Abstract
BACKGROUND & AIMS
Patients with hepatopulmonary syndrome (HPS) are prioritized for liver transplantation (given exception points) due to their high pre- and post-transplantation mortality. However, few studies have evaluated the outcomes of these patients.
METHODS
We performed a retrospective cohort study using data submitted to the United Network for Organ Sharing in a study of the effects of room-air oxygenation on pre- and post-transplantation outcomes of patients with HPS. We identified thresholds associated with post-transplantation survival using cubic spline analysis and compared overall survival times of patients with and without HPS.
RESULTS
From 2002 through 2012, nine hundred and seventy-three patients on the liver transplant waitlist received HPS exception points. There was no association between oxygenation and waitlist mortality among patients with HPS exception points. Transplant recipients with more severe hypoxemia had increased risk of death after liver transplantation. Rates of 3-year unadjusted post-transplantation survival were 84% for patients with PaO2 of 44.1–54.0 mm Hg vs 68% for those with PaO2 ≤ 44.0 mm Hg. In multivariable Cox models, transplant recipients with an initial room-air PaO2 ≤ 44.0 mm Hg had significant increases in post-transplantation mortality (hazard ratio = 1.58; 95% confidence interval [CI]: 1.15–2.18) compared with those with a PaO2 of 44.1–54.0 mm Hg. Overall mortality was significantly lower among waitlist candidates with HPS exception points than those without (hazard ratio = 0.82; 95% CI: 0.70–0.96), possibly because patients with HPS have a reduced risk of pre-transplantation mortality and similar rate of post-transplantation survival.
CONCLUSIONS
Although there was no association between pre-transplantation oxygenation and waitlist survival in patients with HPS Model for End-Stage Liver Disease exception points, a pre-transplantation room-air PaO2 ≤ 44.0 mm Hg was associated with increased post-transplantation mortality. HPS Model for End-Stage Liver Disease exception patients had lower overall mortality compared with others awaiting liver transplantation, suggesting that the appropriateness of the HPS exception policy should be reassessed.
Keywords: UNOS, MELD, Allocation, Gas Exchange
Hepatopulmonary syndrome (HPS) is a pulmonary vascular disorder characterized by altered gas exchange due to intrapulmonary vascular dilatations occurring in the setting of hepatic dysfunction, usually with portal hypertension.1,2 It is a common complication found in up to 32% of patients1–4 with portal hypertension and cirrhosis and is associated with worse health-related quality of life, functional class, and a doubling in the risk of death among patients evaluated for liver transplantation.5
The current US liver transplant allocation system prioritizes patients based on medical urgency using the Model for End-Stage Liver Disease (MELD) score, derived from measurements of serum bilirubin, international normalized ratio, and creatinine. Although the MELD score correlates with 3-month survival due to end-stage liver disease,6,7 it does not account for post-transplantation outcomes or consider complications that influence outcomes independent of severity of liver disease.1–4,8,9 As a result, waitlist candidates with certain conditions, including HPS and hypoxemia (defined as partial pressure of oxygen in arterial blood [PaO2] <60 mm Hg), who are thought to have increased post-transplantation mortality, have been eligible for exception points to increase their waitlist priority.
There have been conflicting reports on the association between pre-transplantation oxygenation and post-transplantation mortality in HPS.1–4,10,11 However, published studies have limited sample sizes (<75) and HPS MELD exception oxygenation cut points are derived from a small cohort of patients.8 To address the impact of HPS MELD exception policy on outcomes, we examined the relationship between oxygenation and outcomes in a large cohort of patients who received HPS exception points and compared survival in HPS vs non-HPS patients.
Materials and Methods
All analyses used Organ Procurement and Transplant Network (OPTN)/United Network for Organ Sharing (UNOS) data from February 27, 2002 until December 14, 2012. The start date of February 27, 2002 was the inception of MELD-based allocation, and the first date waitlist candidates with HPS could receive MELD exception points. Follow-up time for waitlist candidates with HPS waitlisted before this date began on the date of the first approved MELD exception after the start date.
Study Sample
The HPS cohort included all adult (age 18 years or older) waitlist candidates registered for their first liver transplant who applied for an HPS exception on or after February 27, 2002, had documented HPS based on data provided in the exception narrative (Table 1), and at least 1 exception application approved. These criteria were used because <5% of exception applications included the primary data to meet strict HPS diagnostic criteria.1,4,5,8,12–15 We excluded patients with portopulmonary hypertension miscoded as HPS. Each exception narrative was reviewed by a single investigator (SB) with a random sample receiving a secondary review (DG). Waitlist candidates might have been listed before implementation of MELD-based allocation.
Table 1.
Inclusion Criteria Defining Waitlist Candidates With HPS Based on Exception Narrative Data
| Criteria | Data requirements |
|---|---|
| Strict HPS criteria1,4–6,10–12 | Alveolar-arterial gradient ≥15 mm Hg, or ≥20 mm Hg if age older than 60 y |
| Intrapulmonary shunting on transthoracic echocardiogram or >6% shunt fraction on macroaggregated albumin scan | |
| No evidence of severe restrictive or obstructive pulmonary disease1,4–6,10–12 | |
| Hypoxia/hypoxemia + intrapulmonary shunting | Hypoxemia defined as:
|
| Intrapulmonary shunting (right→left bubbles on echocardiogram after 3 cardiac cycles and/or free text stating “intrapulmonary shunting”) | |
| No evidence of concurrent cardiopulmonary disease | |
| HPS defined by transplantation center | Statement that patient met HPS diagnostic criteria without specific objective data |
The non-HPS cohort included all adult waitlist candidates registered for their first transplantation on or after February 27, 2002. We excluded patients who received non-HPS exceptions to create a comparison group whose waitlist priority was based on laboratory MELD score (this included the 63 excluded portopulmonary hypertension exception patients misclassified as HPS). Secondary analyses were restricted to a more focused non-HPS comparator group whose laboratory MELD score at waitlisting (determining waitlist priority) was 21–23, as HPS exception patients initially receive 22 MELD points.16
Outcomes
Our main outcome was patient survival. Pre-transplantation death was defined by UNOS removal code of “died” and UNOS removal code “too sick to transplant” or “other” in the setting of a confirmed Social Security Death Master File death date within 90 days of waitlist removal.16,17 Death within a short time from waitlist removal is reflective of severity of illness and viewed as equivalent to dying on the waitlist.16,17
Statistical Analysis
Pre-transplantation oxygenation and out-comes in HPS
We first fit competing risk Cox regression models to evaluate pre-transplantation survival, considering transplantation as a competing risk, as it influences the probability of waitlist removal for death or clinical deterioration.18 Death on the waitlist or within 90 days of removal was the outcome,18–20 and all other outcomes were censored (eg, condition improved). We categorized HPS patients using room-air PaO2 at the time of initial exception approval using previously defined PaO2 cut points (ie, <50 mm Hg, 50–59 mm Hg, and ≥60 mm Hg). We estimated the PaO2 of patients with room-air pulse oximetry only using formulas described previously.15,21–23 We analyzed the entire HPS cohort and, secondarily, the restricted cohort with confirmed PaO2 values.
Potential covariates considered were sex, race/ethnicity, age, and laboratory MELD score at exception approval, blood type, serum albumin at listing, primary diagnosis (as defined by UNOS coding), and UNOS region. We used robust standard errors to account for correlation due to patient clustering by UNOS region.24 We evaluated time period of exception (2002–2004, post-MELD implementation; 2005–2009, after a national consensus conference proposed more formalized MELD exception policies25; and 2010–2012, simplified application process).
We evaluated post-transplantation mortality by analyzing the subset of HPS exception deceased donor transplant recipients (98% of all HPS transplant recipients) in order to adjust for key donor factors associated with post-transplantation survival. We fit Cox regression models that evaluated race/ethnicity, age, final laboratory MELD score, and serum albumin at transplantation, blood type, diagnosis, UNOS region, time period of exception, and donor-risk index.26
We determined optimal PaO2 cut points to predict post-transplantation mortality to refine HPS exception policies. We evaluated the subset with documented PaO2 values4,8,11,14,27 using cubic splines, a statistical method to evaluate for thresholds (inflection points or knots) in the relationship between an exposure (oxygenation) and outcome (death).28 Cubic splines are a superior method for modeling the relationship between a continuous exposure and an outcome that does not follow a simple linear relationship, and tests for the best model fit based on inflection points in the data. We fit unadjusted cubic spline models with 2, 3, and 4 knots to identify inflection points in the data, and chose final cut points based on the best model fit.28–30 We then refit the pre- and post-transplantation survival models, as mentioned, using the new PaO2 categories, and compared model fit using the Akaike Information criterion (AIC). The AIC is a measure of the relative quality of the statistical model and can be used to identify and compare the fit of a model, with the optimal fitted model having the minimum value of AIC.
HPS vs non-HPS waitlist outcomes
The demographic and clinical variables between HPS and non-HPS patients were compared using Fisher’s exact tests and χ2 tests for categorical variables and 2-sample t tests or Wilcoxon rank-sum tests for continuous variables, depending on the distribution of the data. We fit multistate survival models to compare overall survival in HPS vs non-HPS patients. These models are considered the best approach to studying outcomes in transplantation candidates and account for transitions from pre-transplantation to post-transplantation states, with transplantation considered an intervening state rather than a censor or competing risk.31 We assumed proportional baseline hazards and fit Cox regression models as Markov proportional hazard models.32 The transition state of transplantation was fit as an interaction term to account for variable survival time in the pre- vs post-transplantation states.32 Survival time for HPS patients was analyzed 2 ways: time from listing or from receipt of exception points. To then determine if differences in overall survival were due to differences in pre- and/or post-transplant survival, we fit competing risk Cox (pre-transplantation) and Cox (post-transplantation) models, described here, with listing laboratory MELD score in the pre-transplantation model, and laboratory MELD score at transplantation in the post-transplantation model.
Covariates were selected for inclusion in final multivariable models if they were associated with the outcome (P > .2) or confounded the relationship between the primary exposure and the outcome by changing the hazard ratio (HR) by 10%.
Institutional Review Board Approval was obtained from the University of Pennsylvania and the University of Texas-Houston. All statistical analyses were performed using Stata 13.0 software (College Station, TX).
Results
From February 27, 2002 through December 14, 2012, one thousand and seventy-five waitlist candidates submitted at least one HPS exception applications (including resubmission or renewals required every 3 months by UNOS). Of these 1,075 applying for an exception, 973 (90.5%) had at least one application approved and were included in the HPS cohort—868 (89.2%) had a room-air arterial blood gas PaO2 value (Supplementary Figure 1; Supplementary Table 1). The demographics were similar between those with vs without room-air PaO2 data (data not shown).
Several demographic and clinical variables were significantly different in the HPS cohort in comparison with the non-HPS cohort (n = 59,619; Table 2), with HPS exception patients being significantly more likely to be female and white, with significantly lower laboratory MELD scores at listing. Non-HPS patients were significantly more likely to have had ascites or any hepatic decompensation event before waitlisting, with statistically higher but numerically similar ages at listing.
Table 2.
Baseline Clinical and Demographic Characteristics of All HPS and Non-HPS Waitlist Candidates
| Variable | HPS (n = 973) | Non-HPS (n = 59,619) | P value |
|---|---|---|---|
| Age at listing, median (IQR) | 53 (48–58) | 54 (48–60) | <.001 |
| Female sex, n (%) | 447 (45.9) | 21,881 (36.7) | <.001 |
| Race/ethnicity, n (%) | <.001 | ||
| White | 788 (81.0) | 43,693 (73.3) | |
| Black | 26 (2.7) | 4739 (8.0) | |
| Hispanic | 129 (13.3) | 8879 (14.9) | |
| Asian | 21 (2.2) | 1668 (2.8) | |
| Other | 9 (0.9) | 641 (1.1) | |
| Primary diagnosis, n (%) | <.001 | ||
| HCV | 447 (45.9) | 23,623 (39.6) | |
| NASH/cryptogenic | 198 (20.4) | 10,458 (17.5) | |
| EtOH | 175 (18.0) | 12,526 (21.0) | |
| Other | 64 (6.6) | 2554 (4.3) | |
| Autoimmune | 45 (4.6) | 3130 (5.3) | |
| Cholestatic | 31 (3.2) | 5682 (9.5) | |
| HBV | 13 (1.3) | 1645 (2.8) | |
| Listing laboratory MELD score, median (IQR) | 13 (11–16) | 16 (12–22) | <.001 |
| Listing laboratory MELD score category | <.001 | ||
| <15 | 610 (62.7) | 24,224 (40.6) | |
| 15–20 | 321 (33.0) | 17,526 (29.4) | |
| >20 | 42 (4.3) | 17,869 (30.0) | |
| History of ascites before listing, n (%) | 661 (67.9) | 48,618 (81.6) | <.001 |
| History of any hepatic decompensation event, n (%) | 700 (71.9) | 49,362 (82.8) | <.001 |
| Blood type, n (%) | .09 | ||
| O | 477 (49.0) | 27,526 (46.2) | |
| A | 360 (37.0) | 22,530 (37.8) | |
| B | 111 (11.4) | 7260 (12.2) | |
| AB | 25 (2.6) | 2293 (3.9) |
HBV, hepatitis B virus; HCV, hepatitis C virus; NASH, nonalcoholic steatohepatitis.
HPS Outcomes and Oxygenation
Overall, 86 (8.8%) HPS patients died while listed or within 90 days of de-listing, and 739 (86.0%) received transplants. Of the remaining 148 with HPS exceptions, 76 were still on the waitlist and the other 72 were removed due to improved clinical conditions, refused transplantation, or “other” reasons. Median time from exception approval to deceased donor transplantation was 55 days (interquartile range [IQR], 21–127 days). In univariable and multivariable competing risk models, there was no association between oxygenation and pre-transplantation waitlist survival (data not shown).
The 1-, 3-, and 5-year post-transplantation patient survival rates of all HPS transplant recipients were 91% (95% CI: 88%–93%), 81% (95% CI: 78%–84%), and 76% (95% CI: 71%–79%), respectively, with similar results when restricted to the cohort with documented PaO2 values.
The multivariable model results were unchanged when we analyzed the entire cohort, or the restricted cohort with documented PaO2 values. We present the results of the restricted cohort given the study goal of identifying clinically important PaO2 cut points in the oxygenation–post-transplantation outcome relationship.
When compared with recipients with a PaO2 of 50–59 mm Hg, transplant recipients with PaO2 <50 mm Hg had significantly worse survival (log-rank test P = .02; Table 3). In multivariable models, pre-transplantation room-air oxygenation was significantly associated with post-transplantation survival. In pairwise comparisons, with recipients with a PaO2 of 50–59 mm Hg as the reference, only those with a PaO2 <50 mm Hg had significantly increased post-transplantation mortality (HR = 1.56; Table 4).
Table 3.
Unadjusted 1-, 3-, and 5-Year Post-Transplantation Survival Rates of HPS Transplant Recipients by PaO2 Category and HPS vs Non-HPS Transplant Recipients
| PaO2 category | 1-Year survival, 95% CI | 3-Year survival, 95% CI | 5-Year survival, 95% CI |
|---|---|---|---|
| Standard categories | |||
| <50 mm Hg | 87.2 (81.1–91.5) | 75.0 (66.6–81.5) | 69.3 (59.6–77.1) |
| 50–59 mm Hg | 93.1 (89.8–95.4) | 85.9 (81.0–89.6) | 80.1 (73.4–85.0) |
| 60–69 mm Hg | 87.0 (77.8–92.6) | 79.8 (68.9–87.2) | 77.7 (66.3–85.7) |
| Cubic spline categoriesa | |||
| ≤44.0 mm Hg | 84.4 (72.2–91.6) | 68.1 (52.9–79.4) | 59.0 (40.9–73.3) |
| 44.1–54.0 mm Hg | 91.8 (87.4–94.7) | 83.6 (77.4–88.2) | 77.9 (70.3–83.7) |
| 54.1–61.0 mm Hg | 92.5 (88.3–95.3) | 86.4 (80.3–90.7) | 81.7 (74.0–87.3) |
| ≥61.1 mm Hg | 84.8 (73.6–91.5) | 70.6 (57.3–80.5) | 68.4 (54.8–78.7) |
| HPS vs non-HPS recipients | |||
| All HPS recipients | 90.6 (88.1–92.6) | 81.2 (77.6–84.3) | 75.5 (71.2–79.3) |
| HPS lowest riskb | 92.3 (89.4–94.4) | 84.7 (80.6–88.1) | 79.7 (74.6–83.9) |
| Non-HPS DDLT recipients | 88.7 (88.3–89.1) | 80.7 (80.2–81.3) | 74.3 (73.6–75.1) |
DDLT, deceased donor liver transplant.
Cubic spline categories determined by fitting cubic spline logistic regression models for the binary outcome of post-transplantation mortality (yes/no), with pre-transplantation room-air PaO2 as a continuous variable. Cut points determined based on the best model fit. The 15 transplant recipients with a PaO2 >70 mm Hg were excluded.
Lowest-risk HPS transplant recipients defined as HPS transplant recipients with the best post-transplantation outcomes based on the cubic spline analysis—those with an initial room-air PaO2 of 44.1–61.0 mm Hg.
Table 4.
Post-Transplantation Survival of HPS Transplant Recipients Based on Pre-transplantation Room-Air Oxygenation
| Variable | Standard PaO2 categoriesa
|
Cubic spline PaO2 categoriesb
|
||
|---|---|---|---|---|
| Multivariable HR (95% CI) | P value | Multivariable HR (95% CI) | P value | |
| Standard PaO2 categories | .04 | |||
| <50 mm Hg (n = 175) | 1.56 (1.02–2.38) | |||
| 50–59 mm Hg (n = 347) | 1 | |||
| 60–69 mm Hg (n = 88) | 1.51 (0.88–2.58) | |||
| Cubic spline PaO2 categories | .01 | |||
| ≤44.0 mm Hg (n = 65) | 1.58 (1.15–2.18) | |||
| 44.1–54.0 mm Hg (n = 242) | 1 | |||
| 54.1–61.0 mm Hg (n = 250) | 0.74 (0.55–1.01) | |||
| ≥61.1 mm Hg (n = 50) | 1.56 (0.77–3.17) | |||
| Age at transplantationc | 1.11 (0.88–1.39) | .38 | 1.09 (0.82–1.45) | .56 |
| Primary diagnosis | .02 | .02 | ||
| Hepatitis C | 1 | 1 | ||
| Alcohol | 0.86 (0.50–1.46) | 0.85 (0.47–1.52) | ||
| Hepatitis B | 0.54 (0.07–4.27) | 0.50 (0.30–0.85) | ||
| Nonalcoholic steatohepatitis/cryptogenic | 0.63 (0.36–1.10) | 0.62 (0.39–0.99) | ||
| Cholestatic | 0.18 (0.02–1.34) | 0.18 (0.07–0.49) | ||
| Autoimmune | 0.73 (0.29–1.85) | 0.73 (0.22–2.46) | ||
| Other | 2.23 (1.18–4.24) | 2.22 (1.35–3.66) | ||
| Donor risk index | 2.33 (1.51–3.59) | <.001 | 2.39 (1.87–3.04) | <.001 |
| Serum albumin at transplantation | 0.79 (0.56–1.10) | .20 | 0.78 (0.53–1.13) | .20 |
| Blood type | .04 | .03 | ||
| O | 1 | 1 | ||
| A | 1.06 (0.70–1.60) | 1.09 (0.76–1.54) | ||
| B | 0.71 (0.33–1.52) | 0.67 (0.40–1.13) | ||
| AB | 1.01 (0.36–2.86) | 1.08 (0.50–2.32) | ||
| Race/ethnicity | .06 | .009 | ||
| White | 1 | 1 | ||
| Black | 1.49 (0.58–3.83) | 1.35 (0.81–2.25) | ||
| Hispanic | 0.78 (0.42–1.45) | 0.79 (0.50–1.26) | ||
| Asian | 0.77 (0.10–5.80) | 0.81 (0.25–2.65) | ||
| Other | 1.25 (0.17–9.23) | 1.16 (0.30–4.50) | ||
AIC of model 1292.12. Final model did not include final laboratory MELD score, male sex, time period of transplantation, UNOS region, or final blood type, which were not significant in univariable models (P > .3), and were not confounders (did not change HR for PaO2 category by 10%). When compared with the reference PaO2 category of 50 to 59 mm Hg, the only group with a significantly increased risk of post-transplantation mortality in pairwise comparisons was the ≤50 mm Hg group (P = .04).
AIC of model 1288.18. Final model did not include final laboratory MELD score, time period of transplantation, UNOS region as they were not significant in univariable models (P > .3), and were not confounders (did not change HR for PaO2 category by 10%). Hazard ratio for male sex in final model: 0.86, 95% CI: 0.69–1.08; P = .19. The 15 transplant recipients with a PaO2 >70 mm Hg were excluded. When compared with the reference PaO2 category of 44.1–54.0 mm Hg, the only group with a significantly increased risk of post-transplantation mortality in pairwise comparisons was the ≤44.0 mm Hg group (P = .005).
Hazard for every increase in 10 years at transplantation.
Evaluation of New PaO2 Cut Points
Cubic splines models evaluated for thresholds of the relationship between oxygenation (exposure) and post-transplantation mortality (outcome) given the lack of association between oxygenation and pre-transplantation survival.28 We only analyzed the cohort with confirmed PaO2 data for whom we are confident of the accuracy of their oxygenation status. The best-fit cubic spline model had 3 knots (4 PaO2 categories): ≤44.0 mm Hg, 44.1–54.0 mm Hg, 54.1–61.0 mm Hg, and ≥61.1 mm Hg (similar knots obtained with inclusion of patients with imputed PaO2). The demographic, clinical, and laboratory characteristics of patients in these 4 groups were similar (data not shown). The distribution of the PaO2 values of these 4 categories is as follows: ≤44.0 mm Hg: median, 41; IQR, 38–43; overall range, 25–44.0; 44.1–54.0 mm Hg: median, 50.3; IQR, 48–53; overall range, 45.0–54.0); 54.1–61.0 mm Hg: median, 57.0; IQR, 56.0–59.0; overall range, 54.7–61.0; and ≥61.1 mm Hg: median, 66.3; IQR, 63.3–69.0; overall range, 61.6–69.9.
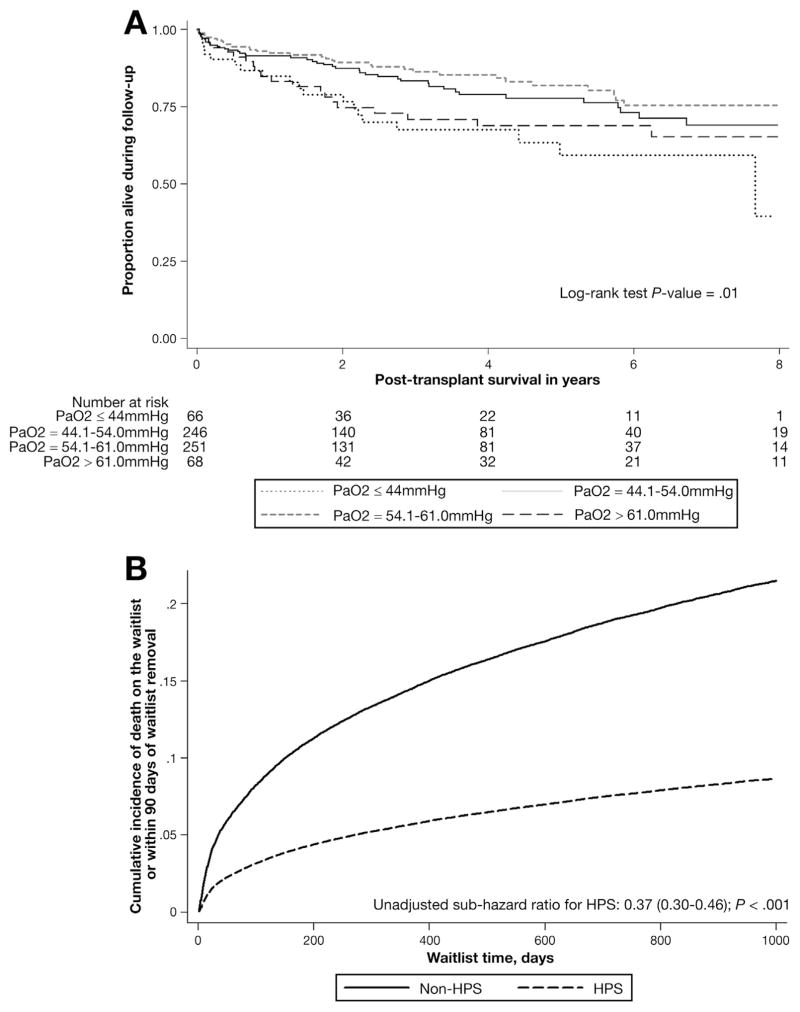
There was no association between cubic spline PaO2 category and waitlist survival (Supplementary Table 2). There were significant differences in unadjusted post-transplantation patient survival when analyzed by cubic spline PaO2 category (log-rank test P = .01; Figure 1A), with the lowest post-transplantation survival in recipients with a PaO2 ≤ 44.0 mm Hg. In multivariable Cox models, there was a significant association between room-air oxygenation and post-transplantation survival. However, in pairwise comparisons, only recipients with a PaO2 ≤ 44.0 mm Hg had significantly increased post-transplantation mortality when compared with recipients with a PaO2 of 44.1–54.0 mm Hg (HR = 1.58; 95% CI, 1.15–2.18; Table 4). These results were unchanged with exclusion of the HPS cohort with a PaO2 >60 mm Hg who did not meet automatic criteria for HPS exception points based on their PaO2 value.
Figure 1.
(A) Post-transplantation patient survival of HPS transplant recipients based on room-air PaO2. (B) Competing risk curves for pre-transplantation waitlist survival in HPS vs non-HPS waitlist candidates.
The Cox model using the cubic spline PaO2 cut points had superior model fit and performance, as determined by a lower AIC33 (cubic spline model AIC 1288.18 vs standard model AIC 1292.12). The discrimination of 1-, 3-, and 5-year unadjusted post-transplantation patient survival was superior using the cubic spline PaO2 cut points Table 3).
Overall Survival of HPS vs Non-HPS Waitlist Candidates
In the multi-state model evaluating overall survival time, accounting for transplantation as an intervention rather than an outcome, HPS waitlist candidates had a significantly decreased risk of dying (HR = 0.82; 95% CI: 0.70–0.96; Table 5), which was even more pronounced when restricted to the non-HPS cohort with a listing MELD of 21–23 (HR = 0.53; 95% CI: 0.44–0.65). The results were unchanged when HPS survival time was based on time from listing or time from initial HPS exception approval.
Table 5.
Multi-State Cox Regression Model Evaluating Overall Patient Survival of HPS vs Non-HPS Waitlist Candidates
| Variable | Multivariable HR (95% CI) | P valuea |
|---|---|---|
| HPS | 0.82 (0.70–0.96) | .01 |
| Age at listingb | 1.27 (1.25–1.30) | <.001 |
| Male sex | 0.97 (0.92–1.02) | .29 |
| Race/ethnicity | <.001 | |
| White | 1 | |
| Black | 1.14 (1.08–1.20) | |
| Hispanic | 0.98 (0.92–1.06) | |
| Asian | 0.97 (0.90–1.04) | |
| Other | 1.05 (0.89–1.25) | |
| Primary diagnosis | <.001 | |
| Hepatitis C | 1 | |
| Alcohol | 0.87 (0.83–0.91) | |
| Hepatitis B | 0.84 (0.77–0.92) | |
| NASH/cryptogenic | 0.88 (0.83–0.93) | |
| Cholestatic | 0.77 (0.69–0.86) | |
| Autoimmune | 0.87 (0.81–0.93) | |
| Other | 1.20 (1.08–1.33) | |
| Initial serum albumin | 0.61 (0.56–0.65) | <.001 |
| Ascites before listing | 1.49 (1.39–1.59) | <.001 |
| Receipt of transplantationc | 0.52 (0.50–0.54) | <.001 |
| Blood type | <.001 | |
| O | 1 | |
| A | 1.00 (0.96–1.04) | |
| B | 0.90 (0.85–0.96) | |
| AB | 0.86 (0.78–0.95) |
P value for multivariable hazard ratio.
Hazard for every increase in 10 years at transplantation.
Transition state of transplantation fit as an interaction term in the multi-state Cox model.33
Differences in Pre- and Post-Transplant HPS vs Non-HPS Survival
The difference in overall survival was due to an increased risk of pre-transplantation mortality in non-HPS patients (Figure 1B; multivariable competing risk model results shown in Table 6). Specifically, a significantly greater proportion of non-HPS waitlist candidates died on the waitlist or within 90 days of waitlist removal (20% vs 9%; P < .001), and a greater proportion of HPS waitlist candidates received transplants (73% vs 43%; P < .001). The waitlist mortality of non-HPS patients was numerically higher than that of HPS patients in all regions, and was statistically significantly higher in 7 of 11 UNOS regions: 2, 3, 4, 5, 8, 10, and 11. Also, the native laboratory score at transplantation in non-HPS transplant recipients was significantly higher than those with HPS: 23 (range, 17–30) vs 14 (range, 12–17); P < .001. Notably, based on available OPTN/UNOS data, which captures some but not all complications of end-stage liver disease, only 49.9% (352 of 706) of HPS transplant recipients had clear evidence of clinical indications for transplantation aside from HPS (defined as a laboratory MELD score ≥15 at listing, moderate ascites at listing, or encephalopathy beyond grade 1 at listing34–36) compared with 89.9% (23,098 of 25,704) non-HPS transplant recipients (P < .001).
Table 6.
Competing Risk Model Evaluating Risk of Pre-transplantation Death on the Waitlist or Within 90 Days of De-Listing of HPS vs Non-HPS Waitlist Candidatesa
| Variable | Multivariable SHR (95% CI) | P valueb |
|---|---|---|
| HPS | 0.41 (0.31–0.55) | <.001 |
| Age at listingc | 1.27 (1.23–1.31) | <.001 |
| Male sex | 0.85 (0.79–0.92) | <.001 |
| Race/ethnicity | <.001 | |
| White | 1 | |
| Black | 0.86 (0.78–0.95) | |
| Hispanic | 1.10 (0.98–1.25) | |
| Asian | 1.05 (0.97–1.12) | |
| Other | 1.12 (0.92–1.35) | |
| Initial laboratory MELD scored | 1.03 (1.02–1.03) | <.001 |
| Primary diagnosis | <.001 | |
| Hepatitis C | 1 | |
| Alcohol | 0.80 (0.77–0.83) | |
| Hepatitis B | 0.82 (0.74–0.91) | |
| NASH/cryptogenic | 0.80 (0.74–0.87) | |
| Cholestatic | 0.74 (0.67–0.82) | |
| Autoimmune | 0.81 (0.75–0.87) | |
| Other | 1.00 (0.89–1.12) | |
| Ascites before listing | 1.23 (1.12–1.34) | <.001 |
| Blood type | ||
| O | 1 | <.001 |
| A | 0.97 (0.92–1.02) | |
| B | 0.79 (0.73–0.86) | |
| AB | 0.49 (0.44–0.55) | |
| Initial serum albumin | 0.74 (0.69–0.79) | <.001 |
NASH, nonalcoholic steatohepatitis.
Competing risk Cox regression model, treating transplantation as a competing risk. Results reported as sub-hazard ratios (SHRs) instead of hazard ratios.
P value for multivariable hazard ratio.
Hazard for every increase in 10 years at listing.
SHR represents increased risk for every 1 MELD point.
Overall unadjusted 1, 3, and 5-year post-transplantation patient survival rates were not significantly different in HPS vs non-HPS transplant recipients (log-rank test P value= .33; Table 3).
In multivariable Cox regression models post-transplantation survival was not statistically different in HPS vs non-HPS transplant recipients (HR = 1.12; 95% CI: 0.98–1.27; full model results not shown), with similar results when restricted to the non-HPS MELD 21–23 cohort. These results were unchanged with exclusion of the HPS cohort with a PaO2 >60 mm Hg, who did not meet automatic criteria for HPS exception points based on their PaO2 value.
Discussion
In this study, we present the largest analysis of liver transplant waitlist candidates with HPS to date. Our findings confirm that waitlisted liver transplant patients with exceptions for HPS can receive transplants with outcomes similar to other liver transplant candidates. However, our observation that transplant recipients with HPS and marked hypoxemia (room-air PaO2 ≤ 44.0 mm Hg) have worse post-transplantation survival refutes recent reports and demonstrates an association between pre-transplantation room-air oxygenation and post-transplantation mortality.10,11 Although these data need to be validated in future cohorts, they suggest that increasing hypoxemia is or can be a marker of worsened post-transplantation outcomes in HPS transplant recipients meeting automatic MELD exception criteria. Candidates with HPS exception points had decreased pre-transplantation mortality compared with non-HPS patients, due to a greater chance of receiving a transplant and better overall survival. This suggests that current exception policy might overprioritize waitlisted HPS patients. Although the increased priority was instituted, in part, to avoid compromising post-transplantation outcomes,14,25 striking a balance between pre-transplantation waitlist mortality and post-transplantation outcomes exposes a limitation in current MELD-based prioritization. As the UNOS Liver and Intestine Committee is currently reexamining exception point policies, these data can be used to potentially modify exception policies for waitlist candidates with HPS to better reflect the relationship between room-air oxygenation and pre- and post-transplantation outcomes.
In 2002, liver allocation transitioned from a system that accounted for severity of illness and waiting time, to one based solely on medical urgency using the MELD score.6,7 Although the MELD score predicts 90-day pre-transplantation mortality, it is an imperfect measure of overall survival and poorly predicts post-transplantation outcomes or survival benefit.37 Since the inception of MELD-based allocation, UNOS policy has dictated that liver transplantation candidates with certain conditions are eligible to receive exception points, either through standardized protocols (ie, hepatocellular carcinoma) or a case-by-case peer-review process. The most common indication for MELD exception points is hepatocellular carcinoma, developed based on data demonstrating that tumor burden was strongly associated with post-transplantation outcomes.38 Therefore, increased waitlist priority is given to ensure expedited transplant to maximize post-transplantation outcomes (Milan criteria).38,39 The HPS exception policy was predicated on this philosophy as well. However, although there are robust data with which to derive and validate hepatocellular carcinoma criteria to predict post-transplantation survival, the largest prior study evaluating the association between room-air oxygenation and post-transplantation survival in HPS derived from 75 patients. In addition, the previously defined oxygenation cut off to define higher-risk HPS transplant recipients was developed in a cohort of 24 transplant recipients.8 Our study provides the most robust data to date on the relationship between pre-transplantation room-air oxygenation and post-transplantation survival in HPS patients.
One earlier report of outcomes of HPS exception patients used OPTN/UNOS data from February 27, 2002 through March 1, 2007 showed similar results.9 However, our analysis expands the size, depth, and granularity of this work. First, we reviewed each exception narrative in detail, thereby confirming HPS status, allowing us to exclude patients who were misclassified by UNOS coding as having HPS. Second, we extracted detailed data related to HPS in order to evaluate the association between oxygenation and post-transplantation outcomes in the largest cohort of HPS patients. Lastly, our sample size was nearly 4 times larger.
Although the data presented here demonstrate that current exception policy provides access to transplantation for HPS patients and results in excellent post-transplantation outcomes, it raises several issues. First, in contrast to recent reports, we find an association between pre-transplantation room-air oxygenation and post-transplantation survival, with a decline in post-transplantation survival for those with an initial room-air PaO2 ≤ 44.0 mm Hg. This finding extends results from a small prospective study early in the MELD era and can define a lower limit of PaO2 for awarding HPS MELD exception points.8 However, these data must be taken in context, as the 5-year post-transplantation patient survival in HPS patients with the lowest values of PaO2 is still at or above a threshold many would consider acceptable for a transplant recipient. Therefore, the transplant community must decide what degree of hypoxemia makes a patient too high risk.40 The low risk of waitlist dropout, combined with excellent post-transplantation outcomes in those with less severe hypoxemia, suggest that it might be possible to optimize post-transplantation outcomes for patients with HPS without disadvantaging the broader transplant population by decreasing the initial number of exception points for HPS patients, while offering additional priority to those whose PaO2 values decline toward higher-risk values. Such a policy modification would increase pre-transplantation waiting times and delay transplantation, which can result in HPS patients developing progressive hypoxemia, which could adversely impact both pre- and post-transplantation survival. We did find that HPS MELD exception patients waitlisted in “high-MELD” regions (UNOS regions 1, 5, and 9) with longer waiting times had increased pre-transplantation waitlist removal for death or clinical deterioration (14.5% vs 6.8%; P < .001) compared with all other regions. However, these data do not allow us to determine if increased waiting time resulted in progressive hypoxemia, and whether HPS was the cause of pre-transplantation death or waitlist removal, and requires additional investigation.
We also find that despite estimates that 5%–15% of liver waitlist candidates have HPS meriting an automatic MELD exception (PaO2 < 60 mm Hg41) and up to 30% have HPS overall based on the alveolar-arterial gradient, <2% of waitlist candidates applied for an HPS exception. This observation suggests that HPS remains an under-recognized complication of liver disease, despite its impact on patient survival. Third, although nearly all of the HPS waitlist candidates we analyzed had room-air oxygenation data documented in the OPTN dataset, a substantial number had incomplete data to stringently phenotype HPS (severity of gas exchange abnormalities using the alveolar-arterial gradient, degree of intrapulmonary shunting) or fully characterize pulmonary function. Since 2010, the HPS MELD exception process has been streamlined and applications now require only a PaO2 measure with simple acknowledgement that other criteria for HPS are met (portal hypertension, intrapulmonary shunt, and no evidence of underlying pulmonary disease).41 These observations underscore important challenges in optimizing the current HPS MELD exception policy.
Our study has limitations. First, we were unable to employ the strict criteria defining HPS used in prospective multi-center studies.5 However, we are confident that most, if not all, of the patients had HPS based on the data documenting hypoxemia and shunting in nearly 90% of patients. Second, our HPS cohort only included patients who applied and received MELD exception points.5,27,42 This might have led to the inclusion of some HPS patients in the non-HPS cohort. However, we do not believe this explains our results in comparison with those of the Pulmonary Vascular Complications in Liver Disease Study Group,14 as they analyzed overall survival of patients evaluated for transplantation, only 55% of whom were waitlisted, as opposed to our cohort who, by definition, was waitlisted. Third, room-air oxygenation data were unavailable in approximately 10% of the cohort. The results were unchanged both with exclusion of this cohort and in sensitivity analyses where they were recoded into different oxygenation categories (data not shown). Fourth, we could not definitively determine whether progressive respiratory failure contributed to the increased mortality in the cohort with the lowest PaO2 values. However, HPS transplant recipients with a PaO2 ≤ 44.0 mm Hg were significantly more likely to die (29.2% [19 of 65] vs 18.2% [102 of 560]; P = .03) and have respiratory failure as the cause of death based on OPTN/UNOS coding when compared with the other HPS transplant recipients who died post-transplantation (36.9% [7 of 19] vs 10.8% [11 of 102]; P = .003). In addition, 4 of 7 deaths attributable to respiratory failure in the lowest PaO2 group occurred within the first 70 days of transplantation. Lastly, we were unable to explain with the available OPTN/UNOS data why the HPS patients with the highest values of PaO2 had increased post-transplantation mortality. These patients might, in fact, have been misclassified as HPS, and instead had other cardiopulmonary disease (eg, chronic obstructive pulmonary disease) causing hypoxia and increased mortality, or they might have had other comorbidities contributing to an increased pre-transplantation mortality that prompted the transplantation center to apply for exception points. However, these possibilities could not be ascertained in OPTN/UNOS data and require additional study.
In conclusion, we find that HPS patients granted MELD exceptions have superior overall survival relative to non-HPS patients due to less waitlist drop out in the pre-transplantation period. Post-transplantation survival in HPS vs non-HPS patients is not different, but significantly declines in HPS patients with more severe hypoxemia at initial evaluation based on available OPTN/UNOS data. Reevaluation of UNOS policy for HPS MELD exceptions might be appropriate to optimize outcomes for patients with HPS without disadvantaging the broader transplantation population. An increase rather than decrease in data collected regarding these patients is needed to guide policy.
Supplementary Material
Supplementary Figure 1. Flow diagram of inclusion of liver transplant waitlist candidates submitting an HPS exception application from 2002–2012.
Supplementary Table 1. Data That Could Be Extracted From Narratives of 973 Waitlist Candidates With Approved HPS Exceptions
Supplementary Table 2. Pre–Liver Transplantation HPS Waitlist Candidates Dying on the Waitlist or Within 90 Days of De-Listing for Waitlist Candidates With HPS MELD Exceptions
Acknowledgments
Funding
Steven Kawut received funding from National Institutes of Health (NIH) grants K24 HL 103844, HL 116886. David Goldberg received funding from NIH K08 DK098272-01A1. This work was supported in part by Health Resources and Services Administration contract 234-2005-37011C. The content is the responsibility of the authors alone and does not necessarily reflect the views or policies of the Department of Health and Human Services, nor does mention of trade names, commercial products, or organizations imply endorsement by the US Government.
Abbreviations used in this paper
- AIC
Akaike Information Criterion
- CI
confidence interval
- HPS
hepatopulmonary syndrome
- HR
hazard ratio
- IQR
interquartile range
- MELD
Model for End-Stage Liver Disease
- OPTN
Organ Procurement and Transplant Network
- PaO2
partial pressure of oxygen in arterial blood
- UNOS
United Network for Organ Sharing
Footnotes
Note: To access the supplementary material accompanying this article, visit the online version of Gastroenterology at www.gastrojournal.org, and at http://dx.doi.org/10.1053/j.gastro.2014.01.005.
Conflicts of interest
The authors disclose no conflicts.
References
- 1.Krowka MJ, Wiseman GA, Burnett OL, et al. Hepatopulmonary syndrome: a prospective study of relationships between severity of liver disease, PaO(2) response to 100% oxygen, and brain uptake after (99m) Tc MAA lung scanning. Chest. 2000;118:615–624. doi: 10.1378/chest.118.3.615. [DOI] [PubMed] [Google Scholar]
- 2.Swanson KL, Wiesner RH, Krowka MJ. Natural history of hepatopulmonary syndrome: Impact of liver transplantation. Hepatology. 2005;41:1122–1129. doi: 10.1002/hep.20658. [DOI] [PubMed] [Google Scholar]
- 3.Krowka MJ, Fallon MB. Liver transplantation for hepatopulmonary syndrome (HPS): what is the MESSAGE? Am J Transplant. 2008;8:911–912. doi: 10.1111/j.1600-6143.2008.02190.x. [DOI] [PubMed] [Google Scholar]
- 4.Krowka MJ, Mandell MS, Ramsay MA, et al. Hepatopulmonary syndrome and portopulmonary hypertension: a report of the multicenter liver transplant database. Liver Transpl. 2004;10:174–182. doi: 10.1002/lt.20016. [DOI] [PubMed] [Google Scholar]
- 5.Fallon MB, Krowka MJ, Brown RS, et al. Impact of hepatopulmonary syndrome on quality of life and survival in liver transplant candidates. Gastroenterology. 2008;135:1168–1175. doi: 10.1053/j.gastro.2008.06.038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Kamath PS, Wiesner RH, Malinchoc M, et al. A model to predict survival in patients with end-stage liver disease. Hepatology. 2001;33:464–470. doi: 10.1053/jhep.2001.22172. [DOI] [PubMed] [Google Scholar]
- 7.Wiesner R, Edwards E, Freeman R, et al. Model for end-stage liver disease (MELD) and allocation of donor livers. Gastroenterology. 2003;124:91–96. doi: 10.1053/gast.2003.50016. [DOI] [PubMed] [Google Scholar]
- 8.Arguedas MR, Abrams GA, Krowka MJ, Fallon MB. Prospective evaluation of outcomes and predictors of mortality in patients with hepatopulmonary syndrome undergoing liver transplantation. Hepatology. 2003;37:192–197. doi: 10.1053/jhep.2003.50023. [DOI] [PubMed] [Google Scholar]
- 9.Sulieman BM, Hunsicker LG, Katz DA, Voigt MD. OPTN policy regarding prioritization of patients with hepatopulmonary syndrome: does it provide equitable organ allocation? Am J Transplant. 2008;8:954–964. doi: 10.1111/j.1600-6143.2007.02124.x. [DOI] [PubMed] [Google Scholar]
- 10.Gupta S, Castel H, Rao RV, et al. Improved survival after liver transplantation in patients with hepatopulmonary syndrome. Am J Transplant. 2010;10:354–363. doi: 10.1111/j.1600-6143.2009.02822.x. [DOI] [PubMed] [Google Scholar]
- 11.Iyer VN, Swanson KL, Cartin-Ceba R, et al. Hepatopulmonary syndrome: favorable outcomes in the MELD exception era. Hepatology. 2013;57:2427–2435. doi: 10.1002/hep.26070. [DOI] [PubMed] [Google Scholar]
- 12.Abrams GA, Nanda NC, Dubovsky EV, et al. Use of macroaggregated albumin lung perfusion scan to diagnose hepatopulmonary syndrome: a new approach. Gastroenterology. 1998;114:305–310. doi: 10.1016/s0016-5085(98)70481-0. [DOI] [PubMed] [Google Scholar]
- 13.Hoeper MM, Krowka MJ, Strassburg CP. Portopulmonary hypertension and hepatopulmonary syndrome. Lancet. 2004;363(9419):1461–1468. doi: 10.1016/S0140-6736(04)16107-2. [DOI] [PubMed] [Google Scholar]
- 14.Fallon MB, Mulligan DC, Gish RG, Krowka MJ. Model for end-stage liver disease (MELD) exception for hepatopulmonary syndrome. Liver Transpl. 2006;12(Suppl 3):S105–S107. doi: 10.1002/lt.20971. [DOI] [PubMed] [Google Scholar]
- 15.Arguedas MR, Singh H, Faulk DK, Fallon MB. Utility of pulse oximetry screening for hepatopulmonary syndrome. Clin Gastroenterol Hepatol. 2007;5:749–754. doi: 10.1016/j.cgh.2006.12.003. [DOI] [PubMed] [Google Scholar]
- 16.Goldberg D, French B, Abt P, et al. Increasing disparity in waitlist mortality rates with increased model for end-stage liver disease scores for candidates with he-patocellular carcinoma versus candidates without hepatocellular carcinoma. Liver Transpl. 2012;18:434–443. doi: 10.1002/lt.23394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Moylan CA, Brady CW, Johnson JL, et al. Disparities in liver transplantation before and after introduction of the MELD score. JAMA. 2008;300:2371–2378. doi: 10.1001/jama.2008.720. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Kim WR, Therneau TM, Benson JT, et al. Deaths on the liver transplant waiting list: an analysis of competing risks. Hepatology. 2006;43:345–351. doi: 10.1002/hep.21025. [DOI] [PubMed] [Google Scholar]
- 19.Satagopan JM, Ben-Porat L, Berwick M, et al. A note on competing risks in survival data analysis. Br J Cancer. 2004;91:1229–1235. doi: 10.1038/sj.bjc.6602102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Fine JP, Gray RJ. A proportional hazards model for the subdistribution of a competing risk. J Am Stat Assoc. 1999;94(446):496–509. [Google Scholar]
- 21.Roughton FJ, Severinghaus JW. Accurate determination of O2 dissociation curve of human blood above 98. 7 percent saturation with data on O2 solubility in unmodified human blood from 0 degrees to 37 degrees C. J Appl Physiol. 1973;35:861–869. doi: 10.1152/jappl.1973.35.6.861. [DOI] [PubMed] [Google Scholar]
- 22.Cornell University. [Accessed July 13, 2013];Oxygen saturation, calculated. Available at: http://www-users.med.cornell.edu/~spon/picu/calc/o2satcal.htm.
- 23.Abrams GA, Sanders MK, Fallon MB. Utility of pulse oximetry in the detection of arterial hypoxemia in liver transplant candidates. Liver Transpl. 2002;8:391–396. doi: 10.1053/jlts.2002.32252. [DOI] [PubMed] [Google Scholar]
- 24.French B, Heagerty PJ. Analysis of longitudinal data to evaluate a policy change. Stat Med. 2008;27:5005–5025. doi: 10.1002/sim.3340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Freeman RB, Jr, Gish RG, Harper A, et al. Model for end-stage liver disease (MELD) exception guidelines: results and recommendations from the MELD Exception Study Group and Conference (MESSAGE) for the approval of patients who need liver transplantation with diseases not considered by the standard MELD formula. Liver Transpl. 2006;12(Suppl 3):S128–S136. doi: 10.1002/lt.20979. [DOI] [PubMed] [Google Scholar]
- 26.Feng S, Goodrich NP, Bragg-Gresham JL, et al. Characteristics associated with liver graft failure: the concept of a donor risk index. Am J Transplant. 2006;6:783–790. doi: 10.1111/j.1600-6143.2006.01242.x. [DOI] [PubMed] [Google Scholar]
- 27.Schenk P, Schoniger-Hekele M, Fuhrmann V, et al. Prognostic significance of the hepatopulmonary syndrome in patients with cirrhosis. Gastroenterology. 2003;125:1042–1052. doi: 10.1016/s0016-5085(03)01207-1. [DOI] [PubMed] [Google Scholar]
- 28.Molinari N, Daures JP, Durand JF. Regression splines for threshold selection in survival data analysis. Stat Med. 2001;20:237–247. doi: 10.1002/1097-0258(20010130)20:2<237::aid-sim654>3.0.co;2-i. [DOI] [PubMed] [Google Scholar]
- 29.Harrell FE, Jr, Lee KL, Pollock BG. Regression models in clinical studies: determining relationships between predictors and response. J Natl Cancer Inst. 1988;80:1198–1202. doi: 10.1093/jnci/80.15.1198. [DOI] [PubMed] [Google Scholar]
- 30.Tsugawa Y, Mukamal KJ, Davis RB, et al. Should the hemoglobin A(1c) diagnostic cutoff differ between blacks and whites? A cross-sectional study. Ann Intern Med. 2012;157:153–159. doi: 10.7326/0003-4819-157-3-201208070-00004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Bailey RC, Jia-Yeong Lin M, Krakauer H. Time-to-event modeling of competing risks with intervening states in transplantation. Am J Transplant. 2003;3:192–202. doi: 10.1034/j.1600-6143.2003.30203.x. [DOI] [PubMed] [Google Scholar]
- 32.Putter H, Fiocco M, Geskus RB. Tutorial in biostatistics: competing risks and multi-state models. Stat Med. 2007;26:2389–2430. doi: 10.1002/sim.2712. [DOI] [PubMed] [Google Scholar]
- 33.Posada D, Buckley TR. Model selection and model averaging in phylogenetics: advantages of akaike information criterion and bayesian approaches over likelihood ratio tests. Syst Biol. 2004;53:793–808. doi: 10.1080/10635150490522304. [DOI] [PubMed] [Google Scholar]
- 34.Somsouk M, Kornfield R, Vittinghoff E, et al. Moderate ascites identifies patients with low model for end-stage liver disease scores awaiting liver transplantation who have a high mortality risk. Liver Transpl. 2011;17:129–136. doi: 10.1002/lt.22218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.O’Leary JG, Lepe R, Davis GL. Indications for liver transplantation. Gastroenterology. 2008;134:1764–1776. doi: 10.1053/j.gastro.2008.02.028. [DOI] [PubMed] [Google Scholar]
- 36.Merion RM, Schaubel DE, Dykstra DM, et al. The survival benefit of liver transplantation. Am J Transplant. 2005;5:307–313. doi: 10.1111/j.1600-6143.2004.00703.x. [DOI] [PubMed] [Google Scholar]
- 37.Schaubel DE, Guidinger MK, Biggins SW, et al. Survival benefit-based deceased-donor liver allocation. Am J Transplant. 2009;9(Pt 2):970–981. doi: 10.1111/j.1600-6143.2009.02571.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Mazzaferro V, Regalia E, Doci R, et al. Liver transplantation for the treatment of small hepatocellular carcinomas in patients with cirrhosis. N Engl J Med. 1996;334:693–699. doi: 10.1056/NEJM199603143341104. [DOI] [PubMed] [Google Scholar]
- 39.Bruix J, Sherman M. Management of hepatocellular carcinoma: an update. Hepatology. 2011;53:1020–1022. doi: 10.1002/hep.24199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Volk ML, Vijan S, Marrero JA. A novel model measuring the harm of transplanting hepatocellular carcinoma exceeding Milan criteria. Am J Transplant. 2008;8:839–846. doi: 10.1111/j.1600-6143.2007.02138.x. [DOI] [PubMed] [Google Scholar]
- 41. [Accessed September 2, 2013];OPTN/UNOS policies and bylaws. Available at: http://optn.transplant.hrsa.gov/PoliciesandBylaws2/policies/pdfs/policy_8.pdf.
- 42.Fritz JS, Fallon MB, Kawut SM. Pulmonary vascular complications of liver disease. Am J Respir Crit Care Med. 2013;187:133–143. doi: 10.1164/rccm.201209-1583CI. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary Figure 1. Flow diagram of inclusion of liver transplant waitlist candidates submitting an HPS exception application from 2002–2012.
Supplementary Table 1. Data That Could Be Extracted From Narratives of 973 Waitlist Candidates With Approved HPS Exceptions
Supplementary Table 2. Pre–Liver Transplantation HPS Waitlist Candidates Dying on the Waitlist or Within 90 Days of De-Listing for Waitlist Candidates With HPS MELD Exceptions