Abstract
Autoimmune diabetes is characterized by autoantigen-specific T cell-mediated destruction of pancreatic islet beta cells, and CD8+ T cells are key players during this process. We assessed whether the bitransgenic RIP-CD80 x RIP-LCMV-GP (RIP-CD80GP) mice may be a versatile antigen-specific model of inducible CD8+ T cell-mediated autoimmune diabetes. Antigen-encoding DNA, peptide-loaded dendritic cells and antigen plus incomplete Freund's adjuvant were used for vaccination. Of 14 pancreatic proteins tested by DNA vaccination, murine pre-proinsulin 2 (100% of mice; median time after vaccination, 60 days) and islet-specific glucose-6-phosphatase catalytic subunit-related protein (IGRP) (77%, 58 days) could induce diabetes. Vaccination with DNA encoding for zinc transporter 8, Ia-2, Ia-2β, glutamic acid decarboxylase 67 (Gad67), chromogranin A, insulinoma amyloid polypeptide and homeobox protein Nkx-2.2 induced diabetes development in 25–33% of mice. Vaccination with DNA encoding for Gad65, secretogranin 5, pancreas/duodenum homeobox protein 1 (Pdx1), carboxyl ester lipase, glucagon and control hepatitis B surface antigen (HBsAg) induced diabetes in <20% of mice. Diabetes induction efficiency could be increased by DNA vaccination with a vector encoding a ubiquitin–antigen fusion construct. Diabetic mice had florid T cell islet infiltration. CD8+ T cell targets of IGRP were identified with a peptide library-based enzyme-linked immunospot assay, and diabetes could also be induced by vaccination with major histocompatibility complex (MHC) class I-restricted IGRP peptides loaded on mature dendritic cells. Vaccination with antigen plus incomplete Freund's adjuvant, which can prevent diabetes in other models, led to rapid diabetes development in the RIP-CD80GP mouse. We conclude that RIP-CD80GP mice are a versatile model of antigen specific autoimmune diabetes and may complement existing mouse models of autoimmune diabetes for evaluating CD8+ T cell-targeted prevention strategies.
Keywords: antigens/peptides/epitopes, autoimmunity, cytotoxic T cells, diabetes
Introduction
In type 1 diabetes (T1D), a self-directed immune response leads to the destruction of insulin-producing beta cells in the pancreatic islets. There is good evidence from animal models of disease that autoreactive T cells are the critical effectors of beta cell destruction 1–4. Moreover, studies with samples from cadaveric T1D donors show that pancreatic infiltrates contain and are sometimes dominated by CD8+ T cells 5–10, and include beta cell antigen-specific cells 11. Furthermore, insulin-directed CD8+ T cells cloned from T1D patients can efficiently kill autoantigen-expressing cells 12.
The relevance of CD8+ T cells in human T1D is currently poorly represented in animal models. The rat insulin promoter (RIP)-CD80 mouse model, established almost two decades ago, transgenically expresses the CD80 co-stimulatory molecule on islet beta cells to facilitate a break in self-tolerance when CD8+ T cells directed against a beta cell-specific self-protein are present 13. Sole vaccination with plasmid DNA encoding for murine or human insulin is sufficient to induce CD8+ T cell-mediated beta cell destruction and diabetes development 14. Diabetes induction upon DNA vaccination with insulin is independent of CD4+ T cells in this model, but depends critically upon interferon (IFN)-γ-secreting diabetogenic CD8+ T cells that specifically recognize the Kb-restricted insulin A-chain epitope A12–21 15. RIP-CD80 mice that are cross-bred to RIP-lymphocytic choriomeningitis virus glycoprotein (LCMV-GP) 16 transgenic mice (RIP-CD80GP) can additionally be rendered diabetic by vaccination with a plasmid encoding for the neo-self-antigen LCMV-GP and also show a higher sensitivity towards insulin DNA vaccination 17.
In this study, we examined the versatility of the RIP-CD80GP mouse as a model of antigen-induced CD8+ T cell-mediated autoimmune diabetes. We screened a set of additional islet proteins for their potential to induce diabetes upon DNA vaccination, multiple antigen vaccination strategies and exemplarily tested targeting to the antigen processing machinery.
Material and methods
Mice
The generation of RIP-murine CD80 (B7·1) transgenic mice back-crossed for >15 generations to the C57BL/6 (H-2b) background, as well as the generation of RIP-LCMV-GP on the C57BL/6 background, has been described previously 13,16. For experimental studies, heterozygous F1 animals (RIP-CD80 × RIP-LCMV-GP), single transgenic RIP-CD80 or C57BL/6 were used. All animal studies were conducted with institutional board approval in accordance with the Federal German Animal Protection Law (55·2-1-54-2531-154-08; 24-9168·11-1/2010-39).
Construction of DNA expression vectors
Antigens were selected on the basis of known targets of autoantibodies in patients with type 1 diabetes and their pancreatic islet-specific expression and abundance 18. cDNA of pancreatic islet antigens was generated by reverse transcription–polymerase chain reaction (RT–PCR) from C57BL/6 murine islet cell RNA. Subsequently, for each antigen encoding cDNA PCR primers containing appropriate restriction sites, as well as a uniform Kozak consensus, GTAGGCATG were used to allow ligation of the PCR product into the multiple cloning site of pcDNA3·1V5HisB (Invitrogen, Carlsbad, CA, USA). NotI/XbaI restriction sites were used for cloning of insulinoma amyloid polypeptide (IAPP) (gene ID: 15874 Iapp; 93 amino acids), chromogranin A (ChgA) (gene ID: 12652 Chga; 463aa), islet-specific glucose-6-phosphatase catalytic subunit-related protein (IGRP) (gene ID: 14378 G6pc2; 355aa), homeobox protein Nkx-2.2 (Nkx2.2) (gene ID: 18088 Nkx2-2; 273aa), glutamic acid decarboxylase 67 (Gad67) (gene ID: 14415 Gad1; 593aa), carboxyl ester lipase (Cel) (gene ID: 12613 Cel; 599aa), pancreas duodenum homeobox protein 1 (Pdx-1) (gene ID: 18609 Pdx1; 284aa), pre-proinsulin 2 (ppIns) (gene ID: 16334 Ins2; 110aa), Gad65 (gene ID: 14417 Gad2; 585aa), insulinoma-associated protein 2 (Ia-2) intracellular region (i.c.) (gene ID: 19275 Ptprn; 381) and Ia-2β i.c. (gene ID: 19276 Ptprn2; 379). NotI/BstBI restriction sites were used for cloning of hepatitis B surface antigen (HBsAg) (HBsAg AS1-AS226 EF103285), zinc transporter 8 (ZnT8) (gene ID: 239436 Slc30a8; 367aa), secretogranin 5 (Scg5) (gene ID: 20394 Scg5; 212aa), LCMV-GP (LCMV-GP AS1-AS498, NM _AF186080), and glucagon (gene ID: 14526 Gcg; 180aa). All antigen-encoding vectors used in this study are based on the pcDNA3·1 V5HisB backbone except for pCI/ppinsN110A, which was kindly provided by Reinhold Schirmbeck, Ulm, Germany, and uses the pCI backbone. The construction of pCI/ppinsN110A has been described previously 19. For cloning of Ia-2 and Ia-2β i.c. constructs, start codons were introduced into the sense primers accordingly. The Ia-2 i.c. construct encodes for Ia-2 amino acids 599-979, the Ia-2β i.c. construct encodes for amino acids 623-1001. For construction of ubiquitin–ZnT8 (Ubi–ZnT8) or immunoglobulin G–signal peptide (IgG-SP) fusion containing plasmids, ubiquitin (gene ID: 22190 Ubc; 76aa) or IgG-SP (gene ID: 692179 aa1-21) encoding DNA was PCR amplified from murine islet cDNA with primers that allow homologous recombination of ubiquitin or IgG-SP with the above-mentioned ZnT8 construct upstream of ZnT8. For the Ubi–ZnT8-encoding construct the anti-sense primer was designed to remove the start codon of ZnT8 and to introduce a G76V mutation in ubiquitin, resulting in stable ubiquitin fusion to ZnT8 after translation. Subsequently, Red/ET recombination technology 20 was carried out using recombineering proficient competent Escherichia coli GB2005 harbouring the pSC101-BAD-gbaA plasmid, as described previously 21. Primer details are listed in the Supporting information. Construct-driven expression of protein was tested by transfection in HEK293FT cells and Western blot (Supporting information).
Vaccination and follow-up
For DNA vaccination, 50 μg of plasmid DNA (dissolved in 50 μl saline) was administered intramuscularly into each tibialis anterior muscle of 10–12-week-old male or female mice. No pretreatment or adjuvants were used. For peptide vaccination, bone marrow cells were isolated from B6·129S7-Ragtm1Mom/J mice, cultivated in medium containing interleukin (IL)-4 and granulocyte–macrophage colony-stimulating factor (GM-CSF) and stimulated with lipopolysaccharide (LPS) from day 5. On day 6, fractions of cells were incubated for 1 h in medium containing 10 μM of the respective peptides (0·1 μM in the case of LCMV-GP33–41). Cells were washed in phosphate-buffered saline (PBS) and injected intraperitoneally at 200 000 cells/per mouse. LCMV-GP33–41 (KAVYNFATM), LCMV-GP276–286 (SGVENPGGYCL), IGRP225–233 (LRLFGIDLL), IGRP241–249 (KWCANPDWI) and LCMV-NP396–404 (FQPQNGQFI) were synthesized by Bio-Synthesis (Lewisville, TX, USA). For immunization with antigen and incomplete Freund's adjuvant (IFA), mice (n = 24) were injected subcutaneously with 100 μg insulin (human recombinant; Sigma-Aldrich, St Louis, MO, USA, 91077C) dissolved in water (1 mg/ml) and emulsified 1:1 in IFA (Sigma-Aldrich, F5506) or with IFA alone at 4, 5, 6 and 7 weeks of age. At age 11 weeks, the mice received 100 μg ppIns2 DNA as described or were left untreated. In all mice, urine glucose levels were measured twice weekly (Diastix; Bayer HealthCare LLC, Mishawaka, IN, USA). Diabetes was defined as two consecutive urine glucose values >5·5 mmol/l and blood glucose levels >13·9 mmol/l (Glucometer Elite; Bayer Diagnostics). If not stated differently, mice were followed for 140 days post-final vaccination.
Histology
Pancreatic cryosections (5 μm) were fixed in 4% formalin and stained for c-peptide or glucagon using polyclonal rabbit anti-c-peptide (Cell Signaling, Boston, MA, USA) or polyclonal rabbit anti-glucagon (Dako, Glostrup, Denmark) followed by Alexa Fluor 488-labelled polyclonal goat anti-rabbit IgG (Invitrogen) antibodies. Subsequently detection of CD4 or CD8 was carried out using rat anti-CD4 (clone RM4-5) or rat anti-CD8 antibodies (clone 53-6·7; both BD Pharmingen, San Diego, CA, USA) followed by staining with Alexa Fluor 568-labelled polyclonal goat anti-rat IgG secondary antibodies (Invitrogen). 4′-6-Diamidino-2-phenylindole (DAPI) staining of DNA was used to visualize cell nuclei. All images were acquired with a Leica SP5 upright Laser Scanning confocal microscope.
Enzyme-linked immunospot (ELISPOT) assays
IFN-γ ELISPOT assays were carried out with purified CD8+ T cells from vaccinated mice and T cell-depleted splenocytes as antigen-presenting cells according to the previously described protocol 22. In short, CD8+ T cells were pre-enriched from cells of spleen, popliteal and pancreatic lymph nodes using magnetic-activated cell sorting (MACS) technology and CD8+ T cells were sorted to high purity on a BD Aria II Sorp. For antigen presentation, splenocytes from healthy RIP-CD80 mice were freshly isolated and depleted of T cells using biotinylated anti-CD3 (clone 145-2C11), anti-CD4 (clone GK1·5) and anti-CD8 antibodies (clone 53-6·7, all eBioscience, San Diego, CA, USA) followed by streptavidin coupled microbeads and MACS LD separation columns (Milteyni Biotech, Bergisch Gladbach, Germany). Per well, 1·8 × 105 antigen-presenting cells were used together with 3 × 104 purified viable CD8+ T cells. IGRP 15mer library peptides were synthesized in screening grade by Mimotopes (Clayton, Victoria, Australia), peptides were dissolved with dimethylsulphoxide (DMSO) and used at a concentration of 10 μg/ml. GP33–41 (KAVYNFATM) and ppIns101–110 (A12–21) (SLYQLENYCN) peptide were purchased from PANATecs (Tübingen, Germany) at >98% purity and used in the assay at a final concentration of 10 μg/ml. Stimulation with anti-CD3/anti-CD28/anti-CD137 (Invitrogen) coupled beads was used as positive control. Incubation was for 24 h in 96-well ELISPOT MultiScreen Filter plates (Merck Millipore, Darmstadt, Germany) and IFN-γ-specific antibody pairs from U-Cytech (Utrecht, the Netherlands) were used for spot detection. Plates were processed according to the manufacturer's instructions and as described previously 22.
Statistical analysis
Prism 5·03 GraphPad software was used for statistical analyses. Kaplan–Meier time-to-event analysis was used for estimating diabetes-free survival in mice. Comparisons of survival curves between groups were made using the log-rank test. For all analyses, a two-tailed P-value <0·05 was considered significant. P-values were not corrected for multiple comparisons.
Results
DNA vaccination identifies IGRP as diabetogenic antigen in RIP-CD80GP mice
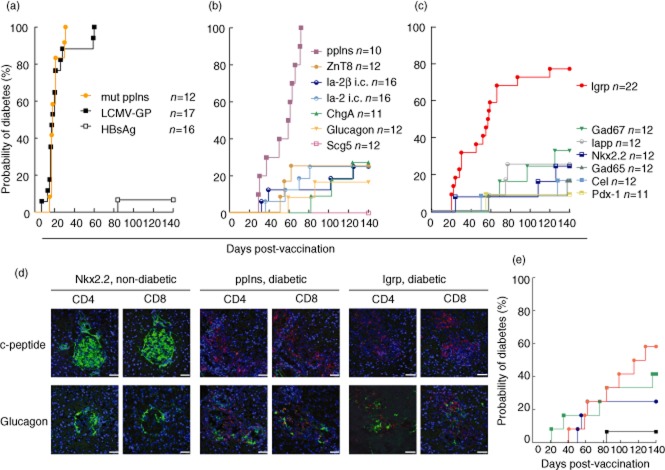
As reported previously, vaccination with a construct encoding for mutated murine pre-proinsulin 2 (mut ppIns, n = 12 mice) or the model autoantigen LCMV-GP (n = 17 mice) led to diabetes in all vaccinated RIP-CD80GP mice after a median of 18 days (Fig. 1a). In contrast, only one of 16 mice developed diabetes upon vaccination with a vector encoding for the non-endogenously expressed HBsAg.
Figure 1.
Diabetes induction by vaccination in RIP-CD80GP mice. Ten to 12-week-old mice were vaccinated with DNA vectors encoding for established (mutated ppIns, LCMV-GP) and control [hepatitis B surface antigen (HBsAg)] antigens in this model; (a), secretory granule-associated islet proteins (b) or other islet proteins (c) and followed for diabetes development shown by life table analysis. Numbers of mice vaccinated per group are indicated. Immunohistological images (d) of islets from vaccinated non-diabetic mice (Nkx2·2-vaccinated, 140 days post-vaccination), and diabetic mice at diabetes onset (ppIns-vaccinated – 61 days after vaccination, IGRP-vaccinated – 45 days after vaccination). C-peptide or glucagon-positive cells are depicted in green; CD4-or CD8-positive cells are shown in red. Scale bars = 50 μm. Vaccination was also performed with antigen targeted to the antigen processing machinery (e). Ten to 12-week-old RIP-CD80GP mice were vaccinated with standard zinc transporter 8 (ZnT8) encoding DNA vector (blue line, n = 12), vectors encoding for ubiquitin-ZnT8 (red line, n = 12) or immunoglobulin (Ig)G-signal peptide-ZnT8 (green line, n = 12) fusion constructs, or vector encoding control HBsAg (black line, n = 16). Mice were followed for diabetes development shown as a life table analysis.
A further 14 antigens found in pancreatic islets were tested. Seven of these were secretory granule proteins (Fig. 1b). DNA vaccination with vectors encoding for wild-type pre-proinsulin 2 induced diabetes in all mice (ppIns; n = 10; P < 0·0001 compared to HBsAg control) at a median of 60 days post-vaccination. Of note, vectors encoding for wild-type and mutated pre-proinsulin 2 also induced diabetes in single transgenic RIP-CD80 mice, although with delayed disease onset and at a lower frequency (40 and 50%, respectively, data not shown). In RIP-CD80GP, cases of diabetes were also observed in mice vaccinated with constructs encoding for ZnT8 (three of 12 mice), the intracellular domain of the receptor tyrosine phosphatase family members Ia-2 or Ia-2β (Ia-2 i.c.; Ia-2β i.c.; each four of 16 mice), ChgA (three of 11 mice) or the α-cell peptide hormone glucagon (two of 12 mice). Frequencies of diabetes were not, however, different to that of the HBsAg control group. Vaccination with Scg5, which is highly and specifically expressed in beta cells, resulted in no cases of diabetes.
Among the seven non-secretory granule antigens, vaccination with plasmid DNA encoding for the islet-specific glucose-6-phosphatase IGRP induced diabetes from as early as 20 days post-vaccination; 17 (77%) of 22 IGRP-vaccinated animals developed diabetes within the observation period with a diabetes onset comparable to ppIns (median 58 days; P < 0·0001 compared to HBsAg control; Fig. 1c). Additionally, diabetes development was observed in 25–33% of mice vaccinated with constructs encoding for Gad67 (four of 12 mice), IAPP (three of 12 mice), Nkx 2·2 (three of 12 mice), Gad65 (two of 12 mice), Cel (two of 12 mice) and Pdx-1 (one of 12 mice). Again, frequencies of diabetes were not different to that of the HBsAg control group.
For both ppIns and IGRP-vaccinated mice, florid lymphocyte infiltrates were observed in pancreatic islets along with reduced beta cells within islets at diabetes onset (Fig. 1d). Infiltrates contained both CD8+ and CD4+ T cells. Islets were unaffected and remained free of lymphocytes in non-diabetic mice immunized with a low diabetes-inducing DNA and killed 140 days after vaccination.
In order to increase diabetogenicity of the low diabetes-inducing antigens, we targeted antigen delivery to the antigen processing compartments. To this end, we used homologous recombination to either insert a non-cleavable ubiquitin or IgG signal peptide upstream of ZnT8 in the ZnT8-encoding DNA vaccination vector. Five of 12 mice developed diabetes using endoplasmic reticulum (ER)-targeting (P = 0·03 versus HBsAg control) and seven of 12 mice (P = 0·003 versus HBsAg control) upon DNA vaccination with the vector encoding for the ubiquitin fusion construct (Fig. 1e). We did not test HBsAg with ubiquitin or IgG signal peptide and cannot exclude that the approach may increase diabetes induction by irrelevant antigen.
Identification of IGRP CD8+ T cell targets in DNA-vaccinated mice
CD8+ T cells from spleens and lymph nodes of IGRP DNA-vaccinated diabetic mice were tested against an IGRP 15mer peptide library to identify peptides targeted by CD8+ T cells upon DNA vaccination with IGRP encoding vector. Strong responses could be detected towards the adjacent 15mer peptides IGRP237–251, IGRP241–255 that contain the H-2Db restricted 9mer epitope IGRP241–249 (Fig. 2a). Additionally, lower responses were detected against the peptide IGRP245–259 that partially overlaps the same epitope and against IGRP273–287, which does not contain any previously described epitopes of IGRP. CD8+ T cell responses could also be detected towards the insulin epitope ppIns101–110 (A12–21), as well as against the dominant LCMV-GP epitope GP33–41, suggesting that antigen-spreading had occurred within these mice. Separately, non-transgenic C57BL/6 mice were immunized with IGRP-encoding plasmid and splenocytes tested for IGRP reactivity 7 weeks post-vaccination. These mice showed low responses (<10 spots per 100 000 CD8+ T cells) to only few IGRP peptides, suggesting that the strong responses observed in IGRP-vaccinated, diabetic RIP-CD80GP mice had been amplified in the islet pathology region.
Figure 2.

CD8+ T cell targets in RIP-CD80GP mice. (a) Identification of IGRP peptide regions targeted by CD8+ T cells in IGRP vector-vaccinated diabetic mice. Representative data of an interferon (IFN)-γ enzyme-linked immunospot (ELISPOT) assay performed with sorted CD8+ T cells isolated from pooled splenocytes, popliteal and pancreatic lymph node cells of two mice are shown (black bars). T cell-depleted splenocytes from non-vaccinated RIP-CD80 mice loaded with peptides of an overlapping IGRP 15mer peptide library were used for antigen presentation. As controls, cells were incubated with peptide solvent [dimethylsulphoxide (DMSO)], polyclonal T cell stimulatory anti-CD3/anti-CD28/anti-CD137-coated beads and splenocytes loaded with immunodominant epitopes of the insulin A chain (A12–21) and LCMV-GP (GP33–41). Number of spots per 100 000 CD8+ T cells (filled bars) and in the absence of T cells (open bars) is shown, as are the numbers of spots observed 7 weeks post-IGRP vector vaccination in non-transgenic C57BL/6 mice (grey bars). (b) Diabetes induction upon vaccination with bone marrow-derived dendritic cells loaded with peptide. Ten to 12-week-old mice were injected intraperitoneally with 200 000 BMDC pulsed with major histocompatibility complex (MHC) class I-restricted peptides of LCMV-GP (GP33–41, black line, n = 10; GP276–286, blue line, n = 11), LCMV-NP (NP396–404, grey line, n = 12) or IGRP (IGRP225–233, red line, n = 12; IGRP241–249, green line, n = 11) and followed for diabetes development shown as a life table analysis.
Summarizing, these data suggest that in RIP-CD80GP mice, IGRP241–249 is targeted by CD8+ T cells upon vaccination with IGRP encoding DNA and we identify IGRP273–287 as new target region of IGRP directed CD8+ T cells.
Diabetes can be induced with IGRP-peptide loaded BMDCs
IGRP225–233 and IGRP241–249 have been described as H-2Db-restricted epitopes of IGRP 23 and may represent targets of CD8+ T cells in RIP-CD80GP mice. We applied a bone marrow-derived dendritic cell (BMDC) vaccination approach to examine whether diabetes can be initiated by BMDCs pulsed with either of the two epitopes. As reported previously 24, all mice (n = 10) vaccinated with BMDCs loaded with immunodominant peptide GP33–41 of the model autoantigen LCMV-GP developed diabetes after a median of 8 days (Fig. 2b). Mice vaccinated with BMDC/GP276–286 (n = 11) developed diabetes after a median of 53 days. Vaccination with BMDC/IGRP225–233 induced diabetes in eight of 12 mice (P = 0·025 compared to BMDC/NP396–404-vaccinated control mice), demonstrating that vaccination with a major histocompatibility complex (MHC) class I-restricted IGRP epitope can suffice to induce diabetes development in RIP-CD80GP mice. Vaccination with BMDC/IGRP241–249 led to diabetes development in two mice.
Insulin-IFA immunization does not prevent but induces diabetes in RIP-CD80GP mice
A model in which disease can be induced antigen-specifically offers the opportunity to study antigen-specific disease pre-and intervention. Insulin plus IFA injection can prevent autoimmune diabetes in non-obese diabetic (NOD) mice if given at age 4–10 weeks 25,26. Young mice received either four vehicle/IFA or insulin/IFA immunizations before standard DNA vaccination with ppIns encoding vector. Six of seven mice that received vehicle/IFA immunization and all five mice that received insulin/IFA immunization developed diabetes after a median of 62 and 63 days after ppIns DNA vaccination, indicating no protection by insulin/IFA immunization (Fig. 3). Control mice that received either vehicle/IFA or insulin/IFA treatment without subsequent ppIns DNA vaccination confirmed that insulin/IFA immunization could induce diabetes in RIP-CD80GP mice; none of seven vehicle/IFA-immunized mice and four of five insulin/IFA-immunized mice developed diabetes (P = 0·0036).
Figure 3.

Insulin/incomplete Freund's adjuvant (IFA) immunization in RIP-CD80GP mice. Mice received four weekly insulin/IFA (solid lines, n = 10) or vehicle/IFA (dashed lines, n = 14) immunizations at 4–7 weeks of age. Half the mice from each group were vaccinated at 10 weeks of age (21 days post-final IFA vaccination) with pre-proinsulin (red lines) encoding DNA vector; the other half remained untreated (black lines). All animals were followed for diabetes development shown as a life table analysis.
Discussion
Experimental models of antigen-induced autoimmune diabetes are few, and most involve adoptive transfer of transgenic T cells. In this study, we expand the versatility of the RIP-CD80GP mouse as an experimental antigen-induced autoimmune diabetes model. We show that in addition to insulin and the transgenically expressed LCMV-GP protein, the beta cell antigen IGRP strongly induces diabetes in this model in a manner consistent with CD8+ T cell-mediated disease. Moreover, we show that antigen vaccination strategies can be tuned so that other proteins, including antigens relevant to human T1D, can also induce diabetes in this model. A variety of immunization strategies were effective in inducing diabetes, demonstrating robustness of disease induction and, similar to spontaneous models of autoimmune diabetes and human T1D, immunization with a single antigen led to spreading of T cell responses to other beta cell antigens.
Similar to the NOD mouse, both insulin and IGRP appear to be strong antigen targets in this experimental model, suggesting that they can induce diabetes on diverse MHC backgrounds. Studies have shown that the insulin antigen is likely to be an essential target and IGRP a non-essential target for diabetes in the NOD mouse 27–29. Of note, we saw that insulin-and LCMV-GP-specific CD8+ T cell responses were also generated by vaccination with IGRP. Thus, it is possible that targeting insulin and/or the LCMV-GP may also be required for diabetes development in the RIP-CD80GP model. We did not test vaccination with multiple antigens, nor did we examine T cell responses in mice developing diabetes after vaccination with minor antigens, both of which may have been helpful to understand whether insulin is a required target in the RIP-CD80GP model or whether the differences in diabetes observed between antigens reflect variation in antigenicity or the ability of effector T cells to destroy beta cells.
A range of vaccination strategies was able to induce diabetes in this model. DNA vaccination with model LCMV-GP antigen and insulin had been demonstrated previously to induce diabetes, as had immunization with the LCMV-GP peptide-loaded DCs 17,24. We extended the peptide-loaded BMDC to also include IGRP peptides as diabetes inducers. For the peptide-loaded DC vaccination, the peptides with greatest diabetes induction potential did not correlate with the major CD8+ T cell peptide responses observed after DNA vaccination. It is possible, therefore, that the vaccination strategies tested result in diverse immunodominant epitopes. We also found that vaccination with insulin plus IFA, a treatment that protects against diabetes development in the NOD mouse, induced diabetes in this model. It is unclear why insulin plus IFA induced disease in this model and protects against disease in the NOD mouse. Insulin plus IFA is known to induce a strong antibody response 25 and probably a transient inflammatory response. It is therefore possible that the transgenic RIP-CD80GP islets are sensitive to humoral immune factors that promote beta cell death and activation of CD8+ T cells at the pancreas. Additionally, it has been described that peptides emulsified in IFA can trigger effector cell immunity and, in particular, vaccination with longer peptides or short proteins (as is the case for insulin) can promote cytotoxic T cell responses 30. Thus, the RIP-CD80GP mouse could represent a stringent model with respect to identifying strategies that interfere with diabetes induction. Identification of antigen delivery modes that could prevent the development of diabetes by antigen DNA vaccination in this model are expected to have powerful tolerance-inducing mechanisms.
Along with the versatility of the model demonstrated in the study, the RIP-CD80GP model has limitations. Diabetes induction is strongly facilitated by the transgenic expression of co-stimulatory molecules CD80 specifically on beta cells. This renders the model susceptible to spontaneous diabetes development in 5–16% of older mice (17 and own observations), and low frequencies of diabetes can be induced by vaccination with irrelevant antigen, possibly as a result of generalized inflammation. Nevertheless, experimental antigen-specific models have distinct benefits. The inducing antigen is known, thereby providing the ability to intervene against the primary disease-inducing antigen and monitor responses to this antigen. Such models have provided preclinical evaluation of therapies in immune-mediated diseases such as multiple sclerosis and allergy 31,32. The RIP-CD80GP model also has the advantage that peptides targeted by CD8+ T cells can be used to induce and potentially drive disease and therefore strategies to interfere with CD8+ T cell-mediated disease can be tested. Finally, in contrast to the NOD mouse and more similar to man, diabetes could be induced equally in both males and females. We therefore propose the experimental autoimmune diabetes RIP-CD80GP mouse to be considered in the repertoire of autoimmune disease models that should be used for preclinical evaluation of immune tolerance-inducing therapies.
Acknowledgments
The authors would like to thank Dr Angela Hommel for her support with confocal microscopy and Dr Anne Eugster for discussion. The work was supported by funding from the German Federal Ministry of Education and Research (BMBF) to the German Center for Diabetes Research (DZD e.V.), by the DFG Research Center and Cluster of Excellence – Center for Regenerative Therapies Dresden (FZ 111), by the NIH/DFG Research Career Transition Award Program to Dr Kerstin Adler (KO 3418/1-1) and by the foundation ‘Das zuckerkranke Kind’.
Disclosure
The authors declare no conflicts of interest.
Supporting information
Additional Supporting information may be found in the online version of this article at the publisher's web-site:
Verification of DNA vector-driven antigen expression in mammalian cells was verified exemplarily for four secretory granule antigens and six non-secretory granule antigens. Lysates of human embryonic kidney (HEK) 293 cells transfected with pcDNA 3·1 V5HISB DNA vectors encoding the indicated proteins were separated via sodium dodecyl sulphate-polyacrylamide gel electrophoresis (SDSPAGE) and analysed using immunoblotting with V5-tag directed antibodies. Equal amounts of lysates were loaded. Blots show empty vector-transfected controls (left lanes, −) and antigen-transfected samples (right lanes, +) for each antigen. Protein standards in kDa are shown for each respective sample.
References
- 1.Utsugi T, Yoon JW, Park BJ, et al. Major histocompatibility complex class I-restricted infiltration and destruction of pancreatic islets by NOD mouse-derived beta-cell cytotoxic CD8+ T-cell clones in vivo. Diabetes. 1996;45:1121–1131. doi: 10.2337/diab.45.8.1121. [DOI] [PubMed] [Google Scholar]
- 2.Wong FS, Visintin I, Wen L, Flavell RA, Janeway CA., Jr CD8 T cell clones from young nonobese diabetic (NOD) islets can transfer rapid onset of diabetes in NOD mice in the absence of CD4 cells. J Exp Med. 1996;183:67–76. doi: 10.1084/jem.183.1.67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Zekzer D, Wong FS, Ayalon O, et al. GAD-reactive CD4+ Th1 cells induce diabetes in NOD/SCID mice. J Clin Invest. 1998;101:68–73. doi: 10.1172/JCI119878. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Graser RT, DiLorenzo TP, Wang F, et al. Identification of a CD8 T cell that can independently mediate autoimmune diabetes development in the complete absence of CD4 T cell helper functions. J Immunol. 2000;164:3913–3918. doi: 10.4049/jimmunol.164.7.3913. [DOI] [PubMed] [Google Scholar]
- 5.Bottazzo GF, Dean BM, McNally JM, MacKay EH, Swift PG, Gamble DR. In situ characterization of autoimmune phenomena and expression of HLA molecules in the pancreas in diabetic insulitis. N Engl J Med. 1985;313:353–360. doi: 10.1056/NEJM198508083130604. [DOI] [PubMed] [Google Scholar]
- 6.Foulis AK, McGill M, Farquharson MA. Insulitis in type 1 (insulin-dependent) diabetes mellitus in man – macrophages, lymphocytes, and interferon-gamma containing cells. J Pathol. 1991;165:97–103. doi: 10.1002/path.1711650203. [DOI] [PubMed] [Google Scholar]
- 7.Hanninen A, Jalkanen S, Salmi M, Toikkanen S, Nikolakaros G, Simell O. Macrophages, T cell receptor usage, and endothelial cell activation in the pancreas at the onset of insulin-dependent diabetes mellitus. J Clin Invest. 1992;90:1901–1910. doi: 10.1172/JCI116067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Itoh N, Hanafusa T, Miyazaki A, et al. Mononuclear cell infiltration and its relation to the expression of major histocompatibility complex antigens and adhesion molecules in pancreas biopsy specimens from newly diagnosed insulin-dependent diabetes mellitus patients. J Clin Invest. 1993;92:2313–2322. doi: 10.1172/JCI116835. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Santamaria P, Nakhleh RE, Sutherland DE, Barbosa JJ. Characterization of T lymphocytes infiltrating human pancreas allograft affected by isletitis and recurrent diabetes. Diabetes. 1992;41:53–61. doi: 10.2337/diab.41.1.53. [DOI] [PubMed] [Google Scholar]
- 10.Somoza N, Vargas F, Roura-Mir C, et al. Pancreas in recent onset insulin-dependent diabetes mellitus. Changes in HLA, adhesion molecules and autoantigens, restricted T cell receptor V beta usage, and cytokine profile. J Immunol. 1994;153:1360–1377. [PubMed] [Google Scholar]
- 11.Coppieters KT, Dotta F, Amirian N, et al. Demonstration of islet-autoreactive CD8 T cells in insulitic lesions from recent onset and long-term type 1 diabetes patients. J Exp Med. 2012;209:51–60. doi: 10.1084/jem.20111187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Skowera A, Ellis RJ, Varela-Calvino R, et al. CTLs are targeted to kill beta cells in patients with type 1 diabetes through recognition of a glucose-regulated preproinsulin epitope. J Clin Invest. 2008;118:3390–3402. doi: 10.1172/JCI35449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Harlan DM, Hengartner H, Huang ML, et al. Mice expressing both B7-1 and viral glycoprotein on pancreatic beta cells along with glycoprotein-specific transgenic T cells develop diabetes due to a breakdown of T-lymphocyte unresponsiveness. Proc Natl Acad Sci USA. 1994;91:3137–3141. doi: 10.1073/pnas.91.8.3137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Karges W, Pechhold K, Al Dahouk S, et al. Induction of autoimmune diabetes through insulin (but not GAD65) DNA vaccination in nonobese diabetic and in RIP-B7·1 mice. Diabetes. 2002;51:3237–3244. doi: 10.2337/diabetes.51.11.3237. [DOI] [PubMed] [Google Scholar]
- 15.Rajasalu T, Brosi H, Schuster C, et al. Deficiency in B7-H1 (PD-L1)/PD-1 coinhibition triggers pancreatic beta-cell destruction by insulin-specific, murine CD8 T-cells. Diabetes. 2010;59:1966–1973. doi: 10.2337/db09-1135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Ohashi PS, Oehen S, Buerki K, et al. Ablation of ‘tolerance’ and induction of diabetes by virus infection in viral antigen transgenic mice. Cell. 1991;65:305–317. doi: 10.1016/0092-8674(91)90164-t. [DOI] [PubMed] [Google Scholar]
- 17.Pechhold K, Karges W, Blum C, Boehm BO, Harlan DM. Beta cell-specific CD80 (B7-1) expression disrupts tissue protection from autoantigen-specific CTL-mediated diabetes. J Autoimmun. 2003;20:1–13. doi: 10.1016/s0896-8411(02)00105-1. [DOI] [PubMed] [Google Scholar]
- 18.Wenzlau JM, Hutton JC, Davidson HW. New antigenic targets in type 1 diabetes. Curr Opin Endocrinol Diabetes Obes. 2008;15:315–320. doi: 10.1097/MED.0b013e328308192b. [DOI] [PubMed] [Google Scholar]
- 19.Rajasalu T, Brosi H, Schuster C, et al. Deficiency in B7-H1 (PD-L1)/PD-1 coinhibition triggers pancreatic beta-cell destruction by insulin-specific, murine CD8 T-cells. Diabetes. 2010;59:1966–1973. doi: 10.2337/db09-1135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Zhang Y, Buchholz F, Muyrers JP, Stewart AF. A new logic for DNA engineering using recombination in Escherichia coli. Nat Genet. 1998;20:123–128. doi: 10.1038/2417. [DOI] [PubMed] [Google Scholar]
- 21.Fu J, Wenzel SC, Perlova O, et al. Efficient transfer of two large secondary metabolite pathway gene clusters into heterologous hosts by transposition. Nucleic Acids Res. 2008;36:e113. doi: 10.1093/nar/gkn499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Fuchs YF, Adler K, Bonifacio E. Beta-cell autoimmunity. Methods Mol Biol. 2012;933:265–274. doi: 10.1007/978-1-62703-068-7_17. [DOI] [PubMed] [Google Scholar]
- 23.Han B, Serra P, Amrani A, et al. Prevention of diabetes by manipulation of anti-IGRP autoimmunity: high efficiency of a low-affinity peptide. Nat Med. 2005;11:645–652. doi: 10.1038/nm1250. [DOI] [PubMed] [Google Scholar]
- 24.Pechhold K, Chakrabarty S, Harlan DM. Cytotoxic T cell-mediated diabetes in RIP-CD80 transgenic mice: autoantigen peptide sensitivity and fine specificity. Ann NY Acad Sci. 2007;1103:132–142. doi: 10.1196/annals.1394.008. [DOI] [PubMed] [Google Scholar]
- 25.Koczwara K, Schenker M, Schmid S, Kredel K, Ziegler AG, Bonifacio E. Characterization of antibody responses to endogenous and exogenous antigen in the nonobese diabetic mouse. Clin Immunol. 2003;106:155–162. doi: 10.1016/s1521-6616(02)00040-2. [DOI] [PubMed] [Google Scholar]
- 26.Muir A, Peck A, Clare-Salzler M, et al. Insulin immunization of nonobese diabetic mice induces a protective insulitis characterized by diminished intraislet interferon-gamma transcription. J Clin Invest. 1995;95:628–634. doi: 10.1172/JCI117707. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Krishnamurthy B, Dudek NL, McKenzie MD, et al. Responses against islet antigens in NOD mice are prevented by tolerance to proinsulin but not IGRP. J Clin Invest. 2006;116:3258–3265. doi: 10.1172/JCI29602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Oeser JK, Parekh VV, Wang Y, et al. Deletion of the G6pc2 gene encoding the islet-specific glucose-6-phosphatase catalytic subunit-related protein does not affect the progression or incidence of type 1 diabetes in NOD/ShiLtJ mice. Diabetes. 2011;60:2922–2927. doi: 10.2337/db11-0220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Nakayama M, Abiru N, Moriyama H, et al. Prime role for an insulin epitope in the development of type 1 diabetes in NOD mice. Nature. 2005;435:220–223. doi: 10.1038/nature03523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Bijker MS, Eeden van den SJ, Franken KL, Melief CJ, Offringa R, Burg van der SH. CD8+ CTL priming by exact peptide epitopes in incomplete Freund's adjuvant induces a vanishing CTL response, whereas long peptides induce sustained CTL reactivity. J Immunol. 2007;179:5033–5040. doi: 10.4049/jimmunol.179.8.5033. [DOI] [PubMed] [Google Scholar]
- 31.Goverman J. Autoimmune T cell responses in the central nervous system. Nat Rev Immunol. 2009;9:393–407. doi: 10.1038/nri2550. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Corazza N, Kaufmann T. Novel insights into mechanisms of food allergy and allergic airway inflammation using experimental mouse models. Allergy. 2012;67:1483–1490. doi: 10.1111/all.12065. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Verification of DNA vector-driven antigen expression in mammalian cells was verified exemplarily for four secretory granule antigens and six non-secretory granule antigens. Lysates of human embryonic kidney (HEK) 293 cells transfected with pcDNA 3·1 V5HISB DNA vectors encoding the indicated proteins were separated via sodium dodecyl sulphate-polyacrylamide gel electrophoresis (SDSPAGE) and analysed using immunoblotting with V5-tag directed antibodies. Equal amounts of lysates were loaded. Blots show empty vector-transfected controls (left lanes, −) and antigen-transfected samples (right lanes, +) for each antigen. Protein standards in kDa are shown for each respective sample.