.George S, Xia T, Rallo R, Zhao Y, Ji Z, Lin S, et al. Use of a high-throughput screening approach coupled with in vivo zebrafish embryo screening to develop hazard ranking for engineered nanomaterials. ACS Nano 2011;5:1805–1817 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Bai W, Tian W, Zhang Z, He X, Ma Y, Liu N, et al. Effects of copper nanoparticles on the development of zebrafish embryos. J Nanosci Nanotechnol 2010;10:8670–8676 [DOI] [PubMed] [Google Scholar]
- 33.Zhu X, Tian S, Cai Z. Toxicity assessment of iron oxide nanoparticles in zebrafish (Danio rerio) early life stages. PLoS One 2012;7:e46286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Lee KJ, Browning LM, Nallathamby PD, Osgood CJ, Xu XH. Silver nanoparticles induce developmental stage-specific embryonic phenotypes in zebrafish. Nanoscale 2013;5:11625–11636 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Ali S, Champagne DL, Alia A, Richardson MK. Large-scale analysis of acute ethanol exposure in zebrafish development: a critical time window and resilience. PLoS One 2011;6:e20037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Sylvain NJ, Brewster DL, Ali DW. Zebrafish embryos exposed to alcohol undergo abnormal development of motor neurons and muscle fibers. Neurotoxicol Teratol 2010;32:472–480 [DOI] [PubMed] [Google Scholar]
- 37.McCarthy N, Wetherill L, Lovely CB, Swartz ME, Foroud TM, Eberhart JK. Pdgfra protects against ethanol-induced craniofacial defects in a zebrafish model of FASD. Development 2013;140:3254–3265 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Selderslaghs IW, Van Rompay AR, De CW, Witters HE. Development of a screening assay to identify teratogenic and embryotoxic chemicals using the zebrafish embryo. Reprod Toxicol 2009;28:308–320 [DOI] [PubMed] [Google Scholar]
- 39.Van den Bulck K, Hill A, Mesens N, Diekman H, De SL, Lammens L. Zebrafish developmental toxicity assay: a fishy solution to reproductive toxicity screening, or just a red herring? Reprod Toxicol 2011;32:213–219 [DOI] [PubMed] [Google Scholar]
- 40.Strecker R, Weigt S, Braunbeck T. Cartilage and bone malformations in the head of zebrafish (Danio rerio) embryos following exposure to disulfiram and acetic acid hydrazide. Toxicol Appl Pharmacol 2013;268:221–231 [DOI] [PubMed] [Google Scholar]
- 41.Chromcova L, Stepanova S, Plhalova L, Praskova E, Svobodova Z. Effect of four selected carrier solvents on embryonal stages of Danio rerio. Neuro Endocrinol Lett 2012;3:60–65 [PubMed] [Google Scholar]
- 42.Teixidó E, Piqué E, Gómez-Catalán J, Llobet JM. Assessment of developmental delay in the zebrafish embryo teratogenicity assay. Toxicol In Vitro 2013;27:469–478 [DOI] [PubMed] [Google Scholar]
- 43.Panzica-Kelly JM, Zhang CX, Augustine-Rauch K. Zebrafish embryo developmental toxicology assay. Methods Mol Biol 2012;889:25–50 [DOI] [PubMed] [Google Scholar]
- 44.Praskova E, Zivna D, Stepanova S, Sevcikova M, Blahova J, Marsalek P, Siroka Z, Voslarova E, Svobodova Z. Acute toxicity of acetylsalicylic acid to juvenile and embryonic stages of Danio rerio. Neuro Endocrinol Lett 2012;3:72–76 [PubMed] [Google Scholar]
- 45.Schock EN, Ford WC, Midgley KJ, Fader JG, Giavasis MN, McWhorter ML. The effects of carbaryl on the development of zebrafish (Danio rerio) embryos. Zebrafish 2012;9:169–178 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Maes J, Verlooy L, Buenafe OE, de Witte PA, Esguerra CV, Crawford AD. Evaluation of 14 organic solvents and carriers for screening applications in zebrafish embryos and larvae. PLoS One 2012;7:e43850. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Akhtar MT, Ali S, Rashidi H, van der Kooy F, Verpoorte R, Richardson MK. Developmental effects of cannabinoids on zebrafish larvae. Zebrafish 2013;10:283–293 [DOI] [PubMed] [Google Scholar]
- 48.Kimmel CB, Ballard WW, Kimmel SR, Ullmann B, Schilling TF. Stages of embryonic development of the zebrafish. Dev Dyn 1995;203:253–310 [DOI] [PubMed] [Google Scholar]
- 49.Zon LI, Peterson RT. In vivo drug discovery in the zebrafish. Nat Rev Drug Discov 2005;4:35–44 [DOI] [PubMed] [Google Scholar]
- 50.Dooley K, Zon LI. Zebrafish: a model system for the study of human disease. Curr Opin Genet Dev 2000;10:252–256 [DOI] [PubMed] [Google Scholar]
- 51.Voelker D, Vess C, Tillmann M, Nagel R, Otto GW, Geisler R, et al. Differential gene expression as a toxicant-sensitive endpoint in zebrafish embryos and larvae. Aquat Toxicol 2007;81:355–364 [DOI] [PubMed] [Google Scholar]
- 52.Burne T, Scott E, van SB, Hilliard M, Reinhard J, Claudianos C, et al. Big ideas for small brains: what can psychiatry learn from worms, flies, bees and fish? Mol Psychiatry 2011;16:7–16 [DOI] [PubMed] [Google Scholar]
- 53.Guo S. Using zebrafish to assess the impact of drugs on neural development and function. Expert Opin Drug Discov 2009;4:715–726 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Guo S. Linking genes to brain, behavior and neurological diseases: what can we learn from zebrafish? Genes Brain Behav 2004;3:63–74 [DOI] [PubMed] [Google Scholar]
- 55.Morris JA. Zebrafish: a model system to examine the neurodevelopmental basis of schizophrenia. Prog Brain Res 2009;179:97–106 [DOI] [PubMed] [Google Scholar]
- 56.Pogoda HM, Hammerschmidt M. How to make a teleost adenohypophysis: molecular pathways of pituitary development in zebrafish. Mol Cell Endocrinol 2009;312:2–13 [DOI] [PubMed] [Google Scholar]
- 57.Postlethwait JH, Woods IG, Ngo-Hazelett P, Yan YL, Kelly PD, Chu F, et al. Zebrafish comparative genomics and the origins of vertebrate chromosomes. Genome Res 2000;10:1890–1902 [DOI] [PubMed] [Google Scholar]
- 58.Rodriguez F, Lopez JC, Vargas JP, Broglio C, Gomez Y, Salas C. Spatial memory and hippocampal pallium through vertebrate evolution: insights from reptiles and teleost fish. Brain Res Bull 2002;57:499–503 [DOI] [PubMed] [Google Scholar]
- 59.Schaaf MJ, Champagne D, van L I, van W, Meijer AH, Meijer OC, et al. Discovery of a functional glucocorticoid receptor beta-isoform in zebrafish. Endocrinology 2008;149:1591–1599 [DOI] [PubMed] [Google Scholar]
- 60.Sison M, Cawker J, Buske C, Gerlai R. Fishing for genes influencing vertebrate behavior: zebrafish making headway. Lab Anim (NY) 2006;35:33–39 [DOI] [PubMed] [Google Scholar]
- 61.Tropepe V, Sive HL. Can zebrafish be used as a model to study the neurodevelopmental causes of autism? Genes Brain Behav 2003;2:268–281 [DOI] [PubMed] [Google Scholar]
- 62.Veldman MB, Lin S. Zebrafish as a developmental model organism for pediatric research. Pediatr Res 2008;64:470–476 [DOI] [PubMed] [Google Scholar]
- 63.Steenbergen PJ, Richardson MK, Champagne DL. The use of the zebrafish model in stress research. Prog Neuropsychopharmacol Biol Psychiatry 2010;35:1432–1451 [DOI] [PubMed] [Google Scholar]
- 64.Howe K, Clark MD, Torroja CF, Torrance J, Berthelot C, Muffato M, et al. The zebrafish reference genome sequence and its relationship to the human genome. Nature 2013;496:498–503 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Dahm R, Geisler R. Learning from small fry: the zebrafish as a genetic model organism for aquaculture fish species. Mar Biotechnol (NY) 2006;8:329–345 [DOI] [PubMed] [Google Scholar]
- 66.Grunwald DJ, Eisen JS. Headwaters of the zebrafish—emergence of a new model vertebrate. Nat Rev Genet 2002;3:717–724 [DOI] [PubMed] [Google Scholar]
- 67.Hu N, Yost HJ, Clark EB. Cardiac morphology and blood pressure in the adult zebrafish. Anat Rec 2001;264:1–12 [DOI] [PubMed] [Google Scholar]
- 68.Peterson KJ, Lyons JB, Nowak KS, Takacs CM, Wargo MJ, McPeek MA. Estimating metazoan divergence times with a molecular clock. Proc Natl Acad Sci U S A 2004;101:6536–6541 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Lee KJ, Nallathamby PD, Browning LM, Osgood CJ, Xu XH. In vivo imaging of transport and biocompatibility of single silver nanoparticles in early development of zebrafish embryos. ACS Nano 2007;1:133–143 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Henn K, Braunbeck T. Dechorionation as a tool to improve the fish embryo toxicity test (FET) with the zebrafish (Danio rerio). Comp Biochem Physiol C Toxicol Pharmacol 2011;153:91–98 [DOI] [PubMed] [Google Scholar]
- 71.Ali S, Champagne DL, Richardson MK. Behavioral profiling of zebrafish embryos exposed to a panel of 60 water-soluble compounds. Behav Brain Res 2012;228:272–283 [DOI] [PubMed] [Google Scholar]
- 72.Wielhouwer EM, Ali S, Al-Afandi A, Blom MT, Olde Riekerink MB, Poelma C, Westerweel J, Oonk J, Vrouwe EX, Buesink W, Vanmil HG, Chicken J, van't OR, Richardson MK. Zebrafish embryo development in a microfluidic flow-through system. Lab Chip 2011;11:1815–1824 [DOI] [PubMed] [Google Scholar]
- 73.United States Environmental Protection Agency. Ecological Effects Test Guidelines: OPPTS 850.1075: Fish Acute Toxicity Test, Freshwater and Marine; 1996;1–11 [Google Scholar]
- 74.Fraysse B, Mons R, Garric J. Development of a zebrafish 4-day embryo-larval bioassay to assess toxicity of chemicals. Ecotoxicol Environ Saf 2006;63:253–267 [DOI] [PubMed] [Google Scholar]
- 75.Arslanova D, Yang T, Xu X, Wong ST, Augelli-Szafran CE, Xia W. Phenotypic analysis of images of zebrafish treated with Alzheimer's gamma-secretase inhibitors. BMC Biotechnol 2010;10:24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Hermsen SA, van den Brandhof EJ, van der Ven LT, Piersma AH. Relative embryotoxicity of two classes of chemicals in a modified zebrafish embryotoxicity test and comparison with their in vivo potencies. Toxicol In Vitro 2011;25:745–753 [DOI] [PubMed] [Google Scholar]
- 77.Sawle AD, Wit E, Whale G, Cossins AR. An information-rich alternative, chemicals testing strategy using a high definition toxicogenomics and zebrafish (Danio rerio) embryos. Toxicol Sci 2010;118:128–139 [DOI] [PubMed] [Google Scholar]
- 78.Yang F, Zhang Q, Guo H, Zhang S. Evaluation of cytotoxicity, genotoxicity and teratogenicity of marine sediments from Qingdao coastal areas using in vitro fish cell assay, comet assay and zebrafish embryo test. Toxicol In Vitro 2010;24:2003–2011 [DOI] [PubMed] [Google Scholar]
- 79.Song C, Gao NY, Gao HW. Transmembrane distribution of kanamycin and chloramphenicol: insights into the cytotoxicity of antibacterial drugs. Mol Biosyst 2010;6:1901–1910 [DOI] [PubMed] [Google Scholar]
- 80.Shibata S. A drug over the millennia: pharmacognosy, chemistry, and pharmacology of licorice. Yakugaku Zasshi 2000;120:849–862 [DOI] [PubMed] [Google Scholar]
- 81.Jurand A. Teratogenic activity of lithium carbonate: an experimental update. Teratology 1988;38:101–111 [DOI] [PubMed] [Google Scholar]
- 82.Holdiness MR. Teratology of the antituberculosis drugs. Early Hum Dev 1987;15:61–74 [DOI] [PubMed] [Google Scholar]
- 83.Berkenstadt M, Weisz B, Cuckle H, Di-Castro M, Guetta E, Barkai G. Chromosomal abnormalities and birth defects among couples with colchicine treated familial Mediterranean fever. Am J Obstet Gynecol 2005;193:1513–1516 [DOI] [PubMed] [Google Scholar]
- 84.Diav-Citrin O, Shechtman S, Schwartz V, Avgil-Tsadok M, Finkel-Pekarsky V, Wajnberg R, et al. Pregnancy outcome after in utero exposure to colchicine. Am J Obstet Gynecol 2010;203:144–146 [DOI] [PubMed] [Google Scholar]
- 85.McKim JM, Eaton JG, Holcombe GW. Metal toxicity to embryos and larvae of eight species of freshwater fish-II: copper. Bull Environ Contam Toxicol 1978;19:608–616 [DOI] [PubMed] [Google Scholar]
- 86.Shazili NA, Pascoe D. Variable sensitivity of rainbow trout (Salmo gairdneri) eggs and alevins to heavy metals. Bull Environ Contam Toxicol 1986;36:468–474 [DOI] [PubMed] [Google Scholar]
- 87.O'Neil MJ, Heckelman PE, Koch CB, Roman KJ. The Merck Index: An encyclopedia of chemicals, drugs, and biologicals. 14th ed; Merck Research Laboratories, Whitehouse Station, NJ, 2006; 1–1156 [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.