Abstract
BACKGROUND & AIMS
Patients with eosinophilic esophagitis (EoE) often become dysphagic from the combination of organ fibrosis and motor abnormalities. We investigated mechanisms of dysphagia, assessing the response of human esophageal fibroblasts (HEF), muscle cells (HEMC), and esophageal muscle strips to eosinophil-derived products.
METHODS
Biopsies were collected via endoscopy from the upper, middle and lower thirds of the esophagus of 18 patients with EoE and 21 individuals undergoing endoscopy for other reasons (controls). Primary cultures of esophageal fibroblasts and muscle cells were derived from 12 freshly resected human esophagectomy specimens. Eosinophil distribution was investigated by histologic analyses of full-thickness esophageal tissue. Active secretion of EoE-related mediators was assessed from medium underlying mucosal biopsy cultures. We quantified production of fibronectin and collagen I by HEF and HEMC in response to eosinophil products. We also measured expression of ICAM1 and VCAM1 by, and adhesion of human eosinophils to, HEF and HEMC. Eosinophil products were tested in an esophageal muscle contraction assay.
RESULTS
Activated eosinophils were present in all esophageal layers. Significantly higher concentrations of eosinophil-related mediators were spontaneously secreted in mucosal biopsies from patients with EoE than controls. Exposure of HEF and HEMC to increasing concentrations of eosinophil products or co-culture with eosinophils caused HEF and HEMC to increase secretion of fibronectin and collagen I; this was inhibited by blocking transforming growth factor (TGF)β1 and p38 mitogen-activated protein kinase (MAKP) signaling. Eosinophil binding to HEF and HEMC increased following incubation of mesenchymal cells with eosinophil-derived products, and decreased following blockade of TGFβ1 and p38MAPK blockade. Eosinophil products reduced electrical field-induced contraction of esophageal muscle strips, but not acetylcholine-induced contraction.
CONCLUSION
In an analysis of tissues samples from patients with EoE, we linked the presence and activation state of eosinophils in EoE with altered fibrogenesis and motility of esophageal fibroblasts and muscle cells. This process might contribute to the development of dysphagia.
Keywords: Primary human cells, transmural disease, swallowing, immune regulation
INTRODUCTION
EoE is a clinicopathological condition characterized by dysphagia, chest pain and food impaction 1, symptoms associated with distinct but interrelated mechanisms: inflammation, fibrotic structural changes and motor abnormalities 2–5. Evaluation of the esophagus in EoE using impedance planimetry, barium radiography and endoscopic ultrasound show decreased distensibility 6, decreased luminal diameter 7 as well as smooth muscle dysfunction 8, 9. While progress has been made to elucidate EoE pathogenesis, understanding of the mechanisms underlying fibrosis and dysmotility is still limited.
EoE likely arises from an allergic response to environmental factors in susceptible individuals 1, and is typically associated with a Th2-immune response, with increased expression of interleukin (IL)-4, IL-5, IL-13 or eotaxins 10. EoE is apparently a disorder driven by multiple distinct mechanisms. IL-5 and IL-13 recruit and activate eosinophils, leading to secretion of transforming growth factor (TGF)-β1 10, 11; IL-4 and IL-13 activate fibroblasts to secrete enhanced amounts of extracellular matrix (ECM) and IL-13 activates epithelial cells to secrete eotaxins, powerful eosinophil chemoattractants 12. TGF-β1 is the main driver of fibrosis across all organs by inducing excessive ECM secretion by mesenchymal cells; it is secreted by essentially all cell types present in the esophagus, and in EoE its amount and phosphorylated signaling molecules SMAD2/3 are increased in the mucosa and submucosa 3, and it may promote epithelial-to-mesenchymal transition 13.
While different cell types are implicated in EoE pathogenesis, the eosinophil is still believed to play a central role. This is supported by its increased number and activation state in the mucosa, its ability to secrete virtually all mediators found in EoE, and its release of potent biologically active molecules, such as major basic proteins (MBP) 14.
The above pathogenic paradigms are based almost exclusively on animal models, immortalized cell lines and superficial biopsy specimens. Strikingly, functional data derived from primary human esophageal cells beyond the esophageal epithelium are limited, which considerably hampers understanding of EoE pathogenesis. As pointed out by Aceves 15, major challenges remain in understanding mechanisms of tissue remodeling in EoE, with a need for new approaches that unveil events occurring beneath the esophageal epithelium. To fill this gap, we used primary human cells and show that mediators present in EoE mucosa and human eosinophils activate esophageal mesenchymal cells, differentiate them into a pro-fibrogenic phenotype, and alter neurogenic esophageal smooth muscle contraction. These findings offer novel insights into the pathogenesis of fibrosis and dysmotility, the two major complications of EoE.
MATERIALS AND METHODS
Patient population and procurement of endoscopic and surgical tissues
Endoscopic mucosal biopsies were obtained from the upper, middle and lower thirds of the esophagus of patients with a history compatible with EoE and patients requiring endoscopic examination for other reasons 16. EoE was diagnosed based on guideline criteria 1, namely >15 eos/HPF and no response to PPI therapy for 8 weeks. One group included subjects with EoE (n=18) and another group included subjects without GERD symptoms and free of microscopic or macroscopic inflammation (n=21) (Detailed demographic and clinical characteristics of both patient populations is shown in Supplemental Table 2). None of the patients had endoscopic or histologic findings indicative of Barrett’s esophagus. Three biopsies were obtained from each third of the esophagus and at least 2 cm above the gastroesophageal junction and used for in vitro studies; additional biopsies were taken for histologic evaluation by two gastrointestinal pathologists (J.G. and J.L.) unaware of the clinical or endoscopic diagnosis. Patients with achalasia, severe systemic diseases, or on current chemotherapeutic drugs were excluded. The study protocol was approved by the Institutional Review Board of the Cleveland Clinic.
Cell lines
Primary cultures of esophageal fibroblasts and muscle cells were derived from 12 freshly resected human esophagectomy specimens. The mean age of the patients (11 males a 2 female) was 62.6±9.5 years, and indication for surgery was esophageal squamous carcinoma in 9 and adenocarcinoma in 3; all patients were treated prior to resection with standard institutional protocols. Cell isolation and culture were performed as previously described (Supplemental Materials). Mucosal explants gave rise to cells with typical fibroblast morphology and termed human esophageal fibroblasts (HEF); the muscle tissue was minced, enzymatically digested, filtered and the resulting cell suspension plated resulting in a monolayer of spindle-like cells termed human esophageal muscle cells (HEMC). Additional cells and cells lines included: 1) The differentiated human eosinophil myelocyte cell line AML14.3D10; 2) Mature human mast cells (both kindly provided by Dr. Fred Hsieh, Department of Pathobiology, Cleveland Clinic; 3) The human MOLT-4 T cell line; 4) The human monocytic cell line THP-1; 5) Human peripheral blood T cells; 6) Human peripheral blood monocytes. Details of isolation and culture are described in the Supplemental Materials.
Cat esophageal circular muscle contraction assay
Two-millimeter-wide circular muscle strips were prepared and mounted in 1 ml chambers as previously described 18 Strips were stretched to 2.5g to bring them near conditions of optimum force development and equilibrated for 2 hours while being perfused continuously with oxygenated physiologic salt solution at 37°C equilibrated with 95% O2 and 5% CO2 at 37°C, pH 7.4. After full equilibration, strips were stimulated with square wave pulses of supramaximal voltage, 0.5ms, 2Hz, 10-s trains, delivered by a stimulator (Grass Instruments, model S48; Astro-Med Inc., W. Warwick, RI) using electrodes placed on either side of the strip. This stimulation produces maximal esophageal contraction by inducing the release of acetylcholine from intramural neurons 18. To study the effect on the contraction in response to electrical field stimulation, the strips were incubated with a pre-established concentration of cytokines, growth factors, eosinophil sonicates or eosinophil products for 2h, and contraction in response to electrical field stimulation measured. All experiments were performed in triplicate, using 3 strips/cat from 3 different cats.
Additional methods can be found in Supplemental Materials.
RESULTS
Histopathologic evaluation of a full thickness eosinophilic esophagitis specimen
The number and activation state of eosinophils in all layers of a full thickness EoE specimen were evaluated and compared to those in a normal esophagus. Histologic evaluation of H&E- and EPX-stained tissue revealed typical findings of EoE, with eosinophils scattered throughout the esophageal wall (Fig. 1): their density was highest in the epithelium, but they were also abundant in the submucosa, muscle and adventitia with a median density of >15 eos/HPF in all layers (Supplemental Fig. 1). Supporting the transmural nature of EoE, eosinophils were found in contact with mucosal fibroblasts and muscularis propria muscle cells. EPX staining confirmed that the eosinophils in EoE tissue were activated, with degranulation and presence of microabscesses 19. The normal control esophagus was essentially devoid of eosinophils.
Figure 1. Eosinophil infiltration and activation state of in all layers of a surgically resected full thickness human EoE specimen compared to those in the normal esophagus.
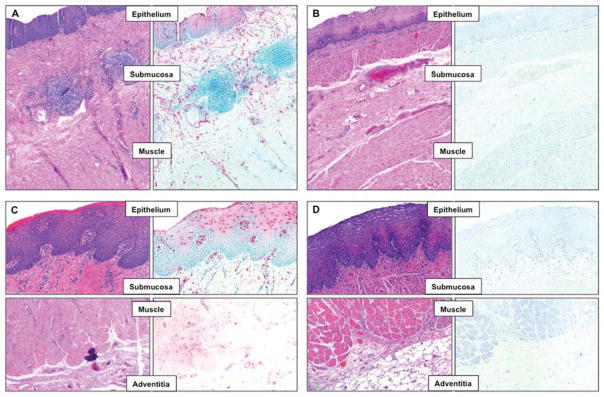
Histologic evaluation of hematoxylin & eosin (H&E; left side of each panel) and eosinophil peroxidase (EPX; right side of each panel) stained esophageal tissue. In the EoE esophagus (Panel A and C) activated eosinophils were found scattered throughout the esophageal wall with the highest density in the epithelium. The normal control esophagus (Panel B and D) was essentially devoid of eosinophils, even in the deeper tissue layers. Panel A and B: 50X magnification; Panel C and D: 160X magnification.
Active production of IL-5, IL-6, IL-13, eotaxin-1 and of TGF-β1 in EoE mucosal biopsy specimens
Using an organ culture system, we investigated the secretion of cytokines and growth factors associated with inflammation, eosinophilia and fibrosis in mucosal biopsies obtained from EoE and control subjects. IL-5, IL-6, IL-13, eotaxin-1 and TGF-β1 were actively secreted in the undernatants and their levels were significantly increased in EoE compared to controls (Supplemental Table 1), while IL-4 and eotaxin 3 were not detected.
ECM secretion in response to EoE-associated cytokines
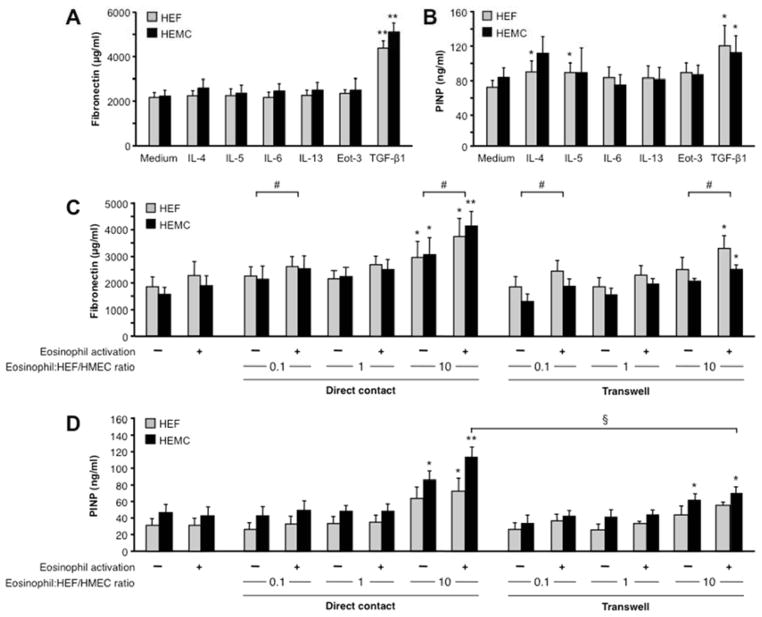
We investigated whether HEF and HEMC could upregulate ECM production when exposed to cytokines associated with EoE, and we focused on the secretion of fibronectin and collagen I, both produced in large amounts during fibrogenesis. HEF and HEMC spontaneously produced substantial levels of FN and collagen I that increased after stimulation with TGF-β1 (Fig. 2A and B). No significant differences were detected between cytokine-activated HEF and HEMC, but IL-4 and IL-5 only upregulated collagen secretion by HEF.
Figure 2. Secretion of ECM by HEF and HEMC in response to cytokines and co-culture with human eosinophils.
2A and B: HEF and HEMC were cultured for 72 h with or without the presence of cytokines known to be involved in the pathogenesis of EoE. FN secretion (2A) and PINP (2B) were measured using ELISA and RIA, respectively. TGF-β1 enhanced both FN and PINP secretion in HEF and HEMC, while IL-4 and IL-5 increased only PINP in HEF. N=5–8 for FN and PINP. *p<0.05, **p<0.01.
2C and D: HEF and HEMC were co-cultured with the human eosinophil cell line AML14.3D10 for 72h with and without eosinophil activation by IL-3, IL-5 and GM-CSF. With increasing eosinophil numbers HEF and HEMC increased secretion of FN (2C) and PINP (2D). Eosinophil activation enhanced matrix secretion by HEF and HEMC, and inhibition of direct contact in a transwell system reduced the matrix secretion. N=4–6 for FN and PINP. *p<0.05, **p<0.01 compared to no eosinophils; #p<0.05 for activated versus non-activated eosinophils; §p<0.05 for transwell versus no transwell.
Eosinophil-induced esophageal mesenchymal cell ECM and cytokine production
Aside from eosinophil-specific mediators, such as MBP, essentially all cytokines and growth factors found in EoE can be produced by eosinophils. As eosinophils are present throughout the esophageal wall in EoE in close proximity to mesenchymal cells (Fig. 1), we investigated whether contact of human eosinophils with esophageal mesenchymal cells would induce ECM and cytokine secretion. Co-culture of increasing numbers of the human eosinophil cell line AML14.3D10 with HEF and HEMC led to a progressive increase in FN and collagen I secretion, an effect further enhanced when eosinophils were pre-activated by IL-3, IL-5 and GM-CSF combined (Fig. 2C and D). Eosinophils alone did not secrete FN or collagen I (data not shown).
To determine whether the increase in ECM secretion is strictly dependent on cell-cell contact a transwell system was used to prevent physical interaction. With increasing numbers of eosinophils again an increase in FN and collagen I secretion by the HEF and HEMC was noted (Fig. 2C and D), but the effect was less pronounced compared to direct cell-cell contact. No difference was noted between the HEF and HEMC response to eosinophil co-culture.
Next, we investigated whether ECM production by HEF and HEMC is an eosinophil-specific response by co-culturing HEF and HEMC with other immune cell types found in EoE tissue, namely T cells, primary human monocytes, THP-1 human monocytic cells and primary human cord blood-derived mast cells (HCMCs). In addition to human eosinophils, co-culture with primary human monocytes and HCMCs also significantly increased secretion of FN by HEF and HEMC (Supplemental Fig. 3).
Finally, we assessed the secretion of cytokines induced by eosinophil and HEF/HEMC co-culture. Upon direct contact of HEF or HEMC with eosinophils an increase in IL-6, eotaxin-1 and TGF-β1 was noted compared to all cells alone. IL-13 was only detected when eosinophils were in contact with HEF or HEMC (Supplemental Fig. 4), and IL-4 and IL-5 were not detected (data not shown). The secretion of IL-6 was analyzed in further detail. Its increase was enhanced upon contact with eosinophils pre-activated with IL-3, IL-5 and GM-CSF compared to non-activated eosinophils, and was reduced when a transwell system was used (Supplemental Fig. 5), similarly to the results of the ECM secretion experiments.
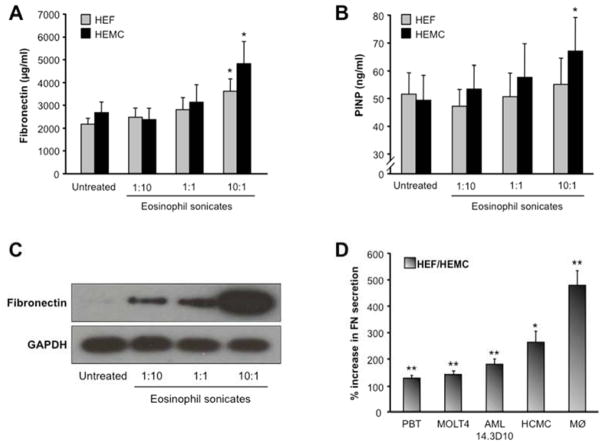
Eosinophil sonicate-induced esophageal mesenchymal cell ECM production
After establishing that whole eosinophils are potent inducers of ECM secretion by esophageal mesenchymal cells, we wondered which eosinophil products are involved in this fibrogenic response. We exposed HEF and HEMC to increasing concentrations of eosinophil sonicates and observed a dose-dependent augmentation in FN and collagen I production (Fig. 3A and B), a response confirmed by immunoblotting (Fig. 3C). Interestingly, native human MBP used in concentrations from 0.03 to 3 μM did not increase FN secretion by HEF (data not shown). No significant differences in ECM production were noted comparing sonicate-activated HEF and HEMC (Fig. 3A and B).
Figure 3. Secretion of ECM by HEF and HEMC in response to eosinophil sonicates.
HEF and HEMC were co-cultured for 72 h with sonicates of the human cell line AML14.3D10. With increasing concentrations of the eosinophil sonicates HEF and HEMC increased secretion of FN (3A) and PINP (3B). N=4–8 for FN and PINP. *p<0.05 compared to untreated. 3C: immunoblot showing that incubation with eosinophil sonicates enhances the intracellular amounts of FN in HEF in a dose-dependent manner. Figure representative of 3 experiments. 3D compares the effect of AML14.3D10 cells on HEF and HEMC with sonicates of human peripheral blood T-cells (PBT), the T-cell line MOLT4, human cord blood derived mast cells (HCMC) and peripheral blood monocytes (PBM). N=4–5 for HEF and HEMC. *p<0.05, **p<0.01 compared to untreated HEF and HEMC.
To investigate whether the effects observed using eosinophil sonicates were cell-specific, HEF and HEMC were incubated with sonicates derived from PBT, MOLT4 T cells, primary human monocytes, THP-1 cells and HCMCs. All sonicates increased FN secretion by HEF and HEMC, with the strongest effect exerted by monocyte sonicates (Fig. 3D).
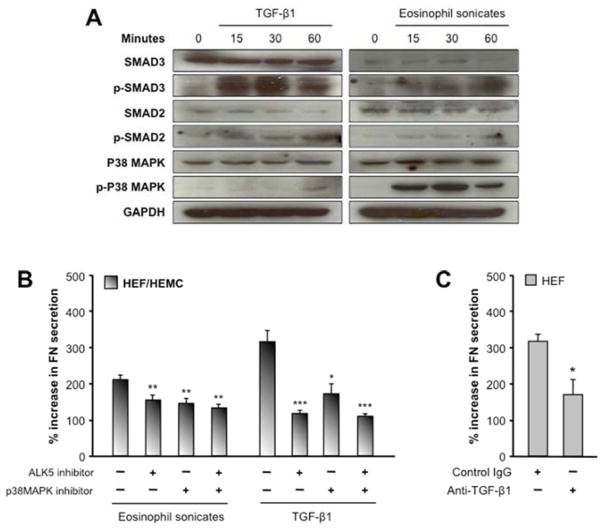
Eosinophil product-induced ECM production by HEF and HEMC is TGF-β1- and p38MAPK-dependent
TGF-β1 was practically the only mediator consistently inducing ECM production by HEF and HEMC (Fig. 2). In view of the pro-fibrotic effect of eosinophil sonicates we assessed whether they were using the canonical TGF-β1 signaling pathway involving SMAD2, SMAD3 and p38MAPK. Both eosinophil sonicates and TGF-β1 led to rapid and sustained phosphorylation of SMAD2, SMAD3 and p38MAPK, without differences between HEF and HEMC (Fig. 4A). To substantiate these findings, we incubated HEF and HEMC with the TGF-β1 receptor signaling inhibitor ALK5 (SB431542; 10μM) and the p38MAPK inhibitor SB203580 (10μM) for 1 hour before exposure to TGF-β1 or eosinophil sonicates. Blockade of ALK5 or p38MAPK alone or in combination markedly reduced the TGF-β1-and eosinophil sonicate-induced FN secretion by HEF and HEMC (Fig. 4B). To further implicate TGF-β1 in the pro-fibrogenic response of EoE, we neutralized TGF-β1 activity in eosinophil sonicates prior to co-culture with HEF and observed a significant reduction of the eosinophil sonicate-induced FN secretion by HEF (Fig. 4C). In the same experiments inhibition of PI3 kinase failed to alter ECM secretion by HEF or HEMC (data not shown).
Figure 4. Activation and blockade of signaling pathways in HEF and HEMC in response to TGF-β1 and eosinophil sonicates.
4A: HEF and HEMC were incubated with TGF-β1 or eosinophil sonicates for 0 to 60 minutes. Both TGF-β1 and eosinophil sonicates activate SMAD2, SMAD3 and p38MAPK signaling. Figure representative of 8 experiments for both HEF and HEMC.
4B: Blockade of p38MAPK and ALK5 signaling reduces the FN secretion by HEF and HEMC in response to TGF-β1 and eosinophil sonicates. HEF and HEMC were stimulated for 72 h with TGF-β1 or eosinophil sonicates; p38MAPK was inhibited by SB203580 and ALK5 by SB431542. Inhibition of both pathways, alone or combined, reduced FN secretion by HEF and HEMC in response to TGF-β1 or eosinophil sonicates. N=7–8 for HEF and HEMC. *p<0.05, **p<0.01, *p<0.001 compared to no inhibitors. Comparable results found by adding TGF-β1 neutralizing antibodies to the eosinophil sonicates (4C). N=3 for HEF. *p<0.05.
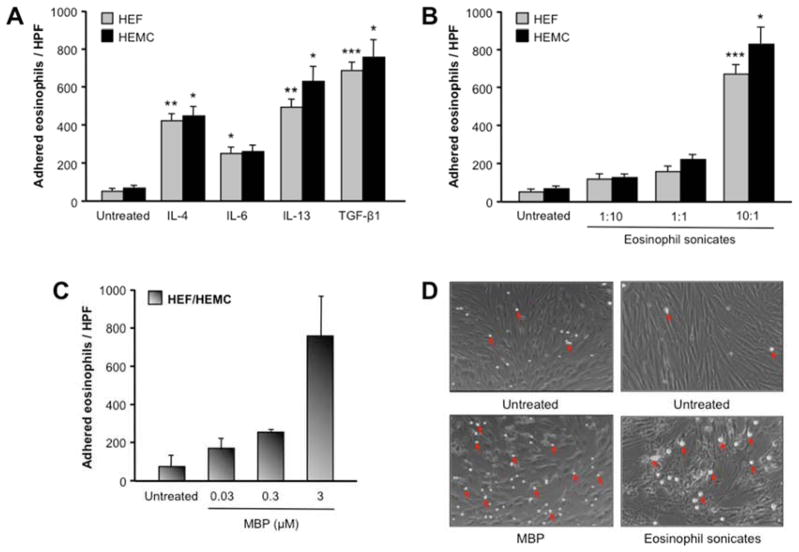
Increased adhesion of eosinophils to esophageal mesenchymal cells by EoE-associated mediators
After establishing the pro-fibrotic effect of eosinophils, eosinophil products and eosinophil-derived TGF-β1 on HEF and HEMC, we investigated mechanisms underlying this eosinophil-mesenchymal cell interaction. We generated HEF and HEMC monolayers and assessed their adhesiveness to human eosinophils. Untreated HEF and HEMC adhered only low numbers of eosinophils (Fig. 5). However, pre-incubation of HEF or HEMC with IL-4, IL-6, IL-13, TGF-β1, eosinophil sonicates or native human MBP-1 remarkably augmented the number of adherent eosinophils to both HEF and HEMC (Fig. 5). To investigate whether this effect was specific to eosinophils, we used the same mediators and measured the adhesion of PBT to HEF. All mediators that increased eosinophil adhesion also increased adhesion of PBT to HEF, and pre-incubation of HEF with PBT and MOLT-4 T cell sonicates increased adhesion of eosinophils to HEF (data not shown).
Figure 5. Adhesion of eosinophils to HEF and HEMC in response to cytokines.
5A: HEF and HEMC were cultured for 72 h with or without cytokines involved in the pathogenesis of EoE, and adherence of AML14.3D10 cells to HEF or HEMC was measured using fluorescent labeling with calcein. IL-4, IL-6, IL-13 and TGF-β1 markedly enhanced the adherence of eosinophils to HEF and HEMC. N=8–9 for HEF and HEMC. *p<0.05, **p<0.01, ***p<0.001 compared to untreated HEF or HEMC.
5B and C: Eosinophil sonicates and human MBP increased adherence of eosinophils to HEF and HEMC. N=8–9 for HEF and HEMC for the eosinophil sonicates; N=2 combined HEF and HEMC for the MBP. *p<0.05, ***p<0.001 compared to untreated HEF or HEMC.
5D depicts representative brightfield images of human monocytes (arrows) adherent to untreated and treated HEF monolayers. Magnification 100x.
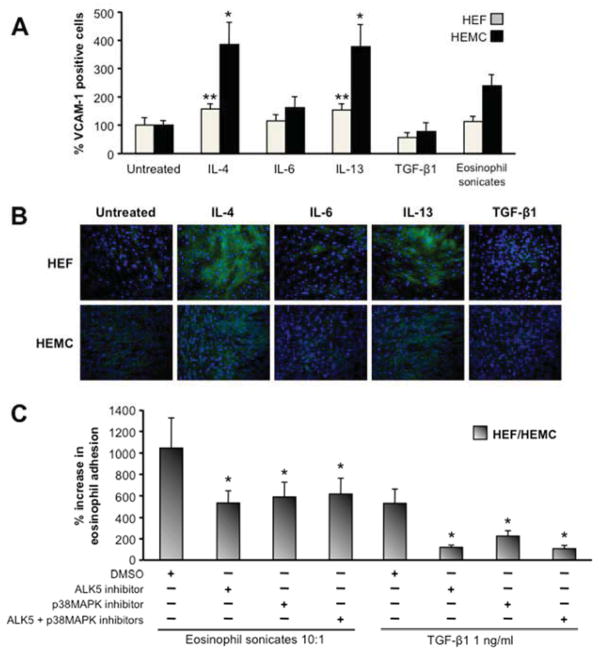
To investigate the increased adhesion of eosinophils to mesenchymal cells we next measured VCAM-1 and ICAM-1 expression by HEF and HEMC, before and after exposure to mediators that enhance eosinophil adhesion. Exposure to IL-4 and IL-13, but not to IL-6, TGF-β1 or eosinophil sonicates significantly increased VCAM-1 surface expression in HEF and HEMC (Fig. 6A). These results were confirmed with immunocytochemistry (Fig. 6B). The expression of ICAM-1 remained unchanged after incubation of HEF or HEMC with the same mediators or eosinophil sonicates (data not shown).
Figure 6. Expression of VCAM-1 by HEF and HEMC in response to cytokines and eosinophil sonicates and after blockade of signaling pathways.
6A: HEF and HEMC were cultured for 72 h with or without cytokines involved in the pathogenesis of EoE or eosinophil sonicates, and expression of VCAM-1 was measured by flow cytometry. IL-4 and IL-13 enhanced the expression of VCAM-1 in HEF and HEMC, while TGF-β1 decreased the amount of VCAM-1 positive HEF. N=5 for HEF and HEMC. *p<0.05, **p<0.01 compared to untreated HEF or HEMC.
6B: HEF and HEMC were plated on glass slides and incubated as for 6A. VCAM-1 expression was assessed via fluorescent microscopy after staining with a specific anti-VCAM-1 antibody. IL-4 and IL-13 increased the expression of VCAM-1 in HEF and HEMC. Figure representative of 6 experiments. Magnification 100x.
6C: Blockade of p38MAPK and ALK5 signaling reduces eosinophil adhesiveness of HEF and HEMC in response to TGF-β1 and eosinophil sonicates. HEF and HEMC were stimulated as for 6A, and p38MAPK inhibited by SB203580 and ALK5 by SB431542. Inhibition of both pathways, alone or combined, reduced the adhesion of AML14.3D10 cells to HEF and HEMC in response to TGF-β1 or eosinophil sonicates. N=5–7 for HEF and HEMC. *p<0.05 compared to no signaling inhibitor.
As TGF-β1 and eosinophil sonicates had the strongest effect on eosinophil adhesion, and given the above results indicating a dominant role of eosinophil-derived TGF-β1 in mesenchymal cell activation, we blocked TGF-β1 (with the ALK5-inhibitor SB431542) and p38MAPK signaling (with SB203580) in the cell adhesion assay. Inhibition of ALK5 or p38MAPK alone or in combination dramatically reduced the adhesion of eosinophils to eosinophil sonicate-exposed HEF and HEMC and completely abrogated the increased adhesion induced by TGF-β1 (Fig. 6C).
Effect of cytokines, growth factors and esophageal cell culture supernatants on esophageal muscle contraction
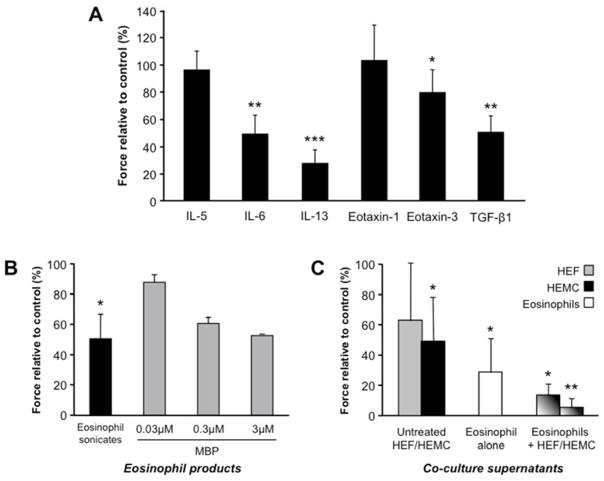
The clinical symptoms found in EoE may be related not only to organ fibrosis but also motility abnormalities. We previously reported that cytokines can inhibit contraction of esophageal smooth muscle cells 18. We therefore tested whether the various mediators implicated in EoE pathogenesis and we detected in our experimental systems had the capacity to alter contraction of cat esophageal circular muscle strips. Two sets of experiments were performed. In the first we used recombinant cytokines of previously identified mediators, and in the second we utilized eosinophil sonicates, native human MBP and supernatants from the co-culture of HEF and HEMCs with human eosinophils. Figure 7A shows the effect of recombinant cytokines on the contraction of esophageal circular muscle strips in response to electrical (i.e. neural) stimulation: IL-6, IL-13, eotaxin-3 and TGF-β1 reduced the amplitude of contraction in response to neural stimulation, while IL-5 and eotaxin-1 had no effect. Figure 7B shows the effect of eosinophil sonicates, native MBP and eosinophil co-culture supernatants on the neurally mediated contraction of esophageal muscle strips in the electrical field. Eosinophil sonicates and MBP induced a decrease in muscle cell contraction in response to electrical stimulation. Incubation with supernatants of eosinophils or HEMC alone, but not HEF alone, caused a mild to moderate reduction in the contractile response. In contrast, the supernatants of the eosinophil and HEF/HEMC co-cultures almost entirely abolished muscle contraction (Fig. 7C). Contraction of muscle strips returned to normal after wash out of the supernatants. None of the cytokines, growth factors, eosinophil products or supernatants influenced the acetylcholine-induced muscle contraction (data not shown), and hence the interaction of acetylcholine with the muscle receptors.
Figure 7. Effect of cytokines, growth factors and primary esophageal cell culture supernatants on esophageal smooth muscle contraction.
Cat esophageal circular muscle strips were incubated with recombinant cytokines (7A), eosinophil sonicates or human MBP (7B) as well as supernatants from co-cultures of HEF and HEMC with human eosinophils (7C). In response to electrical stimulation IL-6, IL-13, eotaxin-3 and TGF-β1 reduced the amplitude of contraction field, while IL-5 and eotaxin-1 had no effect. Eosinophil sonicates and MBP induced a decrease in muscle cell contraction in response to electrical stimulation. Incubation with supernatants of eosinophils or HEMC alone, but not HEF alone, caused a mild to moderate reduction in the contractile response. In contrast, supernatants of the eosinophil and HEF/HEMC co-cultures almost entirely abolished muscle contraction. Contraction of all muscle strips returned to normal after wash out of the supernatants. N=6–9 for cytokines and sonicates, N=2 for MBP and N=3 for co-culture supernatants. *p<0.05, **p<0.01, ***p<0.001 compared to untreated.
DISCUSSION
Information on tissue eosinophil distribution and the role of eosinophils in mediating fibrosis and motor abnormalities complicating human EoE is still limited. We conducted experiments with primary esophageal mesenchymal cells exposed to mediators associated with EoE and mediators known to induce fibrogenic and motility responses, and provide mechanistic evidence for a pathogenic role of eosinophils, eosinophil-derived factors and TGF-β1 in mediating the above clinically important complications.
Knowledge on the distribution of eosinophils in the esophageal wall is restricted to three case reports of full thickness specimens 20–22. Eosinophils were detected in all esophageal layers and reached the parasympathetic ganglion cells of the myenteric plexus, and transmural thickening and fibrosis were evident. We provide a fourth case using the highly sensitive and specific EPX staining for eosinophil identification 19. Our findings not only confirm the transmural nature of the disease, but also emphasize the interface of eosinophils with fibroblasts and muscle cells, making these mesenchymal cells direct targets of activated eosinophils and their products. It is difficult to extrapolate two-dimensional ex vivo tissue findings to biological outcomes of in vitro cell-cell interactions. Nevertheless, this functional scenario is amply supported by our results, underscoring the importance of mesenchymal cells in EoE pathogenesis, both in respect to fibrosis and dysmotility. Although the majority of studies have focused on the role of epithelium in EoE, there is disconnect between levels of epithelial eosinophilia and symptom severity, and a poor correlation between response to treatment and epithelial eosinophil count 23, 24. These discrepancies suggest that eosinophils acting only in the mucosa cannot explain the whole EoE pathophysiology, and eosinophils in the deeper esophageal layers may play a greater and complementary role than previously thought.
Initially we assessed which mediators were actively secreted in esophageal mucosal biopsies. This information is crucial as prior studies have been limited to whole tissue protein or gene expression alone, which limits the relevance for functional interpretation. We found an elevated secretion of IL-5, IL-6, IL-13, eotaxin-1 and TGF-β1 in EoE compared to control samples, with a close correlation of their levels with eosinophil numbers, eosinophil degranulation and the presence of microabscesses. This implicates the combined presence, distribution and activation of eosinophils in the expression of those cytokines.
To understand the effect of the ex vivo detected cytokines on local mesenchymal cells we tested those mediators on HEF and HEMC. In addition, we also tested other mediators reported in the EoE literature as well as mediators known to stimulate fibrogenesis and muscle contractility. Both cell types spontaneously secreted FN and collagen I and, while IL-4 and IL-5 increased collagen I secretion only in HEF, TGF-β1 potently upregulated their production by both HEF and HEMC. This shows selectivity in the induction of ECM by EoE-associated mediators, and reinforces the dominant role of TGF-β1 in EoE fibrogenesis. However, exposure to TGF-β1 failed to stimulate esophageal mesenchymal cells to produce TSLP, a cytokine eliciting basophil responses in EoE (Supplemental ref. 14).
Since eosinophils are known to be critical for fibrogenesis, we investigated whether eosinophil-mesenchymal interactions could stimulate ECM production by primary human esophageal mesenchymal cells. This was the case, and this effect was reduced by preventing direct cell-cell contact, indicating that both physical contact and soluble mediator secretion are involved in eosinophil-mediated ECM production by esophageal mesenchymal cells. This effect was not restricted to eosinophils, as T-cells, monocytes and mast cells were also able to increase ECM production upon contact with HEF and HEMC. This is compatible with a report showing that mast cells are increased in EoE, express TGF-β1 and increase contractility of smooth muscle cells 25. These and our own findings suggest a combined role for various immune cells in EoE fibrogenesis and dysmotility, underscoring the complex nature of cell infiltration in this condition. We also found that eosinophil sonicates can activate all major signaling TGF-β1 pathways in esophageal mesenchymal cells. In fact, blocking TGF-β1 signaling or p38MAPK signaling, or neutralizing biologically active TGF-β1 greatly reduced, but not completely eliminated, the increase in ECM production induced by TGF-β1 and eosinophil sonicates. These results highlight the prominent, though not exclusive, role of TGF-β1 in eosinophil-induced esophageal fibrosis.
The intimate contact of eosinophils to fibroblasts and muscle cells seen in EoE tissue is compatible with a functional interaction. This assumption was firmly supported by the finding that IL-4, IL-6, IL-13, TGF-β1, native human MBP and eosinophil sonicates dramatically increased the adhesion of eosinophils to mesenchymal cell monolayers. The powerful action of MPB was particularly intriguing considering that it failed to upregulate FN secretion by HEF, further highlighting the selective actions of individual eosinophil products. As in the case for ECM production, this effect was not unique for eosinophils as T-cell sonicates and EoE-related cytokines increased adhesion as well. The fact that non-eosinophil cells are also able to induce matrix production and leukocyte adhesion is intriguing, and suggests that other cells types present in lower numbers in EoE tissue may also be involved, to a less degree, in EoE pathogenic events.
IL-4 and IL-13 induced the expression of VCAM-1, but not ICAM-1, implying a selective cell adhesion molecule repertoire for leukocyte-mesenchymal cell interaction in EoE. Although TGF-β1 failed to upregulated VCAM-1 expression, blocking TGF-β1 or p38MAPK signaling markedly reduced the adhesion increased by TGF-β1 or eosinophil sonicates. Together, these results show that EoE-associated mediators, eosinophils and their products, in addition to promoting fibrogenesis, also lead to retention of eosinophils in EoE tissue, an event of particular pathogenic relevance in regard to the chronic nature of EoE. Thus, although multiple mediators are seemingly involved in esophageal fibrogenesis, eosinophil-derived TGF-β1 appears to be a dominant drive in this process. Additionally, HEF and HEMC responded similarly to all pro-fibrogenic stimuli, supporting a combined contribution to the transmural involvement of EoE.
Scarce information is available to explain the motility abnormalities in EoE. Mice deficient in the IL-13 decoy receptor IL-13α2, and therefore highly susceptible to IL-13-mediated responses, have hypercontractile responses to acetylcholine 26. Overexpression of IL-5 or IL-13 impairs carbachol-induced longitudinal esophageal contractility in experimental EoE, an effect independent of esophageal eosinophilia 27. While these reports imply separate mechanisms for fibrosis and motility abnormalities, our results suggest that these mechanisms may be combined and likely concomitant in EoE pathogenesis. In fact, esophageal muscle cells respond to various pro-fibrogenic stimuli and eosinophil products, and even mediators expressed in the esophageal epithelium can diffuse to the deeper muscle layers and impact on muscle function 28.
This led us to investigate the effect of mediators detected in the esophageal biopsy cultures in a neurogenic muscle contraction assay. A significantly reduced muscle contraction was observed upon incubation of the muscle strips with IL-6, IL-13, eotaxin-3 and TGF-β1. In addition, eosinophil sonicates and MPB reduced the muscle contractility in the electrical field, implying that both EoE-related cytokines and eosinophil products affect muscle contractility. Interestingly, the acetylcholine-induced muscle contraction remained unaffected, demonstrating that eosinophil products and EoE-related mediators affect the release of ACh from the neuromuscular junction rather than the response of the muscle to ACh.
We also established that eosinophil-mesenchymal cell interaction can lead to a remarkable increase in the secretion of IL-6, eotaxin-1 and TGF-β1, indicating that, in addition to epithelial derived mediators, deeper layers of the esophageal wall, namely the submucosa and muscle, are capable of releasing cytokines that affect muscle function 18, 29. Co-culturing the supernatants of eosinophil-mesenchymal cell cultures essentially abolished the neurogenic esophageal muscle contraction, without affecting contraction induced by direct muscle stimulation. This is compatible with a selective effect on neurons, but not on muscle cells. These results indicate that eosinophils and EoE-related products can alter esophageal muscle contraction and that the muscle itself upon contact with eosinophils can release cytokines that influence its function. These data provide a direct link of the motility abnormalities found in human EoE with eosinophils, inflammatory infiltrates and mesenchymal cell interaction.
In summary, using primary human experimental systems, we show a dual direct and indirect involvement of eosinophil-derived mediators in the fibrosis and dysmotility that characterize EoE. Thus, mediators relevant to EoE pathogenesis derive not only from the epithelial layer, but also from the submucosa and the muscle. Since in vivo they are most likely produced concomitantly, their effects should manifest at the same time, suggesting that fibrosis and dysmotility in EoE are concurrent events rather than separate or sequential to one another. While our study was focused primarily on the effect of eosinophils on esophageal mesenchymal cells, this represents a limitation, as complementary effects mediated by other infiltrating leukocytes and their products must occur in EoE. TGF-β1 appears to predominate in these processes, but EoE with its complications is still a multiple mediator disease, a concept that needs to be taken into account for developing novel pathophysiology-based therapeutic approaches.
Supplementary Material
Acknowledgments
Grant support: This work was supported by grants from the Deutsche Forschungsgemeinschaft (RI 1735/2-1 to F.R.), National Institutes of Health (1T32DK083251 to F.R., DK57030 to P.B., DK50984 and DK069854 to C.F.), Mayo Clinic Foundation (to J.J.L.) and from Astra Zeneca and Takeda (to G.F.). The authors acknowledge the support of the Department of Pathology of the Cleveland Clinic Foundation. Tissue samples were provided by the Human Tissue Procurement Facility of the Cleveland Clinic Foundation, Cleveland, Ohio.
Abbreviations
- ACh
Acetylcholine
- Col I
Collagen I
- ECM
Extracellular matrix
- EoE
Eosinophilic esophagitis
- EPX
Eosinophil peroxidase
- FN
Fibronectin
- H&E
Hematoxylin & eosin
- HCMC
Human cord blood derived mast cells
- HEF
Human esophageal fibroblasts
- HEMC
Human esohageal muscle cells
- ICAM
Intercellular adhesion molecule
- IL
Interleukin
- MBP
Major basic protein
- PBT
Peripheral blood T-cells
- TGF
Transforming growth factor
- VCAM
Vascular cell adhesion molecule
Footnotes
Disclosures: None by all authors
Author contribution
Florian Rieder: study concept and design; acquisition of data; drafting of the manuscript; critical revision of the manuscript for important intellectual content; obtained funding; analysis and interpretation of data; statistical analysis.
Ilche Nonevski: study design; acquisition of data; critical revision of the manuscript for important intellectual content; drafting of the manuscript; technical support.
Jie Ma: acquisition of data; technical and material support.
Zhufeng Ouyang: acquisition of data; analysis and interpretation of data; administrative and technical support.
Gail West: acquisition of data; analysis and interpretation of data; technical and material support.
Cheryl Protheroe: acquisition of data; analysis and interpretation of data; critical revision of the manuscript for important intellectual content; technical and material support.
Giovanni DePetris: acquisition of data; analysis and interpretation of data; technical and material support.
Anja Schirbel: acquisition of data; analysis and interpretation of data; technical and material support.
James Lapinski: acquisition of data; analysis and interpretation of data; technical and material support.
John Goldblum: study design; acquisition of data; analysis and interpretation of data; technical and material support.
Tracey Bonfield: acquisition of data; analysis and interpretation of data; technical and material support.
Rocio Lopez: study design; analysis and interpretation of data; critical revision of the manuscript for important intellectual content; technical and material support.
Karen Harnett: acquisition of data; analysis and interpretation of data; technical and material support.
Ikuo Hirano: acquisition of data; analysis and interpretation of data; critical revision of the manuscript for important intellectual content; technical and material support.
Gary Falk: study concept and design; acquisition of data; obtained funding; analysis and interpretation of data; critical revision of the manuscript for important intellectual content; technical and material support.
Piero Biancani: study concept and design; acquisition of data; obtained funding; analysis and interpretation of data; critical revision of the manuscript for important intellectual content; technical and material support.
Claudio Fiocchi: study concept and design; analysis and interpretation of data; drafting of the manuscript; critical revision of the manuscript for important intellectual content; obtained funding; administrative, technical and material support; study supervision.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors maybe discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Dellon ES, Gonsalves N, Hirano I, et al. ACG Clinical Guideline: Evidenced Based Approach to the Diagnosis and Management of Esophageal Eosinophilia and Eosinophilic Esophagitis (EoE) Am J Gastroenterol. 2013;108:679–692. doi: 10.1038/ajg.2013.71. [DOI] [PubMed] [Google Scholar]
- 2.Chehade M, Sampson HA, Morotti RA, et al. Esophageal subepithelial fibrosis in children with eosinophilic esophagitis. J Pediatr Gastroenterol Nutr. 2007;45:319–328. doi: 10.1097/MPG.0b013e31806ab384. [DOI] [PubMed] [Google Scholar]
- 3.Aceves SS, Newbury RO, Dohil R, et al. Esophageal remodeling in pediatric eosinophilic esophagitis. J Allergy Clin Immunol. 2007;119:206–212. doi: 10.1016/j.jaci.2006.10.016. [DOI] [PubMed] [Google Scholar]
- 4.Nurko S, Rosen R, Furuta GT. Esophageal dysmotility in children with eosinophilic esophagitis: a study using prolonged esophageal manometry. Am J Gastroenterol. 2009;104:3050–3057. doi: 10.1038/ajg.2009.543. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Hejazi RA, Reddymasu SC, Sostarich S, et al. Disturbances of esophageal motility in eosinophilic esophagitis: a case series. Dysphagia. 2010;25:231–237. doi: 10.1007/s00455-009-9248-6. [DOI] [PubMed] [Google Scholar]
- 6.Kwiatek MA, Hirano I, Kahrilas PJ, et al. Mechanical properties of the esophagus in eosinophilic esophagitis. Gastroenterology. 2011;140:82–90. doi: 10.1053/j.gastro.2010.09.037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Lee J, Huprich J, Kujath C, et al. Esophageal diameter is decreased in some patients with eosinophilic esophagitis and might increase with topical corticosteroid therapy. Clin Gastroenterol Hepatol. 2012;10:481–486. doi: 10.1016/j.cgh.2011.12.042. [DOI] [PubMed] [Google Scholar]
- 8.Roman S, Hirano I, Kwiatek MA, et al. Manometric features of eosinophilic esophagitis in esophageal pressure topography. Neurogastroenterol Motil. 2011;23:208–214. e111. doi: 10.1111/j.1365-2982.2010.01633.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Korsapati H, Babaei A, Bhargava V, et al. Dysfunction of the longitudinal muscles of the oesophagus in eosinophilic oesophagitis. Gut. 2009;58:1056–1062. doi: 10.1136/gut.2008.168146. [DOI] [PubMed] [Google Scholar]
- 10.Rothenberg ME. Biology and treatment of eosinophilic esophagitis. Gastroenterology. 2009;137:1238–1249. doi: 10.1053/j.gastro.2009.07.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Mishra A, Rothenberg ME. Intratracheal IL-13 induces eosinophilic esophagitis by an IL-5, eotaxin-1, and STAT6-dependent mechanism. Gastroenterology. 2003;125:1419–1427. doi: 10.1016/j.gastro.2003.07.007. [DOI] [PubMed] [Google Scholar]
- 12.Rothenberg ME, Hogan SP. The eosinophil. Annu Rev Immunol. 2006;24:147–174. doi: 10.1146/annurev.immunol.24.021605.090720. [DOI] [PubMed] [Google Scholar]
- 13.Kagalwalla AF, Akhtar N, Woodruff SA, et al. Eosinophilic esophagitis: epithelial mesenchymal transition contributes to esophageal remodeling and reverses with treatment. J Allergy Clin Immunol. 2012;129:1387–1396. e1387. doi: 10.1016/j.jaci.2012.03.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Plager DA, Stuart S, Gleich GJ. Human eosinophil granule major basic protein and its novel homolog. Allergy. 1998;53:33–40. doi: 10.1111/j.1398-9995.1998.tb04937.x. [DOI] [PubMed] [Google Scholar]
- 15.Aceves SS. Tissue remodeling in patients with eosinophilic esophagitis: what lies beneath the surface? J Allergy Clin Immunol. 2011;128:1047–1049. doi: 10.1016/j.jaci.2011.09.026. [DOI] [PubMed] [Google Scholar]
- 16.Rieder F, Cheng L, Harnett KM, et al. Gastroesophageal reflux disease-associated esophagitis induces endogenous cytokine production leading to motor abnormalities. Gastroenterology. 2007;132:154–165. doi: 10.1053/j.gastro.2006.10.009. [DOI] [PubMed] [Google Scholar]
- 17.Strong SA, Pizarro TT, Klein JS, et al. Proinflammatory cytokines differentially modulate their own expression in human intestinal mucosal mesenchymal cells. Gastroenterology. 1998;114:1244–1256. doi: 10.1016/s0016-5085(98)70431-7. [DOI] [PubMed] [Google Scholar]
- 18.Cao W, Cheng L, Behar J, et al. Proinflammatory cytokines alter/reduce esophageal circular muscle contraction in experimental cat esophagitis. Am J Physiol Gastrointest Liver Physiol. 2004;287:G1131–1139. doi: 10.1152/ajpgi.00216.2004. [DOI] [PubMed] [Google Scholar]
- 19.Protheroe C, Woodruff SA, de Petris G, et al. A novel histologic scoring system to evaluate mucosal biopsies from patients with eosinophilic esophagitis. Clin Gastroenterol Hepatol. 2009;7:749–755. e711. doi: 10.1016/j.cgh.2009.03.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Fontillon M, Lucendo AJ. Transmural eosinophilic infiltration and fibrosis in a patient with non-traumatic Boerhaave’s syndrome due to eosinophilic esophagitis. Am J Gastroenterol. 2012;107:1762. doi: 10.1038/ajg.2012.226. [DOI] [PubMed] [Google Scholar]
- 21.Nicholson AG, Li D, Pastorino U, et al. Full thickness eosinophilia in oesophageal leiomyomatosis and idiopathic eosinophilic oesophagitis. A common allergic inflammatory profile? J Pathol. 1997;183:233–236. doi: 10.1002/(SICI)1096-9896(199710)183:2<233::AID-PATH936>3.0.CO;2-Z. [DOI] [PubMed] [Google Scholar]
- 22.Saffari H, Peterson KA, Fang JC, et al. Patchy eosinophil distributions in an esophagectomy specimen from a patient with eosinophilic esophagitis: Implications for endoscopic biopsy. J Allergy Clin Immunol. 2012;130:798–800. doi: 10.1016/j.jaci.2012.03.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Straumann A, Conus S, Grzonka P, et al. Anti-interleukin-5 antibody treatment (mepolizumab) in active eosinophilic oesophagitis: a randomised, placebo-controlled, double-blind trial. Gut. 2010;59:21–30. doi: 10.1136/gut.2009.178558. [DOI] [PubMed] [Google Scholar]
- 24.Spergel JM, Rothenberg ME, Collins MH, et al. Reslizumab in children and adolescents with eosinophilic esophagitis: results of a double-blind, randomized, placebo-controlled trial. J Allergy Clin Immunol. 2012;129:456–463. 463 e451–453. doi: 10.1016/j.jaci.2011.11.044. [DOI] [PubMed] [Google Scholar]
- 25.Aceves SS, Chen D, Newbury RO, et al. Mast cells infiltrate the esophageal smooth muscle in patients with eosinophilic esophagitis, express TGF-beta1, and increase esophageal smooth muscle contraction. J Allergy Clin Immunol. 2010;126:1198–1204. e1194. doi: 10.1016/j.jaci.2010.08.050. [DOI] [PubMed] [Google Scholar]
- 26.Morimoto M, Morimoto M, Zhao A, Madden KB, et al. Functional importance of regional differences in localized gene expression of receptors for IL-13 in murine gut. J Immunol. 2006;176:491–495. doi: 10.4049/jimmunol.176.1.491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Mavi P, Rajavelu P, Rayapudi M, et al. Esophageal functional impairments in experimental eosinophilic esophagitis. Am J Physiol Gastrointest Liver Physiol. 2012;302:G1347–1355. doi: 10.1152/ajpgi.00013.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Cheng L, Cao W, Fiocchi C, et al. HCl-induced inflammatory mediators in cat esophageal mucosa and inflammatory mediators in esophageal circular muscle in an in vitro model of esophagitis. Am J Physiol Gastrointest Liver Physiol. 2006;290:G1307–1317. doi: 10.1152/ajpgi.00576.2005. [DOI] [PubMed] [Google Scholar]
- 29.Cao W, Cheng L, Behar J, et al. IL-1beta signaling in cat lower esophageal sphincter circular muscle. Am J Physiol Gastrointest Liver Physiol. 2006;291:G672–680. doi: 10.1152/ajpgi.00110.2006. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.