Abstract
Background
Encephalopathic neonates undergoing therapeutic hypothermia have increased risk for coagulopathy secondary to perinatal asphyxia and effects of cooling on the coagulation enzyme cascade. Thromboelastography (TEG) allows for a comprehensive assessment of coagulation that can be regulated for temperature. TEG has not been previously evaluated in newborns undergoing hypothermia treatment.
Methods
Encephalopathic neonates treated with systemic hypothermia were enrolled in this prospective observational study. Daily blood specimens were collected for standard coagulation tests and platelet counts during hypothermia and after rewarming. Concurrent TEG assays were performed at 33.5°C and 37.0°C for comparison.
Results
A total of 48 paired TEGs from 24 subjects were performed. Mean (± SD) birthweight was 3.2±0.7 Kg, gestational age 38.4±1.4 weeks, and 40% were male. TEG results differed significantly between assays performed at 37.0°C versus 33.5°C, indicating more impaired coagulation at 33.5°C. TEG parameters K, α, MA and CI were significantly associated with clinical bleeding (p<0.05). These remained significant (except for MA) after controlling for transfusion therapy.
Conclusions
TEG results are affected by temperature, consistent with the known association of hypothermia with coagulopathy. Several TEG parameters are predictive of clinical bleeding in newborns undergoing hypothermia. Selected cutpoints to predict bleeding risk are temperature dependent.
INTRODUCTION
Neonates with hypoxic ischemic encephalopathy (HIE) are at increased risk for coagulopathy (1, 2). Systemic oxygen deprivation impacting the liver and bone marrow impairs the synthesis of coagulation factors and platelets (3–6). It is also well established that a hypothermic environment exacerbates coagulation disturbances by decreasing enzymatic activity involved in the coagulation cascade (7–12). This phenomenon is increasingly important as therapeutic hypothermia is the current standard of care for infants affected by HIE(13–22).
There is wide variability amongst clinicians and institutions with regards to protocols for monitoring coagulation status in newborns undergoing therapeutic hypothermia. This is, in part, due to the uncertainty of whether transfusion therapy should target normalization of standard tests of coagulation versus a more conservative approach of initiating treatment only after clinical bleeding is observed. Algorithms to optimize transfusion therapy to be able to prevent clinical bleeding while minimizing exposure to excessive blood products are lacking. One difficulty arises from the fact that standard coagulation tests (i.e. activated partial thromboplastin time (aPTT), prothrombin time (PT) and international normalized ratio (INR)) are routinely performed at 37.0°C (8). This may not accurately reflect the in vivo condition of a patient undergoing moderate hypothermia who is maintained at a core temperature of 33.5°C (17). Alternative methods to assess coagulation status in this population are needed.
Thromboelastography (TEG) is a functional assay that evaluates the process of clot formation and degradation in a whole blood sample (23–26). In a single test, TEG comprehensively reflects the complex clotting process that involves coagulation factors, cellular components, enzymes, and highly organized feedback mechanisms that maintain equilibrium between clot formation and lysis (1). TEG can be easily calibrated to the temperature of the patient. Despite this advantage that is particularly attractive in neonates undergoing hypothermia, TEG has not been previously evaluated in this population at high risk for coagulopathy.
The aim of this study is to evaluate the utility of TEG in newborns undergoing therapeutic hypothermia. We hypothesized that TEG would demonstrate quantifiable differences when performed under normothermic (37.0°C) versus hypothermic (33.5°C) conditions. Secondarily, we hypothesized that TEG performed at 33.5°C would predict clinical bleeding in this high-risk neonatal population.
METHODS
Study Population
This prospective observational study was conducted at an outborn level 4 neonatal intensive care unit (NICU) in an academic free-standing children’s hospital. All patients meeting established criteria for treatment with hypothermia between August 2011 – July 2012 were approached for enrollment. An additional two patients with encephalopathy secondary to hyperammonemia were treated with hypothermia under an experimental protocol and were also included. All patients underwent whole-body therapeutic hypothermia according to the NICHD Neonatal Research Network protocol (17). Patients were cooled to an esophageal temperature of 33.5°C with a servo-regulated Blanketrol II (Cincinnati Sub-Zero Medical, Cincinnati, OH) cooling blanket. Verbal consent was obtained from the parent(s) of each participant and need for written documentation of informed consent was waived for this minimal risk study. The Children’s National Medical Center Institutional Review Board approved the study and all data was collected in compliance with Health Information Portability and Accountability Act regulations.
Data Collection
Demographic data (including gestational age, birthweight, gender), and clinical data (including presenting characteristics, bleeding and transfusion data) were prospectively collected after enrollment. Significant bleeding was defined as clinically overt bleeding (e.g. pulmonary hemorrhage, gastrointestinal bleeding, gross hematuria, or mucosal bleeding) associated with a decreased hemoglobin by 2g/dL in a twenty-four hour period, bleeding that required blood products or surgical intervention for hemostasis, or bleeding that was found in critical organ systems (i.e. intracranial, pulmonary, or retroperitoneal) (27). Bleeding data were noted for the 24 hour period following each TEG determination. Transfusions with fresh frozen plasma, cryoprecipitate and/or platelets for each corresponding time period were also recorded.
Specimen Collection and Processing
Standard coagulation tests are routinely monitored daily during hypothermia and after rewarming per the CNMC NICU protocol. Up to 3 serial TEGs were performed per subject at 0–24 hours, 24–48, and 48–72 hours of cooling. Whole blood specimens (2.7 mL) were collected from indwelling arterial lines into vials containing 0.3 mL of 3.2% sodium citrate. One milliliter of blood was removed for TEG analysis while the remainder was used for clinically indicated PT, APT and fibrinogen assays (using the STA-R Evolution coagulation analyzer (Diagnostica STAGO, Parsippany NJ) per manufacturer’s instructions). A separate specimen was simultaneously collected to perform routine complete blood counts using the XE-5000, (Sysmex USA, Mundelein IL). Specimens were hand delivered to the central laboratory for processing within 30 minutes of collection.
TEG Determinations
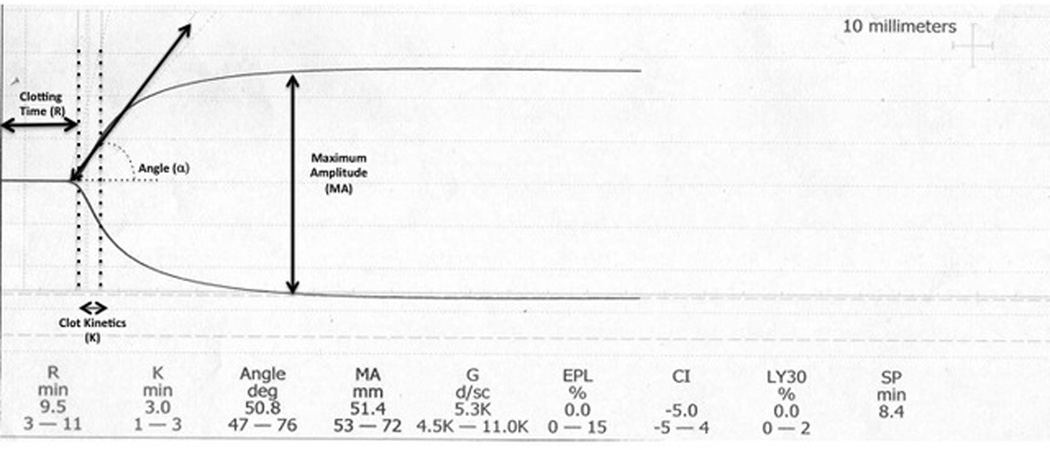
TEG determinations were performed on the dual-channel Haemoscope’s TEG 5000 analyzer (Haemonetics, Niles, IL) after Kaolin activation according to the manufacturer’s instructions. Briefly, 0.36mL of Kaolin activated, citrated whole blood is placed in a heparinase cup which is rotated 4°45’ six times per minute to imitate sluggish venous flow and activate the clotting cascade. A pin is suspended within the sample and as fibrin clot forms between the wall of the cup and the pin, the pin is displaced in synchrony with the cup. The transmitted rotation from the cup to the pin is transduced into a computer analyzer that produces a tracing called a thromboelastograph (Figure1). The four variables directly measured are: 1) Clotting Time (R): the time of latency until initial fibrin formation, 2) Clot Kinetics (K): the speed at which a specific level of clot strength is achieved (20 mm amplitude), 3) Angle (α): the rapidity of clot strengthening, and 4) Maximum Amplitude (MA): the ultimate strength of the clot. Additionally, a coagulation index (CI) is derived from the four directly measured variables. Finally, the LY30 measures the rate at which amplitude decreases 30 minutes after MA is achieved and is representative of clot breakdown or fibrinolysis. Therefore, impaired coagulation is reflected by a higher R, K, LY30 and/or lower MA, α, CI (23, 24). One channel of the TEG analyzer was calibrated to 37.0°C and the second to 33.5°C for simultaneous determination of TEG under normothermic and hypothermic conditions. Six quantitative measures from the TEG trace (R, K, α, MA, CI and LY30) were recorded for each paired TEG. To minimize channel bias, temperature settings of each channel were alternated with each new specimen.
1.
Representative example of thromboelastograph with measured parameters depicted schematically.
Statistical Analysis
Descriptive statistics include mean (± 95% confidence intervals) or median (range) for parametric and non-parametric continuous variables respectively. Categorical variables were expressed as frequencies. Bivariate analyses were performed to assess the effect of temperature on each of the six parameters measured by TEG (R, K, α, MA, CI, LY30) with paired-samples T-tests. To account for intrasubject correlation due to repeated measurements in each subject, temperature effect was further evaluated by a random effects longitudinal regression model with covariables including day of measurement and baseline coagulation status (defined by INR obtained upon admission to the NICU). Logistic regression models were used to explore the role of TEG (and standard tests of coagulation aPTT and INR) to predict clinical bleeding controlling for transfusion therapy. Parameters that were associated with bleeding were further evaluated using receiver operator curve (ROC) analyses in which an area under the curve (AUC) of 1 denotes 100% agreement between predicted and actual outcomes, whereas an AUC of 0.5 signifies no significant model discrimination. Coordinates of the ROCs with significant AUCs were used to determine cut-points with optimal sensitivity and specificity to predict clinical bleeding.
RESULTS
Study Population Characteristics
A total of twenty-four patients were enrolled and received treatment with therapeutic hypothermia either for HIE (n=22) or hyperammonemia (n=2). Characteristics of the study population are summarized in Table 1. Of the twenty-four patients enrolled, seventeen patients demonstrated a total of 27 bleeding events. These events included pulmonary hemorrhage (n=14), GI bleeding (n=17), hematuria (n=3), and mucosal (i.e. profuse umbilical stump and arterial line site) bleeding (n=2).
TABLE 1.
Clinical and Demographic Characteristics of Study Population
| HIE (n= 22) |
Hyperammonemia (n=2) |
|
|---|---|---|
| Birthweight (grams)a | 3273±699 | 3256±65 |
| Gestational Age (weeks)a | 38.74±1.36 | 38.65±0.92 |
| Male Gender n(%) | 11(50) | 0 (0) |
| Cesarean Delivery n(%) | 19 (86) | 1 (50) |
| Sentinel Event n(%) | 7 (32) | |
| Abruption n(%) | 3 (42.8) | N/A |
| Cord accident n(%) | 3 (42.8) | |
| Maternal collapse n(%) | 1 (14.2) | |
| Apgar at 1 minute | 1(1,5) | 8 (8,8) |
| 5 minutes | 3 (1,9) | 9 (9,9) |
| 10 minutes | 4 (2,7) | N/A |
| Presenting pH | 7.01(6.65, 7.35) | N/A |
| Base Deficit | −16 (−34, −6) | N/A |
| Hour of Life Hypothermia Initiated (hours:minutes) | 4:40 (2:4, 5:58) | 89:50 (28:54, 120:45) |
| EEG seizure n(%) | 4 (18.2) | 0 (0) |
Data presented as median (range) except where indicated.
Mean ± standard deviation
Effect of Temperature on TEG
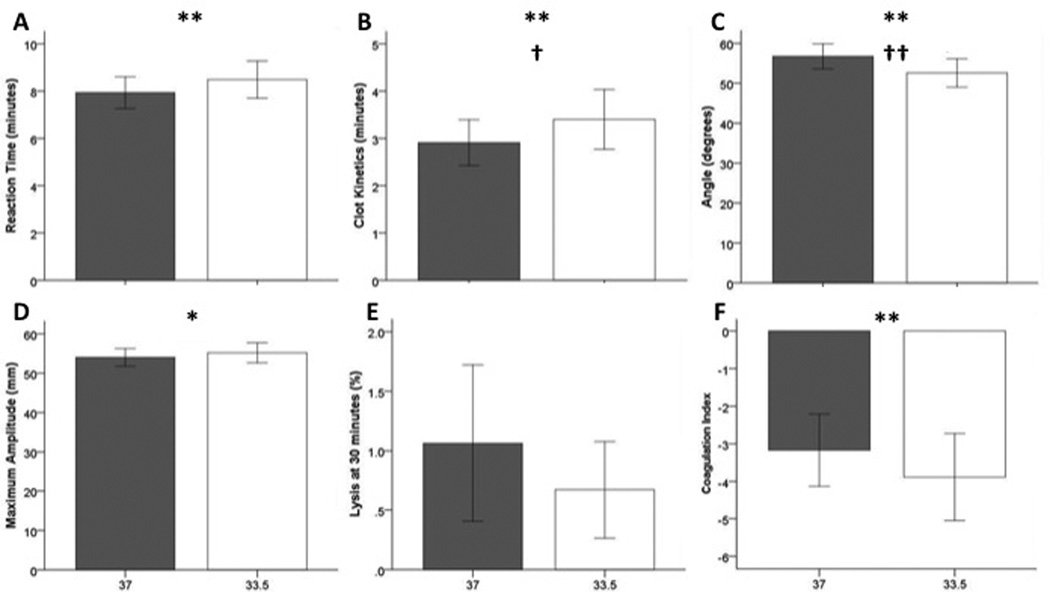
A total of forty-eight paired TEGs were performed at 33.5°C and 37.0°C. Bivariate analyses demonstrated significant differences in TEG R, K, α and CI (p<0.01, Figure 2). Differences in each TEG measure indicated more impaired coagulation in the assays performed at 33.5°C. These differences remained significant for TEG K (β=−0.148, SE 0.068, 95% CI −0.281 to −0.013, p=0.031) and α (β=1.167, SE 0.371, 95% CI 0.440 to 1.894, p=0.002) in the multivariate longitudinal regression models.
2.
Effect of temperature on TEG parameters. The gray bars represent 37°C while the white bars represent 33.5°C. All bars represent mean ± 95% confidence interval. Significant differences by paired T-tests are shown with asterisks (*p<0.05, **p<0.001). Significant adjusted p values are marked with daggers (†p<0.05, ††p<0.01).
TEG to Predict Bleeding
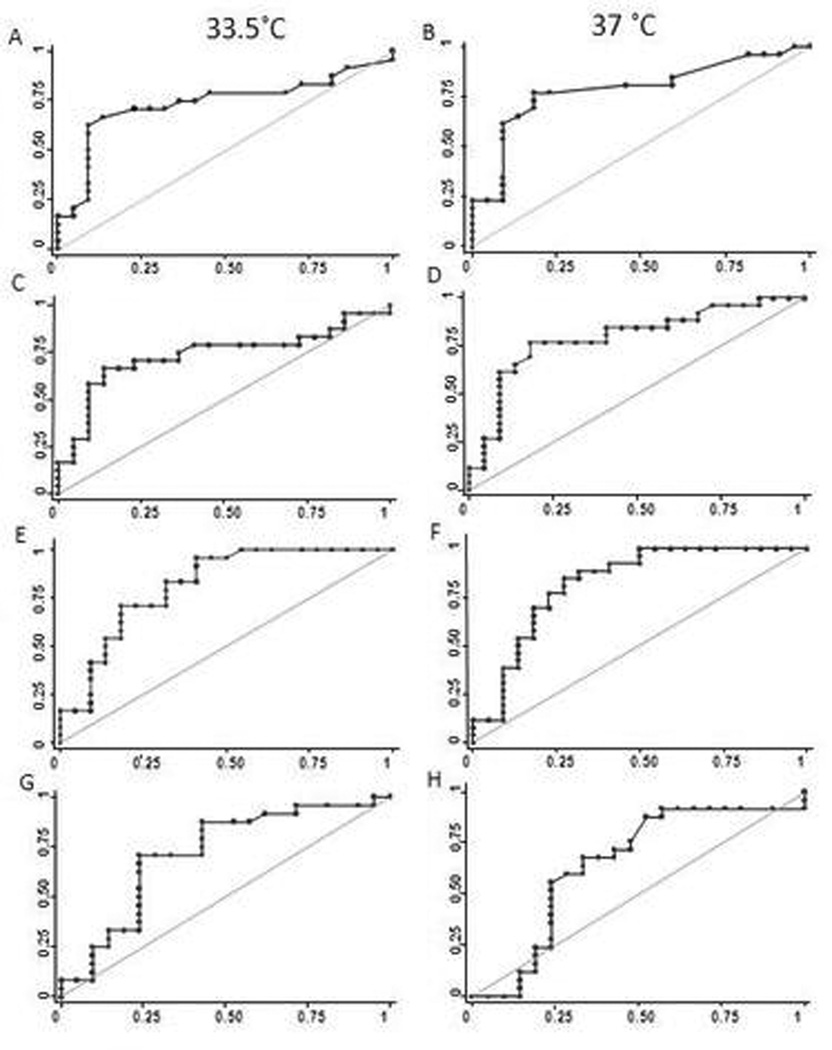
Several TEG parameters including K, α MA and CI demonstrated an association with clinical bleeding (p<0.05). These associations were significant for K, α and CI at 37.0°C and α at 33.5°C after controlling for transfusion therapy. Logistic regression model results are summarized in Table 2. aPTT (p=0.961), INR (p=0.363) and platelet count (p=0.718) were not associated with clinical bleeding. Receiver operator curves demonstrated significant AUCs for TEG K, α and MA at both 33.5°C and 37.0°C, and CI at 33.5°C. (Figure 3). While ROCs were significant at both temperatures, the selected cutpoint differed based on temperature.
Table 2.
Summary of Regression Analysis Results Evaluating the Association of TEG with Clinical Bleeding
| TEG Parameter |
Adjusted Odds Ratio |
Standard Error |
95% CI | P value | |
|---|---|---|---|---|---|
| 37°C | R | 1.075 | 0.273 | 0.654–1.769 | 0.773 |
| K | 4.156 | 2.837 | 1.09–15.845 | 0.037 | |
| α | 0.849 | 0.062 | 0.737–0.979 | 0.025 | |
| MA | 0.770 | 0.110 | 0.582–1.019 | 0.067 | |
| CI | 0.637 | 0.146 | 0.407–0.999 | 0.049 | |
| LY30 | 0.832 | 0.261 | 0.450–1.540 | 0.559 | |
| 33.5°C | R | 1.056 | 0.224 | 0.696–1.601 | 0.798 |
| K | 2.123 | 0.849 | 0.970–4.649 | 0.060 | |
| α | 0.879 | 0.056 | 0.775–0.996 | 0.044 | |
| MA | 0.781 | 0.102 | 0.605–1.007 | 0.057 | |
| CI | 0.732 | 0.101 | 0.521–1.027 | 0.071 | |
| LY30 | 0.392 | 0.348 | 0.691–2.228 | 0.291 |
3.
Receiver operating curves for K, α, MA and CI. The diagonal line represents an Area under the curve (AUC) of 0.5, indicating a non-informative test. Panel A: K performed at 33.5°C. AUC = 0.7377, Cutpoint = 3.4 min, Sensitivity = 62.5% and Specfificity = 90.9%. Panel B: K performed at 37°C. AUC = 0.7876, Cutpoint = 2.6 min, Sensitivity = 76.9%, Specificity = 81.8%. Panel C: α performed at 33.5°C. AUC = 07377, Cutpoint = 52.3 degrees, Sensitivity = 66.7% and Specfificity = 88.4%. Panel D: α performed at 37°C. AUC = 0.7955, Cutpoint = 57.6 degrees, Sensitivity = 76.9%, Specificity = 81.8%. Panel E: MA performed at 33.5°C. AUC = 0.8172, Cutpoint = 56.1 mm, Sensitivity = 70.83% and Specfificity = 81.82. Panel F: MA performed at 37°C. AUC = 0.8217, Cutpoint = 58.1 mm, Sensitivity = 88.46%, Specificity = 61.18%. Panel G: CI performed at 33.5°C. AUC = 0.7173, Cutpoint = −4.6, Sensitivity = 70.83% and Specfificity = 76.19%. Panel H: CI performed at 37°C. AUC = 0.6476, Cutpoint = −3.3, Sensitivity = 68%, Specificity = 66.67%.
DISCUSSION
This is the first study conducted to evaluate the effect of temperature on individual TEG parameters and to evaluate the potential utility of TEG to monitor coagulation status in newborns undergoing therapeutic hypothermia. These data demonstrate quantifiable differences in coagulation status based on temperature, with more impaired coagulation under hypothermic conditions. Thus, standard tests of coagulation performed on blood warmed to 37.0°C may underestimate coagulopathy in patients that are undergoing hypothermia. An association with clinical bleeding could be demonstrated with several TEG parameters while standard tests of coagulation (aPTT and INR) were not associated with bleeding. This provides further evidence that TEG may serve as the preferred method of monitoring coagulation status in this population.
While TEG has been routinely utilized in adult populations for the past 20 years (24–26), it has only recently emerged for use in pediatrics. Early reports have demonstrated its utility in monitoring coagulation status in children requiring cardiac bypass, liver transplant, neurosurgery and extracorporeal membrane oxygenation (28–32). While normative data is available for pediatric patients (33), there is limited data reported in neonates. Only one study reported references ranges for newborns based on umbilical cord blood specimens (23). However, it is unknown whether these values represent normative values for neonates in the first days of life post-partum. The data provided in this study support that further evaluation in neonatal populations, particularly critically ill neonates at high-risk for coagulopathy, is warranted. An advantage of TEG is the ease of calibration for temperature, which is of particular interest in patients undergoing hypothermia. The two variables most influenced by temperature were K and α. These two parameters are the most reflective of clotting factor and enzymatic activity. Under hypothermic conditions, clot kinetics were slower indicating a delay in enzymatic activity and α was narrower indicating lower efficiency in attaining maximum clot strength. Other TEG variables including clot reaction time and coagulation index also trended in a direction consistent with the hypothesis that hypothermia slows enzymatic activity and impairs the coagulation cascade. Of interest, MA, which reflects platelet activity, did not vary based on temperature although there is known cold inhibition on platelet function (34). It should be noted that TEG may be insensitive to mild qualitative platelet dysfunction and likely more sensitive to platelet count (i.e. thrombocytopenia). For example, effects of aspirin and clopidigrel used in Berlin Heart protocols were not readily reflected with regular TEG, and required platelet mapping (35). That LY30 did not appear to vary based on temperature is not surprising as this variable is reflective of a process less dependent on initial enzymatic activity (i.e. LY30 measures clot stability).
Previous multi-center cooling trials did not report increased rates of major hemorrhage or bleeding-related deaths in patients undergoing this therapy. However, consistent findings across studies include thrombocytopenia and prolonged PT/aPTT likely associated with need for transfusion therapy to prevent bleeding risk (13, 17–22). Overall rates of clinical bleeding requiring intervention are not well described by prior studies. Our study suggests that these events may occur frequently in this population. The definition of clinically relevant bleeding used in our study was based on recommendations by the International Society of Thrombosis and Haemostasis (27). While the bleeding events demonstrated in patients enrolled in this study were not life threatening in nature, they all required transfusion therapy or other active intervention (e.g. increase in positive end expiratory pressure in cases of pulmonary hemorrhage).. It is unclear whether transfusion therapy should be targeted to normalizing standard tests of coagulation or conservatively initiating therapy only after clinical bleeding is exhibited. Data from this study suggests that the former approach is problematic given the poor discriminatory ability of standard tests of coagulation to predict clinical bleeding. In an ideal setting transfusion therapy should be targeted to a specific product administered with an appropriate dose to prevent any bleeding. This study provides evidence that TEG may provide a better method to monitor bleeding risk and provide guidance for rational transfusion therapy.
Another benefit of TEG is that it provides a global measure of coagulation in a single test, utilizing a small blood volume (0.36mL). This is of particular interest in the critically ill neonatal population given the frequency of blood sampling is often problematic and can lead to iatrogenic anemia. Each of the current standard tests used to assess coagulation status (PT, aPTT, INR, fibrinogen and platelet count) evaluate one aspect of a complex clotting process. These are often ordered and interpreted as a battery of tests that may be replaceable with the information provided by TEG.
TEG parameters at both at 33.5°C and 37.0°C were found to be predictive of clinical bleeding. Of great importance, however, is the observation of different cutpoints to predict bleeding at the two temperatures. For example, the cutpoint for TEG K performed at 37°C is 2.6 minutes, which actually falls within the normal adult range for this parameter. In contrast, when the assay is performed at 33.5°C (i.e. reflecting the hypothermic environment that the patient is experiencing) the cutpoint for TEG K is higher at 3.4 minutes. Thus for accurate interpretation, TEG assays should be performed under temperature-regulated conditions (ie: at 33.5°C for patients undergoing therapeutic hypothermia). Depending on institutional or laboratory preferences, an alternative strategy would include performing TEG under normothermic conditions and using different interpretive thresholds as provided in this study. Regardless, it is notable that several TEG parameters were more predictive of clinical bleeding than aPTT and INR.
This study has limitations. Although repeated measures provided improved power for statistical analyses, the small number of patients included may have limited our ability to achieve statistical significance where statistical trends were observed. Thus, the association between TEG values and clinical bleeding warrants investigation in a larger population. Sample size limitations also affected our ability to evaluate if these relationships differed between the patients cooled for neonatal encephalopathy versus hyperammonemia. Although animal evidence suggests that elevated ammonia may impact platelet function directly, (36) the coagulation disturbances in this population are likely attributable to the association of hyperammonemia with transaminitis and liver dysfunction reflected by a decrease in hepatic synthetic function (37). These anomalies would be revealed in TEG measures as described. We attempted to define “clinically significant” bleeding events. Commonly, iatrogenic trauma from nasogastric tube placement can lead to bloody gastric residuals, traumatic intubation can lead to bloody endotracheal tube secretions, and urinary catheter placement can lead to gross hematuria. These bleeding events may occur without underlying coagulopathy. These types of bleeding events were excluded unless they were observed repeatedly and met the defined criteria for clinically significant bleeding (i.e. decreased hemoglobin by 2g/dL in 24 hours, bleeding that required blood products for hemostasis or was found in critical organ). Due to obvious feasibility limitations, we were unable to obtain TEG data from healthy newborns for comparison. As previously discussed, normative data for TEG parameters in peripheral blood specimens from neonatal patients is lacking. Thus, the proposed cutpoints to predict bleeding cannot be interpreted in comparison to normative values for neonates.
CONCLUSIONS
TEG clot kinetics and angle are altered by temperature, providing a method to quantify impaired coagulation under hypothermic conditions. Several TEG parameters (K, α, MA, CI) are predictive of clinical bleeding in newborns undergoing therapeutic hypothermia while current standard measurements of coagulopathy (aPTT, INR) were not predictive of bleeding. TEG may be a preferable way to monitor bleeding risk and guide transfusion therapy in this high-risk neonatal population.
ACKNOWLEDGEMENTS
The authors acknowledge Jianping (James) He, M.S. for his data management and statistical support.
This project was financially supported by The Haemonetics Corporation. The sponsors had no role in the design and conduct of the study; in the collection, management, analysis and interpretation of data; or in the preparation, review or approval of the manuscript.
Footnotes
The authors have no financial relationships relevant to this article to disclose.
REFERENCES
- 1.Bauman ME, Cheung PY, Massicotte MP. Hemostasis and platelet dysfunction in asphyxiated neonates. J Pediatr. 2011;158:e35–e39. doi: 10.1016/j.jpeds.2010.11.011. [DOI] [PubMed] [Google Scholar]
- 2.Suzuki S, Morishita S. Hypercoagulability and DIC in high-risk infants. Semin Thromb Hemost. 1998;24:463–466. doi: 10.1055/s-2007-996040. [DOI] [PubMed] [Google Scholar]
- 3.Shah P, Riphagen S, Beyene J, Perlman M. Multiorgan dysfunction in infants with post-asphyxial hypoxic-ischaemic encephalopathy. Arch Dis Child Fetal Neonatal Ed. 2004;89:F152–F155. doi: 10.1136/adc.2002.023093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Sarkar S, Barks JD, Bhagat I, Donn SM. Effects of therapeutic hypothermia on multiorgan dysfunction in asphyxiated newborns: whole-body cooling versus selective head cooling. J Perinatol. 2009;29:558–563. doi: 10.1038/jp.2009.37. [DOI] [PubMed] [Google Scholar]
- 5.Castle V, Andrew M, Kelton J, Giron D, Johnston M, Carter C. Frequency and mechanism of neonatal thrombocytopenia. J Pediatr. 1986;108:749–755. doi: 10.1016/s0022-3476(86)81059-9. [DOI] [PubMed] [Google Scholar]
- 6.Roberts IA, Murray NA. Thrombocytopenia in the newborn. Curr Opin Pediatr. 2003;15:17–23. doi: 10.1097/00008480-200302000-00004. [DOI] [PubMed] [Google Scholar]
- 7.Michelson AD, Barnard MR, Khuri SF, Rohrer MJ, MacGregor H, Valeri CR. The effects of aspirin and hypothermia on platelet function in vivo. Br J Haematol. 1999;104:64–68. doi: 10.1046/j.1365-2141.1999.01146.x. [DOI] [PubMed] [Google Scholar]
- 8.Reed RL, 2nd, Johnson TD, Hudson JD, Fischer RP. The disparity between hypothermic coagulopathy and clotting studies. J Trauma. 1992;33:465–470. doi: 10.1097/00005373-199209000-00022. [DOI] [PubMed] [Google Scholar]
- 9.Reed RL, 2nd, Bracey AW, Jr, Hudson JD, Miller TA, Fischer RP. Hypothermia and blood coagulation: dissociation between enzyme activity and clotting factor levels. Circ Shock. 1990;32:141–152. [PubMed] [Google Scholar]
- 10.Rohrer MJ, Natale AM. Effect of hypothermia on the coagulation cascade. Crit Care Med. 1992;20:1402–1405. doi: 10.1097/00003246-199210000-00007. [DOI] [PubMed] [Google Scholar]
- 11.Straub A, Breuer M, Wendel HP, Peter K, Dietz K, Ziemer G. Critical temperature ranges of hypothermia-induced platelet activation: possible implications for cooling patients in cardiac surgery. Thromb Haemost. 2007;97:608–616. doi: 10.1160/th06-10-0563. [DOI] [PubMed] [Google Scholar]
- 12.Wolberg AS, Meng ZH, Monroe DM, 3rd, Hoffman M. A systematic evaluation of the effect of temperature on coagulation enzyme activity and platelet function. J Trauma. 2004;56:1221–1228. doi: 10.1097/01.ta.0000064328.97941.fc. [DOI] [PubMed] [Google Scholar]
- 13.Jacobs SE, Berg M, Hunt R, Tarnow-Mordi WO, Inder TE, Davis PG. Cooling for newborns with hypoxic ischaemic encephalopathy. Cochrane Database Syst Rev. 2013;1:CD003311. doi: 10.1002/14651858.CD003311.pub3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Shankaran S. Therapeutic hypothermia for neonatal encephalopathy. Curr Treat Options Neurol. 2012;14:608–619. doi: 10.1007/s11940-012-0200-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Shankaran S, Pappas A, McDonald SA, et al. Childhood outcomes after hypothermia for neonatal encephalopathy. N Engl J Med. 2012;366:2085–2092. doi: 10.1056/NEJMoa1112066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Shankaran S, Laptook AR, Tyson JE, et al. Evolution of encephalopathy during whole body hypothermia for neonatal hypoxic-ischemic encephalopathy. J Pediatr. 2012;160:567–572. e563. doi: 10.1016/j.jpeds.2011.09.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Shankaran S, Laptook AR, Ehrenkranz RA, et al. Whole-body hypothermia for neonates with hypoxic-ischemic encephalopathy. N Engl J Med. 2005;353:1574–1584. doi: 10.1056/NEJMcps050929. [DOI] [PubMed] [Google Scholar]
- 18.Azzopardi D, Brocklehurst P, Edwards D, et al. The TOBY Study. Whole body hypothermia for the treatment of perinatal asphyxial encephalopathy: a randomised controlled trial. BMC Pediatr. 2008;8:17. doi: 10.1186/1471-2431-8-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Gunn AJ, Wyatt JS, Whitelaw A, et al. Therapeutic hypothermia changes the prognostic value of clinical evaluation of neonatal encephalopathy. J Pediatr. 2008;152:55–58. doi: 10.1016/j.jpeds.2007.06.003. 58 e51. [DOI] [PubMed] [Google Scholar]
- 20.Wyatt JS, Gluckman PD, Liu PY, et al. Determinants of outcomes after head cooling for neonatal encephalopathy. Pediatrics. 2007;119:912–921. doi: 10.1542/peds.2006-2839. [DOI] [PubMed] [Google Scholar]
- 21.Gluckman PD, Wyatt JS, Azzopardi D, et al. Selective head cooling with mild systemic hypothermia after neonatal encephalopathy: multicentre randomised trial. Lancet. 2005;365:663–670. doi: 10.1016/S0140-6736(05)17946-X. [DOI] [PubMed] [Google Scholar]
- 22.Eicher DJ, Wagner CL, Katikaneni LP, et al. Moderate hypothermia in neonatal encephalopathy: efficacy outcomes. Pediatr Neurol. 2005;32:11–17. doi: 10.1016/j.pediatrneurol.2004.06.014. [DOI] [PubMed] [Google Scholar]
- 23.Edwards RM, Naik-Mathuria BJ, Gay AN, Olutoye OO, Teruya J. Parameters of thromboelastography in healthy newborns. Am J Clin Pathol. 2008;130:99–102. doi: 10.1309/LABNMY41RUD099J2. [DOI] [PubMed] [Google Scholar]
- 24.Luddington RJ. Thrombelastography/thromboelastometry. Clin Lab Haematol. 2005;27:81–90. doi: 10.1111/j.1365-2257.2005.00681.x. [DOI] [PubMed] [Google Scholar]
- 25.Scarpelini S, Rhind SG, Nascimento B, et al. Normal range values for thromboelastography in healthy adult volunteers. Braz J Med Biol Res. 2009;42:1210–1217. doi: 10.1590/s0100-879x2009001200015. [DOI] [PubMed] [Google Scholar]
- 26.Nair SC, Dargaud Y, Chitlur M, Srivastava A. Tests of global haemostasis and their applications in bleeding disorders. Haemophilia 16 Suppl 5. 2010:85–92. doi: 10.1111/j.1365-2516.2010.02304.x. [DOI] [PubMed] [Google Scholar]
- 27.Mitchell LG, Goldenberg NA, Male C, et al. Definition of clinical efficacy and safety outcomes for clinical trials in deep venous thrombosis and pulmonary embolism in children. J Thromb Haemost. 2011;9:1856–1858. doi: 10.1111/j.1538-7836.2011.04433.x. [DOI] [PubMed] [Google Scholar]
- 28.Haizinger B, Gombotz H, Rehak P, Geiselseder G, Mair R. Activated thrombelastogram in neonates and infants with complex congenital heart disease in comparison with healthy children. Br J Anaesth. 2006;97:545–552. doi: 10.1093/bja/ael206. [DOI] [PubMed] [Google Scholar]
- 29.Moganasundram S, Hunt BJ, Sykes K, et al. The relationship among thromboelastography, hemostatic variables, and bleeding after cardiopulmonary bypass surgery in children. Anesth Analg. 2010;110:995–1002. doi: 10.1213/ANE.0b013e3181cd6d20. [DOI] [PubMed] [Google Scholar]
- 30.Alexander DC, Butt WW, Best JD, Donath SM, Monagle PT, Shekerdemian LS. Correlation of thromboelastography with standard tests of anticoagulation in paediatric patients receiving extracorporeal life support. Thromb Res. 2010;125:387–392. doi: 10.1016/j.thromres.2009.07.001. [DOI] [PubMed] [Google Scholar]
- 31.Kang Y, Borland LM, Picone J, Martin LK. Intraoperative coagulation changes in children undergoing liver transplantation. Anesthesiology. 1989;71:44–47. doi: 10.1097/00000542-198907000-00008. [DOI] [PubMed] [Google Scholar]
- 32.Goobie SM, Soriano SG, Zurakowski D, McGowan FX, Rockoff MA. Hemostatic changes in pediatric neurosurgical patients as evaluated by thrombelastograph. Anesth Analg. 2001;93:887–892. doi: 10.1097/00000539-200110000-00017. [DOI] [PubMed] [Google Scholar]
- 33.Chan KL, Summerhayes RG, Ignjatovic V, Horton SB, Monagle PT. Reference values for kaolin-activated thromboelastography in healthy children. Anesth Analg. 2007;105:1610–1613. doi: 10.1213/01.ane.0000287645.26763.be. table of contents. [DOI] [PubMed] [Google Scholar]
- 34.Christensen RD, Sheffield MJ, Lambert DK, Baer VL. Effect of therapeutic hypothermia in neonates with hypoxic-ischemic encephalopathy on platelet function. Neonatology. 2012;101:91–94. doi: 10.1159/000329818. [DOI] [PubMed] [Google Scholar]
- 35.Fraser CD, Jr, Jaquiss RD, Rosenthal DN, et al. Prospective trial of a pediatric ventricular assist device: Supplimentary Material - Protocol - Appendix H: Anticoagulation and platelet inhibition protocol. N Engl J Med. 2012;367 doi: 10.1056/NEJMoa1014164. [DOI] [PubMed] [Google Scholar]
- 36.Shinya H, Matsuo N, Takeyama N, Tanaka T. Hyperammonemia inhibits platelet aggregation in rats. Thrombosis Research. 1996;81:195–201. doi: 10.1016/0049-3848(95)00236-7. [DOI] [PubMed] [Google Scholar]
- 37.Gibson JB, Berry GT. Pathophysiology of metabolic disease of the live. In: Polin RA, Fox WW, Abman SH, editors. Fetal and Neonatal Physiology. Philadelphia: Pa:W.B. Saunders Co; 2004. pp. 1211–1218. [Google Scholar]