An important emerging area in the translational aspects of redox biology and bioenergetics are the new findings that implicate mitochondrial dysfunction as a key player in the etiology of metabolic syndrome and diabetes. The paper in this issue of Free Radical Biology & Medicine, entitled “The mitochondria-targeted antioxidant MitoQ decreases features of the Metabolic Syndrome in ATM+/−/ApoE−/− mice” by Mercer and colleagues shows, in an elegant pre-clinical study, that mitochondrial therapeutics may be beneficial for this important clinical problem. The work is exciting because it not only validates a role for mitochondrial redox signaling in the etiology of metabolic syndrome but also highlights a potential therapeutic agent to treat metabolic syndrome in human subjects.
An alarming public health trend in the developing world has been an increase in obesity due to a combination of socio-economic factors and lifestyle changes [1] with an ever increasing burden on health care costs [2]. Obesity leads to an increased propensity for the development of a complex array of symptoms which have been termed the metabolic syndrome. Metabolic syndrome was originally defined as “Syndrome X” where it was determined that obesity, hypertension, and diabetes correlate with insulin resistance and glucose intolerance [3, 4]. Key clinical features include dyslipidemia, increased incidence of Type II diabetes and atherosclerosis. Clearly, public health measures leading to improved diet and increased exercise must play an important role in combating metabolic syndrome. However, in parallel with such interventions novel therapeutics are needed which prevent or decrease the transition of the risk factors that comprise metabolic syndrome into clinically debilitating conditions such as diabetes. In this context, the paper by Mercer et al uses an animal model to explore the potential of the mitochondrial therapeutic MitoQ, in decreasing important clinical features of metabolic syndrome.
Unfortunately, metabolic syndrome is highly complex and the mechanistic pathways that lead to its pathological effects have not been fully clarified. What is known is that metabolic syndrome is associated with oxidative stress and mitochondrial dysfunction [5–7]. It has been previously shown that ROS induce mitochondrial dysfunction and protein modifications in non-alcoholic steatohepatitis (NASH), which is a common pathology associated with metabolic syndrome [8]. In addition, obesity decreases basal metabolic respiration and induces uncoupling protein-2 in hepatocytes [9]. At first glance, it seems paradoxical that a high calorific intake diet can result in bioenergetic defects. However, high levels of respiratory substrates result in an energetic overload in which mitochondrial oxidative phosphorylation becomes less efficient with an abnormal generation of mitochondrially derived superoxide. This is potentially important since at low levels mitochondrial superoxide or hydrogen peroxide plays a role in retrograde signaling. Loss of control of this mitochondrial redox signaling can then be deleterious through lack of regulation of cell proliferation and differentiation. If persistent and chronic, the formation of deleterious oxidants such as peroxynitrite, hydrogen peroxide, and hydroxyl radical will occur and will damage respiratory proteins, mitochondrial membranes and DNA [10].
The article by Mercer et al uses many of these markers of metabolic and bioenergetic dysfunction as surrogate endpoints for evaluating the effects of MitoQ, the mitochondrial targeted antioxidant selected for this study. MitoQ has been shown to be beneficial in pathologies known to occur downstream of metabolic syndrome such as cardiac hypertrophy [11], diabetic nephropathy [12], and alcohol induced steatohepatitis which also appears to share many common features with NASH [13]. MitoQ is a ubiquinone derivative covalently linked to a ten-carbon chain terminating with a triphenylphosphonium cation [14]. It rapidly accumulates in the mitochondria where it is reduced by complex II of the respiratory chain. It can then potentially modify redox signaling emanating from the organelle. This study for the first time presents MitoQ’s beneficial effects in an in vivo model of metabolic syndrome. The transgenic ATM+/−/ApoE−/− mice was previously used by the authors as a model of metabolic syndrome which also encompasses cardiovascular pathologies. The deletion of ApoE leads to a profound dyslipidemia and the protein kinase ataxia telangiectasia mutated (ATM+/−) gene suppression leads to defects in DNA repair. As a result, these mice develop metabolic syndrome and atherosclerosis when fed a high fat diet. This animal model is then an appropriate choice for the more severe clinical manifestations of metabolic syndrome because it closely mimics the advanced symptoms of human subjects metabolically and pathophysiologically. MitoQ is well tolerated in human subjects [15, 16] which makes it an ideal candidate to explore further in treating metabolic syndrome.
Mercer and colleagues show that administration of MitoQ for 14 weeks decreased adipose tissue and increased carbohydrate utilization relative to fat oxidation in ATM+/−/ApoE−/− mice. Additionally, MitoQ suppressed weight gain over time with no impact on activity or food and water intake. MitoQ also reduced liver steatosis, hypercholesterolemia, and hypertriglyceridemia. This is important in metabolic syndrome because triglyceride (TG) accumulation can induce very-low-density lipoprotein (VLDL) TG and glucose production [17]. L Hepatosteatosis is likely to be a key initiating factor in the development of metabolic syndrome. Interestingly, diet induced steatosis is also associated with hypoxia in the liver and can be related to mitochondrial redox signaling [18–20]. In addition, Mercer et al showed that MitoQ significantly altered lipid composition in livers of ATM+/−/ApoE−/− mice as well as improved glucose and insulin levels.
Next, the authors assessed if MitoQ would exert any anti-atherosclerotic effects. In atherosclerotic plaques, there is decreased blood flow due to the accumulation of fat and cholesterol in arterial vessels. ROS are generated from a number of sources and activate pro-inflammatory signaling pathways. It has been shown that superoxide generated by NAD(P)H oxidase may initiate atherosclerosis by activating smooth muscle cell mitogenic signaling pathways and that attenuation of superoxide decreases atherosclerosis in macrophages and vessel wall cells [21]. However, MitoQ did not decrease atherosclerotic lesion size but did decrease inflammation, cell proliferation, and DNA oxidation within the plaques. This intriguing result suggests that mitochondrial redox signaling is central in mounting an inflammatory response under conditions of hypercholesterolemia. A further interesting possibility is that mtDNA damage constitutes an epigenetic modification of cells in the vasculature during cardiovascular disease [22]. Interestingly, DNA damage could be mediated by a number of reactive species including peroxynitrite which would be an ideal candidate to evaluate in the context of metabolic syndrome. Indeed, MitoQ has been shown to decrease both mitochondrial superoxide and peroxynitrite during cold ischemia/reperfusion of renal cells and rat kidneys [23]. Mutations in mitochondrial DNA (mtDNA) have also been linked to Type II diabetes pathogenesis [24]. One of the most exciting aspects of this study is the finding that MitoQ reduced mtDNA oxidative damage in the livers of ATM+/−/ApoE−/− mice.
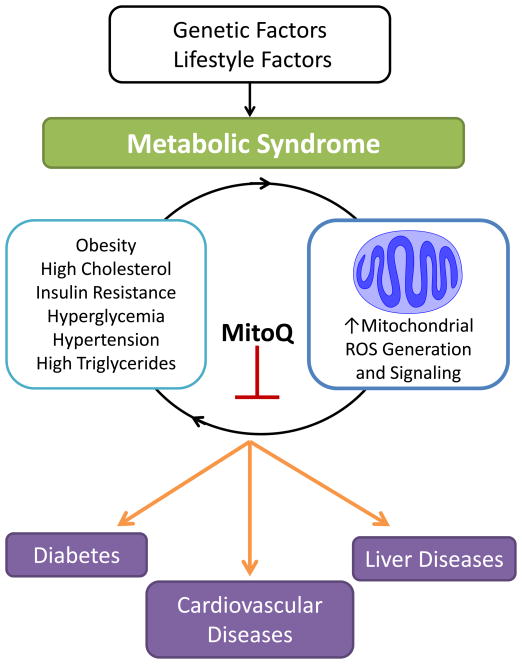
Overall, this comprehensive study suggests that MitoQ modulates mitochondrial redox signaling leading to decreased oxidative damage particularly in the liver as outlined in Figure 1. A combination of genetic and lifestyle factors metabolically stress the individual, in part through a dramatic effect on lipid metabolism, primarily in the liver. Shown in Figure 1 is a hypothetical vicious cycle in which dysfunctional mitochondrial redox signaling increases dyslipidemia and leads to insulin resistance which then further damages mitochondrial function. MitoQ appears to suppress or modify the redox signals which are necessary to sustain this cycle of damage. Naturally, more questions have arisen as a consequence of this study. For example, what are the specific mitochondrial mediators or signals which initiate the metabolic changes underlying the pathology and how are these modulated by MitoQ? How do mitochondria modulate the response to inflammation and in the developing lesion and which cells are implicated in this response? We look forward in anticipation to new insights into the emerging role of bioenergetic dysfunction in the cardiometabolic syndrome.
Figure 1.
Genetic and lifestyle factors contribute to the development of metabolic syndrome. Obesity is the most common feature of metabolic syndrome and can occur with one or more of the following risk factors: high cholesterol, insulin resistance, hyperglycemia, hypertension, and high triglycerides. Reactive oxygen species (ROS) generation and mitochondrial signaling is increased during this time and may the initiate a vicious cycle activating an inflammatory response and exacerbating bioenergetic dysfunction. Ultimately this contributes to diabetes, cardiovascular diseases, and steatosis.
Acknowledgments
The authors are grateful for support from the NIH AA13395, DK075865 (VDU) and NIH T32 Postdoctoral Interdisciplinary Training in Kidney-Related Research Grant DK007545 (TM). This content is solely the responsibility of the authors and does not represent views of the NIH.
References
- 1.Ford ES, Giles WH, Dietz WH. Prevalence of the metabolic syndrome among US adults: findings from the third National Health and Nutrition Examination Survey. JAMA. 2002;287:356–359. doi: 10.1001/jama.287.3.356. [DOI] [PubMed] [Google Scholar]
- 2.Boudreau DM, Malone DC, Raebel MA, Fishman PA, Nichols GA, Feldstein AC, Boscoe AN, Ben-Joseph RH, Magid DJ, Okamoto LJ. Health care utilization and costs by metabolic syndrome risk factors. Metab Syndr Relat Disord. 2009;7:305–314. doi: 10.1089/met.2008.0070. [DOI] [PubMed] [Google Scholar]
- 3.Isomaa B, Almgren P, Tuomi T, Forsén B, Lahti K, Nissén M, Taskinen MR, Groop L. Cardiovascular morbidity and mortality associated with the metabolic syndrome. Diabetes Care. 2001;24:683–689. doi: 10.2337/diacare.24.4.683. [DOI] [PubMed] [Google Scholar]
- 4.Reaven G. Banting Lecture 1988: role of insulin resistance in human disease. Diabetes. 1988;37:1595–1607. doi: 10.2337/diab.37.12.1595. [DOI] [PubMed] [Google Scholar]
- 5.Furukawa S, Fujita T, Shimabukuro M, Iwaki M, Yamada Y, Nakajima Y, Nakayama O, Makishima M, Matsuda M, Shimomura I. Increased oxidative stress in obesity and its impact on metabolic syndrome. J Clin Invest. 2004;114:1752–1761. doi: 10.1172/JCI21625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Kaneki M, Shimizu N, Yamada D, Chang K. Nitrosative stress and pathogenesis of insulin resistance. Antioxid Redox Signal. 2007;9:319–329. doi: 10.1089/ars.2006.1464. [DOI] [PubMed] [Google Scholar]
- 7.Petersen KF, Befroy D, Dufour S, Dziura J, Ariyan C, Rothman DL, DiPietro L, Cline GW, Shulman GI. Mitochondrial dysfunction in the elderly: possible role in insulin resistance. Science. 2003;300:1140–1142. doi: 10.1126/science.1082889. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Mantena SK, Vaughn DP, Andringa KK, Eccleston HB, King AL, Abrams GA, Doeller JE, Kraus DW, Darley-Usmar VM, Bailey SM. High fat diet induces dysregulation of hepatic oxygen gradients and mitochondrial function in vivo. Biochem J. 2009;417:183–193. doi: 10.1042/BJ20080868. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Chavin KD, Yang S, Lin HZ, Chatham J, Chacko VP, Hoek JB, Walajtys-Rode E, Rashid A, Chen CH, Huang CC, Wu TC, Lane MD, Diehl AM. Obesity induces expression of uncoupling protein-2 in hepatocytes and promotes liver ATP depletion. J Biol Chem. 1999;274:5692–5700. doi: 10.1074/jbc.274.9.5692. [DOI] [PubMed] [Google Scholar]
- 10.Murphy MP. How mitochondria produce reactive oxygen species. Biochem J. 2009;417:1–13. doi: 10.1042/BJ20081386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Graham D, Huynh NN, Hamilton CA, Beattie E, Smith RA, Cochemé HM, Murphy MP, Dominiczak AF. Mitochondria-targeted antioxidant MitoQ10 improves endothelial function and attenuates cardiac hypertrophy. Hypertension. 2009;54:322–328. doi: 10.1161/HYPERTENSIONAHA.109.130351. [DOI] [PubMed] [Google Scholar]
- 12.Chacko BK, Reily C, Srivastava A, Johnson MS, Ye Y, Ulasova E, Agarwal A, Zinn KR, Murphy MP, Kalyanaraman B, Darley-Usmar V. Prevention of diabetic nephropathy in Ins2(+/) (AkitaJ) mice by the mitochondria-targeted therapy MitoQ. Biochem J. 2010;432:9–19. doi: 10.1042/BJ20100308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Chacko BK, Srivastava A, Johnson MS, Benavides GA, Chang MJ, Ye Y, Jhala N, Murphy MP, Kalyanaraman B, Darley-Usmar VM. Mitochondria-targeted ubiquinone (MitoQ) decreases ethanol-dependent micro and macro hepatosteatosis. Hepatology. 2011;54:153–163. doi: 10.1002/hep.24377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Kelso GF, Porteous CM, Coulter CV, Hughes G, Porteous WK, Ledgerwood EC, Smith RA, Murphy MP. Selective targeting of a redox-active ubiquinone to mitochondria within cells: antioxidant and antiapoptotic properties. J Biol Chem. 2001;276:4588–4596. doi: 10.1074/jbc.M009093200. [DOI] [PubMed] [Google Scholar]
- 15.Snow BJ, Rolfe FL, Lockhart MM, Frampton CM, O’Sullivan JD, Fung V, Smith RA, Murphy MP, Taylor KM, Group PS. A double-blind, placebo-controlled study to assess the mitochondria-targeted antioxidant MitoQ as a disease-modifying therapy in Parkinson’s disease. Mov Disord. 2010;25:1670–1674. doi: 10.1002/mds.23148. [DOI] [PubMed] [Google Scholar]
- 16.Gane EJ, Weilert F, Orr DW, Keogh GF, Gibson M, Lockhart MM, Frampton CM, Taylor KM, Smith RA, Murphy MP. The mitochondria-targeted anti-oxidant mitoquinone decreases liver damage in a phase II study of hepatitis C patients. Liver Int. 2010;30:1019–1026. doi: 10.1111/j.1478-3231.2010.02250.x. [DOI] [PubMed] [Google Scholar]
- 17.den Boer M, Voshol PJ, Kuipers F, Havekes LM, Romijn JA. Hepatic steatosis: a mediator of the metabolic syndrome. Lessons from animal models. Arterioscler Thromb Vasc Biol. 2004;24:644–649. doi: 10.1161/01.ATV.0000116217.57583.6e. [DOI] [PubMed] [Google Scholar]
- 18.Nath B, Levin I, Csak T, Petrasek J, Mueller C, Kodys K, Catalano D, Mandrekar P, Szabo G. Hepatocyte-specific hypoxia-inducible factor-1α is a determinant of lipid accumulation and liver injury in alcohol-induced steatosis in mice. Hepatology. 2011;53:1526–1537. doi: 10.1002/hep.24256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Simon MC. Mitochondrial reactive oxygen species are required for hypoxic HIF alpha stabilization. Adv Exp Med Biol. 2006;588:165–170. doi: 10.1007/978-0-387-34817-9_15. [DOI] [PubMed] [Google Scholar]
- 20.Simon MC. Coming up for air: HIF-1 and mitochondrial oxygen consumption. Cell Metab. 2006;3:150–151. doi: 10.1016/j.cmet.2006.02.007. [DOI] [PubMed] [Google Scholar]
- 21.Vendrov AE, Hakim ZS, Madamanchi NR, Rojas M, Madamanchi C, Runge MS. Atherosclerosis is attenuated by limiting superoxide generation in both macrophages and vessel wall cells. Arterioscler Thromb Vasc Biol. 2007;27:2714–2721. doi: 10.1161/ATVBAHA.107.152629. [DOI] [PubMed] [Google Scholar]
- 22.Gutierrez J, Ballinger SW, Darley-Usmar VM, Landar A. Free radicals, mitochondria, and oxidized lipids: the emerging role in signal transduction in vascular cells. Circ Res. 2006;99:924–932. doi: 10.1161/01.RES.0000248212.86638.e9. [DOI] [PubMed] [Google Scholar]
- 23.Mitchell T, Rotaru D, Saba H, Smith RA, Murphy MP, MacMillan-Crow LA. The mitochondria-targeted antioxidant mitoquinone protects against cold storage injury of renal tubular cells and rat kidneys. J Pharmacol Exp Ther. 2011;336:682–692. doi: 10.1124/jpet.110.176743. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Rolo AP, Palmeira CM. Diabetes and mitochondrial function: role of hyperglycemia and oxidative stress. Toxicol Appl Pharmacol. 2006;212:167–178. doi: 10.1016/j.taap.2006.01.003. [DOI] [PubMed] [Google Scholar]