Abstract
Down-regulation of soluble or membrane-bound co-stimulatory molecules by RNAi in dendritic cells can prevent the activation of immune responses. Therefore, this study was designed to evaluate the therapeutic efficacy of bone marrow-derived DCs (BMDCs) transduced with lentiviral vectors to permanently expressed shRNA specific for CD40 (CD40LV-DCs) and/or p19 subunit of interleukin (IL)-23 (p19LV-DCs) mRNAs in experimental autoimmune encephalomyelitis (EAE). In-vitro studies showed that double-transduced BMDCs (CD40+p19LV-DCs) resemble tolerogenic DCs due to profound down-regulation of CD40, lower expression of proinflammatory cytokines (IL-6 and IL-12), increased IL-10 production and stronger stimulation of myelin oligodendrocyte glycoprotein (MOG)35–55-specific T cells for production of IL-10 compared with CD40LV-DCs, p19LV-DCs and BMDCs transduced with control lentiviral vector (CoLV-DCs). Moreover, injection of transduced CD40+p19LV-BMDCs in EAE mice resulted in more reduction in clinical score, significant reduction in IL-17 or increased production of IL-10 by mononuclear cells derived from the lymph nodes or spinal cord compared with CoLV-DCs-treated EAE mice. In conclusion, simultaneous knock-down of CD40 and IL-23 production by BMDCs may represent a promising therapeutic tool for the treatment of IL-17-dependent autoimmune diseases, including multiple sclerosis.
Keywords: CD40, dendritic cell, IL-23p19, lentiviral vector, RNAi
Introduction
Multiple sclerosis (MS) is a chronic inflammatory disease affecting the central nervous system (CNS) white matter. The accurate aetiology remains to be established; however, it is deemed that MS is caused by a failure in immune tolerance. It was assumed that activation of autoreactive CD4+ T cells specific for myelin antigens and differentiation to T helper type 1 (Th1) effectors are significant for the development of this disease 1. This widely accepted theory about the pathology of MS was based on data from experimental autoimmune encephalomyelitis (EAE), a rodent model of MS. Th17 cell realization brought attention to interleukin (IL)-17-producing CD4+ T cells that are driven by IL-23 as the pathogenic T cells in EAE 2. Th17 cells were discovered in 2005 3–5. They are considered developmentally distinct lineage from Th1 and Th2 cells. The critical role of Th17 cells in the induction of various types of organ-specific autoimmune diseases such as type 1 diabetes, rheumatoid arthritis and multiple sclerosis (which was previously attributed primarily to Th1 cells) have been well documented 6–8. In this respect the development of Th17 cells is a critical phenomenon in the pathogenesis of EAE, as the lack of IL-17-producing cells can ameliorate EAE 9.
Deviation towards Th17 lymphocytes requires the presence of specific cytokines and membrane-bound co-stimulatory molecules provided by professional antigen-presenting cells, such as dendritic cells (DCs). Among co-stimulatory molecules, the role of IL-6, IL-23 (composed of IL-12 p40-and IL-23-specific p19 subunits) and CD40 signalling in Th17 polarization is prominent 10–12. In this regard, it has been shown that IL-17 production was severely impaired after co-culture of allogeneic CD4+ naive T cells and DCs in the presence of blocking anti-IL-23 antibody. However, it later became clear that IL-23 is involved in the expansion of established Th17 populations and this cytokine alone does not induce differentiation of naive T cell precursors towards this cell type 13. In fact, the development of Th17 cells requires co-ordinate signalling from transforming growth factor (TGF)-β and IL-6 13–15. Of interest, it has been shown that IL-6 production by DCs activated through CD40–CD40L interaction can directly stimulate Th17 deviation from naive CD4+ T cells or can stimulate T cells indirectly to produce IL-21, another important cytokine in Th17 cell differentiation 16–18. Due to the importance of CD40–CD40L interaction in IL-6 induction and Th17 deviation, the lack of CD40–CD40L cross-talk inhibits the development of IL-17-producing cells and consequently the progress of EAE 12.
Considering the importance of CD40-associated IL-6 production and IL-23 production in Th17 cell polarization from naive CD4+ T cells and the maintenance of polarized Th17 cells, respectively, any interference in CD40 or IL-23 expression by DCs can prevent Th17 cell-induced pathological reactions. RNA interference (RNAi) is a technology used for post-transcriptional silencing of gene expression. This method takes advantage of sequence-specificity, simplicity and safety 19. Moreover, a combination of RNAi technology and viral gene delivery systems not only induce permanent and stable knock-down of a gene but has also optimized the low impact of bare RNAi (non-viral gene delivery systems). In this respect, the prevention of immune responses using RNAi after reduction of CD40 levels in DCs has been reported in cardiac transplantation 20, regression of atherosclerosis plaques 21, atopic diseases 22 and autoimmune conditions, including collagen-induced arthritis 23 as well as the impact of IL-23 knock-down by RNAi on asthma 24. It is worth mentioning that, despite the importance of IL-23 in the maintenance of Th17 cells and therefore the preventive and therapeutic potential of IL-23 knock-down DCs in Th17-related autoimmune diseases (such as EAE), there are no published data investigating the efficacy of IL-23 or CD40+ IL-23 knock-down DCs using the RNAi method in autoimmune diseases. Therefore, the aim of this study was to determine the ability of knock-down DCs for IL-23 and CD40 production using shRNA technology in the amelioration of EAE severity.
Materials and methods
Mice
C57BL/6 and 2D2 mice were purchased from the Jackson Laboratory (Bar Harbor, ME, USA). All the mice were bred and kept under pathogen-free conditions in filter-top cages at the animal care facility of the Thomas Jefferson University. All animal studies were performed according to the animal care and use committee in Thomas Jefferson University. C57BL/6 mice with EAE were used as animal models of human multiple sclerosis.
Generation of murine bone marrow-derived dendritic cells (BMDCs)
The BMDCs from C57BL6 mice were generated from BM progenitors as described previously by Inaba et al., with some modifications 25. Briefly, both ends of the femur and tibia bones were cut with scissors, the marrow was flushed out and passed through a nylon mesh to remove small pieces of bone and debris; 6 × 106 cells were counted and plated in 10-cm Petri dishes containing complete RPMI containing 10 ng/ml granulocyte–macrophage colony-stimulating factor (GM-CSF) on day zero. The non-adherent and semi-adherent cells were collected, washed and plated into new Petri dishes on days 3 and 6.
Production of lentiviral vectors
To produce lentiviral vectors expressing CD40-specific shRNA (CD40LV) and/or p19-specific shRNA (p19LV), 3·5 × 106 293FT cells (Invitrogen, Carlsbad, CA, USA) were plated in 10 ml of Dulbecco's modified Eagle's medium (DMEM) supplemented with 10% fetal bovine serum (FBS) and no antibiotics and incubated overnight at 37°C in a 5% CO2 incubator. A DNA cocktail consisting of 5 μg of a transfer vector for CD40 (clone ID: TRCN0000066244, clone name: NM_011611.1-672s1c1; Sigma-Aldrich, St Louis, MO, USA) and/or p19 (clone ID: TRCN0000067120, clone name: NM_031252.1-593s1c1, Sigma-Aldrich) and the three packaging plasmids including pLP1, pLP2 and pMD2.G (2·7, 0·9 and 1·4 μg, respectively; Invitrogen) was added to 293FT cells in the presence of lipofectamine 2000. The culture supernatant containing lentiviral particles was harvested 48 h after transfection.
Transduction of murine BMDCs with lentiviral vectors
To transduce the BMDCs, on day 3 of BMDCs isolation 3 × 106 cells per well were seeded in six-well plates with 3 ml of unconcentrated lentiviral supernatant and with a final polybrene concentration of 8 μg/ml. For co-transduction, equal volumes of CD40LV and p19LV (1·5 ml) were added to the wells. The mock-DCs (untransduced DCs) were also used as control DCs (Cont-DCs). After incubating the cells for 8 h at 37°C and 5% CO2, the whole medium containing LVs was removed and fresh complete RPMI containing 10 ng/ml GM-CSF was added to the cells for 48 h. To select the transduced cells, the optimal concentration of puromycin (2 μg/ml) was added to the plates on days 6 and 8. Transduced DCs were activated with lipopolysaccharide (LPS) (1 μg/ml) for 24 h and stained for surface markers [CD40, CD80, CD86 and major histocompatibility complex (MHCII)], expression of CD40 and p19 subunit of IL-23 at mRNA levels and their supernatants were also collected for cytokine assays. For in-vivo experiments, puromycin-selected BMDCs were pulsed with LPS (1 μg/ml) and myelin oligodendrocyte glycoprotein (MOG)35–55 (5 μg/ml) for 24 h before the injection.
Flow cytometric analysis
Cultured cells were analysed for their surface markers using rat anti-mouse CD11c, MHC class II (I-A/I-E), CD80 and CD86 conjugated with allophycocyanin (APC), fluorescein isothiocyanate (FITC), peridinin chlorophyll cyanin 5·5 (PerCP-Cy5·5), phycoerythrin (PE) Cy7 and PE, respectively. All antibodies were purchased from BD Biosciences (San Jose, CA, USA), and cells were stained according to the manufacturer's instructions. Stained cells were analysed with BD fluorescence activated cell sorter (FACS)Aria (BD Biosciences) using BD FACSDiVa software (BD Biosciences).
RNA isolation, cDNA synthesis and quantitative real-time–polymerase chain reaction (qRT–PCR)
Total RNA was extracted from duplicate samples using the RNeasy kit, according to the manufacturer's instructions (Qiagen, Calencia, CA, USA). Synthesis of cDNA was carried out with high-capacity cDNA synthsis, according to the manufacturer's instructions (Applied Biosystems, Grand Island, NY, USA). Quantitative PCR assay was performed in triplicate for each sample in a final reaction mixture of 20 μl containing 10 μl TaqMan® Universal PCR Master Mix (Applied Biosystems), 9 μl cDNA and 1 μl of each primer. 6-Carboxyfluorescein (FAM)-labelled primers of p19 (IL-23a, Mm00518984_m1), CD40 (Mm00441891_m1) and eukaryotic 18S rRNA as endogenous control (Hs99999901_s1) were used. The PCR conditions were as follows: after an initial denaturation step at 95°C for 10 s, 40 cycles of 5 s at 95°C, 15 s at 55°C and 1 min at 60°C were run on the sequence detection system7000 (Applied Biosystems).
Cytokine and antigen uptake assays
The levels of interferon (IFN)-γ, IL-17, IL-12p70, IL-23, IL-6, IL-10, and GM-CSF in cell culture supernatants were measured by enzyme-linked immunosorbent assay (ELISA) according to R&D Systems' (Minneapolis, MN, USA) and Biolegend Systems' (San Diego, CA, USA) instructions. To check the antigen uptake capacity, 10 × 105 untransduced and transduced DCs were collected and incubated on ice for 10 min. To each sample tube, ovalbumin (OVA)-fluorescein isothiocyanate (FITC) at a final concentration of 2 μg/ml was added. Control tubes were kept on ice and sample tubes were incubated in 37°C for 30 min. After washing, uptake of OVA-FITC was measured by FACS.
Stimulation of MOG35–55-specific T cells by lentiviral transduced BMDCs
MOG35–55-specific CD4+ T cells were enriched from spleens of 2D2 mice by microbeads (Miltenyi Biotec, Auburn, CA, USA). The purity of CD4+ T cells was >90%. T cells (2 × 104) were co-cultured with lentiviral-transduced BMDCs (5 × 103) of C57BL/6 mice in the presence of 20 μg/ml of MOG35–55. After 48 h, culture supernatants were harvested for determination of cytokine levels and cells were pulsed with 0·5 μci [3H]-thymidine for the last 18 h. Thymidine incorporation was measured using a scintillation counter. All proliferation assays were run in triplicate.
Determination of therapeutic efficacy of engineered BMDCs in the EAE model
To induce EAE, each mouse received 200 μl subcutaneously of MOG35–55/complete Freund's adjuvant (CFA) emulsion at two sites on the back. Mice were further injected intraperitoneally with 200 ng pertussis toxin in 200 μl phosphate-buffered saline (PBS) at days 0 and 2. Injected mice were classified into five groups, each group containing five mice. The experimental setups were as follows: group 1, mice injected with CoLV-DCs, served as plasmid control; group 2, mice received PBS; group 3, mice received p19LV-DCs; group 4, mice received CD40LV-DCs; and group 5, mice received co-transduced BMDCs with CD40+p19 LV-DCs or BoLV-DCs. Three injections of 2 × 106 MOG35–55-pulsed engineered BMDCs were administered intravenously on days 3, 5 and 7 and mice were followed-up to day 35 post-immunization. Development of clinical symptoms was scored in a blinded manner using the following clinical scoring system: 0: normal; 0·5: partial loss of tail tonicity; 1: limp tail or waddling gait with tail tonicity; 1·5: limp tail with waddling gait (ataxia); 2: one limb and tail paralysis; 2·5: full paralysis of two limbs; 3: complete paralysis of two hind legs and tail; 3·5: complete paralysis of hind legs and the body between hips; 4: body paralysis above hips or complete paralysis of hind legs and tail and partial front leg paralysis; 4·5: moribund; and 5: death. In addition, mice were killed on day 35 post-immunization and mononuclear cells were prepared from spleens, lymph nodes and spinal cords. Spinal cords were resuspended in 30% Percoll and centrifuged on a discontinuous 30 : 70% Percoll gradient at 500 g for 30 min. Mononuclear cells from spinal cords (SCMCs) were collected from the 30 : 70% interface and stimulated with MOG35–55 (20 μg/ml) for 3 days. Proliferation were measured based on [3H]-thymidine incorporation and the culture supernatants were harvested for determination of IFN-γ, IL-10, GM-CSF and IL-17 concentrations using ELISA assay.
Statistical analysis
Average clinical scores, gene expression, cytokine levels and proliferation assays were analysed by Mann–Whitney U-test to test the differences between treated groups and controls. Differences were considered statistically significant if P < 0·05. All analyses were performed using spss version 17.0 or Graphpad Prism™ version 5.0 software.
Results
In-vitro generation of tolerogenic DCs by transducing lentiviral vectors containing specific shRNAs against CD40 and/or p19IL-23
In order to produce tolerogenic DCs, BMDCs from C57BL/6 were exposed to each of CD40LV, p19LV and CD40LV+ p19LV (BoLV). To check the maturation state of transduced DCs, the expressions of MHCII, CD40, CD86 and CD80 were evaluated by flow cytometry (Fig. 1). After LPS activation, the percentage of positive cells for MHCII, CD86 and CD80 in the transduced CD40LV-, p19LV-and BoLV-DCs was not significantly different compared to those of the CoLV-DC and Cont-DC groups (P > 0·05). However, the percentage of CD40-positive BMDCs in the transduced DCs with CD40LV, p19LV and BoLV (8·32, 9·52 and 4·7%, respectively) was significantly lower than the CoLV-DC (13·2%) or Cont-DC (19·7%) groups (P < 0·05). Our results showed that transduced DCs were maturation-resistant, as the cells expressed CD40 to a limited extent. In addition, the maturation state of transduced DCs was compared to CoLV-DCs and Cont-DCs by checking their endocytic capacity. As shown in Fig. 2, the percentage of FITC-OVA+ cells was not statistically different between CD40LV-DCs, p19LV-DCs, BoLV-DCs (70 ± 4·2, 76 ± 5·6 and 74 ± 5·3%, respectively) and CoLV-DCs or Cont-DC controls (65 ± 5·6 and 79 ± 3·5, respectively; P > 0·05). High enocytic capacity of transduced and untransduced DCs confirmed an immature state of transduced DCs.
Figure 1.
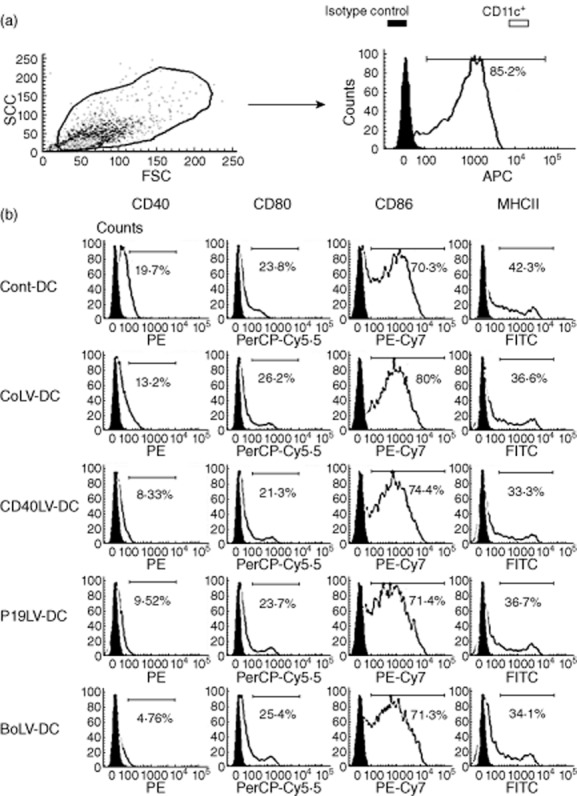
Analysis of dendritic cell (DC) surface phenotype by flow cytometry (a) Cells in forward-(FSC) versus side-scatter (SSC) analysis were gated to determine the percentage of DCs with CD11c surface marker. (b) Engineered bone marrow-derived dendritic cells (BMDCs) were stimulated with lipopolysaccharide (LPS) and checked for expression of major histocompatibility complex (MHC)-II and co-stimulatory molecules. Solid histograms show the background staining with isotype controls monoclonal antibody, and empty histograms represent specific staining of the indicated cell-surface markers. Histograms are representative of five independent experiments.
Figure 2.
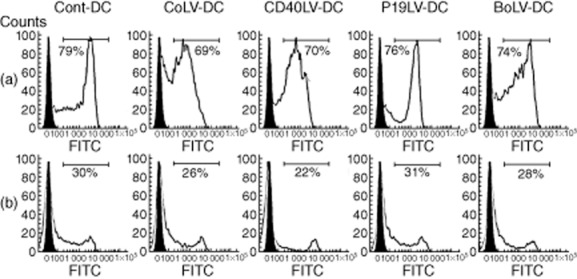
Antigen uptake capacity of engineered bone marrow-derived dendritic cells (BMDCs). BMDCs were incubated in medium containing 1 mg/ml of ovalbumin–fluorescein isothiocyanate (OVA–FITC) for 30 min at 37°C (a) or 4°C (b) and analysed by flow cytometry. Solid histograms show isotype control staining, whereas empty histograms show cells staining either at 37°C or 4°C. Representative experiments are shown.
To explore possible changes in cytokine profile of transduced BMDCs, levels of IL-23, IL-12p70 and IL-10 in culture supernatant of transduced and untransduced BMDCs were evaluated after LPS stimulation. The supernatants were harvested after 6 or 24 h of LPS stimulation for analysis of IL-23 or IL-12p70 and IL-10, respectively. As shown in Fig. 3a, IL-23 was significantly lower in the culture supernatant of p19LV-DCs (199 ± 11 pg/ml) and BoLV-DCs (198 ± 7 pg/ml) compared with CoLV-DCs (271 ± 7 pg/ml; P = 0·029 and P = 0·009, respectively) or Cont-DCs (252 ± 10 pg/ml; P = 0·039 and P = 0·031, respectively). IL-12 levels in LPS-stimulated CD40LV-DC supernatants (238 ± 33 pg/ml) and BoLV-DC supernatants (209 ± 54 pg/ml) have also shown a significant decrease compared with both CoLV-DCs and Cont-DCs (445 ± 28 pg/ml and 527 ± 49 pg/ml, respectively; P < 0·05). However, the levels of IL-10 were significantly higher in the LPS-stimulated BoLV-DC group (1483 ± 72 pg/ml) in comparison with those of CoLV-DCs (714 ± 109 pg/ml, P < 0·05) or Cont-DCs (673 ± 48 pg/ml, P < 0·01; Fig. 3c). In addition, as shown in Fig. 3d, the levels of IL-6 were significantly lower in the culture supernatant of LPS-stimulated CD40LV-DCs (108 ± 14 pg/ml), p19LV-DCs (90 ± 9 pg/ml) and BoLV-DCs (83 ± 8·5 pg/ml) compared with CoLV-DCs (174 ± 15 pg/ml, P < 0·05) and Cont-DCs (224 ± 12 pg/ml, P < 0·05).
Figure 3.
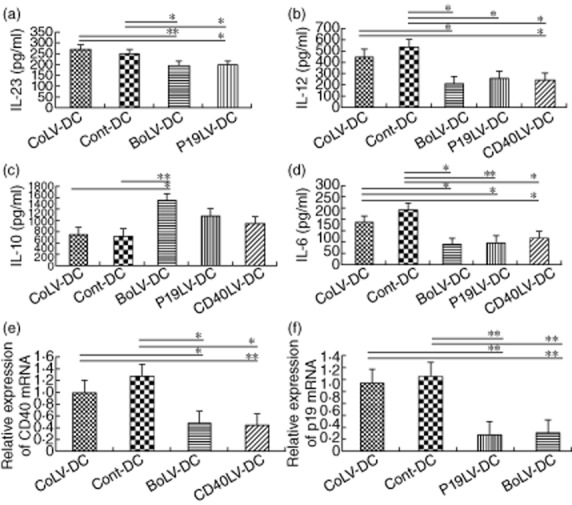
Cytokine production and mRNA measurement by real-time polymerase chain reaction (PCR) in transduced and untransduced dendritic cells (DC)s. Cultures of different bone marrow-derived dendritic cells (BMDCs) were stimulated with lipopolysaccharide (LPS) for 24 h and the levels of interleukin (IL)-23 (a), IL-12p70 (b), IL-10 (c) and IL-6 (d) were determined in their supernatants by enzyme-linked immunosorbent assay (ELISA). Quantitative real-time PCR was used to determine the inhibitory effect of the lentiviral shRNA on CD40 and/or p19 gene expression at mRNA levels. (e) CD40LV-and BMDCs transduced with lentiviral vectors encoding for CD40 and p19-specific shRNA (BoLV) showed significant decrease in the expression of CD40 mRNA compared to BMDCs transduced with the control lentiviral vectors (CoLV-DC) and Cont-DC (control) groups. (f) P19LV and BoLV-transduced DCs had decreased expression of p19 mRNA compared with the CoLV-DC and Cont-DC groups. Bars represent mean values ± standard error of the mean. *P < 0·05; **P < 0·01.
Further to cytokine production, the gene expression levels of CD40 and IL-23p19 subunit were also measured by quantitative reverse transcription–polymerase chain reaction (qRT–PCR) in transduced and control BMDCs. The expression levels of CD40 mRNA showed a significant decrease compared with CoLV-DCs and Cont-DCs (P < 0·05 and P < 0·01, respectively; Fig. 3e). IL-23p19 subunit mRNA levels in p19LV-DCs and BoLV-DCs were also significantly lower than those in control BMDCs (P < 0·01, Fig. 3f).
Inhibition of MOG35–55-specific CD4+ T cell proliferation and cytokine production by co-cultured transduced BMDCs
After LPS stimulation, the ability of transduced CD40LV-DCs, BoLV-DCs and p19LV-DCs in stimulation of MOG35–55-specific CD4+ T cell proliferation were decreased significantly compared with the CoLV-DC (P = 0·0004, P = 0·0008 and P = 0·025, respectively) and Cont-DC groups (P = 0·016, P = 0·016 and P = 0·034, respectively; Fig. 4a). Of interest, in the presence of antigen-pulsed DCs, IFN-γ production was significantly lower in the transduced DCs as opposed to CoLV-DCs and Cont-DCs (P < 0·05, Fig. 4b and Table 1). In addition, MOG35–55-specific CD4+ T cells produced significantly lower levels of GM-CSF in co-culture with transduced p19LV-DCs and BoLV-DCs when compared with control DCs; however, their ability in the production of IL-10 was increased significantly (P < 0·05; Fig. 4c, Table 2 and Fig. 4d, Table 3, respectively).
Figure 4.
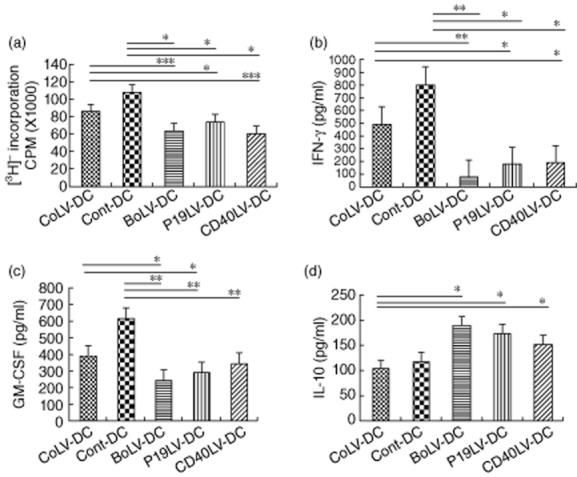
Effects of dendritic cell (DC)s on myelin oligodendrocyte glycoprotein (MOG)35–55-specific CD4+ T cells. (a) Transduced and untransduced DCs were co-cultured with 2D2 splenic T cells at a T cell : DC ratio of 4:1 for 3 days. The ability of DCs to stimulate CD4+ T cells of 2D2 mice was evaluated by [3H]-thymidine uptake. Data presented as the mean values of triplicate cultures ± standard error of the mean. Supernatants of co-cultured CD4+ T cells and engineered bone marrow-derived dendritic cells (BMDCs) or control DCs were checked for interferon (IFN)-γ (b), granulocyte-macrophage colony-stimulating factor (GM-CSF) (c) and IL-10 (d) cytokine levels by enzyme-linked immunosorbent assay (ELISA). *P < 0·05; **P < 0·01.
Table 1.
Interferon (IFN)-γ production by CD4+ T cells co-cultured with transduced dendritic cells (DC)s and their comparison to control DC groups.
|
P-values |
|||
|---|---|---|---|
| Mean ± s.d. (pg/ml) | CoLV-DC | Cont-DC | |
| CD40LV-DCs | 184 ± 32 | 0·015 | 0·01 |
| p19LV-DCs | 173 ± 36 | 0·018 | 0·012 |
| BoLV-DCs | 72 ± 23 | 0·003 | 0·003 |
| CoLV-DCs | 492 ± 19 | 1·0 | 0·005 |
| Cont-DCs | 808 ± 13 | 0·005 | 1·0 |
BoLV = BMDCs transduced with lentiviral vectors encoding for CD40 and p19-specific shRNA; CoLV = BMDCs transduced with control lentiviral vectors; Cont = control; s.d. = standard deviation.
Table 2.
GM-CSF production by CD4+ T cells co-cultured with transduced dendritic cells (DC)s and their comparison to control DC groups.
|
P-values |
|||
|---|---|---|---|
| Mean ± s.d. (pg/ml) | CoLV-DC | Cont-DC | |
| CD40LV-DCs | 345 ± 16 | 0·09 | 0·004 |
| p19LV-DCs | 293 ± 21 | 0·047 | 0·004 |
| BoLV-DCs | 245 ± 26 | 0·044 | 0·006 |
| CoLV-DCs | 392 ± 12 | 1·0 | 0·007 |
| Cont-DCs | 617 ± 17 | 0·007 | 1·0 |
BoLV = BMDCs transduced with lentiviral vectors encoding for CD40 and p19-specific shRNA; CoLV = BMDCs transduced with control lentiviral vectors; Cont = control; s.d. = standard deviation.
Table 3.
Interleukin (IL)-10 production by CD4+ T cells co-cultured with transduced dendritic cells (DC)s and their comparison to control DC groups.
|
P-values |
|||
|---|---|---|---|
| Mean ± s.d. (pg/ml) | CoLV-DC | Cont-DC | |
| CD40LV-DCs | 153 ± 12 | 0·047 | 0·18 |
| p19LV-DCs | 175 ± 11 | 0·02 | 0·095 |
| BoLV-DCs | 190 ± 12 | 0·019 | 0·063 |
| CoLV-DCs | 103 ± 9 | 1·0 | 0·42 |
| Cont-DCs | 119 ± 19 | 0·42 | 1·0 |
BoLV = BMDCs transduced with lentiviral vectors encoding for CD40 and p19-specific shRNA; CoLV = BMDCs transduced with control lentiviral vectors; Cont = control; s.d. = standard deviation.
Therapeutic effect of transduced BMDCs in EAE mice
MOG35–55-immunized mice were treated with transduced BMDCs (2 × 106 cells/100 μl) for three time-points on days 3, 5 and 7 post-immunization. Control mice were also treated with PBS or CoLV-DCs. Mice were checked for disease clinical score after cessation of DC treatment. The results showed that BoLV-DC-, p19LV-DC-and CD40LV-DC-treated EAE mice had a significantly milder disease score when compared to PBS-treated (mean clinical scores for whole follow-up were 0·92 ± 0·36, 1·07 ± 0·21 and 1·27 ± 0·11, respectively; P = 0·009, P = 0·018 and P = 0·036, respectively) or CoLV-DC-treated mice (P = 0·012, P = 0·021 and P = 0·037, respectively; Fig. 5). Although the mean clinical score in BoLV-DC-treated mice was almost consistently lower than other treated groups (Fig. 5), that difference was not statistically significant between BoLV-DC-treated EAE mice and p19LV-DC-treated (P = 0·39) or CD40LV-DC-treated mice (P = 0·09).
Figure 5.
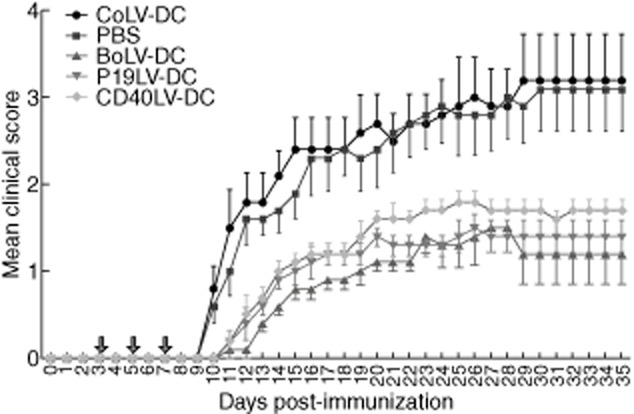
Mean clinical scores in experimental autoimmune encephalomyelitis (EAE) mice after treatment with engineered bone marrow-derived dendritic cells (BMDCs). C57BL/6 mice were injected intravenously three times with transduced DCs or with phosphate-buffered saline (PBS). The mice were checked daily for clinical scores of EAE. Mean clinical scores of EAE mice treated with BMDCs transduced with control lentiviral vectors (CoLV-DCs) (a), BMDCs transduced with lentiviral vectors encoding for CD40 and p19-specific shRNA (BoLV-DCs) (b), p19LV-DCs (c) and CD40LV-DCs (d) were compared to the PBS control group. Arrows show the days of treatment. *P < 0·05; **P < 0·01.
In order to explore the potential effects of transduced BMDCs on the expression of inflammatory (IFN-γ, IL-17 and GM-CSF) or inhibitory (IL-10) cytokines in EAE mice, mononuclear cells of spleens (Fig. 6a), lymph nodes (Fig. 6b) and central nervous systems (CNS) (Fig. 6c) of treated EAE mice were incubated with MOG35–55 and cytokine levels were determined in their culture supernatants. The IFN-γ levels in the supernatant of MOG35–55-stimulated splenocytes and lymph node cells were significantly lower in CD40LV-DCs-treated mice compared to those of CoLV-DC-treated mice (splenocytes: 502 ± 42 pg/ml and 1072 ± 178 pg/ml, respectively, P < 0·01; lymph nodes: 473 ± 22 pg/ml and 847 ± 116 pg/ml, respectively, P < 0·01). In the same manner, p19LV-DCs-treated mice also showed significantly lower levels of IFN-γ as well as GM-CSF in the culture media of MOG35–55-stimulated splenocytes or lymph node-derived cells compared to those of CoLV-DCs (Fig. 6a,b). In addition, unlike the stimulated splenocytes, the levels of IL-17 were significantly lower in the supernatant of lymph node-derived cells from CD40LV-DC-treated (472 ± 146 pg/ml) or p19LV-DC-treated (387 ± 101 pg/ml) mice compared to PBS-treated (793 ± 110 pg/ml; P = 0·014 and P = 0·002, respectively) or CoLV-DC-treated (734 ± 110 pg/ml; P = 0·031 and P = 0·004, respectively) groups. As shown in Fig. 6, similar to the CD40LV-DC-and p19LV-DC-treated mice, the levels of IFN-γ and GM-CSF in the supernatant of stimulated splenocytes or lymph nodes of BoLV-DC-treated mice were significantly lower than those of CoLV-DC-treated mice (IFN-γ in the splenocyte supernatant: 403 ± 39 pg/ml and 1071 ± 178 pg/ml, respectively, P = 0·004; IFN-γ in lymph node supernatant: 250 ± 23 pg/ml and 847 ± 116 pg/ml, respectively, P = 0·002; GM-CSF in the splenocyte supernatant: 264 ± 64 pg/ml and 405 ± 16 pg/ml, respectively, P = 0·021; GM-CSF in the lymph node supernatant: 232 ± 90 pg/ml and 442 ± 46 pg/ml, respectively, P = 0·011) while IL-17 showed merely a significant decrease in the lymph node-derived cell supernatants of BoLV-DC-treated mice compared to CoLV-DC-treated mice (349 ± 69 pg/ml and 734 ± 110 pg/ml, respectively; P = 0·001). Conversely, mice treated with p19LV-DCs or BoLV-DCs showed a significant increase in IL-10 production by splenocytes compared to those of CoLV-DC-treated mice (for p19LV-DCs: 738 ± 180 pg/ml and 347 ± 81 pg/ml, respectively, P = 0·015; for BoLV-DCs: 901 ± 233 pg/ml and 347 ± 81 pg/ml, respectively, P = 0·012), while those differences did not reach significant levels when the supernatant of lymph node-derived cells were compared in p19LV-DC-or BoLV-DC-treated and CoLV-DC-treated mice (Fig. 6a,b). Regarding CNS, it worth mentioning that on day 35 post-immunization, the mean absolute numbers of mononuclear cells in the CNS of BoLV-DC-, p19LV-DC-and CD40LV-DC-treated mice were significantly lower than in the CoLV-DC-treated group (P = 0·005, P = 0·04 and P = 0·003, respectively). Moreover, comparing the cytokine levels in the supernatant of CNS-derived mononuclear cells from different treatment regimens revealed that the levels of IL-17 were decreased significantly in BoLV-DC-treated mice in contrast to those in CoLV-DC-treated mice (612 ± 47 pg/ml and 809 ± 117 pg/ml, respectively; P = 0·023), while a significant difference in the GM-CSF levels was merely detected when p19LV-DC-treated mice were compared with CoLV-DC-treated mice (407 ± 19 pg/ml and 446 ± 22 pg/ml, respectively; P = 0·038). Although IFN-γ levels were decreased and the levels of IL-10 were increased in all mice treated with CD40LV-DCs, p19LV-DCs or BoLV-DCs compared with CoLV-DC-treated mice, those differences were not significant (Fig. 6c). Overall, our results showed a significant decrease in IL-17 production by mononuclear cells derived from lymph nodes and spinal cords of BoLV-DC-treated EAE mice.
Figure 6.
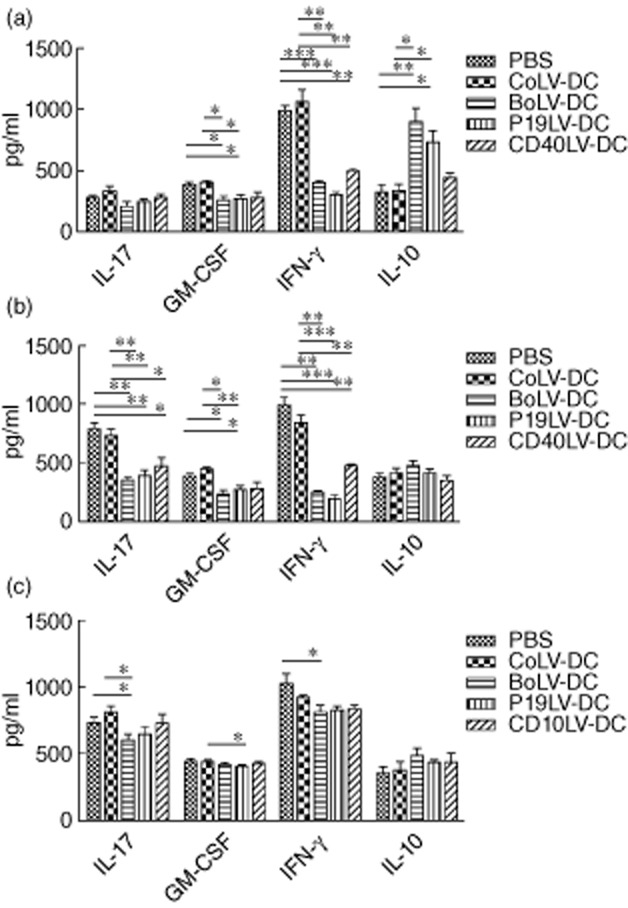
Effects of transduced bone marrow-derived dendritic cells (BMDCs) on interferon (IFN)-γ, interleukin (IL)-17, granulocyte–macrophage colony-stimulating factor (GM-CSF), and IL-10 production by splenocytes (a), lymph node cells (b) and cells infiltrating the central nervous system (CNS) (c) in experimental autoimmune encephalomyelitis (EAE)-treated mice. Mononuclear cells isolated from spleens (a), lymph nodes (b) and CNS (c) were stimulated with myelin oligodendrocyte glycoprotein (MOG)35–55 and their supernatants were checked by enzyme-linked immunosorbent assay (ELISA). *P < 0·05; **P < 0·01; ***P < 0·001.
Discussion
The role of immature DCs in the initiation of tolerance and induction of T cell unresponsiveness has long been recognized 26,27. The absence of secreted or membrane-bound co-stimulatory molecules is one of the most important features of immature or tolerogenic DCs 26,28. Among them, CD40 and IL-23 are two co-stimulatory molecules playing important roles in decision-making between immunity or tolerance and polarization of Th cells 26,29,30. In fact, a lack of CD40 ligation not only prevents DCs increasing the cell surface expression of other co-stimulatory molecules such as CD58 and CD86 31,32, it also inhibits the production of IL-12 and IL-23p19, crucial cytokines in the polarization of immune response towards Th1 (IFN-γ-producer cells) and Th17 (IL-17-producer cells) pathways 33. Moreover, the importance of IL-12/IFN-γ and IL-6/IL-23/IL-17 axes in the immunopathology of different autoimmune diseases has been acknowledged 34–36. In accordance with the above-mentioned data, it has been reported that administration of CD40 siRNA-transfected DCs promotes tolerance in collagen-induced arthritis, a well-known animal model for autoimmune arthritis 37. Interestingly, knock-out mice deficient in p19, or in either subunit of the IL-23 receptor (IL-23R and IL12R-β1), have also developed less severe symptoms of EAE and inflammatory bowel disease, highlighting the importance of IL-23 in the inflammatory pathway 4,38. Therefore, inhibiting CD40 or IL-23 gene expression in DCs can be considered to be a promising approach to make tolerogenic DCs. In fact, tolerogenic DCs have been characterized by normal expression of MHC class II and co-stimulatory molecules CD80 and CD86, low expression of CD40, high levels of IL-10 and low levels of inflammatory cytokines such as IL-12 and IL-23 39. The success of in-vivo DC-based therapy of autoimmune diseases is based on developing stable tolerogenic DCs which protect their immature properties after in-vivo exposure to the inflammatory microenvironment 40. To achieve this goal, in the present study stable tolerogenic DCs were produced by permanent knock-down of CD40 and IL-23 using their specific shRNAs lentiviral vectors, and their therapeutic efficacy was checked in the EAE model.
Our results showed that while the production of IL-6 and IL-12 in BMDCs was reduced significantly after transduction with p19-or CD40-specific shRNA, p19-specific shRNA induced a more significant decrease in IL-6 production by transduced BMDCs (Fig. 3d), a key cytokine in EAE pathogenesis, by induction of Th17 cells. In addition, co-transduction of BMDCs with p19+CD40-specific shRNA encoding lentiviral vectors (BoLV-DCs) showed a more prominent effect in lessening the production of IL-6 and IL-12 when compared with p19LV-DCs and CD40LV-DCs (Fig. 3b,d). Although transduction of BMDCs with lentiviral vectors encoding p19-specific shRNA or CD40-specific shRNA induced a significant increase in IL-10 production by transduced BMDCs, the level of IL-10 production by p19LV-DCs was higher than CD40LV-DCs and co-transduction with both shRNAs induced an even more significant increase in IL-10 production compared with CoLV-DCs (Fig. 3c). In line with our findings, it has been shown that CD40-deficient DCs as well as transfected monocyte-derived DCs from healthy donors with anti-sense oligonucleotides specific to IL-23 produced more IL-10 spontaneously, while the levels of IL-12 in their culture supernatant are lower than normal DCs 41. In addition, determining the cytokine levels in the supernatant of MOG35–55-specific CD4+ T cells after co-culture with transduced BMDCs showed more reduction in IFN-γ production by p19-specific shRNA-transduced BMDCs compared with CD40LV-DCs, although the greatest reduction was observed after co-culture with BoLV-DCs (Fig. 4b). Again, as shown in Fig. 4c, the largest increase in the IL-10 levels was detected after co-culture of MOG35–55-specific CD4+ T cells with co-transduced BMDCs (BoLV-DCs). In this respect, Vaknin-Dembinsky et al. have reported that transduced DCs with p19 anti-sense had a tolerogenic effect on co-cultured CD4+ T cells in which activated T cells acquired regulatory T cell (Treg) properties with increased expression of IL-10 2.
Given that the roles of IL-6 and IL-12 in Th17 and Th1 induction as well as in EAE pathogenesis have been well recognized, on the basis of our in-vitro results it would be conceivable that treatment of EAE mice with BMDCs co-transduced with lentiviral vectors encoding CD40 and p19 shRNA result in a more significant decrease in EAE scores compared to those treated with control vector. Interestingly, administrations of MOG35–55-loaded and transduced BMDCs have shown a considerable decrease in the clinical score of EAE mice (Fig. 5). In line with the results achieved from in-vitro experiments, in comparison to transduced control BMDCs, the strongest impact on the reduction of clinical scores have been observed when co-transduced BMDCs (BoLV-DCs) were administered into EAE mice (0·92 ± 0·36 and 2·42 ± 0·85, respectively; P = 0·012; Fig. 5). Regarding the importance of IL-6 and IL-12 in the pathogenesis of EAE and considering the fact that the lowest levels of those cytokines, as well as the highest levels of IL-10, were observed in the culture media of co-transduced BMDCs (Fig. 3), detecting the lowest clinical score in mice treated with co-transduced BMDCs is rational. In accordance with this finding, although not statistically significant, the levels of secreted IL-17 by mononuclear cells isolated from lymph nodes and CNS of mice treated with BoLV-DCs were lower than other treated groups, while they produced more IL-10 compared with others (Fig. 6). It is also worth mentioning that isolated splenocytes, lymph node cells or CNS-derived mononuclear cells from treated-EAE mice with p19LV-DCs or BoLV-DCs have shown a significant decrease in GM-CSF production compared to CoLV-DCs after in-vitro activation (Fig. 6). Considering the attenuation of EAE after GM-CSF neutralization 42, this finding also provides another explanation for a lower clinical score that observed in EAE mice treated with co-transduced BMDCs or p19-specific shRNA lentiviral-transduced BMDCs compared with CD40-specific shRNA lentiviral-transduced BMDCs.
In conclusion, the results of the present study showed that knock-down DCs for p19 subunit of IL-23 can ameliorate the clinical score of EAE as well as CD40 knock-down DCs. In addition, injection of simultaneously transduced DCs with CD40-specific and p19-specific shRNA encoding lentiviral vectors showed a more profound effect on decreasing EAE severity through down-regulation of IL-12 and IL-6 as well as up-regulation of IL-10 expression by DCs.
Acknowledgments
The present paper was extracted from the thesis written by Ms Tahereh Kalantari to achieve her PhD degree in immunology from the immunology department of Shiraz University of Medical Sciences. This work was partially supported by Shiraz University of Medical Sciences grant no. 90-5727.
Disclosure
No personal, institutional or corporate financial conflicts are involved in the production and publication of this information.
Author contributions
T. K. performed the experiments, M.-H. K., B. C., Y. Y., A. R. and E. K.-S. designed the study and T. K. and E. K.-S. wrote the paper.
References
- 1.Compston A, Coles A. Multiple sclerosis. Lancet. 2008;372:1502–1517. doi: 10.1016/S0140-6736(08)61620-7. [DOI] [PubMed] [Google Scholar]
- 2.Vaknin-Dembinsky A, Balashov K, Weiner HL. IL-23 is increased in dendritic cells in multiple sclerosis and down-regulation of IL-23 by antisense oligos increases dendritic cell IL-10 production. J Immunol. 2006;176:7768–7774. doi: 10.4049/jimmunol.176.12.7768. [DOI] [PubMed] [Google Scholar]
- 3.Harrington LE, Hatton RD, Mangan PR, et al. Interleukin 17–producing CD4+ effector T cells develop via a lineage distinct from the T helper type 1 and 2 lineages. Nat Immunol. 2005;6:1123–1132. doi: 10.1038/ni1254. [DOI] [PubMed] [Google Scholar]
- 4.Langrish CL, Chen Y, Blumenschein WM, et al. IL-23 drives a pathogenic T cell population that induces autoimmune inflammation. J Exp Med. 2005;201:233–240. doi: 10.1084/jem.20041257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Park H, Li Z, Yang XO, et al. A distinct lineage of CD4 T cells regulates tissue inflammation by producing interleukin 17. Nat Immunol. 2005;6:1133–1141. doi: 10.1038/ni1261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Bradshaw EM, Raddassi K, Elyaman W, et al. Monocytes from patients with type 1 diabetes spontaneously secrete proinflammatory cytokines inducing Th17 cells. J Immunol. 2009;183:4432–4439. doi: 10.4049/jimmunol.0900576. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Seki M, Oomizu S, Sakata K-M, et al. Galectin-9 suppresses the generation of Th17, promotes the induction of regulatory T cells, and regulates experimental autoimmune arthritis. Clin Immunol. 2008;127:78–88. doi: 10.1016/j.clim.2008.01.006. [DOI] [PubMed] [Google Scholar]
- 8.Durelli L, Conti L, Clerico M, et al. T-helper 17 cells expand in multiple sclerosis and are inhibited by interferon-β. Ann Neurol. 2009;65:499–509. doi: 10.1002/ana.21652. [DOI] [PubMed] [Google Scholar]
- 9.Batten M, Li J, Yi S, et al. Interleukin 27 limits autoimmune encephalomyelitis by suppressing the development of interleukin 17-producing T cells. Nat Immunol. 2006;7:929–936. doi: 10.1038/ni1375. [DOI] [PubMed] [Google Scholar]
- 10.Segura E, Touzot M, Bohineust A, et al. Human inflammatory dendritic cells induce Th17 cell differentiation. Immunity. 2013;38:336–348. doi: 10.1016/j.immuni.2012.10.018. [DOI] [PubMed] [Google Scholar]
- 11.Perona-Wright G, Jenkins SJ, O'Connor RA, et al. A pivotal role for CD40-mediated IL-6 production by dendritic cells during IL-17 induction in vivo. J Immunol. 2009;182:2808–2815. doi: 10.4049/jimmunol.0803553. [DOI] [PubMed] [Google Scholar]
- 12.Iezzi G, Sonderegger I, Ampenberger F, Schmitz N, Marsland BJ, Kopf M. CD40–CD40L cross-talk integrates strong antigenic signals and microbial stimuli to induce development of IL-17-producing CD4+ T cells. Proc Natl Acad Sci USA. 2009;106:876–881. doi: 10.1073/pnas.0810769106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Bettelli E, Carrier Y, Gao W, et al. Reciprocal developmental pathways for the generation of pathogenic effector TH17 and regulatory T cells. Nature. 2006;441:235–238. doi: 10.1038/nature04753. [DOI] [PubMed] [Google Scholar]
- 14.Veldhoen M, Hocking RJ, Atkins CJ, Locksley RM, Stockinger B. TGFβ in the context of an inflammatory cytokine milieu supports de novo differentiation of IL-17-producing T cells. Immunity. 2006;24:179–189. doi: 10.1016/j.immuni.2006.01.001. [DOI] [PubMed] [Google Scholar]
- 15.Mangan PR, Harrington LE, O'Quinn DB, et al. Transforming growth factor-β induces development of the TH17 lineage. Nature. 2006;441:231–234. doi: 10.1038/nature04754. [DOI] [PubMed] [Google Scholar]
- 16.Korn T, Bettelli E, Gao W, et al. IL-21 initiates an alternative pathway to induce proinflammatory TH17 cells. Nature. 2007;448:484–487. doi: 10.1038/nature05970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Nurieva R, Yang XO, Martinez G, et al. Essential autocrine regulation by IL-21 in the generation of inflammatory T cells. Nature. 2007;448:480–483. doi: 10.1038/nature05969. [DOI] [PubMed] [Google Scholar]
- 18.Zhou L, Ivanov II, Spolski R, et al. IL-6 programs TH-17 cell differentiation by promoting sequential engagement of the IL-21 and IL-23 pathways. Nat Immunol. 2007;8:967–974. doi: 10.1038/ni1488. [DOI] [PubMed] [Google Scholar]
- 19.Grimm D, Kay MA. RNAi and gene therapy: a mutual attraction. Hematology Am Soc Hematol Educ Program. 2007;2007:473–481. doi: 10.1182/asheducation-2007.1.473. [DOI] [PubMed] [Google Scholar]
- 20.Fu W, Zhu J, Qiu Y, Li W. Induction of CD4+CD25+ T cells and control of cardiac allograft rejection by CD40/CD40L costimulatory pathway blockade in mice. Transplant Proc. 2013;45:611–617. doi: 10.1016/j.transproceed.2012.10.044. [DOI] [PubMed] [Google Scholar]
- 21.Wang B, Qian H, Yang H, Xu L, Xu W, Yan J. Regression of atherosclerosis plaques in apolipoprotein E(–)/(–) mice after lentivirus-mediated RNA interference of CD40. Int J Cardiol. 2013;163:34–39. doi: 10.1016/j.ijcard.2011.05.053. [DOI] [PubMed] [Google Scholar]
- 22.Suzuki M, Zheng X, Zhang X, et al. A novel allergen-specific therapy for allergy using CD40-silenced dendritic cells. J Allergy Clin Immunol. 2010;125:737–743. doi: 10.1016/j.jaci.2009.11.042. e736. [DOI] [PubMed] [Google Scholar]
- 23.Zheng X, Suzuki M, Ichim TE, et al. Treatment of autoimmune arthritis using RNA interference-modulated dendritic cells. J Immunol. 2010;184:6457–6464. doi: 10.4049/jimmunol.0901717. [DOI] [PubMed] [Google Scholar]
- 24.Li Y, Sun M, Cheng H, et al. Silencing IL-23 expression by a small hairpin RNA protects against asthma in mice. Exp Mol Med. 2011;43:197–204. doi: 10.3858/emm.2011.43.4.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Inaba K, Steinman RM, Pack MW, et al. Identification of proliferating dendritic cell precursors in mouse blood. J Exp Med. 1992;175:1157–1167. doi: 10.1084/jem.175.5.1157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Koch F, Stanzl U, Jennewein P, et al. High level IL-12 production by murine dendritic cells: upregulation via MHC class II and CD40 molecules and downregulation by IL-4 and IL-10. J Exp Med. 1996;184:741–746. doi: 10.1084/jem.184.2.741. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Jonuleit H, Schmitt E, Schuler G, Knop J, Enk AH. Induction of interleukin 10-producing, nonproliferating CD4(+) T cells with regulatory properties by repetitive stimulation with allogeneic immature human dendritic cells. J Exp Med. 2000;192:1213–1222. doi: 10.1084/jem.192.9.1213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Banchereau J, Steinman RM. Dendritic cells and the control of immunity. Nature. 1998;392:245–252. doi: 10.1038/32588. [DOI] [PubMed] [Google Scholar]
- 29.Mackey MF, Barth R, Noelle RJ. The role of CD40/CD154 interactions in the priming, differentiation, and effector function of helper and cytotoxic T cells. J Leukoc Biol. 1998;63:418–428. doi: 10.1002/jlb.63.4.418. [DOI] [PubMed] [Google Scholar]
- 30.Cho M-L, Kang J-W, Moon Y-M, et al. STAT3 and NF-κB signal pathway is required for IL-23-mediated IL-17 production in spontaneous arthritis animal model IL-1 receptor antagonist-deficient mice. J Immunol. 2006;176:5652–5661. doi: 10.4049/jimmunol.176.9.5652. [DOI] [PubMed] [Google Scholar]
- 31.Caux C, Massacrier C, Vanbervliet B, et al. Activation of human dendritic cells through CD40 cross-linking. J Exp Med. 1994;180:1263–1272. doi: 10.1084/jem.180.4.1263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.McLellan AD, Starling GC, Williams LA, Hock BD, Hart DN. Activation of human peripheral blood dendritic cells induces the CD86 co-stimulatory molecule. Eur J Immunol. 1995;25:2064–2068. doi: 10.1002/eji.1830250739. [DOI] [PubMed] [Google Scholar]
- 33.Krajina T, Leithäuser F, Möller P, Trobonjaca Z, Reimann J. Colonic lamina propria dendritic cells in mice with CD4+ T cell-induced colitis. Eur J Immunol. 2003;33:1073–1083. doi: 10.1002/eji.200323518. [DOI] [PubMed] [Google Scholar]
- 34.Chen Y, Langrish CL, McKenzie B, et al. Anti–IL-23 therapy inhibits multiple inflammatory pathways and ameliorates autoimmune encephalomyelitis. J Clin Invest. 2006;116:1317–1326. doi: 10.1172/JCI25308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Nakae S, Nambu A, Sudo K, Iwakura Y. Suppression of immune induction of collagen-induced arthritis in IL-17-deficient mice. J Immunol. 2003;171:6173–6177. doi: 10.4049/jimmunol.171.11.6173. [DOI] [PubMed] [Google Scholar]
- 36.Weyand CM, Younge BR, Goronzy JJ. IFN-γ and IL-17-the two faces of T cell pathology in giant cell arteritis. Curr Opin Rheumatol. 2011;23:43–49. doi: 10.1097/BOR.0b013e32833ee946. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Zheng X, Suzuki M, Zhang X, et al. RNAi-mediated CD40-CD154 interruption promotes tolerance in autoimmune arthritis. Arthritis Res Ther. 2010;12:R13. doi: 10.1186/ar2914. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Yen D, Cheung J, Scheerens H, et al. IL-23 is essential for T cell-mediated colitis and promotes inflammation via IL-17 and IL-6. J Clin Invest. 2006;116:1310–1316. doi: 10.1172/JCI21404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Anderson AE, Sayers BL, Haniffa MA, et al. Differential regulation of naive and memory CD4+ T cells by alternatively activated dendritic cells. J Leukoc Biol. 2008;84:124–133. doi: 10.1189/jlb.1107744. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Hilkens CM, Isaacs JD, Thomson AW. Development of dendritic cell-based immunotherapy for autoimmunity. Int Rev Immunol. 2010;29:156–183. doi: 10.3109/08830180903281193. [DOI] [PubMed] [Google Scholar]
- 41.Gao J, Madrenas J, Zeng W, et al. CD40-deficient dendritic cells producing interleukin-10, but not interleukin-12, induce T-cell hyporesponsiveness in vitro and prevent acute allograft rejection. Immunology. 1999;98:159–170. doi: 10.1046/j.1365-2567.1999.00863.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.El-Behi M, Ciric B, Dai H, et al. The encephalitogenicity of TH17 cells is dependent on IL-1-and IL-23-induced production of the cytokine GM-CSF. Nat Immunol. 2011;12:568–575. doi: 10.1038/ni.2031. [DOI] [PMC free article] [PubMed] [Google Scholar]


