Abstract
Placenta derived human amniotic epithelial cells (hAEC) are an attractive source of stem cells for the generation of hepatocyte-like cells (HLC) for therapeutic applications to treat liver diseases. During hAEC differentiation into HLC, they become increasingly immunogenic, which may result in immune cell-mediated rejection upon transplantation into allogeneic recipients. Placing cells within devices such as alginate microcapsules can prevent immune cell-mediated rejection. The aim of this study was to investigate the characteristics of HLC generated from hAEC and to examine the effects of encapsulation on HLC viability, gene expression, and function. hAEC were differentiated for 4 weeks and evaluated for hepatocyte-specific gene expression and function. Differentiated cells were encapsulated in barium alginate microcapsules and cultured for 7 days and the effect of encapsulation on cell viability, function, and hepatocyte related gene expression was determined. Differentiated cells performed key functions of hepatocytes including urea synthesis, drug-metabolizing cytochrome P450 (CYP)3A4 activity, indocyanine green (ICG) uptake, low-density lipoprotein (LDL) uptake, and exhibited glutathione antioxidant capacity. A number of hepatocyte-related genes involved in fat, cholesterol, bile acid synthesis, and xenobiotic metabolism were also expressed showing that the hAEC had differentiated into HLC. Upon encapsulation, the HLC remained viable for at least 7 days in culture, continued to express genes involved in fat, cholesterol, bile acid, and xenobiotic metabolism and had glutathione antioxidant capacity. CYP3A4 activity and urea synthesis by the encapsulated HLC were higher than that of monolayer HLC cultures. Functional HLC can be derived from hAEC, and HLC can be encapsulated within alginate microcapsules without losing viability or function in vitro.
Introduction
Liver failure can arise from chronic or acute causes and inherited metabolic disorders. Chronic inflammation from many causes including alcohol abuse, obesity, and hepatotoxic viruses can lead to cirrhosis while acute liver failure can result from drug overdoses [1]. Inherited disorders such as ornithine transcarbamylase (OTC) enzyme deficiencies can lead to reduced urea output and to hyper-ammonia toxicity [2]. The common feature in these diseases is loss or defective hepatocyte function and the only curative therapy is orthotopic liver transplant. However, since there is a severe shortage of suitable donor organs, transplantation of human fetal and adult hepatocytes is being evaluated [3–6]. However, hepatocyte transplantation is limited by the severe shortage of suitable liver resections for cell isolation, the large number of hepatocytes required for transplantation and difficulties associated with hepatocyte culture and cryopreservation [7]. Replacing hepatocytes with hepatocyte-like cells (HLC) generated from stem cells is an alternative strategy to overcome the shortage of hepatocytes.
HLC derived from both embryonic and induced pluripotent stem cells [8–10] have been shown to rescue fulminant hepatic failure when transplanted into the liver of non-obese diabetic, severe combined immune-deficient (SCID) mice [9,10]. There are ethical concerns regarding the use of embryonic stem cells and there is the risk of tumorigenesis with both embryonic and induced pluripotent stem cells. Mesenchymal stem/stromal cells (MSC) from various sources including bone marrow (BM), adipose, and placenta can be used to derive HLC [11–15]. HLC generated from BM-MSC engraft in the livers of normal SCID mice [16] and contribute to regeneration in a murine liver regeneration model [17]. However, MSC from different sources have varied potential for differentiation into HLC [18] and they may fuse with other cells upon transplantation [19]. Thus, ethically acceptable, non-invasive, alternative cell sources are needed.
Human amniotic epithelial cells (hAEC) from the placenta are an important cell source for generating HLC. hAEC are very abundant, have stem cell-like features, and differentiate into the lineages from the three embryonic germ layers including HLC originating from the endoderm [20,21]. Naïve hAEC display some relevant features of hepatic progenitors such as the lineage specification transcription factors FoxA2 and GATA-4 [22]. hAEC also display characteristic expression of mature hepatocyte markers such as albumin, α1-anti-trypsin, and OTC and also express various hepatocyte-related genes [22,23]. Upon differentiation, HLC derived from hAEC express mRNA of liver-enriched transcription factors such as C/EBP-α, CAR, RAR, and PXR [24], nuclear localization of the key transcription factor hepatocyte nuclear factor-4 alpha (HNF-4α), cytochrome P450 (CYP)1A1/2, CYP3A4, and CYP3A7 activity [20,21,25]. These HLC can also synthesize urea and breakdown testosterone [25]. However, expression of genes involved in the metabolism of cholesterol, fat, urea, and xenobiotics and other important functional properties of hepatocytes, such as low-density lipoprotein (LDL) and indocyanine green (ICG) uptake, antioxidative capacity, and bile acid synthesis have not been reported for HLC derived from hAEC.
In a recent study, we found that interferon gamma, a cytokine that is markedly elevated in acute and chronic liver diseases, increased the expression of major histocompatibility complex class IA and II antigens and the co-stimulatory molecule CD40 when applied to HLC obtained from hAEC [22]. These findings suggest that following transplantation, HLC will be recognized by allogeneic T cells and be rejected. One strategy for preventing cell-mediated rejection is to place cells within immunoisolation devices such as microcapsules made from various polymers including agarose, polyethylene glycol, polyacrylates, and alginate [26]. Alginates are the most commonly used polymers for microencapsulation because of their biocompatibility and the ability to cross-link with Ca2+/ Ba2+ at physiological pH [27]. Barium alginate microcapsules have a diameter ranging from 100 to 700 μm and a pore size of ∼250 kDa, thereby allowing the diffusion of nutrients and oxygen, but preventing the entry of immune cells and antibodies [28,29]. Further, microencapsulation creates a three-dimensional environment and this environment has been shown to enhance some hepatocyte-specific functions including detoxification and albumin and α1-anti-trypsin production in human hepatocyte cell lines [30,31].
HLC derived from hAEC have not been encapsulated. Whether the HLC generated from any type of stem cells remain viable and functional following encapsulation has not been extensively evaluated. Here, we characterized HLC derived from hAEC and evaluated their viability, expression of genes associated with the urea cycle, cholesterol, fat, bile acid synthesis, and xenobiotic metabolism, and their ability to function following encapsulation within barium alginate microcapsules.
Materials and Methods
Isolation of hAEC
This study was approved by the Human Research Ethics Committees of Monash Medical Center and The Royal Women's Hospital. Amniotic membranes were collected from healthy women with a normal singleton pregnancy delivered by caesarean section at term (n=8). hAEC were isolated as previously described [32] with minor modifications. Amniotic membranes were cut into small pieces, washed in Hank's Balanced Salt Solution, and digested twice in 0.05% trypsin containing 0.05 mM EDTA (Gibco, Grand Island, NY) for 40 min at 37°C. Trypsin was neutralized with 10% newborn calf serum in DMEM/F12 medium (Gibco). Dissociated cells were centrifuged at 200 g for 10 min, resuspended in DMEM/F12, strained through a 100 μm filter, and red blood cells lysed with hypotonic solution. The viable SSEA4+ population was recovered using a 24% Percoll (Sigma-Aldrich, St Louis, MO) gradient. Cells were washed several times in DMEM/F12 and viability assessed by trypan blue exclusion. Cells were cryopreserved in fetal bovine serum (FBS; Gibco) containing 10% dimethyl sulfoxide. Cells displaying typical cobble stone epithelial morphology in culture and >98% positive for the epithelial markers cytokeratin 7 and 8/18 (Dako, Glostrup, Denmark) were used in subsequent experiments [20,33].
Differentiation into HLC
Cryopreserved hAEC were thawed and plated on type I collagen (Roche, Mannheim, Germany)-coated dishes in DMEM/F12 with 20% FBS and 10 ng/mL epidermal growth factor (EGF) for 3–5 days. To induce differentiation, hAEC were cultured in DMEM/F12 supplemented with 10% FBS, 10% HepG2 cell-conditioned medium, 10 ng/mL EGF, fibroblast growth factor-4 (FGF-4) and hepatocyte growth factor (HGF; growth factors purchased from Invitrogen, Camarillo, CA), and 0.1 μM insulin and dexamethasone (Sigma-Aldrich). After 2 weeks cells were switched to DMEM/F12 supplemented with 10% FBS, 10% HepG2 cell-conditioned medium, 10 ng/mL HGF, 0.1 μM dexamethasone, and 1×insulin-transferrin-selenium (Invitrogen). Medium was replenished on alternate days. Cultures were maintained for a further 2 weeks.
Encapsulation of HLC and culture
HLC were encapsulated in barium alginate microcapsules as described elsewhere [34]. Briefly, HLC were trypsinized, washed thrice in PBS, and mixed with 2.2% ultra-pure alginate solution at a ratio of 1:6. The alginate solution was composed of 60:40 mixture of guluronic acid: mannuronic acid (UP MVG Pronova; FMC Biopolymer, Sandvika, Norway). Encapsulation was performed with an air-driven droplet generator (Steinau, Berlin, Germany) at a pressure of 80 kPa with an air flow rate of 6 L/min. The microcapsules generated were incubated in a 20 mM barium chloride precipitation bath for 2 min followed by three washings in 0.9% saline to remove excess barium. The average diameter of the microcapsules was 600 μm with ∼1,000 HLC in each capsule. Similarly, transformed human hepatocyte HepG2 cells, were also encapsulated for comparison. Encapsulated cells were cultured for a further 7 days in parallel with monolayer HLC and HepG2 cultures that served as controls.
Cell viability assay
Viability of both non-encapsulated and encapsulated cells was determined using 6-carboxyfluorescein diacetate (CFDA, 4.6 μg/mL; Sigma-Aldrich) and propidium iodide (PI, 10 μg/mL; Sigma-Aldrich). Briefly, cells were rinsed with Dulbecco's phosphate-buffered saline (DPBS) and incubated with CFDA for 30 min at 37°C. After several washes with DPBS, PI was added and left for 5 min. Cultured cells were washed and detached from the culture plates using TripLE (Invitrogen). Encapsulated cells were decapsulated in 50 mM EDTA and 10 mM HEPES solution at 37°C for 15 min. Flow cytometry was used to quantitate the numbers of viable (green CFDA): dead (red PI) cells. Analysis was performed on a Canto II (BD Biosciences, San Jose, CA) flow cytometer and data analyzed using FlowJo v10 software (Tree Star Incorporation, Ashland, OR).
Immunocytochemistry
Non-encapsulated and decapsulated cells were fixed in 4% paraformaldehyde for 15 min (HNF-4α) or 100% methanol for 8 min (albumin). Non-specific binding was blocked using CAS block (Invitrogen). Cells were incubated with rabbit anti-human HNF-4α (1:6,000; Cell Signalling Technology, Danvers, MA) or rabbit anti-human albumin (1:500; Abcam, Cambridge, United Kingdom) at 4°C overnight. Cells were washed several times with DPBS and incubated with goat anti-rabbit Alexa Fluor-488 secondary antibody (1:1,000; Invitrogen) for 30 min at room temperature. Primary antibodies were omitted from negative controls. After several washes, cells were mounted in Vectashield with DAPI (Vector Laboratories, Burlingame, CA).
Periodic acid Schiff's stain
To assess glycogen accumulation, non-encapsulated and decapsulated cells were fixed in 100% ethanol for 8 min and rinsed several times with DPBS. Cells were incubated with periodic acid for 7 min and rinsed in water for 5 min followed by 7 min incubation with Schiff's reagent. Cells were counterstained with hematoxylin and mounted with DPX. Reagents were purchased from Sigma-Aldrich.
LDL uptake
LDL receptor and LDL uptake were analyzed using a commercial kit (Cayman Chemicals, Ann Arbor, MI). Non-encapsulated and encapsulated cells were grown in 96-well plates (1×104 cells/well). For LDL uptake, cells were washed in DMEM/F12 and incubated overnight at 37°C with LDL-Dylight 549 (1:100). After 24 h, Hoechst dye (5 μg/mL; Invitrogen) was added to stain the nuclei. LDL uptake is shown by red cytoplasmic staining. Cells were fixed for 10 min with the fixative from the kit. After several washes and blocking non-specific binding, cells were incubated with rabbit anti-LDL receptor antibody (1:100) for 1 h at room temperature. Subsequently, cells were washed and incubated with DyLight 488 (green)-conjugated secondary antibody for 1 h. Secondary antibody was rinsed and the cells mounted with Vectashield (Vector Laboratories).
ICG uptake
ICG (Sigma-Aldrich) was dissolved in DMSO and added to cells at a final concentration of 0.5 mg/mL for 1 h at 37°C. Non-encapsulated and encapsulated HLC were rinsed several times with DPBS and visualized under bright field. Cells were then monitored for a period of 24 h to study the efflux of ICG.
CYP3A4 activity
Non-encapsulated and encapsulated cells were treated with 20 μM rifampicin for 48 h to induce CYP3A4 activity [35] and enzyme activity measured using the P450-Glo assay (Promega, Madison, WI) with 3 μM luciferin-IPA (CYP3A4 specific) as the substrate. An equal volume of detection reagent from the kit was added and readings taken on a luminometer (BMG Labtech, Offenburg, Germany) as per manufacturer's instructions.
Urea synthesis
Non-encapsulated and encapsulated cells were treated with 10 mM ammonium chloride in serum-free DMEM/F12 for 18 h. Media were collected and urea measured by the Quantichrome urea assay kit (Bioassay Systems; Hayward, CA) according to the manufacturer's instructions. Optical density readings were taken at 420 nm using a plate reader (Labsystems, Franklin, MA).
Glutathione
Non-encapsulated and decapsulated cells were plated on 96-well plates (1×104 cells/well). Total and oxidized glutathione were measured using the GSH/GSSG-Glo assay (Promega) according to the manufacturer's instructions. Briefly, cells were lysed with luciferin NT (total glutathione) or luciferin NT with 25 mM NEM buffer (oxidized glutathione) and gently shaken for 5 min. An equal volume of luciferin generation reagent was added and incubated for a further 30 min. Luciferin detection reagent was added and luminescence allowed to stabilize for 20 min and readings taken on a luminometer (BMG Labtech).
Expression of genes associated with urea, fat, cholesterol, and xenobiotic metabolism
Total RNA was extracted using RNeasy mini columns (Qiagen, Hilden, Germany) and quantitated using the NanoDrop1000 spectrophotometer (Thermo Fisher, Wilmington, DE). For urea cycle and CYP3A4 gene expression, HLC were stimulated with ammonium chloride or rifampicin, respectively as described previously. Briefly, 2 μg of total RNA was converted to cDNA using Superscript III (Invitrogen). cDNA was diluted 1:20 and mixed with 250 nM TaqMan probes (Applied Biosystems, Foster City, CA; Supplementary Table S1; Supplementary Data are available online at www.liebertpub.com/scd) and amplified according to parameters given by the supplier. Samples were assayed in duplicate on a 7900HT realtime PCR machine (Applied Biosystems).
Forward and reverse primers for CYP3A4 are also shown in Supplementary Table S1. The annealing temperature for CYP3A4 was 58°C for 30 s and extension at 72°C for 30 s. Data were normalized to 18S rRNA, expressed as a fold change relative to naive hAEC or HLC monolayers grown for a further week and calculated by the 2−ΔΔCt method [36].
Statistical analysis
Data are shown as the mean±SEM from HLC derived from a minimum of n=4–6 hAEC cultures. Cultures were set up in duplicate or triplicate wells for each assay. Comparisons were made using the unpaired t-test (GraphPad Prism V5.04, San Diego, CA) and multiple comparisons by ANOVA followed by Tukey's post hoc test. Significance was accorded when P<0.05.
Results
Characterization of the differentiated cells
hAEC were stimulated with a combination of growth factors (EGF, FGF, and HGF), hepatic inducers (insulin and dexamethasone) and HepG2-conditioned medium in a two-step protocol over a 4-week period and the resulting cells were examined. Hepatocytes synthesize albumin and store glycogen. Albumin protein was present in naive hAEC and increased amounts detected in the differentiated cells (Fig. 1A). Like albumin, glycogen was also detected in naïve hAEC by periodic acid Schiff staining, however, the differentiated cells showed intense violet staining indicative of increased glycogen storage. Unlike the naïve hAEC, which do not express HNF-4α, the HLC showed strong nuclear localization of the transcription factor HNF-4α, which regulates the expression of many hepatocyte-specific genes (Fig. 1A).
FIG. 1.
Characterization of differentiated human amniotic epithelial cells (hAEC). hAEC were differentiated over a 4-week period. Albumin and glycogen continued to be accumulated while the transcription factor hepatocyte nuclear factor-4 alpha (HNF-4α) was found only in the nuclei of stimulated cells; nuclei stained with DAPI is shown in the inset (A). Compared with hAEC, the differentiated cells showed increased expression of ABCA2, CYP7A1 involved in cholesterol metabolism, and ABCB11, encoding a bile acid export pump. Expression of genes involved in fat (SLC27A2) and xenobiotic metabolism (EPHX1) were reduced relative to hAEC. Data were normalized to 18S rRNA, expressed as 2−ΔΔCt relative to naïve hAEC and analyzed by the unpaired t-test (B). Scale bar=100 μm. Color images available online at www.liebertpub.com/scd
We then examined whether the differentiated cells expressed genes linked to cholesterol, fat, and xenobiotic metabolism that are known to be present in mature hepatocytes. Expression of ABCA2 associated with cholesterol transport and CYP7A1 encoding an enzyme that catalyses the first step in the cholesterol catabolic pathway were elevated compared with naïve hAEC (P<0.05 and P<0.01, respectively; Fig. 1B). The membrane transporter ABCA1 was expressed by hAEC but its expression did not alter with differentiation (Supplementary Fig. S1) while APOF that is implicated in cholesterol metabolism was absent in naïve and stimulated hAEC. HSD3B7 and ABCB11/BSEP are involved in the bile acid synthesis pathway that utilizes cholesterol and bile acid export, respectively. HSD3B7 was expressed in naïve and differentiated cells while ABCB11/BSEP expression was elevated compared with naïve hAEC (P<0.05). ACOX2, BAAT, and SCL27A2 regulate fat metabolism. There were no significant changes in ACOX2 and BAAT expression following differentiation (Supplementary Fig. S1) while SLC27A2 was highly suppressed in the differentiated cells (P<0.01). Genes associated with xenobiotic metabolism AKR1C3 and HAMP were elevated but not significantly, whereas EPHX1 mRNA was decreased in the stimulated cells (P<0.001; Fig. 1B).
Functional characterization of the differentiated cells
Next, we examined whether the differentiated cells were able to perform some of the key functions of hepatocytes. LDL receptors were evident in the differentiated cells and these cells were able to take up LDL, whereas the hAEC neither expressed LDL receptors nor showed LDL uptake (Fig. 2A). ICG is taken up by hepatocytes and effluxed unchanged with the bile acids. Differentiated cells showed uptake of ICG unlike the naive hAEC and ICG was effluxed within 24 h. Glutathione levels are indicative of the ability of cells to eliminate free radicals while levels of oxidized glutathione show effective scavenging of free radicals. The differentiated cells had high levels of total glutathione and oxidized glutathione; but these levels were similar to those of hAEC (Fig. 2B). CYP3A4 is a major class of P450 enzymes responsible for drug metabolism in hepatocytes. Following stimulation with the specific inducer rifampicin, CYP3A4 mRNA expression was significantly increased in the differentiated cells compared with naïve hAEC (P<0.01), and CYP3A4 enzyme activity increased concurrently (P<0.0001 vs. hAEC; Fig. 2C). Another important function of hepatocytes is detoxification. We examined the expression of enzymes associated with the removal of ammonia via the urea cycle. Upon stimulation with ammonium chloride, mRNA of the urea cycle enzyme ASS1 was elevated in the differentiated cells compared with naïve hAEC (P<0.05; Fig. 2D). However, there was no significant change in ARG1 and CPS1 expression (Supplementary Fig. S1). Consistent with these findings, hAEC showed some ability to synthesize urea following stimulation with ammonium chloride, whereas the differentiated cells produced higher amounts of urea (P<0.05; Fig. 2D). Collectively, these findings confirm that the hAEC had differentiated into HLC.
FIG. 2.
Functional characteristics of the differentiated cells. Differentiated cells were positive for low-density lipoprotein (LDL) receptors and LDL was present in the cytoplasm in contrast to naïve hAEC. Only differentiated cells took up indocyanine green (ICG) and effluxed the dye (A). Total and oxidized glutathione levels remained similar (B). Differentiated cells showed increased mRNA expression of the drug metabolizing enzyme CYP3A4 and had elevated CYP3A4 enzymatic activity after rifampicin stimulation compared with hAEC (C). Upon stimulation with ammonium chloride, there was increased mRNA expression of the urea cycle gene ASS1 and increased urea synthesis in the differentiated cells relative to naïve hAEC. Data were analyzed by the unpaired t-test (D). Scale bar=100 μm. Color images available online at www.liebertpub.com/scd
We also examined the functional competency of the differentiated cells compared to the transformed human hepatocyte cell line HepG2. ICG was taken up by HepG2 cells and effluxed within 24 h. Total and oxidized glutathione levels were similar. However, CYP3A4 activity in the HepG2 cells was nearly twofold greater while urea production was three times higher (Table 1).
Table 1.
Comparison of Functional Properties of HLC and HepG2 Cells
| Assay | HepG2:HLC | Enc-HepG2:Enc-HLC |
|---|---|---|
| Total glutathione | 1.063 | 0.634 |
| Oxidized glutathione | 0.869 | 1.105 |
| CYP3A4 activity | 1.878 | 1.246 |
| Urea synthesis | 3.247 | 1.310 |
Enc, encapsulation; HLC, hepatocyte-like cells.
Viability and gene expression of encapsulated HLC
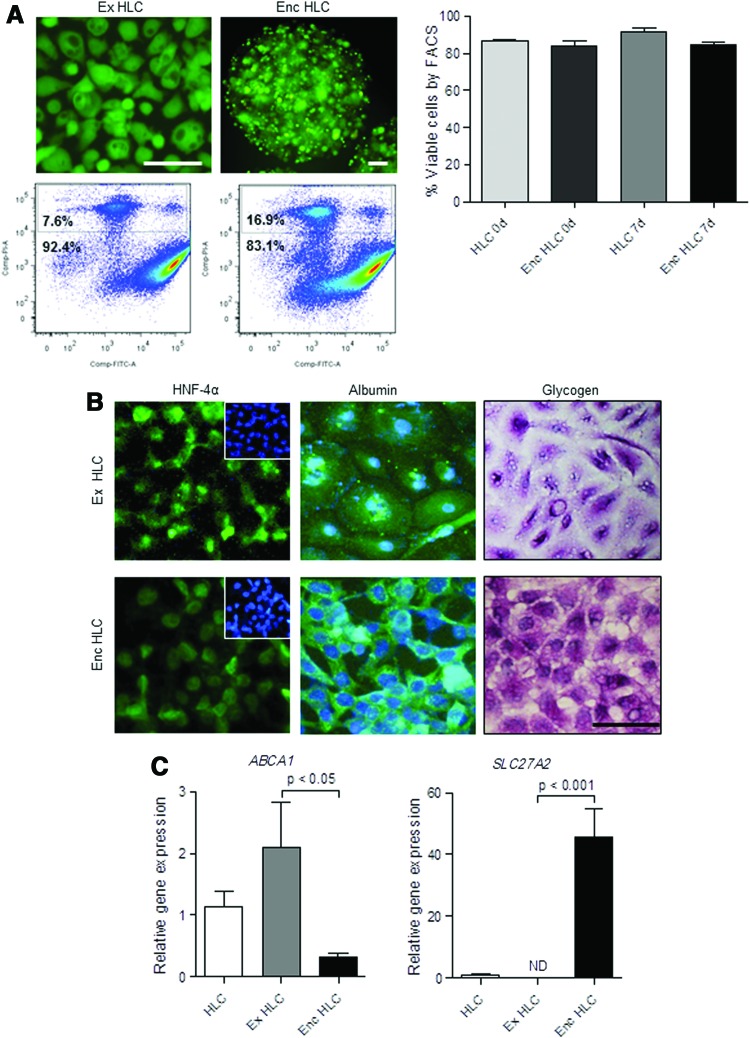
Next, we examined the effects of encapsulation on viability and gene expression in the HLC. HLC encapsulated in barium alginate microcapsules were maintained for 7 days in culture. Initially, we evaluated the viability of monolayer and encapsulated HLC at day 0 using CFDA/PI staining followed by flow cytometry. The percentage of viable cells in monolayer HLC was 86.9±1.0 (mean±SEM) and there was no significant loss of viability immediately following encapsulation (84.1±2.8; Fig. 3A). Similar to monolayer HLC, the encapsulated HLC were viable after 7 days (85.1±1.3). Representative photomicrographs and flow cytometry plots of cells stained with CFDA/PI are shown in Figure 3A. Encapsulated HLC continued to have HNF-4α protein localized in the nuclei after 7 days in culture (Fig. 3B). Similarly, cytoplasmic albumin was abundant and glycogen staining showed intense, cytoplasmic violet coloration in the encapsulated HLC cultures (Fig. 3B).
FIG. 3.
Characterization of encapsulated hepatocyte-like cells (enc HLC). HLC were encapsulated and cultured for 7 days to determine effects on viability and function. Comparisons were made with monolayer cultures extended for 7 days (ex HLC). Enc HLC remained viable as shown by 6-carboxyfluorescein diacetate (CFDA)/propidium iodide (PI) staining and quantitated by flow cytometry (A). HNF-4α localized to the nuclei of enc HLC; DAPI-stained nuclei shown in the inset. Enc HLC had stored albumin and glycogen after 7 days in culture (B). Scale bar=100 μm. Relative to ex HLC, mRNA expression of ABCA1 decreased while SLC27A2 increased in enc HLC; data were analyzed by ANOVA followed by Tukey's post hoc test (C). Color images available online at www.liebertpub.com/scd
We also assessed changes in gene expression in the encapsulated HLC after 7 days in culture. Initially, we determined whether the extended culture period altered the mRNA expression in monolayer cultures. There were no significant changes in the expression of genes linked to fat, cholesterol, and xenobiotic metabolism between monolayer HLC grown for±1 week except for ACOX2 and BAAT (P<0.05 by ANOVA; Supplementary Fig. S2). Following encapsulation, expression of ACOX2 and BAAT remained low, while ABCA1 declined in the encapsulated HLC (P<0.05 vs. extended HLC) and SLC27A2, which was absent in the extended HLC was expressed in the encapsulated HLC (P<0.001; Fig. 3C).
Functional properties of encapsulated HLC
Like the monolayer cultures, the encapsulated HLC showed LDL uptake as shown by the red fluorescence in the cytoplasm. The encapsulated HLC took up ICG (Fig. 4A) and effluxed the dye within 24 h similar to monolayer HLC (data not shown). While total glutathione levels remained unchanged with extended culture, levels declined with encapsulation (P<0.05; Fig. 4B). Oxidized glutathione levels were lower in the encapsulated HLC compared with the extended HLC cultures (P<0.01).
FIG. 4.
Functional characteristics of enc HLC. Enc HLC cultured for 7 days took up LDL and ICG (A). Both total and oxidized glutathione levels in enc HLC grown for 7 days were lower than monolayer HLC cultures extended for 7 days (ex HLC) (B). Rifampicin stimulation (+) and encapsulation led to increased CYP3A4 mRNA expression. HNF-4α, which regulates CYP3A4 mRNA expression was highly elevated in the enc HLC. CYP3A4 enzymatic activity was highest in the enc HLC. *P<0.05 and ***P<0.001, respectively (C). Despite some changes in the mRNA expression of the urea cycle genes ARG1 and CPS1, urea output increased in enc HLC (D). mRNA expression data were normalized to 18S rRNA, expressed as 2−ΔΔCt relative to HLC. Comparisons were made by ANOVA. Scale bar=100 μm. Color images available online at www.liebertpub.com/scd
The encapsulated and extended monolayer HLC responded to rifampicin stimulation showing increased CYP3A4 mRNA expression with the encapsulated HLC having the highest expression levels (P<0.05; Fig. 4C). We then investigated the expression of HNF-4α, one of the main regulators of CYP3A4 expression, and likewise found highly increased HNF-4α mRNA expression in the encapsulated HLC treated with rifampicin (P<0.001; Fig. 4C). Analyzing CYP3A4 activity, the highest levels were seen in the encapsulated HLC (P<0.01 vs. extended monolayer HLC cultures; Fig. 4C).
There was no difference in the mRNA expression of urea cycle enzymes ASS1, ARG1, and CPS1 between HLC monolayer cultures±1 week. While there were changes in mRNA expression with encapsulation (ARG1 decreased while CPS1 was elevated), the relative changes were minor. Urea output was elevated in the encapsulated HLC compared with the extended monolayer cultures (P<0.05 by ANOVA; Fig. 4D).
We then compared the functional aspects of encapsulated HLC with encapsulated HepG2 cells. CYP3A4 activity, urea secretion, and oxidized glutathione levels of encapsulated HLC were similar to encapsulated HepG2 (Table 1). Overall, the encapsulated HLC were functionally closer to the HepG2 cells, suggesting that encapsulation augmented some HLC functions.
Discussion
We showed that hAEC differentiate into HLC, that these HLC remain viable and functional following encapsulation in barium alginate microspheres, and that there was a significant increase in urea output and CYP3A4 activity following encapsulation. HLC were differentiated from hAEC in a similar manner as previously reported [20,22], except for the addition of HepG2-conditioned medium. Studies have shown that when hAEC were co-cultured with mouse hepatocytes expression of albumin, α1 anti-trypsin, and cytochrome P450 enzyme was increased [25]. Conditioned medium from human HepG2 cells, a non-xenogeneic cell source, was also effective since HLC expressed albumin, glycogen, and HNF-4α. The HLC also expressed several mRNAs present in hepatocytes and were able to carry out hepatocyte-specific functions.
The HLC expressed genes linked with cholesterol, fat, and xenobiotic metabolism and importantly, encapsulation did not inhibit their expression. An important functional test was to determine whether the HLC would take up cholesterol and convert it into bile acids. The HLC had abundant LDL receptors and took up LDL. Further, the expression of CYP7A1, which encodes the rate-limiting enzyme in the first step of cholesterol breakdown and ABCA2, a membrane transporter, increased with differentiation, and the expression remained unaltered with culture following encapsulation. CYP7A1 mRNA expression is regulated by HNF-4α [37]. HNF-4α protein was localized to the nuclei of the HLC and it appears that downstream target genes of HNF-4α are being transcribed in the HLC. Moreover, high expression level of ABCB11/BSEP, the bile acid export pump, was maintained in the HLC following encapsulation. In a previous study, we detected bile canaliculi like structures in the HLC derived from hAEC [20]. Collectively, while these data suggest that bile acid is synthesized and secreted, it would be important to confirm the presence of bile acids via HPLC analysis.
Uptake and efflux of ICG is clinically used to evaluate liver functions [38,39]. The monolayer HLC showed uptake of ICG and complete efflux occurred within 24 h. A similar profile in the uptake and efflux of ICG was also seen with the encapsulated HLC. ICG is removed from the liver together with the bile acids, suggesting that functional bile canaliculi may be present in the HLC.
Inflammation and breakdown of drugs and xenobiotics via cytochrome P450 enzymes generate reactive oxygen species (ROS) such as superoxide and hydrogen peroxide. To prevent ROS-mediated damage to hepatocytes, cytoplasmic superoxide, and hydrogen peroxide are inactivated producing oxidized glutathione [40]. The HLC had high levels of oxidized glutathione suggesting that they can efficiently eliminate ROS. Importantly, glutathione incorporates cysteine and prevents cysteine toxicity and generation of lethal ROS [41]. Although not significant, encapsulated HLC had a higher ratio of oxidized glutathione to total glutathione relative to non-encapsulated HLC. This implies that the encapsulated HLC were generating higher levels of ROS and utilizing glutathione to mop-up the ROS. Interestingly, in addition to CYP3A4 activity, higher levels of ROS are generated via urea synthesis and notably both of these functions were elevated in the encapsulated HLC.
One of the important functions of hepatocytes is xenobiotic metabolism and the cytochrome P450 family members are the major phase I enzymes implicated in the process. Studies have shown that HLC derived from hAEC have functional CYP1A2 and CYP3A7 enzymes [21,22,25]. CYP3A4 is highly expressed in the liver and is thought to breakdown almost 50% of all therapeutic drugs [42]. Although CYP3A4 activity significantly increased with differentiation, enzyme activity in the monolayer cultures was only half that of HepG2 cells. These findings are consistent with earlier reports showing that CYP3A4 activity in cultured HLC derived from hAEC does not reach levels seen in the transformed HepG2 cell line or human hepatocytes [22,24,25]. Although not ideal HepG2 cells are commonly used for comparison in lieu of human hepatocytes, which are difficult to source and culture. The underlying cause of low CYP3A4 activity is unclear but it may be partly attributed to lower expression of CYP3A4 mRNA due to epigenetic regulation. However, CYP3A4 mRNA expression was responsive to rifampicin stimulation with encapsulated HLC showing the strongest upregulation of CYP3A4 mRNA. HLC derived from hAEC express transcription factors such as PXR, CAR, and RAR [24], which regulate CYP3A4 expression. CYP3A4 is also a downstream target of the transcription factor HNF-4α [43]. As reported in the encapsulated, transformed human hepatocyte cell line Huh7 [44], we found highly elevated HNF-4α mRNA levels in the encapsulated HLC and nuclear HNF-4α protein, which may have contributed toward increased CYP3A4 mRNA expression and subsequently CYP3A4 protein and activity. Indeed, CYP3A4 activity in the encapsulated HLC was close to that of encapsulated HepG2 cells. Previous studies reported elevated CYP3A4 activity in hepatocytes grown on matrices or encapsulated to create a three-dimensional environment [45–47], and that the increase in CYP3A4 activity could be due to increased cell to cell contact, additional extracellular matrix and recovery of cell polarity within in the microcapsule [44,48]. Thus, encapsulated HLC may also be suitable as an in vitro drug screening tool.
Earlier studies revealed the presence of OTC, an important enzyme of the urea cycle in HLC derived from hAEC, and that urea was produced [22,25]. Encapsulated HLC had significantly enhanced levels of urea synthesis relative to monolayer HLC. Similar findings have been observed in human hepatocytes, which showed significant increases in urea synthesis upon encapsulation [46]; however, the mechanism underlying increased urea synthesis with encapsulation remains to be elucidated.
Collectively, our data suggest that gene expression and the functional properties of the HLC were retained following encapsulation and their culture for at least 7 days. Microencapsulation is a strategy used to prevent the rejection of grafted tissues [49]. Transplantation of encapsulated islet cells to treat type 1 diabetes is currently being evaluated clinically [50]. Evaluating the effects of encapsulation on HLC function is an important first step in assessing whether encapsulated HLC could provide an alternative approach to the transplantation of naïve stem cells into the liver or spleen, which would subsequently differentiate into HLC. Studies have shown that hAEC delivered into the murine liver differentiated into HLC expressing HNF-4α, CYP genes, hepatocyte-enriched transcription factors, and hepatic transporter proteins and could rescue a murine retrosine model of liver regeneration [25]. hAEC also rescued mice with intermediate maple syrup urine disease with improvement in peripheral metabolites [51,52]. Unlike naïve hAEC transplantation, it may be feasible to graft encapsulated HLC into an extra hepatic site such as the peritoneal cavity. This would allow the delivery of large numbers of HLC, which may be able to provide immediate support to liver function. Indeed, a recent study showed that encapsulated HLC generated from umbilical cord blood cells and grafted into the peritoneal cavity was able to rescue rats with acute hepatic failure [53]. HLC derived from hAEC secrete some of the immunomodulatory factors produced by the parental cells, but soluble HLA-G was significantly lower [22]. In addition to reduced immunomodulation, it may be important to prevent cell–cell contact-mediated interactions with allogeneic immune cells by means such as encapsulation. The HLC were found to have increased HLA Class IA and II antigens and co-stimulatory molecules compared with naïve hAEC and directly interacted with allogeneic T cells stimulating their proliferation in vitro [22]. Such interaction in vivo would lead to cytotoxic outcomes for the HLC.
However, the diffusion of nutrients and oxygen to the core of the microcapsule remains a key challenge in cell encapsulation. Insufficient nutrient and oxygen diffusion to the core of the capsules can activate hypoxia-inducible factor-1 alpha (HIF-1α) and lead to eventual necrotic cell death [54]. In this study, we found that encapsulated HLC remained viable for a week in culture suggesting sufficient diffusion of nutrients and oxygen to the core of the microcapsules. However, the effects of longer term in vitro culture and importantly the effects in vivo on viability, gene expression, and function need careful assessment. In conclusion, we have shown that HLC derived from hAEC can be encapsulated within alginate microcapsules without loss of viability and with improvement to key functions such as CYP3A4 activity and urea synthesis suggesting that further evaluation in animal models is warranted.
Supplementary Material
Acknowledgments
Study funded by a SMART grant from the Australian Regenerative Medicine Institute. U.M. is supported by a Researcher Accelerator Award from Monash University and B.K. by NHMRC grant no. 509178, KAIMRC grant no. RC08/114, and KACST grant no. ARP-29-186. V. Vaghjiani and U.M. are supported by the Victorian Government's Operational Infrastructure Support Program.
Author Disclosure Statement
The authors have no conflicts of interest financial or otherwise.
References
- 1.Lee WM. (2012). Acute liver failure. Semin Respir Crit Care Med 33:36–45 [DOI] [PubMed] [Google Scholar]
- 2.Hansen K. and Horslen S. (2008). Metabolic liver disease in children. Liver Transpl 14:713–733 [DOI] [PubMed] [Google Scholar]
- 3.Bilir BM, Guinette D, Karrer F, Kumpe DA, Krysl J, Stephens J, McGavran L, Ostrowska A. and Durham J. (2000). Hepatocyte transplantation in acute liver failure. Liver Transpl 6:32–40 [DOI] [PubMed] [Google Scholar]
- 4.Strom SC, Chowdhury JR. and Fox IJ. (1999). Hepatocyte transplantation for the treatment of human disease. Semin Liver Dis 19:39–48 [DOI] [PubMed] [Google Scholar]
- 5.Habibullah CM, Syed IH, Qamar A. and Taher-Uz Z. (1994). Human fetal hepatocyte transplantation in patients with fulminant hepatic failure. Transplantation 58:951–952 [DOI] [PubMed] [Google Scholar]
- 6.Horslen SP, McCowan TC, Goertzen TC, Warkentin PI, Cai HB, Strom SC. and Fox IJ. (2003). Isolated hepatocyte transplantation in an infant with a severe urea cycle disorder. Pediatrics 111:1262–1267 [DOI] [PubMed] [Google Scholar]
- 7.Soltys KA, Soto-Gutierrez A, Nagaya M, Baskin KM, Deutsch M, Ito R, Shneider BL, Squires R, Vockley J, et al. (2010). Barriers to the successful treatment of liver disease by hepatocyte transplantation. J Hepatol 53:769–774 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Hannan NR, Segeritz CP, Touboul T. and Vallier L. (2013). Production of hepatocyte-like cells from human pluripotent stem cells. Nat Protoc 8:430–437 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Touboul T, Hannan NR, Corbineau S, Martinez A, Martinet C, Branchereau S, Mainot S, Strick-Marchand H, Pedersen R, et al. (2010). Generation of functional hepatocytes from human embryonic stem cells under chemically defined conditions that recapitulate liver development. Hepatology 51:1754–1765 [DOI] [PubMed] [Google Scholar]
- 10.Basma H, Soto-Gutierrez A, Yannam GR, Liu L, Ito R, Yamamoto T, Ellis E, Carson SD, Sato S, et al. (2009). Differentiation and transplantation of human embryonic stem cell-derived hepatocytes. Gastroenterology 136:990–999 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Alison MR, Poulsom R, Jeffery R, Dhillon AP, Quaglia A, Jacob J, Novelli M, Prentice G, Williamson J. and Wright NA. (2000). Hepatocytes from non-hepatic adult stem cells. Nature 406:257. [DOI] [PubMed] [Google Scholar]
- 12.Banas A, Teratani T, Yamamoto Y, Tokuhara M, Takeshita F, Quinn G, Okochi H. and Ochiya T. (2007). Adipose tissue-derived mesenchymal stem cells as a source of human hepatocytes. Hepatology 46:219–228 [DOI] [PubMed] [Google Scholar]
- 13.Tamagawa T, Oi S, Ishiwata I, Ishikawa H. and Nakamura Y. (2007). Differentiation of mesenchymal cells derived from human amniotic membranes into hepatocyte-like cells in vitro. Hum Cell 20:77–84 [DOI] [PubMed] [Google Scholar]
- 14.Anzalone R, Lo Iacono M, Corrao S, Magno F, Loria T, Cappello F, Zummo G, Farina F. and La Rocca G. (2010). New emerging potentials for human Wharton's jelly mesenchymal stem cells: immunological features and hepatocyte-like differentiative capacity. Stem Cells Dev 19:423–438 [DOI] [PubMed] [Google Scholar]
- 15.Vaghjiani V, Vaithilingam V, Tuch B, Sievert W. and Manuelpillai U. (2013). Deriving hepatocyte-like cells from placental cells for transplantation. Curr Stem Cell Res Ther 8:15–24 [DOI] [PubMed] [Google Scholar]
- 16.Stock P, Bruckner S, Ebensing S, Hempel M, Dollinger MM. and Christ B. (2010). The generation of hepatocytes from mesenchymal stem cells and engraftment into murine liver. Nat Protoc 5:617–627 [DOI] [PubMed] [Google Scholar]
- 17.Aurich I, Mueller LP, Aurich H, Luetzkendorf J, Tisljar K, Dollinger MM, Schormann W, Walldorf J, Hengstler JG, Fleig WE. and Christ B. (2007). Functional integration of hepatocytes derived from human mesenchymal stem cells into mouse livers. Gut 56:405–415 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Wu XB. and Tao R. (2012). Hepatocyte differentiation of mesenchymal stem cells. Hepatobiliary Pancreat Dis Int 11:360–371 [DOI] [PubMed] [Google Scholar]
- 19.Charbord P. (2010). Bone marrow mesenchymal stem cells: historical overview and concepts. Hum Gene Ther 21:1045–1056 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Ilancheran S, Michalska A, Peh G, Wallace EM, Pera M. and Manuelpillai U. (2007). Stem cells derived from human fetal membranes display multilineage differentiation potential. Biol Reprod 77:577–588 [DOI] [PubMed] [Google Scholar]
- 21.Miki T, Lehmann T, Cai H, Stolz DB. and Strom SC. (2005). Stem cell characteristics of amniotic epithelial cells. Stem Cells 23:1549–1559 [DOI] [PubMed] [Google Scholar]
- 22.Tee JY, Vaghjiani V, Liu YH, Murthi P, Chan J. and Manuelpillai U. (2013). Immunogenicity and immunomodulatory properties of hepatocyte-like cells derived from human amniotic epithelial cells. Curr Stem Cell Res Ther 8:91–99 [DOI] [PubMed] [Google Scholar]
- 23.Takashima S, Ise H, Zhao P, Akaike T. and Nikaido T. (2004). Human amniotic epithelial cells possess hepatocyte-like characteristics and functions. Cell Struct Funct 29:73–84 [DOI] [PubMed] [Google Scholar]
- 24.Miki T, Marongiu F, Ellis EC, Dorko K, Mitamura K, Ranade A, Gramignoli R, Davila J. and Strom SC. (2009). Production of hepatocyte-like cells from human amnion. Methods Mol Biol 481:155–168 [DOI] [PubMed] [Google Scholar]
- 25.Marongiu F, Gramignoli R, Dorko K, Miki T, Ranade AR, Paola Serra M, Doratiotto S, Sini M, Sharma S, et al. (2011). Hepatic differentiation of amniotic epithelial cells. Hepatology 53:1719–1729 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Opara EC. and Kendall WF., Jr. (2002). Immunoisolation techniques for islet cell transplantation. Expert Opin Biol Ther 2:503–511 [DOI] [PubMed] [Google Scholar]
- 27.Santos E, Zarate J, Orive G, Hernandez RM. and Pedraz JL. (2010). Biomaterials in cell microencapsulation. Adv Exp Med Biol 670:5–21 [DOI] [PubMed] [Google Scholar]
- 28.de Vos P, Faas MM, Strand B. and Calafiore R. (2006). Alginate-based microcapsules for immunoisolation of pancreatic islets. Biomaterials 27:5603–5617 [DOI] [PubMed] [Google Scholar]
- 29.Vaithilingam V, Kollarikova G, Qi M, Lacik I, Oberholzer J, Guillemin GJ. and Tuch BE. (2011). Effect of prolonged gelling time on the intrinsic properties of barium alginate microcapsules and its biocompatibility. J Microencapsul 28:499–507 [DOI] [PubMed] [Google Scholar]
- 30.Nakamura K, Mizutani R, Sanbe A, Enosawa S, Kasahara M, Nakagawa A, Ejiri Y, Murayama N, Miyamoto Y, et al. (2011). Evaluation of drug toxicity with hepatocytes cultured in a micro-space cell culture system. J Biosci Bioeng 111:78–84 [DOI] [PubMed] [Google Scholar]
- 31.Selden C, Shariat A, McCloskey P, Ryder T, Roberts E. and Hodgson H. (1999). Three-dimensional in vitro cell culture leads to a marked upregulation of cell function in human hepatocyte cell lines—an important tool for the development of a bioartificial liver machine. Ann N Y Acad Sci 875:353–363 [DOI] [PubMed] [Google Scholar]
- 32.Miki T, Marongiu F, Ellis E. and S CS. (2007). Isolation of amniotic epithelial stem cells. Curr Protoc Stem Cell Biol Chapter 1:Unit 1E3. [DOI] [PubMed] [Google Scholar]
- 33.Pratama G, Vaghjiani V, Tee JY, Liu YH, Chan J, Tan C, Murthi P, Gargett C. and Manuelpillai U. (2011). Changes in culture expanded human amniotic epithelial cells: implications for potential therapeutic applications. PLoS One 6:e26136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Foster JL, Williams G, Williams LJ. and Tuch BE. (2007). Differentiation of transplanted microencapsulated fetal pancreatic cells. Transplantation 83:1440–1448 [DOI] [PubMed] [Google Scholar]
- 35.Rae JM, Johnson MD, Lippman ME. and Flockhart DA. (2001). Rifampin is a selective, pleiotropic inducer of drug metabolism genes in human hepatocytes: studies with cDNA and oligonucleotide expression arrays. J Pharmacol Exp Ther 299:849–857 [PubMed] [Google Scholar]
- 36.Livak KJ. and Schmittgen TD. (2001). Analysis of relative gene expression data using real-time quantitative PCR and the 2(-Delta Delta C(T)) Method. Methods 25:402–408 [DOI] [PubMed] [Google Scholar]
- 37.Crestani M, Sadeghpour A, Stroup D, Galli G. and Chiang JY. (1998). Transcriptional activation of the cholesterol 7alpha-hydroxylase gene (CYP7A) by nuclear hormone receptors. J Lipid Res 39:2192–2200 [PubMed] [Google Scholar]
- 38.Faybik P. and Hetz H. (2006). Plasma disappearance rate of indocyanine green in liver dysfunction. Transplant Proc 38:801–802 [DOI] [PubMed] [Google Scholar]
- 39.Tsubono T, Todo S, Jabbour N, Mizoe A, Warty V, Demetris AJ. and Starzl TE. (1996). Indocyanine green elimination test in orthotopic liver recipients. Hepatology 24:1165–1171 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Sies H. (1999). Glutathione and its role in cellular functions. Free Radic Biol Med 27:916–921 [DOI] [PubMed] [Google Scholar]
- 41.Garcia-Ruiz C. and Fernandez-Checa JC. (2007). Redox regulation of hepatocyte apoptosis. J Gastroenterol Hepatol 22 (Suppl 1):S38–S42 [DOI] [PubMed] [Google Scholar]
- 42.Nebert DW. and Russell DW. (2002). Clinical importance of the cytochromes P450. Lancet 360:1155–1162 [DOI] [PubMed] [Google Scholar]
- 43.Lim YP, Kuo SC, Lai ML. and Huang JD. (2009). Inhibition of CYP3A4 expression by ketoconazole is mediated by the disruption of pregnane X receptor, steroid receptor coactivator-1, and hepatocyte nuclear factor 4alpha interaction. Pharmacogenet Genomics 19:11–24 [DOI] [PubMed] [Google Scholar]
- 44.Sainz B, Jr., TenCate V. and Uprichard SL. (2009). Three-dimensional Huh7 cell culture system for the study of Hepatitis C virus infection. Virol J 6:103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Cheng N, Wauthier E. and Reid LM. (2008). Mature human hepatocytes from ex vivo differentiation of alginate-encapsulated hepatoblasts. Tissue Eng Part A 14:1–7 [DOI] [PubMed] [Google Scholar]
- 46.Miranda JP, Rodrigues A, Tostoes RM, Leite S, Zimmerman H, Carrondo MJ. and Alves PM. (2010). Extending hepatocyte functionality for drug-testing applications using high-viscosity alginate-encapsulated three-dimensional cultures in bioreactors. Tissue Eng Part C Methods 16:1223–1232 [DOI] [PubMed] [Google Scholar]
- 47.Chia SM, Leong KW, Li J, Xu X, Zeng K, Er PN, Gao S. and Yu H. (2000). Hepatocyte encapsulation for enhanced cellular functions. Tissue Eng 6:481–495 [DOI] [PubMed] [Google Scholar]
- 48.Elkayam T, Amitay-Shaprut S, Dvir-Ginzberg M, Harel T. and Cohen S. (2006). Enhancing the drug metabolism activities of C3A—a human hepatocyte cell line—by tissue engineering within alginate scaffolds. Tissue Eng 12:1357–1368 [DOI] [PubMed] [Google Scholar]
- 49.Krishnamurthy NV. and Gimi B. (2011). Encapsulated cell grafts to treat cellular deficiencies and dysfunction. Crit Rev Biomed Eng 39:473–491 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Tuch BE, Keogh GW, Williams LJ, Wu W, Foster JL, Vaithilingam V. and Philips R. (2009). Safety and viability of microencapsulated human islets transplanted into diabetic humans. Diabetes Care 32:1887–1889 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Skvorak KJ, Dorko K, Marongiu F, Tahan V, Hansel MC, Gramignoli R, Arning E, Bottiglieri T, Gibson KM. and Strom SC. (2013). Improved amino acid, bioenergetic metabolite and neurotransmitter profiles following human amnion epithelial cell transplant in intermediate maple syrup urine disease mice. Mol Genet Metab 109:132–138 [DOI] [PubMed] [Google Scholar]
- 52.Skvorak KJ, Dorko K, Marongiu F, Tahan V, Hansel MC, Gramignoli R, Gibson KM. and Strom SC. (2013). Placental stem cell correction of murine intermediate maple syrup urine disease. Hepatology 57:1017–1023 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Zhang FT, Wan HJ, Li MH, Ye J, Yin MJ, Huang CQ. and Yu J. (2011). Transplantation of microencapsulated umbilical-cord-blood-derived hepatic-like cells for treatment of hepatic failure. World J Gastroenterol 17:938–945 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Sahai S, McFarland R, Skiles ML, Sullivan D, Williams A. and Blanchette JO. (2012). Tracking hypoxic signaling in encapsulated stem cells. Tissue Eng Part C Methods 18:557–565 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.