Abstract
Background
Determining a disease's impact on life quality is important in clinical decision making, research, and resource allocation. Determinants of quality of life (QOL) in morphea are poorly understood.
Objective
We sought to ascertain demographic and clinical variables correlated with negative impact on self-reported QOL in morphea.
Methods
We conducted a cross-sectional survey of the Morphea in Adults and Children cohort.
Results
Symptoms (pruritus and pain) and functional impairment were correlated with decreased QOL in children and adults. This was true in both sexes and was independent of subtype and age. Patient-reported QOL correlated with physician-based measures of disease severity in adults, but not in children. Patients with linear and generalized morphea had the greatest impact on QOL.
Limitations
Small sample size is a limitation.
Conclusion
Symptoms and functional impairment were determinants of impaired life quality in both children and adults independent of morphea subtype. These results suggest that clinicians should consider suppressing the accumulation of new lesions (when rapidly accumulating) and symptoms (pain and pruritus) in the treatment of patients with morphea.
Keywords: life quality or quality of life, localized scleroderma, morphea, Morphea in Adults and Children cohort, outcomes or disease outcomes
Morphea (localized scleroderma) is an inflammatory skin disorder characterized by sclerosis of the dermis and subcutaneous tissue that may cause functional and cosmetic impairment.1-4 Most outcome studies in morphea have focused on physician-based or imaging measures, such as skin thickness, dermal collagen content, or the ability to pinch move skin.5-8 Little is known about disease-related quality of life (QOL) or demographic and clinical features associated with patient-reported outcomes.
Most QOL studies in morphea have focused on children and report mild to moderate impact.9-11 However, current studies have small sample size and limited information about patient demographics or clinical features. Even fewer studies examine the impact of morphea in adults. To date, Kroft et al12,13 are the only authors to report the psychological and physical impact of morphea in adults; however, their cohort comprises only 74 patients. This gap in knowledge may hinder optimal patient care because physician-based measures may not adequately reflect patients' perceptions of severity and therapeutic outcomes.
This study, the Morphea in Adults and Children (MAC) cohort, was designed to examine demographic, clinical, and autoimmune features of morphea. By studying patients prospectively, we aimed to define the effect of such features on clinical outcomes. The objective of this study was to determine impact of morphea on life quality using the Dermatology Life Quality Index (DLQI) and Children's DLQI (CDLQI). We examined the association among patient demographics, clinical characteristics, and disease-specific variables on QOL in 277 pediatric and adult patients.
Methods
Patients and procedures
Patients
The institutional review board-approved MAC cohort contained 322 adults (≥ 18 years old at enrollment) and children (≤ 17 years old at enrollment) as of March 2012 and is in compliance with the Declaration of Helsinki principles. Patients were recruited September 2007 through March 2012. Inclusion criteria were: eligibility for enrollment in MAC cohort, age 4 years or older at enrollment, presence of sufficient information for analysis, determinate morphea subtype, and English language/literacy skills. Of 322 patients, 45 were excluded for: age younger than 4 years (2), language/literacy skills (4), insufficient data on variables of interest (28), and indeterminate morphea subtype (11). The cohort was designed to capture prevalent and incident cases of morphea. Patients were recruited from within the University of Texas Southwestern Medical Center system encompassing 2 dedicated pediatric care facilities, a county hospital, and a faculty-based clinic practice. Patients were also enrolled through regional referrals.
Variables
The MAC database contains the following domains: demographic, clinical, medical history, immunologic, immunogenetic, DLQI for adults age 18 years or older or CDLQI for children age 17 years or younger, physician-based determinants of disease severity (modified Rodnan skin score [mRSS] and Localized Scleroderma Cutaneous Assessment Tool [LoSCAT]),6,14 and functional status (presence/absence of limited range of motion, contracture, limb length discrepancy). The availability of the recently validated LoSCA6 allowed comparison of patient-based and physician-based evaluations. The mRSS was included because the LoSCAT was not available at the inception of the cohort and is the only clinical score available for all patients in the MAC cohort. The LoSCAT was administered beginning with patient number 182. These variables were assessed through direct physician interview/examination and validated questionnaires. Data are abstracted using a comprehensive clinical report form. This form (designed before the study) includes the DLQI and CDLQI questionnaires. Medical records are reviewed for confirmation of patient-reported findings. Based on criteria established by Laxer and Zulian15 (Table I), all patients are assigned subtypes and scores by a single clinician with expertise in morphea (H. J.).
Table I. Preliminary proposed classification of morphea.
| Main group | Subtype | Description |
|---|---|---|
| (1) Circumscribed morphea (single or multiple) |
|
Oval or round circumscribed areas of induration limited to epidermis and dermis |
| Oval or round circumscribed deep induration of the skin involving subcutaneous tissue extending to fascia and may involve underlying muscle. The lesions can be single or multiple | ||
| (2) Linear morphea |
|
Linear induration involving dermis, subcutaneous tissue and, sometimes, muscle and underlying bone and affecting the limbs and the trunk. |
| En coup de sabre. Linear induration that affects the face and the scalp and sometimes involves muscle and underlying bone. | ||
| Parry Romberg or progressive hemifacial atrophy. Loss of tissue on 1 side of the face. Both may involve dermis, subcutaneous tissue, muscle, and/or bone. | ||
| (3) Generalized morphea | Induration of the skin starting as individual plaques (≥4 and >3 cm) that become confluent and involve at least 2 of 7 anatomic sites. | |
| (4) Pansclerotic morphea* | Circumferential involvement of limb(s) affecting the skin, subcutaneous tissue, muscle, and bone. | |
| (5) Mixed morphea | Combination of ≥ 2 of the previous subtypes. |
Adapted from Laxer and Zulian.15
Disease-related QOL over the preceding 7 days was measured with the 10-item validated DLQI, adapted with permission from Dr Finlay. Administration of the DLQI versus the CDLQI was determined by prior publications in which the DLQI was validated for adults age 18 years or older.16 Children were given either cartoon or text versions of the CDLQI as appropriate for their age.17,18, DLQI and CDLQI were chosen despite their unidimensional features because they were the only measures validated for all age groups across the MAC cohort. Further, the DLQI is still considered a valuable tool for determining patient-based outcomes, as it accurately reflects symptoms and feelings, leisure, school/work, personal relationships, and treatments similar to multidimensional instruments.16,19,20 DLQI and CDLQI (range 0-30, with 30 reflecting greatest effect) were calculated for each patient and subdivided according to previously published cutoff values: 0 to 1 (no effect); 2 to 5 (small effect); 6 to 10 (moderate effect); 11 to 20 (very large effect); and 21 to 30 (extremely large effect).21
Physical symptoms of itch, pain, numbness/tingling, and sensation of skin tightness were assessed on a visual analog scale of 1 to 10, with 10 representing greatest severity over the preceding 7 days. Fatigue was specifically queried on the review of systems sheet. Visual analog scales for patient-reported symptoms have been extensively validated for skin disorders, including prior studies with morphea (Impact of Chronic Skin Disease on Daily Life).22-24
Statistical analysis
Demographics were reported as proportions for categorical variables and means ± SD for continuous variables. Analysis of association between demographic, clinical, and physician-based measures and DLQI and CDLQI scores was accomplished with the analysis of variance and Pearson correlations (weak correlation: r < 0.2; moderate correlation: 0.2 ≤ r < 0.6; strong correlation: 0.6 ≤ r ≤ 1). A P value less than .05 was considered statistically significant. All statistical analyses were performed using software (SAS, Version 9.2, SAS Institute Inc, Cary, NC).
Results
Demographics
Tables II and III Mean age of onset in adults was 37.4 ± 20.0 years and in children was 8.9 ± 3.9 years. The most frequently represented subtypes were linear and generalized (Table III). Predominant subtypes differed by age: most children had linear subtype, whereas adults predominantly had generalized. Of the 68 adults with linear morphea, 30 (44.11%) had onset in childhood. Results of the cross-sectional study examining impact on life quality in adults with pediatric onset morphea in the MAC cohort are available in a prior publication.25
Table II. Demographic characteristics of 277 patients with morphea.
| Characteristic | Value |
|---|---|
| Total patients | 277 |
| Children (age ≤ 17 y at enrollment) | 75 |
| Adults (age ≥ 18 y at enrollment) | 202 |
| Age at onset, overall, y | |
| Mean (SB) (±SD) | 29.7 ± 21.4 |
| Median (range) | 24 (4-78) |
| Gender | |
| N (%) | 233 (84.12) |
| F:M ratio | 5.30:1 |
| F:M (age ≤ 17 y) ratio | 2.95:1 |
| F:M (age ≥ 18 y) ratio | 7.08:1 |
| Ethnicity, N (%) | |
| White | 198 (71.48) |
| Hispanic/Latino | 48 (17.33) |
| Asian | 9 (3.25) |
| African American | 9 (3.25) |
| Other | 13 (4.69) |
F, Female; M, male.
Table III. Morphea subtype distribution, N (%) of patients.
| Group | Total | Plaque | Linear | Generalized | Mixed |
|---|---|---|---|---|---|
| Overall | 277 | 29 (10.47) | 137 (49.46) | 99 (35.74) | 12 (4.33) |
| Children | 75 | 2 (2.67) | 69 (92.00) | 3 (4.00) | 1 (1.33) |
| Adults | 202 | 27 (13.37) | 68 (33.66) | 96 (47.52) | 11 (5.45) |
Impact of morphea subtype on QOL
Fig 1 provides details of morphea subtype and associated DLQI and CDLQI scores. Of 202 adults, 75 (37.13%) experienced moderate or greater effect on QOL (DLQI > 5), with the greatest QOL impairment seen with generalized morphea. Mean DLQIs among adults were: generalized (6.58 ± 6.14), linear (5.96 ± 6.06), and plaque (3.56 ± 4.67). However, there was no correlation between subtype and DLQI (F value = 1.97, P = .12).
Fig 1.
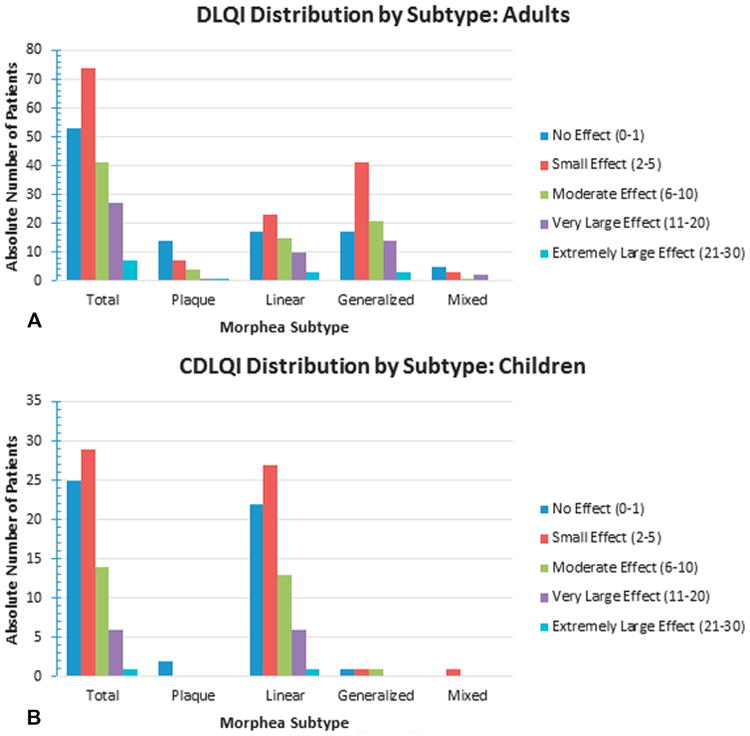
Absolute number of patients with each major subtype experiencing no, small, moderate, very large, or extremely large effect on quality of life as measured by Dermatology Life Quality Index (DLQI) (A) and Children's DLQI (B).
Among 75 children, 21 (28%) had moderate or greater impact on QOL (all but 1 had linear morphea). Mean CDLQIs were: generalized (4.67 ± 5.03), linear (4.71 ± 5.11), and plaque (0.50 ± 0.71). There was no correlation between subtype and CDLQI (F value = 0.45, P = .72). Overall, fewer children had moderate or greater impact on QOL compared with adults.
Association of disease-related symptoms and QOL
Patient-perceived lesion pain, numbness/tingling, itch, and skin tightness were assessed on a visual analog scale (Table IV). A designation of 2 or higher indicated presence of the symptom. Overall, 78.3% of patients reported at least 1 symptom, most commonly itch followed by pain. For adults, all 4 symptoms were associated with negative impact on QOL. For children, pain, itch, and tightness were associated with poorer life quality.
Table IV. Disease-related symptoms and relationship with quality of life.
| Pain | Numbness/tingling | Itch | Tightness | |
|---|---|---|---|---|
| Overall | ||||
| Mean VAS, ±SD | 2.78 ± 2.60 | 2.36 ± 2.33 | 3.82 ± 3.07 | 2.60 ± 2.65 |
| Median | 1 | 1 | 3 | 1 |
| Range | 1-10 | 1-10 | 1-10 | 1-10 |
| Score ≥2, n | 117 | 98 | 169 | 97 |
| Adults | ||||
| Mean VAS, ±SD | 2.91 ± 2.68 | 2.47 ± 2.38 | 3.96 ± 3.17 | 2.76 ± 2.79 |
| Median | 1 | 1 | 3 | 1 |
| Range | 1-10 | 1-10 | 1-10 | 1-10 |
| Score ≥2, n | 91 | 78 | 128 | 76 |
| Children | ||||
| Mean VAS, ±SD | 2.42 ± 2.34 | 2.06 ± 2.18 | 3.42 ± 2.76 | 2.17 ± 2.19 |
| Median | 1 | 1 | 2 | 1 |
| Range | 1-10 | 1-10 | 1-10 | 1-9 |
| Score ≥2, n | 26 | 20 | 41 | 21 |
| DLQI correlation | ||||
| Adults | r = 0.46 | r = 0.36 | r = 0.38 | r = 0.49 |
| P < .0001 | P < .0001 | P < .0001 | P < .0001 | |
| Children | r = 0.48 | r = 0.15 | r = 0.41 | r = 0.29 |
| P < .0001 | P = .20 | P < .0005 | P = .02 |
DLQI, Dermatology Life Quality Index; VAS, visual analog scale.
Relationship between physician assessment of disease severity and QOL
Table V In adults, increasing mRSS, LoSSI, and LoSDI scores correlated with greater impact on QOL. In children, there was no correlation between physician-based measures and CDLQI.
Table V. Correlation of Children's Dermatology Life Quality Index and Dermatology Life Quality Index with physician assessment of disease severity.
| mRSS | LoSSI | LoSDI | |
|---|---|---|---|
| Overall | |||
| Mean ± SD | 6.04 ± 5.05 | 16.01 ± 20.68 | 15.98 ± 14.82 |
| Median | 4 | 8 | 11 |
| Range | 1-37 | 0-132 | 0-68 |
| Adults | |||
| Mean ± SD | 6.18 ± 5.31 | 18.75 ± 22.96 | 17.44 ± 16.10 |
| Median | 4 | 11 | 12 |
| Range | 1-37 | 0-132 | 0-68 |
| Children | |||
| Mean ± SD | 5.67 ± 4.29 | 8.44 ± 8.80 | 11.95 ± 9.59 |
| Median | 4 | 6 | 8 |
| Range | 1-21 | 0-45 | 2-42 |
| CDLQI and DLQI correlation | |||
| Adults | r = 0.32 | r = 0.27 | r = 0.36 |
| P < .0001 | P < .005 | P = .0001 | |
| Children | r = −0.03 | r = −0.05 | r = 0.09 |
| P = .84 | P =.76 | P = .59 | |
CDLQI, Children's Dermatology Life Quality Index; DLQI, Dermatology Life Quality Index; mRSS, modified Rodnan skin score.
Correlation of disease duration, number of affected body segments, and lesion location and QOL
Mean overall disease duration was 6.6 ±11 years (median 3 years, range 0-83 years). Adults had greater disease duration and affected body segments than children (adults: mean duration 7.8 ± 12.1 years, median 3 years; children: mean duration 2.7 ± 3.1 years, median 2 years). Disease duration and DLQI and CDLQI show a trend toward an inverse relationship (adults: r = −0.10, P = .17; children: r = −0.05, P= .70).
Overall, mean number of affected body segments was 3.75 ± 4.00 and range of 1 to 33 (adults: 3.93 ± 4.23, range 1-33; children: 3.26 ± 3.28, range 1-19). There was correlation with DLQI but not CDLQI (adults: r = 0.21, P = .003, n = 202; children: r = 0.15, P = .21, n = 75). Cosmetically sensitive areas were identified as the face and neck (hands and arms were considered functionally sensitive). Number of patients with lesions in cosmetically sensitive sites and mean DLQI and CDLQI scores were adults (34 of 202), 5.59 ± 5.98 and children (16 of 75), 4.44 ± 4.70. Cosmetically sensitive location did not correlate with DLQI and CDLQI (adults: F value = 0.09, P = .77; children: F value = 0.02, P = .89).
The presence of lesions on extremities was defined as “functionally sensitive.” Mean DLQI scores were: adults: n = 46, 7.61 ± 5.93; children: n = 14, 6.64 ±7.55. Those with lesions in functionally sensitive areas had greater DLQI than those with cosmetically sensitive lesions. There was significant correlation with DLQI but not CDLQI (adults: F value = 5.16, P= .02; children: F value = 2.98, P= .09).
Association of gender and QOL
Women comprise the majority of patients with morphea (n = 233, 84.12%). Among adults, women had a trend toward worse QOL compared with men. Among children, the reverse was true (adults: women 6.12 ± 6.10 vs men 4.04 ± 4.75; children: girls 4.00 ± 3.80 vs boys 6.32 ± 7.41). (Adults: F value = 2.68, P = .10; children: F value = 3.12, P = .08.)
Correlation of comorbidities and QOL
Presence of comorbidities (N = 51) did not correlate with DLQI (adults: r = 0.04, P = .57; children: r = 0.18, P = .12).
Discussion
Current studies on morphea outcomes are predominantly physician-based assessments of cutaneous changes and functional impairment.5-7 Patient-based outcomes are critical for clinical decision making and research, yet the impact of morphea on patients is poorly understood. This cross-sectional study examined the association of clinical features, symptoms, and physician-based outcomes on patients' health-related QOL. One third of patients overall had moderate or greater (>5 DLQI) impairment of QOL related to morphea. Patients with morphea on average have similar impact as patients with basal cell carcinoma, discoid lupus, psoriasis, acne, and vitiligo.16,17
Morphea is considered a largely asymptomatic disorder. Although clinical signs, such as functional impairment, are accepted indications for systemic immunosuppressives,26 current guidelines do not consider symptoms in grading severity or instituting systemic treatment. In fact, a recent consensus treatment plan for pediatric morphea based criteria for aggressive treatment solely on clinical findings.26 Our findings challenge this notion, as greater than 75% of patients reported at least 1 symptom. Moreover, the presence of any symptoms was associated with greater patient-perceived negative impact on life quality for both adults and children. Our results are similar to those of Kroft et al,12,13 who noted fatigue, pain, and itch were significant correlates of DLQI. They also reported patients with generalized morphea have anxiety and depression comparable with that of psychiatric outpatients.12
Fatigue was not prominent in our cohort. Interestingly, our analysis demonstrated a significant correlation between itch and lesion activity (as measured by the LoSSI component of the LoSCAT), suggesting this symptom might be a useful marker of active disease in addition to presence of erythema, peripheral induration, and new or expanding lesions. These findings suggest symptoms should play a more prominent role in therapeutic decision making.
Number of affected body segments was associated with greater impairment of life quality in adults (but not children). Recent studies describe a subgroup of patients with progressive disease over years resulting in acquisition of an ever greater number of lesions.25 It appears that adult patients perceive negative sequelae not only from active disease, but also from the permanent residua of active lesions. Thus, disease progression–manifested by increasing numbers of affected body segments–should be an indication for aggressive therapy. Further, damage from morphea (pigmentary alteration, dermal or subcutaneous atrophy, and central sclerosis) may impair QOL even without functional deficits, thus indicating the need to improve support and therapy offered to patients with inactive morphea.
Patient-perceived disease impact (DLQI) has not previously been compared with physician-based measures in adults, and only limited studies exist in children.11 In this study, mRSS, LoSSI, and LoSDI correlated with DLQI but not CDLQI, suggesting the validity of DLQI in capturing aspects of morphea important to adults but not necessarily children. One interpretation of this lack of correlation among children may be that this group is unable to express the impact on life quality of morphea via the CDLQI. A recent study by Baildam et al9 implemented 4 instruments (Child Health Assessment Questionnaire, CDLQI, Child Quality of Life Questionnaire, and Child Health Questionnaire) and found moderate impact of morphea on QOL of pediatric patients. This implies that adults and children do not experience their diseases in the same way, and other patient- and parent-based instruments should be investigated in pediatric morphea. The importance of developing more accurate assessment instruments is highlighted by our recent finding that adults with pediatric-onset morphea tend to have continued impact on QOL (especially with higher number of lesions or functional impairment).25
Although this study represents evaluation of a larger, prospective cohort of patients than previous studies, limitations do exist. There were relatively few patients representing some morphea subtypes and clinical variables of interest. In the case of these small groups, results should be interpreted with caution. Although newer multidimensional life quality measures exist (Skindex and SF-36), the DLQI and CDLQI are shorter and have been validated across pediatric and adult groups.
Our results have important implications for practice, largely because they underscore aspects of morphea that are significant to patients but may be underappreciated by clinicians. These include the presence of symptoms such as itch and pain in all age groups. In addition, distress over the presence of increasing numbers of lesions and the permanent damage of formerly active lesions was also prominent in adults. In addition to assessing disease activity, subtype, and functional impairment, providers should also consider these aspects of morphea in their clinical decision making. Further research is needed to address not only treatment of active lesions but also the permanent functional and cosmetic sequelae of lesions.
Acknowledgments
Research for this article was supported by National Institutes of Health (NIH) Grant No. K23AR056303-4. This work was conducted with support from UT-STAR, NIH/National Center for Research Resources (NCRR)/NCATS Grant No. UL1RR024982. The content is solely the responsibility of the authors and does not necessarily represent the official views of UT-STAR, the University of Texas Southwestern Medical Center at Dallas and its affiliated academic and health care centers, the NCRR, or the NIH.
The authors thank Rose Ann Cannon and Stephanie Saxton-Daniels, MD.
Abbreviations used
- CDLQI
Children's Dermatology Life Quality Index
- DLQI
Dermatology Life Quality Index
- LoSCAT
Localized Scleroderma Cutaneous Assessment Tool
- MAC
Morphea in Adults and Children
- mRSS
modified Rodnan skin score
- QOL
quality of life
Footnotes
Conflicts of interest: None declared.
Reprints not available from the authors.
References
- 1.Zulian F, Vallongo C, Woo P, Russo R, Ruperto N, Harper J, et al. Localized scleroderma in childhood is not just a skin disease. Arthritis Rheum. 2005;52:2873–81. doi: 10.1002/art.21264. [DOI] [PubMed] [Google Scholar]
- 2.Leitenberger JJ, Cayce RL, Haley RW, Adams-Huet B, Bergstresser PR, Jacobe HT. Distinct autoimmune syndromes in morphea: a review of 245 adult and pediatric cases. Arch Dermatol. 2009;145:545–50. doi: 10.1001/archdermatol.2009.79. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Uziel Y, Krafchik BR, Silverman ED, Thorner PS, Laxer RM. Localized scleroderma in childhood: a report of 30 cases. Semin Arthritis Rheum. 1994;23:328–40. doi: 10.1016/0049-0172(94)90028-0. [DOI] [PubMed] [Google Scholar]
- 4.Christen-Zaech S, Hakim MD, Afsar FS, Paller AS. Pediatric morphea (localized scleroderma): review of 136 patients. J Am Acad Dermatol. 2008;59:385–96. doi: 10.1016/j.jaad.2008.05.005. [DOI] [PubMed] [Google Scholar]
- 5.Bendeck SE, Jacobe HT. Ultrasound as an outcome measure to assess disease activity in disorders of skin thickening: an example of the use of radiologic techniques to assess skin disease. Dermatol Ther. 2007;20:86–92. doi: 10.1111/j.1529-8019.2007.00116.x. [DOI] [PubMed] [Google Scholar]
- 6.Arkachaisri T, Vilaiyuk S, Li S, O'Neil KM, Pope E, Higgins GC, et al. The localized scleroderma skin severity index and physician global assessment of disease activity: a work in progress toward development of localized scleroderma outcome measures. J Rheumatol. 2009;36:2819–29. doi: 10.3899/jrheum.081284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Li SC, Liebling MS. The use of Doppler ultrasound to evaluate lesions of localized scleroderma. Curr Rheumatol Rep. 2009;11:205–11. doi: 10.1007/s11926-009-0028-y. [DOI] [PubMed] [Google Scholar]
- 8.Nezafati KA, Cayce RL, Susa JS, Setiawan AT, Tirkes T, Bendeck SE, et al. 14-MHz ultrasonography as an outcome measure in morphea (localized scleroderma) Arch Dermatol. 2011;147:1112–5. doi: 10.1001/archdermatol.2011.243. [DOI] [PubMed] [Google Scholar]
- 9.Baildam EM, Ennis H, Foster HE, Shaw L, Chieng AS, Kelly J, et al. Influence of childhood scleroderma on physical function and quality of life. J Rheumatol. 2011;38:167–73. doi: 10.3899/jrheum.100447. [DOI] [PubMed] [Google Scholar]
- 10.Orzechowski NM, Davis DM, Mason TG, III, Crowson CS, Reed AM. Health-related quality of life in children and adolescents with juvenile localized scleroderma. Rheumatology (Oxford) 2009;48:670–2. doi: 10.1093/rheumatology/kep059. [DOI] [PubMed] [Google Scholar]
- 11.Uziel Y, Laxer RM, Krafchik BR, Yeung RS, Feldman BM. Children with morphea have normal self-perception. J Pediatr. 2000;137:727–30. doi: 10.1067/mpd.2000.108564. [DOI] [PubMed] [Google Scholar]
- 12.Kroft EB, de Jong EM, Evers AW. Psychological distress in patients with morphea and eosinophilic fasciitis. Arch Dermatol. 2009;145:1017–22. doi: 10.1001/archdermatol.2009.202. [DOI] [PubMed] [Google Scholar]
- 13.Kroft EB, de Jong EM, Evers AW. Physical burden of symptoms in patients with localized scleroderma and eosinophilic fasciitis. Arch Dermatol. 2008;144:1394–5. doi: 10.1001/archderm.144.10.1394. [DOI] [PubMed] [Google Scholar]
- 14.Furst DE, Clements PJ, Steen VD, Medsger TA, Jr, Masi AT, D'Angelo WA, et al. The modified Rodnan skin score is an accurate reflection of skin biopsy thickness in systemic sclerosis. J Rheumatol. 1998;25:84–8. [PubMed] [Google Scholar]
- 15.Laxer RM, Zulian F. Localized scleroderma. Curr Opin Rheumatol. 2006;18:606–13. doi: 10.1097/01.bor.0000245727.40630.c3. [DOI] [PubMed] [Google Scholar]
- 16.Lewis V, Finlay AY. 10 Years' experience of the Dermatology Life Quality Index (DLQI) J Investig Dermatol Symp Proc. 2004;9:169–80. doi: 10.1111/j.1087-0024.2004.09113.x. [DOI] [PubMed] [Google Scholar]
- 17.Lewis-Jones MS, Finlay AY. The Children's Dermatology Life Quality Index (CDLQI): initial validation and practical use. Br J Dermatol. 1995;132:942–9. doi: 10.1111/j.1365-2133.1995.tb16953.x. [DOI] [PubMed] [Google Scholar]
- 18.Holme SA, Man I, Sharpe JL, Dykes PJ, Lewis-Jones MS, Finlay AY. The Children's Dermatology Life Quality Index: validation of the cartoon version. Br J Dermatol. 2003;148:285–90. doi: 10.1046/j.1365-2133.2003.05157.x. [DOI] [PubMed] [Google Scholar]
- 19.Basra MK, Fenech R, Gatt RM, Salek MS, Finlay AY. The Dermatology Life Quality Index 1994-2007: a comprehensive review of validation data and clinical results. Br J Dermatol. 2008;159:997–1035. doi: 10.1111/j.1365-2133.2008.08832.x. [DOI] [PubMed] [Google Scholar]
- 20.Finlay AY, Basra MK, Piguet V, Salek MS. Dermatology Life Quality Index (DLQI): a paradigm shift to patient-centered outcomes. J Invest Dermatol. 2012;132:2464–5. doi: 10.1038/jid.2012.147. [DOI] [PubMed] [Google Scholar]
- 21.Finlay AY, Khan GK. Dermatology Life Quality Index (DLQI)—a simple practical measure for routine clinical use. Clin Exp Dermatol. 1994;19:210–6. doi: 10.1111/j.1365-2230.1994.tb01167.x. [DOI] [PubMed] [Google Scholar]
- 22.Evers AW, Duller P, van de Kerkhof PC, van der Valk PG, de Jong EM, Gerritsen MJ, et al. The Impact of Chronic Skin Disease on Daily Life (ISDL): a generic and dermatology-specific health instrument. Br J Dermatol. 2008;158:101–8. doi: 10.1111/j.1365-2133.2007.08296.x. [DOI] [PubMed] [Google Scholar]
- 23.Goreshi R, Chock M, Foering K, Feng R, Okawa J, Rose M, et al. Quality of life in dermatomyositis. J Am Acad Dermatol. 2011;65:1107–16. doi: 10.1016/j.jaad.2010.10.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Gniadecki R, Robertson D, Molta CT, Freundlich B, Pedersen R, Li W, et al. Self-reported health outcomes in patients with psoriasis and psoriatic arthritis randomized to two etanercept regimens. J Eur Acad Dermatol Venereol. 2012;26:1436–43. doi: 10.1111/j.1468-3083.2011.04308.x. [DOI] [PubMed] [Google Scholar]
- 25.Saxton-Daniels S, Jacobe HT. An evaluation of long-term outcomes in adults with pediatric-onset morphea. Arch Dermatol. 2010;146:1044–5. doi: 10.1001/archdermatol.2010.239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Li SC, Torok KS, Pope E, Dedeoglu F, Hong S, Jacobe HT, et al. Development of consensus treatment plans for juvenile localized scleroderma. Arthritis Care Res. 2012;64:1175–85. doi: 10.1002/acr.21687. [DOI] [PMC free article] [PubMed] [Google Scholar]


