Recent articles in Science by Liu and Imlay (2013), and Keren and colleagues (2013) question whether bactericidal antibiotics in Escherichia coli act via the production of reactive oxygen species (ROS). This hypothesis contradicts the view, proposed by Kohanski and colleagues (2007), that bactericidal antibiotics kill bacteria through hydroxyl radical-mediated DNA damage. Kohanski and colleagues (2007) came to this conclusion because in E. coli, bactericidal compounds, but not bacteriostatic compounds, lead to the quenching of intrinsic hydroxyphenyl fluorescein (HPF) dye fluorescence. Furthermore, the authors assumed that this quenching was due to the production of hydroxyl radicals. This belief in an ROS-mediated bactericidal mechanism has now been challenged (Keren et al., 2013; Liu and Imlay, 2013) through recent observations where bactericidal activity was not disrupted in the absence of oxygen, bactericidal antibiotics do not induce hydrogen peroxide formation and no oxidative stress response is provoked. Liu and Imlay (2013) also proposed that the observed HPF quenching by bactericidal antibiotics is due to oxidation of the dye by high-valence iron (FeO2+) initially formed by the Fenton reaction rather than the hydroxyl radical decomposition product.
Pseudomonas putida is resistant to the bactericidal activity of ampicillin and the bacteriostatic activity of chloramphenicol. This resistance is achieved via TtgABC efflux pump-mediated extrusion of the antibiotics (Godoy et al., 2010). Global transcriptional array experiments were carried out and show that similarly, the oxidative stress programme is not initiated in these cells when exposed to the antibiotics (Fernandez et al., 2012). HPF dye fluorescence quenching was also observed, but only in the presence of ampicillin and not chloramphenicol (Molina-Santiago et al., 2013). In a TtgABC-deficient mutant, the burst of HPF fluorescence quenching was enhanced. When a secondary antibiotic efflux pump, named TtgGHI (Fernandez et al., 2012), was induced by indole, a decrease in fluorescence quenching was observed (Molina-Santiago et al., 2013) – thus confirming that efflux pump-mediated extrusion of antibiotics influence the Fenton reaction (Fig. 1). Therefore, the hypothesis performed by Liu and Imlay (2013) and Keren and colleagues (2013) where ROS are not exclusively responsible for antimicrobial activity produced by bactericidal compounds can be expanded to other gammaproteobacteria according to our results. Future research is required to determine whether the high-valence FeO2+ formed in the presence of bactericidal compounds enhances killing in addition to the direct inhibition of cell wall assembly, protein synthesis or interference with DNA metabolism.
Figure 1.
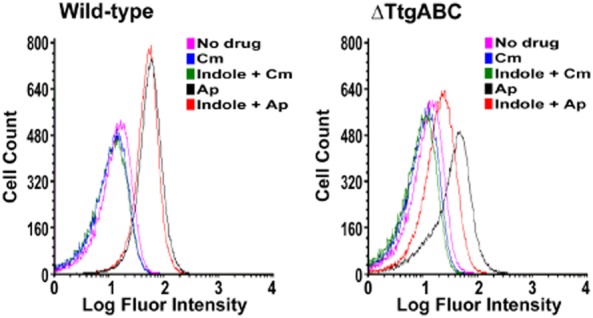
HPF fluorescence quenching in P. putida DOT-T1E and DOT-T1E-18 (ΔTtgABC) in the presence and absence of 300 μM indole following exposure to 300 or 150 μg ml−1 chloramphenicol (Cm), or 800 or 200 μg ml−1 ampicillin (Ap) respectively. Pink, no drug; blue, Luria-Bertani (LB) + chloramphenicol; green, indole + chloramphenicol; black, LB + Ap; red, indole + Ap.
Conflict of interest
None declared.
Funding Information
No funding information provided.
References
- Fernandez M, Conde S, Torre de la J, Molina-Santiago C, Ramos JL, Duque E. Mechanisms of resistance to chloramphenicol in Pseudomonas putida KT2440. Antimicrob Agents Chemother. 2012;56:1001–1009. doi: 10.1128/AAC.05398-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Godoy P, Molina-Henares AJ, Torre de la J, Duque E, Ramos JL. Characterization of the RND family of multidrug efflux pumps: in silico to in vivo confirmation of four functionally distinct subgroups. Microb Biotechnol. 2010;3:691–700. doi: 10.1111/j.1751-7915.2010.00189.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Keren I, Wu Y, Inocencio J, Mulcahy LR, Lewis K. Killing by bactericidal antibiotics does not depend on reactive oxygen species. Science. 2013;339:1213–1216. doi: 10.1126/science.1232688. [DOI] [PubMed] [Google Scholar]
- Kohanski MA, Dwyer DJ, Hayete B, Lawrence CA, Collins JJ. A common mechanism of cellular death induced by bactericidal antibiotics. Cell. 2007;130:797–810. doi: 10.1016/j.cell.2007.06.049. [DOI] [PubMed] [Google Scholar]
- Liu Y, Imlay JA. Cell death from antibiotics without the involvement of reactive oxygen species. Science. 2013;339:1210–1213. doi: 10.1126/science.1232751. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Molina-Santiago C, Daddaoua A, Fillet S, Duque E, Ramos JL. Interspecies signaling: Pseudomonas putida efflux pump TtgGHI is activated by indole to increase antibiotic resistance. Environ Microbiol. 2013 doi: 10.1111/1462-2920.12368. doi: 10.1111/1462-2920.12368. [DOI] [PubMed] [Google Scholar]


