Abstract
Transgenic expression of bacterial nitroreductase (NTR) facilitates chemically-inducible targeted cell ablation. In zebrafish, the NTR system enables studies of cell function and cellular regeneration. Metronidazole (MTZ) has become the most commonly used prodrug substrate for eliciting cell loss in NTR-expressing transgenic zebrafish due to the cell-specific nature of its cytotoxic derivatives. Unfortunately, MTZ treatments required for effective cell ablation border toxic effects, and, thus, likely incur undesirable nonspecific effects. Here, we tested whether a triple mutant variant of NTR, previously shown to display improved activity in bacterial assays, can solve this issue by promoting cell ablation in zebrafish using reduced prodrug treatment regimens. We generated several complementary transgenic zebrafish lines expressing either wild-type or mutant NTR (mutNTR) in specific neural cell types, and assayed prodrug-induced cell ablation kinetics using confocal time series imaging and plate reader-based quantification of fluorescent reporters expressed in targeted cell types. The results show that cell ablation can be achieved in mutNTR expressing transgenic lines with markedly shortened prodrug exposure times and/or at lower prodrug concentrations. The mutNTR variant characterized here can circumvent problematic nonspecific/toxic effects arising from low prodrug conversion efficiency, thus increasing the effectiveness and versatility of this selective cell ablation methodology.
Introduction
Nitroreductase (NTR)-mediated cell ablation is a technique facilitating studies of cell function and, perhaps more importantly, cellular regeneration.1,2 This approach provides a novel means to investigate the mechanisms shaping how individual stem cell niches detect and respond to selective cell loss. Targeted cell ablation can be accomplished using other methodologies as well, but it typically has been limited to select contexts (e.g., hair cell regeneration3). A key advantage of the NTR system is the ability to target any cell type of interest that can be labeled with standard transgenic techniques. In effect, this increases the number of cellular paradigms which are amenable to regeneration studies and, therefore, the types of degenerative disorders that can be physiologically modeled with this strategy. More broadly, cell-specific ablation methods enable mechanisms governing the regeneration of individual cell types to be delineated from those that regulate the regrowth of entire tissues or multi-tissue structures (e.g., limb). A greater understanding of how to control the activity of discrete stem cell niches will aid efforts to develop targeted therapeutic strategies for enhancing regenerative capacities in humans.
The Escherichia coli gene nfsB encodes an NTR flavoprotein. NTR was initially developed as a means to target and kill tumorigenic cells via its capacity to reduce quinone and nitroaromatic substrates to cytotoxic products.4,5 Depending on the “prodrug” substrate used, the cytotoxin can act selectively, eliminating only NTR-expressing cells, or nonselectively by killing nearby cells through a ‘bystander’ effect. NTR has been transgenically expressed in a variety of experimental model species where the use of selective prodrugs facilitates inducible targeted cell ablation studies.6–8 Similarly, we adapted the NTR system to zebrafish.9 In the context of this highly regenerative species, transgenic NTR expression facilitates cell-specific regeneration studies.1 To date, this system has been deployed in several different cell and tissue types in both larval and adult zebrafish.9–18 Importantly, prodrugs are easily administered in aqueous media; thus, cell ablation can be induced in thousands of fish simultaneously, facilitating large-scale screens.19 Accordingly, we recently developed a true high-throughput screening (HTS) methodology for identifying factors that regulate regeneration.20
The most common prodrug used in combination with NTR-expressing zebrafish is Metronidazole (MTZ), which promotes selective cell ablation.9,13 The duration and concentration of MTZ treatment required for cell ablation differs according to cell type, presumably due to factors such as enzyme expression level, cellular metabolic rates, and accessibility (i.e., depth in tissues). For example, rod photoreceptors can be ablated in as low as 2.5 mM MTZ for 24 h,20 while macrophages require 15 h in 5 mM MTZ,18 and a 3-day treatment in 5–10 mM MTZ was used to ablate dopaminergic neurons.21 Treatment in 10 mM MTZ for 24 h was used in the initial reports,9,13 and has become a standard regimen for NTR-mediated ablation of multiple cell types.2,9–13,15,22,23 However, 24 h/10 mM MTZ treatments are problematic. For instance, nonspecific apoptosis within the superficial telencephalon has been noted.13 Furthermore, while treatment in 10 mM MTZ has been reported to cause no obvious defects, lethality is observed after treatment with 15–20 mM MTZ.9 These results demonstrate that the 24 h/10 mM MTZ treatment regimen, while showing a few obvious nonspecific effects, is near the border of general toxicity. Moreover, since transgenic fish expressing this moiety are now being incorporated into HTS assays,20 24 h MTZ incubation times are inconvenient for concurrent/subsequent pharmacological treatments. An improved NTR system in which the concentration and/or duration of prodrug treatment can be reduced could circumvent issues of general toxicity and those attending the demands of HTS assays.
Using a phage-based selection assay, Guise et al. leveraged directed evolution methods to identify mutant versions of NTR with improved activity toward the prodrug substrate CB1954 (5-[aziridin-1-yl]-2,4-dinitrobenzamide).24 CB1954 is a commonly used prodrug within the context of studies related to cancer therapeutics due to its nonselective nature.25,26 In particular, Guise et al. described a triple mutant (T41Q/N71S/F124T) that conferred a nearly 50-fold improvement in catalytic efficiency for CB1954 over wild-type NTR (wtNTR). The triple mutant NTR (mutNTR) has been shown to be effective at reducing the selective prodrug substrate MTZ in zebrafish as well.27 However, direct comparisons to wtNTR have not yet been reported with regard to CB1954 or MTZ conversion efficacy. Here, we have tested whether the triple mutNTR improves prodrug-induced cell ablation kinetics using analogous NTR and NTR mutant expressing transgenic zebrafish lines. The data show that cell ablation in NTR mutant expressing lines can be achieved with shorter exposure times and/or lower prodrug concentrations. We find that this mutant increases the utility of MTZ for selective cell ablation in zebrafish, and also facilitates the use of CB1954-induced nonspecific ablation as a means to model broader injury paradigms.
Results
Short-term treatment with MTZ results in less lethality
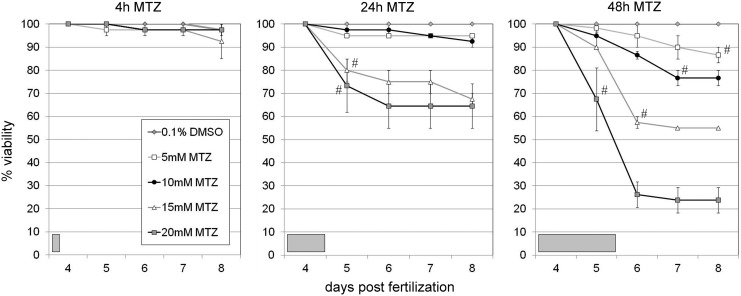
Previous results indicated that treating fish with 10 mM MTZ for 24 h was generally nontoxic, yet a slight increase in concentration to 15–20 mM MTZ resulted in lethality.9 These data suggest that the common 10 mM/24 h MTZ treatment regimen used in zebrafish ablation experiments is near generally toxic levels. In order to more explicitly establish toxicological parameters for MTZ, we treated wild-type zebrafish larvae with varying concentrations of MTZ (5, 10, 15, and 20 mM) for three durations (4, 24, 48 h) and recorded viability through 8 days postfertilization (dpf). Prodrug exposure was initiated at 4 dpf, after major organ systems are established and functional.28 We found that treatment for 4 h at all concentrations tested resulted in no detectable lethality (Fig. 1). However, the 24 h treatment regimen induced significant lethality (20–50%) at 15 and 20 mM MTZ, while 10 mM showed only a limited degree of lethality (5–10%) that was not statistically different than 5 mM or untreated controls (Fig. 1). Continuous treatment for 48 h indicated that MTZ can be toxic in the 5–20 mM range on extended exposure: 15 and 20 mM MTZ showed ∼50–80% lethality while 10 mM yielded ∼25% lethality, all significantly different from controls (Fig. 1). Interestingly, the 5 mM MTZ treatment was largely indistinguishable from vehicle-treated controls except after a longer exposure, suggesting that this concentration is preferable. These results indicate that long-term ≥24 h 10 mM MTZ treatment regimens are borderline toxic, but that short-term (4 h) treatments are nontoxic across a range spanning 5–20 mM MTZ. A similar assay assessing viability over a longer time frame (to 14 dpf) supported these findings: 24 h treatments in 15–20 mM MTZ yielded the highest lethality, and 24 h exposures were associated with greater lethality than 4 h regimens overall (Supplementary Fig. S1; Supplementary Data are available online at www.liebertpub.com/zeb). Taken together, these data suggest that identifying improved NTR variants which enable the concentration and/or duration of MTZ treatment regimens to be reduced would be desirable, as this would help reduce the possibility of nonspecific effects, and to distinguish toxicity-associated phenotypes from those induced by NTR-mediated loss of specific cell types.
FIG. 1.
MTZ-induced toxicity/lethality. Wild-type (nontransgenic) larvae were treated at 4 dpf with the indicated concentrations of MTZ for 4, 24, or 48 h (shaded boxes, lower left). The number of viable fish was counted each day through 8 dpf; the percent of viable fish for each day is shown. Data are averages from two to three trials; error bars are SEM. Data were analyzed by chi-square analysis: no significant differences (χ2>0.05) were evident between conditions after 4 h treatments (left panel); # marks the first data point in each data series that is significantly different (χ2≤0.05) from corresponding untreated control data points (0.1% DMSO), with all subsequent data points in each series also being significantly different; all other data points are not significantly different from untreated controls. DMSO, dimethyl sulfoxide; dpf, days postfertilization; MTZ, metronidazole.
mutNTR provides improved MTZ-induced cell ablation of spinal motor neurons
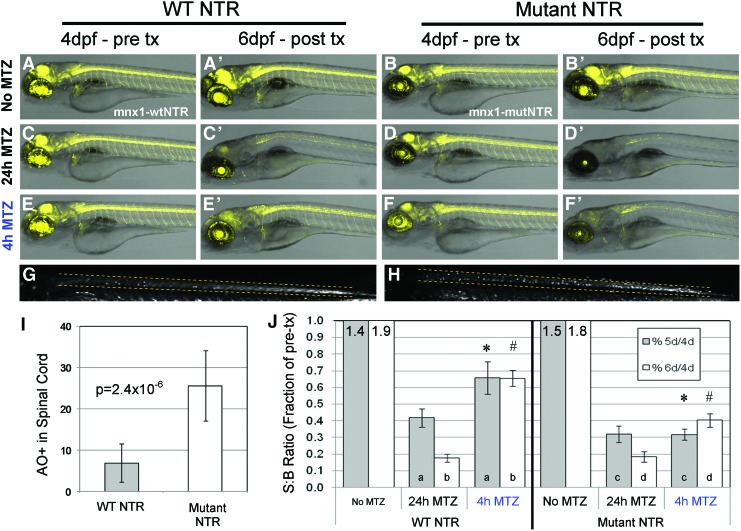
In order to directly compare wtNTR with the T41Q/N71S/F124T triple mutNTR, we established analogous stable transgenic lines expressing these variants in equivalent cellular subpopulations. For initial tests, a short 125 bp element from the zebrafish mnx1 enhancer region was used to drive expression in spinal motor neurons and undefined neuronal subsets in the eye and brain.29,30 Bicstronic transgenes were designed to express equimolar amounts of NTR and a fluorescent reporter, TagYFP (see Materials and Methods section for full transgene details). For brevity, these lines are abbreviated here as mnx1:wtNTR and mnx1:mutNTR (collectively, mnx1:NTR; Supplementary Fig. S2 and Table 1 for full transgene and allele designations). For initial comparisons of wtNTR and mutNTR expressing larvae, we performed time series confocal imaging studies to visualize the loss of TagYFP labeled cells after MTZ treatments in individual fish over time. For both mnx1:NTR lines, use of the standard treatment regimen of 10 mM MTZ for 24 h (starting at 4 dpf) yielded near total ablation of TagYFP-labeled cells (6 dpf; Fig. 2A–D time series). We next tested whether shorter MTZ exposure times were sufficient for cell ablation.
Table 1.
Abbreviations and Full Designations of Transgenic Zebrafish Lines Used in This Study
| Line | Transgenic line designationsa | Figure |
|---|---|---|
| isl1-wtNTR-03 | Tg(2xNRSE-isl1-Mmu.Fos:KalTA4,5xUAS-ADV.E1b:GAP-YFP-2A-Eco.NfsB)lmc003 | 4, 6, S3 |
| isl1-mutNTR-04 | Tg(2xNRSE-isl1-Mmu.Fos:KalTA4,5xUAS-ADV.E1b:GAP-YFP-2A-Eco.NfsBT41Q/N71S/F124T)lmc004 | 4, 6, S3 |
| isl1-wtNTR-05 | Tg(2xNRSE-isl1-Mmu.Fos:KalTA4,5xUAS-ADV.E1b:GAP-YFP-2A-Eco.NfsB)lmc005 | 3, S3 |
| isl1-mutNTR-06 | Tg(2xNRSE-isl1-Mmu.Fos:KalTA4,5xUAS-ADV.E1b:GAP-YFP-2A-Eco.NfsBT41Q/N71S/F124T)lmc006 | 3, S3 |
| mnx1-wtNTR | Tg(2xNRSE-2xMnx1-Mmu.Fos:KalTA4,5xUAS-ADV.E1b:GAP-YFP-2A-Eco.NfsB)lmc007 | 2, S2 |
| mnx1-mutNTR | Tg(2xNRSE-2xMnx1-Mmu.Fos:KalTA4,5xUAS-ADV.E1b:GAP-YFP-2A-Eco.NfsBT41Q/N71S/F124T)lmc008 | 2, S2 |
Line designations listed are based on earlier ZFIN nomenclature modifications. Original published31 designation of line lmc003 was Tg(2xNRSE-CREST1-cfos:KalTA4, 5xUAS-E1b:YFP-2A-nfsB)lmc003.
NRSE, neuron-restrictive silencer element; NTR, nitroreductase; wtNTR, wild-type NTR.
FIG. 2.
Improved MTZ-induced ablation of spinal motor neurons by mutNTR. (A–F’) Comparison of ablation efficiency between complementary wtNTR (A, C, E: mnx1:wtNTR line) and mutNTR (B, D, F: mnx1:mutNTR line) expressing transgenic lines using confocal time series imaging. Pretreatment images of labeled spinal motor neuron and uncharacterized brain neuron subpopulations were captured at 4 dpf (A–F), post-treatment images at 6 dpf, thus at ≥1 day after the indicated MTZ treatments (A’–F’). (A, A’, B, B’) Control larvae treated in 0.1% DMSO for 24 h yielded no decrease in YFP expression. (C, C’, D, D’) Larvae treated in 10 mM MTZ for 24 h (with 1 day of recovery) yielded a loss of fluorescence in labeled cell types, demonstrating that both NTR types are competent to induce ablation under these conditions. (E, E’, F, F’) Larvae treated in 10 mM MTZ for 4 h showed little, if any, effect on spinal motor neurons expressing wtNTR (E, E’). In contrast, appreciable ablation of labeled cells was evident in mutNTR expressing fish (F, F’). (G, H) Acridine Orange labeling of apoptotic cells after 4 h of MTZ and 6 h of recovery, spinal cord regions labeled by dashed lines; (G) mnx1:wtNTR, (H) mnx1:mutNTR showed increased numbers of dying cells (bright dots) in mutNTR expressing larvae. (I) Quantification of Acridine Orange-labeled apoptotic cells in spinal cord regions after 4 h of MTZ or vehicle control, and 6 h recovery. (J) Quantification of TagYFP fluorescence by plate reader after MTZ treatment of mnx1:wtNTR and mnx1:mutNTR lines; S:B ratios were calculated from individual larvae at 4 dpf (pretreatment), 5 dpf and 6 dpf, then plotted as the fraction of individual pretreatment values. Data were analyzed by Student's t-tests: all MTZ-treated samples yielded significant differences (p≤0.05) when compared with corresponding untreated (No MTZ) controls, and there was no significant difference between wild-type and mutant 24 h MTZ treatment data at 6 days, or mutant 4 h MTZ data at 5 and 6 days; p-values between data pairs: *=3.4×10−3, #=3.8×10−4, a,b,d ≤0.05, c>0.05. All error bars denote SEM. NTR, nitroreductase; mutNTR, mutant NTR; S:B, signal to background; wtNTR, wild-type NTR. Color images available online at www.liebertpub.com/zeb
When 4 dpf mnx1:NTR transgenic lines were treated with 10 mM MTZ for only 4 h, wtNTR expressing larvae showed little evidence of labeled cell ablation at 6 dpf, with only partial ablation of tectal and other brain cells yet little effect on motor neurons (Fig. 2E time series). In contrast, mutNTR expressing fish demonstrated appreciable cell ablation throughout the spinal cord and brain (Fig. 2F time series). In support of these data, Acridine Orange labeling after 4 h 10 mM MTZ and 6 h of recovery demonstrated a significant increase in apoptotic cells within the spinal cords of larvae expressing mutNTR compared with wtNTR (Fig. 2G–I). Taken together, these data strongly suggest that the triple mutNTR can convert the prodrug MTZ at a faster kinetic rate than wtNTR in transgenic zebrafish. This results in robust MTZ-induced cell ablation in mutNTR expressing larvae after exposure times as short as 4 h, a regimen that is largely ineffective in wtNTR expressing fish.
To confirm our time series confocal imaging results, we employed a plate reader-based assay that our group had previously developed to quantify changes in fluorescent signals over time in living zebrafish larvae.20 This method is useful for comparing fluorescent signals between individual larvae arrayed in multiwell plates, and is sensitive enough to detect changes in small numbers of labeled cells, for example, loss and regeneration of pancreatic beta cells.20 To assess cell ablation, a reading was obtained before MTZ exposure for all assayed larvae at 4 dpf. These pretreatment scans served as the baseline for subsequent reads, such that the assay was internally controlled per each subject; this aspect addresses variability both within a given line (i.e., differences between siblings)20 and between “paired” transgenic lines, thereby enabling ablation performance comparisons between wtNTR and mutNTR expressing lines. After 4 or 24 h treatments with 10 mM MTZ (or dimethyl sulfoxide [DMSO] as a control), larvae were allowed to recover in regular media. Post-treatment readings were acquired at 5 and 6 dpf. For the analysis, post-treatment reads were normalized to corresponding pretreatment values (4 dpf) to determine relative gains and losses in fluorescence per individual fish, and these values were then averaged across like conditions. The data show that a significant loss in fluorescence is observed for both lines after 24 h exposure to 10 mM MTZ (Fig. 2J). The mnx1:wtNTR line demonstrated a slight (but significant) reduction in fluorescence after 4 h in 10 mM MTZ, but not to the level of the 24 h treatment (Fig. 2J); this is likely due to partial ablation of labeled cells in the eye and brain (as seen in Fig. 2E’). In contrast, the level of fluorescence observed for the mnx1:mutNTR line after 4 h 10 mM MTZ treatment was more significantly reduced than that observed with wtNTR (Fig. 2J, right panel). Taken together, these data from two independent assays provide significant evidence that the mutNTR has increased activity in vivo for conversion of the prodrug MTZ into cytotoxic derivatives.
mutNTR provides improved MTZ-induced cell ablation of spinal interneurons
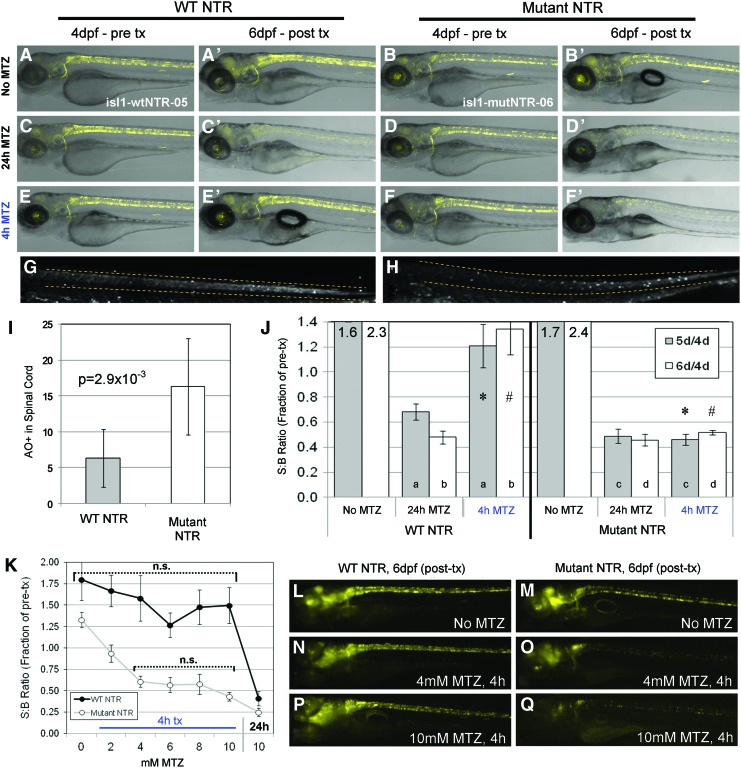
To test possible improved in vivo effectiveness of mutNTR for MTZ-induced ablation on other cell types, we established another analogous set of transgenic lines expressing NTR variants and TagYFP. These lines employed the CREST1 enhancer from the islet1 gene, which we and others had previously shown can function to drive expression in cranial motor neurons.31,32 Here, we identified lines that yielded an alternative expression pattern (Supplementary Fig. S3C, C’, D, D’). Early on, at 2 dpf, expression is evident in cranial motor neurons and varying numbers of spinal interneurons, with the latter identified by location and morphology33 (Supplementary Fig. S3C, D). Later in development, at 4 dpf, cranial expression expands to encompass much of the brain and spinal interneuron expression levels increase (Supplementary Fig. S3C’, D’). For brevity, these lines are abbreviated here as isl1:wtNTR-5 and isl1:mutNTR-6 (allele numbers are used delineate these lines from a third transgenic set discussed next; see Table 1 for full transgene designation). These lines were used for a series of assays akin to those performed earlier, but focused on spinal interneuron ablation (Fig. 3A–J). As earlier, standard incubation in 10 mM MTZ for 24 h resulted in near total ablation of labeled cells in both lines when assessed with time series imaging (Fig. 3A–D time series). In contrast, while incubation in 10 mM MTZ for only 4 h yielded no outwardly discernible cell ablation in isl1:wtNTR-5 lines, 4 h treatments produced losses indistinguishable from the 24 h exposure in isl1:mutNTR-6 lines (Fig. 3E, F time series). Acridine Orange labeling at 5 dpf showed a quantifiable increase in apoptotic cells within the spinal cords of larvae expressing the mutNTR compared with wtNTR after 4 h treatments the previous day (Fig. 3G–I).
FIG. 3.
Improved MTZ-induced ablation of interneurons by mutNTR. (A–F’) Comparison of ablation efficiency between complementary wtNTR (A, C, E: isl1-wtNTR-05 line) and mutNTR (B, D, F: isl1-mutNTR-06 line) expressing transgenic lines using confocal time series imaging. Pretreatment images of labeled spinal interneuron subpopulations were captured at 4 dpf (A–F) and at 6 dpf, following the indicated MTZ treatments (A’–F’). (A, A’, B, B’) Control larvae exposed to 0.1% DMSO for 24 h showed no decreases in YFP expression. (C, C’, D, D’) Larvae treated with 10 mM MTZ for 24 h (with 1 day of recovery) yielded a loss of fluorescence in labeled cell types, again demonstrating that both NTR types are competent to induce ablation under these conditions. (E, E’, F, F’) Larvae treated with 10 mM MTZ for 4 h revealed little to no effect on labeled interneurons expressing wtNTR (E, E’); however, ablation of mutNTR expressing interneurons (F, F’) occurred equivalently to 24 h MTZ-treated larvae (compare C’ and D’ with F’). (G, H) Acridine Orange labeling of apoptotic cells after 4 h MTZ exposure and 24 h of recovery, spinal cord regions indicated by dashed lines; (G) isl1-wtNTR-05, (H) isl1-mutNTR-06. (I) Quantification of Acridine Orange labeled apoptotic cells. (J) Quantification of TagYFP fluorescence by plate reader after MTZ treatment of isl1-wtNTR-05 and isl1-mutNTR-06 lines; S:B ratios were calculated from individual larvae at 4 dpf (pretreatment), 5 dpf and 6 dpf, then plotted as the fraction of individual pretreatment values. Data were analyzed by Student's t-tests: All MTZ-treated samples yielded significant (p≤0.05) differences when compared with corresponding untreated (No MTZ) controls, and there was no significant difference between wild-type and mutant 24 h MTZ treatment data at 6 days; p-values between data pairs: *=3.2×10−4, #=4.9×10−4, a,b≤0.05, c,d>0.05. (K) Quantification of TagYFP fluorescence by plate reader (as in panel J) after MTZ titration; S:B ratios were plotted as the fraction of post-treatment (6 dpf) over pretreatment (4 dpf) values, at indicated MTZ concentrations. Data were analyzed by Student's t-tests: all 4 h treatment data points between wtNTR and mutNTR were significantly different; within wild-type and mutant datasets, data points that are not significantly (n.s.) different from the 4 h/10 mM MTZ treatment data are indicated with brackets. (L–Q) Representative images of 6 dpf larvae after the plate reader assay in panel (K). (L, N, P) isl1-wtNTR-05, (M, O, Q) isl1-mutNTR-06; (L, M) No MTZ, (N, O) treated 4 h with 4 mM MTZ, (P, Q) treated 4 h with 10 mM MTZ. All error bars denote SEM. Color images available online at www.liebertpub.com/zeb
Similar results were obtained in plate reader-based quantification assays (Fig. 3J). A decrease in fluorescence was observed for each line after 24 h of 10 mM MTZ. Importantly, after 4 h treatments, the mutNTR line could demonstrate reductions in fluorescence that were statistically comparable to losses evident after 24 h treatments. Conversely, the wtNTR line showed only a limited loss of fluorescence, which was significantly less effective than the 24 h exposure. Thus, these results were comparable to spinal motor neuron ablation assays (mnx1:NTR, see Fig. 2A–J), providing further evidence that the catalytic activity of the mutNTR toward the prodrug substrate MTZ is enhanced.
To test whether the triple mutNTR would facilitate reducing the concentration of MTZ required for cell ablation, we performed a titration experiment. At 4 dpf, isl1:wtNTR-5 and isl1:mutNTR-6 larvae were exposed to five different concentrations (2–10 mM at 2 mM intervals) of MTZ for a total of 4 h and then allowed to recover in standard media. To assess cell ablation effectiveness, plate reader quantifications of fluorescence (Fig. 3K) were performed as discussed earlier. We found that with the mutNTR, the concentration of MTZ could be titrated and still yield this effect after a 4 h treatment. In particular, a reduction in fluorescence statistically comparable to a 4 h/10 mM MTZ treatment could be observed using as low as 4 mM MTZ (Fig. 3K); representative images of isl1:mutNTR-6 larvae from this condition indicated a partial ablation of labeled cell types (Fig. 3L–Q). Thus far, results from two different spinal neuron cell types (motor neurons and interneurons) indicate that the mutNTR is a more effective tool to achieve cell ablation with either a lower duration or a concentration of MTZ, which we have shown to be better for larval health (Fig. 1) and, therefore, better in general for overall assay optimization.
mutNTR provides improved MTZ-induced ablation of cranial motor neurons
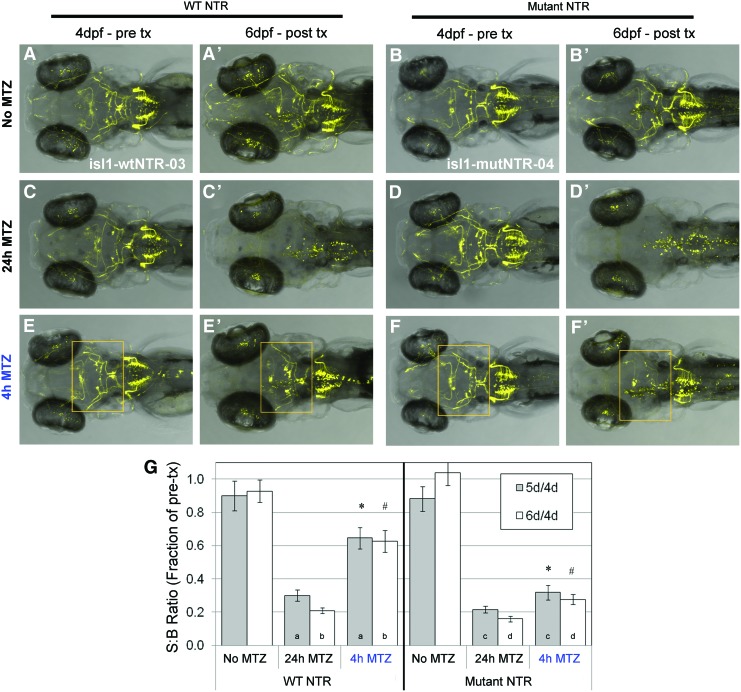
We next turned to evaluating the capacity of the mutNTR to improve ablation of less accessible cellular targets. For this study, transgenic lines expressing NTR variants and TagYFP specifically in cranial motor neuron were utilized, as these neurons are relatively deeply situated and, thus, a more stringent test of NTR effectiveness. We previously established31 a transgenic line in which the CREST1 enhancer of isl1 was used to drive the expression of wtNTR in cranial motor neurons (Supplementary Fig. S3A), abbreviated here as isl1:wtNTR-3. Here, we generated an analogous line bearing the mutNTR (Supplementary Fig. S3B), isl1:mutNTR-4.
Consistent with earlier observations, ablation of the majority of labeled cells was achieved in both lines using the standard 24 h/10 mM MTZ treatment regimen (Fig. 4A–D), indicating that MTZ can penetrate to the level of these cells. However, use of the 4 h/10 mM MTZ treatment yielded very little cell ablation in the isl1:wtNTR-3 line (Fig. 4E, E’), with only a slight drop in fluorescence arising from axonal projections. In contrast, the isl1:mutNTR-4 line achieved robust ablation of all anterior cranial motor neuron subsets (nuclei III-VII, see Supplementary Fig. S2E for details) and a loss of most axonal projections in this region (Fig. 4F, F’, boxed regions). For reasons that remain unclear, vagal motor neurons (region X, Supplementary Fig. S3E) were more resistant to MTZ-induced ablation, despite the more superficial location of this nucleus.34 Data from these imaging studies were further supported by plate reader assays, with the mutNTR expressing line showing a much greater loss of fluorescence than the wtNTR control lines after a 4 h/10 mM MTZ treatment (Fig. 4G). Taken together, these results indicate that the ablation of deeply situated cell types can be more efficiently induced by the mutNTR.
FIG. 4.
Improved MTZ-induced ablation of cranial motor neurons by mutNTR. (A–F’) Comparison of ablation efficiency between complementary wtNTR (A, C, E: isl1-wtNTR-03 line) and mutNTR (B, D, F: isl1-mutNTR-04 line) expressing transgenic lines by confocal time series imaging. Pretreatment images of cranial motor neuron subpopulations in individual larvae were captured at 4 dpf (A–F) and at 6 dpf, after MTZ treatment (A’–F’). (A, A’, B, B’) Control larvae treated with 0.1% DMSO for 24 h yielded no decrease in YFP expression. (C, C’, D, D’) Larvae treated with 10 mM MTZ for 24 h (with 1 day of recovery) showed a loss of fluorescence in labeled cell types, demonstrating that both NTR types are competent to induce ablation under these conditions. (E, E’, F, F’) Larvae exposed to 10 mM MTZ for 4 h revealed little to no effect on labeled motor neurons expressing wtNTR (E, E’); in contrast, this treatment induces ablation of a subset of neurons expressing mutNTR (F, F’; orange boxes). (G) Quantification of TagYFP fluorescence by plate reader after MTZ treatment of isl1-wtNTR-03 and isl1-mutNTR-04 lines; S:B ratios were calculated from individual larvae at 4 dpf (pretreatment), 5 dpf and 6 dpf, then plotted as the fraction of individual pretreatment values. Data were analyzed by Student's t-tests: All MTZ-treated samples yielded significant (p≤0.05) differences when compared with corresponding untreated (No MTZ) controls, and there was no significant difference between wild-type and mutant 24 h MTZ treatment data at 6 days; p-values between data pairs: *=3.9×10−4, #=8.6×10−5, a,b,c,d<0.05. All error bars denote SEM. Color images available online at www.liebertpub.com/zeb
mutNTR provides improved CB1954-induced ablation
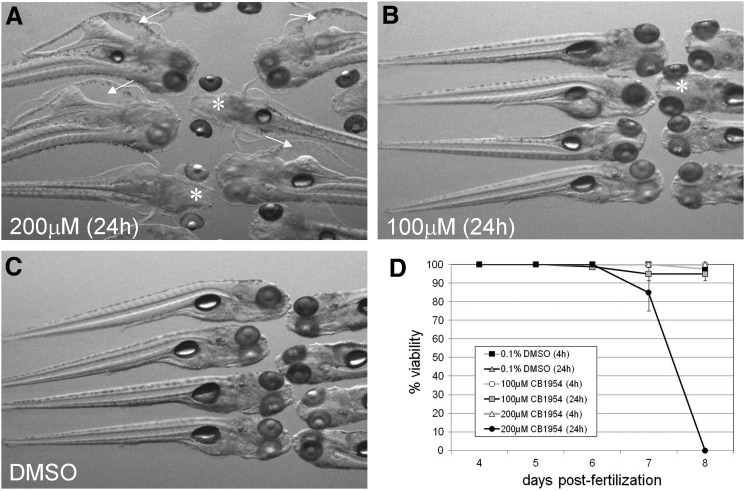
In order to more fully evaluate the capabilities of the mutNTR, we also assessed cell ablation induced by the prodrug CB1954. This prodrug is much less commonly used in the zebrafish community, likely due to potential “bystander effects” where prodrug exposure induces the death of NTR-expressing and nearby nontargeted cells as well.25,26 The only published report using CB1954 in zebrafish established 250 μM as the effective concentration for NTR-mediated cell ablation.13 However, we observed gross morphological abnormalities (severe edema of the pericardial sac and hydrocephaly) in larvae treated with 200 μM CB1954 for 24 h after 1 full day of recovery (i.e., 6 dpf, after treatment at 4 dpf; Fig. 5A); it should be noted that these defects are not present immediately after the treatment (i.e., 5 dpf). Treatment with 100 μM CB1954 yielded limited evidence of physical defects, for example, mild hydrocephaly in only a fraction of treated larvae, while most larvae appeared indistinguishable from DMSO controls (Fig. 5B, C). Moreover, 200 μM treatments resulted in 100% lethality 3 days after CB1954 exposure (8 dpf), whereas 100 μM treatments were statistically equivalent to vehicle controls (Fig. 5D). Interestingly, fish treated with 100–200 μM CB1954 for 4 h yielded no readily observable morphological defects (data not shown) and demonstrated no lethality for approximately 3 days post-treatment (Fig. 5D), again indicating that shortened prodrug exposures are generally less toxic to larvae.
FIG. 5.
CB1954 treatment induces morphological defects and lethality. (A–C) Images of wild-type larvae treated for 24 h in 200 μM CB1954 (A), 100 μM CB1954 (B), or DMSO control (C). Larvae were treated from 4 to 5 dpf, then imaged at 6 dpf (i.e., after 1 day of recovery). Arrows indicate edema, and asterisks indicate hydrocephalus. (D) CB1954-induced toxicity/lethality: Wild-type larvae at 4 dpf were treated in the indicated concentrations of CB1954 for 4 or 24 h. The number of viable fish was counted each day during and after treatment (for approximately 8 dpf); the percent of viable fish at each time point is plotted. Data are averages from two to three trials; error bars denote SEM.
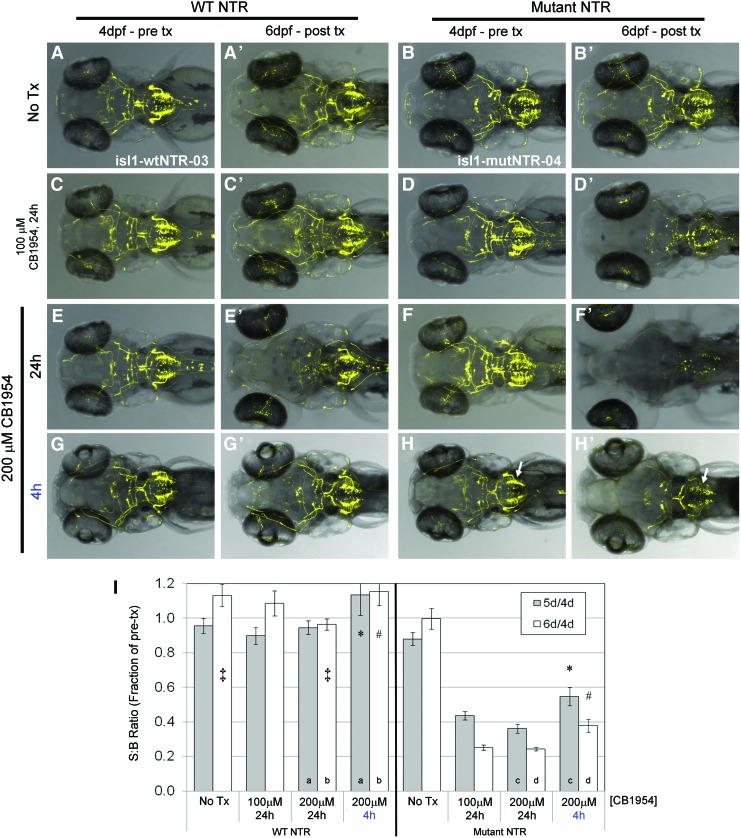
To test the effectiveness of CB1954-mediated cell ablation, we again utilized the cranial motor neuron lines (isl1:wtNTR-3 and isl1:mutNTR-4). Incubation in 100 μM CB1954 for 24 h followed by 1 day of recovery yielded no ablation with wtNTR, while the mutNTR demonstrated partial ablation (Fig. 6A–D time series) similar to that observed with short-term treatment in MTZ (Fig. 4F’). Treatment with 200 μM CB1954 for 24 h followed by 1 day of recovery resulted in the aforementioned morphological defects in both lines (Fig. 6E, F time series). Interestingly, only a small degree of cell ablation was observed in the isl1:wtNTR-3 line (Fig. 6E’), while the isl1:mutNTR-4 line demonstrated near complete ablation of cranial motor neurons under these conditions (Fig. 6F’). In addition, although a 4 h/200 μM CB1954 treatment had little to no effect in the isl1:wtNTR-3 line (Fig. 6G’), mutNTR expressing larvae showed evidence of partial ablation of all cranial neuron subsets (Fig. 6H’), including vagal motor neurons (region X, Supplementary Fig. S3E), which had proved refractory to MTZ-induced ablation. In agreement with our pilot studies, 4 h treatments produced no consistent developmental defects; a very slight edema in the head region was rarely observed (Fig. 6H’). These results were confirmed using the plate reader assay (Fig. 6I), indicating that mutNTR is a more efficient variant for inducing CB1954-mediated cell ablation in zebrafish. Further, these data suggest that CB1954 may be a viable prodrug for inducing broader injury paradigms studies when used in conjunction with mutNTR expressing zebrafish.
FIG. 6.
Improved CB1954-induced ablation of cranial motor neurons by mutNTR. (A–H’) Comparison of ablation efficiency between complementary wtNTR (A, C, E, G: isl1-wtNTR-03 line) and mutNTR (B, D, F, H: isl1-mutNTR-04 line) expressing transgenic lines by confocal time series imaging. Pretreatment images of cranial motor neuron subpopulations in individual larvae were captured at 4 dpf (A–H) and at 6 dpf (A’–H’), after indicated CB1954 treatments. (A, A’, B, B’) Control larvae treated in DMSO showed no decrease in YFP expression. (C, C’, D, D’) Larvae exposed to 100 μM MTZ for 24 h (with 1 day of recovery) revealed little effect on wtNTR cells (C, C’), while numbers of mutNTR expressing cells were clearly reduced (D, D’). (E, E’, F, F’) Larvae treated with 200 μM MTZ for 24 h (with 1 day of recovery) showed morphological defects in both lines (hydrocephalus in E’ and F’), while interestingly, wtNTR expressing neurons were largely unaffected (E, E’) and near total ablation of labeled motor neurons expressing mutNTR was evident (F, F’). (G, G’, H, H’) Larvae treated with 200 μM MTZ for 4 h, which does not cause morphological defects in either line (G’, H’), yielded no appreciable loss of wtNTR cells (G, G’) and partial ablation of mutNTR cells (H, H’; arrows indicate “group X” neurons). (I) Quantification of TagYFP fluorescence by plate reader after MTZ treatment of isl1-wtNTR-03 and isl1-mutNTR-04 lines; S:B ratios were calculated from individual larvae at 4 dpf (pretreatment), 5 dpf, and 6 dpf, then plotted as the fraction of individual pretreatment values. Data were analyzed by Student's t-tests: wtNTR, CB1954-treated samples yielded no significant differences (p>0.05) to corresponding untreated controls (No Tx) except where indicated (‡=0.03); for mutNTR, all CB1954-treated samples were significantly different from corresponding untreated controls; p-values between data pairs: *=1.5×10−4, #=1.6×10−8, a>0.05, b,c,d ≤0.05. All error bars denote SEM. Color images available online at www.liebertpub.com/zeb
Discussion
NTR/prodrug-mediated cell ablation has become a popular methodology in the zebrafish community, where it facilitates studies of cell function and/or cell-specific regeneration. As its use becomes more widespread, it is important to better understand the advantages and limitations of this system. The effectiveness of prodrug-induced cell ablation is dependent on multiple factors, including the subtype and location of targeted cells, transgene expression level, and age of the fish during the assay period. Further, we have noted that perdurance of fragmented fluorescent reporters can complicate visual detection of prodrug-induced cell death with this system.1 Moreover, some cell types may be inherently more labile than others, and the expression level of the NTR transgene will vary according to the promoter/enhancer utilized and developmental stage under investigation. Despite this, we note that treating zebrafish with 10 mM MTZ for 24 h is widely practiced, and seems to have been adopted as a standard protocol. However, data presented here and those of earlier investigators suggest that this treatment regimen incurs undesirable consequences13 and is near toxic levels (Fig. 1). Obviously, establishing methods to reduce effective prodrug concentrations and/or minimize exposure durations would help improve the usefulness of this system. Ultimately, what is needed is a means to effectively induce ablation of NTR expressing cells without compromising viability and/or causing unintended bystander effects. One method of facilitating this goal is to identify NTR variants with improved catalytic activity toward useful prodrug substrates.
Our results indicate that a triple mutant of the E. coli NTR enzyme greatly facilitates prodrug-induced cell ablation in transgenic zebrafish. This mutant was originally identified as having an increased catalytic activity toward the prodrug CB1954 in bacterial and cell cultures.24 Here, using assays involving multiple neuronal cell types, we show that this variant achieves robust cell ablation after brief MTZ exposure times (i.e., 4 h; Figs. 2–4) and at reduced MTZ concentrations (Fig. 3). Both of these modifications to MTZ treatment regimens serve to eliminate evidence of toxicity and off-site effects. These optimized parameters will greatly improve studies of cell regeneration after NTR-mediated ablation, as a reduced treatment duration provides a quicker window for regeneration to commence. Further, a lower dose of prodrug should be much more easily washed out than a higher dose, which may remain in tissues (especially those that are deeply situated) and, therefore, inhibit regeneration. While many ablation/regeneration studies in zebrafish involve assays at embryonic, larval, or juvenile stages, the NTR system has also been utilized in adult zebrafish.10,15,22,35 Use of the NTR mutant could similarly improve cell ablation assays in adult fish, whose greater complexity would make them likely more susceptible to prodrug-induced side effects that may not be readily observable. A recent report featured a transgenic line in which the mutNTR was transgenically expressed in skin cells27; to achieve ablation, adult fish were incubated in 2.5 mM MTZ for 3 days. In this report, it was not obvious whether use of the mutNTR was necessary to overcome limitations of wtNTR, but the 3 day treatment regimen suggests that this may have been the case.
In addition to solving toxicity/specificity issues, shortened exposures facilitate the application of commonly used molecular manipulations in conjunction with NTR-mediated ablation. For example, a microinjection of mRNA or morpholino-modified oligonucleotides into early-stage embryos are widely applied methods to examine the effects of overexpressing or “knocking-down” specific genes, respectively. However, the effects of these manipulations are transient, usually lasting only a few days postinjection/electroporation. Therefore, the window to assess these perturbations in combination with NTR-mediated cell ablation is hindered by the need for a 24 h prodrug regimen. In contrast, by achieving effective cell ablation after only 4 h of prodrug treatment, the NTR mutant facilitates a wider window to assess the combined effects of cell ablation and targeted molecular manipulations.
Techniques developed to monitor regenerative responses to targeted cell loss further benefit from mutNTR-facilitated reductions in the time required to induce ablation. Both high-resolution time lapse imaging of resident stem cell populations1,36 and high-throughput chemical screening methods20 are facilitated by temporally delimited inductions of cell death. For instance, chemical screens for factors that modulate cell loss and/or cell replacement can better avoid potential chemical cross-reactivity issues by introducing prodrugs and test compounds sequentially—a paradigm that 4 h MTZ treatments allow to occur on a same-day basis, thus increasing test compound exposure times. In addition, if cell loss is better synchronized after 4 h exposures, a possibility that our results are consistent with, both of these techniques can better account for the kinetics of regenerative responses.
Another option that the mutNTR enables is use of the prodrug CB1954 in zebrafish. This prodrug is not as commonly used as MTZ, most likely due to the potential of inducing a “bystander effect” in which neighboring (nontargeted) cells are killed by diffusible metabolites. Alternatively, however, one could envision leveraging CB1954-induced cell/tissue loss to model broader injury paradigms, for example, traumatic brain injury. Unfortunately, we found that a previously utilized CB1954 treatment regimen (250 μM for 24 h)13 resulted in delayed morphological deformities and lethality (Fig. 5). Together, our findings suggest that CB1954-induced ablation is highly inefficient when applied in combination with wtNTR expressing zebrafish (e.g., Fig. 6). Results with mutNTR expressing lines were markedly different, with near total ablation of cranial motor neurons after a 24 h treatment in 200 μM CB1954 and partial ablation at 100 μM (Fig. 6). Of greater interest are our results using a 4 h treatment of 200 μM CB1954: (1) in wild-type larvae, this treatment yielded neither lethality nor readily observable morphological defects (Fig. 5), and (2) this regimen yielded partial ablation of cranial motor neurons (Fig. 6). These results indicate that CB1954 may be more effectively employed in combination with the mutNTR expressing fish. These results are not entirely surprising, as the mutant was isolated from a directed evolution screen for bacterial mutants displaying greater enzymatic activity toward this particular prodrug.24 While our lab has not assessed the “bystander effect,” it is noteworthy that the NTR system was originally developed as an anti-tumor therapy.5 Since the field of tumor biology has recently expanded to zebrafish,37,38 including assays of metastasis,39 the mutNTR/CB1954 ablation paradigm could be deployed to assess tumor-ablating properties in vivo.
In summary, the NTR system of inducible targeted cell ablation has many potential uses beyond cancer therapeutics. In model systems where it has been successfully deployed, it facilitates the elucidation of cell function (e.g., neural subcircuits). In zebrafish and other robustly regenerative species (axolotl, xenopus, planaria, etc.), it also promotes extending regenerative biology studies to a wide range of individual cellular subtypes. Here, we have provided evidence that that system can be improved in an effort to overcome current limitations. Future improvements can be directed toward aspects of the system that were not addressed here, for example, reporter perdurance, or more effective prodrugs, in an effort to develop an optimized inducible cell ablation platform. To facilitate widespread adaptation of improved NTR variants, such as the triple mutNTR characterized here, we plan to generate a series of corresponding bicistronic “effector/reporter” lines (e.g., UAS). Such resources will be useful in conjunction with the large number of Gal4 and other “driver” lines that have been developed in the zebrafish community.
Materials and Methods
Ethics statement
This study was carried out in accordance with the recommendations in the Guide for the Care and Use of Laboratory Animals of the National Institutes of Health. An animal use protocol was approved by the Institutional Animal Care and Use Committee (Approval Identification No. BR10-12-391) of Georgia Regents University, which has an Animal Welfare Assurance on file in the Office of Laboratory Animal Welfare (Assurance No. A3307-01). Using approved anesthetics, all efforts were made to minimize discomfort and suffering during experimental procedures.
Zebrafish husbandry and larvae maintenance
Adult zebrafish were maintained on a 14 h/10 h light/dark schedule within a recirculating system (Aquatic Habitats) at Georgia Regents University. Embryos were generated from pairwise crosses of adult fish, and maintained at 28.5°C in fish water (FW: 250 mg/L sodium bicarbonate, 100 mg/L instant ocean salts, pH=7). After ∼24 h of development, embryos were maintained in FW containing 0.2 mM (0.003%) phenylthiourea (PTU) to inhibit melanization.
Assessment of prodrug-induced lethality
Prodrugs were prepared as follows: MTZ (MP Biomedicals) was resuspended to 20 mM in FW containing 0.1% DMSO, then diluted to lower concentrations (15, 10, 5 mM) in FW containing 0.1% DMSO, which was used as a negative control; CB-1954 (Sigma) was dissolved in DMSO to a stock concentration of 100 mM, then diluted in FW to 100–200 μM (with 0.1% and 0.2% DMSO serving as negative controls, respectively). Wild-type zebrafish larvae were arrayed in six-well dishes at 4 dpf (20 larvae per well), then treated in diluted prodrugs or control solutions (8 mL per well) for the indicated times (4, 24, and 48 h), after which the larvae were washed thrice in FW. Each day (approximately 8 dpf), media was exchanged, and viability was assessed and quantified by examination through a dissecting microscope: Viable fish were minimally defined by a beating heart and a response to stimulus, that is, a gentle shake of the plate, or prodding with a pipet tip. For long-term assays to 14 dpf (Supplementary Fig. S1), wild-type larvae at 5 dpf (90 per condition, in 6 cm dishes) were treated with the indicated concentrations of MTZ for 4 or 24 h; at 6 dpf, larvae were transferred to a 1 L tank, where they were fed paramecia daily. The number of viable fish was counted each day through 14 dpf.
Plasmid construction and production of transgenic lines
Plasmids designed to coexpress NTR and a fluorescent reporter in specific neuronal cell subtypes were made in the style of a “self-reporting” Gal4:UAS construct, as previously detailed.31 Full cloning details are available on request. Briefly, an optimized Gal4 driver (KalTA4)40 was placed downstream of a 2×tandem repeat of the neuron-restrictive silencer element (NRSE),31 a cell-specific enhancer, and a cfos minimal promoter. In the same construct, a bicistronic message coding for both TagYFP (Evrogen) and an NTR variant was placed downstream of a 5xUAS Gal4 binding element.41 To promote equimolar expression of TagYFP and NTR without physically fusing them, separate coding sequences were linked by a T2A viral peptide sequence.41 Cell-specific enhancers were amplified from genomic DNA (AB strain) by PCR and subcloned between the NRSE and cfos promoter. For expression in spinal motor neurons, a 125bp enhancer element from mnx129,30 was utilized; the resulting construct featured a 2×tandem repeat of the 125bp element (for plasmid diagram, see Supplementary Fig. S2). For expression in cranial motor neurons, the CREST1 enhancer from islet132 was used (for plasmid diagram, see Supplementary Fig. S3). The triple mutation (T41Q/N71S/F124T) of NTR was introduced into wtNTR expressing mnx1 and CREST1 constructs by subcloning into a BlpI flanked fragment bearing all three mutants. All constructs utilized mini-Tol2 elements to promote efficient transgenesis.42 Full plasmid cloning details are available on request. Transgenic lines were derived by a microinjection of plasmid constructs along with Tol2 transposase mRNA into embryos, followed by selection and propagation of fluorescent offspring. The CREST1 line bearing wtNTR has previously appeared 31 as Tg(2xNRSE-CREST1-cfos:KalTA4, 5xUAS-E1b:YFP-2A-nfsB)lmc003, the ZFIN designation is Tg(2xNRSE-isl1-Mmu.Fos:KalTA4,5xUAS-ADV.E1b:GAP-YFP-2A-Eco.NfsB)lmc003 (see Table 1 for full transgene names and abbreviations used here).
Cell ablation assays
NTR-bearing lines were out-crossed to AB stock lines such that all comparisons of wtNTR and mutNTR were performed between heterozygous transgenic progeny. After 24 h of development, larvae were maintained in FW+PTU at all times. Larvae with comparable levels of fluorescence (and therefore similar levels of NTR) were selected by microscopic observation at 3–4 dpf; all subsequent assays (i.e., confocal imaging and plate reader, detailed next) were performed in a longitudinal manner by which each individual fish served as its own internal control with regard to variations in transgene expression—in this manner, variability within a single line (e.g., among siblings) and between different transgenic lines was controlled for, as detailed further in the text. All cell ablation assays started at 4 dpf, with a collection of pretreatment data (images or fluorescence values, as detailed next) followed by incubation in prodrugs for the indicated times and concentrations. To stop prodrug treatments, larvae were washed four times in FW+PTU; it should be noted that for each assay, all prodrug treatments were begun simultaneously, with differing treatment durations (e.g., 4 and 24 h) defined by the timing of the prodrug washout. Typically, cell ablation data were collected at both 5 and 6 dpf; since the loss of fluorescence (and therefore cell ablation) was often more evident at 6 dpf, we chose to display images at this timepoint in all figures incorporating confocal microscopy.
Confocal imaging and plate reader assays
Larvae were anesthetized in FW+PTU containing either tricaine methansulfonate (0.2 mg/mL; Argent) or Eugenol (50 ng/mL; Sigma). Confocal imaging was performed on an Olympus FV1000 upright confocal microscope as previously described.36 Fluorescence was quantified using a TECAN Infinite M1000 plate reader as previously described20; on each day, data were collected from both transgenic (n=12 per condition) and nontransgenic larvae, the latter of which were used to define background fluorescence values. Fluorescence values in transgenic larvae (i.e., Signal) were divided by an averaged background value (i.e., from each corresponding day of data collection) to generate signal-to-background (S:B) ratios. For each experiment, S:B ratios obtained at 4 dpf (before prodrug treatment) for each individual larvae assayed served as the baseline for subsequent readings (at 5 and 6 dpf), such that all measurements were internally controlled per each subject. Plate reader data are, thus, presented in figures as the fraction of the pretreatment (e.g., 4 dpf/5 dpf and 4 dpf/6 dpf) values for each condition.
Apoptotic cell labeling and quantification
To label apoptotic cells, larvae were incubated in Acridine Orange (1 mg/mL in FW+PTU; Fisher Scientific) for 15 min, followed by several washes in FW+PTU. Acridine Orange-labeled cells were quantified from confocal images by manually counting brightly fluorescent cells within the presumptive spinal cord (i.e., the slightly autoflurorescent dorsal region indicated in Figs. 2G, H and 3G, H).
Statistical analyses
Lethality assays were analyzed by chi-square tests: data from prodrug-treated samples were compared with untreated controls, with significantly different data defined as χ2 <0.05. All other data were analyzed by Student's t-tests, with significantly different data defined as p<0.05.
Supplementary Material
Acknowledgments
The authors thank the members of the Mumm lab and Luminomics for helpful discussions during the course of this work, and the lab of David Kozlowski for maintenance of the Zebrafish facility at Georgia Regents University. They also thank their reviewers for helpful suggestions that improved this article.
Disclosure Statement
JSM and MTS have a financial interest in Luminomics, Inc., a small biotechnology company that uses the NTR transgene-based system of targeted cellular ablation as a platform for studying cell-specific regeneration using models of degenerative and autoimmune diseases. JSM has a patent on the use of prodrug converting enzyme systems in zebrafish (United States Patent 7514595). JRM and MTS are salaried employees of Luminomics. JSM receives consulting fees from Luminomics. No competing financial interests exist for ZZ.
References
- 1.White DT, Mumm JS. The nitroreductase system of inducible targeted ablation facilitates cell-specific regenerative studies in zebrafish. Methods 2013;62:232–240 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Curado S, Stainier DYR, Anderson RM. Nitroreductase-mediated cell/tissue ablation in zebrafish: a spatially and temporally controlled ablation method with applications in developmental and regeneration studies. Nat Protoc 2008;3:948–954 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Mackenzie SM, Raible DW. Proliferative regeneration of zebrafish lateral line hair cells after different ototoxic insults. PLoS One 2012;7:e47257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Zenno S, Koike H, Tanokura M, Saigo K. Gene cloning, purification, and characterization of NfsB, a minor oxygen-insensitive nitroreductase from Escherichia coli, similar in biochemical properties to FRase I, the major flavin reductase in Vibrio fischeri. J Biochem 1996;120:736–744 [DOI] [PubMed] [Google Scholar]
- 5.Bridgewater JA, Springer CJ, Knox RJ, Minton NP, Michael NP, Collins MK. Expression of the bacterial nitroreductase enzyme in mammalian cells renders them selectively sensitive to killing by the prodrug CB1954. Eur J Cancer 1995;31A:2362–2370 [DOI] [PubMed] [Google Scholar]
- 6.Kaya F, Mannioui A, Chesneau A, Sekizar S, Maillard E, Ballagny C, et al. Live imaging of targeted cell ablation in Xenopus: a new model to study demyelination and repair. J Neurosci 2012;32:12885–12895 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Isles AR, Ma D, Milsom C, Skynner MJ, Cui W, Clark J, et al. Conditional ablation of neurones in transgenic mice. J Neurobiol 2001;47:183–193 [DOI] [PubMed] [Google Scholar]
- 8.Choi RY, Engbretson GA, Solessio EC, Jones GA, Coughlin A, Aleksic I, et al. Cone degeneration following rod ablation in a reversible model of retinal degeneration. Invest Ophthalmol Vis Sci 2011;52:364–373 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Curado S, Anderson RM, Jungblut B, Mumm J, Schroeter E, Stainier DYR. Conditional targeted cell ablation in zebrafish: a new tool for regeneration studies. Dev Dyn 2007;236:1025–1035 [DOI] [PubMed] [Google Scholar]
- 10.Zhou W, Hildebrandt F. Inducible podocyte injury and proteinuria in transgenic zebrafish. J Am Soc Nephrol 2012;23:1039–1047 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Zhao X, Ellingsen S, Fjose A. Labelling and targeted ablation of specific bipolar cell types in the zebrafish retina. BMC Neurosci 2009;10:107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.White YAR, Woods DC, Wood AW. A transgenic zebrafish model of targeted oocyte ablation and de novo oogenesis. Dev Dyn 2011;240:1929–1937 [DOI] [PubMed] [Google Scholar]
- 13.Pisharath H. Validation of nitroreductase, a prodrug-activating enzyme, mediated cell death in embryonic zebrafish (Danio rerio). Comp Med 2007;57:241–246 [PubMed] [Google Scholar]
- 14.Montgomery JE, Parsons MJ, Hyde DR. A novel model of retinal ablation demonstrates that the extent of rod cell death regulates the origin of the regenerated zebrafish rod photoreceptors. J Comp Neurol 2010;518:800–814 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Li X, Montgomery J, Cheng W, Noh JH, Hyde DR, Li L. Pineal photoreceptor cells are required for maintaining the circadian rhythms of behavioral visual sensitivity in zebrafish. PLoS One 2012;7:e40508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Hu S, Lin P, Liao C, Gong H, Lin G, Kawakami K, et al. Nitroreductase-mediated Gonadal Dysgenesis for Infertility Control of Genetically Modified Zebrafish. Mar Biotechnol 2010;12:569–578 [DOI] [PubMed] [Google Scholar]
- 17.Hsu C, Hou M, Hong J, Wu J, Her GM. Inducible male infertility by targeted cell ablation in zebrafish testis. Mar Biotechnol 2010;12:466–478 [DOI] [PubMed] [Google Scholar]
- 18.Gray C, Loynes CA, Whyte MKB, Crossman DC, Renshaw SA, Chico TJA. Simultaneous intravital imaging of macrophage and neutrophil behaviour during inflammation using a novel transgenic zebrafish. Thromb Haemost 2011;105:811–819 [DOI] [PubMed] [Google Scholar]
- 19.Andersson O, Adams BA, Yoo D, Ellis GC, Gut P, Anderson RM, et al. Adenosine signaling promotes regeneration of pancreatic β cells in vivo. Cell Metab 2012;15:885–894 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Walker SL, Ariga J, Mathias JR, Coothankandaswamy V, Xie X, Distel M, et al. Automated reporter quantification in vivo: high-throughput screening method for reporter-based assays in zebrafish. PLoS One 2012;7:e29916. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Lambert AM, Bonkowsky JL, Masino MA. The conserved dopaminergic diencephalospinal tract mediates vertebrate locomotor development in zebrafish larvae. J Neurosci 2012;32:13488–13500 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Singh SP, Holdway JE, Poss KD. Regeneration of amputated zebrafish fin rays from de novo osteoblasts. Dev Cell 2012;22:879–886 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Davison JM, Akitake CM, Goll MG, Rhee JM, Gosse N, Baier H, et al. Transactivation from Gal4-VP16 transgenic insertions for tissue-specific cell labeling and ablation in zebrafish. Dev Biol 2007;304:811–824 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Guise CP, Grove JI, Hyde EI, Searle PF. Direct positive selection for improved nitroreductase variants using SOS triggering of bacteriophage lambda lytic cycle. Gene Ther 2007;14:690–698 [DOI] [PubMed] [Google Scholar]
- 25.Friedlos F, Court S, Ford M, Denny WA, Springer C. Gene-directed enzyme prodrug therapy: quantitative bystander cytotoxicity and DNA damage induced by CB1954 in cells expressing bacterial nitroreductase. Gene Ther 1998;5:105–112 [DOI] [PubMed] [Google Scholar]
- 26.Bridgewater JA, Knox RJ, Pitts JD, Collins MK, Springer CJ. The bystander effect of the nitroreductase/CB1954 enzyme/prodrug system is due to a cell-permeable metabolite. Hum Gene Ther 1997;8:709–717 [DOI] [PubMed] [Google Scholar]
- 27.Chen C, Chu C, Chen T, Lee S, Shen C, Hsiao C. Establishment of a transgenic zebrafish line for superficial skin ablation and functional validation of apoptosis modulators in vivo. PLoS One 2011;6:e20654. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Kimmel CB, Ballard WW, Kimmel SR, Ullmann B, Schilling TF. Stages of embryonic development of the zebrafish. Dev Dyn 1995;203:253–310 [DOI] [PubMed] [Google Scholar]
- 29.Nakano T, Windrem M, Zappavigna V, Goldman SA. Identification of a conserved 125 base-pair Hb9 enhancer that specifies gene expression to spinal motor neurons. Dev Biol 2005;283:474–485 [DOI] [PubMed] [Google Scholar]
- 30.Zelenchuk TA, Brusés JL. In Vivo labeling of zebrafish motor neurons using an mnx1 enhancer and Gal4/UAS. Genesis 2011;49:546–554 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Xie X, Mathias J, Smith M, Walker S, Teng Y, Distel M, et al. Silencer-delimited transgenesis: NRSE/RE1 sequences promote neural-specific transgene expression in a NRSF/REST-dependent manner. BMC Biol 2012;10:93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Uemura O, Okada Y, Ando H, Guedj M, Higashijima S, Shimazaki T, et al. Comparative functional genomics revealed conservation and diversification of three enhancers of the isl1 gene for motor and sensory neuron-specific expression. Dev Biol 2005;278:587–606 [DOI] [PubMed] [Google Scholar]
- 33.Higashijima S, Schaefer M, Fetcho JR. Neurotransmitter properties of spinal interneurons in embryonic and larval zebrafish. J Comp Neurol 2004;480:19–37 [DOI] [PubMed] [Google Scholar]
- 34.Higashijima S, Hotta Y, Okamoto H. Visualization of cranial motor neurons in live transgenic zebrafish expressing green fluorescent protein under the control of the islet-1 promoter/enhancer. J Neurosci 2000;20:206–218 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Chung A, Kim P, Kim S, Kim E, Kim D, Jeong I, et al. Generation of demyelination models by targeted ablation of oligodendrocytes in the zebrafish CNS. Mol Cells 2013;36:82–87 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Ariga J, Walker SL, Mumm JS. Multicolor time-lapse imaging of transgenic zebrafish: visualizing retinal stem cells activated by targeted neuronal cell ablation. J Vis Exp 2010;43:2093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Taylor AM, Zon LI. Zebrafish tumor assays: the state of transplantation. Zebrafish 2009;6:339–346 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Ceol CJ, Houvras Y, White RM, Zon LI. Melanoma biology and the promise of zebrafish. Zebrafish 2008;5:247–255 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Shao J, Teng Y, Padia R, Hong S, Noh H, Xie X, et al. COP1 and GSK3β Cooperate to promote c-Jun degradation and inhibit breast cancer cell tumorigenesis. Neoplasia 2013;15:1075–1085 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Distel M, Wullimann MF, Köster RW. Optimized Gal4 genetics for permanent gene expression mapping in zebrafish. Proc Natl Acad Sci U S A 2009;106:13365–13370 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Provost E, Rhee J, Leach SD. Viral 2A peptides allow expression of multiple proteins from a single ORF in transgenic zebrafish embryos. Genesis 2007;45:625–629 [DOI] [PubMed] [Google Scholar]
- 42.Urasaki A, Morvan G, Kawakami K. Functional dissection of the Tol2 transposable element identified the minimal cis-sequence and a highly repetitive sequence in the subterminal region essential for transposition. Genetics 2006;174:639–649 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.