Abstract
Anti-neutrophil cytoplasmic antibody-associated vasculitis (AAV) treatment strategy is based on immunosuppressive agents. Little information is available concerning mycophenolic acid (MPA) and the area under the curve (AUC) in patients treated for AAV. We evaluated the variations in pharmacokinetics for MPA in patients with AAV and the relationship between MPA–AUC and markers of the disease. MPA blood concentrations were measured through the enzyme-multiplied immunotechnique (C0, C30, C1, C2, C3, C4, C6 and C9) to determine the AUC. Eighteen patients were included in the study. The median (range) MPA AUC0–12 was 50·55 (30·9–105·4) mg/h/l. The highest coefficient of determination between MPA AUC and single concentrations was observed with C3 (P < 0·0001) and C2 (P < 0·0001) and with C4 (P < 0·0005) or C0 (P < 0·001). Using linear regression, the best estimation of MPA AUC was provided by a model including C30, C2 and C4: AUC = 8·5 + 0·77 C30 + 4·0 C2 + 1·7 C4 (P < 0·0001). Moreover, there was a significant relationship between MPA AUC0-12 and lymphocyte count (P < 0·01), especially CD19 (P < 0·005), CD8 (P < 0·05) and CD56 (P < 0·05). Our results confirm the interindividual variability of MPA AUC in patients treated with MMF in AAV and support a personalized therapy according to blood levels of MPA.
Keywords: anti-neutrophil cytoplasmic antibody-associated vasculitis, mycophenolate mofetil, pharmacokinetics
Introduction
The treatment of anti-neutrophil cytoplasmic antibody-associated vasculitis (AAV) is based on immunosuppressive agents. In most cases, cyclophosphamide and corticosteroids are used as gold standard induction, and recent findings have promoted the use of azathioprine to maintain remission 1. However, mycophenolate mofetil (MMF) has proved to be an alternative therapy for induction or maintenance of remission in patients with AAV with or without renal involvement and is used off-label as a second line of treatment 2–6.
MMF was first introduced in solid organ transplantation and, more recently, prescribed in autoimmune diseases (AID) such as systemic lupus erythematosus (SLE) [7–10], rheumatoid arthritis, psoriatic arthritis, polymyositis, dermatomyositis, inclusion body myositis and systemic sclerosis 11,12. Initially the recommended dose of MMF for solid organ recipients was 1 g MMF twice daily, regardless of weight 13. More recent pharmacokinetic studies have reported high interindividual variability of mycophenolic acid (MPA) plasma concentrations for a given dose, depending on co-medication, renal function and genetic polymorphism 14–18. Therapeutic drug monitoring (TDM) has therefore been recommended particularly in renal graft recipients, because of potential interaction with cyclosporin.
MPA is the active metabolite of MMF and a patient's exposure to MPA is considered to be predictive of the therapeutic response: an area under the plasma concentration versus time curve from 0 to 12 h (AUC0–12) of 30–60 mg/h/l [when measured with a high-performance liquid chromatography (HPLC) technique] 19 or 35–70 mg/h/l [when measured with an enzyme-multiplied immunotechnique (EMIT)] has been proposed for renal graft recipients 20. Only one AID study involving SLE has focused upon the AUC0–12 that should be aimed at to achieve low disease activity. The target range was 35 mg/h/l using the EMIT technique 21. Otherwise, practitioners generally consider that the dose and the target AUC0–12 range should be the same in AID as in renal graft recipients.
As undertaken previously with SLE 22, we conducted a prospective study to evaluate interindividual clinical and biological variations in MPA pharmacokinetics in patients treated for AAV in our institution. The aim was to predict MPA pharmacokinetics through a limited sampling strategy (LSS), and to investigate potential relationships between MPA blood concentrations and immune response, disease activity, co-medication, side effects and biochemical parameters.
Material and methods
Patients
All patients included in the study were treated at the local University Hospital of Tours (Departments of Nephrology and Clinical Immunology and Internal Medicine). Diagnosis of AAV was based on the American College of Rheumatology 1990 classification criteria or the Chapel Hill Consensus Conference 1994 for the classification of vasculitis. Patients treated with MMF (Cellcept®; Roche, Nutley, NJ, USA) for at least 2 weeks were eligible for the pharmacokinetics study. Patients who were on dialysis or who had received kidney transplantation were not excluded.
On the day of the study all patients underwent clinical examination. All clinical signs potentially related to a flare-up of AAV were examined (cutaneous, rheumatological, neurological, pulmonary and cardiac signs). Total blood cell count, C-reactive protein, liver function, fasting plasma albumin and creatinine serum concentrations were measured by standard immunospectrophotometric methods. Creatinine clearance was estimated according to the Modification of Diet in Renal Disease Study (MDRD) formula 23. Urinary sediment and proteinuria were measured. Disease activity was assessed with the Birmingham Vasculitis Activity Score (BVAS) 2003 24. Anti-neutrophil cytoplasmic antibodies (ANCA) were also screened using indirect immunofluorescence (Inova Kit; Inova Diagnostics, Inc., San Diego, CA, USA); then antibody specificity for proteinase 3 (PR3) or myeloperoxidase (MPO) was confirmed by enzyme-linked immunosorbent assay (ELISA) (ELISA ImmunoCAP Phadia 250; Phadia, Vienna, Austria). Patients were asked to report any gastrointestinal symptoms or past medical history of tumour or infection. All co-medications taken by patients were also reported.
Ethics information
All patients gave written informed consent to participate in this study. In our centre, MPA blood concentrations are checked routinely (mainly trough concentrations). Therefore, ethical approval was not sought.
Pharmacokinetics
After 12 h overnight fasting, each patient had blood samples (5 ml) drawn on ethylenediamine tetraacetic acid (EDTA) immediately before intake of MMF and 30 min (C30), 1 (C1), 2 (C2), 3 (C3), 4 (C4), 6 (C6) and 9 h (C9) after treatment intake. The enterohepatic cycle was thus taken into account. The dose of MMF was not modified for the study and previous dose modifications were reported. Daily treatment was given at the same time as MMF. MPA blood concentrations were measured using the EMIT technique (EMIT–MPA; Dade-Behring Diagnostics, Paris La Défense, France). Free MPA fractions were not measured.
The area under the curve (AUC) from 0 to 9 h (AUC0–9) was calculated by the trapezoidal method. The AUC from 0 to 12 h (AUC0–12) was estimated using the same method, considering the C0 level as the C12 level. The dose-normalized AUC0–12 was calculated by dividing the AUC0–12 by the MMF intake dose.
Predictive linear models of AUC based on MPA concentration were developed using the multiple linear regression approach based on a limited number of MPA concentrations. Multiple linear regression models of AUC trapezoid estimates (independent variable) with each concentration (dependent variable) were based on equations in the form AUC = β0 + β1C1 + β2C2 + βnCn, where β are coefficients to be estimated and n is the number of samples. Only the first five samples collected were used for the model in order to make it acceptable in clinical practice.
Immune response study protocol
Total blood count (LH 785 Beckman Coulter®; Brea, CA, USA), complement system (Siemens nephelometry kit, Marburg, Germany), serum protein electrophoresis, serum immunoglobulin (Ig) levels (Nephelometry DN ProSpec®, Marburg, Germany) and lymphocyte immunophenotyping (flow cytometry; Beckman Coulter EPICS XL MCL®) were performed after 12-h fasting and before intake of mycophenolate mofetil and standard medication.
Statistical analyses
The results are expressed as median and (range). Spearman's rank coefficient was used to test correlations. Continuous variables were compared with the Mann–Whitney–Wilcoxon test. A P-value of less than 0·05 was considered significant.
Models linking AUC and concentrations were analysed using r software (version 2·14·1; R Foundation, Vienna, Austria) and were compared using a Fisher–Snedecor (F) test. The best model was the one that provided no significant F-test when compared to more a complicated model. A P-value less than 0·05 was considered significant. Similarly, this model was the one that provided the highest significance in R2 coefficient.
Results
Patient characteristics
Between 2005 and 2011, 99 patients were treated for AAV at our institution, of whom 26 were treated with MMF. One patient declined to participate, six were lost to follow-up and one was excluded because he had taken his treatment before the first blood sample was drawn on the day of the study. Thus, a total of 18 patients were enrolled. The characteristics of the patients enrolled are shown in Table 1. All patients were Caucasian. Eight patients had granulomatosis with polyangiitis, nine patients had microscopic polyangiitis and one had x-ANCA-associated vasculitis. There was no patient with eosinophilic granulomatosis with polyangiitis. Four patients had received a kidney transplant, one 2 weeks before the study, one 7 years before, another 10 years before and the other 20 years before the study. Two patients were on dialysis. Eight patients had been treated previously with cyclophosphamide (CYC), two patients with azathioprine, two patients with intravenous immunoglobulins, two patients with rituximab (RTX) and one patient with plasma exchange. The median (range) time interval between previous use of CYC and the study was 65 (1–135) months. All patients had received steroids as treatment for vasculitis and only two of them were not on steroids on the day of the pharmacokinetics study.
Table 1.
Personal, clinical, biochemical and pharmacokinetic characteristics of patients from whom mycophenolic acid trough levels were collected
| Characteristic | n = 18 | |
|---|---|---|
| Personal characteristics | ||
| Gender (female/male) | 8/10 | |
| Age (years) | 66 (30–86) | |
| Weight (kg) | 68·5 (46–97) | |
| Caucasian | 18 | |
| ANCA vasculitis | ||
| Disease duration (years) | 5·76 (0·14–14·34) | |
| BVAS score | 0 | |
| ANCA specificity (UI/ml) | PR3-ANCA (8/18) | 21·50 (0–40·00) |
| MPO-ANCA (9/18) | 3·40 (0·10–18·00) | |
| x-ANCA (1/18) | – | |
| Biochemical | ||
| Creatinine (mg/l) (n = 16)* | 14·6 (9·3–36·3) | |
| eGFR (MDRD formula) (n = 16)* | 45·5 (13·0–75·0) | |
| Proteinuria (g/l) (n = 16)* | 0·23 (0·09–1·39) | |
| Serum albumin (g/l) | 41·0 (34·0–45·0) | |
| Haemoglobin (g/dl) | 118 (84–146) | |
| White blood cells (cells/μl) | 7450 (4800−10400) | |
| Neutrophils (cells/μl) | 5135 (2420–8790) | |
| Lymphocytes (cells/μl) | 1210 (330–2480) | |
| AST (IU/l) | 17 (12–52) | |
| CRP (mg/l) | 2·3 (0–16·9) | |
| Pharmacokinetic | ||
| C0 (mg/l) | 2·40 (0·5–10·8) | |
| AUC0–9 (mg/h/l) | 41·00 (24·6–82·6) | |
| AUC0–12 (mg/h/l) | 50·55 (30·9–105·4) | |
| Dose normalized AUC0–12 (mg/h/l) | 40·75 (20·6–98·8) | |
Median values and ranges are shown. eGFR: estimated glomerular filtration rate; AST: aspartate aminotransferase; CRP: C-reactive protein; AUC: area under the curve; MDRD: Modification of Diet in Renal Disease Study; ANCA: anti-neutrophil cytoplasmic antibodies; BVAS: Birmingham Vasculitis Activity Score; PR3: proteinase 3.
Without patients on dialysis.
MMF treatment and side effects
Patients had received MMF for 1·84 (15 days–10·3 years) years at a dose of 1·50 (0·50–2·00) g/day in two doses. MMF was used in 14 patients as maintenance therapy and in four patients to prevent allograft rejection. The MMF dose had been modified in 11 patients prior to the study. The reasons for dose modification included intestinal side effects (n = 1), low AUC (n = 2), high AUC (n = 3), disease relapses (n = 3), steroid tapering (n = 1) and introduction of sirolimus (n = 1).
Pharmacokinetics
The pharmacokinetics profiles for the 18 patients are shown in Fig. 1. As shown in Table 1, the median (range) MPA AUC0–9 was 41·00 (24·6–82·6) mg/h/l. The median (range) MPA AUC0–12 was 50·55 (30·9–105·4) mg/h/l. The median (range) dose-normalized MPA AUC was 40·75 (20·6–98·8) mg/h/l. No significant difference was observed for patients who had received a kidney transplant (P = 0·65) or for patients on dialysis (P = 0·47) compared to the remaining patients.
Figure 1.
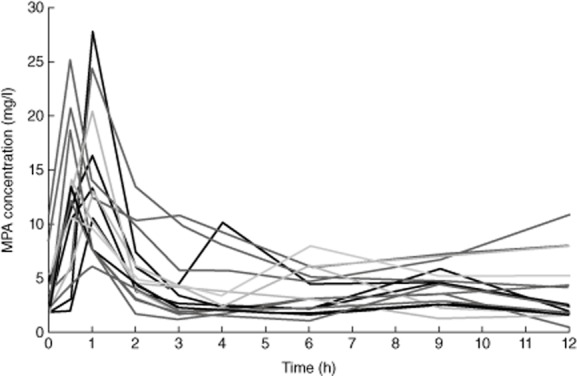
Mycophenolic acid (MPA) concentrations in 18 patients treated with mycophenolate mofetil in anti-neutrophil cytoplasmic antibodies (ANCA)-associated vasculitis.
Except for haemoglobin (r = −0·57; P < 0·05), there was no significant relationship between MPA and clinical or biochemical parameters (Supporting information, Table S1).
Limited-sample strategy model building
As shown in Table 2, the most significant coefficient of determination between MPA AUC and a single blood concentration was observed first with C3 (R2 = 0·819, P < 0·0001) and C2 (R2 = 0·784, P < 0·0001) and then with C4 (R2 = 0·591, P < 0·0005) or C0 (R2 = 0·519, P < 0·001). Using linear regression, the best linear model predicting AUC by MPA concentrations included concentrations at 30 min and 2 and 4 h: AUC = 8·5 + 0·77 C30 + 4·0 C2 + 1·7 C4 (R2 = 0·9292, P < 0·0001). All other three-point models including C30, C2, C3 or C4 also showed a satisfactory correlation.
Table 2.
Limited sample stratification of mycophenolic acid (MPA) concentration
| Concentration | R2 | SSR | P | Multicollinearity |
|---|---|---|---|---|
| CO | 0·519 | 2965 | 0·0008 | – |
| C30 | 0·284 | 4410 | 0·0229 | – |
| C1 | 0·102 | 5529 | 0·1962 | – |
| C2 | 0·784 | 1331 | <0·0001 | – |
| C3 | 0·819 | 1118 | <0·0001 | – |
| C4 | 0·591 | 2519 | 0·0002 | – |
| C0–C30 | 0·695 | 1880 | 0·0001 | |
| C0–C2 | 0·842 | 973,2 | <0·0001 | No |
| C0–C3 | 0·859 | 868 | <0·0001 | No |
| C0–C4 | 0·695 | 1880 | 0·0001 | No |
| C30–C2 | 0·893 | 658 | <0·0001 | No |
| C30–C3 | 0·878 | 750 | <0·0001 | No |
| C30–C4 | 0·645 | 2186 | 0·0004 | No |
| C2–C3 | 0·847 | 951 | <0·0001 | Yes |
| C2–C4 | 0·869 | 803 | <0·0001 | No |
| C3–C4 | 0·825 | 1078 | <0·0001 | No |
| C0–C30–C2 | 0·894 | 653 | <0·0001 | No |
| C0–C30–C3 | 0·882 | 725 | <0·0001 | No |
| C0–C30–C4 | 0·698 | 1857 | 0·0006 | No |
| C0–C2–C3 | 0·88 | 740 | <0·0001 | Yes |
| C0–C2–C4 | 0·889 | 682 | <0·0001 | No |
| C0–C3–C4 | 0·86 | 860 | <0·0001 | No |
| C30–C2–C3 | 0·919 | 496 | <0·0001 | Yes |
| C30–C2–C4 | 0·928 | 441 | <0·0001 | No |
| C30–C3–C4 | 0·878 | 749 | <0·0001 | No |
| C2–C3–C4 | 0·872 | 786 | <0·0001 | Yes |
SSR: Sum of Squares due to Regression.
Relationship between immune parameters and MPA
Total white cell blood count was 7450 leucocytes/μl (4800–10400); 5135 neutrophils/μl (2420–8790) and 1210 lymphocytes/μl (330–2480) (Table 1). As shown in Table 3, the median (range) serum immunoglobulin levels were IgA 1·25 g/l (0·53–2·76); IgG 7·12 g/l (2·93–14·20); IgM 0·42 g/l (0·09–2·69). Immunophenotyping of lymphocytes revealed CD19+ lymphocytes 71/mm3 (1–541); CD3+ lymphocytes 955/mm3 (250–1846); CD4+ lymphocytes 718/mm3 (149–1555); CD8+ lymphocytes 346/mm3 (94–943); CD56+ lymphocytes 106/mm3 (23–498), and the CD4/CD8 ratio was 1·88 (0·48–5·98). Complement system fractions were C3 0·98 g/l (0·74–1·51) and C4 0·26 g/l (0·13–0·42).
Table 3.
Correlation tests between patient immune response parameters and mycophenolic acid (MPA) pharmacokinetics [area under the curve (AUCT0–T12)]
| Immune response parameters | n = 18 | r | P |
|---|---|---|---|
| Immunoglobulins | |||
| IgA (g/l) | 1·25 (0·53–2·76) | −0·243 | 0·348 |
| IgG (g/l) | 7·12 (2·93−14·20) | −0·213 | 0·410 |
| IgM (g/l) | 0·42 (0·09–2·69) | −0·300 | 0·243 |
| Lymphocyte subsets | |||
| CD19 (mm3) | 71 (1–541) | −0·63 | <0·005 |
| CD3 (mm3) | 955 (250–1846) | −0·17 | 0·498 |
| CD4 (mm3) | 718 (149–1555) | −0·24 | 0·330 |
| CD8 (mm3) | 346 (94–943) | −0·53 | <0·05 |
| CD56 (mm3) | 106 (23–498) | −0·56 | <0·05 |
| CD4/CD8 ratio | 1·88 (0·48–5·98) | 0·168 | 0·503 |
| Complement system fractions | |||
| C3 (g/l) | 0·98 (0·74–1·51) | −0·375 | 0·126 |
| C4 (g/l) | 0·26 (0·13–0·42) | −0·184 | 0·465 |
Ig: immunoglobulin; r: Spearman's rank coefficient.
There was a significant correlation between MPA AUC0–12 and total lymphocyte count (r = −0·59, P < 0·01). This was more pronounced for CD19+ lymphocytes (r = −0·63, P < 0·005), CD8+ lymphocytes (r = −0·53, P < 0·05) and CD56+ lymphocytes (r = −0·56, P < 0·05), whereas no correlation was observed with other subtypes of lymphocytes including CD3+ lymphocytes (r = −0·17, P = 0·498), CD4+ lymphocytes (r = −0·24, P = 0·330) or the CD4/CD8 ratio (r = 0·168, P = 0·503) (Fig. 2). Moreover, no correlation was found between MPA AUC and leucocytes, neutrophils, immunoglobulin levels or complement system fractions (Table 3 and Table S1).
Figure 2.
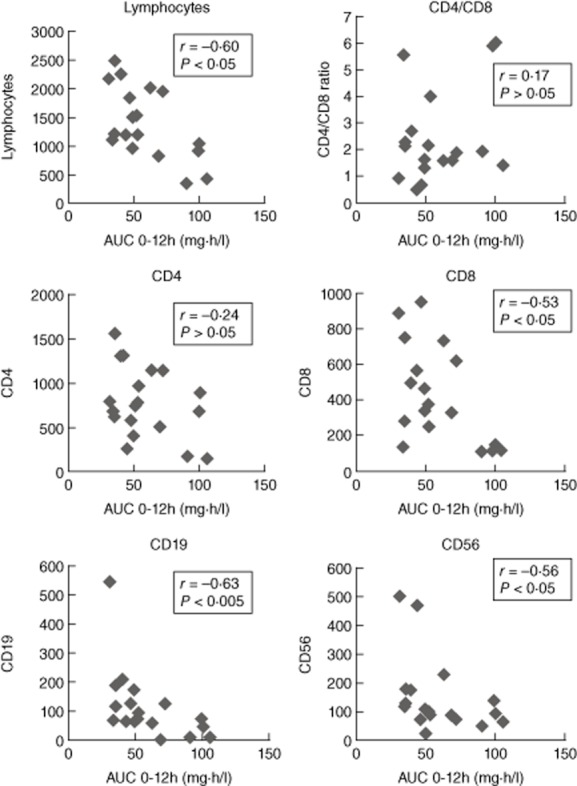
Correlations between mycophenolic acid (MPA) [area under the curve (AUC)0–12] and lymphocyte subpopulations in 18 patients treated with mycophenolate mofetil.
Patients (n = 4) who had MPA AUC more than 75 mg/h/l had fewer than 1100 lymphocytes (P < 0·01), fewer than 200 CD8+ lymphocytes (P < 0·005), fewer than 50 CD19+ lymphocytes (P < 0·05) and fewer than 150 CD56+ lymphocytes (P > 0·05) (Fig. 2).
Patients who had previously received CYC had fewer CD3+ lymphocytes (P < 0·05) and fewer CD4+ lymphocytes (P < 0·05), but there was no significant difference in CD8+ lymphocyte count, CD19+ lymphocyte count or CD56+ lymphocyte count (data not shown), compared to other patients.
No significant difference in lymphocyte count was observed for patients who had received a kidney transplant or for patients on dialysis (data not shown).
Relationship between disease activity and MPA
On the day of the study, the median (range) disease duration was 5·76 (0·14–14·34) years, nine patients had ANCA MPO specificity 3·40 (0·10–18·00) UI/ml, eight patients had ANCA PR3 specificity 21·50 (0–40·00) UI/ml and one had x-ANCA. The BVAS score median value was 0 (0–5). Proteinuria was detected in all patients [median 0·23 (0·09–1·39) g/l)] and four of them had microscopic haematuria.
There was no correlation between AUC and ANCA PR3 specificity level (r = −0·14; P = 0·729) or with ANCA MPO specificity level (r = −0·4352; P = 0·242) or with BVAS score (r = 0·01; P = 0·96) (Supporting information, Table S1).
Discussion
In this study we showed an interindividual variability of MPA pharmacokinetics, and proposed a limited sample strategy (LSS) to evaluate MPA AUC that was correlated with peripheral lymphocyte count of patients treated with MMF in AAV.
We founded a high level of interindividual variability of MPA pharmacokinetics in patients with AAV, a finding that confirms the interpatient variability in MMF exposure. We did not find MPA AUC to be associated with demographic, clinical or biochemical parameters or with the dose of MMF. Such variability was reported recently to be associated with genetic polymorphisms including UGT1A7, UGT1A8, UGT1A9, UGT2B7 and IMPDH1 14,25–29. Previous studies were contradictory concerning the relationship between MPA AUC and renal function 15,29–31. Our findings support the absence of association between MPA AUC and renal function in AAV. Recent publications also suggest that MMF exposure could be influenced by co-medication such as proton-pump inhibitor 32–34. It thus appears that drug monitoring is needed in the follow-up of patients with AAV treated with MMF.
Clinicians may therefore need a limited sampling strategy to evaluate MPA AUC. We previously showed a correlation between MPA trough levels and AUC0–4 in patients with SLE 22. Another study reported a relationship between MPA 12-h trough level and AUC0–12 in patients with AID 35. In the present study, we did not find a strongly significant association between the trough plasma concentration and the AUC, which is in accordance with previous evaluation of the MPA AUC in renal transplant patients 36. We also showed that a single blood concentration approximately predicts MPA AUC in AAV and that at least three blood concentrations are needed to predict it with accuracy. The best models to estimate it always include C30 and C2. In our study, the most accurate model was obtained with C30, C2 and C4, the same as for Barraclough et al., who recently published a LSS incorporating concentration measurements at the same three time-points for the simultaneous estimation of tacrolimus, mycophenolic acid and unbound prednisolone exposure in kidney transplant recipients 37. It is notable that three-point models, including C0 or C3 instead of C4, also provide very good estimations.
Our study is the first pharmacological study to evaluate a possible influence of MMF pharmacokinetics on the immune system in patients with AAV. Our results suggest an influence of MPA concentrations on the total peripheral lymphocyte count, particularly on cytotoxic cells such as CD8 T lymphocytes and natural killer cells. CD8 T lymphocytes are known to be expanded in patients with active AAV 38. Moreover, a marked CD8 T lymphocyte expansion was shown to be associated with a poorer prognosis and a higher number of flare-ups and relapses of the disease, whereas ANCA title is not 39,40. The role of natural killer (NK) cells in vasculitis has been less fully described, but they might be associated with an increase in Toll-like receptor (TLR) activation that could result in disease reactivation 41,42. Interestingly, MMF may be a more powerful inhibitor of NK cells than cyclosporin, tacrolimus and methotrexate 43. In our population, all patients with a MPA AUC0–12 above 75 mg/h/l had fewer than 200 CD8 T lymphocytes and fewer than 200 CD56+ lymphocytes. There are no guidelines on the target range of MPA AUC in patients with AAV. Only one previous study advised a MPA trough level between 3·5 and 4·5 mg/l (EMIT) as the target range upon combined analysis of efficacy and safety 44. Therefore, a MPA AUC0–12 measured by the EMIT technique above 75 mg/h/l could be a target range in patients with AAV. This is somewhat higher than in renal transplant recipients. With regard to adverse events, there have been contradictory results concerning a hypothetical relationship between MPA exposure and toxicity in kidney transplant recipients 45. The 2011 Transplantation Society consensus meeting therefore gave no upper limit to the target range and it was advised to tailor the treatment to the individual relationship between MPA exposure and toxicity 45. Nevertheless, we should avoid MPA toxicity, particularly gastrointestinal events and anaemia.
Our study has several limitations. First, it involved a heterogeneous population in terms of renal status, duration of MMF treatment and circumstances of MMF initiation. However, pharmacokinetic and immunological parameters were recorded at the same time as MPA concentrations, and medical records were reviewed carefully for each patient. Moreover, we used the EMIT assay, which quantifies MPA as well as its active metabolite MPA-acyl glucuronide, whereas the HPLC technique allows more specific measurement of MPA only 46,47. Secondly, as in previous studies on the pharmacokinetics of MMF in AAV 35,48,49, our population included fewer than 30 patients and thus multivariate analyses regarding MMF and co-medication were not possible. Thirdly, we did not study the effect of steroids on lymphocyte count; such effect cannot be excluded. However, Tornatore et al. reported that lymphocyte count returned to baseline by 8–12 h after treatment with methylprednisolone 50. Gluhovschi et al. reported that CD3, CD3CD4, CD3CD8 lymphocyte subsets showed no change after 7 days of oral prednisone (1 mg/kg/day in two divided doses) in 16 patients with primary chronic glomerulonephritis 51. Lastly, it must be emphasized that except for cyclophosphamide we studied only the current dosage of MMF and immunosuppressive drugs on lymphocyte count. Further longitudinal studies, in which the cumulative dose of MMF and the potential effect of other immunosuppressive drugs will be taken into account, are needed to confirm these results.
In conclusion, this study adds support to the therapeutic drug monitoring of MMF and personalized therapy in AAV because of the intervariability of MPA pharmacokinetics. Such monitoring in AAV patients can be performed with only three concentrations.
Acknowledgments
None.
Disclosures
The authors declare no conflicts of interest.
Author contributions
B. C., P. G., F. D., C. B., D. D., M. F., P. S., N. R., G. G., E. D., Y. L., H. N., G. P., J.-M. H. and M. B. were involved in the conception or design or analysis and interpretation of data, or both; B. C., P. G., F. D., C. B., J.-M. H., L. G. and M. B. were involved in drafting the article or revising it. B. C., P. G., C. B., E. D., F. M., J.-M. H., L. G. and M. B. provided intellectual content of critical importance to the work described.
Supporting information
Additional Supporting information may be found in the online version of this article at the publisher's web-site:
Correlation tests between patient characteristics and mycophenolic acid (MPA) pharmacokinetics [area under the curve (AUC)0–12].
References
- 1.Hiemstra TF, Walsh M, Mahr A, et al. Mycophenolate mofetil vs azathioprine for remission maintenance in antineutrophil cytoplasmic antibody-associated vasculitis: a randomized controlled trial. JAMA. 2010;304:2381–2388. doi: 10.1001/jama.2010.1658. [DOI] [PubMed] [Google Scholar]
- 2.Iatrou C, Zerbala S, Revela I, et al. Mycophenolate mofetil as maintenance therapy in patients with vasculitis and renal involvement. Clin Nephrol. 2009;72:31–37. doi: 10.5414/cnp72031. [DOI] [PubMed] [Google Scholar]
- 3.Silva F, Specks U, Kalra S, et al. Mycophenolate mofetil for induction and maintenance of remission in microscopic polyangiitis with mild to moderate renal involvement – a prospective, open-label pilot trial. Clin J Am Soc Nephrol. 2010;5:445–453. doi: 10.2215/CJN.06010809. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Joy MS, Hogan SL, Jennette JC, et al. A pilot study using mycophenolate mofetil in relapsing or resistant ANCA small vessel vasculitis. Nephrol Dial Transplant. 2005;20:2725–2732. doi: 10.1093/ndt/gfi117. [DOI] [PubMed] [Google Scholar]
- 5.Langford CA, Talar-Williams C, Sneller MC. Mycophenolate mofetil for remission maintenance in the treatment of Wegener's granulomatosis. Arthritis Rheum. 2004;51:278–283. doi: 10.1002/art.20240. [DOI] [PubMed] [Google Scholar]
- 6.Nowack R, Göbel U, Klooker P, et al. Mycophenolate mofetil for maintenance therapy of Wegener's granulomatosis and microscopic polyangiitis: a pilot study in 11 patients with renal involvement. J Am Soc Nephrol. 1999;10:1965–1971. doi: 10.1681/ASN.V1091965. [DOI] [PubMed] [Google Scholar]
- 7.Ginzler EM, Dooley MA, Aranow C, et al. Mycophenolate mofetil or intravenous cyclophosphamide for lupus nephritis. N Engl J Med. 2005;353:2219–2228. doi: 10.1056/NEJMoa043731. [DOI] [PubMed] [Google Scholar]
- 8.Chan TM, Li FK, Tang CS, et al. Efficacy of mycophenolate mofetil in patients with diffuse proliferative lupus nephritis. Hong Kong–Guangzhou Nephrology Study Group. N Engl J Med. 2000;343:1156–1162. doi: 10.1056/NEJM200010193431604. [DOI] [PubMed] [Google Scholar]
- 9.Moore RA, Derry S. Systematic review and meta-analysis of randomised trials and cohort studies of mycophenolate mofetil in lupus nephritis. Arthritis Res Ther. 2006;8:R182. doi: 10.1186/ar2093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Walsh M, James M, Jayne D, et al. Mycophenolate mofetil for induction therapy of lupus nephritis: a systematic review and meta-analysis. Clin J Am Soc Nephrol. 2007;2:968–975. doi: 10.2215/CJN.01200307. [DOI] [PubMed] [Google Scholar]
- 11.Iaccarino L, Rampudda M, Canova M, et al. Mycophenolate mofetil: what is its place in the treatment of autoimmune rheumatic diseases? Autoimmun Rev. 2007;6:190–195. doi: 10.1016/j.autrev.2006.11.001. [DOI] [PubMed] [Google Scholar]
- 12.Eskin-Schwartz M, David M, Mimouni D. Mycophenolate mofetil for the management of autoimmune bullous diseases. Dermatol Clin. 2011;29:555–559. doi: 10.1016/j.det.2011.06.012. [DOI] [PubMed] [Google Scholar]
- 13.Halloran P, Mathew T, Tomlanovich S, et al. Mycophenolate mofetil in renal allograft recipients: a pooled efficacy analysis of three randomized, double-blind, clinical studies in prevention of rejection. The International Mycophenolate Mofetil Renal Transplant Study Groups. Transplantation. 1997;63:39–47. doi: 10.1097/00007890-199701150-00008. [DOI] [PubMed] [Google Scholar]
- 14.Tett SE, Saint-Marcoux F, Staatz CE, et al. Mycophenolate, clinical pharmacokinetics, formulations, and methods for assessing drug exposure. Transplant Rev (Orlando) 2011;25:47–57. doi: 10.1016/j.trre.2010.06.001. [DOI] [PubMed] [Google Scholar]
- 15.Hest van RM, Mathot RAA, Pescovitz MD, et al. Explaining variability in mycophenolic acid exposure to optimize mycophenolate mofetil dosing: a population pharmacokinetic meta-analysis of mycophenolic acid in renal transplant recipients. J Am Soc Nephrol. 2006;17:871–880. doi: 10.1681/ASN.2005101070. [DOI] [PubMed] [Google Scholar]
- 16.Le Meur Y, Büchler M, Thierry A, et al. Individualized mycophenolate mofetil dosing based on drug exposure significantly improves patient outcomes after renal transplantation. Am J Transplant. 2007;7:2496–2503. doi: 10.1111/j.1600-6143.2007.01983.x. [DOI] [PubMed] [Google Scholar]
- 17.Büchler M, Lebranchu Y, Bénéton M, et al. Higher exposure to mycophenolic acid with sirolimus than with cyclosporine cotreatment. Clin Pharmacol Ther. 2005;78:34–42. doi: 10.1016/j.clpt.2005.03.005. [DOI] [PubMed] [Google Scholar]
- 18.Gelder van T. Mycophenolate blood level monitoring: recent progress. Am J Transplant. 2009;9:1495–1499. doi: 10.1111/j.1600-6143.2009.02678.x. [DOI] [PubMed] [Google Scholar]
- 19.Gelder van T, Le Meur Y, Shaw LM, et al. Therapeutic drug monitoring of mycophenolate mofetil in transplantation. Ther Drug Monit. 2006;28:145–154. doi: 10.1097/01.ftd.0000199358.80013.bd. [DOI] [PubMed] [Google Scholar]
- 20.Weber LT, Shipkova M, Armstrong VW, et al. Comparison of the Emit immunoassay with HPLC for therapeutic drug monitoring of mycophenolic acid in pediatric renal-transplant recipients on mycophenolate mofetil therapy. Clin Chem. 2002;48:517–525. [PubMed] [Google Scholar]
- 21.Zahr N, Arnaud L, Marquet P, et al. Mycophenolic acid area under the curve correlates with disease activity in lupus patients treated with mycophenolate mofetil. Arthritis Rheum. 2010;62:2047–2054. doi: 10.1002/art.27495. [DOI] [PubMed] [Google Scholar]
- 22.Roland M, Barbet C, Paintaud G, et al. Mycophenolate mofetil in patients with systemic lupus erythematosus: a prospective pharmacokinetic study. Lupus. 2009;18:441–447. doi: 10.1177/0961203308098631. [DOI] [PubMed] [Google Scholar]
- 23.Levey AS, Bosch JP, Lewis JB, et al. A more accurate method to estimate glomerular filtration rate from serum creatinine: a new prediction equation. Modification of Diet in Renal Disease Study Group. Ann Intern Med. 1999;130:461–470. doi: 10.7326/0003-4819-130-6-199903160-00002. [DOI] [PubMed] [Google Scholar]
- 24.Merkel PA, Cuthbertson DD, Hellmich B, et al. Comparison of disease activity measures for anti-neutrophil cytoplasmic autoantibody (ANCA)-associated vasculitis. Ann Rheum Dis. 2009;68:103–106. doi: 10.1136/ard.2008.097758. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Barraclough KA, Isbel NM, Franklin ME, et al. Evaluation of limited sampling strategies for mycophenolic acid after mycophenolate mofetil intake in adult kidney transplant recipients. Ther Drug Monit. 2010;32:723–733. doi: 10.1097/FTD.0b013e3181fc8fbb. [DOI] [PubMed] [Google Scholar]
- 26.Sombogaard F, Schaik van RHN, Mathot RA, et al. Interpatient variability in IMPDH activity in MMF-treated renal transplant patients is correlated with IMPDH type II 3757T > C polymorphism. Pharmacogenet. Genomics. 2009;19:626–634. doi: 10.1097/FPC.0b013e32832f5f1b. [DOI] [PubMed] [Google Scholar]
- 27.Schaik van RHN, Agteren van M, Fijter de JW, et al. UGT1A9-275T>A/-2152C>T polymorphisms correlate with low MPA exposure and acute rejection in MMF/tacrolimus-treated kidney transplant patients. Clin Pharmacol Ther. 2009;86:319–327. doi: 10.1038/clpt.2009.83. [DOI] [PubMed] [Google Scholar]
- 28.Sánchez-Fructuoso AI, Maestro ML, Calvo N, et al. The prevalence of uridine diphosphate-glucuronosyltransferase 1A9 (UGT1A9) gene promoter region single-nucleotide polymorphisms T-275A and C-2152T and its influence on mycophenolic acid pharmacokinetics in stable renal transplant patients. Transplant Proc. 2009;41:2313–2316. doi: 10.1016/j.transproceed.2009.06.038. [DOI] [PubMed] [Google Scholar]
- 29.Winter de BCM, Mathot RAA, Sombogaard F, et al. Differences in clearance of mycophenolic acid among renal transplant recipients, hematopoietic stem cell transplant recipients, and patients with autoimmune disease. Ther Drug Monit. 2010;32:606–614. doi: 10.1097/FTD.0b013e3181efd715. [DOI] [PubMed] [Google Scholar]
- 30.Mino Y, Naito T, Shimoyama K, et al. Pharmacokinetic variability of mycophenolic acid and its glucuronide in systemic lupus erythematosus patients in remission maintenance phase. Biol Pharm Bull. 2011;34:755–759. doi: 10.1248/bpb.34.755. [DOI] [PubMed] [Google Scholar]
- 31.Tornatore KM, Sudchada P, Dole K, et al. Mycophenolic acid pharmacokinetics during maintenance immunosuppression in African American and Caucasian renal transplant recipients. J Clin Pharmacol. 2011;51:1213–1222. doi: 10.1177/0091270010382909. [DOI] [PubMed] [Google Scholar]
- 32.Miura M, Satoh S, Niioka T, et al. Limited sampling strategy for simultaneous estimation of the area under the concentration–time curve of tacrolimus and mycophenolic acid in adult renal transplant recipients. Ther Drug Monit. 2008;30:52–59. doi: 10.1097/FTD.0b013e31815f5416. [DOI] [PubMed] [Google Scholar]
- 33.Rupprecht K, Schmidt C, Raspé A, et al. Bioavailability of mycophenolate mofetil and enteric-coated mycophenolate sodium is differentially affected by pantoprazole in healthy volunteers. J Clin Pharmacol. 2009;49:1196–1201. doi: 10.1177/0091270009344988. [DOI] [PubMed] [Google Scholar]
- 34.Kofler S, Deutsch M-A, Bigdeli AK, et al. Proton pump inhibitor co-medication reduces mycophenolate acid drug exposure in heart transplant recipients. J Heart Lung Transplant. 2009;28:605–611. doi: 10.1016/j.healun.2009.03.006. [DOI] [PubMed] [Google Scholar]
- 35.Neumann I, Haidinger M, Jäger H, et al. Pharmacokinetics of mycophenolate mofetil in patients with autoimmune diseases compared renal transplant recipients. J Am Soc Nephrol. 2003;14:721–727. doi: 10.1097/01.asn.0000051598.12824.da. [DOI] [PubMed] [Google Scholar]
- 36.Le Guellec C, Bourgoin H, Büchler M, et al. Population pharmacokinetics and Bayesian estimation of mycophenolic acid concentrations in stable renal transplant patients. Clin Pharmacokinet. 2004;43:253–266. doi: 10.2165/00003088-200443040-00004. [DOI] [PubMed] [Google Scholar]
- 37.Barraclough KA, Isbel NM, Johnson DW, et al. A limited sampling strategy for the simultaneous estimation of tacrolimus, mycophenolic acid and unbound prednisolone exposure in adult kidney transplant recipients. Nephrology (Carlton) 17:294–299. doi: 10.1111/j.1440-1797.2011.01560.x. [DOI] [PubMed] [Google Scholar]
- 38.Iking-Konert C, Vogl T, Prior B, et al. Expression of CD57 on CD8+ T lymphocytes of patients with Wegener's granulomatosis and microscopic polyangiitis: evidence for continuous activation of CD8+ cells. Clin Exp Rheumatol. 2009;27(Suppl 52):S19–24. [PubMed] [Google Scholar]
- 39.McKinney EF, Lyons PA, Carr EJ, et al. A CD8+ T cell transcription signature predicts prognosis in autoimmune disease. Nat Med. 2010;16:586–591. doi: 10.1038/nm.2130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Savage COS. Pathogenesis of anti-neutrophil cytoplasmic autoantibody (ANCA)-associated vasculitis. Clin Exp Immunol. 2011;164(Suppl 1):23–26. doi: 10.1111/j.1365-2249.2011.04362.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Tadema H, Abdulahad WH, Stegeman CA, et al. Increased expression of Toll-like receptors by monocytes and natural killer cells in ANCA-associated vasculitis. PLOS ONE. 2011;6:e24315. doi: 10.1371/journal.pone.0024315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Lamprecht P, Wieczorek S, Epplen JT, et al. Granuloma formation in ANCA-associated vasculitides. APMIS Suppl. 2009;127:32–36. doi: 10.1111/j.1600-0463.2009.02474.x. [DOI] [PubMed] [Google Scholar]
- 43.Ohata K, Espinoza JL, Lu X, et al. Mycophenolic acid inhibits natural killer cell proliferation and cytotoxic function: a possible disadvantage of including mycophenolate mofetil in the graft-versus-host disease prophylaxis regimen. Biol Blood Marrow Transplant. 2011;17:205–213. doi: 10.1016/j.bbmt.2010.08.014. [DOI] [PubMed] [Google Scholar]
- 44.Neumann I, Fuhrmann H, Fang I-F, et al. Association between mycophenolic acid 12-h trough levels and clinical endpoints in patients with autoimmune disease on mycophenolate mofetil. Nephrol Dial Transplant. 2008;23:3514–3520. doi: 10.1093/ndt/gfn360. [DOI] [PubMed] [Google Scholar]
- 45.Le Meur Y, Borrows R, Pescovitz MD, et al. Therapeutic drug monitoring of mycophenolates in kidney transplantation: report of The Transplantation Society consensus meeting. Transplant Rev (Orlando) 2011;25:58–64. doi: 10.1016/j.trre.2011.01.002. [DOI] [PubMed] [Google Scholar]
- 46.Prémaud A, Rousseau A, Le Meur Y, et al. Comparison of liquid chromatography–tandem mass spectrometry with a commercial enzyme-multiplied immunoassay for the determination of plasma MPA in renal transplant recipients and consequences for therapeutic drug monitoring. Ther Drug Monit. 2004;26:609–619. doi: 10.1097/00007691-200412000-00005. [DOI] [PubMed] [Google Scholar]
- 47.Yeung JS, Wang W, Chan L. Determination of mycophenolic acid level: comparison of high-performance liquid chromatography with homogeneous enzyme-immunoassay. Transplant Proc. 1999;31:1214–1215. doi: 10.1016/s0041-1345(98)01968-x. [DOI] [PubMed] [Google Scholar]
- 48.Joy MS, Hilliard T, Hu Y, et al. Influence of clinical and demographic variables on mycophenolic acid pharmacokinetics in antineutrophil cytoplasmic antibody-associated vasculitis. Ann Pharmacother. 2009;43:1020–1027. doi: 10.1345/aph.1L699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Winter de BCM, Gelder van T. Therapeutic drug monitoring for mycophenolic acid in patients with autoimmune diseases. Nephrol Dial Transplant. 2008;11:3386–3388. doi: 10.1093/ndt/gfn497. [DOI] [PubMed] [Google Scholar]
- 50.Tornatore KM, Venuto RC, Logue G, Davis PJ. CD4+ and CD8+ lymphocyte and cortisol response patterns in elderly and young males after methylprednisolone exposure. J Med. 1998;29:159–183. [PubMed] [Google Scholar]
- 51.Gluhovschi C, Gluhovschi G, Herman D, et al. The effect of steroids on lymphocyte profile in primary chronic glomerulonephritis. Empirical or tailored therapy? Int Immunopharmacol. 2007;7:1265–1270. doi: 10.1016/j.intimp.2007.05.012. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Correlation tests between patient characteristics and mycophenolic acid (MPA) pharmacokinetics [area under the curve (AUC)0–12].


