Abstract
Inflammatory bowel diseases are characterized by a deregulated immune response targeting the gut bacterial flora. Mucosal-associated invariant T (MAIT) cells are major histocompatibility complex (MHC) class Ib-restricted innate-like lymphocytes with anti-bacterial functions. They display an effector/memory phenotype and are found in large numbers in the blood, mucosae and liver. They have also been implicated in inflammatory diseases such as multiple sclerosis. Therefore, we aimed to analyse the possible involvement of MAIT cells in Crohn's disease (CD) and ulcerative colitis (UC). To this end, a phenotypical and functional analysis of MAIT cells isolated from the blood of healthy subjects, CD and UC patients was undertaken. MAIT cells were also quantified in ileal biopsies of CD patients. The frequency of blood MAIT cells was specifically reduced in IBD patients compared with healthy donors, whereas it was dramatically greater in the inflamed versus healthy tissue. MAIT cells were activated as they expressed significantly more the Ki67 antigen, and this was accompanied by phenotypical changes such as increased expression of natural killer (NK)G2D and B and T lymphocyte attenuator (BTLA). Finally, in-vitro-activated MAIT cells from CD and UC patients secreted significantly more interleukin (IL)-17, together with a decreased interferon (IFN)-γ in CD but an increased IL-22 in UC. These data show that MAIT cells are activated in IBD, which results in an increased recruitment towards the inflamed tissues, an altered phenotype and a switch in the pattern of cytokine secretion. This is the first demonstration that MAIT cells are immune players in IBD, whose precise functions in this context need to be addressed.
Keywords: immune system, inflammatory bowel diseases, innate T cells, MHC class I-like
Introduction
The uncontrolled immune response is a major contributor to the pathogenesis of inflammatory bowel diseases (IBD), such as Crohn's disease (CD) and ulcerative colitis (UC). The identification of the cellular immune players involved represents a major objective to decipher the pathophysiological mechanisms of these pathologies, and may suggest future therapeutic strategies to treat these chronic inflammatory conditions. CD is the most-studied IBD, both in humans and with the help of animal models. There is a consensus that the disease is mainly initiated and driven by CD4+ T cells targeting the gut commensal microbiota. Initially described as a T helper type 1 (Th1)-like immune response, characterized by the production of interleukin (IL)-12, tumour necrosis factor (TNF)-α and interferon (IFN)-γ, recent data strongly suggest that IL-17-producing Th17 CD4+ T cells are strong contributors to the disease; furthermore, a diminished activity of regulatory T cells may also be involved. The situation is less clear in the case of UC: the disease is also dependent upon the intestinal bacterial flora, but the inappropriate immune response is mainly Th2-like, characterized by the secretion of IL-5 and IL-13 by a peculiar subset of CD1d-restricted natural killer (NK) T cells that do not express the semi-invariant Vα24-Jα18 T cell receptor (TCR)-α chain (type II NK T cells) 1–3.
Mucosal-associated invariant T (MAIT) cells constitute a unique subset of major histocompatibility complex (MHC) class Ib-restricted, innate-like T lymphocytes 4. They express a semi-invariant TCR repertoire (made of an invariant Vα7.2-Jα33 TCRα chain) and recognize the MHC class Ib molecule MHC-related 1 (MR1) 5,6. Most MAIT cells are of a CD8α (either αα or αβ) phenotype, with a minority of CD4–CD8– cells; CD4+ MAIT cells are rare. MAIT cells are relatively abundant in human peripheral blood, where they usually represent 1–4% of total TCR-αβ+ T cells 7. However, consistent with their phenotype of effector/memory cells with tissue-homing properties CD45RA–CCR7–CD62L–CCR6+, MAIT cells are also found in peripheral tissues, such as the mucosae of the lung and the intestine and are particularly abundant in the human liver, where they can make up to 50% of T cells 8. MAIT cells express high levels of CD26 and CD161, and are positive for retinoid-related orphan receptor (ROR)γt, three hallmarks of IL-17-producing cells. Consistently, in-vitro activation with phorbol myristate acetate (PMA) and ionomycin enables production of IL-17A, together with IFN-γ, TNF-α, granzyme B (GrzB) and IL-2. MAIT cells development is dependent upon the presence of the microbial flora, and mature MAIT cells are activated in the presence of various (but not all) bacteria and fungi, in an MR1-dependent manner 9. Recently, metabolites of vitamin B2 have been identified as bacterial ligands for MR1, which may explain this broad reactivity of MAIT cells 10. The role of this innate T cell subset is still enigmatic: MAIT cells are recruited in the lungs of patients presenting bacterial respiratory infections, and are implicated in the clearance of mycobacteria in a mouse model 9,11. However, they are also apparently involved in non-infectious inflammatory diseases or viral infections: in particular, CD161hiCD8α+ T cells similar (and most probably identical) to MAIT cells are recruited both in demyelinating lesions of patients with multiple sclerosis, as well as in the inflammatory tissues of hepatitis C virus (HCV)-infected patients 12,13. Their role in these conditions is not known, although it was reported recently that MAIT cells are pathogenic in a mouse model of rheumatoid arthritis 14.
All the elements described above prompted us to investigate the possible implication of MAIT cells in the pathogenesis of IBD. We undertook this analysis and found that they are activated in patients with IBD, accumulate in the inflamed mucosa and secrete higher levels of IL-17 and/or IL-22 than their normal counterparts. Therefore, innate-like MAIT cells may be involved in these inflammatory diseases, a finding that adds a new piece to the puzzle of IBD pathophysiology.
Materials and methods
Human samples
Forty patients with moderate to severely active IBD (CD, n = 31; UC, n = 9) were included in this prospective study. Characteristics of the patients are given in Table 1. Blood from healthy donors was obtained from the Etablissement Français du Sang, according to standard regulations. Intestinal biopsies were obtained from the Department of Pathology (CHU Amiens, France). All patients gave written informed consent to this study, according to ethical guidelines.
Table 1.
Characteristics of the patients included in the study.
| Patients with CD (n = 31) | Patients with UC (n = 9) | Healthy donors (n = 44) | |
|---|---|---|---|
| Age (years) (mean) | 39 | 33 | 31 |
| Sex (M/F) | 16/15 | 2/7 | 26/18 |
| Disease location (Montréal classification) | L1 n = 5 | E1 n = 0 | |
| L2 n = 1 | E2 n = 6 | ||
| L3 n = 25 | E3 n = 3 | ||
| L4 n = 4 | |||
| P n = 7 | |||
| Ongoing treatments | |||
| 5-AZA | 3 | 1 | |
| Corticosteroids | 3 | 3 | |
| Immunosuppressors | 3 | 3 | |
| Anti-TNF | 16 | 7 | |
| Clinical activity (active/remission) | 11/20 | 3/6 | |
| Crohn's Disease Activity Index (active/remission) | 221/84, 3 | n.a. | |
| Partial Mayo Clinic score (active/remission) | n.a. | 4·3/1·5 | |
| CRP (active/remission) | 45, 8/8, 1 | 20·1/<3 | |
| Endoscopy | 18 | 5 | |
| Surgery (yes/no) | 16/15 | 0/9 | |
CD = Crohn's disease; UC = ulcerative colitis; TNF = tumour necrosis factor; CRP = C-reactive protein; AZA = azathioprine; n.a. = not applicable; M/F = male/female.
Flow cytometry
Peripheral blood mononuclear cells were obtained after a standard gradient centrifugation technique (Lymphoprep; GE Healthcare, Little Chalfont, UK). Cell stainings were performed according to standard techniques. The following antibodies were used: anti-CD3-physoerythrin (PE)-cyanin 5 (Cy5), anti-CD4-allophycocyanin (APC)-H7, anti-CD8-Pacific blue, anti-CD161-APC, anti-BTLA-PE, anti-NKG2D-PE and anti-Ki67-fluorescein isothiocyanate (FITC) (BD Pharmingen, San Diego, CA, USA), anti-TCR-γδ-FITC (Beckman Coulter, Brea, CA, USA) and anti-Vα7.2 (3C10)-biotin. 3C10 staining was detected with streptavidin-PE-Cy7 (BD Pharmingen). For Ki67 intracellular staining, the Cytofix/Cytoperm™ Fixation/Permeabilization Kit was used according to the manufacturer's specification (BD Biosciences, Franklin Lakes, NJ, USA).
Acquisitions were performed on the FACS Canto II (BD Biosciences) and data were analysed with FlowJo software (Tree Star, Ashland, OR, USA).
Confocal microscopy
The detection of MAIT cells in biopsies was performed on acetone-fixed frozen sections. The antibody panel included anti-CD3 (polyclonal rabbit; Dako, Glostrup, Denmark), anti-IL-18Rα (polyclonal goat; R&D Systems, Minneapolis, MN, USA) and anti-Vα7.2 (3C10) detected by the respective secondary antibodies: donkey anti-rabbit-Cy5 (Jackson Immunoresearch, West Grove, PA, USA), donkey anti-goat-Cy3 (Jackson Immunoresearch), goat anti-mouse-A488 (Invitrogen, Carlsbad, CA, USA). The frequency of MAIT cells was calculated as follows: %MAIT cells = (number of CD3+IL-18Rα+Vα7.2+ cells)/(number of CD3+ cells). For each sample, a screening was performed to select fields that contain a sufficient number of CD3+ T cells, which were selected for further analysis. A minimum of 300 CD3+ cells/sample were counted to reach statistical significance. Analyses were performed on the LSM 710 (Zeiss, Jena, Germany).
In-vitro MAIT cell activation and cytokine quantification
CD3+CD161hiVα7.2+ MAIT cells were sorted on a FACS Aria II (Becton Dickinson). Purity was routinely >95%. MAIT cells from healthy donors, CD and UC were stimulated with phorbol myristate acetate (0·25 μg/ml) and ionomycin (1 μg/ml). Supernatants were harvested after 36 h of stimulation and cytokine levels were measured by the human Th1/Th2/Th9/Th17/Th22 13plex FlowCytomix Multiplex kit (eBioscience, San Diego, CA, USA), according to the supplier's recommendations.
Statistical analysis
All data were analysed using GraphPad Prism (GraphPad, San Diego, CA, USA). Differences between groups were analysed using the Mann–Whitney U-test, with significance defined as P < 0·05.
Ethical considerations
Written informed consents were obtained for each patient included in the study. This study was performed in agreement with guidelines edited by the local ethics committee.
Results
Peripheral blood MAIT cells are less abundant in patients with CD and UC
MAIT cells can be defined and identified as CD161hiVα7.2+CD3+ lymphocytes 7. To investigate their potential involvement in IBD, we first measured and compared their frequency among CD3+TCRγδneg lymphocytes in the peripheral blood of patients with CD, UC and healthy donors (Table 1, Fig. 1a). We observed that the proportion of MAIT cells decreases significantly both in CD (mean 1·6% ± 0·2; P < 0·0001) and UC (mean 1·55% ± 0·55; P = 0·0019), compared with controls (mean 3·4% ± 0·3) (Fig. 1a,b).
Figure 1.
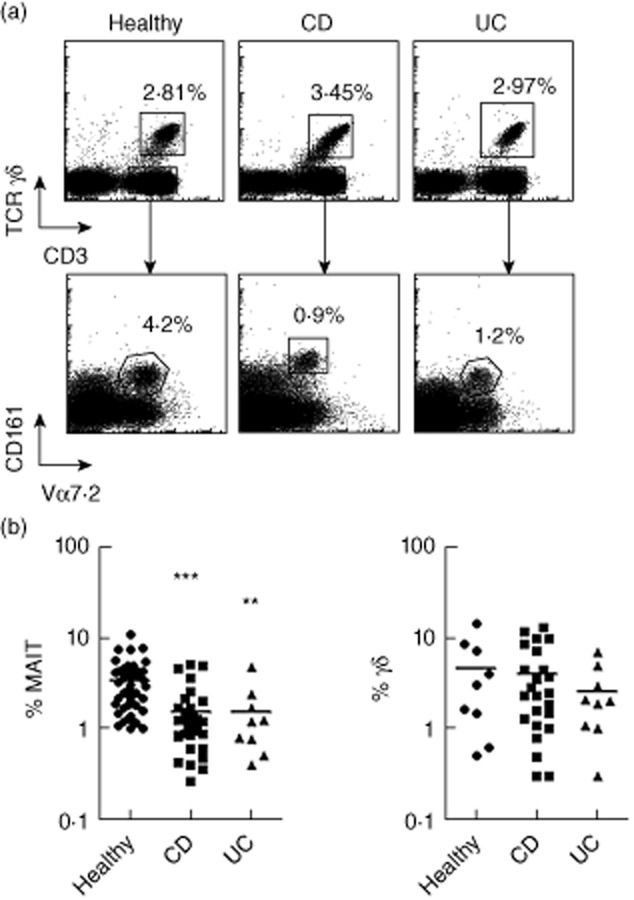
Blood mucosal-associated invariant T (MAIT) cells are less abundant in Crohn's disease (CD) and ulcerative colitis (UC). The proportion of blood MAIT and γδ T cells in CD, UC and healthy donors was analysed by flow cytometry. (a) Total MAIT cells are defined as T cell receptor (TCR)γδ- Vα7.2+CD161hi cells, γδ T cells as CD3+TCRγδ+ cells. One representative example for each group is shown. (b) Percentage of MAIT cells (left panel) or γδ T cells (right panel) [analysed as in (a)] among CD3+ T cells, in healthy donors (n = 46), CD (n = 31) and UC (n = 9) patients. Statistical analysis of mean ± standard error of the mean (s.e.m.). **P < 0·05; ***P < 0·005.
As controls, we analysed the proportion of TCR-γδ+ lymphocytes, which constitute another population of effector/memory innate-like T cells. TCR-γδ+ lymphocytes are increased in the peripheral blood of patients with active CD, whereas it remains steady under remission 15. In contrast with MAIT cells, the frequency of TCR-γδ+ lymphocytes within peripheral blood CD3+ T cells was comparable between our groups of patients (Fig. 1b). This apparent discrepancy might reflect the fact that a majority of our patients are in remission, while patients with active disease had mainly moderate activity. Nevertheless, the decrease in blood MAIT cells observed in our cohort of IBD patients seems specific to this T cell population.
Most MAIT cells display either a CD8α+ or CD4–CD8– double-negative (DN) phenotype, whereas a small proportion express the CD4 molecule. As the putative difference between these subsets of MAIT cells is not known, we analysed more precisely their behaviour in IBD. We observed that blood CD8α+ MAIT cells were decreased both in CD (mean 0·76 ± 0·17; P < 0·001) and UC patients (mean 0·74 ± 0·33 P < 0·05) compared to healthy controls (2·1 ± 0·38) (Fig. 2a,b). DN MAIT cells were also decreased, but the results did not reach statistical significance. The frequency of CD4+ cells among MAIT cells was relatively higher in IBD patients compared to controls, owing to the decrease in CD8+ cells. However, the frequency of CD4+ MAIT cells among T cells was similar between the three groups. We conclude that CD8α+ and, to a lesser extent, DN MAIT cells, are specifically less abundant in the blood of IBD patients, regardless of the precise subtype (CD or UC) of the disease.
Figure 2.
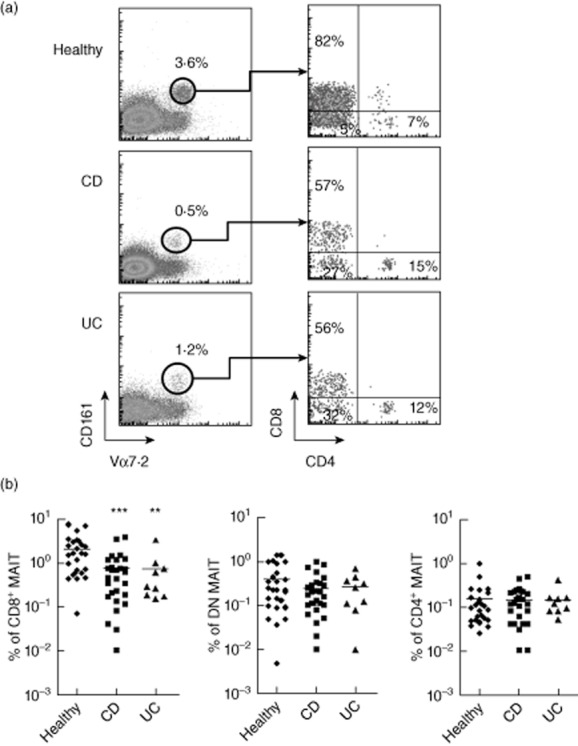
CD8+ mucosal-associated invariant T (MAIT) cells are specifically decreased in blood of inflammatory bowel diseases (IBD) patients. The proportion of CD8+, CD4+ and CD4–CD8– [double-negative (DN)] blood MAIT cells were analysed in IBD versus healthy donors. (a) One representative example per group of the proportion of CD8+, CD4+ and DN cells among CD3+Vα7.2+CD161hi MAIT cells. (b) Percentage of CD8+ (left), CD4+ (middle) and DN (right) MAIT cells among CD3+ T cells in the blood of healthy, Crohn's disease (CD) and ulcerative colitis (UC) patients. Data are analysed from the same set of patients as in Fig. 1. Statistical analysis of mean ± standard error of the mean (s.e.m.). **P < 0·05; *** P < 0·005.
We next asked whether the extent of MAIT cells disappearance from the blood reflected the disease activity. We separated the patients into two groups according to clinical activity [measured by the Crohn's Disease Activity Index (CDAI)], endoscopic score [measured by the Crohn's Disease Index of Severity (CDEIS)] or biological activity [defined as a positive C-reactive protein (CRP)]. We found no correlations between MAIT cells involvement and disease activity as measured by these three distinct parameters (Supporting information, Fig. S1 a–c). We also analysed whether a previous surgery in the context of the IBD had any influence on MAIT cells frequency, but found no correlations (Supporting information, Fig. S1 d).
Most patients enrolled into the study were undergoing specific treatment, including immunosuppressive drugs such as corticoids, azathioprine or TNF inhibitors. As we do not know the impact of these drugs on MAIT cells in vivo, this could represent important confounding factors. However, the frequency of MAIT cells was not correlated with the presence or absence of a specific treatment such as TNF inhibitors or corticoids (Supporting information, Fig. S1 e,f). The number of patients receiving azathioprine treatment was too small to yield any informative data.
MAIT cells accumulate in the inflamed mucosa of CD patients
Several hypotheses could explain this decreased number of blood MAIT cells in patients with IBD, such as impaired development, increased apoptosis or redistribution to peripheral tissues. We first focused upon the latter possibility, because several studies have shown an accumulation of MAIT cells in inflamed or infected tissues, such as the lung in tuberculosis 11. We compared the distribution of CD3+IL18Rα+Vα7.2+ MAIT cells in healthy versus inflamed ileal biopsies from 11 CD patients (Fig. 3a). In healthy tissues, they represented an average of 1·5% ± 0·3 of CD3+ cells, a result close to previously published data 8. Conversely, we observed a strong accumulation of MAIT cells in the inflamed intestine, where they represented approximately 6·6% ± 1·4 (P = 0·002) of total T cells (Fig. 3b). Therefore, an accumulation of MAIT cells in the inflamed mucosa correlated with, and was hypothetically the cause of, their decreased frequency in the peripheral blood of patients with IBD.
Figure 3.
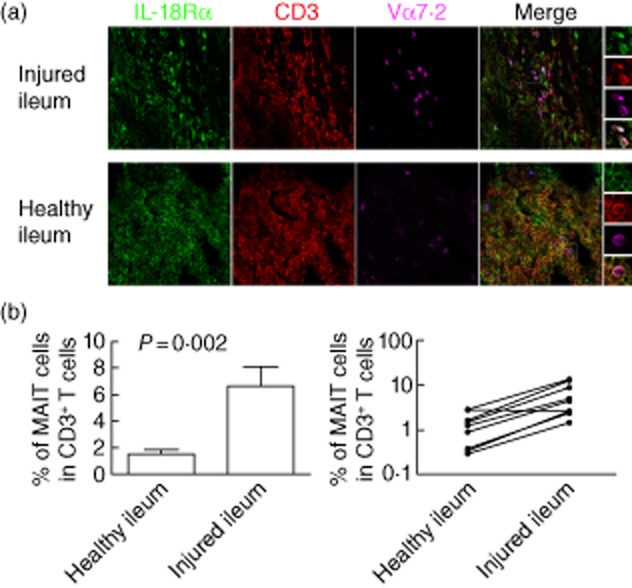
Mucosal-associated invariant T (MAIT) cells accumulation in the inflamed ileon of patients with Crohn's disease (CD). Ileal biopsies of patients with CD were triple-stained with interleukin (IL)-18Rα (green), anti-CD3 (red) and anti-Vα7.2 (magenta) antibodies. This staining strategy allows the identification of CD3+Vα7.2+IL18Rα+ MAIT cells 8. (a) Representative example of MAIT cells in the inflamed (upper panel) versus healthy ileum (lower panel). The right panel demonstrates triple staining of analysed MAIT cells. (b) Percentage of MAIT cells [analysed as in (a)] among total CD3+ T cells in the inflamed versus healthy ileum of CD patients. The left panel shows the mean ± standard error of the mean (s.e.m.). of the percentage of MAIT cells among T cells in healthy versus injured ileum of 11 CD patients. The right panel shows the paired data obtained for each patient analysed. Analyses were performed with the ×63 objective of a LSM 710 confocal microscope (Zeiss).
In-vivo activation of MAIT cells in IBD
The redistribution of MAIT cells in patients with IBD suggests that they are activated in vivo. To confirm this hypothesis, we looked at their proliferative status. In healthy subjects, MAIT cells are hypoproliferative in vitro and in vivo, compared with mainstream T cells 8. We analysed the intracellular expression of the proliferation marker Ki67 in blood MAIT cells from IBD patients. As shown in Fig. 4a,b, the frequency of MAIT cells expressing Ki67 was markedly higher in patients with CD that in healthy donors, reaching 3% ± 0·4 positive cells versus 0·7% ± 0·1 (P < 0·01). This increase implicated both the CD8+ and the DN subset of MAIT cells. Although we could analyse two only patients with UC, they also showed a dramatic increase in Ki67 expression (Fig. 4a,b). Therefore, there is an increased proportion of cycling MAIT cells in the blood of diseased patients, confirming that they are activated in vivo.
Figure 4.
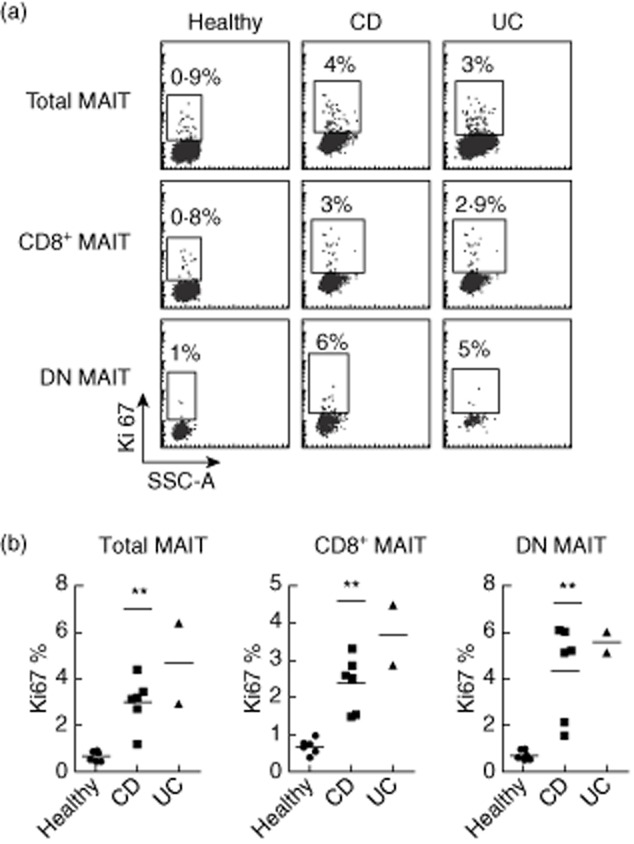
Blood mucosal-associated invariant T (MAIT) cells are more proliferative in Crohn's disease (CD) and ulcerative colitis (UC). (a) Representative Ki67 intracellular staining of MAIT cells (analysed as in Fig. 1) among total CD3+, CD8+ or double-negative (DN) T cells from healthy donors, CD and UC patients. (b) Percentage of proliferating (Ki67+) MAIT cells analysed as in (a) in the different groups (controls n = 6, CD n = 6, UC n = 2). Statistical analysis of mean ± standard error of the mean (s.e.m.). **P < 0·05.
MAIT cells in IBD show increased NKG2D and BTLA expression
MAIT cells activation in this state of chronic inflammation might impact their phenotype and functions. Indeed, several studies have shown various phenotypical alterations among lymphocyte subsets in the course of IBD, which may be related to the pathophysiology of the diseases. In particular, pioneering studies by the Allez laboratory demonstrated the involvement of NKG2D induced at the surface of CD4+ T cells in CD 16,17. As this co-stimulatory molecule is expressed by MAIT cells 8, we studied its expression in patients with IBD. We found NKG2D to be highly up-regulated in IBD patients, both in CD and UC, regardless of co-receptor expression (Fig. 5a,b), compared to its dimmer expression in controls. We then searched for other phenotypical features of MAIT cells in patients with IBD. We found that expression of the inhibitory BTLA receptor was also increased on blood MAIT cells from CD and UC (Fig. 5c,d). Not surprisingly, the increased expression of these two molecules was not restricted to MAIT cells, however, as it could also be observed on TCR-αβ+ CD8 and TCR-γδ+ T cells (data not shown). Nonetheless, these changes in MAIT cells phenotype are highly suggestive of a chronic state of activation in patients with IBD, which probably impact their functions and/or responsiveness to various stimuli.
Figure 5.

Blood mucosal-associated invariant T (MAIT) cells from inflammatory bowel diseases (IBD) patients up-regulate natural killer (NK)G2D and B and T lymphocyte attenuator (BTLA) expression. (a,c) Representative example of NKG2D (a) and BTLA (c) expression by total, CD8+ or double-negative (DN) MAIT cells among healthy donors, Crohn's disease (CD) and ulcerative colitis (UC) patients. The dotted lines represent staining with an isotype-matched control. (b,d) Percentages of NKG2D-positive (b) and BTLA-positive (d) MAIT cells among the different groups (controls n = 23, CD n = 28, UC n = 12). Statistical analysis of mean ± standard error of the mean (s.e.m.). ***P < 0·005.
MAIT cells cytokine secretion pattern is affected in IBD
As the above results demonstrate MAIT cell activation during IBD, they should probably also translate into functional consequences. MAIT cells produce chiefly IFN-γ, TNF-α, IL-2 and IL-17 when stimulated in vitro. We reasoned that activated MAIT cells might produce either more proinflammatory cytokines or, conversely, might switch to the secretion of a different set of soluble factors. To investigate this matter, we purified MAIT cells from blood and, using a multiplex assay, analysed their cytokine secretion pattern after in-vitro stimulation with PMA and ionomycin. As already described 8, MAIT cells in healthy donors display apparent ‘inflammatory’ functions with a mixed Th1/Th17 profile (Fig. 6). We found no evidence for production of Th2-like, Th9-like or immune-regulatory cytokines by MAIT cells isolated from patients with IBD (data not shown). Similar ranges of IL-2 and TNF-α were produced by the different groups compared. By contrast, MAIT cells from both CD and UC patients secreted significantly more IL-17 than healthy donors (Fig. 6). Interestingly, this increased IL-17 production was accompanied by a decreased IFN-γ secretion in CD patients only, whereas in UC we observed a small but detectable production of IL-22. Therefore, MAIT cells from patients with IBD display a cytokine secretion pattern which probably reflects their chronic activation state. Furthermore, the differences observed between patients with MC and UC suggest that MAIT cells adapt their precise functions according to the context of their activation.
Figure 6.
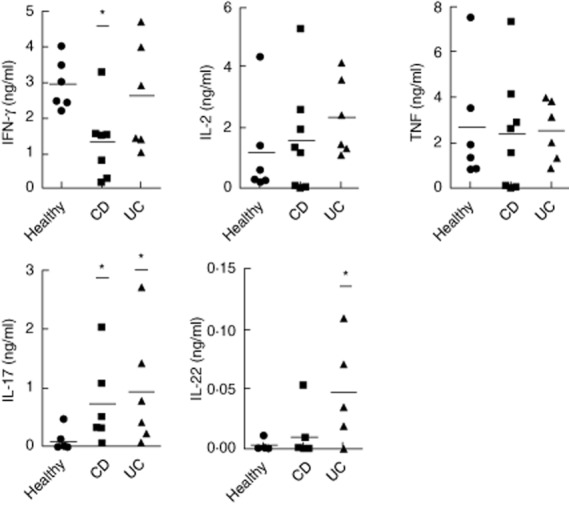
Altered and distinct pattern of cytokine secretion by blood mucosal-associated invariant T (MAIT) from Crohn's disease (CD) and ulcerative colitis (UC) patients. CD3+Vα7.2+CD161hi blood MAIT cells from healthy donors (n = 5), CD (n = 8) and UC (n = 6) patients were fluorescence activated cell sorted (FACS) with a purity >95%; 105 sorted cells were plated and stimulated for 36 h with phorbol myristate acetate (PMA) and ionomycin. The amount of 13 different cytokines in the cell culture supernatants was quantified by a multiplex bead-based assay. Results for interleukin (IL)-2, tumour necrosis factor (TNF)α, interferon (IFN)-γ, IL-17 and IL-22 are shown. Statistical analysis of mean ± standard error of the mean (s.e.m.). *P < 0·05.
Discussion
The involvement of the immune system in the pathophysiology of IBD is a matter of numerous investigations 18. Herein, we brought a new piece to the puzzle: we show for the first time that MAIT cells, a recently described subset of evolutionary conserved, non-conventional, MHC class Ib-restricted T cells, are activated in patients with IBD, accumulate in the inflamed mucosa, and display increased cytokine secretion capacities. These data broaden our picture of the immune players in IBD and open new trails of investigation, but also raise a number of questions.
The first issue in our view is the question of where and how are MAIT cells activated, i.e. by an MR1-dependent or -independent process. From the data obtained and presented in this paper, we would argue that both mechanisms may be involved. Indeed, MAIT cells are effector/memory cells and express tissue-targeting molecules such as α4β7 and CCR6; therefore, inflammatory mediators produced within the mucosa might attract them non-specifically. This would explain the activation of MAIT cells observed both in CD and UC, which are distinct diseases with different pathophysiological mechanisms; this is also in line with published data suggesting or demonstrating the involvement of MAIT cells in other situations, such as respiratory infectious diseases, HIV and HCV infection, multiple sclerosis or cancer 9,12,13,19–22. However, the activation of MAIT lymphocytes, reflected in the increased proportion of Ki67+ cycling cells, probably involves cognate interaction with their restricting molecule, MR1. Indeed, we show here that activated MAIT cells in IBD patients display elevated levels of the BTLA protein, which has been shown to be regulated in lymphocytes stimulated through their TCR. During cytomegalovirus (CMV) infection, CD8+ conventional T cells transiently up-regulate BTLA, which returns to lower levels during the memory phase 23. The high level of BTLA found on MAIT cells might reflect a chronic stimulation in the course of IBD. In this hypothesis, this up-regulation might play a role in regulating MAIT cell functions through interactions with herpes virus entry mediator (HVEM), the BTLA ligand. If MAIT cells are activated in an MR1-dependent manner in these situations, it raises the question of the antigen(s) presented by MR1. Le Bourhis et al. showed that diverse microorganisms can stimulate MAIT cells in an MR1-dependent manner, probably through direct presentation of microbial-derived antigens 9. It has been proposed recently that metabolites of specific microbial vitamins are responsible for this broad pattern of MAIT cells reactivity 10. One hypothesis would be that, in IBD, either the dysbiosis and/or the alterations of the mucosal barrier result in the presentation of commensals-derived antigens by MR1, which do not take place in physiological situations. Alternatively, MR1 might present an endogenous ligand induced or up-regulated by ‘stress’ or ‘danger’ signals. A third possibility would be that MR1 present identical ligands in physiology and pathology, but that the inflammatory milieu provides an additional signal, either through co-stimulation or humoral factors, that permit MAIT cell activation. Indeed, we observed a strong up-regulation of NKG2D on the surface of MAIT cells in IBD. It is relatively well known that NKG2D expression is regulated at least in part by cytokines, such as IL-1, which increases its expression 24,25. It is extremely plausible that inflammatory mediators produced by the mucosa up-regulate NKG2D expression, which is a strong co-stimulator of T cell functions. As NKG2D ligands, such as MHC class I chain-related gene A (MICA), are themselves expressed strongly in the inflamed tissues in IBD, they might provide a signal sufficient to activate MAIT cells and increase their functions, akin to the situation demonstrated with a subset of CD4+ T cells in CD 16. New experiments are required to resolve this important issue.
The second important question is obviously related to the role of MAIT cells in IBD. Normal MAIT cells secrete proinflammatory cytokines, and show enhanced levels of IL-17 secretion in IBD patients. It is therefore tempting to speculate that they display inflammatory properties and, as such, are pathogenic in chronic inflammation. However, alternative hypothesis can be proposed: first, IL-17 is not always pathogenic and has been shown to play protective functions in several models of intestinal inflammation. Indeed, a recent clinical trial using a human neutralizing antibody to IL-17A in CD paradoxically resulted in exacerbation of the disease 26. Secondly, in CD patients, MAIT cells exhibit reduced IFN-γ production, which in several studies has been shown to be the major effector cytokines involved in IBD. Therefore, this altered pattern of cytokine secretion by MAIT cells may actually reflect a switch in their functions to a less inflammatory phenotype 27. Thirdly, MAIT cells produce some IL-22 in UC patients, which is not the case either in healthy subjects nor in CD patients; IL-22 may also have protective functions for the mucosa 26. We tried to address this issue by analysing MAIT cell frequency and phenotype, according to the treatment received by the patients as well as their clinical scores. Surprisingly, we did not find any correlations within our groups. The situation is therefore different than in multiple sclerosis, where the frequency and the activation of MAIT cells seems to be correlated at least in part with the activity of the disease 13. Conversely, other comparable situations have already been described. In AIDS patients, the frequency of MAIT cells decreases in the blood permanently, even after successful anti-retroviral therapy 21,22. Similarly, the number of MAIT cells in the blood of patients with coeliac disease is diminished, and does not recover under a gluten-free diet 28. One explanation for the lack of correlation between disease activity and MAIT cell numbers could be related to the dysbiosis in IBD. Hence, it has been suggested that the composition of the gut microbiota is, at least in part, permanently altered in cohorts of IBD patients, both in CD and UC, irrespective of disease activity 29,30. If the MAIT cell activation we observed is dependent upon this dysbiosis, then it could be permanent and explain our results. Finally, other possible biases can be enumerated, which could explain these findings. First, there is a poor correlation between clinical and endoscopic scores in CD 31,32, in part because of the frequent occurrence of irritable bowel syndrome in patients with almost normal endoscopic pattern. Unfortunately, we could not obtain the endoscopic data from all patients in this pilot study. Secondly, most of our patients showed a moderate intensity of their diseases, and therefore might not reflect the situation in strongly active disease. Thirdly, the low number of patients studied, especially in the UC group, might also explain the low power of statistical data with respect to this matter. New controlled studies are therefore required to address the question of the role of MAIT cells in IBD and the possible correlations between their involvement and the course of the diseases.
Acknowledgments
We would like to thank Professor K. Lassoued and J. P. Marolleau (Amiens, France) for support, as well as the staff from the Hepatogastroenterology and Pathology departments of the CHU of Amiens. We also thank the patients who participated in the study. This work was supported by the Association François Aupetit (AFA), the Avenir program from Inserm, the Association pour la Recherche sur le Cancer (ARC), the Ligue contre le Cancer (Comité du Nord) and the Conseil Régional de Picardie.
Disclosure
None.
Supporting information
Additional Supporting information may be found in the online version of this article at the publisher's web-site:
Influence of disease activity (a-c), surgery (d) and treatment (e,f) on the frequency of mucosal-associated invariant T (MAIT) cells among peripheral blood T cells.
References
- 1.Fuss IJ, Heller F, Boirivant M, et al. Nonclassical CD1d-restricted NK T cells that produce IL-13 characterize an atypical Th2 response in ulcerative colitis. J Clin Invest. 2004;113:1490–1497. doi: 10.1172/JCI19836. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Fuss IJ, Strober W. The role of IL-13 and NK T cells in experimental and human ulcerative colitis. Mucosal Immunol. 2008;1(Suppl. 1):S31–33. doi: 10.1038/mi.2008.40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Heller F, Florian P, Bojarski C, et al. Interleukin-13 is the key effector Th2 cytokine in ulcerative colitis that affects epithelial tight junctions, apoptosis, and cell restitution. Gastroenterology. 2005;129:550–564. doi: 10.1016/j.gastro.2005.05.002. [DOI] [PubMed] [Google Scholar]
- 4.Treiner E, Duban L, Moura IC, Hansen T, Gilfillan S, Lantz O. Mucosal-associated invariant T (MAIT) cells: an evolutionarily conserved T cell subset. Microbes Infect. 2005;7:552–559. doi: 10.1016/j.micinf.2004.12.013. [DOI] [PubMed] [Google Scholar]
- 5.Tilloy F, Treiner E, Park SH, et al. An invariant T cell receptor alpha chain defines a novel TAP-independent major histocompatibility complex class Ib-restricted alpha/beta T cell subpopulation in mammals. J Exp Med. 1999;189:1907–1921. doi: 10.1084/jem.189.12.1907. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Treiner E, Duban L, Bahram S, et al. Selection of evolutionarily conserved mucosal-associated invariant T cells by MR1. Nature. 2003;422:164–169. doi: 10.1038/nature01433. [DOI] [PubMed] [Google Scholar]
- 7.Martin E, Treiner E, Duban L, et al. Stepwise development of MAIT cells in mouse and human. PLOS Biol. 2009;7:e54. doi: 10.1371/journal.pbio.1000054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Dusseaux M, Martin E, Serriari N, et al. Human MAIT cells are xenobiotic-resistant, tissue-targeted, CD161hi IL-17-secreting T cells. Blood. 2011;117:1250–1259. doi: 10.1182/blood-2010-08-303339. [DOI] [PubMed] [Google Scholar]
- 9.Le Bourhis L, Martin E, Peguillet I, et al. Antimicrobial activity of mucosal-associated invariant T cells. Nat Immunol. 2010;11:701–708. doi: 10.1038/ni.1890. [DOI] [PubMed] [Google Scholar]
- 10.Kjer-Nielsen L, Patel O, Corbett AJ, et al. MR1 presents microbial vitamin B metabolites to MAIT cells. Nature. 2012;491:717–723. doi: 10.1038/nature11605. [DOI] [PubMed] [Google Scholar]
- 11.Le Bourhis L, Guerri L, Dusseaux M, Martin E, Soudais C, Lantz O. Mucosal-associated invariant T cells: unconventional development and function. Trends Immunol. 2011;32:212–218. doi: 10.1016/j.it.2011.02.005. [DOI] [PubMed] [Google Scholar]
- 12.Billerbeck E, Kang YH, Walker L, et al. Analysis of CD161 expression on human CD8+ T cells defines a distinct functional subset with tissue-homing properties. Proc Natl Acad Sci USA. 2010;107:3006–3011. doi: 10.1073/pnas.0914839107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Annibali V, Ristori G, Angelini DF, et al. CD161(high)CD8+T cells bear pathogenetic potential in multiple sclerosis. Brain. 2011;134:542–554. doi: 10.1093/brain/awq354. [DOI] [PubMed] [Google Scholar]
- 14.Chiba A, Tajima R, Tomi C, Miyazaki Y, Yamamura T, Miyake S. Mucosal-associated invariant T cells promote inflammation and exacerbate disease in murine models of arthritis. Arthritis Rheum. 2012;64:153–161. doi: 10.1002/art.33314. [DOI] [PubMed] [Google Scholar]
- 15.Giacomelli R, Parzanese I, Frieri G, et al. Increase of circulating gamma/delta T lymphocytes in the peripheral blood of patients affected by active inflammatory bowel disease. Clin Exp Immunol. 1994;98:83–88. doi: 10.1111/j.1365-2249.1994.tb06611.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Pariente B, Mocan I, Camus M, et al. Activation of the receptor NKG2D leads to production of Th17 cytokines in CD4+ T cells of patients with Crohn's disease. Gastroenterology. 2011;141:217–226. doi: 10.1053/j.gastro.2011.03.061. 26 e1–2. [DOI] [PubMed] [Google Scholar]
- 17.Allez M, Tieng V, Nakazawa A, et al. CD4+NKG2D+ T cells in Crohn's disease mediate inflammatory and cytotoxic responses through MICA interactions. Gastroenterology. 2007;132:2346–2358. doi: 10.1053/j.gastro.2007.03.025. [DOI] [PubMed] [Google Scholar]
- 18.Kaser A, Zeissig S, Blumberg RS. Inflammatory bowel disease. Annu Rev Immunol. 2010;28:573–621. doi: 10.1146/annurev-immunol-030409-101225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Gold MC, Cerri S, Smyk-Pearson S, et al. Human mucosal associated invariant T cells detect bacterially infected cells. PLoS Biol. 2010;8:e1000407. doi: 10.1371/journal.pbio.1000407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Peterfalvi A, Gomori E, Magyarlaki T, et al. Invariant Valpha7.2-Jalpha33 TCR is expressed in human kidney and brain tumors indicating infiltration by mucosal-associated invariant T (MAIT) cells. Int Immunol. 2008;20:1517–1525. doi: 10.1093/intimm/dxn111. [DOI] [PubMed] [Google Scholar]
- 21.Leeansyah E, Ganesh A, Quigley MF, et al. Activation, exhaustion and persistent decline of the anti-microbial MR1-restricted MAIT cell population in chronic HIV-1 infection. Blood. 2013;121:1124–1135. doi: 10.1182/blood-2012-07-445429. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Cosgrove C, Ussher JE, Rauch A, et al. Early and non-reversible decrease of CD161++/MAIT cells in HIV infection. Blood. 2013;121:951–956. doi: 10.1182/blood-2012-06-436436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Serriari NE, Gondois-Rey F, Guillaume Y, et al. B and T lymphocyte attenuator is highly expressed on CMV-specific T cells during infection and regulates their function. J Immunol. 2010;185:3140–3148. doi: 10.4049/jimmunol.0902487. [DOI] [PubMed] [Google Scholar]
- 24.Zhang C, Zhang J, Niu J, Zhang J, Tian Z. Interleukin-15 improves cytotoxicity of natural killer cells via up-regulating NKG2D and cytotoxic effector molecule expression as well as STAT1 and ERK1/2 phosphorylation. Cytokine. 2008;42:128–136. doi: 10.1016/j.cyto.2008.01.003. [DOI] [PubMed] [Google Scholar]
- 25.Zhang C, Zhang J, Niu J, Zhou Z, Zhang J, Tian Z. Interleukin-12 improves cytotoxicity of natural killer cells via upregulated expression of NKG2D. Hum Immunol. 2008;69:490–500. doi: 10.1016/j.humimm.2008.06.004. [DOI] [PubMed] [Google Scholar]
- 26.Marwaha AK, Leung NJ, McMurchy AN, Levings MK. TH17 cells in autoimmunity and immunodeficiency: protective or pathogenic? Front Immunol. 2012;3:129. doi: 10.3389/fimmu.2012.00129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Strober W, Fuss IJ. Proinflammatory cytokines in the pathogenesis of inflammatory bowel diseases. Gastroenterology. 2011;140:1756–1767. doi: 10.1053/j.gastro.2011.02.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Dunne MR, Elliott L, Hussey S, et al. Persistent changes in circulating and intestinal gammadelta T cell subsets, invariant natural killer T cells and mucosal-associated invariant T cells in children and adults with coeliac disease. PLOS ONE. 2013;8:e76008. doi: 10.1371/journal.pone.0076008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Rajilic-Stojanovic M, Shanahan F, Guarner F, de Vos WM. Phylogenetic analysis of dysbiosis in ulcerative colitis during remission. Inflamm Bowel Dis. 2013;19:481–488. doi: 10.1097/MIB.0b013e31827fec6d. [DOI] [PubMed] [Google Scholar]
- 30.Andoh A, Kuzuoka H, Tsujikawa T, et al. Multicenter analysis of fecal microbiota profiles in Japanese patients with Crohn's disease. J Gastroenterol. 2012;47:1298–1307. doi: 10.1007/s00535-012-0605-0. [DOI] [PubMed] [Google Scholar]
- 31.Schoepfer AM, Beglinger C, Straumann A, et al. Fecal calprotectin correlates more closely with the Simple Endoscopic Score for Crohn's disease (SES-CD) than CRP, blood leukocytes, and the CDAI. Am J Gastroenterol. 2010;105:162–169. doi: 10.1038/ajg.2009.545. [DOI] [PubMed] [Google Scholar]
- 32.Regueiro M, Kip KE, Schraut W, et al. Crohn's disease activity index does not correlate with endoscopic recurrence one year after ileocolonic resection. Inflamm Bowel Dis. 2011;17:118–126. doi: 10.1002/ibd.21355. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Influence of disease activity (a-c), surgery (d) and treatment (e,f) on the frequency of mucosal-associated invariant T (MAIT) cells among peripheral blood T cells.


