Abstract
Glycogen is the main energetic polymer of glucose in vertebrate animals and plays a crucial role in whole body metabolism as well as in cellular metabolism. Many methods to detect glycogen already exist but only a few are quantitative. We describe here a method using the Abcam Glycogen assay kit, which is based on specific degradation of glycogen to glucose by glucoamylase. Glucose is then specifically oxidized to a product that reacts with the OxiRed probe to produce fluorescence. Titration is accurate, sensitive and can be achieved on cell extracts or tissue sections. However, in contrast to other techniques, it does not give information about the distribution of glycogen in the cell. As an example of this technique, we describe here the titration of glycogen in two cell lines, Chinese hamster lung fibroblast CCL39 and human colon carcinoma LS174, incubated in normoxia (21% O2) versus hypoxia (1% O2). We hypothesized that hypoxia is a signal that prepares cells to synthesize and store glycogen in order to survive1.
Keywords: Basic Protocol, Issue 81, Glycogen, Glucoamylase, Fluorescence, Oxidation, Periodic Acid Shiff staining (PAS)
Introduction
Glycogen is a multibranched polymer of glucose residues, which is present in the cytoplasm of many cell types. It is one of the main forms of energy storage in cells and plays an important role in glucose metabolism. Most mammalian cells are able to produce and store glycogen, which can be rapidly degraded into glucose to promote glycolysis and ATP production during metabolic stress. Hepatocytes produce massive amounts of glycogen to regulate the blood sugar level thereby providing a continuous supply of glucose to the body. In contrast, the concentration of glycogen in other cells (muscles, red blood cells, etc.) is relatively low. However, locally, these quantities are sufficient to provide energy in the short-term when the cells are suddenly exposed to an environment deprived of nutrients.
Glycogen synthesis and glycogen breakdown follows the same steps in all tissues (Figure 1). Firstly, glucose enters cells by glucose transporters (GLUTs), and is rapidly converted from glucose-6-phosphate (G6P) into glucose-1-phosphate (G1P) by phosphoglucomutase. G1P is then modified into UDP-glucose, and the carbon C1 of UDP-glucose is attached to a tyrosine residue of glycogenin, the anchoring protein of glycogen. This molecule, considered a glycogen primer, is extended by attachment of the UDP-glucose to the terminal glucose by an α(1→4) bond via the glycogen synthase. Finally, when a linear chain of over 11 glucose residues is formed, the branching enzyme transfers a terminal oligosaccharide constituted by a minimum of 6 glucose residues to another glucose of the chain nearby through an α(1→6) linkage. The repetition of this process gives a massive fractal structure containing branches that form a helix with 6.5 glucose per turn. Glycogen can be reversely hydrolyzed to glucose by the concerted action of debranching enzymes that hydrolyze the α(1→6) bound and glycogen phosphorylase that hydrolyzes the α(1→4) glycosidic bound between the last residue of glucose of a branch and the glycogen molecule. This reaction called glycogenolysis is activated by increasing levels of AMP (reflecting ATP consumption), and inhibited by glucose and ATP2,3.
By electron microscopy, glycogen molecules have been described in many cell types as β free particles (or glycogen monoparticles) of 15-30 nm in diameter. In specific cell types such as hepatocytes, β particles can be assembled into a complex to form rosettes, also known as α particles that vary in diameter from 80 nm to a maximum of 200 nm4. The way these β particles are bonded to form larger clusters of α particles is still not fully elucidated. Some evidence tends to prove that β particles can be bonded by covalent bonding5, hydrogen bonding, or even through protein-protein interactions6. The amount of glycogen stored in the cells depends on many parameters: (I) the amount of glycogenin in the cell that initiates glycogen synthesis; (II) the activity of glycogen synthase and phosphorylase regulated by protein phosphorylation/dephosphorylation; (III) the concentration of glucose in the cells, which is dependent on several parameters such as the supply of glucose from the vascular system and glucose uptake by cells. Glycogen stores are tightly regulated by allosteric regulation of biosynthetic hormones through intermediate metabolites, by hormones regulating energy metabolism, and by nutrient sensing signaling pathways7.
It is important to be able to quantify glycogen in biological samples to better understand the importance of glycogen metabolism at the whole body and cellular level. We describe here a precise, reproducible and convenient biochemical in vitro assay for glycogen. This technique is based on the quantification glucose fluorescence before and after specific hydrolysis of glycogen.
Other methods exist to estimate the level of glycogen in the cells, but most of them are not quantitative. One of the first techniques described for quantification of glycogen in cells was based on measurement of [14C]-glucose incorporation into glycogen8,9. The use of radioactivity makes this process more difficult to handle but it has the advantage of providing the rate of glucose incorporation into glycogen and in distinguishing between the distribution of glucose residues on the outer branches and in the core of the molecule (it also requires an additional β-amylolysis and a chromatographic step). Another technique was developed more recently and is based on the incorporation of 2-NBDG (2-{N-[7-nitrobenz-2-oxa-1,3-diazol-4-yl] amino}-2-deoxyglucose), a 2-deoxyglucose fluorescent derivative, into glycogen10. The measured fluorescence intensity reflects the amount of glycogen produced and can be measured with a fluorescence reader. Distribution of fluorescence in the cell can also be evaluated by confocal microscopy.
Among the other nonquantitative techniques, Periodic Acid-Schiff staining (PAS) is perhaps the most common. It can be used for the detection of glycogen in fixed cells, tissue sections in paraffin or frozen. This histological technique colors non specifically polysaccharides, glycolipids, glycoproteins, cellulose and neutral mucins in purple. The specificity of this test can be increased by treatment of fixed cells or tissue sections with diastase, which specifically digests glycogen. Thereafter, the level of glycogen can be qualitatively estimated by comparing unhydrolyzed samples (all carbohydrate modified macromolecules) to hydrolyzed samples (carbohydrate modified macromolecules except glycogen). PAS staining and microscopic analysis, unlike biochemical assays of glycogen, provides information concerning the distribution of glycogen in the cell, which can be diffused or concentrated in a certain part of the cell. However, even though PAS staining estimates differences in glycogen accumulation between different conditions, it is not quantitative11.
A monoclonal mouse antibody originally made using mandibular condylar cartilage as the antigen has been shown to react with glycogen in cells and with purified glycogen in vitro12. As this antibody specifically recognizes glycogen-related sugar chains, it is a useful tool for the detection of glycogen by immunohistochemistry in a more specific way than the PAS staining.
Electron microscopy is another technique that allows visualization of grains of glycogen in cells and evaluation of the degree of glycogen storage. In fact, glycogen β particles are easily recognizable with an electron microscopy as electron dense granules1.
Protocol
Biochemical Titration of Glycogen
1. Cell Lysis
Seed cells at a concentration of 0.5-2 x 106 per 100 mm diameter dish.
Treatment: incubate cells 24, 48, or 72 hr in low oxygen concentration (hypoxia) in a Bug-Box anaerobic work station (Ruskinn Technology Biotrace International Plc, Bridgend, UK) set at 1% or 0.1% O2, 94% or 94.9% N2, and 5% CO2. In parallel, incubate cells in normoxia (21% O2, 5% CO2). Change medium (25 mM glucose-containing medium) every 24 hr to minimize variations in the glucose concentration during the time of experiment.
After treatment, wash cells 2x with PBS to remove traces of glucose from culture medium. Scrape the cells in cold PBS.
Centrifuge the solution at 1,000 rpm for 5 min, discard the supernatant and wash the pellet once with PBS. The pellet can be frozen at -20 °C before proceeding further.
Resuspend the pellet in 100 μl of distilled water. Add 100 μl of the Glycogen Hydrolysis Buffer provided with the Glycogen Assay Kit (Abcam). Boil the lysate at 95 °C for 5 min to inactivate enzymes.
Vortex and centrifuge the lysate at 13,000 rpm for 10 min to remove the insoluble products. Keep the supernatant.
To normalize glycogen levels to the protein content between samples, quantification of proteins can be done with 25-35 μl of the supernatant using the Bicinchoninic Acid assay (BCA-Interchim).
Some of the lysate can be used to measure the free glucose concentration inside the cells.
2. Hydrolysis of Glycogen
Mix 50 μl of the supernatant (step 1) with 1 μl of the Hydrolysis Enzyme Mix. This mixture contains glucoamylase. Incubate for 30 min at room temperature.
Prepare the calibration curve by diluting the standard glycogen (2 mg/ml) supplied with the kit to a solution of 10 μg/ml in the hydrolysis buffer. Next, prepare six separate tubes with 0, 4, 8, 12, 16, and 20 μl of 10 μg/ml standard and adjust each tube to a final volume of 50 μl with hydrolysis buffer to give a concentration of glycogen of 0, 0.04, 0.08, 0.12, 0.16, and 0.2 μg/ml. Add 1 μl of Hydrolysis Enzyme Mix in the standards and incubate for 30 min at room temperature.
The background glucose concentration of the cell line, given by value for the free glucose concentration, must be subtracted from the value for the total glucose concentration (glucose from the glycogen hydrolysis + free-glucose) to determine the level of glycogen in the cell.
3. Development and Reading of Fluorescence
For each condition, prepare one tube (tube 1) to measure free glucose concentration and two tubes (tubes 2 and 3) to measure total glucose concentration (each tube with a different volume of hydrolyzed glycogen).
Add 15 μl of supernatant extracted at the end of step 1 in tube 1. Add 35 μl of hydrolysis buffer to complete to a final volume of 50 μl. Obtained fluorescence will give the free glucose concentration.
Add X μl of hydrolyzed glycogen (obtained in step 2) in tube 2. Adjust to a final volume of 50 μl. Sample volume (X μl) has to be adjusted according to cell density and/or cell type in order to obtain fluorescence values that are not beyond concentrations of the calibration curve.
Add 2X μl of hydrolyzed glycogen in tube 3. Adjust to a final volume of 50 μl.
Prepare a development solution for samples and standards by mixing for each tube 48.7 μl of Development Buffer, 1 μl of Development Enzyme Mix and 0.3 μl of OxiRed Probe (supplied in the Glycogen Assay Kit). For example, for 2 conditions, prepare a mix for 12 tubes (6 for standards, 2 for free glucose and 4 for total glucose fluorescence).
Add 50 μl of this mix to each tube and incubate for 30 min at room temperature in the dark.
Measure the fluorescence at an excitation wavelength of 535 nm, an emission wavelength of 587 nm as recommended, and a slit of 3 nm for excitation and emission.
Representative Results
A low level of oxygen (hypoxia) in tumors signals to tumor cells the need to store energy to handle subsequent nutrient depletion, so as to survive. As glycogen is the main energetic polymer of glucose in mammalian cells, we studied the regulation of glycogen storage in hypoxia. The calculation and standardization of the concentration of glycogen in cell lysates must be performed on the raw data of fluorescence as shown in Tables 1, 2, and 3. The biochemical assay for glycogen confirmed that the electron-dense aggregates observed on electron micrographs of CCL39 cells (Figure 2A) were glycogen particles (Figure 2B). The accumulation of glycogen in hypoxia is dependent on the transcription factor Hypoxia-Inducible Factor-1 (HIF-1) (Figure 3), the major transcription factor involved in cellular adaptation to hypoxia. Finally, we demonstrate in different cancer and noncancer cell lines that stored glycogen can be rapidly metabolized by the cell in less than 6 hr (Figure 4A), and that the use of glycogen protects against cell death under conditions of glucose starvation (Figure 4B)1.
Table 1.
| glu. (μg) | 0 | 0.04 | 0.08 | 0.12 | 0.16 | 0.2 | |
| Raw Data Fluorescence (RDF) | fluo. | 60 | 88.8 | 106.7 | 121.8 | 148 | 172.1 |
| RDF - Background (fluorescence for 0 μg in standard curve) | correction | 0 | 28.8 | 46.7 | 61.8 | 88 | 112.1 |
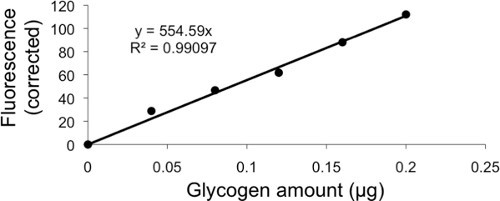
| |||||||
| Raw Data Fluo. (RDF) | RDF - Background | (correction*1000) / slope standard curve | Volume of sample used to titrate glycogen | Raw data protein concen-tration | γ * volume sample | Glucose (ng) / protein total | |
| Total glucose concentration 1 | fluo. | correction | glucose (ng) | volume sample | γ (μg/μl) | protein total | glucose (ng/μg Pr.) |
| Sample 1 (CCL39-NX 1) | 65.8 | 5.8 | 10.5 | 20 | 0.07 | 1.4 | 7.5 |
| Sample 2 (CCL39-NX 2) | 64.1 | 4.1 | 7.4 | 20 | 0.11 | 2.2 | 3.4 |
| Sample 3 (CCL 39 HX 48 hr 1) | 177.3 | 117.3 | 211.5 | 4 | 0.22 | 0.88 | 240.3 |
Table 1.5.
| Sample 4 (CCL 39 HX 48h 2) | 174.2 | 114.2 | 205.9 | 4 | 0.24 | 0.96 | 214.5 |
| Sample 5 (CCL 39 HX 72 hr 1) | 164.7 | 104.7 | 188.8 | 2 | 0.22 | 0.44 | 429.1 |
| Sample 6 (CCL 39 HX 72 hr 2) | 174.7 | 114.7 | 206.8 | 2 | 0.24 | 0.48 | 430.9 |
| Raw Data Fluo. (RDF) | RDF - Background | (correction*1000) / slope standard curve | Volume of sample used to titrate glycogen | Raw data protein concen-tration | γ * volume sample | Glucose (ng) / protein total | |
| Total glucose concentration 2 | fluo. | correction | glucose (ng) | volume sample | γ (μg/μl) | protein total | glucose (ng/μg Pr.) |
| Sample 1 (CCL39-NX 1) | 63 | 3 | 5.4 | 10 | 0.07 | 0.7 | 7.7 |
| Sample 2 (CCL39-NX 2) | 61.2 | 1.2 | 2.2 | 10 | 0.11 | 1.1 | 2.0 |
| Sample 3 (CCL 39 HX 48 hr 1) | 121.6 | 61.6 | 111.1 | 2 | 0.22 | 0.44 | 252.4 |
| Sample 4 (CCL 39 HX 48 hr 2) | 111.9 | 51.9 | 93.6 | 2 | 0.24 | 0.48 | 195.0 |
| Sample 5 (CCL 39 HX 72 hr 1) | 110.3 | 50.3 | 90.7 | 1 | 0.22 | 0.22 | 412.3 |
| Sample 6 (CCL 39 HX 72 hr 2) | 117.8 | 57.8 | 104.2 | 1 | 0.24 | 0.24 | 434.3 |
Table 2.
| free glucose | fluo. | correc-tion | glucose (ng) | volume sample | γ (μg/μl) | protein total | glucose (ng/μg Pr.) | negative values are Considered as 0 |
| Sample 1 (CCL39-NX 1) | 60.2 | 0.2 | 0.4 | 15 | 0.07 | 1.05 | 0.3 | 0.3 |
| Sample 2 (CCL39-NX 2) | 55.3 | -4.7 | -8.5 | 15 | 0.11 | 1.65 | -5.1 | 0 |
| Sample 3 (CCL 39 HX 48 hr 1) | 56.5 | -3.5 | -6.3 | 15 | 0.22 | 3.3 | -1.9 | 0 |
| Sample 4 (CCL 39 HX 48 hr 2) | 56.7 | -3.3 | -6.0 | 15 | 0.24 | 3.6 | -1.7 | 0 |
| Sample 5 (CCL 39 HX 72 hr 1) | 57.5 | -2.5 | -4.5 | 15 | 0.22 | 3.3 | -1.4 | 0 |
| Sample 6 (CCL 39 HX 72 hr 2) | 56.9 | -3.1 | -5.6 | 15 | 0.24 | 3.6 | -1.6 | 0 |
Table 3.
| Total glucose concen-tration 1 | Total glucose concen-tration 2 | Total glucose concen-tration average | Free glucose concen-tration | Glycogen (ng/μg Pr.) (total average glucose - free glucose) | Average glycogen (between dupli-cates) | Stan-dard error | |
| Sample 1 (CCL39-NX 1) | 7.5 | 7.7 | 7.6 | 0.3 | 7.3 | 5.0 | 3.3 |
| Sample 2 (CCL39-NX 2) | 3.4 | 2.0 | 2.7 | 0.0 | 2.7 | ||
| Sample 3 (CCL 39 HX 48 hr 1) | 240.3 | 252.4 | 246.4 | 0.0 | 246.4 | 225.6 | 29.5 |
| Sample 4 (CCL 39 HX 48 hr 2) | 214.5 | 195.0 | 204.7 | 0.0 | 204.7 | ||
| Sample 5 (CCL 39 HX 72 hr 1) | 429.1 | 412.3 | 420.7 | 0.0 | 420.7 | 426.6 | 8.4 |
| Sample 6 (CCL 39 HX 72 hr 2) | 430.9 | 434.3 | 432.6 | 0.0 | 432.6 |
Tables 1, 2, 3. Representative calculation and normalization of glycogen content of CCL39 cells from fluorescence row datas. Glycogen was titrated in CCL39 after incubation in normoxia (Nx) or in hypoxia 1% O2 (Hx) for 48 hr or 72 hr. Glycogen measurements have been done in duplicate for each condition. Top part of Table 1 (table and figure on the top) contains calculation and representation of standard curve. Middle part of Table 1 shows how to calculate and normalize free glucose concentration and total glucose concentration (from two different volumes of sample) from fluorescence data. Bottom part of Table 1 indicates how to calculate glycogen concentration from total glucose concentration and free glucose concentration.
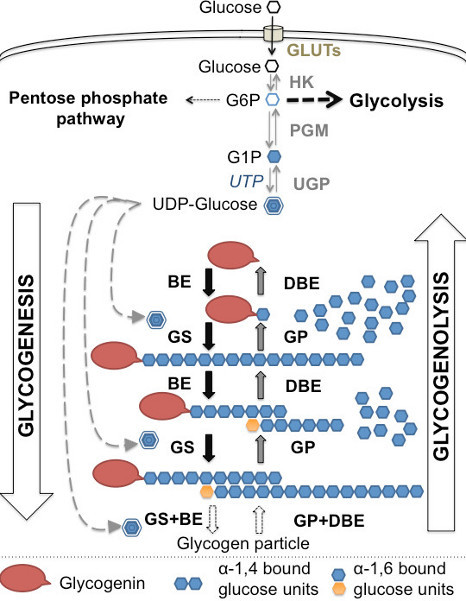 Figure 1. Glycogen metabolism: overview of the synthesis and degradation of glycogen. Glucose enters into the cell cytoplasm through glucose transporters (GLUTs) for transformation into glucose-6-phosphate by hexokinases 1 and 2 (HK). Glucose-6-phosphate (G6P) is at the junction between glycolysis, the pentose phosphate pathway and glycogenesis. Phosphoglucomutase (PGM) is the first enzyme in glycogenesis that catalyzes the conversion of G6P into glucose 1-phosphate (G1P), which is then transformed into UDP-glucose by glucose-1-phosphate urydylyltransferase (UGP). UDP-glucose is used by glycogen synthase (GS) to elongate an anchorage molecule constituted of glycogenin and a glucose residue attached by a branching enzyme (BE). Glycogen synthase and branching enzymes collaborate in the formation of glycogen, also called glycogenesis. The reverse process that hydrolyzes glycogen into G1P via glycogen phosphorylase (GP) and debranching enzymes (DBE) is called glycogenolysis. Adapted from3.
Figure 1. Glycogen metabolism: overview of the synthesis and degradation of glycogen. Glucose enters into the cell cytoplasm through glucose transporters (GLUTs) for transformation into glucose-6-phosphate by hexokinases 1 and 2 (HK). Glucose-6-phosphate (G6P) is at the junction between glycolysis, the pentose phosphate pathway and glycogenesis. Phosphoglucomutase (PGM) is the first enzyme in glycogenesis that catalyzes the conversion of G6P into glucose 1-phosphate (G1P), which is then transformed into UDP-glucose by glucose-1-phosphate urydylyltransferase (UGP). UDP-glucose is used by glycogen synthase (GS) to elongate an anchorage molecule constituted of glycogenin and a glucose residue attached by a branching enzyme (BE). Glycogen synthase and branching enzymes collaborate in the formation of glycogen, also called glycogenesis. The reverse process that hydrolyzes glycogen into G1P via glycogen phosphorylase (GP) and debranching enzymes (DBE) is called glycogenolysis. Adapted from3.
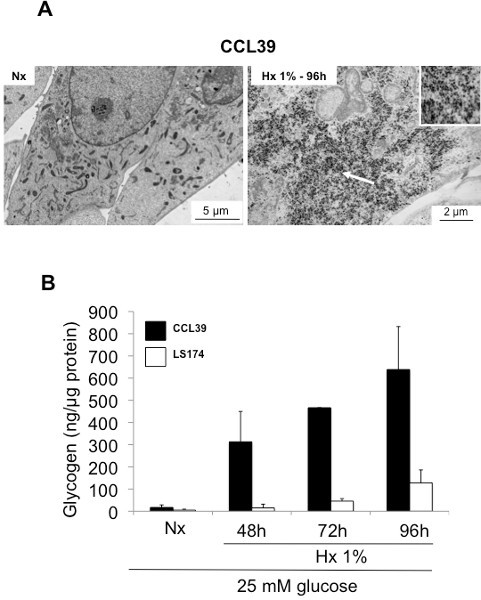 Figure 2. Accumulation of glycogen in hypoxia in non cancer cells and cancer cells. (A) Electron micrografts of CCL39 in normoxia (Nx)(left panel) and hypoxia 1% O2 (Hx 1% 96 hr) (right panel). Arrows denote aggregates of glycogen particles. (B) Quantitation of the amount of glycogen in CCL39 (black bars) and LS174 (white bars) cells grown in normoxia (Nx) or hypoxia 1% O2 (Hx 1%) for 48, 72, and 96 hr in a 25 mM glucose-containing medium.
Figure 2. Accumulation of glycogen in hypoxia in non cancer cells and cancer cells. (A) Electron micrografts of CCL39 in normoxia (Nx)(left panel) and hypoxia 1% O2 (Hx 1% 96 hr) (right panel). Arrows denote aggregates of glycogen particles. (B) Quantitation of the amount of glycogen in CCL39 (black bars) and LS174 (white bars) cells grown in normoxia (Nx) or hypoxia 1% O2 (Hx 1%) for 48, 72, and 96 hr in a 25 mM glucose-containing medium.
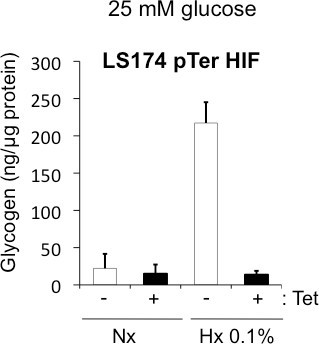 Figure 3. Accumulation of glycogen in hypoxia is dependent on HIF-1. Quantification of the glycogen amount in LS174 pTerHIF-1α. These cells are inducible clones expressing a shRNA against HIF-1α in condition of tetracycline. Cells grew in normoxia (Nx) (white bars) or hypoxia 0.1% O2 (Hx 0.1% - black bars) in the absence (-) or presence (+) of tetracycline (Tet).
Figure 3. Accumulation of glycogen in hypoxia is dependent on HIF-1. Quantification of the glycogen amount in LS174 pTerHIF-1α. These cells are inducible clones expressing a shRNA against HIF-1α in condition of tetracycline. Cells grew in normoxia (Nx) (white bars) or hypoxia 0.1% O2 (Hx 0.1% - black bars) in the absence (-) or presence (+) of tetracycline (Tet).
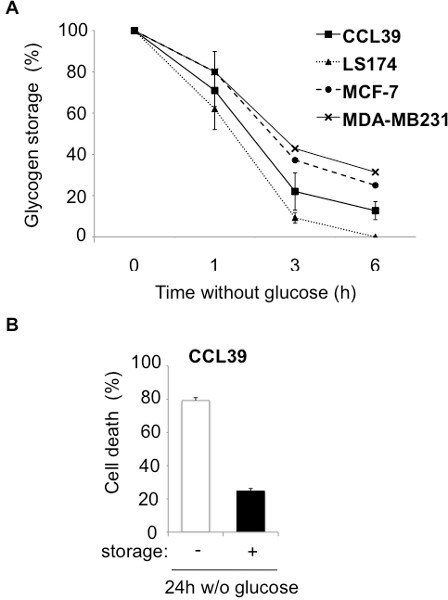 Figure 4. Glycogen storage protects from cell death. (A), CCL39 (■), LS174 (▲), MCF-7 (●), and MDA-MB231 (x) cells were subjected to 1% O2 hypoxia for 96 hr and then cultured in glucose-free medium for 6 hr. The glycogen concentration was measured just before removal of glucose and represents 100%. Glycogen was then measured at 1, 3, and 6 hr. The results are representative of at least three separate experiments. (B) Measurement of cell death in CCL39 cells. Cells were subjected to either normoxia (- storage) or hypoxia (+ storage) for 96 hr and incubated in glucose-free medium in hypoxia for 24 hr before measuring cell death.
Figure 4. Glycogen storage protects from cell death. (A), CCL39 (■), LS174 (▲), MCF-7 (●), and MDA-MB231 (x) cells were subjected to 1% O2 hypoxia for 96 hr and then cultured in glucose-free medium for 6 hr. The glycogen concentration was measured just before removal of glucose and represents 100%. Glycogen was then measured at 1, 3, and 6 hr. The results are representative of at least three separate experiments. (B) Measurement of cell death in CCL39 cells. Cells were subjected to either normoxia (- storage) or hypoxia (+ storage) for 96 hr and incubated in glucose-free medium in hypoxia for 24 hr before measuring cell death.
Discussion
Biochemical titration of glycogen in vitro allows an accurate quantification of cells glycogen content. Comparing to some other techniques (PAS, immunofluorescence with a glycogen antibody, etc.), this titration is very specific, sensitive and reproducible. Moreover, the method is convenient since it requires no radioactivity but a fluorescence spectrometer. However, this technique is purely quantitative and does not provide information about glycogen distribution in the cell.
The technique described in this manuscript is achieved on cell extracts but it can also be achieved on tissue sections with an appropriate method for extraction of glycogen from tissues. This assay is used for in vitro applications and due to an indirect measurement of glycogen (after hydrolysis into glucose), it can not be developed to follow glycogen stores in vivo.
PAS staining is already widely used to diagnose many glycogen storage diseases (von Gierke's disease, Cori's disease, McArdle's disease, etc.). It is also used to detect fungal infections or to discriminate between different subtypes of tumors. In theory, PAS staining could be coupled to an image analyzer to determine the glycogen concentration. In practice, this technique is difficult to perform and is less appropriate than the method described here for the following reasons. Firstly, as PAS positive staining (violet) overlaps with the negative staining (Hematoxylin-blue), it is technically difficult to exclude the negative signal without removing the positive signal. In addition, PAS staining is not reproducible enough to quantify glycogen, because the intensity of the color can depend on the time of fixation, efficacy of coloration, washing, etc. Finally, this biochemical methodology gives an average concentration of glycogen for a large number of cells, while PAS staining focuses on a specific field and therefore is less representative of the overall glycogen concentration.
The biochemical assay of glycogen could provide very accurate and informative data on the level of glycogen in tissue. These data could for example be hypothetically correlated with disease progression, or may help to understand the effects of treatment on glycogen metabolism. On the other hand, this technique requires several steps (protein quantitation, hydrolysis of glycogen and a minimum of three fluorescence readings per sample), and seems less practical than the PAS staining. For a better understanding of the physiological or pathophysiological metabolism (cancer, etc.), it is important to precisely quantify the glucose (and ATP) provided by glycogen. However, glycogen quantification alone is not sufficient to understand glycogen metabolism and must be coupled with microscopy to determine the distribution of glycogen in cells.
It is important to point out that despite the accuracy and reproducibility of this assay, a small variation in the protein content can lead to large variations of normalized values of glycogen. In addition, although there is a linear increase in the fluorescence with glycogen concentration, the linearity is not maintained at high concentration for which the signal drops dramatically. Thus, we recommend not to take into account the fluorescence values beyond concentrations of the calibration curve. We therefore recommend performing two readings with two different sample volumes. In this way the fluorescence reflects the glycogen in the cells and is not offset by a decrease in fluorescence.
Disclosures
The authors declare no conflict of interest.
Acknowledgments
We are grateful to the Dr. Thierry Pourcher for allowing us to use the fluorescence spectrometer and for his help. The laboratory is funded by the Ligue Nationale Contre le Cancer (équipe labellisée), the Association pour la Recherche contre le Cancer, the Institut National du Cancer (INCa), the Agence Nationale pour la Recherche, METOXIA (EU program FP7), the Centre A. Lacassagne, the Centre National de la Recherche Scientifique, the Institut National de la Santé et de la Recherche Médicale and the University of Nice (http://www.unice.fr/isdbc/). We thank Dr. M Christiane Brahimi-Horn for critical reading and editorial correction.
References
- Pelletier J, et al. Glycogen Synthesis is Induced in Hypoxia by the Hypoxia-Inducible Factor and Promotes Cancer Cell Survival. Front Oncol. 2012;2 doi: 10.3389/fonc.2012.00018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alonso MD, Lomako J, Lomako WM, Whelan WJ. A new look at the biogenesis of glycogen. FASEB J. 1995;9:1126–1137. doi: 10.1096/fasebj.9.12.7672505. [DOI] [PubMed] [Google Scholar]
- Bollen M, Keppens S, Stalmans W. Specific features of glycogen metabolism in the liver. Biochem. J. 1998;336(Pt 1):19–31. doi: 10.1042/bj3360019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parker GJ, Koay A, Gilbert-Wilson R, Waddington LJ, Stapleton D. AMP-activated protein kinase does not associate with glycogen alpha-particles from rat liver. Biochem. Biophys. Res. Commun. 2007;362:811–815. doi: 10.1016/j.bbrc.2007.08.080. [DOI] [PubMed] [Google Scholar]
- Sullivan MA, et al. Nature of alpha and beta particles in glycogen using molecular size distributions. Biomacromolecules. 2010;11:1094–1100. doi: 10.1021/bm100074p. [DOI] [PubMed] [Google Scholar]
- Chee NP, Geddes R. The structure of liver glycogen. FEBS Lett. 1977;73:164–166. doi: 10.1016/0014-5793(77)80972-1. [DOI] [PubMed] [Google Scholar]
- Roach PJ, Depaoli-Roach AA, Hurley TD, Tagliabracci VS. Glycogen and its metabolism: some new developments and old themes. Biochem. J. 2012;441:763–787. doi: 10.1042/BJ20111416. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moses SW, Bashan N, Gutman A. Glycogen metabolism in the normal red blood cell. Blood. 1972;40:836–843. [PubMed] [Google Scholar]
- Agbanyo M, Taylor NF. Incorporation of 3-deoxy-3-fluoro-D-glucose into glycogen and trehalose in fat body and flight muscle in Locusta migratoria. Biosci. Rep. 1986;6:309–316. doi: 10.1007/BF01115160. [DOI] [PubMed] [Google Scholar]
- Louzao MC, et al. "Fluorescent glycogen" formation with sensibility for in vivo and in vitro detection. Glycoconj. J. 2008;25:503–510. doi: 10.1007/s10719-007-9075-7. [DOI] [PubMed] [Google Scholar]
- Sheehan DCH, B B. Theory and practice of histotechnology. 2nd edition. Batelle Press; 1980. [Google Scholar]
- Baba O. Production of monoclonal antibody that recognizes glycogen and its application for immunohistochemistry. Kokubyo Gakkai Zasshi. 1993;60:264–287. doi: 10.5357/koubyou.60.264. [DOI] [PubMed] [Google Scholar]


