Abstract
Dental pulp stem cells (DPSCs) remain quiescent until activated in response to severe dental pulp damage. Once activated, they exit quiescence and enter regenerative odontogenesis, producing reparative dentin. The factors and signaling molecules that control the quiescence/activation and commitment to differentiation of human DPSCs are not known. In this study, we determined that the inhibition of insulin-like growth factor 1 receptor (IGF-1R) and p38 mitogen-activated protein kinase (p38 MAPK) signaling commonly activates DPSCs and promotes their exit from the G0 phase of the cell cycle as well as from the pyronin Ylow stem cell compartment. The inhibition of these two pathways, however, inversely determines DPSC fate. In contrast to p38 MAPK inhibitors, IGF-1R inhibitors enhance dental pulp cell sphere-forming capacity and reduce the cells' colony-forming capacity without inducing cell death. The inverse cellular changes initiated by IGF-1R and p38 MAPK inhibitors were accompanied by inverse changes in the levels of active signal transducer and activator of transcription 3 (STAT3) factor, inactive glycogen synthase kinase 3, and matrix extracellular phosphoglycoprotein, a marker of early odontoblast differentiation. Our data suggest that there is cross talk between the IGF-1R and p38 MAPK signaling pathways in DPSCs and that the signals provided by these pathways converge at STAT3 and inversely regulate its activity to maintain quiescence or to promote self-renewal and differentiation of the cells. We propose a working model that explains the possible interactions between IGF-1R and p38 MAPK at the molecular level and describes the cellular consequences of these interactions. This model may inspire further fundamental study and stimulate research on the clinical applications of DPSC in cellular therapy and tissue regeneration.
Introduction
Human dental pulp stem cells (DPSCs) reside in the soft part of teeth, the dental pulp, where they are surrounded by protective hard tissues, dentin and enamel in the crown and dentin and cementum in the root region. Dental pulp tissue contains a heterogeneous population of cells that include dentin-forming odontoblasts, fibroblasts, neurons, and cells of the vascular and immune systems [1,2]. Histologically, dental pulp is organized in zones. The outermost zone, the odontogenesis zone, is composed of mitotically arrested odontoblasts that secrete predentin that matures into dentin at the periphery of the pulp. Central to this zone is the cell-free zone, through which capillaries and nerve fibers enter the pulp chamber via root channels. Finally, adjacent to the central pulp lays the cell-rich zone, which contains fibroblasts, nerves, immune cells, and undifferentiated mesenchymal cells. The latter are thought to be the precursors of odontoblasts and are known as DPSCs [2–4]. Odontoblasts are the only cells that have regenerative capacity and that can restore dentin in response to bacterial decay or mechanical damage [5]. Earlier studies showed that intense damage to mature dental pulp stimulates the division and migration of cells from the center of the dental pulp to its periphery, where they undergo odontoblast-like differentiation, replacing dead odontoblasts and producing reparative dentin [4,6,7]. These observations indicate that newly differentiating odontoblasts originate within the highly vascularized and innervated central zone of the pulp. Later, this zone was shown to contain multipotent DPSCs [8].
DPSCs originate from neural crest cells [9–11] that acquire dental competence as multipotent stem cells (SCs) [12]. First reported in 2000 [8], the existence of DPSCs has been confirmed by many laboratories, including ours [13]; however, the exact area of the dental pulp in which they are located is still not well established. A recent study by Martens et al. [14] confirmed earlier findings [4,12,15,16] that DPSCs occupy the prevascular niche and, in developing teeth, the cervical niche located near the cementum/dentin zone. A study based on the mRNA expression levels of DPSC markers, including CD166, CD146, and CD105, concluded that in rat molars, “coronal pulp harbors more SCs than the other regions” [17]. A study by Ishikawa et al. [18,19] determined that 5-bromo-2′-deoxyuridine (BrdU)-retaining cells expressing the mesenchymal stem cell marker CD146 were associated with vessels located in the central region of adult rat dental pulp. These label retaining cells (LRCs) possessed proliferative capacity and were responsible for the regeneration of damaged odontoblasts.
Localized in the protective environment of the niche, SCs integrate systemic and local signals that drive them from reversible quiescence into the cell cycle. The asymmetric division of SC produces a SC daughter to maintain the stem cell pool and a transient amplifying (TA) daughter that differentiates after a limited number of divisions. Odontoblasts committed to differentiation express an Msx1 homeobox protein [20] that is downregulated as cells enter the early differentiation stage, which is marked by the appearance of matrix extracellular phosphoglycoprotein (MEPE) [21,22]. MEPE must be downregulated for the late differentiation markers, including dentin sialoprotein (DSP), to be upregulated [1,21–24]. These markers regulate the mineralization process. The final stage of odontoblast differentiation is indicated by the presence of calcified von Kossa stain-positive nodules.
The homeostasis, growth, and repair of tissues depend on the existence of a balance between cellular quiescence and the proliferation of SCs [25]. The factors that control this balance are currently being identified in hematopoietic, nervous, and epithelial tissues, but the factors that control the quiescence and activation of DPSCs still await discovery. It has been shown, however, that tumor necrosis factor alpha (TNFα) and other inflammatory factors can activate odontoblastic precursors and stimulate their proliferation and differentiation in response to bacterial infection [16,26,27]. Other studies have shown that the downstream targets of TNFα, the p38 mitogen-activated protein kinase (p38 MAPK) [27–29] and the insulin-like growth factor 1 receptor (IGF-1R) [30–32], also control odontoblast differentiation [33–35] and development [36,37]. However, studies on modulation of DPSC are limited. Here, we determined that p38 MAPK and IGF-1R signaling are required to maintain DPSC quiescence and to regulate the differentiation of these cells, although in opposite manners. Importantly, we identified signal transducer and activator of transcription 3 (STAT3) as a converging point that integrates signals from p38 MAPK and IGF-1R pathways and regulates human DPSC responses.
Materials and Methods
Tissue samples and cell cultures
Wisdom or premolar teeth were extracted for orthodontic reasons from young adults at the Dental Clinic of the University Hospital of Lille. Patients agreed to donate their teeth for research by signing a “no opposition” agreement. The storage and use of human biological samples were declared and performed according to the local Person's Protection Committee and the ethical rules approved by the Department of Health of France (File No. DC-2008-642). Dental pulp tissue was snap-frozen for immunohistochemical analysis, sliced and placed in culture for ex vivo study, or enzymatically dissociated for 1 h at 37°C in a solution containing collagenase type I (3 mg/mL) and dispase (4 mg/mL), both purchased from Invitrogen. The digested tissue was filtered through a 70-μm pore strainer (BD Biosciences), and the obtained cell suspension was rinsed and plated at the indicated densities in the standard odontoblast growth medium [Dulbecco's modified Eagle medium (DMEM) Glutamax supplemented with 1 mg/mL glucose, 1% penicillin/streptomycin solution (Invitrogen), 10% fetal calf serum (FCS; Lonza), and 100 μM vitamin C (Sigma-Aldrich)]. The cells were cultured at 37°C under 5% CO2 and re-fed every 3 days with the fresh medium. Inhibitors of IGF-1R (sc204008; 125 nM) and of STAT3 (Stattic5-sc20281; 5 μM; Santa Cruz Biotech.) and of p38 MAPK (PD169316; 250 nM; Calbiochem) were added to the cell culture medium for the indicated times. The solvents in which the inhibitors were dissolved were added at the same concentrations to the control cultures. Colony-forming units (CFUs) and sphere-forming units (SFUs) were calculated as the percentage of the colonies or spheres of, respectively, 200 and 4,000 seeded live cells formed, as described previously [13]. Cell proliferation was monitored on 96-well plates using IncuCyte 2011A Kinetic Imaging System and software (Essen BioScience).
Ex vivo cultures of dental pulp
Freshly isolated pulp tissue was sectioned under sterile conditions using a scalpel. Slices were placed in the upper chambers of transwell plates and covered with the medium placed in the lower chamber. Inhibitors at nontoxic doses (sc204008; 125 nM) for IGF-1R and PD169316 (250 nM for p38) were added to this lower chamber for 5 days. The medium and inhibitors were changed twice during this period, after which the tissue was snap-frozen and used for immunohistochemistry.
Flow cytometry and cell sorting
Cells were trypsinized and resuspended at a density of 1×106 cells/mL in phosphate-buffered saline containing 2% bovine serum albumin. Aliquots of the cell suspension (100 μL) were incubated with primary antibodies (Abs) for 30 min at 4°C in the dark and with secondary fluorochrome-conjugated Abs for another 30 min if needed. A minimum of 1×105 cells were analyzed with a Cyan-ADP (Beckman Coulter, Inc.) instrument; the data were processed using Summit 4.3 software (Beckman Coulter, Inc.). PE-conjugated monoclonal Abs against CD271 were from Mitenyi Biotech. CD166-FITC conjugate was from BD Pharmingen, CD105-FITC from Immunotools, and Ki67-FITC conjugate was from Santa Cruz Biotech. CD57 (HNK1) Ab was a gift from Dr. Elisabeth Dupin, Institut de la Vision, Paris, France.
Propidium iodide/Ki67 assay
Cells were adjusted to a concentration of 106 cells/mL in DMEM with 10% FCS, and the assay was performed as previously described [38]. Propidium iodide (PI)- and Ki67-negative cells within the G0/G1 fraction were considered to represent quiescent cells in the G0 phase of the cell cycle.
DiI and BrdU staining and cell sorting
Cells were resuspended in serum-free DMEM and stained with 3 μL of Vybrant DiI cell-labeling solution (Invitrogen) per 106 cells for 25 min at 37°C. After rinsing, the cells were re-plated in the standard medium. The 5% of cells with the highest and lowest DiI staining indices were sorted by fluorescence-activated cell sorting using a Beckman Coulter Altra instrument. The sorted cells were used for further analysis. For BrdU incorporation, DiI-labeled cells were pulsed with 0.5 μM BrdU (Roche®) 24 h later and allowed to incorporate BrdU for 6 days. The label was chased for another 6 days by growing the labeled cells in a label-free medium. Staining with anti-BrdU Ab was performed according to the supplier's recommendations. The pyronin Y (PY)/Hoechst 33342 assay was performed with 5×105 cells, as described previously [38]. Flow cytometry analysis of the total population before and after PY staining permitted the gating and identification of cells with low and high PY (FL2) fluorescence intensities.
Immunohistochemistry
Dental pulp samples were embedded in Tissue-Tek OCT (Sakura Finetechnical) and cryosectioned. Sections (8 μm) were placed on Superfrost plus (Thermo Scientific) glass slides, air-dried, and fixed in the paraformaldehyde (PAF) solution (Sigma-Aldrich), and immunohistochemistry was performed according to the standard procedures. Abs against CD271 (p75NTR) and p38 MAPK were from Abcam and Cell Signaling Tech., respectively. Negative controls were performed by replacing the primary Abs with irrelevant Abs of the same isotype. Hoechst 33342 (1 μg/mL) was used for nuclear counterstaining. All slides were mounted under coverslips with the Vectashield Mounting Medium (Vector Laboratories) and photographed using a Leica® fluorescence microscope (Leica). To quantify the proportion of Ki67-positive cells, we manually counted the total number of Ki67-positive and Hoechst 33342-positive nuclei present on 5–7 images obtained in three independent experiments per condition. The percentage of Ki67-positive nuclei was calculated using the following formula: number of Ki67 − positive/Hoechst 33342 − positive nuclei×100.
von Kossa staining
To detect mineralization, cells were incubated in the odontoblast growth medium supplemented with 10 mM β-glycerol phosphate (βGP) for the indicated times, fixed in PAF for 10 min, and stained as described previously [13].
Western blot analysis
Western blot analysis was performed using ready-to-use NuPAGE® 4–12% Bis–Tris polyacrylamide gels (Invitrogen) according to the manufacturer's instructions. Blots were probed with primary Abs to actin (Sigma-Aldrich), cyclin D1, phosphorylation of cAMP-responsive-element-binding protein 1 (pCREB), c-Myc, Bcl-2, MEPE, DSP (Santa Cruz Biotech.), p38, pp38, pSTAT3, phosphorylation of ATF2 (pATF2), and phosphorylation of glycogen synthase kinase 3 (pGSK3; Cell Signaling Technology) followed by horseradish peroxidase-conjugated secondary Ab (Bio-Rad). Immunodetection was performed using an ECL-Prim chemiluminescence kit from Amersham and a LAS4000 apparatus. The results were integrated using Gel Analyst software® (Claravision).
Statistical analysis
Results are expressed as the mean±standard error of the mean of at least two independent experiments. Comparisons between means were assessed using Student's t test for unpaired data. If unequal variance was observed, Welch's correction was applied. Comparisons between several groups were assessed using a one-way analysis of variance followed by Dunnett's multiple comparison test, using an appropriate control group as the reference. The statistical analyses were performed using the GraphPad Prism 4.0 software. A P value of<0.05 was considered significant.
Results
Inactivation of IGF-1R and p38 MAPK induces cycling of quiescent human dental pulp cells ex vivo
IGF-1R is known to activate various downstream targets, including AKT/mTOR, extracellular signal-regulated kinases (ERK1/2), p38 MAPK pathways, and the Janus kinase (JAK)/STAT3 pathway [32,39–41], all of which have been shown to activate quiescent SCs [38,42–44]. Interestingly, p38 MAPK, a serine/threonine kinase that is activated in response to stress and inflammatory cytokines [45], controls the quiescence of SC via several mechanisms [46–49], including the inactivation of GSK3, an important effector of the Wnt self-renewing pathway [50]. To investigate the role of IGF-1R and p38 MAPK in dental pulp cell (DPC) biology, we first performed immunohistochemical analysis of snap-frozen human dental pulp tissue to localize the expression of p38 MAPK and IGF-1R in this tissue (Fig. 1A). p38 MAPK was detected in the nuclei of the majority of DPCs. To the best of our knowledge, this is the first study to localize p38 MAPK in human dental pulp. In agreement with an earlier study by Götz et al. [51], IGF-1R was detected in undifferentiated odontoblasts of the odontogenic zone at the dental pulp periphery. The observed nuclear localization of p38 MAPK represents the active form of this kinase [52,53] and suggests that p38 MAPK is constitutively active in DPCs and, as such, may contribute to their quiescence. IGF-1R expression by the mitotically quiescent cells suggests that in addition to its known function in controlling the cell cycle, cell survival, motility, and attachment [54], IGF-1R may also function to maintain odontoblasts quiescence and preserve their undifferentiated phenotype.
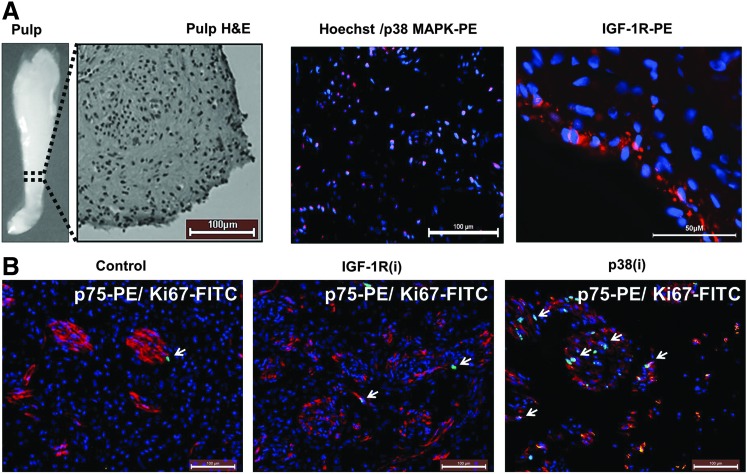
FIG. 1.
p38 mitogen-activated protein kinase (p38 MAPK) and insulin-like growth factor 1 receptor (IGF-1R) inhibitors induce human dental pulp proliferation ex vivo. Dental pulps were removed from extracted premolar teeth, immediately dissected, and placed on transwell plates in the medium supplemented with the IGF-1R or p38 MAPK inhibitors (i) sc204008 or PD169316 (125 and 250 nM, respectively; see “Materials and Methods” for details) for 5 days. (A) An example of one pulp and hematoxylin–eosin staining of one part of the pulp (broken line) and a representative immunohistochemistry image showing p38 MAPK within the nuclei of dental pulp cells and IGF-1R at the pulp periphery (red). Nuclei are stained with Hoechst 33342 (blue). (B) Untreated (control) pulp sections stained with anti-Ki67 antibody to detect cycling cells (green) and with anti-p75NTR to detect neurons and neural crest stem cells (red). Treatment with IGF-1R inhibitor [IGF-13(i)] and p38 MAPK inhibitor [p38(i)] induces Ki67 staining (arrows) in some cells in the central pulp. Nuclei are stained with Hoechst 33342 (blue). Representative image of 4–7 images from three independent experiments.
If p38 MAPK and IGF-1R, which functions upstream of p38 MAPK, maintain the quiescence of DPCs, their inactivation should provoke the exit of the cells from quiescence and their entry into the cell cycle. Cycling cells are detectable by positive staining with Ki67 Ab; this Ab does not recognize resting G0 cells. We incubated slices of freshly extracted dental pulp in the odontoblast growth medium supplemented with 125 nM sc204008 and 250 nM PD169316, inhibitors of IGF-1R and p38 MAPK, respectively, for 5 days. Representative images from these ex vivo studies (Fig. 1B) show that IGF-1R inhibitor [IGF-1R(i)] and p38 MAPK inhibitor [p38(i)] induced the appearance of Ki67-positive cells. The quantitation by manual counting of the Ki67-positive cells per total number of cells showed that, compared to control, 3.2-fold more cells began cycling in response to IGF-1R(i) and 14.8-fold more cells entered the cell cycle in response to p38(i) (Supplementary Table S1; Supplementary Data are available online at www.liebertpub.com/scd), indicating that the inactivation of IGF-1R and p38 MAPK selectively stimulates the division of some quiescent DPCs. Interestingly, most of the cells that entered the cell cycle were located near or within CD271- or p75 neurotrophin receptor (p75NTR)-positive nerve bundles penetrated by blood vessels where the niche for multipotent DPSCs was found [14,15,55].
IGF-1R and p38 MAPK inhibitors decrease the pool of quiescent DPSCs in vitro
To substantiate the ex vivo data, we performed several functional tests to characterize the Ki67-positive cells in vitro. The findings obtained using a PI/Ki67 assay (example shown in Fig. 2A), which distinguishes cycling cells from quiescent SCs resting in the G0 phase of the cell cycle, are summarized in Table 1. As seen, IGF-1R(i) and p38(i) diminished the proportion of G0 cells by 57% and 53%, respectively, compared to control cells and stimulated the transition of cells from the G0/G1 to the S/G2/M phase of the cell cycle. Interestingly, neither IGF-1R(i) nor p38(i) increased the subG1 fraction of dead cells, suggesting that under our experimental conditions, these inhibitors do not significantly induce the death of DPCs relative to the control. This was confirmed by AnnexinV/7-AAD and trypan blue assays (Supplementary Fig. S1A, B). Thus, it appears that the inactivation of either the IGF-1R or the p38 MAPK signaling pathway stimulates the cycling of quiescent DPSCs in vitro.
FIG. 2.
Dental pulp contains a small fraction of quiescent cells. (A) Cell cycle profile of human dental pulp stem cells stained with propidium iodide (PI) (left panel) and the corresponding profile (right panel) of cells double-stained with PI and anti-Ki67FITC antibody to illustrate the presence of cells in the G0 phase of the cell cycle (rectangle). (B) Double DiI (red) and 5-bromo-2′-deoxyuridine (BrdU, green) staining of human dental pulp cells illustrating the presence of noncycling or slowly cycling label retaining cells. Details of this assay are given in the “Materials and Methods” section. Nuclei are stained with Hoechst 33342 (blue), ***p<0.001. (C) Sphere-forming units (SFU) as a percentage (%) of sorted DiI-positive (DiI+) and Di1-negative (DiI−) cells illustrating that DiI+ cells possess a sphere-forming capacity that is superior to that of DiI− cells and characteristic of stem cells. (D) Exposure to IGF-1R(i) and p38(i) stimulated DiI dilution. Flow cytometry histogram overlays show DiI+ cells at time 0 (clear curve) and after 7 days (shaded curve) of culturing with or without inhibitors. Vertical line indicates window for DiI− cells. The number of DiI+ cells in the control is considered as 100%. (E) Representative flow cytometry histograms of pyronin Y (PY)-stained cells. The upper panels show the size and structure of the cells. (PYlow) cells (in blue) are of small size (forward scatter-FS) in structure (side scatter-SS). The lower panels show that the number of PYlow cells diminishes upon treatment with the IGF-1R and p38 MAPK inhibitors (i) sc204008 or PD169316 (125 and 250 nM, respectively) for 24 h. The numbers indicate the percentage of PYlow cells with a low RNA content characteristic of stem cells. PYlow cells were gated according to the profile of unstained cells.
Table 1.
Insulin-Like Growth Factor 1 Receptor and p38 Mitogen-Activated Protein Kinase Inhibitors Diminish the Fraction of Human Dental Pulp Cells in G0 of the Cell Cycle
| Sub G1 | G0 | G1 | S | G2/M | |
|---|---|---|---|---|---|
| Control | 0.1±0.1 | 4.9±0.41 | 75.9±7.9 | 4.7±1.4 | 16.6±6.6 |
| IGF-1R(i) | 0.1±0.01 | 2.1±0.43 | 70.4±9.1 | 6.0±0.8 | 23.0±8.4 |
| p38(i) | 0.1±0.1 | 2.3±0.39 | 68.1±10.7 | 6.6±1.2 | 24.6±9.4 |
Human dental pulp propidium iodide Ki67 cell cycle analysis was used to identify the G0 fraction of quiescent cells. Please note that neither IGF-1R inhibitor [IGF-1R(i)] nor p38 inhibitor [p38(i)] (125 nM sc204008 and 250 nM PD169316, respectively) induced cell death (subG1 fraction) during 24 h of treatment.
IGF-1R, insulin-like growth factor 1 receptor.
To confirm that the inhibition of IGF-1R and p38 MAPK signaling affects the stem cell compartment in dental pulp cultures, we determine the expression of DPSC and neural crest stem cell surface markers. The primary DPC cultures used in our experiments expressed 86.4%±6.02 and 81.03%±8.11 of CD166 and CD105 DPSC markers, respectively, but consistent with our previous study [13], only a very few cells expressed CD271 (0.23±0.2) and CD57 (1.39±1.5) neural crest stem cell markers. Since the expression of surface markers is condition dependent, we did not relay on them in defining SCs and used a vital DiI dye-based functional assay to distinguish quiescent LRCs from the cycling population. Cycling cells dilute this dye by half with each cell division and thus rapidly shed the label. Figure 2B shows a representative image of DPCs labeled simultaneously with DiI and BrdU. BrdU incorporation is another method of LRC assay [19]. This double labeling ensured the accurate identification of quiescent LRCs and/or of slowly cycling cells possessing SC attributes. To further confirm the stemness of LRCs, we sorted DiIhigh LRC and DiIlow cycling cells and determined their sphere-forming capacity, another trait that defines SCs. In agreement with our previous results [13], DiIhigh cells showed a greater ability to form spheres than DiIlow cells (Fig. 2C), indicating that the DiIhigh cell population is enriched for DPSCs. Flow cytometric analysis of DPCs consistently showed that 24 h treatment with IGF-1R or p38 MAPK inhibitors significantly decreased the number of DiIhigh LRCs in relation to the control (Fig. 2D). This finding corroborates our G0 results and demonstrates that both inhibitors stimulate the division of quiescent/slowly cycling LRC DPSCs.
SCs that exit the quiescent state divide to reproduce themselves and to generate TA progeny that are committed to differentiating. Quiescent SCs were shown to have low metabolic activity, as reflected, among other properties, by their low content of cellular RNA resulting in poor labeling by PY, an RNA-binding vital dye [38,56,57]. The results of PY assays provided additional evidence that IGF-1R(i) and p38(i) impinge on the DPSC compartment. Exponentially growing dental pulp primary cultures contained 3.81–11.96% PYlow cells. These cells are small and of low granularity, as determined by their low forward and side scatter profiles, respectively (Fig. 2E). IGF-1R and p38 MAPK inhibitors decreased persistently the size of the PYlow fraction to 72% and 62% of controls, respectively (example in Fig. 2E), demonstrating that during IGF-1R or p38 MAPK inhibition, a small number of cells exit the PYlow pool and enter the PYhigh pool of metabolically active cells. Again, these data confirm that IGF-1R and p38 MAPK inhibitors act on quiescent DPSCs and stimulate their metabolic activity, a characteristic of cycling TA cells.
Inactivation of IGF-1R and p38 MAPK stimulates DPC proliferation with different kinetics and inversely and differentially regulates stem and TA cell compartments
The proliferation profile of the DPC total population in response to IGF-1R and p38 MAPK inhibitors confirmed their pro-proliferative action but also revealed differences between them. Figure 3A shows that short 24–48 h treatment with IGF-1R(i) did not inhibit, and p38(i) increased, the proliferation of DPCs compared to that of untreated cultures. Prolonged exposure (144 h) to IGF-1R(i), however, reduced proliferation, whereas prolonged exposure to p38(i) continued to stimulate the DPC proliferative activity (Fig. 3A). The stimulation of proliferation following both short and prolonged inhibition of p38 MAPK is consistent with the known ability of p38 MAPK to induce quiescence [46]. It is likely that the short-term effect is reversible because prolonged inhibition sustained the effect. In contrast, the inhibition of proliferation by short and prolonged treatment with IGF-1R(i) reveals a paradoxical ability of IGF-1R to support quiescence and proliferation.
FIG. 3.
IGF-1R and 38 MAPK inhibitors stimulate dental pulp stem cell propagation but instigate different fates. (A) Proliferation curves obtained using IncuCyte live imaging and software showing the percentage of confluency after 6 days of every day treatment with IGF-1R or p38 MAPK inhibitors (125 nM sc204008 and 250 nM PD169316, respectively). The curve inside the box zooms on the first 3 days of treatment with inhibitors. *p<0.05, ***p<0.001. (B) Representative images of spheres formed by cells treated with IGF-1R or p38 MAPK inhibitors (i) (125 nM sc204008 and 250 nM PD169316, respectively). (C) SFUs. (D) Colony-forming units (CFUs). Please note the inverse effect of the inhibitors on (C) and (D). Sphere- and colony-forming capacities reflect, respectively, the size of stem cell and stem cell plus transient amplifying cell pools. **p<0.01, ***p<0.001.
To resolve the paradoxical effect of IGF-1R and p38 MAPK inhibitors, we assumed that they act differently on the SC and TA cell compartments. Whether the SCs self-renew or proceed to their differentiation fate can be estimated by the measurement of SFU values, which reflect the size of the SC compartment. The data in Fig. 3B and C demonstrate that exposure of DPCs to IGF-1R and p38 MAPK inhibitors at the time of seeding affects the sphere-forming capacity of the cells in opposite ways. Surprisingly, IGF-1R(i) augmented and p38(i) decreased the number of spheres formed, implying that IGF-1R(i) expands the SC pool, whereas p38(i) reduces it. Thus, the observed differences in the mitotic activity of DPSC in response to each of the inhibitors have distinct biological consequences.
The exit of SCs from their compartment and their entry into the destined-to-differentiate TA cell compartment reduces their pool and self-renewing activity. The number of cells capable to initiate a colony is a measure of the proliferative potential of SCs and their TA daughters together. The CFU values shown in Fig. 3D indicate that, in contrast to their effects on the sphere-forming ability, IGF-1R(i) significantly decreased (P<0.001) and p38(i) significantly augmented (P<0.01) the number of colonies. The increased CFUs and decreased SFUs observed after p38 MAPK inactivation suggest that such inactivation stimulates cycling and at the same time increases the exit of sphere-forming DPSCs from the SC compartment, thus depleting this compartment. In contrast, the inactivation of IGF-1R seems to prevent the exit of sphere-forming DPSCs from the SC compartment and, consequently, to prevent the exhaustion of DPSCs.
This conclusion was strongly supported by the results of CFU assays performed with sorted PYlow DPSCs and their PYhigh progeny. The differences in the growth rates of untreated and IGF-1R(i)- and p38(i)-treated PYlow cultures were easily noticeable. Fig. 4A shows how strongly PYlow DPSC responded to p38(i). IGF-1R(i) was less powerful, but it also stimulated proliferation, whereas untreated PYlow cells grew very slowly. Sorted PYlow control cells remained quiescent for 3 weeks and did not form colonies at the time of scoring (after 2 weeks). In continuously IGF-1R(i) and p38(i) treated cultures, colonies were visible 1 week earlier. IGF-1R(i) stimulated colony formation to a lesser extent than p38(i) (Fig. 4B). In contrast, untreated and p38(i)-treated PYhigh cells grew much faster. However, IGF-1R(i) treatment of cycling PYhigh cells limited the growth to spindle-like, stem-like cells with a high nuclear/cytoplasmic ratio, clearly restricting the growth of the fibroblast-like TA cells that were visible in the control and p38(i)-treated cultures. Importantly, PYhigh cells treated with p38(i) produced more colonies than control PYhigh cells, and those treated with IGF-1R(i) produced fewer colonies than controls. This finding indicates that the SC and the TA cell compartments, as a whole, are enlarged in response to p38(i). IGF-1R(i), however, appears to stimulate only the SC pool because colonies in PYhigh cultures treated with IGF-1R(i) exhibited an SC-like morphology.
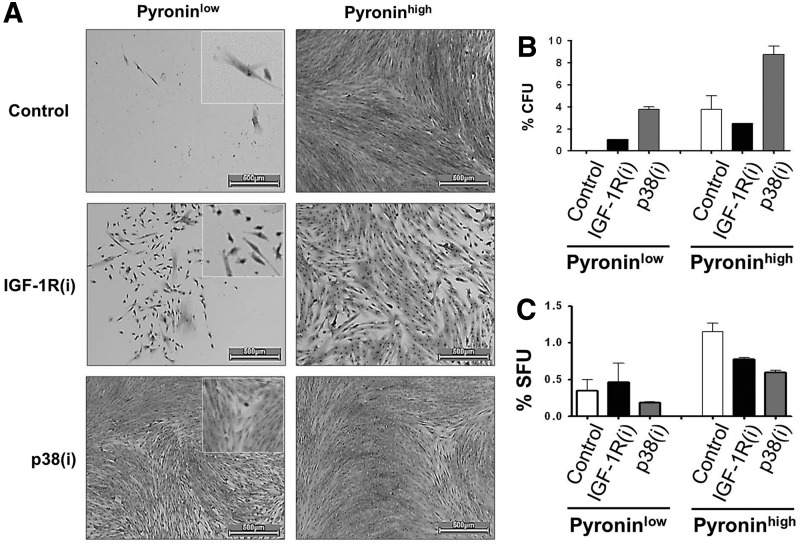
FIG. 4.
IGF-1R and p38 MAPK inhibitors diminish the proportion of PYlow human dental pulp stem cells. (A) Sorted PYlow and PYhigh cells respond differently to IGF-1R(i) and p38(i). The morphology and colonies of sorted PYlow and PYhigh cells after prolonged (2 weeks) treatment with the IGF-1R and p38 MAPK inhibitors (i) sc204008 or PD169316 (125 and 250 nM, respectively). The enlarged images (squares) show cells with undifferentiated morphology and high nuclear-to-cytoplasmic ratios. PYhigh, rapidly proliferating cells, have a fibroblastic morphology classically associated with differentiating odontoblasts. Scale bar=500 μm. Please note the marked difference in the subpopulations of cells after different treatments. (B) CFUs of PYlow and PYhigh subsets of human dental pulp cells treated every 3 days for 2 weeks. The PYlow control cells had not formed colonies at the time of scoring (2 weeks). (C) SFUs of the same of PYlow and PYhigh subsets treated with inhibitors at the time of seeding and scored after 1 week of culturing. Although PYlow slow growing subset of dental pulp cells produced less first-generation spheres, they retained higher self-renewing potential than fast growing PYhigh cells (see text).
This conclusion was substantiated by the results of SFU assays, which indicated that PYlow cells produced more first-generation spheres in the presence of IGF-1R(i) than in the presence of p38(i) (Fig. 4C) and had a 10.8-fold higher self-renewing capacity than PYhigh cells, as judged by the percentage of spheres in the second generation (1.3%PYlow/0.12%PYhigh). The delayed growth of PYlow cells is consistent with their properties of quiescent cells and suggests that they represent a pool of long-term quiescent SCs as opposed to the short-term poised SCs [25,58]. The PYhigh population apparently contained a subpopulation of cycling SCs that diminishes upon IGF-1R and p38 inhibitor treatment. Taken together, these data demonstrate that both IGF-1R(i) and p38(i) activate the slow-growing PYlow cells of the SC compartment. In addition, p38(i) may specifically accelerate division of SCs in this compartment while simultaneously feeding them into the PYhigh fast-growing, destined-to-differentiate TA cell subset. In contrast, by repressing the well-known pro-proliferation activity of IGF-1R [40], IGF-1R(i) clearly blocks the development of the PYhigh cell compartment.
IGF-1R and p38 MAPK inhibitors activate DPSCs by targeting different signaling pathways
Thus far, we have established that IGF-1R and p38 MAPK signals can maintain DPSC quiescence and induce cell cycling, two antagonistic cellular functions. How do these two pathways interact to regulate these opposite cellular activities? p38 MAPK is a downstream target of the IGF-1R. This suggests that IGF-1R(i) affects odontoblast quiescence through p38 MAPK signaling. To investigate this possibility, we cultured DPCs simultaneously with both IGF-1R and p38 MAPK inhibitors and measured the effects of such treatment on the colony-forming capacity of the cells. We found that the treatment with p38(i) enhanced the CFU values of IGF-1R(i)-treated cultures to the same level as treatment with p38(i) alone (Fig. 5A, B), suggesting that, in fact, p38(i) overcomes the inhibitory effect of IGF-1R(i). The well-established pro-proliferative activity of IGF-1R is mediated by its intracellular downstream targets, which include the PI3K/AKTmTOR signaling pathway [40,42]. To determine which, if any, of the intracellular downstream targets of IGF-1R may be affected by the p38 MAPK signals, we double inhibited this pathway and the PI3K/AKT pathway with LY294002 or mTOR with rapamycin. We found that mTOR inhibitors, but not PI3K/AKT inhibitors, reduced CFU values. Intriguingly, p38(i) alone or in combination with any of the other inhibitors always resulted in a similar increase in CFUs (Fig. 5A, B), demonstrating a prevailing action of p38 MAPK. The colonies formed in the presence of PI3K/AKT or mTOR inhibitors were smaller, however, than those formed in the presence of p38(i) alone, indicating that, conversely to p38 MAPK, PI3K/AKT/mTOR signaling may regulate the proliferative potential of DPCs. mTOR in particular seemed to contribute to the colony growth because double inhibition with LY294002 and rapamycin stopped cell division before colonies developed to the 50-cell stage. Taken together, these data suggest that IGF-1R may control stem cell quiescence via p38 MAPK signaling and proliferation potential via PI3K/AKT/mTOR signaling. The inactivation of p38 MAPK appears to have a regulatory effect on both of these cellular processes.
FIG. 5.
Inhibition of p38 MAPK has a dominant effect on colony-forming capacity and colony size. (A) Representative plates containing human dental pulp cells and illustrating the effect of the addition of inhibitors alone or in combination with any of three other inhibitors during colony formation. Inhibitors were refreshed every 3 days for 2 weeks. Please note that the colony-forming capacity of the cells was increased whenever p38 MAPK inhibitor was added. Simultaneous treatment with PI3K/AKT and mTOR inhibitors totally repressed colony formation. (B) Quantitative graphs of the results shown in (A). **p<0.01, ***p<0.001.
Inactivation of IGF-1R and p38 MAPK inversely regulate STAT3 and MEPE-related DPC differentiation and mineralization
To gain insight into the mechanistic links between cellular responses and molecular events, we generated profiles of the phosphorylated proteins that regulate cell growth and cycling activity in control, IGF-1R(i)- and p38(i)-treated dental pulp cultures. Western blotting (Fig. 6A) of IGF-1R(i)- and p38(i)-treated cells shows a set of proteins that were affected by the treatment. We found that IGF-1R(i) mainly stimulated the pCREB and pGSK3β, suggesting that in the absence of active IGF-1R that normally phosphorylates CREB and GSK3, other signaling pathways upregulate pCREB and pGSK3, perhaps the active p38 MAPK pathway, which is known to phosphorylate CREB [59,60] and inactivate GSK3 [50,61]. Consistently, the inactivation of p38 MAPK had no effect on these targets (Fig. 6A). In accord with the capability of IGF-1R(i) and p38(i) to drive cells out of cellular quiescence, these two inhibitors upregulated the expression of c-Myc and cyclin D1, which drive the cell cycle, and of Bcl-2, a pro-survival factor, to comparable levels, suggesting that the IGF-1R and p38 MAPK signaling pathways converge at this level of regulation.
FIG. 6.
IGF-1R and p38 MAPK inhibitors inversely affect signal transducer and activator of transcription 3 (STAT3) and matrix extracellular phosphoglycoprotein (MEPE) expression and dental pulp cell differentiation. (A) Western blot of dental pulp cell lysates reacted with antibodies against indicated phosphorylated (p) proteins and with antibodies against the proliferation and odontoblast differentiation markers. Cells used for western blots were treated with inhibitors for 24 h. β-Actin staining was used as a control for equal loading. (B) Western blot of dental pulp cell lysates reacted with antibodies against phosphorylated (p) p38 MAPK and its downstream substrate ATF2. IGF-1R(i), p38(i), and 5 μM STAT3(i) (Stattic 5-sc202818; Santa Cruz Biotch) were added for 30 min. (C) von Kossa staining of human dental pulp cells treated with a differentiation factor, 10 mM β-glycerol phosphate (βGP), and with IGF-1R or p38 MAPK inhibitors (sc204008 or PD169316, 125 or 250 nM, respectively, refreshed every 3 days) for 30 days.
Interestingly, p38(i) primarily upregulated STAT3 phosphorylation at the Y705 residue (pSTAT3705) (Fig. 6A). The increased phosphorylation of STAT3 was blocked by IGF-1R(i), confirming that STAT3 is a downstream substrate of IGF-1R signaling [32] and suggesting that active p38 MAPK interferes with this signaling. As shown in Fig. 6A, IGF-1R(i) and p38(i) differentially regulated also the expression of MEPE, a marker of early odontoblast differentiation [22,62]. Treatment of cells with p38(i), which induced pSTAT3705, reduced MEPE levels; conversely, treatment with IGF-1R(i) repressed pSTAT3705 and upregulated MEPE expression. The inverse relationship between pSTAT3 and MEPE was evident in all the experiments in which we were able to pharmacologically manipulate the activity of p38 MAPK (Supplementary Fig. S2A) or to knock down its expression using siRNA (Supplementary Fig. S2B). Moreover, inhibition of pSTAT3705 by STAT3 inhibitor induced MEPE (Supplementary Fig. S2C). Taken together, these data provide strong evidence that STAT3 inhibits MEPE expression. Because pSTAT3705 has been shown to control stem cell self-renewal [63–65], its presence at higher levels in p38(i)-treated cells may provide a link between p38 MAPK, IGF-1R and the quiescence of DPSCs. Figure 6B shows that both inhibitors rapidly repressed phosphorylation of p38 MAPK (pp38 MAPK). Inactivation of STAT3 by its specific inhibitor sc202818 reversed this repression and, consequently, upregulated pATF2, a p38 MAPK downstream substrate. This confirms, to some extent, that pSTAT3705 negatively feedback-regulates p38 MAPK.
The SC-derived TA cells have a limited proliferative capacity and stop cycling after several rounds of replication to enter the differentiation process [66,67]. MEPE is a prerequisite for late odontoblast differentiation [22,62]. IGF-1R(i), which stimulated MEPE production, also consistently stimulated the production of DSP, a marker of late odontoblast differentiation. As evidenced by the appearance of von Kossa-positive mineralization nodules (Fig. 6C) and the expression of alkaline phosphatase (Supplementary Fig. S3), a marker of mineralization [68,69], IGF-1R(i)-treated DPCs entered terminal differentiation in the presence of βGP, an odontoblast differentiation-inducing factor [23,70]. In contrast, p38(i), which stimulated proliferation and reduced MEPE levels, did not affect the levels of DSP, and cells treated with p38(i) did not initiate the mineralization process (Fig. 6C and Supplementary Fig. S3). These results suggest that odontoblast proliferation requires the constant inactivation of p38 MAPK and only a temporal inactivation of IGF-1R. The repression of IGF-1R-signaling and the activation of p38 MAPK, however, appear to be necessary for odontoblast differentiation. Taken together, these data provide strong evidence that STAT3 integrates IGF-1R- and p38-mediated signals to determine DPSC fate.
Altogether, our data demonstrate that although IGF-1R and p38 MAPK inactivation impinge on different downstream targets, their signals are integrated within the cell at STAT3 to activate the cycling of quiescent DPSC and to determine their fate.
Discussion
DPSCs, which are normally quiescent, enter the cell cycle in response to odontoblast injury in vivo [4] and can proliferate and differentiate in vitro [12,13,71]. The actual factors that regulate the transition of DPSCs from the quiescent to the active state, however, are not well defined, and we have a limited understanding of how the self-renewal and differentiation of these cells is regulated. Given the well-established role of p38 MAPK in mediating inflammatory responses [72] and its roles in dentinogenesis [27,35] and in the control of cellular quiescence [46], we investigated whether p38 MAPK regulates the fate of human DPSCs and whether this fate also depends on IGF-1R signaling pathways upstream of p38 MAPK that control the cellular reprogramming and pluripotency of SCs [40].
We determined that integrated p38 MAPK and IGF-1R signals inversely control the STAT3 activity to maintain human DPSC quiescence and activation. Consistent with the proposed roles of p38 MAPK and IGF-1R in the maintenance of cellular quiescence, we determined that the majority of mitotically arrested DPCs in vivo express the nuclear active [52,53] form of p38 MAPK in all zones of dental pulp. In agreement with the results of an earlier study [51], however, IGF-1R was detected mainly at the pulp periphery, implying that it may have a specific function in the committed odontoblasts that are present in this region [12,73]. As expected of G0-arrested cells, DPCs were almost exclusively Ki67-negative. Inactivation of IGF-1R and p38 MAPK by their respective inhibitors, sc204008 and PD169316, induced Ki67 in DPCs ex vivo and decreased the quiescence of PYlow and G0-arrested human DPSCs cells in vitro, demonstrating that p38 MAPK and IGF-1R are responsible for the mitotic quiescence of these cells. The identity of DPSCs was corroborated by their phenotype, stem cell activity, and localization of Ki67-positive cells perfectly matching the position of BrdU-positive DPSCs reported by Téclès et al. [4] and Ishikawa et al. [19] and the position of Oct4-positive pluripotent DPSCs reported in another study [74]. Taken together, our data establish that IGF-1R and p38 MAPK signaling pathways control human DPSC quiescence and demonstrate that these pathways must be inactivated for mitotic division to occur in DPSCs.
Several studies have identified other factors that promote DPSC expansion in vitro, including leukemia inhibitory factor, which activates JAK/STAT3 signaling [75]. Interestingly, STAT3 is a central molecule on which the signals from many cytokine and growth factor receptors, including IGF-1R and p38 MAPK, converge [32,40,76,77]. STAT3 stimulates embryonic and somatic stem cell self-renewal [32,63,64,78] by promoting Klf4 expression, which affects Oct4, Sox 2, and c-Myc, Yamanaka pluripotency and cellular reprogramming factors (see refs. [79–81]). STAT3 also cooperates with GSK3 inhibitors to maintain pluripotency and self-renewal by blocking embryonic stem cell (ESC) differentiation [80,81]. We observed that p38 MAPK inhibitors upregulated STAT3Y705 and propagated DPCs without affecting the levels of pGSK3, an inactive form of GSK3. Together with the size of the stem cell pool, arrested differentiation, and the increase in the clonogenic capacity of the cells, this finding implies that p38 MAPK maintains DPSC quiescence and prevents the exhaustion of the stem cell pool by inhibiting STAT3 activity. The inactivation of STAT3 permits MEPE expression and DPC differentiation. In contrast to p38(i), IGF-1R(i) downregulated STAT3Y705 but increased pGSK3 levels and the size of the stem cell compartment while reducing the clonogenic potential of DPCs. This activity suggests that STAT3 stimulates DPSC propagation and pGSK3, as in ESC [80], represses DPSC commitment to differentiation. Because both IGF-1R(i) and p38(i) induced DPSC proliferation but p38(i) overcomes the inhibitory effect of IGF-1R(i) on the clonogenic potential of DPSCs as of endothelial progenitors [82], we propose that p38 MAPK constitutively inhibits IGF-1R-mediated STAT3 activity and inactivates GSK3 directly ([61] and our data) to maintain DPSCs as undifferentiated quiescent cells. IGF-1R, even if present, would be unable to activate STAT3 and cause DPSCs to exit from quiescence; hence, IGF-1R also maintains DPSC quiescence.
The observed transient stimulation of DPC proliferation by IGF-1R(i) implies that active STAT3 may repress p38 MAPK via a feedback effect. Indeed, inhibition of the STAT3 activity increased the level of pp38 MAPK and its substrate, ATF2 as well as MEPE early marker of odontoblast differentiation. How, then, can IGF-1R simultaneously stimulate cell proliferation and maintain the quiescence of DPSCs, and how do p38 MAPK and IGF-1R exert their opposite effects on DPSC proliferation and differentiation? To consolidate our results, we propose a working model (Fig. 7) that explains how IGF-1R and p38 MAPK signaling pathways may interact to regulate DPSC. In building the model, we assumed that adult human DPSCs, like ESCs [80,83], self-renew and generate undifferentiated progeny due to the inactivation of GSK3 and the increased activity of STAT3 transcription factor. We also assumed that the p38 MAPK signaling pathway represses the STAT3 activity not by repressing all the functions of IGF-1R but, rather, by specifically repressing the function of its downstream STAT3-activating substrate, most likely JAK [32,41,42], which has recently been shown to stimulate odontoblasts proliferation and to inhibit odontoblasts differentiation [75].
FIG. 7.
A working model of cross talk between IGF-1R and p38 MAPK signaling pathways integrating cellular and molecular events that control the self-renewal and differentiation of dental pulp stem cells. Please see the text for details. SCQ, quiescent stem cell; SCA, activated stem cell; TA, transient amplifying committed progenitor; MEPE, matrix extracellular phosphoglycoprotein, a marker of early odontoblast differentiation; ASARM, proteolytic fragment of MEPE that inhibits DSPP (dentin sialophosphoprotein) expression [22]; DSP, dentin sialoprotein, a DSPP processing product associated with late odontoblast differentiation and mineralization; pSTAT3, phosphorylated signal transducer and activator of transcription 3; pGSK3, phosphorylated glycogen synthase kinase 3; JAK, Janus kinase; IGF-1R, insulin growth factor 1 receptor. Arrows indicate activation, and perpendicular lines indicate inhibition. The bent arrow indicates a self-renewing function. The model is based on results reported in this study (black fonts and solid lines) and in the literature (gray fonts and broken lines).
The importance of p38 MAPK in controlling quiescence has been recognized previously [46,49]. Interestingly, the inhibition of p38 stimulates the expansion of hematopoietic stem cells (HSC) in vitro and increases their repopulation potential in vivo [84]. In contrast, reactive oxygen species-induced p38 MAPK signaling impairs HSC self-renewal [85,86], demonstrating a dual activity of p38 MAPK through which it may either promote or inhibit cellular quiescence [59,61]. The mechanism for the observed duality of action of p38 MAPK is not clear, but its control of cell quiescence may involve the inhibition of GSK3 [50,61,87] and STAT3, as work of others [50,59,77] and our data imply. Inactive GSK3 stimulates ESC propagation and inhibits ESC differentiation [80]. This previous finding is consistent with our results demonstrating the proliferative activity of DPSCs in the presence of an IGF-1R inhibitor. However, in the absence of STAT3, which promotes stem cell pluripotency, cycling DPSCs, like ESCs [80], could not maintain their propagation by the inactivation of GSK3 alone and would eventually succumb to differentiation. Indeed, the inhibition of IGF-1R stimulated MEPE expression, suggesting that STAT3 negatively regulates MEPE and odontoblast differentiation.
In summary, we show that IGF-1R and p38 MAPK interact to balance DPSC quiescence and activation and that their signals are integrated to inversely regulate STAT3 and perhaps the GSK3 activity. We consider that p38 MAPK has a dominant effect and that it inactivates both STAT3 and GSK3 to maintain DPSC quiescence and sustain their self-renewal while inhibiting their commitment to differentiation. By inhibiting STAT3, however, p38 MAPK can stimulate MEPE expression and the differentiation of committed DPSCs. The IGF-1R signaling dominated by p38 MAPK also maintains DPSC quiescence, but when STAT3 is activated in a feedback reaction, p38 MAPK is inhibited. The presence of IGF-1R at the pulp periphery suggests that IGF-1R blocks odontoblast proliferation and differentiation. Due to their location, cells at the periphery are predisposed to respond rapidly to factors released by infected or damaged pulp. A precedent for a mechanism linking IGF-1R expression to cellular quiescence has been described in pancreatic tumor cells, which exhibit a growth factor-independent phenotype that enables them to rapidly re-enter the cell cycle [88]. We believe that our study opens an avenue for further investigation of DPSC expansion and differentiation in vitro and that it may also advance our understanding of the mechanisms controlling reparative dentinogenesis in inflamed and damaged teeth in vivo.
Supplementary Material
Acknowledgments
This work was supported by La Fondation pour la Recherche Médicale (FRM), which provided a PhD fellowship to J.V. for 2 years, and by the Institut Nationale de la Santé et de la Recherche Médicale (INSERM). We acknowledge Marie-Helene Gevaert, Histology Laboratory, University Lille 2, and Nathalie Jouy, BiCell-IFR 114, for their greatly appreciated help with frozen sections and hematoxylin–eosin (H&E) staining and with flow cytometry analyses, respectively, and Dr. Gilbert Nafash from the Department of Dentistry at the University Lille 2 for providing us with extracted teeth.
Author Disclosure Statement
The authors have no conflicts of interest to disclose.
References
- 1.Ruch JV, Lesot H. and Bègue-Kirn C. (1995). Odontoblast differentiation. Int J Dev Biol 39:51–68 [PubMed] [Google Scholar]
- 2.Goldberg M. (2008). In vitro and in vivo studies on the toxicity of dental resin components: a review. Clin Oral Investig 12:1–8 [DOI] [PubMed] [Google Scholar]
- 3.Tomaszewska JM, Miskowiak B, Matthews-Brzozowska T. and Wierzbicki P. (2013). Characteristics of dental pulp in human upper first premolar teeth based on immunohistochemical and morphometric examinations. Folia Histochem Cytobiol 51:149–155 [DOI] [PubMed] [Google Scholar]
- 4.Téclès O, Laurent P, Zygouritsas S, Burger A-S, Camps J, Dejou J. and About I. (2005). Activation of human dental pulp progenitor/stem cells in response to odontoblast injury. Arch Oral Biol 50:103–108 [DOI] [PubMed] [Google Scholar]
- 5.Cooper PR, Takahashi Y, Graham LW, Simon S, Imazato S. and Smith AJ. (2010). Inflammation–regeneration interplay in the dentine–pulp complex. J Dent 38:687–697 [DOI] [PubMed] [Google Scholar]
- 6.Goldberg M. and Smith AJ. (2004). Cells and extracellular matrices of dentin and pulp: a biological basis for repair and tissue engineering. Crit Rev Oral Biol Med 15:13–27 [DOI] [PubMed] [Google Scholar]
- 7.Fitzgerald M, Chiego DJ, Jr., and Heys DR. (1990). Autoradiographic analysis of odontoblast replacement following pulp exposure in primate teeth. Arch Oral Biol 35:707–715 [DOI] [PubMed] [Google Scholar]
- 8.Gronthos S, Mankani M, Brahim J, Robey PG. and Shi S. (2000). Postnatal human dental pulp stem cells (DPSCs) in vitro and in vivo. Proc Natl Acad Sci U S A 97:13625–13630 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Chai Y, Jiang X, Ito Y, Bringas P, Jr., Han J, Rowitch DH, Soriano P, McMahon AP. and Sucov HM. (2000). Fate of the mammalian cranial neural crest during tooth and mandibular morphogenesis. Dev Camb Engl 127:1671–1679 [DOI] [PubMed] [Google Scholar]
- 10.Dupin E. and Coelho-Aguiar JM. (2013). Isolation and differentiation properties of neural crest stem cells. Cytometry A 83A:38–47 [DOI] [PubMed] [Google Scholar]
- 11.Gopinathan G, Kolokythas A, Luan X. and Diekwisch TGH. (2013). Epigenetic marks define the lineage and differentiation potential of two distinct neural crest-derived intermediate odontogenic progenitor populations. Stem Cells Dev 22:1763–1778 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Sloan AJ. and Smith AJ. (2007). Stem cells and the dental pulp: potential roles in dentine regeneration and repair. Oral Dis 13:151–157 [DOI] [PubMed] [Google Scholar]
- 13.Stevens A, Zuliani T, Olejnik C, LeRoy H, Obriot H, Kerr-Conte J, Formstecher P, Bailliez Y. and Polakowska RR. (2008). Human dental pulp stem cells differentiate into neural crest-derived melanocytes and have label-retaining and sphere-forming abilities. Stem Cells Dev 17:1175–1184 [DOI] [PubMed] [Google Scholar]
- 14.Martens W, Wolfs E, Struys T, Politis C, Bronckaers A. and Lambrichts I. (2012). Expression pattern of basal markers in human dental pulp stem cells and tissue. Cells Tissues Organs 196:490–500 [DOI] [PubMed] [Google Scholar]
- 15.Shi S. and Gronthos S. (2003). Perivascular niche of postnatal mesenchymal stem cells in human bone marrow and dental pulp. J Bone Miner Res 18:696–704 [DOI] [PubMed] [Google Scholar]
- 16.Mitsiadis TA, Feki A, Papaccio G. and Catón J. (2011). Dental pulp stem cells, niches, and notch signaling in tooth injury. Adv Dent Res 23:275–279 [DOI] [PubMed] [Google Scholar]
- 17.Kaneko T, Arayatrakoollikit U, Yamanaka Y, Ito T. and Okiji T. (2013). Immunohistochemical and gene expression analysis of stem-cell-associated markers in rat dental pulp. Cell Tissue Res 351:425–432 [DOI] [PubMed] [Google Scholar]
- 18.Ishikawa Y, Ida-Yonemochi H, Nakakura-Ohshima K. and Ohshima H. (2012). The relationship between cell proliferation and differentiation and mapping of putative dental pulp stem/progenitor cells during mouse molar development by chasing BrdU-labeling. Cell Tissue Res 348:95–107 [DOI] [PubMed] [Google Scholar]
- 19.Ishikawa Y, Ida-Yonemochi H, Suzuki H, Nakakura-Ohshima K, Jung H-S, Honda MJ, Ishii Y, Watanabe N. and Ohshima H. (2010). Mapping of BrdU label-retaining dental pulp cells in growing teeth and their regenerative capacity after injuries. Histochem Cell Biol 134:227–241 [DOI] [PubMed] [Google Scholar]
- 20.Han J, Ito Y, Yeo JY, Sucov HM, Maas R. and Chai Y. (2003). Cranial neural crest-derived mesenchymal proliferation is regulated by msx1-mediated p19ink4d expression during odontogenesis. Dev Biol 261:183–196 [DOI] [PubMed] [Google Scholar]
- 21.Liu H, Li W, Shi S, Habelitz S, Gao C. and Denbesten P. (2005). MEPE is downregulated as dental pulp stem cells differentiate. Arch Oral Biol 50:923–928 [DOI] [PubMed] [Google Scholar]
- 22.Wang H, Kawashima N, Iwata T, Xu J, Takahashi S, Sugiyama T. and Suda H. (2010). Differentiation of odontoblasts is negatively regulated by MEPE via its C-terminal fragment. Biochem Biophys Res Commun 398:406–412 [DOI] [PubMed] [Google Scholar]
- 23.Liu M, Sun Y, Liu Y, Yuan M, Zhang Z. and Hu W. (2012). Modulation of the differentiation of dental pulp stem cells by different concentrations of β-glycerophosphate. Molecules 17:1219–1232 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Six N, Septier D, Chaussain-Miller C, Blacher R, DenBesten P. and Goldberg M. (2007). Dentonin, a MEPE fragment, initiates pulp-healing response to injury. J Dent Res 86:780–785 [DOI] [PubMed] [Google Scholar]
- 25.Trumpp A, Essers M. and Wilson A. (2010). Awakening dormant haematopoietic stem cells. Nat Rev Immunol 10:201–209 [DOI] [PubMed] [Google Scholar]
- 26.Catón J, Bostanci N, Remboutsika E, De Bari C. and Mitsiadis TA. (2011). Future dentistry: cell therapy meets tooth and periodontal repair and regeneration. J Cell Mol Med 15:1054–1065 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Paula-Silva FWG, Ghosh A, Silva LAB. and Kapila YL. (2009). TNF-alpha promotes an odontoblastic phenotype in dental pulp cells. J Dent Res 88:339–344 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Schindler JF, Monahan JB. and Smith WG. (2007). p38 pathway kinases as anti-inflammatory drug targets. J Dent Res 86:800–811 [DOI] [PubMed] [Google Scholar]
- 29.Peifer C, Wagner G. and Laufer S. (2006). New approaches to the treatment of inflammatory disorders small molecule inhibitors of p38 MAP kinase. Curr Top Med Chem 6:113–149 [DOI] [PubMed] [Google Scholar]
- 30.Shalita-Chesner M, Katz J, Shemer J. and Werner H. (2001). Regulation of insulin-like growth factor-I receptor gene expression by tumor necrosis factor-alpha and interferon-gamma. Mol Cell Endocrinol 176:1–12 [DOI] [PubMed] [Google Scholar]
- 31.Thesleff I. and Mikkola M. (2002). The role of growth factors in tooth development. Int Rev Cytol 217:93–135 [DOI] [PubMed] [Google Scholar]
- 32.Zong CS, Chan J, Levy DE, Horvath C, Sadowski HB. and Wang L-H. (2000). Mechanism of STAT3 activation by insulin-like growth factor I receptor. J Biol Chem 275:15099–15105 [DOI] [PubMed] [Google Scholar]
- 33.Lovschall H, Fejerskov O. and Flyvbjerg A. (2001). Pulp-capping with recombinant human insulin-like growth factor I (rhIGF-I) in rat molars. Adv Dent Res 15:108–112 [DOI] [PubMed] [Google Scholar]
- 34.Martín A, Unda FJ, Bègue-Kirn C, Ruch J. and Aréchaga JV. (1998). Effects of aFGF, bFGF, TGFbeta1 and IGF-I on odontoblast differentiation in vitro. Eur J Oral Sci 106Suppl 1:117–121 [DOI] [PubMed] [Google Scholar]
- 35.Simon S, Smith AJ, Berdal A, Lumley PJ. and Cooper PR. (2010). The MAP kinase pathway is involved in odontoblast stimulation via p38 phosphorylation. J Endod 36:256–259 [DOI] [PubMed] [Google Scholar]
- 36.Joseph BK, Savage NW, Daley TJ. and Young WG. (1996). In situ hybridization evidence for a paracrine/autocrine role for insulin-like growth factor-I in tooth development. Growth Factors 13:11–17 [DOI] [PubMed] [Google Scholar]
- 37.Werner H. and Katz J. (2004). The emerging role of the insulin-like growth factors in oral biology. J Dent Res 83:832–836 [DOI] [PubMed] [Google Scholar]
- 38.Touil Y, Zuliani T, Wolowczuk I, Kuranda K, Prochazkova J, Andrieux J, Le Roy H, Mortier L, Vandomme J, et al. (2013). The PI3K/AKT signaling pathway controls the quiescence of the low-rhodamine123-retention cell compartment enriched for melanoma stem cell activity. Stem Cells 31:641–651 [DOI] [PubMed] [Google Scholar]
- 39.Li L. and Bhatia R. (2011). Stem cell quiescence. Clin Cancer Res 17:4936–4941 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Li Y. and Geng Y-J. (2010). A potential role for insulin-like growth factor signaling in induction of pluripotent stem cell formation. Growth Horm IGF Res 20:391–398 [DOI] [PubMed] [Google Scholar]
- 41.Himpe E. and Kooijman R. (2009). Insulin-like growth factor-I receptor signal transduction and the janus kinase/signal transducer and activator of transcription (JAK-STAT) pathway. BioFactors 35:76–81 [DOI] [PubMed] [Google Scholar]
- 42.Siddle K. (2011). Signalling by insulin and IGF receptors: supporting acts and new players. J Mol Endocrinol 47:R1–R10 [DOI] [PubMed] [Google Scholar]
- 43.Lin KK, Rossi L, Boles NC, Hall BE, George TC. and Goodell MA. (2011). CD81 is essential for the re-entry of hematopoietic stem cells to quiescence following stress-induced proliferation via deactivation of the Akt pathway. PLoS Biol 9:e1001148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Chell JM. and Brand AH. (2010). Nutrition-responsive glia control exit of neural stem cells from quiescence. Cell 143:1161–1173 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Nebreda AR. and Porras A. (2000). p38 MAP kinases: beyond the stress response. Trends Biochem Sci 25:257–260 [DOI] [PubMed] [Google Scholar]
- 46.Sosa MS, Avivar-Valderas A, Bragado P, Wen H-c. andAguirre-Ghiso JA. (2011). ERK1/2 and p38/signaling in tumor cell quiescence: opportunities to control dormant residual disease. Clin Cancer Res 17:5850–5857 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Ossowski L. and Aguirre-Ghiso JA. (2010). Dormancy of metastatic melanoma. Pigment Cell Melanoma Res 23:41–56 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Miyamoto K, Araki K, Naka K, Arai F, Takubo K, Yamazaki S, Matsuoka S, Miyamoto T, Ito K. and Ohmura M. (2007). Foxo3a is essential for maintenance of the hematopoietic stem cell pool. Cell Stem Cell 1:101–112 [DOI] [PubMed] [Google Scholar]
- 49.Oeztuerk-Winder F. and Ventura J. (2012). The many faces of p38 mitogen-activated protein kinase in progenitor/stem cell differentiation. Biochem J 445:1–10 [DOI] [PubMed] [Google Scholar]
- 50.Bikkavilli RK, Feigin ME. and Malbon CC. (2008). p38 mitogen-activated protein kinase regulates canonical Wnt-beta-catenin signaling by inactivation of GSK3beta. J Cell Sci 121:3598–3607 [DOI] [PubMed] [Google Scholar]
- 51.Götz W, Heinen M, Lossdörfer S. and Jäger A. (2006). Immunohistochemical localization of components of the insulin-like growth factor system in human permanent teeth. Arch Oral Biol 51:387–395 [DOI] [PubMed] [Google Scholar]
- 52.Wood CD, Thornton TM, Sabio G, Davis RA. and Rincon M. (2009). Nuclear localization of p38 MAPK in response to DNA damage. Int J Biol Sci 5:428–437 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Gong X, Ming X, Deng P. and Jiang Y. (2010). Mechanisms regulating the nuclear translocation of p38 MAP kinase. J Cell Biochem 110:1420–1429 [DOI] [PubMed] [Google Scholar]
- 54.Youngren JF. (2007). Regulation of insulin receptor function. Cell Mol Life Sci 64:873–891 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Giordano G, La Monaca G, Annibali S, Cicconetti A. and Ottolenghi L. (2011). Stem cells from oral niches: a review. Ann Stomatol (Roma) 2:3–8 [PMC free article] [PubMed] [Google Scholar]
- 56.Ladd AC, Pyatt R, Gothot A, Rice S, McMahel J, Traycoff CM. and Srour EF. (1997). Orderly process of sequential cytokine stimulation is required for activation and maximal proliferation of primitive human bone marrow CD34+ hematopoietic progenitor cells residing in G0. Blood 90:658–668 [PubMed] [Google Scholar]
- 57.Hüttmann A, Liu SL, Boyd AW. and Li CL. (2001). Functional heterogeneity within rhodamine123(lo) Hoechst33342(lo/sp) primitive hemopoietic stem cells revealed by pyronin Y. Exp Hematol 29:1109–1116 [DOI] [PubMed] [Google Scholar]
- 58.Cheung TH. and Rando TA. (2013). Molecular regulation of stem cell quiescence. Nat Rev Mol Cell Biol 14:329–340 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Faust D, Schmitt C, Oesch F, Oesch-Bartlomowicz B, Schreck I, Weiss C. and Dietrich C. (2012). Differential p38-dependent signalling in response to cellular stress and mitogenic stimulation in fibroblasts. Cell Commun Signal 10:6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Delghandi MP, Johannessen M. and Moens U. (2005). The cAMP signalling pathway activates CREB through PKA, p38 and MSK1 in NIH 3T3 cells. Cell Signal 17:1343–1351 [DOI] [PubMed] [Google Scholar]
- 61.Thornton TM. (2009). Non-classical P38 map kinase functions: cell cycle checkpoints and survival. Int J Biol Sci 5:44–52 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Goldberg M, Lacerda-Pinheiro S, Priam F, Jegat N, Six N, Bonnefoix M, Septier D, Chaussain-Miller C, Veis A, Denbesten P. and Poliard A. (2008). Matricellular molecules and odontoblast progenitors as tools for dentin repair and regeneration. Clin Oral Investig 12:109–112 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Hall J, Guo G, Wray J, Eyres I, Nichols J, Grotewold L, Morfopoulou S, Humphreys P, Mansfield W, et al. (2009). Oct4 and LIF/Stat3 additively induce Krüppel factors to sustain embryonic stem cell self-renewal. Cell Stem Cell 5:597–609 [DOI] [PubMed] [Google Scholar]
- 64.Matsuda T, Nakamura T, Nakao K, Arai T, Katsuki M, Heike T. and Yokota T. (1999). STAT3 activation is sufficient to maintain an undifferentiated state of mouse embryonic stem cells. EMBO J 18:4261–4269 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Ying Q-L, Wray J, Nichols J, Batlle-Morera L, Doble B, Woodgett J, Cohen P. and Smith A. (2008). The ground state of embryonic stem cell self-renewal. Nature 453:519–523 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Coller HA. (2011). The essence of quiescence. Science 334:1074–1075 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Watt FM. (2011). Stem cells: on the front line. J Cell Sci 124:3527–3528 [DOI] [PubMed] [Google Scholar]
- 68.Larmas M. (2001). Odontoblast function seen as the response of dentinal tissue to dental caries. Adv Dent Res 15:68–71 [DOI] [PubMed] [Google Scholar]
- 69.Orimo H. (2010). The mechanism of mineralization and the role of alkaline phosphatase in health and disease. J Nippon Med Sch 77:4–12 [DOI] [PubMed] [Google Scholar]
- 70.About I, Bottero MJ, de Denato P, Camps J, Franquin JC. and Mitsiadis TA. (2000). Human dentin production in vitro. Exp Cell Res 258:33–41 [DOI] [PubMed] [Google Scholar]
- 71.Gronthos S, Arthur A, Bartold PM. and Shi S. (2011). A method to isolate and culture expand human dental pulp stem cells. Methods Mol Biol Clifton NJ 698:107–121 [DOI] [PubMed] [Google Scholar]
- 72.Clark AR. and Dean JL. (2012). The p38 MAPK pathway in rheumatoid arthritis: a sideways look. Open Rheumatol J 6:209–219 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Ruch JV. (1998). Odontoblast commitment and differentiation. Biochem Cell Biol 76:923–938 [PubMed] [Google Scholar]
- 74.Lizier NF, Kerkis A, Gomes CM, Hebling J, Oliveira CF, Caplan AI. and Kerkis I. (2012). Scaling-up of dental pulp stem cells isolated from multiple niches. PLoS One 7:e39885. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Zhou Y, Qian M, Liang Y, Liu Y, Yang X, Jiang T. and Wang Y. (2011). Effects of leukemia inhibitory factor on proliferation and odontoblastic differentiation of human dental pulp cells. J Endod 37:819–824 [DOI] [PubMed] [Google Scholar]
- 76.Bode JG, Ehlting C. and Häussinger D. (2012). The macrophage response towards LPS and its control through the p38MAPK–STAT3 axis. Cell Signal 24:1185–1194 [DOI] [PubMed] [Google Scholar]
- 77.Souma Y, Nishida T, Serada S, Iwahori K, Takahashi T, Fujimoto M, Ripley B, Nakajima K, Miyazaki Y, et al. (2012). Antiproliferative effect of SOCS-1 through the suppression of STAT3 and p38 MAPK activation in gastric cancer cells. Int J Cancer 131:1287–1296 [DOI] [PubMed] [Google Scholar]
- 78.Hirai H, Karian P. and Kikyo N. (2011). Regulation of embryonic stem cell self-renewal and pluripotency by leukaemia inhibitory factor. Biochem J 438:11–23 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Li Y-Q. (2010). Master stem cell transcription factors and signaling regulation. Cell Reprogramm 12:3–13 [DOI] [PubMed] [Google Scholar]
- 80.Wray J, Kalkan T, Gomez-Lopez S, Eckardt D, Cook A, Kemler R. and Smith A. (2011). Inhibition of glycogen synthase kinase-3 alleviates Tcf3 repression of the pluripotency network and increases embryonic stem cell resistance to differentiation. Nat Cell Biol 13:838–845 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Sato N, Meijer L, Skaltsounis L, Greengard P. and Brivanlou AH. (2004). Maintenance of pluripotency in human and mouse embryonic stem cells through activation of Wnt signaling by a pharmacological GSK-3-specific inhibitor. Nat Med 10:55–63 [DOI] [PubMed] [Google Scholar]
- 82.Humpert PM, Djuric Z, Zeuge U, Oikonomou D, Seregin Y, Laine K, Eckstein V, Nawroth PP. and Bierhaus A. (2008). Insulin stimulates the clonogenic potential of angiogenic endothelial progenitor cells by IGF-1 receptor-dependent signaling. Mol Med 14:301–308 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Graham JR, Tullai JW. and Cooper GM. (2010). GSK-3 represses growth factor-inducible genes by inhibiting NF-κB in quiescent cells. J Biol Chem 285:4472–4480 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Wang Y, Kellner J, Liu L. and Zhou D. (2011). Inhibition of p38 mitogen-activated protein kinase promotes ex vivo hematopoietic stem cell expansion. Stem Cells Dev 20:1143–1152 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Ito K, Hirao A, Arai F, Takubo K, Matsuoka S, Miyamoto K, Ohmura M, Naka K, Hosokawa K, Ikeda Y. and Suda T. (2006). Reactive oxygen species act through p38 MAPK to limit the lifespan of hematopoietic stem cells. Nat Med 12:446–451 [DOI] [PubMed] [Google Scholar]
- 86.Liu J. and Finkel T. (2006). Stem cell aging: what bleach can teach. Nat Med 12:383–384 [DOI] [PubMed] [Google Scholar]
- 87.Tullai JW, Chen J, Schaffer ME, Kamenetsky E, Kasif S. and Cooper GM. (2007). Glycogen synthase kinase-3 represses cyclic amp response element-binding protein (CREB)-targeted immediate early genes in quiescent cells. J Biol Chem 282:9482–9491 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Nair PN, De Armond DT, Adamo ML, Strodel WE. and Freeman JW. (2001). Aberrant expression and activation of insulin-like growth factor-1 receptor (IGF-1R) are mediated by an induction of IGF-1R promoter activity and stabilization of IGF-1R mRNA and contributes to growth factor independence and increased survival of the pancreatic cancer cell line MIA PaCa-2. Oncogene 20:8203–8214 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.