Abstract
Obesity has been associated with accelerated biological ageing and immunosenescence. As the prevalence of childhood obesity is increasing, we wanted to determine if associations between obesity and immunosenescence would manifest in children. We studied 123 Mexican American adolescents aged 10–14 (mean 12·3 ± 0·7) years, with body weights ranging from 30·1 to 115·2 kg (mean 52·5 ± 14·5 kg). Blood samples were obtained to determine proportions of naive, central memory (CM), effector memory (EM), senescent and early, intermediate and highly differentiated subsets of CD4+ and CD8+ T cells. Overweight and obese children had significantly lowered proportions of early CD8+ T cells (B = −11·55 and –5·51%, respectively) compared to healthy weight. Overweight children also had more EM (B = +7·53%), late (B = +8·90%) and senescent (B = +4·86%) CD8+ T cells than healthy weight children, while obese children had more intermediate CD8+ (B = +4·59%), EM CD8+ (B = +5·49%), late CD4+ (B = +2·01%) and senescent CD4+ (B = +0·98%) T cells compared to healthy weight children. These findings withstood adjustment for potentially confounding variables, including age, gender and latent cytomegalovirus and Epstein–Barr virus infections. We conclude that excess body mass, even in adolescence, may accelerate immunosenescence and predispose children to increased risks of incurring immune-related health problems in adulthood.
Keywords: adolescents, herpes viruses, immunosenescence, obesity, overweight
Introduction
The percentage of children and adolescents classified as obese has tripled over the last three decades. This has resulted in an increased incidence of obesity-related diseases and health problems seen typically in adults manifesting as early as adolescence, with pre-diabetes 1, type 2 diabetes 2, high blood pressure 3, metabolic syndrome 4 and high cholesterol becoming more prevalent among teenagers. Moreover, childhood obesity is a predisposition to adult obesity 5, and could consequently accelerate the onset of obesity-related disorders in later life. This is supported by evidence linking childhood obesity with lasting detrimental cardiac structural changes, sleep apnoea and thromboembolic disease in adults 6, but also psychological disorders such as depression and poor quality of life 7.
Obesity has been associated with increased incidences of respiratory viral infections 8 and impaired vaccine efficacy 9, and was identified as a risk factor for the 2009 H1N1 influenza pandemic 10. Persistent viral infections such as adenovirus-36 and herpes simplex virus-1 (HSV-1) are also more prevalent among obese adults and children 11,12, indicating that obesity may be associated with an impaired ability to defend against invading pathogens. As many of these changes in immunity are analogous to those seen with ageing, it is possible that obesity is linked with the early onset of immunosenescence – a canopy term that has been used to describe the biological ageing of the human immune system. Immunosenescence, and the associated ‘immune risk profile’, has a number of hallmark features, including the accumulation of late-differentiated subsets of T lymphocytes, lowered proportions of naive T cells, shortened leucocyte telomeres and seropositivity for latent cytomegalovirus (CMV) and Epstein–Barr virus (EBV) infections 13. These systemic immune alterations have important clinical implications and have been associated with poor vaccine efficacy, impaired immune vigilance and greater morbidity and mortality as a result of infectious disease 14.
No study, to our knowledge, has examined the relationship between obesity and T cell differentiation associated with immunosenescence. Obesity may impact upon T cell differentiation due to elevations in systemic low-grade inflammation 15, increased viral prevalence and load 11,12, heightened levels of oxidative stress 16, psychological and psychosocial factors such as depression and poor quality of life 17 and/or to increased serum concentrations of the appetite regulating hormone leptin, which has been shown to increase T cell activation and proliferation 18. However, whether or not these associations with immunosenescence will manifest in children who, by definition, have been obese for a relatively short period of time, is not known. Identifying links between adiposity and T cell differentiation in children would provide a strong indication that obesity accelerates immunosenescence early in life, thus predisposing children to greater levels of immune impairment as adults.
The aim of this study was to investigate the relationships between excess body mass and T cell differentiation indicative of immunosenescence in a cohort of Mexican American adolescent children, while adjusting for potential confounders such as psychosocial factors and latent CMV and EBV infections. Moreover, as viruses such as adenovirus-36 and HSV-1 have been associated with obesity 11,12, we were interested to determine if CMV and EBV infections (key features of immunosenescence and the ‘immune risk profile’) are also more prevalent among obese children.
Methods and procedure
Participants
We studied 123 Mexican American children (67 females) aged 10–14 (mean 12·3 ± 0·7) years recruited from an urban charter school in the city of Houston, TX with a 95% prevalence of Mexican Americans. None of the subjects were on any medication, and were free of any infectious illness for 6 weeks prior to the study. Prior to the inclusion of their child in the study, parents signed a written consent form on behalf of their child along with a written assent provided by the subjects. Ethical approval was granted by the Institutional Review Board for Human Subjects at Baylor College of Medicine. Subject physical characteristics are shown in Table 1.
Table 1.
Physical characteristics of the participants in relation to their weight classifications (mean ± standard error of the mean). Significant differences from the healthy weight classification (*P < 0·001) and the obese classification (#P < 0·01).
| All subjects (n = 123) | Healthy weight (n = 65) | Overweight (n = 22) | Obese (n = 36) | |
|---|---|---|---|---|
| Age (years) | 12·3 ± 0·7 | 12·4 ± 0·7 | 12·2 ± 0·6 | 12·1 ± 0·6 |
| Female frequency (%) | 54% | 58·5% | 54·5% | 47·2% |
| Body mass (kg) | 52·5 ± 14·5 | 43·1 ± 7·3# | 54·5 ± 6·7*,# | 69·0 ± 12·7 |
| BMI (kg/m2) | 22·6 ± 5·3 | 18·7 ± 1·9# | 23·3 ± 1·0*,# | 29·2 ± 4·2 |
| zBMI | 0·9 ± 1·1 | 0·1 ± 0·7# | 1·4 ± 0·1*,# | 2·1 ± 0·3 |
| BMI percentile | 73·0 ± 27·6 | 53·5 ± 24·6# | 91·3 ± 2·3* | 97·7 ± 1·4 |
| % Body fat | 26·5 ± 11·1 | 19·6 ± 6·8# | 27·9 ± 6·8*,# | 38·6 ± 10·2 |
| % Overweight | 24·5 ± 29·7 | 2·7 ± 10·4# | 28·8 ± 4·5*,# | 62·4 ± 22·7 |
| Serum leptin (ng/ml) | 27·0 ± 22·0 | 15·4 ± 7·7# | 24·5 ± 11·5# | 49·5 ± 26·8 |
| CMV seropositivity | 27·4% | 28·6% | 29·4% | 18·2% |
| EBV seropositivity | 50·9% | 47·3% | 35·3% | 60·6% |
| PEDS QL physical health score | 86·7 ± 16·3 | 87·8 ± 16·2 | 88·8 ± 12·4 | 83·4 ± 18·1 |
| PEDS QL psychosocial health score | 80·6 ± 19·3 | 82·0 ± 23·0 | 79·9 ± 15·2 | 78·8 ± 13·9 |
| PEDS QL total score | 82·8 ± 20·1 | 83·9 ± 23·0 | 83·0 ± 13·2 | 80·5 ± 18·0 |
BMI = body mass index; zBMI = standardized BMI; CMV = cytomegalovirus; EBV = Epstein–Barr virus; PEDS = Pediatric Quality of Life Inventory.
Procedures
Height, weight and percentage of body fat were assessed by measuring the subcutaneous fat over the right triceps muscle equidistant between the acromion and the olecranon. To calculate body mass index (BMI) and standardized BMI (zBMI), weight classifications were determined using the Centers for Disease Control and Prevention (CDC) guidelines growth charts 19. Intravenous blood samples were collected from an antecubital vein in 10 ml-Vacutainer® tubes (BD Vacutainer™; Franklin Lakes, NJ, USA) spray-coated with lithium heparin. Serum samples were collected in 10-ml serum-separating tubes SST™ (BD Vacutainer™) coated with clotting agents and stored frozen at −80°C for further analysis.
Flow cytometry
Mononuclear cells were isolated by density gradient centrifugation method using Histopaque 1077 (Sigma Aldrich, St Louis, MO, USA) and labelled with a combination of monoclonal antibodies, as described in Table 2 20–24. No differences were found for peripheral blood mononuclear cells (PBMC) yield (∼2 × 106 cells/ml of blood) or viability post-isolation (>98%) among the participant groups (P > 0·05). Antibody staining and flow cytometry procedures were performed as described previously 25 PBMC phenotypes were assessed on a BD Accuri C6 flow cytometer equipped with a blue laser emitting light at a fixed wavelength of 488 nm and a red laser-emitting light at a fixed wavelength of 640 nm. The cells were identified and gated electronically using the forward- and side-light-scatter mode and CFlow software (CFlow® software version 2; BD Biosciences, San Jose, BA, USA). Lymphocytes were identified and gated electronically. Fluorescent signals were collected in logarithmic mode (6 decade logarithmic amplifier). For each sample, 50 000 CD3+/CD4+ or CD3+/CD8+ events were collected for analysis (Fig. 1). Following acquisition, flow cytometry standard (FCS) files were transferred to a third-party software program (FCS Express version 3·0; De Novo, Los Angeles, CA, USA) for analysis. The percentage of all lymphocytes and lymphocyte subsets expressing surface markers of interest was tabulated. Total lymphocyte subset numbers were calculated by multiplying the percentage of lymphocytes expressing the surface markers of interest by the total lymphocyte count.
Table 2.
Combination of monoclonal antibodies used in the characterization of the different T cell phenotypes
| T cell phenotypes | T cell subtypes | References |
|---|---|---|
| CD28+/CD27+ | Early dif. | Appay et al. 2002 20 |
| CD28–/CD27+ | Intermediate dif. | |
| CD28−/CD27− | Late dif. | |
| CD28−/CD57+ | Senescent | Bandres 2000 21 |
| CD45RA+/CCR7+ | Naive (N) | Sallusto et al. 2004 23 |
| CD45RA−/CCR7+ | Central memory (CM) | Geginat et al. 2003 22 |
| CD45RA−/CCR7− | Effector memory (EM) | |
| CD45RA+/CCR7− | TEMRA |
TEMRA = T effector memory cells.
Figure 1.
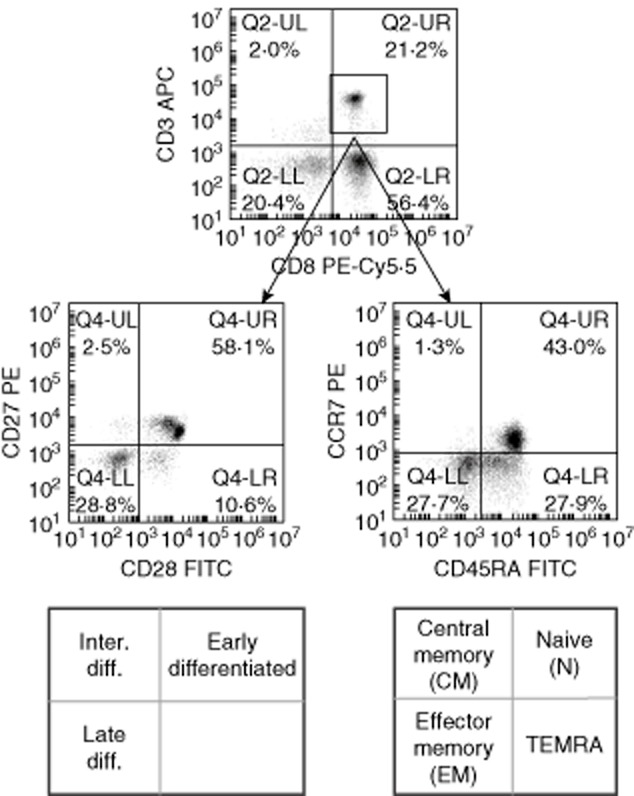
Representative samples depicting the gating strategy used to identify CD8TL subsets (numbers indicate %). CD3+/CD8+ T lymphocytes (CD8TLs) subsets were identified by CD3 and CD8 expression and either CD27/CD28 (left row) or CD45RA/CCR7 (right row) expression.
Determination of plasma leptin levels and latent viral status
Serum samples were analysed in duplicate for immunoglobulin (Ig)G antibodies anti-CMV and anti-EBV using separate commercially available enzyme-linked immunosorbent assay (ELISA) kits (GenWay Biotech, San Diego, CA, USA). Plasma leptin concentrations were also determined by ELISA (Invitrogen, Carlsbad, CA, USA). All samples were analysed in duplicate following the manufacturer's instructions.
Quality of life assessment
All participants completed the Pediatric Quality of Life Inventory™ (PedsQL 4·0). Physical health scores along with psychosocial health scores and total quality of life scores were obtained from the self-reported itemized questionnaire. Items were graded on a five-point scale from 0, corresponding to ‘never a problem’ to 4, ‘almost always a problem’. Finally, items were reverse-scored and linearly transformed to a 0–100 scale where higher scores attest to a greater health-related quality of life.
Statistical methods
All data were assessed for assumptions of normality using the Shapiro–Wilk test and constant error variance prior to formal statistical testing. Participants were categorized into one of three weight classifications based on their zBMI scores: (i) healthy weight, (ii) overweight or (iii) obese. χ2 tests were used to detect any differences in gender distribution and latent viral status among the weight classifications, while differences in physical characteristics among the body weight classifications were identified using a one-way analysis of variance (anova).
Generalized linear models were used to detect relationships between the independent (i.e. body weight classification, BMI, percentage body fat, plasma leptin concentration, latent viral status) variables and the proportions of specific T cell subsets in blood. Fractional logit models, which are generalized linear models with a logit link function and binomial distribution and robust estimators, were used to analyse the initial univariate associations between each individual variable and the proportions of each T cell subsets as the dependent variable. The fractional logit modelling approach allow for evaluation of variables that have fractional values ranging from 0 to 1 (i.e. proportions). All variables found to have a significant univariate relationship were included in multivariable fractional logit models to determine if the relationship still existed after adjusting for all other significant factors. The effects size of each independent variable (B) on the composition of blood T cell subsets were reported as an average change in the proportion of T cells with a particular surface phenotype for a single-unit change in the independent variable.
Statistical significance was set at P < 0·05. All values are presented as the mean ± standard error of the mean (s.e.m.). All statistical analyses were performed using the Statistical Package for the Social Sciences (spss version 17·0; SPSS, Inc., Chicago, IL, USA).
Results
The physical characteristics of all subjects are presented in Table 1. Differences in body composition were seen among the weight groups (P < 0·001); however, no significant differences in age, gender, viral status or PEDS QL scores were observed among the three body weight categories (P > 0·05).
Univariate associations between body weight and blood T cell subset composition
The individual variables found to be associated with the proportions of T cell subsets in blood are presented in the Table 3. Obese subjects had, on average, 4·01 and 7·60% fewer early (P < 0·05) CD4+ and CD8+ T cells, respectively, and 3·37 and 4·51% more EM and intermediate CD8+ T cells (P < 0·05) than subjects of healthy weight. Obese subjects also had a greater proportion of senescent and late CD4+ T cells (+0·97 and +2·00%, P < 0·05, respectively) than subjects of healthy weight. Overweight subjects had fewer early (−12·05%, P = 0·001), more EM (+6·04%, P < 0·001) and higher proportions of senescent and late CD8+ T cells (+5·42%, P < 0·05 and +10·10%, P < 0·01, respectively) than subjects of normal weight. An increase of 1% body fat was associated with a modest but significantly greater proportion of EM CD4+ T cells (+0·17%, P < 0·05) and intermediate CD8+ T cells (+0·06%, P < 0·01). No effect of body weight classification was observed on the total T cell numbers (P > 0·05).
Table 3.
Univariate associations: unadjusted direct relation between the different factors and the proportions of T cell subsets. Significant factors are listed according to their effect size on the proportions of T cell subsets.
| T cell subtype | Factors | Effect size* | P-value |
|---|---|---|---|
| Early CD4+ (CD28+/CD27+) | At risk | −4·43% | 0·084 |
| Obese | −4·01% | 0·050 | |
| zBMI | −1·80% | 0·038 | |
| % Body fat | −0·15% | 0·083 | |
| Early CD8+ (CD28+/CD27+) | Overweight | −12·05% | 0·001 |
| Gender (female) | +7·78% | 0·004 | |
| Obese | −7·60% | 0·011 | |
| EBV status (positive) | −5·93% | 0·031 | |
| CMV titre | −5·29% | 0·006 | |
| CMV status (positive) | −5·24% | 0·098 | |
| zBMI | −3·46% | 0·008 | |
| Intermediate CD8+ (CD28−/CD27+) | Obese | +4·51% | 0·001 |
| Age | −4·16% | 0·001 | |
| CMV status (positive) | −3·64% | 0·013 | |
| CMV titre | −1·76% | 0·053 | |
| zBMI | +1·66% | 0·006 | |
| % Body fat | +0·06% | 0·004 | |
| Leptin concentration | +0·05% | 0·094 | |
| EM CD8+ (CD45RA−/CCR7−) | Overweight | +6·04% | 0·001 |
| Obese | +3·37% | 0·025 | |
| Age | −1·73% | 0·090 | |
| zBMI | +1·14% | 0·083 | |
| Late CD4+ (CD28−/CD27−) | Obese | +2·00% | 0·003 |
| Overweight | +1·95% | 0·020 | |
| CMV titre | +1·21% | 0·022 | |
| zBMI | +0·66% | 0·023 | |
| % Body fat | +0·05% | 0·068 | |
| Late CD8+ (CD28−/CD27−) | Overweight | +10·10% | 0·003 |
| Gender (female) | −6·23% | 0·012 | |
| CMV status (positive) | +5·61% | 0·049 | |
| CMV titre | +5·02% | 0·001 | |
| Age | −3·77% | 0·040 | |
| TEMRA CD8+ (CD45RA+/CCR7−) | CMV status (positive) | +5·70% | 0·031 |
| Gender (female) | −4·80% | 0·041 | |
| Age | −4·58% | 0·007 | |
| CMV titre | +3·13% | 0·045 | |
| Senescent CD4+ (CD28−/CD57+) | Overweight | +0·97% | 0·096 |
| Obese | +0·97% | 0·037 | |
| CMV titre | +0·53% | 0·073 | |
| Senescent CD8+ (CD28−/C57+) | Overweight | +5·42% | 0·038 |
| Gender (female) | −5·16% | 0·005 | |
| CMV status (positive) | +4·41% | 0·037 | |
| CMV titre | +3·75% | 0·003 | |
| Age | −2·96% | 0·030 | |
| EBV titre | +1·56% | 0·086 |
Effect size is the average difference (+/−) in % of the phenotype for a 1-unit difference in the respective factor. BMI = body mass index; zBMI = standardized BMI; CMV = cytomegalovirus; EBV = Epstein–Barr virus; EM = effector memory; TEMRA = T effector memory cells.
Associations were found between CMV seropositivity and lower proportions of intermediate (−3·64%, P < 0·05) and greater proportions of late, T effector memory cells (TEMRA) and senescent CD8+ T cells (+5·61%, P < 0·05; +5·70%, P < 0·05 and +4·41%, P < 0·05, respectively). Furthermore, while EBV seropositivity was associated significantly with lower proportions of early CD8+ T cells (−5·93%, P < 0·05), a similar, although non-significant, trend was seen with CMV seropositivity (−5·24%, P = 0·098). Quality of life was not associated with blood T cell subset composition (P > 0·05).
Multivariate associations between body weight and blood T cell subset composition
Individual variables that were found to be associated significantly with the composition of T cell subsets in peripheral blood (Table 3) were included in the respective multivariable model for each subset. In these models, T cell subset proportions were included as dependent variables, and effects were adjusted for all other significantly related independent variables in the model. The independent variables associated with the composition of T cell subsets in blood after controlling for all other significantly related factors are shown in Table 4. Overweight and obese subjects still had, on average, 11·55 and 5·51% fewer early CD8+ T cells (P < 0·05), respectively, compared to subjects of normal weight. Age-adjusted body mass was also associated with altered proportions of differentiated T cells, as overweight and obese subjects had, on average, 7·53% (P < 0·001) and 5·49% (P < 0·05) more EM CD8+ T cells, respectively, and 1·90% (P < 0·05) and 2·01% (P < 0·05) more late CD4+ T cells, respectively, than subjects of normal weight. Finally, overweight subjects had, on average, 8·90% more late and 4·86% more senescent CD8+ T cells than subjects of normal weight (P < 0·01). Obese subjects also had 0·98% more senescent CD4+ T-cells (P < 0·05) compared to subjects of normal weight. All associations reported here withstood adjustment for all other variables that were found to have a significant relationship with the particular T cell subset proportions, including latent CMV and EBV infections (seropositivity) and viral load (using IgG antibody titres as a surrogate measure). Differences in the composition of blood T cell subsets among the body weight classifications are shown in Fig. 2.
Table 4.
Multivariate associations: all factor effects controlled for the other significant factors shown for the proportions of T cell subsets. Significant factors are listed according to their effect size on the proportions of T cell subsets.
| T cell subtype | Factors | Effect size* | P-value |
|---|---|---|---|
| N CD4+ (CD45RA+/CCR7+) | EBV status (positive) | −4·03% | 0·098 |
| Age | −3·50% | 0·050 | |
| N CD8+ (CD45RA+/CCR7+) | Gender (female) | +5·31% | 0·050 |
| Early CD8+ (CD28+/CD27+) | Overweight | −11·55% | 0·001 |
| CMV titre | −7·78% | 0·019 | |
| Gender (female) | +6·33% | 0·011 | |
| EBV status (positive) | −5·83% | 0·020 | |
| Obese | −5·51% | 0·047 | |
| Intermediate CD8+ (CD28−/CD27+) | Obese | +4·59% | 0·010 |
| Age | −3·81% | 0·000 | |
| EM CD4+ (CD45RA−/CCR7−) | Age | +5·29% | 0·001 |
| % Body fat | +0·21% | 0·003 | |
| EM CD8+ (CD45RA−/CCR7−) | Overweight | +7·53% | 0·001 |
| Obese | +5·49% | 0·025 | |
| Age | −1·56% | 0·098 | |
| Late CD4+ (CD28−/CD27−) | Obese | +2·01% | 0·020 |
| Overweight | +1·90% | 0·022 | |
| Late CD8+ (CD28−/CD27−) | Overweight | +8·90% | 0·004 |
| Gender (female) | −6·78% | 0·002 | |
| CMV titre | +3·95% | 0·011 | |
| Age | −3·78% | 0·015 | |
| TEMRA CD8+ (CD45RA+/CCR7−) | Age | −4·78% | 0·0036 |
| Gender (female) | −4·63% | 0·036 | |
| Senescent CD4+ (CD28−/CD57+) | Obese | +0·98% | 0·032 |
| CMV titre | +0·52% | 0·049 | |
| Senescent CD8+ (CD28−/C57+) | Gender (female) | −6·17% | 0·001 |
| Overweight | +4·86% | 0·029 | |
| CMV titre | +4·32% | 0·050 | |
| Age | −3·09% | 0·005 | |
| EBV titre | +1·90% | 0·008 |
Effect size is the average difference (+/−) in % of the phenotype for a 1-unit difference in the respective factor. CMV = cytomegalovirus; EBV = Epstein–Barr virus; EM = effector memory; N = naive; TEMRA = T effector memory cells.
Figure 2.
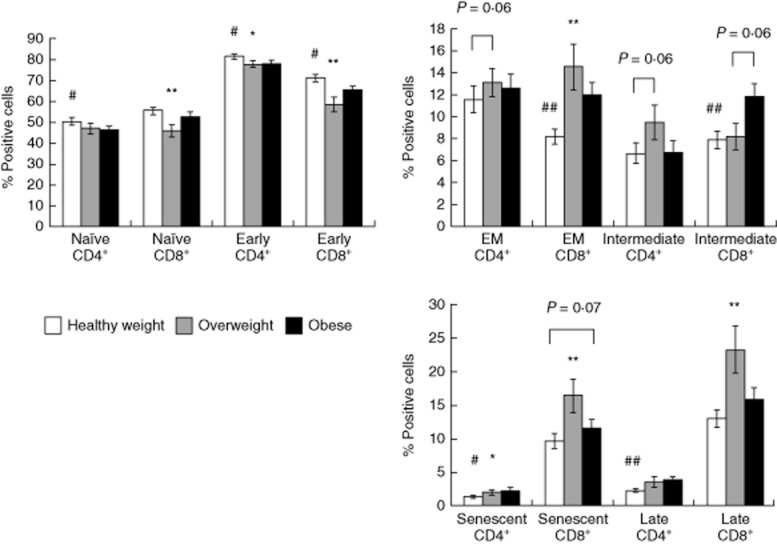
The impact of weight classifications on the blood T cell subsets (in %). Values are mean ± standard error of the mean. Statistically significant differences from the ‘healthy weight’ group is indicated by *P < 0·05 and **P < 0·01. Statistically significant differences from the ‘obese’ group are indicated by #P < 0·05 and ##P < 0·01.
Other factors associated with blood T cell subset composition after adjustment for body mass variables
In addition to the body mass variables, several other factors such as latent viral infections and sex (male or female) were found to be associated with the composition of T cell subsets in blood (Table 4). Subjects infected with EBV had fewer early CD8+ T cells (−5·83%, P < 0·05) than seronegative subjects. A similar, although non-significant, trend was observed between EBV seropositivity and the proportions of naive CD4+ T cells (−4·03%, P = 0·098). Anti-CMV antibody titres within the CMV-seropositive subject group were associated with increased proportions of senescent CD4+ (+0·52%, P < 0·05) and CD8+ T cells (+4·32%, P < 0·05), along with increased proportions of late CD8+ T cells (+3·95%, P < 0·05). Similarly, an increase in 1 unit of anti-EBV antibody titre was associated with an increase of 1·90% of senescent CD8+ T cells in EBV-seropositive subjects (P < 0·05). Sex (male or female) was also associated with the proportions of naive and early CD8+ T cells when all other significant variables were controlled for. Females had, on average, 5·31% (P < 0·05) more naive CD8+ T cells and 6·3% (P < 0·05) more early CD8+ T cells than males. Females had consistently fewer senescent and late CD8+ T cells (−6·17%, P < 0·01 and −6·78%, P < 0·05, respectively) and fewer TEMRA CD8+ T cells (−4·63%, P < 0·05) than males. All associations reported here withstood adjustment for all other variables found to have a significant relationship with the particular T cell subset proportions.
Discussion
The main finding of this study is that excess body mass was associated with T cell differentiation indicative of immunosenescence in adolescent children. Proportions of naive and early-differentiated cells among the CD4+ and CD8+ T cell compartments were substantially lower in those children with excess body mass compared to healthy weight children. Moreover, overweight and obese children had greater proportions of mature, effector-memory CD8+ T cells and late differentiated CD4+ T cells. These findings withstood adjustment for psychosocial factors (quality of life), age, gender and latent CMV and EBV infections, all of which have been shown previously to have a profound influence on the composition of T cell subsets in blood 26. These data indicate that excess body mass, even in adolescence, may accelerate ageing of the immune system and we speculate that this might also predispose children to the early acquisition of an immune risk profile in adulthood.
Blood T cell populations with an increased composition of cells exhibiting an effector-memory and senescent phenotype, particularly within the CD8+ T cell compartment, are signature features of the immune risk profile (IRP) 26. Although the IRP can predict morbidity and mortality in elderly people 27, identifying factors responsible for altering IRP components in children had not been determined previously. The present data demonstrate clear differences in the composition of T cell subsets in blood among children of different body weight classifications. Children with excess body mass (either overweight or obese) had lower proportions of naive and early differentiated CD4+ and CD8+ T cells and greater proportions of intermediate and effector-memory CD8+ T cells than children of normal weight. However, within the CD4+ T cell compartment, proportions of naive cells were lowered and senescent cells elevated only in the obese children. These results suggest that excess body mass may have a deleterious impact on the composition of the blood T cell population, by increasing late differentiated and senescent T cell numbers and reducing the naive CD4+ T cell pool.
Although our overall hypothesis that excess body mass would be associated with increased T cell differentiation was accepted, there were some unexpected findings. For instance, we anticipated T cell differentiation to be associated positively with excess body mass in a stepwise manner; however, overweight children had significantly greater proportions of late differentiated CD8+ T cells than both obese and healthy weight children. Similarly, overweight children had fewer naive CD8+ T cells than both obese and healthy weight children, whereas no differences were found between obese and healthy weight children for proportions of late or naive CD8+ T cells. As the participants in this study were of adolescent age, possible variations in pubertal maturation among the weight classifications may be responsible for these unexpected findings. Another possibility is that more metabolically healthy children were included in the obese compared to the overweight group. Metabolically healthy but obese (MHO) individuals, despite having excess body fat, display a metabolically healthy profile, including high levels of insulin sensitivity and clinically normal lipid and inflammation profiles 28. These individuals are not as susceptible to excessive inflammation 29 and cardiovascular disease as overweight individuals who have metabolic disorder 30. Future studies should determine the impact of metabolic disorder on the currently observed relationship between excess body mass and T cell differentiation.
All associations between excess body mass and T cell differentiation reported here withstood adjustment for latent CMV and EBV infections indicating that, even in children, adiposity is an independent predictor of T cell subset composition. Moreover, although adenovirus 36 and HSV-1 have been found to be more prevalent in obese compared to healthy weight individuals 11,31, CMV and EBV infections were similar across the weight classifications and not related to any of the body mass variables measured in this study. This indicates that viral infections and adiposity may impact T cell subset composition via different mechanisms. Although no study to date has assessed the impact of excess body mass on the composition of T cells subsets in blood, a few studies have proposed mechanisms by which obesity might accelerate immunosenescence. For instance, short telomeres are indicative of advanced biological ageing and BMI is known to be correlated inversely with mean telomere length in blood leucocytes 32. Furthermore, while both telomere length 33 and obesity 34 are known predictors of cardiovascular disease (CVD), senescent T cells have been shown to accumulate during the development of CVD 35 suggesting potential links between obesity, immunosenescence and CVD.
Although the impact of latent CMV and EBV infections on the composition of blood T cell subsets has been studied extensively in adults 36–38, this is the first study to our knowledge to examine these relationships in children. We were interested to determine if associations between latent viral infections and T cell differentiation would manifest in children who, by definition, are likely to have been carrying the virus for a relatively shorter period of time than adults. Not surprisingly, we found that CMV and EBV infections are also associated with increased levels of T cell differentiation in children although, at least in adolescents, EBV and not CMV seropositivity is associated with lower proportions of naive and early differentiated T cells. Moreover, within the CMV and/or EBV seropositive subjects, anti-viral antibody titres, as an indication of viral load, were associated positively with the proportions of late differentiated CD8+ T cells (CMV titres) and senescent CD4+ (CMV titres) and CD8+ (CMV and EBV titres) T cells in blood. High anti-CMV antibody titres have been associated with viral reactivation 39, inflammation and mortality 40. As EBV but not CMV serostatus was associated with lower proportions of naive CD4+ T cells and early differentiated CD8+ T cells, it could be speculated that primary EBV infection has a stronger initial impact on T cell differentiation, while multiple reactivations of CMV may be required before discernible alterations in the composition of blood T cell subsets are observed. Another possibility is that most subjects may have acquired EBV at an earlier age than those who contracted CMV. Although it is not currently possible to determine when primary infection occurred, many individuals infected with EBV remain CMV seronegative, while most people with CMV also have a latent EBV infection 37. This would indicate that primary EBV infection precedes primary CMV infection and that most individuals will harbour EBV for a longer period of time than CMV.
We also found that adolescent males exhibited greater levels of T cell differentiation compared to females of similar age, and that these differences between the sexes withstood adjustment for all other measured confounding factors. While some gender-related differences in immune response have been documented, including greater humoral and cellular immune responses in women 41 and a greater T cell responses to mitogens 42, few studies have assessed sex-associated differences in the composition of T cell subsets in blood 43. We found that females had more naive and early differentiated T cells, but less TEMRA, late differentiated and senescent CD8+ T cells than males. The mechanisms behind the sex-associated difference remains to be identified; however, it is plausible that sex hormones play a role in either accelerating T cell differentiation in males or negating it in females. Indeed, it has been shown that oestrogen activates the gene coding for telomerase, an enzyme responsible for extending chromosome telomere lengths, indicating that female sex hormones may help to delay the onset of immunosenescence. Future studies should determine the role of sex hormones on age-related changes in the composition of blood T cell subsets in both males and females, particularly during adolescence when hormones are in flux. A limitation of this study is that vaccination records were not obtained. It is possible, therefore, that differences in the composition of certain T cell subsets among the subject groups could be due to vaccination history.
We were surprised to find that neither physical or psychological health were associated with the composition of T cell subsets in blood, particularly because psychological stress is known to be associated with the accumulation of highly differentiated T cells in adults 44 and also in children with clinically depressed parents 45. It should be noted, however, that the quality of life scores reported by the subjects were relatively high and did not differ among the weight classification groups. As the cohort used in this study were polarized toward low self-reported stress scores, it is likely that a more heterogeneous group will be required to adequately determine relationships between quality of life and T cell differentiation in paediatric populations.
In conclusion, this study reports for the first time relationships between excess body mass and T cell differentiation associated with immunosenescence in adolescents. As these findings withstood adjustment for a large number of potentially confounding variables, including age, quality of life, sex differences and latent viral infections, we suggest that excess body mass is an independent predictor of T cell differentiation. Moreover, given that discernible associations between excess body mass and the composition of blood T cell subsets are evident even in children, we speculate that childhood obesity increases the risk of incurring immune-related health problems in adulthood. Future research should examine the effects of weight loss interventions on the composition of blood T cell subsets in both children and adults, in order to determine if lowering body fat levels can positively alter the composition of blood T cell subsets.
Disclosure
The authors have no conflicts of interest to disclose. The authors have no competing interests.
References
- 1.Rhodes ET, Goran MI, Lieu TA, et al. Health-related quality of life in adolescents with or at risk for type 2 diabetes mellitus. J Pediatr. 2012;160:911–917. doi: 10.1016/j.jpeds.2011.11.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.American Diabetes Association. Type 2 diabetes in children and adolescents. Diabetes Care. 2000;23:381–389. doi: 10.2337/diacare.23.3.381. [DOI] [PubMed] [Google Scholar]
- 3.van Emmerik NM, Renders CM, van de Veer M, et al. High cardiovascular risk in severely obese young children and adolescents. Arch Dis Child. 2012;97:818–821. doi: 10.1136/archdischild-2012-301877. [DOI] [PubMed] [Google Scholar]
- 4.Cook S, Weitzman M, Auinger P, Nguyen M, Dietz WH. Prevalence of a metabolic syndrome phenotype in adolescents: findings from the third National Health and Nutrition Examination Survey, 1988–1994. Arch Pediatr Adolesc Med. 2003;157:821–827. doi: 10.1001/archpedi.157.8.821. [DOI] [PubMed] [Google Scholar]
- 5.Freedman DS, Khan LK, Serdula MK, Dietz WH, Srinivasan SR, Berenson GS. The relation of childhood BMI to adult adiposity: the Bogalusa Heart Study. Pediatrics. 2005;115:22–27. doi: 10.1542/peds.2004-0220. [DOI] [PubMed] [Google Scholar]
- 6.Dhuper S, Abdullah RA, Weichbrod L, Mahdi E, Cohen HW. Association of obesity and hypertension with left ventricular geometry and function in children and adolescents. Obesity (Silver Spring) 2011;19:128–133. doi: 10.1038/oby.2010.134. [DOI] [PubMed] [Google Scholar]
- 7.Strauss RS, Pollack HA. Social marginalization of overweight children. Arch Pediatr Adolesc Med. 2003;157:746–752. doi: 10.1001/archpedi.157.8.746. [DOI] [PubMed] [Google Scholar]
- 8.Jedrychowski W, Maugeri U, Flak E, Mroz E, Bianchi I. Predisposition to acute respiratory infections among overweight preadolescent children: an epidemiologic study in Poland. Public Health. 1998;112:189–195. doi: 10.1038/sj.ph.1900438. [DOI] [PubMed] [Google Scholar]
- 9.Sheridan PA, Paich HA, Handy J, et al. Obesity is associated with impaired immune response to influenza vaccination in humans. Int J Obes (Lond) 2011;36:1072–1077. doi: 10.1038/ijo.2011.208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Louie JK, Acosta M, Samuel MC, et al. A novel risk factor for a novel virus: obesity and 2009 pandemic influenza A (H1N1) Clin Infect Dis. 2011;52:301–312. doi: 10.1093/cid/ciq152. [DOI] [PubMed] [Google Scholar]
- 11.Gabbert C, Donohue M, Arnold J, Schwimmer JB. Adenovirus 36 and obesity in children and adolescents. Pediatrics. 2010;126:721–726. doi: 10.1542/peds.2009-3362. [DOI] [PubMed] [Google Scholar]
- 12.Karjala Z, Neal D, Rohrer J. Association between HSV1 seropositivity and obesity: data from the National Health and Nutritional Examination Survey, 2007–2008. PLOS ONE. 2011;6:e19092. doi: 10.1371/journal.pone.0019092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Pawelec G. Hallmarks of human ‘immunosenescence’: adaptation or dysregulation? Immun Ageing. 2012;9:15. doi: 10.1186/1742-4933-9-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Wikby A, Maxson P, Olsson J, Johansson B, Ferguson FG. Changes in CD8 and CD4 lymphocyte subsets, T cell proliferation responses and non-survival in the very old: the Swedish longitudinal OCTO-immune study. Mech Ageing Dev. 1998;102:187–198. doi: 10.1016/s0047-6374(97)00151-6. [DOI] [PubMed] [Google Scholar]
- 15.Tam CS, Clement K, Baur LA, Tordjman J. Obesity and low-grade inflammation: a paediatric perspective. Obes Rev. 2010;11:118–126. doi: 10.1111/j.1467-789X.2009.00674.x. [DOI] [PubMed] [Google Scholar]
- 16.Codoner-Franch P, Valls-Belles V, Arilla-Codoner A, Alonso-Iglesias E. Oxidant mechanisms in childhood obesity: the link between inflammation and oxidative stress. Transl Res. 2011;158:369–384. doi: 10.1016/j.trsl.2011.08.004. [DOI] [PubMed] [Google Scholar]
- 17.Jia H, Lubetkin EI. The impact of obesity on health-related quality-of-life in the general adult US population. J Public Health (Oxf) 2005;27:156–164. doi: 10.1093/pubmed/fdi025. [DOI] [PubMed] [Google Scholar]
- 18.Martin-Romero C, Santos-Alvarez J, Goberna R, Sanchez-Margalet V. Human leptin enhances activation and proliferation of human circulating T lymphocytes. Cell Immunol. 2000;199:15–24. doi: 10.1006/cimm.1999.1594. [DOI] [PubMed] [Google Scholar]
- 19.Centers for Disease Control and Prevention (CDC) Table for calculated Body Mass Index values for selected heights and weights for ages 2 to 20. Atlanta, GA: CDC; 2000. [Google Scholar]
- 20.Appay V, Dunbar PR, Callan M, et al. Memory CD8+ T cells vary in differentiation phenotype in different persistent virus infections. Nat Med. 2002;8:379–385. doi: 10.1038/nm0402-379. [DOI] [PubMed] [Google Scholar]
- 21.Bandres E, Merino J, Vazquez B, et al. The increase of IFN-gamma production through aging correlates with the expanded CD8(+high)CD28(–)CD57(+) subpopulation. Clin Immunol. 2000;96:230–235. doi: 10.1006/clim.2000.4894. [DOI] [PubMed] [Google Scholar]
- 22.Geginat J, Lanzavecchia A, Sallusto F. Proliferation and differentiation potential of human CD8+ memory T-cell subsets in response to antigen or homeostatic cytokines. Blood. 2003;101:4260–4266. doi: 10.1182/blood-2002-11-3577. [DOI] [PubMed] [Google Scholar]
- 23.Sallusto F, Geginat J, Lanzavecchia A. Central memory and effector memory T cell subsets: function, generation, and maintenance. Annu Rev Immunol. 2004;22:745–763. doi: 10.1146/annurev.immunol.22.012703.104702. [DOI] [PubMed] [Google Scholar]
- 24.Sallusto F, Lenig D, Forster R, Lipp M, Lanzavecchia A. Two subsets of memory T lymphocytes with distinct homing potentials and effector functions. Nature. 1999;401:708–712. doi: 10.1038/44385. [DOI] [PubMed] [Google Scholar]
- 25.Spielmann G, McFarlin BK, O'Connor DP, Smith PJ, Pircher H, Simpson RJ. Aerobic fitness is associated with lower proportions of senescent blood T-cells in man. Brain Behav Immun. 2011;25:1521–1529. doi: 10.1016/j.bbi.2011.07.226. [DOI] [PubMed] [Google Scholar]
- 26.Wikby A, Mansson IA, Johansson B, Strindhall J, Nilsson SE. The immune risk profile is associated with age and gender: findings from three Swedish population studies of individuals 20–100 years of age. Biogerontology. 2008;9:299–308. doi: 10.1007/s10522-008-9138-6. [DOI] [PubMed] [Google Scholar]
- 27.Pawelec G, Larbi A, Derhovanessian E. Senescence of the human immune system. J Comp Pathol. 2010;142(Suppl. 1):S39–44. doi: 10.1016/j.jcpa.2009.09.005. [DOI] [PubMed] [Google Scholar]
- 28.Karelis AD, Faraj M, Bastard JP, et al. The metabolically healthy but obese individual presents a favorable inflammation profile. J Clin Endocrinol Metab. 2005;90:4145–4150. doi: 10.1210/jc.2005-0482. [DOI] [PubMed] [Google Scholar]
- 29.Shin MJ, Hyun YJ, Kim OY, Kim JY, Jang Y, Lee JH. Weight loss effect on inflammation and LDL oxidation in metabolically healthy but obese (MHO) individuals: low inflammation and LDL oxidation in MHO women. Int J Obes (Lond) 2006;30:1529–1534. doi: 10.1038/sj.ijo.0803304. [DOI] [PubMed] [Google Scholar]
- 30.Meigs JB, Wilson PW, Fox CS, et al. Body mass index, metabolic syndrome, and risk of type 2 diabetes or cardiovascular disease. J Clin Endocrinol Metab. 2006;91:2906–2912. doi: 10.1210/jc.2006-0594. [DOI] [PubMed] [Google Scholar]
- 31.Fernandez-Real JM, Ferri MJ, Vendrell J, Ricart W. Burden of infection and fat mass in healthy middle-aged men. Obesity (Silver Spring) 2007;15:245–252. doi: 10.1038/oby.2007.541. [DOI] [PubMed] [Google Scholar]
- 32.Valdes AM, Andrew T, Gardner JP, et al. Obesity, cigarette smoking, and telomere length in women. Lancet. 2005;366:662–664. doi: 10.1016/S0140-6736(05)66630-5. [DOI] [PubMed] [Google Scholar]
- 33.Nakashima H, Ozono R, Suyama C, Sueda T, Kambe M, Oshima T. Telomere attrition in white blood cell correlating with cardiovascular damage. Hypertens Res. 2004;27:319–325. doi: 10.1291/hypres.27.319. [DOI] [PubMed] [Google Scholar]
- 34.Mathieu P, Lemieux I, Despres JP. Obesity, inflammation, and cardiovascular risk. Clin Pharmacol Ther. 2010;87:407–416. doi: 10.1038/clpt.2009.311. [DOI] [PubMed] [Google Scholar]
- 35.Brouilette SW, Moore JS, McMahon AD, et al. Telomere length, risk of coronary heart disease, and statin treatment in the West of Scotland Primary Prevention Study: a nested case–control study. Lancet. 2007;369:107–114. doi: 10.1016/S0140-6736(07)60071-3. [DOI] [PubMed] [Google Scholar]
- 36.Almanzar G, Schwaiger S, Jenewein B, et al. Long-term cytomegalovirus infection leads to significant changes in the composition of the CD8+ T-cell repertoire, which may be the basis for an imbalance in the cytokine production profile in elderly persons. J Virol. 2005;79:3675–3683. doi: 10.1128/JVI.79.6.3675-3683.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Derhovanessian E, Maier AB, Hahnel K, et al. Infection with cytomegalovirus but not herpes simplex virus induces the accumulation of late-differentiated CD4+ and CD8+ T-cells in humans. J Gen Virol. 2011;92:2746–2756. doi: 10.1099/vir.0.036004-0. [DOI] [PubMed] [Google Scholar]
- 38.Pawelec G, Akbar A, Beverley P, et al. Immunosenescence and cytomegalovirus: where do we stand after a decade? Immun Ageing. 2010;7:13. doi: 10.1186/1742-4933-7-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Bennett JM, Glaser R, Malarkey WB, Beversdorf DQ, Peng J, Kiecolt-Glaser JK. Inflammation and reactivation of latent herpesviruses in older adults. Brain Behav Immun. 2012;26:739–746. doi: 10.1016/j.bbi.2011.11.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Roberts ET, Haan MN, Dowd JB, Aiello AE. Cytomegalovirus antibody levels, inflammation, and mortality among elderly Latinos over 9 years of follow-up. Am J Epidemiol. 2010;172:363–371. doi: 10.1093/aje/kwq177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Zen M, Ghirardello A, Iaccarino L, et al. Hormones, immune response, and pregnancy in healthy women and SLE patients. Swiss Med Wkly. 2010;140:187–201. doi: 10.4414/smw.2010.12597. [DOI] [PubMed] [Google Scholar]
- 42.Santoli D, Trinchieri G, Zmijewski CM, Koprowski H. HLA-related control of spontaneous and antibody-dependent cell-mediated cytotoxic activity in humans. J Immunol. 1976;117:765–770. [PubMed] [Google Scholar]
- 43.Yan J, Greer JM, Hull R, et al. The effect of ageing on human lymphocyte subsets: comparison of males and females. Immun Ageing. 2010;7:4. doi: 10.1186/1742-4933-7-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Bosch JA, Fischer JE, Fischer JC. Psychologically adverse work conditions are associated with CD8+ T cell differentiation indicative of immunesenescence. Brain Behav Immun. 2009;23:527–534. doi: 10.1016/j.bbi.2009.02.002. [DOI] [PubMed] [Google Scholar]
- 45.Caserta MT, O'Connor TG, Wyman PA, et al. The associations between psychosocial stress and the frequency of illness, and innate and adaptive immune function in children. Brain Behav Immun. 2008;22:933–940. doi: 10.1016/j.bbi.2008.01.007. [DOI] [PMC free article] [PubMed] [Google Scholar]


