Abstract
The majority of gastrointestinal stromal tumors (GIST) are characterized by activating mutations of KIT, an HSP90 client protein. Further secondary resistance mutations within KIT limit clinical responses to tyrosine kinase inhibitors, such as imatinib. The dependence of KIT and its mutated forms on HSP90 suggests that HSP90 inhibition might be a valuable treatment option for GIST, which would be equally effective on imatinib-sensitive and -resistant clones. We investigated the activity of AT13387, a potent HSP90 inhibitor currently being evaluated in clinical trials, in both in vitro and in vivo GIST models. AT13387 inhibited the proliferation of imatinib-sensitive (GIST882, GIST-T1) and -resistant (GIST430, GIST48) cell lines, including those resistant to the geldanamycin analogue HSP90 inhibitor, 17-AAG. Treatment with AT13387 resulted in depletion of HSP90 client proteins, KIT and AKT, along with their phospho-forms in imatinib-sensitive and -resistant cell lines, irrespective of KIT mutation. KIT signaling was ablated, whereas HSP70, a marker of HSP90 inhibition, was induced. In vivo, antitumor activity of AT13387 was showed in both the imatinib-sensitive, GIST-PSW, xenograft model and a newly characterized imatinib-resistant, GIST430, xenograft model. Induction of HSP70, depletion of phospho-KIT and inhibition of KIT signaling were seen in tumors from both models after treatment with AT13387. A combination of imatinib and AT13387 treatment in the imatinib-resistant GIST430 model significantly enhanced tumor growth inhibition over either of the monotherapies. Importantly, the combination of AT13387 and imatinib was well tolerated. These results suggest AT13387 is an excellent candidate for clinical testing in GIST in combination with imatinib.
Introduction
Gastrointestinal stromal tumors (GIST) are mesenchy-mal tumors that are frequently characterized by expression of the receptor tyrosine kinase KIT (1, 2). About 85% of advanced GISTs have activating mutations in KIT, whereas another 5% harbor mutations in platelet-derived growth factor receptor alpha (PDGFRA; ref. 3). Typically first-line treatment for inoperable GIST is imatinib, but 20% of patients with GIST do not respond to this treatment with response apparently related, in part, to the type and position of the primary activating mutation (4, 5). Those that respond ultimately develop resistance to treatment largely through secondary mutations in KIT (6). Resistance tends to be highly heterogeneous with different mutations observed within the same patient at different metastatic sites and even within the same tumor nodule (7). The second-line treatment, sunitinib, is active against some of the imatinib-resistant forms of KIT, but eventually the vast majority of patients will also develop resistance to sunitinib. Because of the extensive inter- and intralesional heterogeneity of resistance mutations within individual patients it has been suggested that tyrosine kinase inhibitors (TKI) are unlikely to have curative potential in this disease (2).
KIT and PDGFRA are clients for HSP90, a key chaperone and an attractive target in many cancers (2, 8, 9). Mutated forms of the proteins are also dependent on the chaperone for their stability (10). It has been suggested that use of HSP90 inhibitors could be a good strategy for treatment in GIST, as they would target both imatinib-sensitive and -resistant GIST, irrespective of the type of KIT mutation (11). Inhibition of HSP90 has previously been shown to bring about degradation of KIT, with the geldanamycin analogues 17-AAG, IPI-493, and retaspimycin (IPI-504) having shown antitumor activity in a number of GIST and mast cell tumor models (10–14). The geldanamycins have been extensively studied and tested in the clinic, however they have clinical limitations (15). 17-AAG has shown hepatotoxicity and formulation issues while a phase III GIST trial with retaspimycin was terminated because of toxicity (16). Hence, there is a need for safer and possibly more effective HSP90 inhibitors to test in this disease.
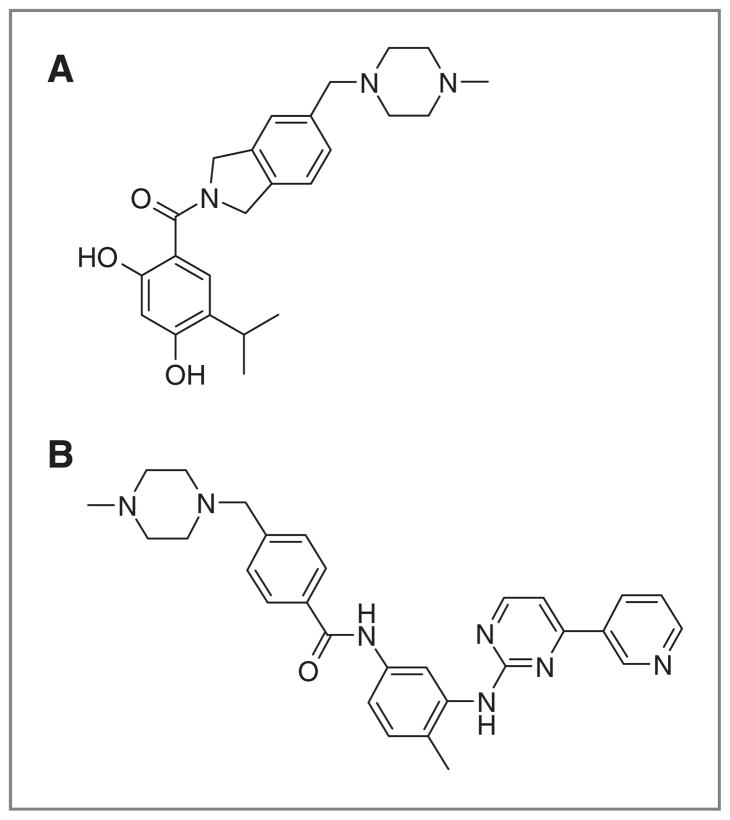
Here, we report on the activity of the non-geldanamycin HSP90 inhibitor, AT13387 (Fig. 1A), in GIST in vitro and in vivo models. AT13387 is a potent small-molecule HSP90 inhibitor, which is currently being evaluated in the clinic in a phase II GIST trial (ClinicalTrials.gov Identifier: NCT01294202) in combination with imatinib (Fig. 1B). It was discovered using a fragment-based approach and has significant antitumor activity in a number of in vitro and in vivo cancer models (17, 18). Here, we show that this inhibitor is active in both imatinib-sensitive and -resistant GIST models and that combination with imatinib in an imatinib-resistant model enhances tumor growth inhibition over either of the monotherapies. These data support the current clinical testing of AT13387 in this disease.
Figure 1.
Chemical structures of AT13387 (A) and imatinib (B).
Materials and Methods
Materials
AT13387 was synthesized at Astex Pharmaceuticals and stored as a lyophilized powder. Synthesis of AT13387 is as described by Woodhead and colleagues (18). Imatinib mesylate and sunitinib malate were purchased from Sequoia Research Products Ltd. All other reagents were purchased from Sigma unless otherwise stated.
Cell culture and reagents
The human GIST cell lines, GIST882, GIST430, and GIST48 described in (11) and GIST-T1 in (19) were used. GIST48B (20) and GIST430A were derived from GIST48 and GIST430, respectively, grown in the presence of 500 nmol/L 17-AAG. GIST882 and GIST430 were grown in RPMI-1640 and Iscove’s Modified Dulbecco’s Medium, respectively, both supplemented with 15% FBS. GIST48 was grown in F10 medium supplemented with 15% FBS, 3 μg/mL bovine pituitary extract, and MITO+ Serum Extender (BD Biosciences). Cells were maintained at 37°C in a humidified atmosphere of 5% CO2. All cell culture reagents were purchased from Invitrogen unless otherwise stated. GIST882, GIST430, GIST48, GIST-T1, GIST48B, and GIST430A cell lines and GIST430 xenograft tumors were validated by genomic PCR for the continued presence of known KIT mutations and by single-nucleotide polymorphism profiles (21–23). All lines were cultured in vitro for no more than 6 months after PCR and single-nucleotide polymorphism validations. Response of GIST882, GIST48, and GIST430 to imatinib was monitored regularlyto ensure the cellline characteristics did not alter.
Proliferation assays
Viability studies were carried out using the CellTiter-Glo luminescent assay (Promega) as described previously (11) or by using Alamar Blue (Invitrogen) as described previously (24) but with slight modifications. Briefly, 105 cells were seeded in 200 μL of complete culture medium per well into flat-bottomed 96-well plates 1 day before the drug treatment. Cells were incubated with compound in 0.1% (v/v) dimethyl sulfoxide (DMSO) for up to 7 days before viability was assessed by using Alamar Blue.
Western blot samples and analysis
Cells were seeded into 6-well plates at 106 cells per well and incubated overnight at 37°C followed by treatment with AT13387 or imatinib for the indicated time. Floating and adherent cells were collected and prepared as described previously (25). Samples were resolved by SDS-PAGE on a NuPage Novex (4%–12% Bis-Tris gel) system, transferred to nitrocellulose membranes, and incubated with primary antibodies specific for: KIT, phos-pho-KIT; AKT, phospho-AKT (p-AKT); cleaved PARP, (ClPARP); ERK, phospho-ERK (p-ERK); S6, phospho-S6 (p-S6); HSP90 (Cell Signaling Technologies); actin (Abcam); or HSP70 (Enzo Life Sciences or Santa Cruz Technologies) followed by either infrared-dye-labeled anti-rabbit or anti-mouse antibodies (Licor Bioscience). Blots were scanned to detect infrared fluorescence on the Odyssey infrared imaging system (Licor Biotechnology) and, where relevant, signal intensity was determined.
Xenograft efficacy studies
The GIST430 xenograft study was conducted in United Kingdom. The care and treatment of experimental animals were in accordance with the United Kingdom Coordinating Committee for Cancer Research guidelines (26) and with United Kingdom Animals (Scientific Procedures) Act 1986 (27). GIST430 xenografts were prepared by subcutaneously injecting 5 × 106 cells in 50 μL serum-free medium mixed with 50 μL Matrigel (~10 mg/mL, BD Biosciences) into the right flanks of BALB/c severe combined immunodeficient (SCID) mice (Harlan). Tumor burden was estimated from caliper measurements and by applying the formula for an ellipsoid.
The GIST-PSW (human GIST, with KIT exon 11 harboring p.K558_G565delinsR mutation) study was conducted in Belgium as approved by the committee of animal ethics of the Catholic University of Leuven, Belgium. GIST-PSW tumor pieces were grafted bilaterally into nu/nu NMRI mice (Janvier Laboratories) as described previously (28). Tumor burden was estimated from caliper measurements and by applying the formula for a cuboid.
Drug treatment of the mice was started when tumors were well-established. GIST430 tumors developed in approximately 3 weeks and animals bearing tumors in the range of 92 and 303 mm3 were included in the study (N = 8 per group). GIST-PSW tumors required approximately 9 weeks to establish. Animals bearing 1 or 2 tumors between 2 and 2,737 mm3 were divided into 4 groups of 7 animals. All drug-treated animals harbored 2 tumors (N =14). Control groups included 2 animals with 2 tumors and 5 with only 1 tumor (N = 9). AT13387 was dissolved in 17.5% (w/v) hydroxypropyl-β-cyclodextrin and administered intraperitoneally at 70 mg/kg once a week for 4 weeks except for the first dose in GIST-PSW study, which was 80 mg/kg. Imatinib was dissolved in sterile water and given orally at 50 mg/kg (13) for 3 or 4 weeks. Both AT13387 and imatinib were given at 10 mL/kg. Control animals received 17.5% (w/v) 2-hydroxypropyl-β-cyclodextrin intraperitoneally.
A statistically significant (one-way ANOVA with Dunnett multiple comparison test or unpaired t test conducted on GraphPad Prism version 3.02) slowing of the relative increase in xenograft volume or regression of tumors compared with the control group was used to characterize efficacy. Statistically significant differences were defined as P < 0.05. Tolerability was estimated by monitoring body weight loss and survival over the course of the study.
Analysis of pharmacodynamic markers in tumor sample
Tumor-bearing animals were sacrificed at various time points after single or multiple doses of AT13387 or ima-tinib. Tumors (typically 4 per treatment or time point) were snap-frozen in liquid nitrogen and analysis of phar-macodynamic markers in tumor samples was carried out as described previously (25, 29) with the same antibodies used in vitro. Total and phospho-KIT levels in the same tumor lysates were also quantified using Phospho (Tyr721)/Total c-Kit Whole Cell Lysate Kit and SECTOR PR 400 reader from Meso Scale Discovery (MSD) according to manufacturer’s instruction.
Histologic analyses
Tumor and liver samples were embedded in paraffin and sections were stained with hematoxylin and eosin to aid histologic interpretation. Tumor sections were also immunostained for KIT (polyclonal antibody, DAKO Corp.) as previously described (30).
Results
AT13387 inhibits proliferation in GIST cell lines
AT13387 is a potent, novel HSP90 inhibitor that has previously been shown to have antiproliferative activity in a wide range of different cell lines (17, 18). Here, AT13387 was tested against a range of GIST cell lines with different activating and secondary resistance mutations (Table 1). AT13387 was shown to inhibit proliferation, of all imatinib-sensitive (GIST-T1, GIST882), imatinib-resistant (GIST430), and multiple TKI-resistant (GIST48) lines with sub-100 nmol/L potencies (Table 1, Supplementary Fig. S1). The imati-nib-resistant GIST cells were derived from patients progressing on imatinib and harbored secondary KIT mutations in addition to the primary activating mutations found in both sensitive and resistant lines (Table 1; ref. 11). In addition, AT13387 also inhibited proliferation of 17-AAG–resistant cell lines, GIST430A and GIST48B (IC50 values of 75 and 310 nmol/L, respectively; ref. 20), which were derived by prolonged culturing of GIST430 and GIST48 cells in the presence of 500 nmol/L of the HSP90 inhibitor, 17-AAG.
Table 1.
Cell lines investigated in this study and their response to inhibitors
| Cell line | Primary KIT mutation | Secondary KIT mutation | Other findings | Resistance pharmacology | IC50, nmol/L
|
|||
|---|---|---|---|---|---|---|---|---|
| AT13387 | 17-AAG | Imatinib | Sunitinib | |||||
| GIST882a | p.K642E | 82 | 140 | 111 | 26 | |||
| GIST-T1b | p.V560_Y578del | 36 | 29 | 45 | 25 | |||
| GIST430a | p.V560_L576del | p.V654A | Imatinib-resistant | 34 | 110 | 46%I at 300 nmol/Lc | 37 | |
| GIST430Ab | p.V560_L576del | p.V654A | NQO1-negative | 17-AAG–resistant | 75 | >1,000 | N/D | N/D |
| GIST48a | p.V560D | p.D820A | Imatinib/sunitinib-resistant | 55 | 97 | >1,000 | >10,000 | |
| GIST48Bb | p.V560D | p.D820A | KIT-independent | Imatinib/sunitinib/17-AAG-resistant | 310 | 890 | >1,000 | >1,000 |
Cells were incubated with drugs for 7 days.
Cells were incubated with drugs for 6 days.
Forty-six percent inhibition at 300 nmol/L. See also Supplementary Fig. S1 for typical dose–response curve.
AT13387 inhibits KIT signaling in both imatinib-sensitive and -resistant cell lines
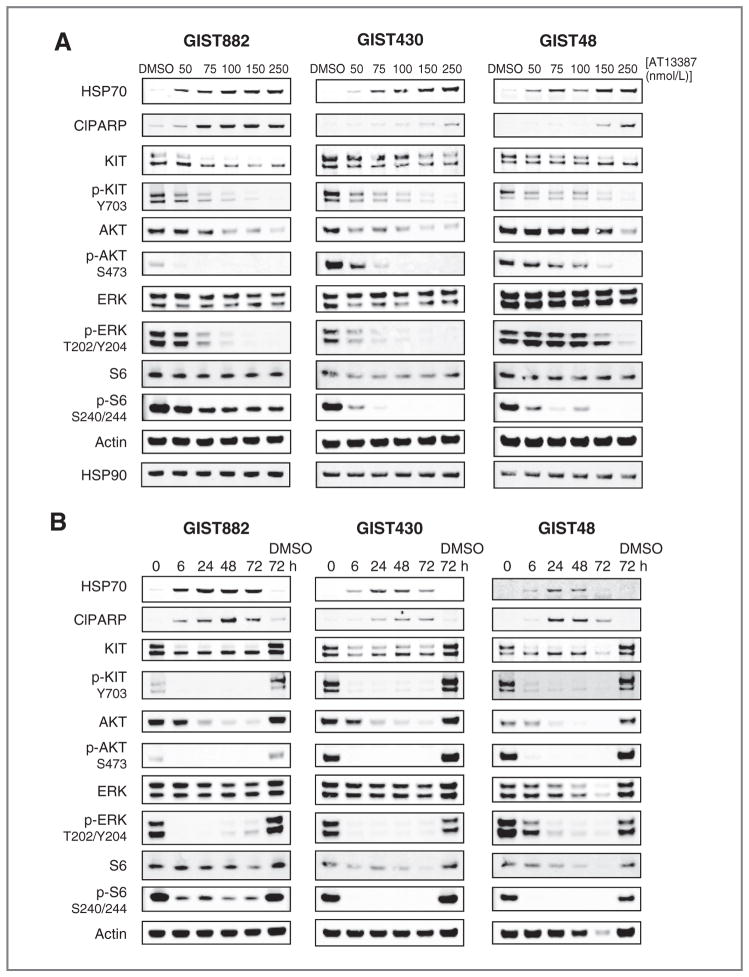
KIT is a client for HSP90 (10). The ability of AT13387 to degrade KIT and therefore inhibit KIT signaling was investigated in 3 cell lines (GIST882, GIST430, and GIST48). Treatment of all 3 cell lines with increasing concentrations of AT13387 depleted HSP90 client proteins, KIT and AKT, and caused significant inhibition of KIT signaling at concentrations of AT13387 above 75 nmol/L for GIST882 and GIST430 and above 100 nmol/L for GIST48 (Fig. 2A), which correlates well with the IC50 values determined by the viability assay for these cell lines (Table 1). A time course evaluating AT13387 treatment for up to 72 hours (Fig. 2B) indicated that effects of HSP90 inhibition such as HSP70 induction and KIT depletion could be seen after 6 hours of treatment and that these effects were sustained for 72 hours by which time significant cell death was observed. Similar effects were seen in all 3 cell lines tested.
Figure 2.
Dose- and time-dependence of AT13387 treatment on HSP90 clients and KIT signaling in GIST cell lines. GIST882, GIST430, and GIST48 cells were treated with the indicated concentrations of AT13387 for 24 hours (A) or 250 nmol/L of AT13387 for 6 to 72 hours (B). Samples were run by SDS-PAGE and immunoblotted with the indicated antibodies.
To compare the effects of AT13387 treatment with imatinib treatment in the 3 cell lines, cells were treated with varying concentrations of AT13387 or imatinib for 24 hours (Fig. 3). As previously, AT13387 treatment induced HSP70, an indicator of HSP90 inhibition, in all 3 cell lines with a concomitant depletion in the levels of the clients KIT, AKT, and their phospho-forms. Levels of the downstream signaling molecules, phospho-ERK and phospho-S6, were also decreased, indicating that KIT signaling was significantly inhibited in all cell lines. In contrast, imatinib significantly inhibited KIT signaling, only in the imatinib-sensitive cell line, GIST882, with little change in the levels of phospho-KIT, phospho-AKT, and phospho-S6 seen in the imatinib-resistant cell lines, GIST430 and GIST48. An increase in cleaved PARP, indicative of apoptosis, was seen in all cells treated with AT13387, whereas again for cells treated with imatinib, an increase in cleaved PARP was only seen for the ima-tinib-sensitive cell line. These data confirm that inhibition of HSP90 leads to inhibition of KIT signaling pathways in GIST cell lines driven by activating mutations in KIT and that this inhibition is irrespective of secondary mutations in KIT, which can render cells resistant to imatinib.
Figure 3.
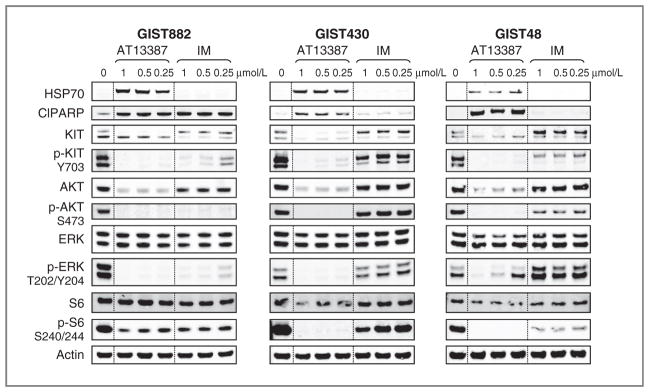
Effects of AT13387 and imatinib on KIT signaling in imatinib-sensitive and -resistant GIST cell lines. GIST882 (A), GIST430 (B), and GIST48 (C) cells were incubated with the indicated concentrations of AT13387 or imatinib (IM) for 24 hours. Samples were run by SDS-PAGE and immunoblotted with the indicated antibodies. This study was typical of at least 3 experiments.
Characterization of the novel imatinib-resistant GIST430 xenograft model
To date, only 2 imatinib-resistant GIST xenograft models have been reported, GIST48 and GIST-BOE (13). Because the GIST430 cell line showed resistance to imatinib in vitro (11), this cell line was a good candidate for establishing a novel imatinib-resistant GIST xenograft model.
GIST430 cells harbor a heterozygous primary mutation in exon 11 (p.V560_L576del) and a secondary resistance mutation in exon 13 (V654A). This cell line failed to develop subcutaneous solid tumors in nude mice with or without Matrigel, or in SCID mice without Matrigel (data not shown). However, when 5 × 106 GIST430 cells were mixed with Matrigel then injected into SCID mice, solid tumors developed in more than 90% of the animals after 19 to 22 days. In 3 independent experiments, the average sizes of all tumors at this time were 146 to 184 mm3. Tumors that were selected for efficacy studies were in the range of 92 and 303 mm3 (57%–60% of the total number of animals inoculated). The vehicle-treated tumors grew exponentially with a doubling time of 5.3 to 7.0 days.
Total KIT expression in tumors was confirmed by Western blotting (Figs. 4 and 5) and immunohistochem-istry, in which uniform total KIT staining was observed (data not shown). Presence of phosphorylated KIT and its downstream signaling through AKT, ERK, and S6 were also confirmed by Western blotting (Figs. 4 and 5).
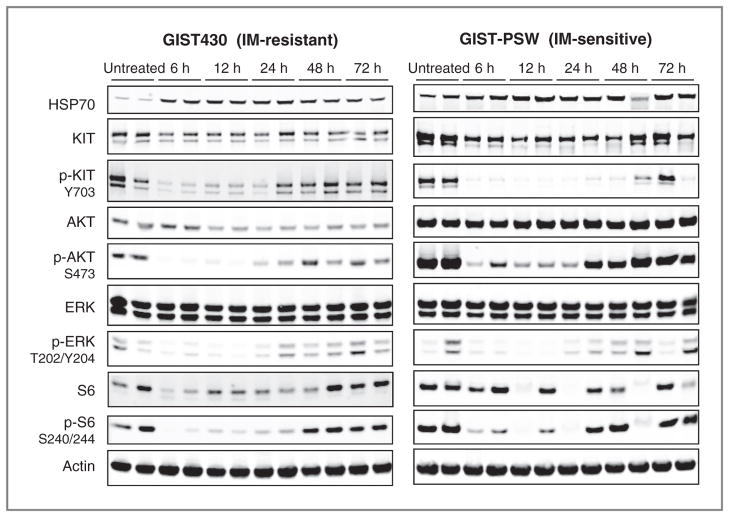
Figure 4.
Duration of effects of AT13387 treatment on KIT signaling in imatinib (IM)-sensitive and -resistant GIST xenografts. Mice bearing GIST-PSW or GIST430 xenograft tumors were treated with AT13387 once at 70 mg/kg. Animals were sacrificed at the indicated time points and protein expression in tumors was evaluated by Western blotting. Two random tumors out of 4 for each time point are shown.
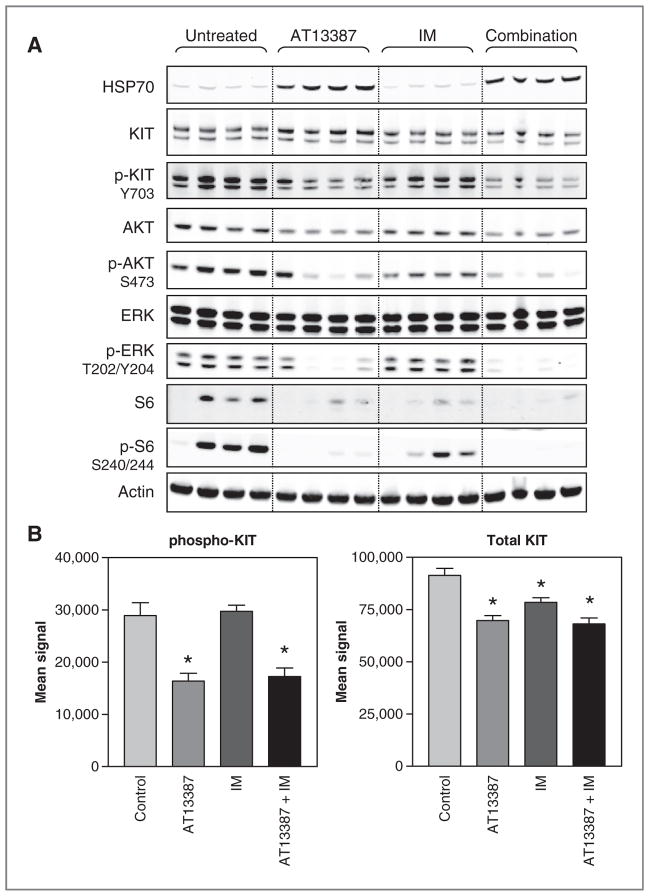
Figure 5.
Comparison of the effects of AT13387 and imatinib dosed individually or in combination on KIT signaling in xenograft tumors. Mice bearing GIST430 xenograft tumors were treated with AT13387 once at 70 mg/kg, imatinib (IM) twice at 50 mg/kg, or the combination of 2 treatments. This represents the day 2 of the efficacy studies. Animals were sacrificed at 24 hours after the first dose and tumors were evaluated by Western blotting (A). Total and phospho-KIT in the same tumor lysates were also analyzed by MSD (B). All data points represent the mean ± SE of 4 tumors from 4 individual animals.*, P < 0.05 versus control.
Sensitivity of the GIST430 xenograft to imatinib was tested by orally dosing the animals with imatinib at 50 mg/kg twice a day as described later.
AT13387 significantly inhibits growth of both imatinib-sensitive and -resistant xenograft models in vivo
The antitumor effects of AT13387 were tested at its maximum-tolerated dose of 70 mg/kg in a once-weekly dose schedule as a single agent as well as in combination with imatinib in both imatinib-sensitive (GIST-PSW) and imatinib-resistant (GIST430) xenograft models (Fig. 6). The GIST430 model has been described earlier. GIST-PSW is an imatinib-sensitive model with a primary KIT mutation in exon 11 (with p.K558_G656delinsR mutation; ref. 28).
Figure 6.
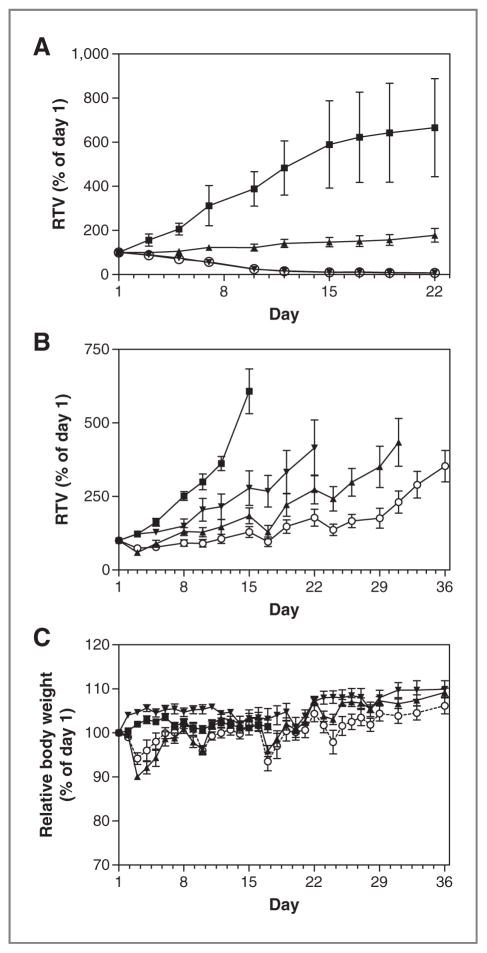
Antitumor activities of AT13387 and imatinib as single agents and in combination against imatinib-sensitive and -resistant GIST xenografts. Mice bearing GIST-PSW (A) and GIST430 (B) xenograft tumors were treated with AT13387 once a week intraperitoneally at 70 mg/kg (▲), imatinib (IM) twice a day orally at 50 mg/kg (▼), or a combination of AT13387 and imatinib (○). Control tumor-bearing animals received vehicle (■). Relative tumor volumes (RTV) are shown. Body weight changes in the GIST430 study are shown in C. All data points are mean ±SE of at least 7 tumors per animals. GIST430 data are representative of 2 studies.
In both xenograft models, AT13387 was given intraper-itoneally at 70 mg/kg once a week. AT13387 inhibited tumor growth in both models (P < 0.001 against the vehicle control; Fig. 6A and B). As expected, twice-daily oral treatment with imatinib at 50 mg/kg caused regression of the imatinib-sensitive GIST-PSW tumors during the 22-day treatment period, but not of the imatinib-resistant GIST430 tumors. Imatinib caused moderate but statistically significant reduction in GIST430 tumor growth (P < 0.001), although growth inhibition was not as marked as that caused by AT13387 treatment. However, combining AT13387 and imatinib enhanced tumor growth inhibition over the maximal effect of either of the monotherapies in the GIST430 xenograft.
Single-agent treatment with AT13387 caused mean body weight loss of 10% after the first dosing in SCID mice but this effect was transient, lasting no more than 6 days (Fig. 6C). Less body weight loss was observed on subsequent dosing. No significant tolerability issues were associated with imatinib monotherapy. Importantly, the combination of AT13387 and imatinib resulted in no increased toxicity in mice with either GIST-PSW or GIST430 tumors (Fig. 6C). In addition, at the end of the GIST430 xenograft study, livers of the animals were analyzed by hematoxylin and eosin staining to assess if any of the treatments had caused liver damage, such as those reported for IPI-493 (13). No histologic differences were observed between the animals in the 4 different treatment groups (data not shown), indicating none of the treatments had caused significant histologic hepatotoxicity.
KIT signaling is inhibited by AT13387 in xenograft tissues
The effect of AT13387 treatment on KIT signaling in xenograft tissue was investigated in both the GIST-PSW and GIST430 xenografts (Fig. 4). Analyses of protein levels by Western blotting in the xenograft tumors from mice treated with a single 70 mg/kg dose of AT13387 showed similar effects on KIT signaling to those seen in cell lines. A reduction in the phospho-forms of the client proteins, KIT and AKT, was seen in parallel with inhibition of downstream signaling through ERK and S6 for at least 24 hours in both models (Fig. 4). HSP70, an indicator of HSP90 inhibition, was induced over the same time period. This confirms that AT13387 brings about ablation of KIT signaling, as a result of HSP90 inhibition, in both imatinib-sensitive and -resistant xenografts with various KIT mutations and suggests that downregulation of signaling for this length of time is sufficient to trigger apoptosis and inhibition of tumor growth.
AT13387 treatment was also compared with imatinib as monotherapy and to the AT13387 and imatinib combination. As expected, imatinib treatment depleted phos-pho-KIT levels in the GIST-PSW tumors at 4 hours after the last dose on day 22, similarly to AT13387 treatment (Supplementary Fig. S2). In contrast, imatinib caused little effect on phospho-KIT and its signaling in GIST430 tumors (Fig. 5), confirming reduced sensitivity of these tumors to imatinib. In both models, the combination of AT13387 and imatinib caused a similar depletion of phos-pho-KIT and inhibition of downstream signaling to that seen with AT13387 alone (Fig. 5, Supplementary Fig. S2).
Discussion
Previous experiments in cell lines and in vivo models have suggested that HSP90 inhibition might be an effective treatment option for GIST (10–14). GIST subclones with various resistance mutations are selected during treatment with the currently available therapies, imatinib and sunitinib, and these can be heterogeneous within the same patient at different metastatic sites and even within the same tumor nodule (7). Imatinib-resistant GIST remains largely KIT-dependent and so targeting KIT via inhibition of its chaperone, HSP90, which is essential for stability of all mutant KIT proteins, is particularly attractive.
AT13387 is a novel, small-molecule inhibitor of HSP90 currently being evaluated in combination with imatinib, in a phase II GIST trial (ClinicalTrials.gov Identifier: NCT01294202). Here, we have shown that AT13387 is effective at inhibiting proliferation and KIT signaling, via the depletion of KIT, in both imatinib-sensitive and -resistant GIST cell lines in vitro. In addition, we have shown that AT13387 has significant antitumor activity in both imatinib-sensitive and -resistant xenograft models. This confirms that HSP90 inhibition is a viable treatment option for KIT-driven GIST regardless of secondary ima-tinib resistance mutations and highlights AT13387 as an excellent candidate for clinical use in this disease.
Inter- and intralesional heterogeneity of resistance mutations often coexist in the same patient (7). In such cases only some lesions may respond to an individual TKI whereas others remain resistant. AT13387 was effective against both the GIST430 and GIST48 cell lines, which have different resistance-inducing KIT mutations (V654A and D820A, respectively) confirming that effective HSP90 inhibition may have a distinct advantage over further TKIs for treatment in GIST once resistance has arisen. In addition to multiple resistant clones, imatinib-sensitive and -resistant clones can also coexist in the same patient (7, 11). A combination of AT13387 and imatinib therefore could provide benefit in targeting this heterogeneity, with imatinib active on the sensitive clones and AT13387 on those that are either sensitive or resistant. In our studies, AT13387 was well tolerated in combination with imatinib and in the imatinib-resistant GIST430 model the combination had improved efficacy over either of the monotherapies, supporting the idea of combining these therapies in the clinic. So far clinical trials have failed to prove that drug combinations achieve better results than a single agent TKI in GIST, but our preclinical data suggest that the combination of the HSP90 inhibitor, AT13387, and imatinib may be more successful.
Interestingly, AT13387 also still inhibited proliferation of cell lines that had developed resistance to 17-AAG, indicating that the resistance mechanism to the geldana-mycin analogues is not universal to all HSP90 inhibitors and can be circumvented by use of an alternative structural class.
Previously, the related geldanamycins, 17-AAG, retas-pimycin and IPI-493 have been tested in GIST models (11, 13, 14), but this class of inhibitors has shown limitations in the clinic due to formulation issues, namely adverse effects of Cremophor (31) or DMSO (32) included in the formulation of poorly soluble 17-AAG and hepa-totoxicity (15, 33). A retaspimycin phase III GIST trial was terminated early due to a number of toxicity issues, including liver failure that possibly led to excess mortality in one trial (16), and recent preclinical data on this drug showed liver damage in mice when combining with imatinib (14). Hence, there is a need for new compounds with a more favorable pharmacologic profile for testing in GIST and particularly for compounds that can be safely administered in combination with the first-line therapy, imatinib. AT13387 appears to be an excellent candidate as its combination with imatinib was well tolerated in mice with no apparent liver damage.
GIST in vivo models have proven difficult to establish and only 2 imatinib-resistant models have been previously described: GIST48, which has a secondary exon 17 resistance mutation, and GIST-BOE, which has a single-copy KIT with a mutation in exon 9 and exhibits relative (dose-dependent) resistance to imatinib (13, 28). Both models are slow growing and have higher success rates when serially transplanted. In contrast, the GIST430 xeno-graft described here can be more readily established by injecting cells cultured in vitro and the resulting tumors have a faster growth rate. The response of our GIST430 xenograft model to imatinib was markedly different to the regressions seen in the imatinib-sensitive GIST-PSW model. This confirms a level of imatinib resistance although a moderate response, rather than complete insensitivity, was observed. This result is consistent with our in vitro data with this cell line, where inhibition of proliferation can be observed at high concentrations of imatinib (Supplementary Fig. S1). Similarly, the previously described GIST-BOE and GIST48 xenografts were only partially resistant to imatinib, with treatment still resulting in stabilization of the xenograft tumors (13). The availability of another GIST xenograft model, with different secondary resistance mutations to the previously established GIST48, adds to the tools available for the future evaluation of potential GIST-active compounds.
GIST is not the only cancer where KIT activation is a key driver of disease (34). The majority of cases of adult systemic mastocytosis is associated with the D816V KIT gain-of-function mutation (35), whereas a proportion of core binding factor acute myeloid leukemias also have mutations at the 816 position or in exon 8 (36, 37). The D816V mutation, positioned in the kinase activation loop, causes KIT activation and confers resistance to imatinib (38). KIT has also been shown to be a therapeutic target in a subset of melanomas where KIT-activating mutations and overexpression have been observed (39, 40). Hence, HSP90 inhibitors such as AT13387 could have further uses in KIT-driven disease.
Overall, the effectiveness of AT13387 against both ima-tinib-sensitive and -resistant GIST and the fact that it is well tolerated in mice in combination with the first-line therapy, imatinib, suggest it is a promising candidate for use in this disease. These data as a whole strongly support the current testing of AT13387 in combination with ima-tinib in GIST clinical studies and validate the novel mechanism of combining an HSP90 inhibitor with TKIs in GIST.
Supplementary Material
Acknowledgments
The authors thank Rachel McMenamin, Jon Lewis, and Brent Graham for their help with cell culture, in vivo work, and scientific discussion and Maria Debiec-Rychter, who has supported the xenograft work in Leuven, Belgium.
Grant Support
J.A. Fletcher is supported in part by funding from GI SPORE 1P50CA127003, the Life Raft Group, the GIST Cancer Research Fund, and the Virginia and Daniel K. Ludwig Trust for Cancer Research.
Footnotes
Supplementary material for this article is available at Molecular Cancer Therapeutics Online (http://mct.aacrjournals.org/).
Disclosure of Potential Conflicts of Interest
T. Smyth, J.E. Curry, A.M. Rodriguez-Lopez, J. Lyons, N.T. Thompson, and N.G. Wallis are employees of Astex Pharmaceuticals. T.V. Looy, A. Wozniak, and P. Schöffski in Leuven, Belgium received research support from Astex Pharmaceuticals. P. Schöffski has also received research grants for GIST translational work from Novartis Pharmaceuticals and Infinity Pharmaceuticals. J.F. Lyons has a relationship with Onyx Pharmaceuticals. A. Wozniak received honoraria from Novartis. No potential conflicts of interest were disclosed by the other authors.
Authors’ Contributions
Conception and design: T. Smyth, T.V. Looy, J.E. Curry, A. Wozniak, J.A. Fletcher, P. Schoffski, J.F. Lyons, N.T. Thompson, N.G. Wallis
Development of methodology: T. Smyth, J.E. Curry, A.M. Rodriguez-Lopez, J.G. Morgan, M.M. Mayeda, P. Schoffski
Acquisition of data (provided animals, acquired and managed patients, provided facilities, etc.): T. Smyth, T.V. Looy, J.E. Curry, A. Wozniak, M. Zhu, M.M. Mayeda, J.A. Fletcher, P. Schoffski
Analysis and interpretation of data (e.g., statistical analysis, biostatistics, computational analysis): T. Smyth, T.V. Looy, J.E. Curry, A. Wozniak, M. Zhu, M.M. Mayeda, J.A. Fletcher, P. Schoffski, N.T. Thompson, N.G. Wallis
Writing, review, and/or revision of the manuscript: T. Smyth, T.V. Looy, J.E. Curry, A. Wozniak, J.A. Fletcher, P. Schoffski, J.F. Lyons, N.T. Thomp-son, N.G. Wallis
Administrative, technical, or material support (i.e., reporting or organizing data, constructing databases): T. Smyth, J.E. Curry, P. Schoffski
Study supervision: J.E. Curry, J.F. Lyons
Development of GIST cell lines: R. Donsky
References
- 1.Antonescu CR. The GIST paradigm: lessons for other kinase-driven cancers. J Pathol. 2011;223:251–61. doi: 10.1002/path.2798. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Hornick JL, Fletcher CD. The role of KIT in the management of patients with gastrointestinal stromal tumors. Hum Pathol. 2007;38:679–87. doi: 10.1016/j.humpath.2007.03.001. [DOI] [PubMed] [Google Scholar]
- 3.Hirota S, Isozaki K, Moriyama Y, Hashimoto K, Nishida T, Ishiguro S, et al. Gain-of-function mutations of c-kit in human gastrointestinal stromal tumors. Science. 1998;279:577–80. doi: 10.1126/science.279.5350.577. [DOI] [PubMed] [Google Scholar]
- 4.Debiec-Rychter M, Dumez H, Judson I, Wasag B, Verweij J, Brown M, et al. Use of c-KIT/PDGFRA mutational analysis to predict the clinical response to imatinib in patients with advanced gastrointestinal stromal tumours entered on phase I and II studies of the EORTC Soft Tissue and Bone Sarcoma Group. Eur J Cancer. 2004;40:689–95. doi: 10.1016/j.ejca.2003.11.025. [DOI] [PubMed] [Google Scholar]
- 5.Heinrich MC, Corless CL, Demetri GD, Blanke CD, von MM, Joensuu H, et al. Kinase mutations and imatinib response in patients with metastatic gastrointestinal stromal tumor. J Clin Oncol. 2003;21:4342–9. doi: 10.1200/JCO.2003.04.190. [DOI] [PubMed] [Google Scholar]
- 6.Gramza AW, Corless CL, Heinrich MC. Resistance to tyrosine kinase inhibitors in gastrointestinal stromal tumors. Clin Cancer Res. 2009;15:7510–8. doi: 10.1158/1078-0432.CCR-09-0190. [DOI] [PubMed] [Google Scholar]
- 7.Liegl B, Kepten I, Le C, Zhu M, Demetri GD, Heinrich MC, et al. Heterogeneity of kinase inhibitor resistance mechanisms in GIST. J Pathol. 2008;216:64–74. doi: 10.1002/path. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Csermely P, Schnaider T, Soti C, Prohaszka Z, Nardai G. The 90-kDa molecular chaperone family: structure, function, and clinical applications. A comprehensive review. Pharmacol Ther. 1998;79:129–68. doi: 10.1016/s0163-7258(98)00013-8. [DOI] [PubMed] [Google Scholar]
- 9.Solit DB, Rosen N. Hsp90: a novel target for cancer therapy. Curr Top Med Chem. 2006;6:1205–14. doi: 10.2174/156802606777812068. [DOI] [PubMed] [Google Scholar]
- 10.Fumo G, Akin C, Metcalfe DD, Neckers L. 17-Allylamino-17-demethoxygeldanamycin (17-AAG) is effective in down-regulating mutated, constitutively activated KIT protein in human mast cells. Blood. 2004;103:1078–84. doi: 10.1182/blood-2003-07-2477. [DOI] [PubMed] [Google Scholar]
- 11.Bauer S, Yu LK, Demetri GD, Fletcher JA. Heat shock protein 90 inhibition in imatinib-resistant gastrointestinal stromal tumor. Cancer Res. 2006;66:9153–61. doi: 10.1158/0008-5472.CAN-06-0165. [DOI] [PubMed] [Google Scholar]
- 12.Dewaele B, Wasag B, Cools J, Sciot R, Prenen H, Vandenberghe P, et al. Activity of dasatinib, a dual SRC/ABL kinase inhibitor, and IPI-504, a heat shock protein 90 inhibitor, against gastrointestinal stromal tumor-associated PDGFRAD842V mutation. Clin Cancer Res. 2008;14:5749–58. doi: 10.1158/1078-0432.CCR-08-0533. [DOI] [PubMed] [Google Scholar]
- 13.Floris G, Sciot R, Wozniak A, Van LT, Wellens J, Faa G, et al. The novel heat shock protein 90 inhibitor, IPI-493, is highly effective in human gastrostrointestinal stromal tumor (GIST) xenografts carrying heterogeneous KIT mutations. Clin Cancer Res. 2011;17:5604–14. doi: 10.1158/1078-0432.CCR-11-0562. [DOI] [PubMed] [Google Scholar]
- 14.Floris G, Debiec-Rychter M, Wozniak A, Stefan C, Normant E, Faa G, et al. The heat shock protein 90 inhibitor IPI-504 induces KIT degradation, tumor shrinkage and cell proliferation arrest in xenografts models of gastrointestinal stromal tumors. Mol Cancer Ther. 2011;10:1897–908. doi: 10.1158/1535-7163.MCT-11-0148. [DOI] [PubMed] [Google Scholar]
- 15.Biamonte MA, Van de Water R, Arndt JW, Scannevin RH, Perret D, Lee WC. Heat shock protein 90: inhibitors in clinical trials. J Med Chem. 2010;53:3–17. doi: 10.1021/jm9004708. [DOI] [PubMed] [Google Scholar]
- 16.Demetri GD, Le Cesne A, Von Mehren M, Chmielowski B, Bauer S, Chow WA, et al. Final results from a phase III study of IPI-504 (retas-pimycin hydrochloride) versus placebo in patients (pts) with gastrointestinal stromal tumors (GIST) following failure of kinase inhibitor therapies [abstract]. Proceedings of the Gastrointestinal Cancers Symposium; 2010 Jan 22; Alexandria, VA: American Society of Clinical Oncology; 2010. [Google Scholar]
- 17.Graham B, Curry J, Smyth T, Fazal L, Feltell R, Harada I, et al. The HSP90 inhibitor, AT13387, displays a long duration of action in vitro and in vivo in non-small cell lung cancer. Cancer Sci. 2011;103:522–7. doi: 10.1111/j.1349-7006.2011.02191.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Woodhead AJ, Angove H, Carr MG, Chessari G, Congreve M, Coyle JE, et al. Discovery of (2,4-dihydroxy-5-isopropylphenyl)-[5-(4-methylpi-perazin-1-ylmethyl)-1,3-dihydroisoindol-2-yl]methanone (AT13387), a novel inhibitor of the molecular chaperone Hsp90 by fragment based drug design. J Med Chem. 2010;53:5956–69. doi: 10.1021/jm100060b. [DOI] [PubMed] [Google Scholar]
- 19.Taguchi T, Sonobe H, Toyonaga S, Yamasaki I, Shuin T, Takano A, et al. Conventional and molecular cytogenetic characterization of a new human cell line, GIST-T1, established from gastrointestinal stro-mal tumor. Lab Invest. 2002;82:663–5. doi: 10.1038/labinvest.3780461. [DOI] [PubMed] [Google Scholar]
- 20.Gunaratnam M, Swank S, Haider SM, Galesa K, Reszka AP, Beltran M, et al. Targeting human gastrointestinal stromal tumor cells with a quadruplex-binding small molecule. J Med Chem. 2009;52:3774–83. doi: 10.1021/jm900424a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Heinrich MC, Corless CL, Blanke CD, Demetri GD, Joensuu H, Roberts PJ, et al. Molecular correlates of imatinib resistance in gastrointestinal stromal tumors. J Clin Oncol. 2006;24:4764–74. doi: 10.1200/JCO.2006.06.2265. [DOI] [PubMed] [Google Scholar]
- 22.Lassus H, Sihto H, Leminen A, Nordling S, Joensuu H, Nupponen NN, et al. Genetic alterations and protein expression of KIT and PDGFRA in serous ovarian carcinoma. Br J Cancer. 2004;91:2048–55. doi: 10.1038/sj.bjc.6602252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.McLean SR, Gana-Weisz M, Hartzoulakis B, Frow R, Whelan J, Sel-wood D, et al. Imatinib binding and cKIT inhibition is abrogated by the cKIT kinase domain I missense mutation Val654Ala. Mol Cancer Ther. 2005;4:2005–15. doi: 10.1158/1535-7163.MCT-05-0070. [DOI] [PubMed] [Google Scholar]
- 24.Squires MS, Feltell RE, Wallis NG, Lewis EJ, Smith DM, Cross DM, et al. Biological characterization of AT7519, a small-molecule inhibitor of cyclin-dependent kinases, in human tumor cell lines. Mol Cancer Ther. 2009;8:324–32. doi: 10.1158/1535-7163.MCT-08-0890. [DOI] [PubMed] [Google Scholar]
- 25.Curry J, Angove H, Fazal L, Lyons J, Reule M, Thompson N, et al. Aurora B kinase inhibition in mitosis: strategies for optimising the use of aurora kinase inhibitors such as AT9283. Cell Cycle. 2009;8:1921–9. doi: 10.4161/cc.8.12.8741. [DOI] [PubMed] [Google Scholar]
- 26.Workman P, Aboagye EO, Balkwill F, Balmain A, Bruder G, Chaplin DJ, et al. Guidelines for the welfare and use of animals in cancer research. Br J Cancer. 2010;102:1555–77. doi: 10.1038/sj.bjc.6605642. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Hollands C. The Animals (scientific procedures) Act 1986. Lancet. 1986;2:32–3. doi: 10.1016/s0140-6736(86)92571-7. [DOI] [PubMed] [Google Scholar]
- 28.Floris G, Debiec-Rychter M, Sciot R, Stefan C, Fieuws S, Machiels K, et al. High efficacy of panobinostat towards human gastrointestinal stromal tumors in a xenograft mouse model. Clin Cancer Res. 2009;15:4066–76. doi: 10.1158/1078-0432.CCR-08-2588. [DOI] [PubMed] [Google Scholar]
- 29.Banerji U, Walton M, Raynaud F, Grimshaw R, Kelland L, Valenti M, et al. Pharmacokinetic-pharmacodynamic relationships for the heat shock protein 90 molecular chaperone inhibitor 17-allylamino, 17-demethoxygeldanamycin in human ovarian cancer xenograft models. Clin Cancer Res. 2005;11:7023–32. doi: 10.1158/1078-0432.CCR-05-0518. [DOI] [PubMed] [Google Scholar]
- 30.Penner CR, Folpe AL, Budnick SD. C-kit expression distinguishes salivary gland adenoid cystic carcinoma from polymorphous low-grade adenocarcinoma. Mod Pathol. 2002;15:687–91. [Google Scholar]
- 31.Modi S, Stopeck AT, Gordon MS, Mendelson D, Solit DB, Bagatell R, et al. Combination of trastuzumab and tanespimycin (17-AAG, KOS-953) is safe and active in trastuzumab-refractory HER-2 overexpres-sing breast cancer: a phase I dose-escalation study. J Clin Oncol. 2007;25:5410–7. doi: 10.1200/JCO.2007.11.7960. [DOI] [PubMed] [Google Scholar]
- 32.Solit DB, Osman I, Polsky D, Panageas KS, Daud A, Goydos JS, et al. Phase II trial of 17-allylamino-17-demethoxygeldanamycin in patients with metastatic melanoma. Clin Cancer Res. 2008;14:8302–7. doi: 10.1158/1078-0432.CCR-08-1002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Solit DB, Ivy SP, Kopil C, Sikorski R, Morris MJ, Slovin SF, et al. Phase I trial of 17-allylamino-17-demethoxygeldanamycin in patients with advanced cancer. Clin Cancer Res. 2007;13:1775–82. doi: 10.1158/1078-0432.CCR-06-1863. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Heinrich MC, Blanke CD, Druker BJ, Corless CL. Inhibition of KIT tyrosine kinase activity: a novel molecular approach to the treatment of KIT-positive malignancies. J Clin Oncol. 2002;20:1692–703. doi: 10.1200/JCO.2002.20.6.1692. [DOI] [PubMed] [Google Scholar]
- 35.Lim KH, Tefferi A, Lasho TL, Finke C, Patnaik M, Butterfield JH, et al. Systemic mastocytosis in 342 consecutive adults: survival studies and prognostic factors. Blood. 2009;113:5727–36. doi: 10.1182/blood-2009-02-205237. [DOI] [PubMed] [Google Scholar]
- 36.Paschka P, Marcucci G, Ruppert AS, Mrozek K, Chen H, Kittles RA, et al. Adverse prognostic significance of KIT mutations in adult acute myeloid leukemia with inv(16) and t(8;21): a Cancer and Leukemia Group B study. J Clin Oncol. 2006;24:3904–11. doi: 10.1200/JCO.2006.06.9500. [DOI] [PubMed] [Google Scholar]
- 37.Schnittger S, Kohl TM, Haferlach T, Kern W, Hiddemann W, Spieker-mann K, et al. KIT-D816 mutations in AML1-ETO-positive AML are associated with impaired event-free and overall survival. Blood. 2006;107:1791–9. doi: 10.1182/blood-2005-04-1466. [DOI] [PubMed] [Google Scholar]
- 38.Ma Y, Zeng S, Metcalfe DD, Akin C, Dimitrijevic S, Butterfield JH, et al. The c-KIT mutation causing human mastocytosis is resistant to STI571 and other KIT kinase inhibitors; kinases with enzymatic site mutations show different inhibitor sensitivity profiles than wild-type kinases and those with regulatory-type mutations. Blood. 2002;99:1741–4. doi: 10.1182/blood.v99.5.1741. [DOI] [PubMed] [Google Scholar]
- 39.Went PT, Dirnhofer S, Bundi M, Mirlacher M, Schraml P, Mangialaio S, et al. Prevalence of KIT expression in human tumors. J Clin Oncol. 2004;22:4514–22. doi: 10.1200/JCO.2004.10.125. [DOI] [PubMed] [Google Scholar]
- 40.Woodman SE, Davies MA. Targeting KIT in melanoma: a paradigm of molecular medicine and targeted therapeutics. Biochem Pharmacol. 2010;80:568–74. doi: 10.1016/j.bcp.2010.04.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.