Abstract
Background
Cutaneous metastases from internal malignancies are uncommon. Moreover, endometrial carcinoma rarely metastasizes to the skin, with a reported prevalence of 0.8%. Here, we report the case of a 62-year-old woman who developed cutaneous metastases from an endometrial carcinoma.
Case presentation
When admitted to our department, the patient underwent a biopsy that showed the presence of cutaneous metastasis in relation to her initial endometrial cancer, diagnosed 3 years earlier. Thereafter, she was treated with a bilateral uterine artery embolization and chemotherapy. The patient had complications and survived 5 months after the diagnosis of the cutaneous metastasis. She died from sepsis.
Conclusion
Cutaneous metastases of the endometrial carcinoma are usually incurable and suggest an unfortunate prognosis where palliation is the mainstay of patient management.
Keywords: Bleeding, Cutaneous metastasis, Endometrial carcinoma, Lesions
Background
Cutaneous metastases may occur as the initial manifestation of internal malignancy or late in the course of the disease. The incidence of cutaneous metastases in internal malignancies has been reported between 0.7% and 10% [1]. Incidence of various tumors metastasizing to the skin correlates with the sex-wise frequency of occurrence of various primary malignancies. As such, lung cancer (1.7 to 3.1%) and breast cancer (23.9%) are the commonest epithelial malignancies metastasizing to the skin in men and women respectively [2-4]. Furthermore, although endometrial carcinoma is the most common gynecological cancer, it rarely metastasizes to the skin, with a reported prevalence of 0.8% [5].
In this paper, we report the case of a 62-year-old woman with cutaneous metastasis of the endometrial cancer and describe the clinical and pathological features. We also discuss the clinical and histopathological criteria for the differential diagnosis of cutaneous paraneoplastic lesions and cutaneous metastases. Moreover, we review the literature to determine and to evaluate both the prognostic significance and the frequency of this disease.
Case presentation
A 62-year-old female, Gravida 2, Para 2, was referred to our department for itching with identifiable pelvic cutaneous lesions. She was menopausal at age 51 years. Clinical examination revealed the presence of cutaneous papulo-bullous lesions located at the site of the laparotomy incision extending down to the vulva and up to the umbilicus (Figure 1). No pelvic mass was palpated on physical examination. A thoraco-abdomino-pelvic computed tomography scan was performed and showed no abnormality.
Figure 1.
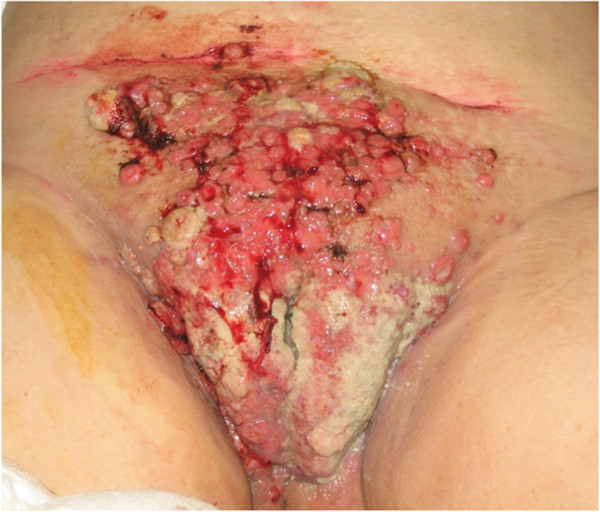
Cutaneous metastasis of the endometrial carcinoma.
The patient’s previous gynecological history revealed that she was diagnosed 3 years earlier with endometrial carcinoma. The histological type of the endometrial adenocarcinoma was endometrioid and defined as Féderation Internationale de Gynécologie et d’Obstétrique stage 1B, grade 2. A total hysterectomy, bilateral salpingo-oophorectomy, and pelvic lymph node dissection were performed. Pelvic lymph nodes were negative (seven nodes). The patient did not undergo any para-aortic lymphadenectomy. External pelvic radiotherapy was prescribed, leading to a complete clinical response. During the last 3 years, the patient had regular check-ups with her oncologist outside our institution.
When admitted to our department, the patient underwent a biopsy that showed the presence of cutaneous metastasis in relation to her initial cancer (Figure 2). Thereafter, she had a bilateral uterine artery embolization because of important vaginal bleeding, and four cycles of salvage chemotherapy with etoposide (VP-16) and cisplatin [6-8].
Figure 2.
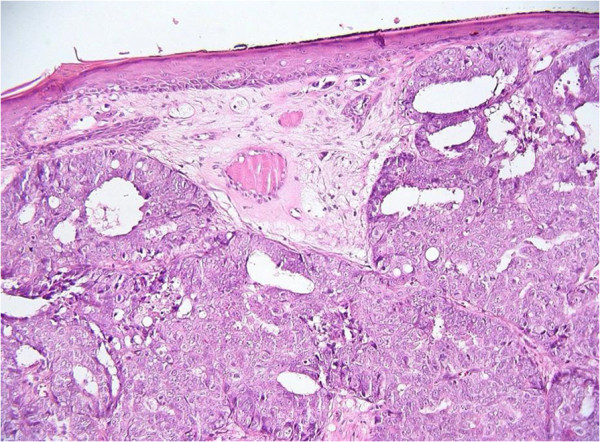
Histopathological findings of the skin nodule. Medium-power photomicrograph (original magnification, ×200; hematoxylin and eosin stain) of the skin nodule, showing a moderately differentiated endometrioid adenocarcinoma.
Three months later, the patient was transfused with 3 units of packed red blood cells because of recurrent massive vaginal bleeding. She then underwent pelvic magnetic resonance imaging, which revealed the presence of a pelvic tumor mass of 15 cm × 8 cm × 4 cm invading the bladder and rectum. However, pelvic computed tomography angiography identified no signs of active arterial bleeding. The last months of the patient were marked by episodes of urinary tract and scar incision infections (extended-spectrum beta-lactamase Escherichia coli and Pseudomonas aeruginosa) treated with antibiotics. The patient died 2 months later from sepsis.
Conclusions
Distinguishing between cutaneous paraneoplastic lesions and those due to invasion of the skin through hematogeneous spread, lymphatic spread or by direct extension from a primary tumor is essential. Paraneoplastic dermatoses follow the evolution of the underlying cancer and are due to the production by the tumor of hormones, cytokines and growth factors [9].
Histopathologically, the type of endometrial carcinoma that disseminated to the skin was endometrioid adenocarcinoma in our case – as the majority of studies that analyze skin metastases from endometrial cancer also report [10]. A mixed Müllerian tumor has also been reported. In these lesions, neoplastic cells appear to be arranged in a gland-like pattern within the dermis with different grades of atypia. Metastatic skin disease from uterine papillary serous carcinoma, an aggressive variant of endometrial carcinoma mimicking serous papillary ovarian carcinoma, has been documented [10]. Clinically, cutaneous metastasis can take any form of lesions including nodules, papules, ulcers, plaques and tumors, with usually four histopathological forms involving the dermis; namely, nodular, infiltrative, diffuse and intravascular [11]. These lesions may be the only manifestation of an underlying visceral cancer.
The frequency of cutaneous metastasis is correlated to the frequency of each malignancy, which is why women with cutaneous metastases most frequently have the following primary malignancies: breast, ovary, oral cavity, lung and large intestine [10]. Globally, cutaneous metastases represent 2% of all cutaneous neoplasms [12].
Although endometrial carcinoma is the most common gynecological cancer, it only rarely metastasizes to the skin, with a reported prevalence of 0.8% [5]. A systematic English-language literature search on PubMed between 1966 and 2013 using the terms ‘endometrial carcinoma’, ‘skin’, ‘cutaneous’, ‘metastasis’ and ‘spread’ identified only 26 cases of cutaneous metastasis in endometrial cancer [13-19]. Most of these reports emphasize the rarity of this pattern of dissemination. Four points illustrated in the current case deserve to be discussed.
The strategy for cancer treatment and management in cutaneous metastases is to determine the tumor origin, which is achievable with tissue biopsy of the metastatic nodule. However, this may prove unhelpful in settings where patients would have visited several hospitals and had surgeries without histological diagnosis or confirmation of excised lesions [14]. Contrarily, in our case, biopsy enabled us to confirm the presence of cutaneous metastasis in relation to the initial endometrial cancer of the patient.
Secondly, in the reported cases, metastasis from endometrial cancer has been most commonly noted at the site of initial surgery [16], such as in our patient. The initial surgical and radiotherapy site must therefore be examined carefully for skin metastasis. More rarely, distant cutaneous sites, including scalp, toes and trunk, have been reported [20,21].
Thirdly, cutaneous metastases may vary in numberfrom a single nodule to greater than 20 lesions, similarly to our case.
Finally, cutaneous metastases of the endometrial carcinoma are associated with poor prognosis and a mean life expectancy reported as approximately 4 to 12 months after the diagnosis of cutaneous metastasis [22,23]. The factor influencing survival is the time elapsed between diagnosis and the appearance of the skin recurrences [24]. Furthermore, to date there are no treatments with proven efficacy because of the rarity of this specific site of metastasis. The treatment for most patients is palliative, and although chemotherapy and radiotherapy are often used in these patients, they are ineffective in many cases [25]. In our case, the patient underwent embolization and etoposide and cisplatin chemotherapy. Etoposide and cisplatin regimen was prescribed given that these two agents are more efficacious than the carboplatin and paclitaxel regimen in the treatment of advanced endometrial cancer, as reported by Olawaiye and colleagues in 2012 [6]. Kim and colleagues [7] and Pierga and colleagues [8] also demonstrated that the combination of etoposide and cisplatin is an effective regimen with an acceptable toxicity in patients with recurrent or metastatic endometrial carcinoma. Moreover, we considered that the patient had a second-line treatment for metastatic endometrial carcinoma, although the standard of care for women who present with locally advanced or who experience relapse with metastatic disease is to proceed with an anthracycline, taxane and platinum combination [26]. However, the disease evolution was rapidly fatal and the patient died 5 months after her admission to our department due to sepsis.
In conclusion, both our case and the other cases reported in the literature demonstrate that the appearance of a cutaneous lesion at the site of initial surgery a few years or months after the diagnosis of endometrial carcinoma could represent widespread dissemination of the primary tumor. These cutaneous metastases herald a poor prognosis and fatal evolution.
Consent
Consent for publication for the patient was sought from the next of kin of the patient. A copy of the written consent is available for review by the Editor-in-Chief of this journal to be published.
Competing interests
The authors declare that they have no competing interests.
Authors’ contributions
DA participated in the treatment and follow-up of the patient, and wrote the article. SN and DA participated in the histopathological diagnosis. NeK, FL, JS, CS and LB participated in the writing of article. All authors read and approved the final manuscript.
Contributor Information
David Atallah, Email: david.atallah@gmail.com.
Nadine el Kassis, Email: nadine.kassis@gmail.com.
Fouad Lutfallah, Email: fouadlutfallah@gmail.com.
Joelle Safi, Email: joelle.safi@gmail.com.
Charbel Salameh, Email: parasympathique@yahoo.com.
Samah Nadiri, Email: samah.nadiri@hdf.usj.edu.lb.
Lina Bejjani, Email: linabejjani@hotmail.com.
References
- Wollina U, Graefe T, Konrad H, Schönlebe J, Koch A, Hansel G, Haroske G, Köstler E. Cutaneous metastases of internal cancer. Acta Derma Venereol APA. 2004;13:9–84. [Google Scholar]
- Marcoval J, Penín RM, Llatjós R, Martínez-Ballarín I. Cutaneous metastasis from lung cancer: retrospective analysis of 30 patients. Australas J Dermatol. 2012;53:288–290. doi: 10.1111/j.1440-0960.2011.00828.x. [DOI] [PubMed] [Google Scholar]
- Krathen RA, Orengo IF, Rosen T. Cutaneous metastasis: a meta-analysis of data. South Med J. 2003;96:164–167. doi: 10.1097/01.SMJ.0000053676.73249.E5. [DOI] [PubMed] [Google Scholar]
- Barbetakis N, Paliouras D, Asteriou C, Samanidis G, Kleontas A, Anestakis D, Kaplanis K, Tsilikas C. Cutaneous skull metastasis from uterine leiomyosarcoma: a case report. World J Surg Oncol. 2009;7:45. doi: 10.1186/1477-7819-7-45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lerner LB, Andrews SJ, Gonzalez JL, Heaney JA, Currie JL. Vulvar metastases secondary to transitional cell carcinoma of the bladder: a case report. J Reprod Med. 1999;44:729–732. [PubMed] [Google Scholar]
- Olawaiye AB, Godoy HE, Shahzad MM, Rauh-Hain JA, Lele SB, Odunsi K. Comparison of outcomes in patients treated with multi-agent regiments of cisplatin, adriamycin, and VP-16 versus carboplatin and paclitaxel for advanced and recurrent endometrial cancer. Eur J Gynaecol Oncol. 2012;33:477–479. [PMC free article] [PubMed] [Google Scholar]
- Kim JH, Lee SJ, Bae JH, Lee SH, Bae SN, Namkoong SE, Park JS. Adjuvant therapy in high-risk early endometrial carcinoma: a retrospective analysis of 46 cases. J Gynecol Oncol. 2008;19:236–240. doi: 10.3802/jgo.2008.19.4.236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pierga JY1, Dieras V, Paraiso D, Dorval T, Palangie T, Beuzeboc P, Jouve M, Scholl SM, Garcia-Giralt E, Pouillart P. Treatment of advanced or recurrent endometrial carcinoma with combination of etoposide, cisplatin, and 5-fluorouracil: a phase II study. Gynecol Oncol. 1996;60:59–63. doi: 10.1006/gyno.1996.0012. [DOI] [PubMed] [Google Scholar]
- da Silva JA, Mesquita Kde C, Igreja AC, Lucas IC, Freitas AF, de Oliveira SM, Costa IM, Campbell IT. Paraneoplastic cutaneous manifestations: concepts and updates. An Bras Dermatol. 2013;88:9–22. doi: 10.1590/S0365-05962013000100001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alcaraz I, Cerroni L, Rütten A, Kutzner H, Requena L. Cutaneous metastases from internal malignancies: a clinicopathologic and immunohistochemical review. Am J Dermatopathol. 2012;34:347–393. doi: 10.1097/DAD.0b013e31823069cf. [DOI] [PubMed] [Google Scholar]
- Fernandez-Flores A. Cutaneous metastases: a study of 78 biopsies from 69 patients. Am J Dermatopathol. 2010;32:222–239. doi: 10.1097/DAD.0b013e3181b348f8. [DOI] [PubMed] [Google Scholar]
- Nashan D, Meiss F, Braun-Falco M, Reichenberger S. Cutaneous metastases from internal malignancies. Dermatol Therapy. 2010;23:567–580. doi: 10.1111/j.1529-8019.2010.01364.x. [DOI] [PubMed] [Google Scholar]
- Giordano G, Gnetti L, Melpignano M. Endometrial carcinoma metastatic to the vulva: a case report and review of the literature. Pathol Res Pract. 2005;201:751–756. doi: 10.1016/j.prp.2005.08.006. [DOI] [PubMed] [Google Scholar]
- Samaila MO, Adesiyun AG, Waziri GD, Koledade KA, Kolawole AO. Cutaneous umbilical metastases in post-menopausal females with gynaecological malignancies. J Turkish-German Gynecol Assoc. 2012;13:204–207. doi: 10.5152/jtgga.2012.29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Temkin SM, Hellman M, Lee YC, Bulafia O. Surgical resection of vulvar metastases of endometrial cancer: a presentation of two cases. J Low Genit Tract Dis. 2007;11:118–121. doi: 10.1097/01.lgt.0000236970.35125.4b. [DOI] [PubMed] [Google Scholar]
- Baydar M, Dikilitas M, Sevinc A, Senel S, Senel F, Aydogdu I. Cutaneous metastasis of endometrial carcinoma with hemorrhagic nodules and papules. Eur J Gynaecol Oncol. 2005;26:464–465. [PubMed] [Google Scholar]
- El M’rabet FZ, Hottinger A, George AC. Cutaneous metastasis of endometrial carcinoma: a case report and literature review. J Clin Gynecol Obstet. 2012;1:19–23. [Google Scholar]
- Stonard CM, Manek S. Cutaneous metastasis from an endometrial carcinoma: a case history and review of the literature. Histopathology. 2003;43:201–203. doi: 10.1046/j.1365-2559.2003.01616.x. [DOI] [PubMed] [Google Scholar]
- Damewood MD, Rosenshein NB, Grumbine FC, Parmley TH. Cutaneous metastasis of endometrial carcinoma. Cancer. 1980;46:1471–1475. doi: 10.1002/1097-0142(19800915)46:6<1471::AID-CNCR2820460629>3.0.CO;2-S. [DOI] [PubMed] [Google Scholar]
- Mandrekas DP, Dimopoulos AM, Moulopoulou D, Papamichalis G, Gougoulakis AG. Distant cutaneous metastasis from carcinoma of the uterus. A case report. Eur J Gynaecol Oncol. 1999;20:212–213. [PubMed] [Google Scholar]
- Giardina VN, Morton BF, Potter GK, Mesa-Tejada R, Waterfield WC. Metastatic endometrial adenocarcinoma to the skin of a toe. Am J Dermatopathol. 1996;18:94–98. doi: 10.1097/00000372-199602000-00016. [DOI] [PubMed] [Google Scholar]
- Elit L, Lukka H, Friedman E. Cutaneous metastasis of papillary serous uterine cancer. Gynecol Oncol. 2001;82:208–211. doi: 10.1006/gyno.2001.6224. [DOI] [PubMed] [Google Scholar]
- Yilmaz Z, Bese T, Demirkiran F. Skin metastasis in ovarian carcinoma. Int J Gynecol Cancer. 2001;16(Suppl 1):414–418. doi: 10.1111/j.1525-1438.2006.00486.x. [DOI] [PubMed] [Google Scholar]
- Cormio G, Capotorto M, Di Vagno G, Cazzolla A, Carriero C, Selvaggi L. Skin metastases in ovarian carcinoma: a report of nine cases and a review of the literature. Gynecol Oncol. 2003;90:682–685. doi: 10.1016/S0090-8258(03)00400-1. [DOI] [PubMed] [Google Scholar]
- Mustafa MS, Al-Nuaim L, Inayat-Ur-Rahman N. Scalp and cranial bone metastasis of endometrial carcinoma: a case report and literature review. Gynecol Oncol. 2001;81:105–109. doi: 10.1006/gyno.2000.6038. [DOI] [PubMed] [Google Scholar]
- Dizon DS, Blessing JA, McMeekin DS, Sharma SK, Disilvestro P, Alvarez RD. Phase II trial of ixabepilone as second-line treatment in advanced endometrial cancer: gynecologic oncology group trial 129-P. J Clin Oncol. 2009;27:3104–3108. doi: 10.1200/JCO.2008.20.6995. [DOI] [PMC free article] [PubMed] [Google Scholar]


