Abstract
Vitamin A and its active metabolite retinoic acid are essential for the development and function of many tissues including the immune system. The induction of mucosal homing receptors on T and B cells by mucosal dendritic cells (DC) depends on the presence of vitamin A. Recent studies indicate that also the differentiation of CD11b+ DC subsets in the mucosa as well as the spleen depend on vitamin A signalling. As CD11b+ DC subsets exert non-redundant functions in anti-bacterial and anti-fungal immune responses, defects in CD11b+ DC differentiation will contribute to the clinical problems observed during vitamin A deficiency.
Keywords: dendritic cell, differentiation, mucosa, vitamin A
Introduction
Vitamin A (retinol) and its active metabolite retinoic acid (RA) have been well known for their crucial role in embryonic development, vision and the nervous system, as well as in regulation and development of the immune system.1 An estimated 190 million children have low levels of serum vitamin A, which is associated with increased susceptibility to infectious diseases.2 Vitamin A supplementation decreases childhood mortality in areas where vitamin A deficiency is endemic,3,4 which is related to the fact that several key features of the mucosal immune system are dependent on the presence of vitamin A. More recently the development of conventional dendritic cells (DC) in both mucosal and lymphoid tissues has been shown to be influenced by vitamin A levels. In this review we will provide a brief update on how vitamin A and RA are regulated and how these factors exert their influence on the mucosal immune system; we will then focus on their effects on murine DC development and the immunological implications of vitamin A deficiency.
Vitamin A metabolism
Dietary uptake of vitamin A is crucial for all vertebrates, because they cannot synthesize vitamin A de novo from food sources.5 Both vegetable and fruit-derived carotenoids and retinyl esters from animal sources are converted to retinol and taken up by enterocytes. Retinol is stored in the liver from where serum levels of retinol are tightly regulated.6,7 Retinol is released into the circulation, bound to retinol-binding protein; and taken up by target cells via a Stra-6-mediated uptake process (Fig. 1). In the cytoplasm, retinol is reversibly oxidized by alcohol dehydrogenases or retinol dehydrogenases to retinal. A subsequent irreversible oxidation step by one of three retinaldehyde dehydrogenases (RALDH1–3) yields all-trans retinoic acid. The RA is transported into the nucleus where it can bind to retinoic acid receptor (RAR) and retinoic X receptor (RXR) heterodimers, which bind to RA response elements present in the promoter regions of various genes.1,8 Binding of RA to these RAR–RXR dimers results in a conformational change, release of co-repressors, recruitment of co-activators and initiation of transcription. Hundreds of genes are regulated by RA, either directly or indirectly and this explains the pleiotropic effects of the agent.9 Because RA has a short half-life and its degradation is regulated by cytochrome P450 26 family members10 it is thought that active RA is generated in the target cell itself or in nearby cells.
Figure 1.
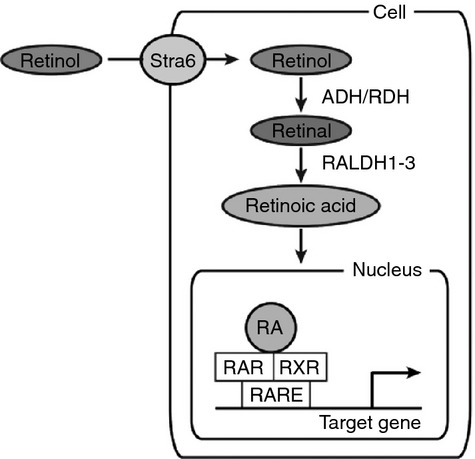
Cellular vitamin A metabolism and signalling. Retinol is taken up from the blood via binding to Stra6. Two enzymatic oxidation steps convert retinol to retinal and subsequently into retinoic acid (RA). Upon transportation into the nucleus, RA binds to retinoic acid receptor (RAR)/ retinoic X receptor (RXR) heterodimers and these complexes regulate transcriptional activity of target genes via binding to retinoic acid response elements (RAREs) in the promoter regions.
Mucosal immunity depends on vitamin A
The mucosal immune system has the important function of preventing infections by intestinal pathogens and maintaining tolerance to the commensal bacterial flora. Migration of immune cells to the mucosal tissues is achieved by the expression of specialized homing receptors. In the small intestines expression of mucosal addressin cell adhesion molecule-1 on endothelial cells together with the chemokine CCL25 leads to specific homing of integrin α4β7 and CCR9-expressing T and B cells, while homing to the large intestine is mediated by α4β7 and α4β1 integrins.11 The imprinting of mucosa-specific homing receptors was shown to be specifically induced by DC derived from mucosal tissues, but not from other peripheral lymphoid organs.12–14 Subsequently, Iwata et al. demonstrated that mucosal DC expressed enzymes (RALDH1 and RALDH2) to convert retinal into RA, which in turn induced gut-homing receptors on T cells. Vitamin A-deficient mice exhibited severely decreased T-cell numbers in mucosal tissues.15 Similarly, RA was shown to be necessary and sufficient for the induction of gut homing of IgA-secreting B cells.16 In addition to DC, epithelial cells in the intestine and stromal cells of the mucosal lymphoid tissues possess the ability to synthesize RA and collaborate with DC to induce homing receptors.15,17,18
In the absence of inflammation, the mucosal immune system stimulates the differentiation of regulatory T (Treg) cells, while specific members of the microflora specifically induce inflammatory T helper type 17 (Th17) T cells.19 In vitro studies have consistently shown that RA enhances Treg and inhibits Th17 induction.20–22 However, since in vivo studies using vitamin A-deficient mice did not show a decrease in Treg cell frequencies, but instead exhibited a decrease in Th17 cells,23,24 the exact role of RA in Treg and Th17 cell development needs to be further defined.
Mucosal DC are thought to gain the capacity to synthesize RA locally in the mucosa. RA itself up-regulates the expression of RALDH2 and thereby stimulates RA production. This positive feedback loop seems to be functional in the gut as RALDH2 expression in DC has been shown to correlate with vitamin A levels in the diet.25 As intestinal epithelial cells highly express RALDH1 and produce RA, conditioning of DC by intestinal epithelial cells leads to RA production by DC.18,26 Also Toll-like receptor ligands and granulocyte–macrophage colony-stimulating factor have been shown to stimulate RA production,27–30 but the in vivo relevance of the flora in the induction of RALDH activity is not completely clear.
Together these studies clearly demonstrate a non-redundant role for vitamin A and RA in the differentiation of gut-homing effector T and B cells by mucosal DC. More recent studies have revealed an important role for vitamin A in the generation of DC, in particular the local differentiation of DC from pre-DC in both the intestines as well as the spleen. In the next paragraphs we will present an overview of DC development and focus on the specific role of RA in this process.
Overview of conventional DC development
Studies conducted in the last 20 years have resulted in the identification of three main types of DC: plasmacytoid DC (pDC), monocyte-derived DC (moDC) and conventional DC (cDC). In this review we will focus on the development of cDC, which is the most important DC cell type to stimulate T-cell responses. Murine cDC are situated in all lymphoid and peripheral tissues and are constantly replenished by precursor cDC (pre-cDC) from the bone marrow as they have a relatively short life-span of 5–7 days.31–33 In the bone marrow pre-cDC differentiate from a common monocyte DC progenitor that generates the monocyte lineage and committed DC progenitors (Fig. 2).32 The committed DC progenitors differentiate into pDC and pre-cDC, which migrate from the bone marrow, via the blood, to lymphoid and non-lymphoid tissues, where they further mature. FMS-like tyrosine kinase 3 ligand (Flt3L) is the key growth and differentiation factor for both pDC and cDC to develop in vivo.34 The cDC lineage is characterized by expression of the transcription factor Zbtb46 and is, although myeloid-derived, clearly separate from the macrophage lineage.35–38 The cDC in lymphoid and nonlymphoid organs can be further subdivided into different subsets with specific phenotypes and functions and differential requirements for transcription factors and growth factors.
Figure 2.
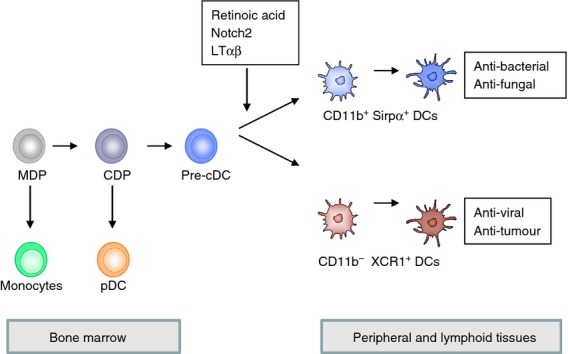
Differentiation of conventional dendritic cell (cDC) subsets. In the bone marrow haematopoietic stem cells give rise to monocyte DC progenitors that can develop into the monocytic lineage and into committed DC progenitors that generate the DC lineage. Committed DC progenitors progress to plasmacytoid DC (pDC) and pre-cDC that enter the blood and seed the peripheral and lymphoid tissues. In these tissues pre-cDC receive signals that stimulate the differentiation in CD11b+ and CD11b− DC subsets.
Conventional DC subsets in lymphoid organs and their development
Whereas the spleen only contains resident cDC that directly differentiate from blood-derived pre-cDC, lymph nodes contain both resident cDC and migratory cDC that enter from the peripheral tissues. Originally, in the mouse different types of CD11c+ cDC in lymphoid organs were discriminated on the basis of CD8 and CD4 expression.39,40 Initial gene expression studies showed that the expression profiles of CD4− CD8− (DN) and CD4+ CD8− DC closely resembled each other, unlike the CD8+ CD4− DC, which showed a distinct gene expression profile.41,42 CD8− DC, which include both CD4+ DC and DN DC, express high levels of CD11b, DCIR2, Sirpα and endothelial cell-selective adhesion molecule (ESAM), in contrast to CD8+ DC, which exhibit expression of CD205 (DEC205), CD24, XCR1 and Clec9a/DNGR1 and low levels of CD11b. More recent studies confirmed the close relationship between CD4+ and DN cDC, but also identified a small population in the CD8+ DC subset that is more related to pDC43 and a monocyte-derived population in the CD8− cDC subset that is characterized by low ESAM expression.44 A similar division into two main DC subtypes has been reported for human DC. The human CD141+ (BDCA-3+) DC subset is homologous to the mouse CD8+ DC and the CD1c (BDCA-1+) DC is comparable to the CD8− DC subset.45–50
Several transcription factor knockout mice have been generated that show deficiencies in one or more cDC subsets (reviewed in refs 51,52). The development of CD8− DC depends on the transcription factors RelB, interferon regulatory factor 2 (IRF2) and IRF4. In contrast the CD8+ DC differentiation is abrogated in IRF8−/−, Batf3−/−, Nfil3−/− and Id2−/− mice, although a recent study showed that CD8+ DC can emerge in the absence of Id2, Nfil3 and Batf3.53–56 How these transcription factors are regulated and drive cDC subset differentiation is not very clear. RelB mediates signalling of the lymphotoxin β receptor and lymphotoxin-αβ (LTαβ) binding to this receptor is required for the development of CD8− DC in the murine spleen.57,58 Recent reports show that the localization of CD4+ DC in the marginal zone and bridging channels of the spleen is regulated by the chemotactic receptor EBI2 and its ligand 7α,25-dihydroxycholesterol.59,60 In EBI2-deficient mice CD4+ DC survival was impaired but the defect could be overcome by lymphotoxin-β receptor signalling, suggesting that the LTαβ signal is provided in the marginal zone bridging channels. In addition, the marginal zone is described to provide Notch signals to CD8− DC.61 Notch signals via the transcription factor RBP-J and RBP-J−/− mice show a specific reduction of CD8− DC.62 Notch2 signalling is essential not only for the generation of splenic CD8− DC, which are characterized by high ESAM expression, but also for the generation of CD103+ CD11b+ DC in the intestines.44
In conclusion, two main cDC subsets are present in lymphoid tissues that are regulated by different sets of transcription factors and growth factors. Most likely the generation of these two DC subsets is induced by stimuli provided in specific niches, but the identity and regulation of these stimuli still require elucidation.
Conventional DC subsets in non-lymphoid organs
Conventional DC in peripheral organs do not express CD8 or CD4 and have mainly been characterized by the expression of CD103 and CD11b. Intestinal CD103+ CD11b+ and CD103+ CD11b− DC derive from pre-cDC in an Flt3L-dependent manner.63,64 Similar to CD8− DC in the spleen, intestinal CD103+ CD11b+ cDC require IRF4 and Notch2.44,63–67 CD103+ CD11b− cDC require IRF8, Id2 and Batf3, but not IRF4 or Notch2 and are thought to be closely related to lymphoid tissue CD8+ cDC.44,63–67 Homologous to intestinal cDC, lung cDC can be divided into CD103+ CD11b− and CD11b+ cDC subsets that are dependent on Batf3 and IRF4, respectively.63,65,68 Dermal cDC are also divided into similar subsets, and although the presence of the CD11b+ cDC subset does not appear to be dependent on IRF4, their migration is affected in the absence of IRF4.69 Together these studies indicate the presence of two cDC subsets in peripheral tissues with considerable homology to the two cDC subsets in the lymphoid tissues and this has been confirmed by gene expression analysis.70 However, the CD11b+ cDC subset shows more heterogeneity and also contains CD64+ macrophages that are dependent on CSF-1R.68,71,72 In conclusion, when only Flt3L-dependent cDC are considered, both peripheral-tissue-resident cDC and lymphoid-tissue-resident cDC can be discriminated into two homologous subsets (Fig. 2).
Vitamin A stimulates the development of splenic and intestinal CD11b+ DC
Recently, we and others have discovered an important role for the vitamin A metabolite RA in the generation of splenic CD8− CD11b+ and intestinal CD103+ CD11b+ cDC subsets.73–75 Mice fed with vitamin A-deficient food or treated with a pan-RAR antagonist showed a significant defect in splenic CD8− CD11b+ DC numbers and specifically in the ESAMhigh population that is dependent on Notch2 signalling. In addition, intestinal CD103+ CD11b+ DC were decreased in vitamin A-deficient mice and in mice treated with the pan-RAR antagonist, whereas CD103+ CD11b− DC that are related to splenic CD8+ DC were not affected or even increased. In contrast, high amounts of RA in the food led to increased numbers of CD8− CD11b+ DC in the spleen and inhibited the generation of CD8+ DC. Since pre-DC numbers were not affected by decreased RA signalling, RA appeared to control the transition of pre-DC to CD8− CD11b+ DC. In accordance, transfer of pre-DC into vitamin A-deficient mice or into mice treated with the pan-RAR antagonist also showed decreased generation of CD8− CD11b+ ESAMhigh DC. Together these results indicate that RA signalling is essential for the commitment of pre-DC to develop into the CD8-CD11b+ DC subset (Fig. 2).
Whereas our studies and those of Klebanoff et al. pointed to a role for RA in the local differentiation of pre-DC into splenic and intestinal DC,73,74 the study by Zeng et al.76 indicated a role for RA in pre-DC differentiation already in the bone marrow. In this study RA induced the mucosal homing receptor α4β7 on bone-marrow-derived B220+ CD11cint DC precursors that preferentially homed to intestinal lamina propria and spleen and could differentiate into pDC and CD103+ DC. Surprisingly, the RA-dependent α4β7+ B220+ DC precursor predominantly developed into intestinal CD103+ CD11b− and splenic CD8+ DC, which is in contrast to the studies by the teams of Beijer and Klebanoff in which the opposite CD11b+ DC subset developed under the control of RA.73,74 How can these different outcomes be reconciled? One of the main differences between these studies is the gating strategy used to define pre-cDC. As RA could potentially have different effects on different types of precursors, the discrepancy in gating strategies could have major implications for the outcome.
Another question is why vitamin A metabolism stimulates cDC subset differentiation specifically in intestinal lamina propria, mesenteric lymph node and spleen and not in other lymph nodes. In the intestines retinol is absorbed by intestinal epithelial cells, which express RALDH1 and produce RA that may locally drive intestinal CD103+ CD11b+ differentiation from pre-cDC. Most of the absorbed retinol is incorporated into chylomicrons and secreted in the intestinal lymph or alternatively, directly secreted into the portal circulation where it is stored in hepatic stellate cells.6 The liver ensures constant retinol levels and stores sufficient amounts of retinol for weeks to months. These blood retinol levels provide the substrate for RA synthesis in the spleen, but why this does not result in vitamin A-dependent DC development in peripheral lymph nodes is still unclear.
Mechanisms of vitamin A-mediated DC differentiation
Retinoic acid stimulates the development of CD8− lymphoid and peripheral CD11b+ DC that also depend on IRF2, IRF4 and RelB transcription factors. On the other hand, CD8+ and CD11b− DC differentiation is guided by IRF8, Id2 and Batf3. Retinoic acid could potentially affect the expression or regulation of these transcription factors, but although specifically RelBhigh DC are affected in vitamin A deficiency, a role for vitamin A in the regulation of this or other transcription factors has not yet been observed.73,74
The Notch signalling pathway is essential for the development of CD11b+ DC in spleen and intestines.44,62 An effect of vitamin A on Notch signalling in spinal cords has been inferred from experiments in RALDH2-deficient mice.77 Notch signalling is initiated by binding of one of four different Notch receptors (Notch1–4) to one of five ligands (delta-like ligand 1, 3 and 4, and jagged 1 and 2). This binding initiates proteolytic cleavages of the intracellular Notch domain by metalloproteinases and γ-secretases. Upon transport to the nucleus, the intracellular Notch domain binds to transcription factors to initiate transcription.78 Vitamin A could potentially affect this process at many levels and could even exert its function at other cell types that provide ligands for Notch receptors.
Alternatively, vitamin A may affect the generation of a specific niche that drives CD11b+ DC differentiation or influence the induction of receptors that induce migration of preDC to this niche. The chemotactic receptor EBI2 guides CD8− DC to the marginal zone and bridging channels of the spleen.59,60 This area may form an essential niche during CD11b+ DC development where they receive LTαβ signals. Vitamin A could potentially stimulate the expression of LTαβ, LTβR, EBI2, or its ligand and thereby could stimulate the development of CD8− DC in the spleen.
Since RAR/RXRs heterodimers control the expression of hundreds of genes and multiple pathways are involved in the differentiation of CD11b+ DC, experimental studies that use conditional knock-outs will be necessary to evaluate the mechanism of RA-driven DC differentiation.
Functions of cDC subsets and implications of vitamin A deficiency
Multiple studies have shed light on the specific functions of the CD8+ and CD11b+ DC subsets and these have revealed important non-redundant functions for both subsets. Mouse CD8+ DC excel in the cross-presentation of cellular material due to the expression of specific receptors for apoptotic and necrotic cells.79,80 CD8+ DC preferentially activate CD8+ T cells, produce interleukin-12 and induce Th1 responses.42,81–83 Recent studies using Batf3-deficient mice that lack CD8+ DC have shown an essential role for this DC subset in the activation of CD8+ T-cell responses to viruses and tumours54 (Fig. 2).
CD8− DC also exert cross-presentation capacity, but this is regulated by the type of pathogen and the expression of uptake receptors.84,85 CD8− DC have a dominant role in CD4 T-cell activation and the induction of B-cell responses.42,59,60,86 More recent studies indicate that the related mucosal CD11b+ DC stimulate Th17 responses, promote innate anti-bacterial responses via the production of IL-23, are the main inducers of Th2 responses, and stimulate B-cell responses.59,60,66–68,86–88
Vitamin A deficiency has been shown to result in a switch from Th2 to Th1 responses, decreased antibody responses, increased respiratory and intestinal infections, and a general increased mortality (reviewed in refs 4,89–91). Recently, the activation of CD4 T cells was shown to be impaired in the absence of vitamin A signalling.74 As CD8− CD11b+ DC are reported to stimulate Th2 and antibody responses, the observed defects in Th2 and antibody responses in vitamin A deficiency may well be the direct consequence of defective CD11b+ DC differentiation. However, direct effects of RA on T-cell and B-cell differentiation have also been described.91,92
Although an early study showed that CD8− DC were effective in cross-presentation of fungal antigens and produce interleukin-23 in response to fungus,85 more recent studies using IRF4-or Notch2-deficient mice have shown that intestinal and lung CD103+ CD11b+ DC stimulate anti-fungal Th17 responses by their capacity to produce IL-6 and IL-23.66,68 In addition, IL-23 production by intestinal CD103+ CD11b+ DC was shown to stimulate innate lymphoid cells to produce IL-22 and to be essential in the innate immune response to the intestinal bacteria Citrobacter rodentium.67 These studies indicate that the DC differentiation defect observed in vitamin A deficiency may well contribute to respiratory and intestinal infections.
Concluding remarks
Vitamin A deficiency due to insufficient dietary intake is a major problem in underdeveloped countries, but vitamin A deficiency can also result from impaired absorption due to malfunction of the intestines.74 Recent studies in mice have elucidated a role for vitamin A in the generation of mucosal and splenic CD11b+ DC subsets that have an important role in the generation of Th2, Th17 and antibody responses, and the generation of anti-bacterial and anti-fungal immune responses. As similar cDC subsets have been identified in humans, vitamin A deficiency is expected to affect mucosal and systemic immune responses via its effects on cDC generation and function as well as via direct effects on effector cells of the innate and adaptive immune system. Restoration of vitamin A status in vitamin A-deficient individuals will be essential for the generation of effective mucosal and systemic immune responses against infections and after vaccinations.
Acknowledgments
J.d.H. was supported by a grant from the Dutch Scientific Research program (NWO grant 836.08.003).
Disclosures
The authors declare no competing financial interests.
References
- 1.Niederreither K, Dolle P. Retinoic acid in development: towards an integrated view. Nat Rev Genet. 2008;9:541–53. doi: 10.1038/nrg2340. [DOI] [PubMed] [Google Scholar]
- 2.WHO. Global Prevalence of Vitamin A Deficiency in Populations at Risk 1995–2005. Geneva: World Health Organization; 2009. WHO Global Database on Vitamin A Deficiency. [Google Scholar]
- 3.Villamor E, Fawzi WW. Effects of vitamin a supplementation on immune responses and correlation with clinical outcomes. Clin Microbiol Rev. 2005;18:446–64. doi: 10.1128/CMR.18.3.446-464.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Sommer A. Vitamin A infectious disease, and childhood mortality: a 2 solution? J Infect Dis. 1993;167:1003–7. doi: 10.1093/infdis/167.5.1003. [DOI] [PubMed] [Google Scholar]
- 5.Theodosiou M, Laudet V, Schubert M. From carrot to clinic: an overview of the retinoic acid signaling pathway. Cell Mol Life Sci. 2010;67:1423–45. doi: 10.1007/s00018-010-0268-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Blomhoff R, Blomhoff HK. Overview of retinoid metabolism and function. J Neurobiol. 2006;66:606–30. doi: 10.1002/neu.20242. [DOI] [PubMed] [Google Scholar]
- 7.Napoli JL. Physiological insights into all-trans-retinoic acid biosynthesis. Biochim Biophys Acta. 2012;1821:152–67. doi: 10.1016/j.bbalip.2011.05.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Samarut E, Rochette-Egly C. Nuclear retinoic acid receptors: conductors of the retinoic acid symphony during development. Mol Cell Endocrinol. 2012;348:348–60. doi: 10.1016/j.mce.2011.03.025. [DOI] [PubMed] [Google Scholar]
- 9.Balmer JE, Blomhoff R. Gene expression regulation by retinoic acid. J Lipid Res. 2002;43:1773–808. doi: 10.1194/jlr.r100015-jlr200. [DOI] [PubMed] [Google Scholar]
- 10.Ross AC, Zolfaghari R. Cytochrome P450s in the regulation of cellular retinoic acid metabolism. Annu Rev Nutr. 2011;31:65–87. doi: 10.1146/annurev-nutr-072610-145127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Sigmundsdottir H, Butcher EC. Environmental cues, dendritic cells and the programming of tissue-selective lymphocyte trafficking. Nat Immunol. 2008;9:981–7. doi: 10.1038/ni.f.208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Stagg AJ, Kamm MA, Knight SC. Intestinal dendritic cells increase T cell expression of α4β7 integrin. Eur J Immunol. 2002;32:1445–54. doi: 10.1002/1521-4141(200205)32:5<1445::AID-IMMU1445>3.0.CO;2-E. [DOI] [PubMed] [Google Scholar]
- 13.Mora JR, Bono MR, Manjunath N, et al. Selective imprinting of gut-homing T cells by Peyer's patch dendritic cells. Nature. 2003;424:88–93. doi: 10.1038/nature01726. [DOI] [PubMed] [Google Scholar]
- 14.Johansson-Lindbom B, Svensson M, Wurbel MA, Malissen B, Marquez G, Agace W. Selective generation of gut tropic T cells in gut-associated lymphoid tissue (GALT): requirement for GALT dendritic cells and adjuvant. J Exp Med. 2003;198:963–9. doi: 10.1084/jem.20031244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Iwata M, Hirakiyama A, Eshima Y, Kagechika H, Kato C, Song SY. Retinoic acid imprints gut-homing specificity on T cells. Immunity. 2004;21:527–38. doi: 10.1016/j.immuni.2004.08.011. [DOI] [PubMed] [Google Scholar]
- 16.Mora JR, Iwata M, Eksteen B, et al. Generation of gut-homing IgA-secreting B cells by intestinal dendritic cells. Science. 2006;314:1157–60. doi: 10.1126/science.1132742. [DOI] [PubMed] [Google Scholar]
- 17.Molenaar R, Greuter M, Marel van der AP, et al. Lymph node stromal cells support dendritic cell-induced gut-homing of T cells. J Immunol. 2009;183:6395–402. doi: 10.4049/jimmunol.0900311. [DOI] [PubMed] [Google Scholar]
- 18.Edele F, Molenaar R, Gutle D, et al. Cutting edge: instructive role of peripheral tissue cells in the imprinting of T cell homing receptor patterns. J Immunol. 2008;181:3745–9. doi: 10.4049/jimmunol.181.6.3745. [DOI] [PubMed] [Google Scholar]
- 19.Ivanov II, Atarashi K, Manel N, et al. Induction of intestinal Th17 cells by segmented filamentous bacteria. Cell. 2009;139:485–98. doi: 10.1016/j.cell.2009.09.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Coombes JL, Siddiqui KR, Arancibia-Carcamo CV, et al. A functionally specialized population of mucosal CD103+ DCs induces Foxp3+ regulatory T cells via a TGF-β and retinoic acid-dependent mechanism. J Exp Med. 2007;204:1757–64. doi: 10.1084/jem.20070590. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Mucida D, Park Y, Kim G, et al. Reciprocal TH17 and regulatory T cell differentiation mediated by retinoic acid. Science. 2007;317:256–60. doi: 10.1126/science.1145697. [DOI] [PubMed] [Google Scholar]
- 22.Sun CM, Hall JA, Blank RB, et al. Small intestine lamina propria dendritic cells promote de novo generation of Foxp3 T reg cells via retinoic acid. J Exp Med. 2007;204:1775–85. doi: 10.1084/jem.20070602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Cha HR, Chang SY, Chang JH, et al. Downregulation of Th17 cells in the small intestine by disruption of gut flora in the absence of retinoic acid. J Immunol. 2010;184:6799–806. doi: 10.4049/jimmunol.0902944. [DOI] [PubMed] [Google Scholar]
- 24.Kang SG, Wang C, Matsumoto S, Kim CH. High and low vitamin A therapies induce distinct FoxP3+ T-cell subsets and effectively control intestinal inflammation. Gastroenterology. 2009;137:1391–402. doi: 10.1053/j.gastro.2009.06.063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Molenaar R, Knippenberg M, Goverse G, et al. Expression of retinaldehyde dehydrogenase enzymes in mucosal dendritic cells and gut-draining lymph node stromal cells is controlled by dietary vitamin A. J Immunol. 2011;186:1934–42. doi: 10.4049/jimmunol.1001672. [DOI] [PubMed] [Google Scholar]
- 26.Villablanca EJ, Wang S, De CJ, et al. MyD88 and retinoic acid signaling pathways interact to modulate gastrointestinal activities of dendritic cells. Gastroenterology. 2011;141:176–85. doi: 10.1053/j.gastro.2011.04.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Yokota A, Takeuchi H, Maeda N, et al. GM-CSF and IL-4 synergistically trigger dendritic cells to acquire retinoic acid-producing capacity. Int Immunol. 2009;21:361–77. doi: 10.1093/intimm/dxp003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Manicassamy S, Ravindran R, Deng J, et al. Toll-like receptor 2-dependent induction of vitamin A-metabolizing enzymes in dendritic cells promotes T regulatory responses and inhibits autoimmunity. Nat Med. 2009;15:401–9. doi: 10.1038/nm.1925. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Uematsu S, Fujimoto K, Jang MH, et al. Regulation of humoral and cellular gut immunity by lamina propria dendritic cells expressing Toll-like receptor 5. Nat Immunol. 2008;9:769–76. doi: 10.1038/ni.1622. [DOI] [PubMed] [Google Scholar]
- 30.Wang S, Villablanca EJ, Calisto De J, et al. MyD88-dependent TLR1/2 signals educate dendritic cells with gut-specific imprinting properties. J Immunol. 2011;187:141–50. doi: 10.4049/jimmunol.1003740. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Naik SH, Metcalf D, Nieuwenhuijze van A, et al. Intrasplenic steady-state dendritic cell precursors that are distinct from monocytes. Nat Immunol. 2006;7:663–71. doi: 10.1038/ni1340. [DOI] [PubMed] [Google Scholar]
- 32.Liu K, Victora GD, Schwickert TA, et al. In vivo analysis of dendritic cell development and homeostasis. Science. 2009;324:392–7. doi: 10.1126/science.1170540. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Liu K, Waskow C, Liu X, Yao K, Hoh J, Nussenzweig M. Origin of dendritic cells in peripheral lymphoid organs of mice. Nat Immunol. 2007;8:578–83. doi: 10.1038/ni1462. [DOI] [PubMed] [Google Scholar]
- 34.McKenna HJ, Stocking KL, Miller RE, et al. Mice lacking flt3 ligand have deficient hematopoiesis affecting hematopoietic progenitor cells, dendritic cells, and natural killer cells. Blood. 2000;95:3489–97. [PubMed] [Google Scholar]
- 35.Schraml BU, Blijswijk van J, Zelenay S, et al. Genetic tracing via DNGR-1 expression history defines dendritic cells as a hematopoietic lineage. Cell. 2013;154:843–58. doi: 10.1016/j.cell.2013.07.014. [DOI] [PubMed] [Google Scholar]
- 36.Meredith MM, Liu K, Darrasse-Jeze G, et al. Expression of the zinc finger transcription factor zDC (Zbtb46, Btbd4) defines the classical dendritic cell lineage. J Exp Med. 2012;209:1153–65. doi: 10.1084/jem.20112675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Satpathy AT, KC W, Albring JC, et al. Zbtb46 expression distinguishes classical dendritic cells and their committed progenitors from other immune lineages. J Exp Med. 2012;209:1135–52. doi: 10.1084/jem.20120030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Naik SH, Perie L, Swart E, et al. Diverse and heritable lineage imprinting of early haematopoietic progenitors. Nature. 2013;496:229–32. doi: 10.1038/nature12013. [DOI] [PubMed] [Google Scholar]
- 39.Vremec D, Zorbas M, Scollay R, et al. The surface phenotype of dendritic cells purified from mouse thymus and spleen: investigation of the CD8 expression by a subpopulation of dendritic cells. J Exp Med. 1992;176:47–58. doi: 10.1084/jem.176.1.47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Vremec D, Pooley J, Hochrein H, Wu L, Shortman K. CD4 and CD8 expression by dendritic cell subtypes in mouse thymus and spleen. J Immunol. 2000;164:2978–86. doi: 10.4049/jimmunol.164.6.2978. [DOI] [PubMed] [Google Scholar]
- 41.Edwards AD, Manickasingham SP, Sporri R, et al. Microbial recognition via Toll-like receptor-dependent and-independent pathways determines the cytokine response of murine dendritic cell subsets to CD40 triggering. J Immunol. 2002;169:3652–60. doi: 10.4049/jimmunol.169.7.3652. [DOI] [PubMed] [Google Scholar]
- 42.Dudziak D, Kamphorst AO, Heidkamp GF, et al. Differential antigen processing by dendritic cell subsets in vivo. Science. 2007;315:107–11. doi: 10.1126/science.1136080. [DOI] [PubMed] [Google Scholar]
- 43.Bar-On L, Birnberg T, Lewis KL, et al. CX3CR1+ CD8α+ dendritic cells are a steady-state population related to plasmacytoid dendritic cells. Proc Natl Acad Sci U S A. 2010;107:14745–50. doi: 10.1073/pnas.1001562107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Lewis KL, Caton ML, Bogunovic M, et al. Notch2 receptor signaling controls functional differentiation of dendritic cells in the spleen and intestine. Immunity. 2011;35:780–91. doi: 10.1016/j.immuni.2011.08.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Jongbloed SL, Kassianos AJ, McDonald KJ, et al. Human CD141+ (BDCA-3)+ dendritic cells (DCs) represent a unique myeloid DC subset that cross-presents necrotic cell antigens. J Exp Med. 2010;207:1247–60. doi: 10.1084/jem.20092140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Bachem A, Guttler S, Hartung E, et al. Superior antigen cross-presentation and XCR1 expression define human CD11c+CD141+ cells as homologues of mouse CD8+ dendritic cells. J Exp Med. 2010;207:1273–81. doi: 10.1084/jem.20100348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Poulin LF, Salio M, Griessinger E, et al. Characterization of human DNGR-1+ BDCA3+ leukocytes as putative equivalents of mouse CD8α+ dendritic cells. J Exp Med. 2010;207:1261–71. doi: 10.1084/jem.20092618. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Crozat K, Guiton R, Contreras V, et al. The XC chemokine receptor 1 is a conserved selective marker of mammalian cells homologous to mouse CD8α+ dendritic cells. J Exp Med. 2010;207:1283–92. doi: 10.1084/jem.20100223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Crozat K, Guiton R, Guilliams M, et al. Comparative genomics as a tool to reveal functional equivalences between human and mouse dendritic cell subsets. Immunol Rev. 2010;234:177–98. doi: 10.1111/j.0105-2896.2009.00868.x. [DOI] [PubMed] [Google Scholar]
- 50.Robbins SH, Walzer T, Dembele D, et al. Novel insights into the relationships between dendritic cell subsets in human and mouse revealed by genome-wide expression profiling. Genome Biol. 2008;9:R17. doi: 10.1186/gb-2008-9-1-r17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Murphy KM. Transcriptional control of dendritic cell development. Adv Immunol. 2013;120:239–67. doi: 10.1016/B978-0-12-417028-5.00009-0. [DOI] [PubMed] [Google Scholar]
- 52.Belz GT, Nutt SL. Transcriptional programming of the dendritic cell network. Nat Rev Immunol. 2012;12:101–13. doi: 10.1038/nri3149. [DOI] [PubMed] [Google Scholar]
- 53.Seillet C, Jackson JT, Markey KA, et al. CD8α+ DCs can be induced in the absence of transcription factors Id2, Nfil3, and Batf3. Blood. 2013;121:1574–83. doi: 10.1182/blood-2012-07-445650. [DOI] [PubMed] [Google Scholar]
- 54.Hildner K, Edelson BT, Purtha WE, et al. Batf3 deficiency reveals a critical role for CD8α+ dendritic cells in cytotoxic T cell immunity. Science. 2008;322:1097–100. doi: 10.1126/science.1164206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Hacker C, Kirsch RD, Ju XS, et al. Transcriptional profiling identifies Id2 function in dendritic cell development. Nat Immunol. 2003;4:380–6. doi: 10.1038/ni903. [DOI] [PubMed] [Google Scholar]
- 56.Kashiwada M, Pham NL, Pewe LL, Harty JT, Rothman PB. NFIL3/E4BP4 is a key transcription factor for CD8α+ dendritic cell development. Blood. 2011;117:6193–7. doi: 10.1182/blood-2010-07-295873. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Wu Q, Wang Y, Wang J, Hedgeman EO, Browning JL, Fu YX. The requirement of membrane lymphotoxin for the presence of dendritic cells in lymphoid tissues. J Exp Med. 1999;190:629–38. doi: 10.1084/jem.190.5.629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Kabashima K, Banks TA, Ansel KM, Lu TT, Ware CF, Cyster JG. Intrinsic lymphotoxin-beta receptor requirement for homeostasis of lymphoid tissue dendritic cells. Immunity. 2005;22:439–50. doi: 10.1016/j.immuni.2005.02.007. [DOI] [PubMed] [Google Scholar]
- 59.Yi T, Cyster JG. EBI2-mediated bridging channel positioning supports splenic dendritic cell homeostasis and particulate antigen capture. Elife. 2013;2:e00757. doi: 10.7554/eLife.00757. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Gatto D, Wood K, Caminschi I, et al. The chemotactic receptor EBI2 regulates the homeostasis, localization and immunological function of splenic dendritic cells. Nat Immunol. 2013;14:876. doi: 10.1038/ni.2555. [DOI] [PubMed] [Google Scholar]
- 61.Sekine C, Moriyama Y, Koyanagi A, et al. Differential regulation of splenic CD8– dendritic cells and marginal zone B cells by Notch ligands. Int Immunol. 2009;21:295–301. doi: 10.1093/intimm/dxn148. [DOI] [PubMed] [Google Scholar]
- 62.Caton ML, Smith-Raska MR, Reizis B. Notch-RBP-J signaling controls the homeostasis of CD8– dendritic cells in the spleen. J Exp Med. 2007;204:1653–64. doi: 10.1084/jem.20062648. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Ginhoux F, Liu K, Helft J, et al. The origin and development of nonlymphoid tissue CD103+ DCs. J Exp Med. 2009;206:3115–30. doi: 10.1084/jem.20091756. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Bogunovic M, Ginhoux F, Helft J, et al. Origin of the lamina propria dendritic cell network. Immunity. 2009;31:513–25. doi: 10.1016/j.immuni.2009.08.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Edelson BT, KC W, Juang R, et al. Peripheral CD103+ dendritic cells form a unified subset developmentally related to CD8α+ conventional dendritic cells. J Exp Med. 2010;207:823–36. doi: 10.1084/jem.20091627. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Persson EK, Uronen-Hansson H, Semmrich M, et al. IRF4 transcription-factor-dependent CD103+CD11b+ dendritic cells drive mucosal T helper 17 cell differentiation. Immunity. 2013;38:958–69. doi: 10.1016/j.immuni.2013.03.009. [DOI] [PubMed] [Google Scholar]
- 67.Satpathy AT, Briseno CG, Lee JS, et al. Notch2-dependent classical dendritic cells orchestrate intestinal immunity to attaching-and-effacing bacterial pathogens. Nat Immunol. 2013;14:937–48. doi: 10.1038/ni.2679. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Schlitzer A, McGovern N, Teo P, et al. IRF4 transcription factor-dependent CD11b+ dendritic cells in human and mouse control mucosal IL-17 cytokine responses. Immunity. 2013;38:970–83. doi: 10.1016/j.immuni.2013.04.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Bajana S, Roach K, Turner S, Paul J, Kovats S. IRF4 promotes cutaneous dendritic cell migration to lymph nodes during homeostasis and inflammation. J Immunol. 2012;189:3368–77. doi: 10.4049/jimmunol.1102613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Miller JC, Brown BD, Shay T, et al. Deciphering the transcriptional network of the dendritic cell lineage. Nat Immunol. 2012;13:888–99. doi: 10.1038/ni.2370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Tamoutounour S, Henri S, Lelouard H, et al. CD64 distinguishes macrophages from dendritic cells in the gut and reveals the Th1-inducing role of mesenteric lymph node macrophages during colitis. Eur J Immunol. 2012;42:3150–66. doi: 10.1002/eji.201242847. [DOI] [PubMed] [Google Scholar]
- 72.Gautier EL, Shay T, Miller J, et al. Gene-expression profiles and transcriptional regulatory pathways that underlie the identity and diversity of mouse tissue macrophages. Nat Immunol. 2012;13:1118–28. doi: 10.1038/ni.2419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Beijer MR, Molenaar R, Goverse G, Mebius RE, Kraal G, Haan den JM. A crucial role for retinoic acid in the development of Notch-dependent murine splenic CD8– CD4– and CD4+ dendritic cells. Eur J Immunol. 2013;43:1608–16. doi: 10.1002/eji.201343325. [DOI] [PubMed] [Google Scholar]
- 74.Klebanoff CA, Spencer SP, Torabi-Parizi P, et al. Retinoic acid controls the homeostasis of pre-cDC-derived splenic and intestinal dendritic cells. J Exp Med. 2013;210:1961–76. doi: 10.1084/jem.20122508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Duriancik DM, Hoag KA. Vitamin A deficiency alters splenic dendritic cell subsets and increases CD8+Gr-1+ memory T lymphocytes in C57BL/6J mice. Cell Immunol. 2010;265:156–63. doi: 10.1016/j.cellimm.2010.08.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Zeng R, Oderup C, Yuan R, et al. Retinoic acid regulates the development of a gut-homing precursor for intestinal dendritic cells. Mucosal Immunol. 2013;6:847–56. doi: 10.1038/mi.2012.123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Paschaki M, Lin SC, Wong RL, Finnell RH, Dolle P, Niederreither K. Retinoic acid-dependent signaling pathways and lineage events in the developing mouse spinal cord. PLoS ONE. 2012;7:e32447. doi: 10.1371/journal.pone.0032447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Radtke F, MacDonald HR, Tacchini-Cottier F. Regulation of innate and adaptive immunity by Notch. Nat Rev Immunol. 2013;13:427–37. doi: 10.1038/nri3445. [DOI] [PubMed] [Google Scholar]
- 79.Haan den JM, Lehar SM, Bevan MJ. CD8+ but not CD8– dendritic cells cross-prime cytotoxic T cells in vivo. J Exp Med. 2000;192:1685–96. doi: 10.1084/jem.192.12.1685. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Sancho D, Joffre OP, Keller AM, et al. Identification of a dendritic cell receptor that couples sensing of necrosis to immunity. Nature. 2009;458:899–903. doi: 10.1038/nature07750. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Maldonado-Lopez R, Smedt De T, Michel P, et al. CD8α+ and CD8α– subclasses of dendritic cells direct the development of distinct T helper cells in vivo. J Exp Med. 1999;189:587–92. doi: 10.1084/jem.189.3.587. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Hochrein H, Shortman K, Vremec D, Scott B, Hertzog P, O'Keeffe M. Differential production of IL-12, IFN-α, and IFN-γ by mouse dendritic cell subsets. J Immunol. 2001;166:5448–55. doi: 10.4049/jimmunol.166.9.5448. [DOI] [PubMed] [Google Scholar]
- 83.Shortman K, Heath WR. The CD8+ dendritic cell subset. Immunol Rev. 2010;234:18–31. doi: 10.1111/j.0105-2896.2009.00870.x. [DOI] [PubMed] [Google Scholar]
- 84.Haan den JM, Bevan MJ. Constitutive versus activation-dependent cross-presentation of immune complexes by CD8+ and CD8− dendritic cells in vivo. J Exp Med. 2002;196:817–27. doi: 10.1084/jem.20020295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Backer R, Leeuwen van F, Kraal G, Haan den JM. CD8– dendritic cells preferentially cross-present Saccharomyces cerevisiae antigens. Eur J Immunol. 2008;38:370–80. doi: 10.1002/eji.200737647. [DOI] [PubMed] [Google Scholar]
- 86.Chappell CP, Draves KE, Giltiay NV, Clark EA. Extrafollicular B cell activation by marginal zone dendritic cells drives T cell-dependent antibody responses. J Exp Med. 2012;209:1825–40. doi: 10.1084/jem.20120774. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Gao Y, Nish SA, Jiang R, et al. Control of T Helper 2 responses by transcription factor IRF4-dependent dendritic cells. Immunity. 2013;39:722–32. doi: 10.1016/j.immuni.2013.08.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Kumamoto Y, Linehan M, Weinstein JS, Laidlaw BJ, Craft JE, Iwasaki A. CD301b dermal dendritic cells drive T helper 2 cell-mediated immunity. Immunity. 2013;39:733–43. doi: 10.1016/j.immuni.2013.08.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Stephensen CB. Vitamin A infection, and immune function. Annu Rev Nutr. 2001;21:167–92. doi: 10.1146/annurev.nutr.21.1.167. [DOI] [PubMed] [Google Scholar]
- 90.Ross AC. Vitamin A and retinoic acid in T cell-related immunity. Am J Clin Nutr. 2012;96:1166S–72S. doi: 10.3945/ajcn.112.034637. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Ross AC, Chen Q, Ma Y. Vitamin A and retinoic acid in the regulation of B-cell development and antibody production. Vitam Horm. 2011;86:103–26. doi: 10.1016/B978-0-12-386960-9.00005-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Iwata M, Eshima Y, Kagechika H. Retinoic acids exert direct effects on T cells to suppress Th1 development and enhance Th2 development via retinoic acid receptors. Int Immunol. 2003;15:1017–25. doi: 10.1093/intimm/dxg101. [DOI] [PubMed] [Google Scholar]


