Abstract
Myasthenia gravis (MG) is an autoimmune-mediated inflammatory disease of the neuromuscular junction. Previous studies of animal MG models have suggested important roles of cytokines in MG pathogenesis, but adequate studies on cytokines in human MG are lacking. Using a multiplex suspension array system, we measured the serum levels of 27 cytokines/chemokines in 47 anti-acetylcholine receptor antibody-positive patients with MG and 20 normal controls (NC) to investigate the contribution of cytokines/chemokines toward MG pathogenesis. Correlations between clinical parameters and cytokine/chemokine levels in patients with MG were also examined. The serum levels of interleukin (IL)-15 (mean ± standard deviation: 6·85 ± 6·97 pg/ml) and vascular endothelial growth factor (VEGF) (96·21 ± 71·60 pg/ml) significantly increased, whereas IL-4 levels (3·57 ± 0·86 pg/ml) decreased in patients with MG compared with NC (IL-15: 4·42 ± 1·55 pg/ml; VEGF: 63·51 ± 32·95 pg/ml; IL-4: 4·15 ± 0·81 pg/ml, P < 0·05). In addition, eight cytokines (IL-4, IL-8, IL-15, eotaxin, macrophage inflammatory protein-1α, macrophage inflammatory protein-1β, VEGF and IL-1b) were significantly changed among MG patients with thymoma, MG patients without thymoma and NC (P < 0·05). Some cytokines, such as IL-4, IL-15, and VEGF, may play roles in the pathogenesis of MG.
Keywords: interleukin-15, interleukin-4, myasthenia gravis, thymoma, vascular endothelial growth factor (VEGF)
Introduction
Myasthenia gravis (MG), an antibody-mediated autoimmune disease of the neuromuscular junction, is characterized by muscle weakness and fatigability 1 and is caused by autoantibodies against muscle nicotinic acetylcholine receptor (AChR). The anti-AChR antibody is produced by T cell-dependent and B cell-mediated pathogenic mechanisms 2, activates the complement system and leads to inflammation of the postsynaptic muscle membrane 3. Cytokines and chemokines regulate immune responses in inflammatory diseases 4. Naive CD4+ T cells can differentiate into T helper type (Th) 1, Th2, Th9, Th17, Th22 or regulatory T cells (Treg) by the actions of differentiation cytokines 5. These T cell subsets promote different inflammatory responses based on their respective cytokine profiles, responses to chemokines and their interactions with other cells. Hence, cytokine/chemokine profiles are likely to be of major importance in the pathogenesis of MG. Moreover, Th1 and Th17 deviations worsen the pathogenesis of experimental autoimmune myasthenia gravis (EAMG), which is an animal model of MG, and, in contrast, Th2 and Treg deviations ameliorate EAMG pathogenesis 6. Although the role of different cytokines and chemokines in the pathogenesis of EAMG has been investigated extensively, adequate studies concerning its role in the pathogenesis of patients with MG are lacking. It has been reported that serum interleukin (IL)-17 levels are increased significantly and associated with the severity of MG as well as correlated with the concentration of anti-AChR antibodies 7,8. Serum IL-22 levels were also decreased, and exhibited a negative correlation between serum IL-22 and anti-AChR antibody levels in patients with MG 8.
In this study, we analysed an intensive cytokines and chemokines profile in the serum of anti-AChR antibody-positive patients with MG and determined if increased cytokine levels are linked to clinical MG parameters.
Materials and methods
Subjects
In this study, 47 Japanese patients with seropositive MG were included; 20 healthy participants (11 women, nine men; mean age 48·5 years, range 33–72 years) served as normal controls (NC). We reviewed the data on gender, age, disease duration, MG Foundation of America (MGFA) classification 9, MG activities of daily living (MGADL) score 10, anti-AChR antibody titre and the presence of thymoma at the time of serum sampling. All patients with MG visited our hospital for their initial MG symptoms, and did not receive immunosuppressive therapy or thymectomy. The followings are clinical characteristics of patients with MG: ocular MG ratio, 12/47 (25·5%); female ratio, 26/47 (55·3%); anti-AChR antibody positivity, 47/47 (100%); thymoma positivity, 14/47 (29·8%); mean age ± standard deviation (s.d.) (range) was 57·1 ± 17·6 (23–84) years; mean disease duration, 15·7 ± 21·2 (0–81) months; mean MGADL score, 6·3 ± 4·4 (1–18); and median MGFA classification, 2 (1–5) (Table 1).
Table 1.
Clinical characteristics of patients with myasthenia gravis.
| No. | Age (years) | Gender | Thymus pathology | MGFA | MGADL | AChR titre (nmol/l) |
|---|---|---|---|---|---|---|
| 1 | 80 | M | n.a. | 1 | 1 | 13 |
| 2 | 69 | M | n.a. | 1 | 3 | 150 |
| 3 | 41 | M | n.a. | 1 | 4 | 18 |
| 4 | 50 | F | n.a. | 1 | 2 | 6·5 |
| 5 | 72 | M | n.a. | 1 | 1 | 2·8 |
| 6 | 62 | F | n.a. | 1 | 4 | 4·5 |
| 7 | 45 | M | n.a. | 1 | 3 | 13 |
| 8 | 56 | M | n.a. | 1 | 4 | 1·7 |
| 9 | 31 | F | n.a. | 1 | 2 | 81 |
| 10 | 67 | F | n.a. | 1 | 3 | 0·8 |
| 11 | 57 | M | n.a. | 1 | 2 | 3·9 |
| 12 | 65 | M | Thymoma | 1 | 2 | 16 |
| 13 | 23 | F | n.a. | 2a | 8 | 200 |
| 14 | 31 | F | Normal thymus | 2a | 9 | 2·9 |
| 15 | 65 | M | n.a. | 2a | 4 | 110 |
| 16 | 66 | M | Involuted thymus | 2a | 5 | 35 |
| 17 | 65 | F | Involuted thymus | 2a | 7 | 50 |
| 18 | 62 | F | Involuted thymus | 2a | 3 | 10 |
| 19 | 78 | F | n.a. | 2a | 6 | 5·9 |
| 20 | 32 | M | Normal thymus | 2a | 5 | 480 |
| 21 | 72 | F | Involuted thymus | 2a | 6 | 47 |
| 22 | 57 | M | Thymoma | 2a | 3 | 160 |
| 23 | 23 | M | Thymoma | 2a | 4 | 39 |
| 24 | 71 | F | Thymoma | 2a | 3 | 13 |
| 25 | 33 | F | Thymoma | 2a | 7 | 43 |
| 26 | 40 | M | Thymoma | 2a | 9 | 34 |
| 27 | 54 | F | Thymoma | 2a | 7 | 8·1 |
| 28 | 53 | F | Thymoma | 2a | 5 | 2·0 |
| 29 | 24 | F | Normal thymus | 2b | 2 | 3·1 |
| 30 | 78 | F | n.a. | 2b | 9 | 14 |
| 31 | 66 | M | Involuted thymus | 2b | 6 | 0·5 |
| 32 | 68 | M | n.a. | 2b | 5 | 110 |
| 33 | 79 | F | n.a. | 2b | 3 | 56 |
| 34 | 76 | F | Thymoma | 2b | 3 | 74 |
| 35 | 50 | F | Thymoma | 2b | 2 | 65 |
| 36 | 31 | M | Thymoma | 2b | 10 | 180 |
| 37 | 74 | F | n.a. | 3b | 10 | 6·7 |
| 38 | 81 | F | n.a. | 3b | 15 | 42 |
| 39 | 61 | F | Involuted thymus | 3b | 7 | 12 |
| 40 | 60 | F | n.a. | 3b | 16 | 3·4 |
| 41 | 40 | M | Thymoma | 3b | 14 | 310 |
| 42 | 50 | M | Thymoma | 3b | 9 | 55 |
| 43 | 31 | F | Thymoma | 3b | 6 | 230 |
| 44 | 84 | F | n.a. | 4b | 15 | 11 |
| 45 | 75 | M | n.a. | 4b | 10 | 51 |
| 46 | 63 | F | Involuted thymus | 4b | 16 | 49 |
| 47 | 71 | M | n.a. | 5 | 18 | 25 |
AChR = acetylcholine receptor; MGADL = myasthenia gravis activities of daily living; MGFA = myasthenia gravis foundation of America; n.a. = not available; M = male; F = female.
All serum samples were obtained before the treatment of MG and were stored at −80°C until analysis.
Ethics approval was granted by the Ethics Committee of the Chiba University School of Medicine, Chiba, Japan. All subjects gave informed consent for their participation.
Serum cytokine and chemokine measurements
All serum samples were diluted fourfold with specific Bio-Plex sample diluents. Samples were simultaneously analysed for 27 different cytokines and chemokines [IL-1 receptor antagonist (IL-1ra); IL-2; IL-4; IL-5; IL-6; IL-7; IL-8; IL-9; IL-10; IL-12; IL-13; IL-15; IL-17; eotaxin; fibroblast growth factor-basic (FGF-basic); granulocyte colony-stimulating factor (G-CSF); granulocyte macrophage colony-stimulating factor (GM-CSF); interferon (IFN)-γ; interferon gamma-induced protein 10 (IP-10); monocyte chemoattractant protein-1 (MCP-1); macrophage inflammatory protein (MIP)-1α; MIP-1β; regulated on activation, normal T cell expressed and secreted (RANTES); tumour necrosis factor-alpha (TNF-α); vascular endothelial growth factor (VEGF); IL-1b; and platelet-derived growth factor-bb (PDGF-bb)] by a multiplexed fluorescent magnetic bead-based immunoassay (Bio-Rad Laboratories, Inc., Hercules, CA, USA), according to the manufacturer's instructions, as reported previously 11. If the serum values were outside the detection range, values were replaced by the lower or upper detection limits.
Correlation between cytokine levels and clinical MG profiles
We analysed the correlations between the serum cytokine levels and clinical profiles, such as anti-AChR antibody titre, type of MG (ocular type or generalized type), MGFA classification, MGADL score and the presence of thymoma in all patients with MG.
Statistical analysis
For baseline variables, the groups were compared using the Mann–Whitney U-test for continuous variables. The Spearman's rank correlation coefficient was used to test the associations.
Results
Serum cytokine and chemokine profiles
Of the 27 different serum cytokines/chemokines, significant differences between all patients with MG and NC were found in the levels of IL-4, IL-15 and VEGF (P < 0·05). IL-15 and VEGF levels were higher and the IL-4 levels were lower in patients with MG than in NC (Table 2 and Fig. 1). IL-5 and IFN-γ levels tended to be lower (P < 0·10) in patients with MG than in NC, but these differences were not significant.
Table 2.
Serum cytokine/chemokine profiles of patients with myasthenia gravis (MG) and normal controls.
| Cytokines/chemokines (pg/ml) | All MG (n = 47) | MG with thymoma (n = 14) | MG without thymoma (n = 33) | Ocular MG (n = 12) | Generalized MG (n = 35) | Normal controls (n = 20) |
|---|---|---|---|---|---|---|
| IL-1ra | 82·06 ± 149·31 | 122·37 ± 266·55 | 64·96 ± 46·60 | 71·35 ± 63·48 | 85·73 ± 169·72 | 59·45 ± 54·65 |
| IL-2 | 14·20 ± 12·31 | 17·50 ± 22·32 | 12·80 ± 2·96 | 13·12 ± 3·50 | 14·57 ± 14·17 | 12·56 ± 2·95 |
| IL-4 | 3·57 ± 0·86* | 3·60 ± 1·08 | 3·56 ± 0·77* | 3·69 ± 0·71 | 3·53 ± 0·91* | 4·15 ± 0·81 |
| IL-5 | 7·72 ± 2·24 | 7·28 ± 2·58 | 7·90 ± 2·09 | 8·18 ± 1·52 | 7·56 ± 2·44 | 9·03 ± 2·48 |
| IL-6 | 49·54 ± 106·33 | 83·12 ± 150·04 | 35·29 ± 80·02 | 46·57 ± 94·30 | 50·55 ± 111·42 | 15·36 ± 31·64 |
| IL-7 | 6·93 ± 2·55 | 6·13 ± 2·36 | 7·28 ± 2·59 | 7·66 ± 2·72 | 6·69 ± 2·48 | 7·82 ± 5·29 |
| IL-8 | 415·50 ± 683·62 | 593·76 ± 755·02* | 339·87 ± 648·39 | 264·04 ± 428·81 | 467·42 ± 749·61 | 123·19 ± 265·86 |
| IL-9 | 7·66 ± 4·76 | 9·39 ± 7·64 | 6·93 ± 2·65 | 7·63 ± 3·22 | 7·67 ± 5·23 | 14·92 ± 31·48 |
| IL-10 | 14·11 ± 49·03 | 30·42 ± 89·83 | 7·19 ± 3·48 | 7·68 ± 4·37 | 16·31 ± 56·81 | 7·91 ± 5·94 |
| IL-12 | 22·26 ± 34·78 | 32·13 ± 63·14 | 18·07 ± 7·64 | 19·06 ± 10·02 | 23·35 ± 39·99 | 16·24 ± 6·67 |
| IL-13 | 5·99 ± 2·77 | 5·66 ± 2·65 | 6·13 ± 2·85 | 6·59 ± 2·76 | 5·78 ± 2·78 | 7·02 ± 3·49 |
| IL-15 | 6·85 ± 6·97* | 8·11 ± 12·12 | 6·31 ± 3·03* | 5·44 ± 2·47 | 7·33 ± 7·93* | 4·42 ± 1·55 |
| IL-17 | 124·28 ± 28·60 | 124·17 ± 34·39 | 124·32 ± 26·37 | 124·62 ± 24·90 | 124·16 ± 30·10 | 123·52 ± 28·75 |
| Eotaxin | 107·20 ± 60·85 | 138·07 ± 89·79* | 94·11 ± 38·12 | 105·15 ± 26·71 | 107·91 ± 69·11 | 85·14 ± 28·75 |
| FGF-basic | 28·46 ± 7·56 | 28·69 ± 9·21 | 28·36 ± 6·90 | 29·76 ± 5·80 | 28·02 ± 8·10 | 26·68 ± 6·73 |
| G-CSF | 23·33 ± 7·23 | 23·16 ± 8·85 | 23·40 ± 6·58 | 25·33 ± 6·62 | 22·64 ± 7·39 | 25·79 ± 7·47 |
| GM-CSF | 7·01 ± 5·42 | 6·19 ± 6·53 | 7·36 ± 4·95 | 6·95 ± 3·98 | 7·03 ± 5·89 | 5·03 ± 2·58 |
| IFN-γ | 77·08 ± 30·81 | 76·94 ± 38·23 | 77·14 ± 27·76 | 78·48 ± 20·97 | 76·60 ± 33·78 | 90·24 ± 31·52 |
| IP-10 | 833·75 ± 726·04 | 886·52 ± 923·90 | 811·37 ± 697·33 | 1009·70 ± 1084·67 | 773·43 ± 624·79 | 633·35 ± 211·54 |
| MCP-1 | 75·48 ± 116·37 | 58·32 ± 23·11 | 82·76 ± 138·08 | 50·87 ± 24·97 | 83·92 ± 133·53 | 44·97 ± 16·57 |
| MIP-1α | 214·47 ± 363·15 | 370·83 ± 424·13*† | 148·14 ± 318·22 | 158·97 ± 271·88 | 233·50 ± 391·22 | 35·02 ± 66·46 |
| MIP-1β | 363·99 ± 348·90 | 512·44 ± 373·60*† | 301·01 ± 323·35 | 342·20 ± 367·90 | 371·46 ± 347·39 | 203·63 ± 235·90 |
| RANTES | 1139·45 ± 0·00 | 1139·45 ± 0·00 | 1139·45 ± 0·00 | 1139·45 ± 0·00 | 1139·45 ± 0·00 | 1139·45 ± 0·00 |
| TNF-α | 73·22 ± 54·05 | 91·38 ± 71·28 | 65·51 ± 43·93 | 72·05 ± 51·13 | 73·62 ± 55·73 | 57·34 ± 18·60 |
| VEGF | 96·21 ± 71·60* | 91·28 ± 44·79* | 98·31 ± 80·87* | 85·76 ± 58·37 | 99·80 ± 76·04* | 63·51 ± 32·95 |
| IL-1b | 5·43 ± 7·78 | 7·96 ± 11·76* | 4·36 ± 5·19 | 4·22 ± 4·37 | 5·85 ± 8·67 | 2·46 ± 2·01 |
| PDGF-bb | 3572·43 ± 1495·82 | 3840·68 ± 1554·50 | 3458·63 ± 1479·82 | 3345·89 ± 1520·82 | 3650·11 ± 1501·52 | 3642·49 ± 1364·90 |
Significantly different from the cytokine levels of normal controls (NC) (P < 0·05).
Significantly different from the cytokine levels of MG without thymoma (P < 0·05). Values indicate mean ± standard deviation (s.d.). IL = interleukin; FGF = fibroblast growth factor; G-CSF = granulocyte colony-stimulating factor; GM-CSF = granulocyte-macrophage colony-stimulating factor; IFN = interferon; IP = IFN-γ-inducible protein; MCP = monocyte chemoattractant protein; MIP = macrophage inflammatory protein; RANTES = regulated on activation, normal T cell expressed and secreted; TNF = tumour necrosis factor; VEGF = vascular endothelial growth factor; PDGF = platelet-derived growth factor.
Figure 1.
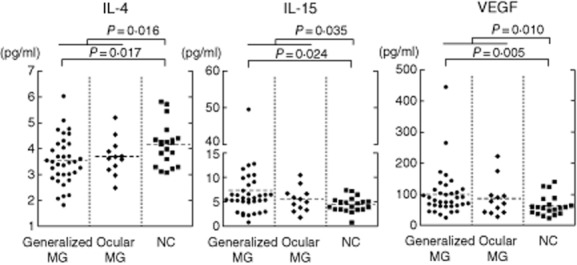
Serum cytokine levels for patients with seropositive myasthenia gravis (MG) and normal controls (NC). Interleukin (IL)-15 and vascular endothelial growth factor (VEGF) levels were significantly higher and IL-4 levels were lower in patients with MG than in NC.
Correlations between serum cytokine levels and clinical profiles of patients with MG
Among the serum cytokines (IL-4, IL-15 and VEGF) that were changed significantly in all patients with MG, significant correlations with clinical findings were not found for the following comparisons: IL-4 levels and anti-AChR antibody titre (r = −0·012) and MGADL score (r = −0·039); IL-15 levels and anti-AChR antibody titre (r = 0·052) and MGADL score (r = 0·238); and VEGF levels and anti-AChR antibody titre (r = −0·075) and MGADL score (r = 0·230).
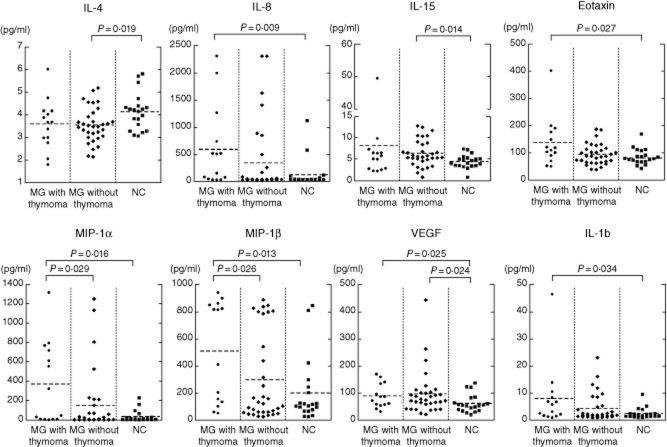
Eight cytokines (IL-4, IL-8, IL-15, eotaxin, MIP-1α, MIP-1β, VEGF and IL-1b) were changed significantly among MG patients with thymoma, MG patients without thymoma and NC (Table 2 and Fig. 2). Conversely, there were no significant differences in 27 cytokines between ocular MG and generalized MG (Table 2 and Fig. 1). There were no correlations between anti-AChR antibody titres or MGFA classification and the levels of the above-described eight cytokines (data not shown).
Figure 2.
Serum cytokine levels for myasthenia gravis (MG) patients with thymoma, MG patients without thymoma and normal controls (NC). Interleukin (IL)-4, IL-8, IL-15, eotaxin, macrophage inflammatory protein (MIP)-1α, MIP-1β, vascular endothelial growth factor (VEGF) and IL-1b levels were significantly changed among MG patients with thymoma, MG patients without thymoma and NC.
Discussion
In this study, we have shown the following significant changes in some serum cytokine levels in the anti-AChR antibody-positive patients with MG compared with those in NC, increased IL-15 and VEGF levels and decreased IL-4 levels in all MG patients, as well as increased IL-8, eotaxin, MIP-1α, MIP-1β and IL-1b levels in MG patients with thymoma.
Although anti-AChR antibodies and the complement system are major causes of neuromuscular junction inflam mation, cytokines also affect the autoimmune responses of patients with MG. To date, only a few analyses of serum cytokines or chemokines in relation to human MG have been reported. These studies have found that serum IL-17 levels were up-regulated and IL-22 levels were down-regulated in patients with MG 7,8. Various cytokines/chemokines and more patients with MG were analysed in this study compared with other previous studies. However, we could not substantiate the IL-17-predominant immune response in patients with MG. The exact reasons for this discrepancy were unclear, but methodological differences in the measurements of cytokines and the different profiles and numbers of patients with MG may play a role.
IL-15, a pleiotropic proinflammatory cytokine with a broad range of biological functions, is expressed in several inflammatory disorders 12; promotes the activation of T cells 13, neutrophils and macrophages; and is critical to dendritic cell function in several model systems 12. In fact, increased IL-15 levels in the circulation and inflamed tissues have been reported in various autoimmune diseases, which may possibly contribute to their pathogenesis 12. A beneficial effect of IL-15 neutralization has been reported in psoriasis models 14. These data hold promise that the anti-IL-15 pathway has a therapeutic potential for autoimmune inflammatory diseases. Regarding MG, previous studies have revealed that IL-15 plays a key role in EAMG pathogenesis. An interesting mechanism by which cytokines may influence EAMG is the cytokine production by muscle cells. Muscle cells produce IL-15 in response to IL-4 stimulation in vitro or EAMG induction in vivo 15. Myocytes of EAMG rats that are induced not only by active immunization but also by the passive transfer of AChR antibodies display enhanced IL-15 production 16. These results suggest that muscle cells contribute to inflammation by releasing IL-15 and thereby worsen the clinical course of EAMG. Hence, IL-15 may be involved in the pathogenesis of MG.
IL-4, a multi-functional pleiotropic cytokine, is produced primarily by activated T cells, mast cells, basophils and eosinophils. IL-4 is known for defining the Th2 phenotype of lymphocytes and regulating cell proliferation, apoptosis and the expression of numerous genes in various cell types 17. A previous study concerning EAMG suggested that IL-4 does not have a pathogenic role and may have a protective function in EAMG; namely, regulatory mechanisms that involve IL-4 contribute to preventing the development of a chronic antibody-mediated autoimmune response to the AChR 18. The development of MG symptoms may result from the disruption of protective function through the down-regulation of serum IL-4 levels in patients with MG, as was observed in this study.
VEGF induces endothelial cell proliferation, promotes cell migration and inhibits apoptosis. It is a key regulator of physiological and pathological angiogenesis 19. In this study, no patients with thymic hyperplasia were involved, and there were no differences in VEGF levels between MG patients with thymoma and MG patients without thymoma. Hence, we could not substantiate whether or not VEGF increased due to the active neoangiogenic processes observed in the MG patients with thymic abnormality 20,21. To date, VEGF levels have not been reported in patients with MG. Increased VEGF levels may reflect inflammation and angiogenesis at the neuromuscular junction in patients with MG. Analyses of the association between VEGF and MG pathogenesis are required.
Increased IL-8, eotaxin, MIP-1α, MIP-1β and IL-1b levels were confirmed in MG patients with thymoma. These cytokine elevations may be correlated with thymoma. To clarify this correlation, cytokine analysis of thymoma patients without MG will be needed.
In summary, our results show a significant increase in IL-15 and VEGF levels and a significant decrease of IL-4 levels in the serum of patients with seropositive MG, as well as a significant increase in IL-8, eotaxin, MIP-1α, MIP-1β and IL-1b levels in MG patients with thymoma. The pathogenic and inflammatory mechanisms at neuromuscular junctions that are exerted by cytokines could be important in patients with MG. Anti-cytokines therapy could have the potential for treating MG. Further studies, including those of patients with seronegative MG, are required to confirm the details of the cytokine profiles of patients with MG.
Acknowledgments
This work was partly supported by research grants from the Ministry of Education, Science, and Technology (to A. U.).
Disclosure
The authors declare that there are no conflicts of interest.
References
- 1.Drachman DB. Myasthenia gravis. N Engl J Med. 1994;330:1797–1810. doi: 10.1056/NEJM199406233302507. [DOI] [PubMed] [Google Scholar]
- 2.Nagvekar N, Moody AM, Moss P, et al. A pathogenetic role for the thymoma in myasthenia gravis. Autosensitization of IL-4-producing T cell clones recognizing extracellular acetylcholine receptor epitopes presented by minority class II isotypes. J Clin Invest. 1998;101:2268–2277. doi: 10.1172/JCI2068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Tüzün E, Scott BG, Goluszko E, Higgs S, Christadoss P. Genetic evidence for involvement of classical complement pathway in induction of experimental autoimmune myasthenia gravis. J Immunol. 2003;171:3847–3854. doi: 10.4049/jimmunol.171.7.3847. [DOI] [PubMed] [Google Scholar]
- 4.Luster AD. Chemokines – chemotactic cytokines that mediate inflammation. N Engl J Med. 1998;338:436–445. doi: 10.1056/NEJM199802123380706. [DOI] [PubMed] [Google Scholar]
- 5.Jutel M, Akdis CA. T-cell subset regulation in atopy. Curr Allergy Asthma Rep. 2011;11:139–145. doi: 10.1007/s11882-011-0178-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Yang H, Zhang Y, Wu M, et al. Suppression of ongoing experimental autoimmune myasthenia gravis by transfer of RelB-silenced bone marrow dentritic cells is associated with a change from a T helper Th17/Th1 to a Th2 and FoxP3+ regulatory T-cell profile. Inflamm Res. 2010;59:197–205. doi: 10.1007/s00011-009-0087-6. [DOI] [PubMed] [Google Scholar]
- 7.Roche JC, Capablo JL, Larrad L, et al. Increased serum interleukin-17 levels in patients with myasthenia gravis. Muscle Nerve. 2011;44:278–280. doi: 10.1002/mus.22070. [DOI] [PubMed] [Google Scholar]
- 8.Zheng S, Dou C, Xin N, et al. Expression of interleukin-22 in myasthenia gravis. Scand J Immunol. 2013;78:98–107. doi: 10.1111/sji.12057. [DOI] [PubMed] [Google Scholar]
- 9.Jaretzki A, 3rd, Barohn RJ, Ernstoff RM, et al. Myasthenia gravis: recommendations for clinical research standards. Task Force of the Medical Scientific Advisory Board of the Myasthenia Gravis Foundation of America. Neurology. 2000;55:16–23. doi: 10.1212/wnl.55.1.16. [DOI] [PubMed] [Google Scholar]
- 10.Wolfe GI, Herbelin L, Nations SP, Foster B, Bryan WW, Barohn RJ. Myasthenia gravis activities of daily living profile. Neurology. 1999;52:1487–1489. doi: 10.1212/wnl.52.7.1487. [DOI] [PubMed] [Google Scholar]
- 11.Uzawa A, Mori M, Arai K, et al. Cytokine and chemokine profiles in neuromyelitis optica: significance of interleukin-6. Mult Scler. 2010;16:1443–1452. doi: 10.1177/1352458510379247. [DOI] [PubMed] [Google Scholar]
- 12.McInnes IB, Gracie JA. Interleukin-15: a new cytokine target for the treatment of inflammatory diseases. Curr Opin Pharmacol. 2004;4:392–397. doi: 10.1016/j.coph.2004.04.003. [DOI] [PubMed] [Google Scholar]
- 13.Rückert R, Brandt K, Bulanova E, Mirghomizadeh F, Paus R, Bulfone-Paus S. Dendritic cell-derived IL-15 controls the induction of CD8 T cell immune responses. Eur J Immunol. 2003;33:3493–3503. doi: 10.1002/eji.200324545. [DOI] [PubMed] [Google Scholar]
- 14.Villadsen LS, Schuurman J, Beurskens F, et al. Resolution of psoriasis upon blockade of IL-15 biological activity in a xenograft mouse model. J Clin Invest. 2003;112:1571–1580. doi: 10.1172/JCI18986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Shandley S, Martinez S, Krolick K. IL-4 receptor as a bridge between the immune system and muscle in experimental myasthenia gravis I: up-regulation of muscle IL-15 by IL-4. Clin Immunol. 2009;132:246–256. doi: 10.1016/j.clim.2009.03.523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Stegall T, Krolick KA. Myocytes respond to both interleukin-4 and interferon-gamma: cytokine responsiveness with the potential to influence the severity and course of experimental myasthenia gravis. Clin Immunol. 2000;94:133–139. doi: 10.1006/clim.1999.4822. [DOI] [PubMed] [Google Scholar]
- 17.Nelms K, Keegan AD, Zamorano J, Ryan JJ, Paul WE. The IL-4 receptor: signaling mechanisms and biologic functions. Annu Rev Immunol. 1999;17:701–738. doi: 10.1146/annurev.immunol.17.1.701. [DOI] [PubMed] [Google Scholar]
- 18.Ostlie N, Milani M, Wang W, Okita D, Conti-Fine BM. Absence of IL-4 facilitates the development of chronic autoimmune myasthenia gravis in C57BL/6 mice. J Immunol. 2003;170:604–612. doi: 10.4049/jimmunol.170.1.604. [DOI] [PubMed] [Google Scholar]
- 19.Robinson CJ, Stringer SE. The splice variants of vascular endothelial growth factor (VEGF) and their receptors. J Cell Sci. 2001;114:853–865. doi: 10.1242/jcs.114.5.853. [DOI] [PubMed] [Google Scholar]
- 20.Berrih-Aknin S, Ruhlmann N, Bismuth J, et al. CCL21 overexpressed on lymphatic vessels drives thymic hyperplasia in myasthenia. Ann Neurol. 2009;66:521–531. doi: 10.1002/ana.21628. [DOI] [PubMed] [Google Scholar]
- 21.Nabarra B, Pontoux C, Godard C, Osborne-Pellegrin M, Ezine S. Neoplastic transformation and angiogenesis in the thymus of transgenic mice expressing SV40 T and t antigen under an L-pyruvate kinase promoter (SV12 mice) Int J Exp Pathol. 2005;86:397–413. doi: 10.1111/j.0959-9673.2005.00451.x. [DOI] [PMC free article] [PubMed] [Google Scholar]