Abstract
Since the original description of the health effects of inhaled occupational manganese (Mn) by Couper in 1837, an extensive literature details the clinical syndrome and pathophysiology of what was thought to be a rare condition. In the last decade, conventional wisdom regarding the clinicopathological effects of Mn has been challenged. Past exposures to Mn were an order of magnitude higher than modern exposures in developed countries; therefore, the clinical syndrome seen in the time of Couper is no longer typical of modern Mn exposed workers. Parkinsonism (rigidity, bradykinesia, rest tremor, and postural instability) is present in 15% of Mn-exposed workers in welding industries, and these parkinsonian signs are associated with reduced health status and quality of life. These parkinsonian signs also overlap considerably with the clinical findings seen in early stages of Parkinson disease (PD); although, molecular imaging suggests that Mn-exposed workers have dopaminergic dysfunction in a pattern unique from PD. Furthermore, geographic information system studies demonstrate that regions of the US with high industrial Mn emissions have an increased incidence of PD and increased PD associated mortality. This review will contrast historical, descriptive human studies in Mn-exposed subjects with more recent data and will suggest a research agenda for the 21st century.
Keywords: Manganese, parkinsonism, Parkinson disease, neurotoxicity, PET, dopamine
Introduction
In 1837 Couper (Couper, 1837) described a clinical syndrome in two patients exposed to Mn oxide through a grinding process to make bleaching powder. One subject was described as having “paraplegia” that did not recover. The second subject had festinating gait, hypophonia, and masked facies, consistent with parkinsonism. The clinical syndrome associated with Mn overexposure was subsequently termed “manganism”. While Parkinson disease (PD) is the most common degenerative cause of parkinsonism, numerous other diseases can cause parkinsonism including manganism. The clinical description of manganism was further characterized by Rodier in 1955 when he described subjects with a rapidly progressive neurobehavioral syndrome characterized by parkinsonism, dystonia, emotional lability, gait impairment, and psychosis. Exposures were as high as 926,000μg Mn/m3 among these subjects. In contrast, modern exposures to Mn, encountered primarily through occupational welding, are several orders of magnitude lower than exposures in the Chilean Mn mines studied by Rodier. (Meeker et al., 2007; RODIER, 1955; Rappaport et al., 1999) The clinical syndrome associated with 21st century Mn exposures may be substantially different than previously described. Numerous reviews have discussed the distinctions between manganism and PD, (Olanow, 2004; Calne et al., 1994; Jankovic, 2005; Perl and Olanow C. W., 2007; Guilarte, 2010) but the data supporting these differences are often based upon very small case series with no reference group for comparison. The primary categories on which this review will focus are clinical symptomatology, imaging, and pathology associated with Mn neurotoxicity.
Clinical Symptomatology
Clinical characteristics stated to be “typical” of Mn-induced parkinsonism include onset of neurobehavioral manifestations, psychosis, low amplitude, rapid postural tremor, early hypokinetic-/hypophonic dysarthria, early gait and balance abnormality, dystonia, action myoclonus, pyramidal signs, and rapid progression initially with stable long term course. (Jankovic, 2005) The data on which these distinctions have been made are mostly the Rodier study and the small study of Taiwanese workers with manganism from a ventilation malfunction. (Wang et al., 1989; RODIER, 1955) These studies involved massive exposures to Mn over a relatively short period of time, several orders of magnitude greater than modern welding or mining exposures. (Meeker et al., 2007; Myers et al., 2003) Most clinical reports of Mn neurotoxicity in the last 40 years describe a parkinsonian predominant phenotype. (Tanaka and Lieben J., 1969; Nelson et al., 1993; Cook et al., 1974; Selikhova et al., 2008; Sikk et al., 2010; Stepens et al., 2010) It is important to note that the “specificity” of the manganism clinical phenotype is very much over stated. Cognitive dysfunction, behavioral/mood disorders, psychosis, and dystonia are all within the spectrum of clinical symptomatology in PD. (Lucking et al., 2000) Only the presence of pyramidal tract dysfunction distinguishes neurotoxicity from high dose Mn exposure from PD, but these signs are not uniformly present. Moreover, there is almost no longitudinal clinical data on Mn-exposed workers. The largest longitudinal dataset is the study of five Taiwanese smelter workers with massive occupational Mn exposure who had follow-up examinations at four and ten years. (Huang et al., 1993; Huang et al., 1998) These workers demonstrated progression in parkinsonism with much of the progression in gait dysfunction and freezing.
To investigate the neurologic signs associated with chronic, low level occupational Mn exposures, we have conducted several clinical studies in welders. Concerns about the neurotoxic effects of welding fumes have increased over recent years. (Harris et al., 2005; Meeker et al., 2007) Mn is commonly found in welding fumes, and many welders are regularly overexposed to the American Conference of Governmental Industrial Hygienists threshold limit value (TLV) of 0.2mg/m3, which was primarily set in regard to pre-clinical neurological effects. (American Conference of Governmental Industrial Hygienists, 1992; Korczynski, 2000) We examined 1,423 Alabama Mn-exposed welders for parkinsonism, calculated the age-adjusted prevalence of parkinsonism using Department of Labor statistics, and compared this prevalence to the prevalence of parkinsonism in Copiah County, MS. (Schoenberg et al., 1985; Susi et al., 2000; Racette et al., 2005) We found that the prevalence of parkinsonism was substantially higher in welders (prevalence ratio 10.19; 95% CI 4.43-23.43) and concluded that this study provided evidence that parkinsonism is more common in welders than in the general population. In a recent study, we investigated the dose-response relationship between cumulative welding exposure and parkinsonism among 811 male shipyard welders, mean age 45.9 (± 12.3), recruited from the International Brotherhood of Boilermakers (IBB). (Racette et al., 2012) Study subjects were examined by a movement disorders specialist using the Unified Parkinson Disease Rating Scale motor subsection 3 (UPDRS3) (Fahn et al., 1987) without knowledge of exposure history. Two reference groups included 59 non-welder trade workers (henceforth termed ‘reference workers’) and 118 newly diagnosed, untreated idiopathic PD patients. Parkinsonism cases among welders were defined as those with UPDRS3 score ≥ 15. (Harris et al., 2011) We used this case definition of parkinsonism since most idiopathic PD patients become symptomatic enough to present for medical attention with UPDRS3 scores ≥ 15. (Parkinson’s Study Group, 1989; Parkinson Study Group, 2004; Fahn et al., 2004) Normal was defined as UPDRS3 < 6. Exposure was classified as intensity weighted, cumulative years of welding, using a previously validated questionnaire. (Hobson et al., 2009) Prevalence ratios (PR) for parkinsonism, adjusted for age, race, smoking, and education, were calculated in relation to quartiles of welding hours. The overall prevalence estimate of parkinsonism was 15.6% in welders compared to 0% in the reference workers. There was a U-shaped dose-response relationship with a modest increase in the prevalence of parkinsonism for the two middle quartiles of total weighted welding years. (Racette et al., 2012) The prevalence of parkinsonism in this population-based cohort was even higher than in our previous cross-sectional study of parkinsonism in welders. (Racette et al., 2005) In the shipyard welder study, UPDRS3 scores for most domains on the UPDRS3 were similar between welders with UPDRS3 ≥ 15 and newly diagnosed PD patients except for higher mean scores for rest tremor and asymmetry in PD patients. This work-site based study of parkinsonism in Mn-exposed welders demonstrated both a high prevalence of parkinsonism compared to reference workers as well as a clinical phenotype that overlaps substantially with PD. However, this cross-sectional study of parkinsonism in Mn-exposed welders does not indicate that these workers have PD. Instead, these findings demonstrate the difficulty in distinguishing occupational parkinsonism from PD in cross-sectional epidemiology studies. Long-term follow-up of these workers will be needed to clarify if the parkinsonian subjects experience progression of their parkinsonism, similar to PD.
Levodopa responsiveness is frequently cited as a distinguishing feature between PD and manganism. Levodopa supplementation replaces dopamine loss from degenerating substantia nigra projections in PD and is a clinical hallmark of PD. However, any condition in which there is loss of these nigral-striatal projections may improve with levodopa supplementation, and there is no gold standard defining levodopa responsiveness. (Constantinescu et al., 2007) Similarly, there is no consensus on the dose of levodopa required to establish that parkinsonism is not responsive to levodopa, but trials of up to 2000mg/day are often used in clinical practice. A commonly cited study of levodopa in manganism randomized four subjects with severe parkinsonism due to manganism to a single dose of 100mg, 200mg, 300mg or placebo and concluded that there was no improvement in clinical measures of parkinsonism. (Lu et al., 1994) A larger study of 13 subjects found no improvement with a longer trial of up to 300mg of levodopa. (Koller et al., 2004) However, we demonstrated improvement in parkinsonism in a patient with manganism due to liver failure with 900mg levodopa per day. Many other studies report lack of efficacy of levodopa in manganism, (Selikhova et al., 2008; Sanotsky et al., 2007; Aggarwal et al., 2006; Sadek et al., 2003) but few provide information on levodopa dose used or quantify clinical signs. Larger (appropriately powered), placebo controlled studies, using higher doses of levodopa are needed to determine degree to which manganism can be distinguished from PD based upon levodopa responsiveness. More importantly, determining levodopa responsiveness has critical clinical implications given the very limited treatment options for parkinsonism associated with Mn exposure.
Brain Imaging and Mn
Molecular Imaging of the Pre-synaptic Dopaminergic System and Mn
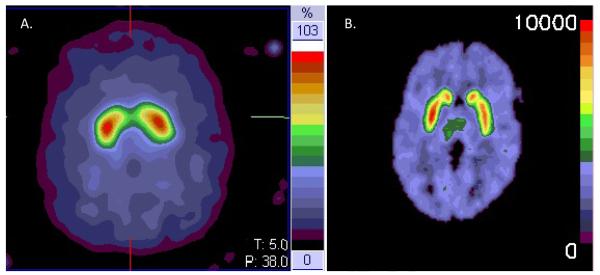
Several single-photon emission computed tomography (SPECT)- or Positron Emission Tomography (PET)-based approaches, using different radiotracers to assess various aspects of pre-synaptic dopaminergic nigrostriatal neurons, (Brooks et al., 2003) have been used to investigate the role of Mn as a nigrostriatal toxin. The three pre-synaptic targets of PET and SPECT radioligands are proteins involved in dopamine synthesis, dopamine reuptake, or vesicular transport of monoamines. Radioligands that bind to the dopamine transporter (DAT), which is responsible for the reuptake of dopamine into dopaminergic nerve terminals, have been used in several Mn studies, (Kim et al., 2002; Sikk et al., 2010; Kim et al., 2007) although the SPECT based methods used in these studies have lower resolution to quantify radioligand binding in subregions of the striatum than PET based methods. (Figure) Dopa decarboxylase is the enzyme responsible for converting L-DOPA to dopamine, and most Mn neurotoxicity studies have used [18F]fluorodopa (FDOPA)(Martin and Perlmutter Joel S., 1994) which labels this enzyme. Vesicular monoamine transporter 2 (VMAT2) is located exclusively in the membranes of pre-synaptic vesicles of monoaminergic neurons where it transports monoamines from the cytosol into synaptic vesicles. (Henry and Scherman D., 1989) Radioligands that label VMAT2 have the theoretical advantage that they are not regulated in dopamine deficiency states and may be the preferred radioligands for dose-response studies. In addition to providing clues to pathophysiology in human neurotoxicity studies, these radioligands have greater sensitivity than clinical examination to detect dysfunction of the nigrostriatal system, and they identify dopaminergic dysfunction in pre-symptomatic relatives several years prior to symptoms in genetic studies of PD. (Brooks, 1991; Shinotoh et al., 1996; Adams et al., 2005; Nandhagopal et al., 2008)
Figure.
A. [123I] Ioflupane SPECT scan [reconstructed resolution approximately 15mm full width half maximum (FHWM)] with normal striatal uptake. Note relatively homogeneous uptake in striatum.
B. [11C]dihydrotetrabenazine (DTBZ) PET scan (reconstructed resolution 4mm FWHM) with normal striatal uptake. Note distinct uptake in caudate and putamen.
FDOPA PET studies in humans with Mn neurotoxicity provide conflicting results. Wolters et al. examined four ferromanganese smelter workers with parkinsonism due to ventilation malfunction. (Wolters et al., 1989) All four subjects had normal FDOPA PET and T1-weighted MRI scans despite blood manganese levels 7-700 times the normal value. Kim et al. described three Mn-exposed subjects with parkinsonism and pre-synaptic dopaminergic dysfunction using molecular imaging (Kim et al., 2002), although one subject had a disease course typical of PD. (Kim et al., 1999a) Several SPECT studies using radioligands that measure DAT have found relatively normal pre-synaptic dopaminergic function. (Kim et al., 2007; Sikk et al., 2010)
To investigate the integrity of the dopamine system in Mn-exposed welders, we performed FDOPA PET scans on 20 asymptomatic welders exposed to Mn containing fumes and compared welder scans to 20 subjects with PD and 20 non-exposed reference subjects. (Criswell et al., 2011) Basal ganglia volumes of interest were identified for each subject and the specific uptake of FDOPA, Ki generated for each region using Patlak graphical analysis with the occipital cortex as the reference region. Repeated measures general linear model analysis demonstrated a significant interaction between diagnostic group and region [F(4, 112) = 15.36, p < 0.001)], indicating that the striatal FDOPA uptake pattern was significantly different between groups. Post hoc analysis demonstrated that caudate Ki’s were significantly lower in asymptomatic welders (0.0098 + 0.0013 min−1) compared to non-exposed reference subjects (0.0112 + 0.0012 min−1, p = 0.002). The regional pattern of uptake in welders was most affected in the caudate > anterior putamen > posterior putamen. This uptake pattern was anatomically reversed from the pattern found in PD subjects. These results were unchanged when the analysis was limited to male welders and reference workers. (Laakso et al., 2002) There was no clear dose-response relationship between FDOPA uptake and welding exposure. This study demonstrates dysfunction in the nigrostriatal dopamine system in active, asymptomatic welders with Mn exposure. The caudate Ki reduction in welders may represent an early (asymptomatic) marker of Mn neurotoxicity and may be distinct from the pattern of dysfunction found in symptomatic PD.
We have also performed FDOPA PET imaging in two subjects with end stage liver disease and Mn deposition on T1 MRI. The first subject had an atypical parkinsonian syndrome with severe parkinsonism (UPDRS3 off medication=45) and early gait impairment. FDOPA PET demonstrated severe loss of FDOPA uptake involving posterior putamen more than anterior putamen and caudate. The second had mild parkinsonism (UPDRS3 =12) and relatively diffuse reduction in FDOPA uptake throughout the striatum. These findings differ from other case-reports using SPECT imaging of the dopamine transporter in end stage liver patients. (Kim et al., 2007) The differential resolution of the imaging methods to quantify radioligand binding in subregions of the striatum or severity of parkinsonism could explain these differences. The molecular imaging of these subjects suggests that Mn neurotoxicity can be associated with pre-synaptic dopaminergic dysfunction and that subjects with presumed Mn neurotoxicity and motor impairment can have a pattern of FDOPA uptake which resembles that seen in PD. The importance of these findings is that Mn appears to be associated with damage to the nigrostriatal system. This evidence provides a basis for considering Mn as an etiologic risk factor and disease modifier of PD. The combination of the Mn-exposed welder and end stage liver imaging data suggests that the preclinical phase of Mn neurotoxicity is associated with preferential caudate dysfunction, but anterior and posterior putamen dysfunction develops as subjects become clinically parkinsonian. Future studies following subjects longitudinally are needed to verify this hypothesis. Moreover, studies of Mn-exposed subjects using a radioligand that measures VMAT2 may provide a more robust dose-response relationship with welding exposure.
Molecular Imaging of the Post-synaptic Dopaminergic System and Mn
There are very limited data investigating the post-synaptic dopaminergic system in Mn-exposed workers. To investigate the post-synaptic dopamine system, four Taiwanese smelter workers with clinical manganism underwent PET with 11C-raclopride and had mildly reduced binding in the caudate and normal binding in the putamen. (Shinotoh et al., 1997) These four subjects represent the entire human Mn neurotoxicity literature on the post-synaptic dopamine system. However, the interpretation of reduced binding in the Taiwanese smelter workers is unclear. Endogenous dopamine competes with raclopride for specific binding sites; therefore, raclopride uptake reflects not only that state of D2-like dopamine receptors but also the amount of endogenous dopamine. Thus baseline studies using raclopride to determine status of D2-like receptors are inherently confounded. Nevertheless, displacement studies using various methods to displace raclopride can be used to calculate the amount of released dopamine from the intervention (like amphetamine administration or a motor task), and this can provide very important data on pre-synaptic dopamine releasable content in the nigrostriatal system. (Guilarte et al., 2008) Imaging with nondisplaceable D2 radioligands would provide more definitive data on the impact of Mn neurotoxicity on the post-synaptic dopaminergic system. Similarly, future studies investigating the extrastriatal dopaminergic system will likely provide critical data to help explain the range of non-motor manifestations associated with Mn neurotoxicity.
MRI imaging and Mn
The classic MRI finding in occupational Mn exposure is bilateral T1-weighted hyperintensities within the globus pallidi with normal T2-imaging. T1 signal changes associated with Mn neurotoxicity can be quantified using the pallidal index (PI), (Krieger et al., 1997; Nelson et al., 1993; Spahr et al., 1996; Kim et al., 1999b) a ratio of signal intensity in globus pallidus compared to a reference white matter region. (Hauser et al., 1996; Burkhard et al., 2003) An increased PI can be seen in asymptomatic Mn-exposed welders, (Kim et al., 1999b) and the intensity of the T1 signal in Mn-exposed workers correlates modestly with cumulative exposure, airborne Mn concentrations, and serum Mn level.(Dietz et al., 2001; Dietz et al., 2000; Kim et al., 1999b; Hauser et al., 1996) These findings may be reversible, emphasizing the potential importance of identifying cases early in the course of exposure. (Pujol et al., 1993) However, in an ex vivo brain MRI study of South African Mn miners, we found that the increased PI on MRI can persist in some Mn-exposed subjects up to 25 years after exposure, (Nelson et al., 2012) suggesting that MRI may serve as a useful measure of chronic exposure in exposure-response studies. Neuropathologic examination of these subjects will be needed to determine if Mn is trapped by microglial cells or whether chronic exposures leads to pathologic changes that alter T1 MRI signal characteristics. In another recent study, we performed a 3.0T MR case-control imaging study on 18 welders and 18 age- and gender-matched, non-exposed reference subjects. (Criswell et al., 2012) Basal ganglia regions of interest were identified for each subject and T1-weighted intensity indices were generated for each region. Intensity indices for all regions were higher in welders than non-exposed reference subjects (p < 0.05). The combined basal ganglia (r = 0.610), caudate (r = 0.645), and posterior putamen (r = 0.511) indices were more correlated with exposure than the pallidal (r = 0.484) index, suggesting that sampling more regions may provide a better biomarker of exposure.
MRI also has the potential to detect neurotoxic brain injury. In the MRI subjects described above, apparent diffusion coefficients (ADC) were generated for each region using diffusion weighted imaging (DWI). DWI is sensitive to the movement of water molecules within tissue. Welder ADC values were lower than non-exposed reference subjects for globus pallidus (p = 0.04) and anterior putamen (p = 0.005). We hypothesize that reduced ADC values in welders may represent restricted diffusion of water secondary to basal ganglia scarring or the deposition of Mn, making DWI a potentially important in vivo marker of Mn associated neurotoxicity. Differences between Mn-exposed welders and controls may not be limited to gray matter structures like the basal ganglia. (Kim et al., 2011) Future studies using functional MRI (fMRI) may prove to be more sensitive to neurotoxic injury although methods to study subcortical structures in neurodegenerative diseases have only recently been published. (Hacker et al., 2012) Two studies task based fMRI (Chang et al., 2010a; Chang et al., 2010b) studies of South Korean welders demonstrate the potential to investigate the pathophysiology of clinical and preclinical abnormalities in Mn-exposed workers. Resting state fMRI may prove a useful tool to quantify preclinical neurotoxic functional connectivity patterns to identify at-risk workers.
Manganese and PD
We have taken a unique approach to investigating PD epidemiology and risk by using GIS to explore the spatial and temporal relationships between PD and environmental risk factors. To identify PD cases, we use Medicare data, the only national health care system in the US. (Wright et al., 2010) Over 98% of Americans over age 65 use Medicare to pay for healthcare. Research identifiable files, which contain demographic, clinical (diagnosis and medical procedure codes), neighborhood residence, and cost data can be purchased through the Center for Medicare Services. These data can be used to study disease rates and can be linked to exposure databases to establish disease-exposure relationships. In one of our initial studies, we calculated age-, race-, and sex- standardized prevalence and incidence of PD in the US at the county level. Cluster analysis supported a nonrandom grouping of county level prevalence and incidence (prevalence: z test statistic = 13.93, p<0.0001; incidence: z test statistic = 12.65, p <0.0001). (Wright et al., 2010) The prevalence/incidence in the Midwestern and Eastern US were an order of magnitude higher than much of the Southern and Western US. To further examine the geographic clustering of PD in the US, we investigated the association between copper, lead, or manganese emissions and PD incidence in the urban US, studying 29 million Medicare beneficiaries. (Willis et al., 2010) Over 35,000 non-mobile (had not moved from their neighborhood) neurologist diagnosed incident PD cases were identified for analysis. Age-, race-, and sex-standardized PD incidence was compared between counties with high cumulative industrial release of copper, manganese, or lead (as reported to the Environmental Protection Agency) and counties with no/low reported release of all three metals. PD incidence (per 100,000) in counties with no/low copper/lead/manganese release was 274.0 (95% confidence interval (95% CI): 226.8, 353.5). Incidence was greater in counties with high manganese release: 489.4 (95% CI: 368.3, 689.5) (RR- 1.78, 95% CI: 1.54, 2.07). This finding was specific to Mn; there was no increased PD incidence in counties with high Zn emissions.
Environmental Mn exposure may also have disease modifying effects in PD. Using the same GIS based methods, we investigated the six year survival in 138,000 incident PD Medicare beneficiaries from 2002-2008 and found that beneficiaries living in counties with high Mn exposure had a 19% greater covariate adjusted mortality compared to PD beneficiaries living in low Mn emission counties (HR, 1.19; 95% CI 1.10-1.29).(Willis et al., 2012) Several other studies support the concept that exposure to Mn can modify the course of PD. First, we performed a case-control study investigating the clinical features in 15 Mn-exposed welders with PD compared to 100 sequentially ascertained PD patients (non-welders) from an academic movement disorders center. While the clinical features of their PD were similar, the age of onset of the welders (46 years) was substantially younger than the control PD patients (63 years; p<0.0001). (Racette et al., 2001) This is consistent with a study using the US National Occupational Mortality Surveillance System to investigate the mortality odds ratio of PD associated with occupational welding. While there was no increased mortality from PD associated with occupational welding, there was an elevated mortality odds ratio (1.77; 95% CI 1.08-2.75) for PD below age 65. Similarly, a GIS-based study of PD prevalence in Hamilton, Ontario, Canada, found a modestly elevated odds of PD and a shift of the prevalence curves to a younger age of diagnosis associated with residence near industrial Mn point source and traffic generated air pollution containing the fuel additive methylcyclopentadienyl manganese tricarbonyl (MMT). (Finkelstein and Jerrett M., 2007; Park et al., 2005) These studies suggest that environmental Mn exposure may modulate PD clinical course by advancing the age of onset and may result in a more severe clinical course with early mortality. Future studies using large PD populations will be needed to follow-up on these findings by investigating dose-response relationships using more detailed exposure assessment methods.
Neuropathology of Mn
There are very limited human autopsy data on subjects with manganism, but pathology affecting primarily basal ganglia appears to be characteristic. Yamada et al. reported a 52 year old man with “increased muscle tone”, weakness, hyperreflexia, and a mood disorder attributed to working in a Mn ore crushing plant. (Yamada et al., 1986) Autopsy demonstrated cell loss in the pallidum with astrocytosis but a normal substantia nigra. Bernheimer et al. reported a patient with a long history of a parkinsonian illness, associated with markedly elevated blood Mn levels that developed while exposed to Mn dioxide in a battery factory. (Bernheimer et al., 1973) Autopsy revealed pallidal atrophy, marked degeneration of the substantia nigra pars compacta, and occasional Lewy bodies in nigral neurons. Older reports comment primarily on cell loss in the putamen and pallidum. (Canavan MM et al., 1934) All of these studies predate the modern immunohistochemical markers for Lewy bodies. Moreover, none provided quantitative cell counts in regions of interest or data from an appropriate reference brain for comparison. Neuropathologic examination of Mn-exposed workers is the most definitive method to understand exposure-response relationships, to understand pathophysiology of Mn neurotoxicity, and to understand the relationship between Mn neurotoxicity and PD.
In order to investigate the neuropathology associated with Mn exposure, we have developed a unique international collaboration that builds on an existing cardiopulmonary autopsy program in South Africa. South Africa contains 80% of the world’s Mn reserves in the Northern Cape Province. The 1973 Occupational Disease in Mines and Works Act gives families of deceased miners the right to a cardiopulmonary autopsy. By adding brain autopsies to this program in the Northern Cape Province, we have been able to study the neuropathologic effects of chronic Mn exposure without a diagnosed movement disorder. (Nelson et al., 2012) Much more work remains to be done in that study, but the potential knowledge to be gained from clinical-neuropathology, imaging-neuropathology, and exposure-pathology correlations is immense. Ultimately, this most informative data will derive from studies that incorporate precise lifetime Mn exposure reconstruction and accurate data on confounding variables.
Future Directions
Manganism in the 21st century appears to be a parkinsonian disorder that may be associated with dopaminergic dysfunction on molecular imaging and pathologic reductions in striatal neurons and astrocytes. Future research combining motor and cognitive outcomes to investigate dose-response relationship should inform exposure limits. Gene-environment interaction studies directed at metal transporter genes have the potential to identify workers at risk for developing clinical neurotoxicity. Future molecular and functional neuroimaging can clarify the pathophysiology of Mn neurotoxicity as well as serve as biomarkers of both exposure and outcomes. While the role of Mn in the pathogenesis of PD remains controversial, the emerging role of Mn as a modifier of PD disease course warrants future study. Finally, pathology studies in Mn-exposed workers will provide the pathologic basis to clinical and imaging studies.
Acknowledgments
Funding Sources This study was supported by the National Institute for Environmental Health Sciences (R01 ES013743, K24 ES017765, P42ES004696, R21ES017504, R01ES021488), the Michael J. Fox Foundation, National Institute of Neurological Disorders and Stroke (NINDS) National Center for Research Resources (NCRR0) and National Institutes of Health (NIH) Roadmap for Medical Research Grant Number UL1 RR024992, the American Parkinson Disease Association, the St. Louis Chapter of the American Parkinson Disease Association). The study sponsors had no involvement in study design; collection, analysis and interpretation of data; the writing of the manuscript; or the decision to submit the manuscript for publication.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Reference List
- Adams JR, van Netten H, Schulzer M, Mak E, McKenzie J, Strongosky A, Sossi V, Ruth TJ, Lee CS, Farrer M, Gasser T, Uitti RJ, Calne DB, Wszolek ZK, Stoessl AJ. PET in LRRK2 mutations: comparison to sporadic Parkinson’s disease and evidence for presymptomatic compensation. Brain. 2005;128:2777–85. doi: 10.1093/brain/awh607. [DOI] [PubMed] [Google Scholar]
- Aggarwal A, Vaidya S, Shah S, Singh J, Desai S, Bhatt M. Reversible Parkinsonism and T1W pallidal hyperintensities in acute liver failure. Mov. Disord. 2006;21:1986–90. doi: 10.1002/mds.21096. [DOI] [PubMed] [Google Scholar]
- Documentation of TLVs. ACGIH; Cincinnati: 1992. American Conference of Governmental Industrial Hygienists. [Google Scholar]
- Bernheimer H, Birkmayer W, Hornykiewicz O, Jellinger K, Seitelberger F. Brain dopamine and the syndromes of Parkinson and Huntington. Clinical, morphological and neurochemical correlations. J. Neurol. Sci. 1973;20:415–55. doi: 10.1016/0022-510x(73)90175-5. [DOI] [PubMed] [Google Scholar]
- Brooks DJ. Detection of preclinical Parkinson’s disease with PET. Geriatrics. 1991;46:25–30. [PubMed] [Google Scholar]
- Brooks DJ, Frey KA, Marek KL, Oakes D, Paty D, Prentice R, Shults CW, Stoessl AJ. Assessment of neuroimaging techniques as biomarkers of the progression of Parkinson’s disease. Exp. Neurol. 2003;184(Suppl 1):S68–79. doi: 10.1016/j.expneurol.2003.08.008. [DOI] [PubMed] [Google Scholar]
- Burkhard PR, Delavelle J, Du PR, Spahr L. Chronic parkinsonism associated with cirrhosis: a distinct subset of acquired hepatocerebral degeneration. Arch. Neurol. 2003;60:521–28. doi: 10.1001/archneur.60.4.521. [DOI] [PubMed] [Google Scholar]
- Calne DB, Chu NS, Huang CC, Lu CS, Olanow W. Manganism and idiopathic parkinsonism: similarities and differences. Neurology. 1994;44:1583–6. doi: 10.1212/wnl.44.9.1583. [DOI] [PubMed] [Google Scholar]
- Canavan MM, Cobb W, Drnovsek B. Chronic manganese poisoning: Report of a case with autopsy. Archives of Neurologic Psychiatry. 1934;32:501–12. [Google Scholar]
- Chang Y, Lee JJ, Seo JH, Song HJ, Kim JH, Bae SJ, Ahn JH, Park SJ, Jeong KS, Kwon YJ, Kim SH, Kim Y. Altered working memory process in the manganese-exposed brain. Neuroimage. 2010a;53:1279–85. doi: 10.1016/j.neuroimage.2010.07.001. [DOI] [PubMed] [Google Scholar]
- Chang Y, Song HJ, Lee JJ, Seo JH, Kim JH, Lee HJ, Kim HJ, Kim Y, Ahn JH, Park SJ, Kwon JH, Jeong KS, Jung DK. Neuroplastic changes within the brains of manganese-exposed welders: recruiting additional neural resources for successful motor performance. Occup. Environ. Med. 2010b;67:809–15. doi: 10.1136/oem.2009.052761. [DOI] [PubMed] [Google Scholar]
- Constantinescu R, Richard I, Kurlan R. Levodopa responsiveness in disorders with parkinsonism: a review of the literature. Mov. Disord. 2007;22:2141–8. doi: 10.1002/mds.21578. [DOI] [PubMed] [Google Scholar]
- Cook DG, Fahn S, Brait KA. Chronic manganese intoxication. Arch. Neurol. 1974;30:59–64. doi: 10.1001/archneur.1974.00490310061010. [DOI] [PubMed] [Google Scholar]
- Couper J. On the effects of black oxide of manganese when inhaled into the lungs. Br Ann Med Pharm Vital Stat Gen Sci. 1837;1:41–2. [Google Scholar]
- Criswell SR, Perlmutter JS, Huang JL, Golchin N, Flores HP, Hobson A, Aschner M, Erikson KM, Checkoway H, Racette BA. Basal ganglia intensity indices and diffusion weighted imaging in manganese-exposed welders. Occup. Environ. Med. 2012;69:437–43. doi: 10.1136/oemed-2011-100119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Criswell SR, Perlmutter JS, Videen TO, Moerlein SM, Flores HP, Birke AM, Racette BA. Reduced uptake of [18F]FDOPA PET in asymptomatic welders with occupational manganese exposure. Neurology. 2011;76:1296–1301. doi: 10.1212/WNL.0b013e3182152830. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dietz MC, Ihrig A, Wrazidlo W, Bader M, Jansen O, Triebig G. Results of magnetic resonance imaging in long-term manganese dioxide-exposed workers. Environ. Res. 2001;85:37–40. doi: 10.1006/enrs.2000.4068. [DOI] [PubMed] [Google Scholar]
- Dietz MC, Wrazidlo W, Ihrig A, Bader M, Triebig G. Magnetic resonance tomography of the brain in workers with chronic occupational manganese dioxide exposure. Rofo. 2000;172:514–20. doi: 10.1055/s-2000-3771. [DOI] [PubMed] [Google Scholar]
- Fahn S, Elton RL, Members of the UPDRS Development Committee . Unified Parkinson’s disease rating scale. In: Fahn S, Marsden CD, Goldstein M, Calne DB, editors. Recent developments in Parkinson’s disease. Macmillan; New York: 1987. pp. 153–63. [Google Scholar]
- Fahn S, Oakes D, Shoulson I, Kieburtz K, Rudolph A, Lang A, Olanow CW, Tanner C, Marek K. Levodopa and the progression of Parkinson’s disease. N. Engl. J. Med. 2004;351:2498–508. doi: 10.1056/NEJMoa033447. [DOI] [PubMed] [Google Scholar]
- Finkelstein MM, Jerrett M. A study of the relationships between Parkinson’s disease and markers of traffic-derived and environmental manganese air pollution in two Canadian cities. Environ. Res. 2007;104:420–32. doi: 10.1016/j.envres.2007.03.002. [DOI] [PubMed] [Google Scholar]
- Guilarte TR. Manganese and Parkinson’s disease: a critical review and new findings. Environ. Health Perspect. 2010;118:1071–80. doi: 10.1289/ehp.0901748. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guilarte TR, Burton NC, McGlothan JL, Verina T, Zhou Y, Alexander M, Pham L, Griswold M, Wong DF, Syversen T, Schneider JS. Impairment of nigrostriatal dopamine neurotransmission by manganese is mediated by pre-synaptic mechanism(s): implications to manganese-induced parkinsonism. J. Neurochem. 2008;107:1236–47. doi: 10.1111/j.1471-4159.2008.05695.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hacker CD, Perlmutter JS, Criswell SR, Ances BM, Snyder A. Resting state functional connectivity of the striatum in Parkinson’s disease. Brain. 2012;135:3699–711. doi: 10.1093/brain/aws281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harris MK, Ewing WM, Longo W, DePasquale C, Mount MD, Hatfield R, Stapleton R. Manganese exposures during shielded metal arc welding (SMAW) in an enclosed space. J. Occup. Environ. Hyg. 2005;2:375–82. doi: 10.1080/15459620591007736. [DOI] [PubMed] [Google Scholar]
- Harris RC, Lundin JI, Criswell SR, Hobson A, Swisher LM, Evanoff BA, Checkoway H, Racette BA. Effects of parkinsonism on health status in welding exposed workers. Parkinsonism Relat Disord. 2011;17:672–6. doi: 10.1016/j.parkreldis.2011.05.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hauser RA, Zesiewicz TA, Martinez C, Rosemurgy AS, Olanow CW. Blood manganese correlates with brain magnetic resonance imaging changes in patients with liver disease. Can. J. Neurol. Sci. 1996;23:95–8. doi: 10.1017/s0317167100038786. [DOI] [PubMed] [Google Scholar]
- Henry JP, Scherman D. Radioligands of the vesicular monoamine transporter and their use as markers of monoamine storage vesicles. Biochem. Pharmacol. 1989;38:2395–404. doi: 10.1016/0006-2952(89)90082-8. [DOI] [PubMed] [Google Scholar]
- Hobson AJ, Sterling DA, Emo B, Evanoff BA, Sterling CS, Good L, Seixas N, Checkoway H, Racette BA. Validity and reliability of an occupational exposure questionnaire for parkinsonism in welders. J Occup. Environ. Hyg. 2009;6:324–31. doi: 10.1080/15459620902836856. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang CC, Chu NS, Lu CS, Chen RS, Calne DB. Long-term progression in chronic manganism: ten years of follow-up. Neurology. 1998;50:698–700. doi: 10.1212/wnl.50.3.698. [DOI] [PubMed] [Google Scholar]
- Huang CC, Lu CS, Chu NS, Hochberg F, Lilienfeld D, Olanow W, Calne DB. Progression after chronic manganese exposure. Neurology. 1993;43:1479–83. doi: 10.1212/wnl.43.8.1479. [DOI] [PubMed] [Google Scholar]
- Jankovic J. Searching for a relationship between manganese and welding and Parkinson’s disease. Neurology. 2005;64:2021–8. doi: 10.1212/01.WNL.0000166916.40902.63. [DOI] [PubMed] [Google Scholar]
- Kim J, Kim JM, Kim YK, Shin JW, Choi SH, Kim SE, Kim Y. Dopamine transporter SPECT of a liver cirrhotic with atypical parkinsonism. Ind. Health. 2007;45:497–500. doi: 10.2486/indhealth.45.497. [DOI] [PubMed] [Google Scholar]
- Kim Y, Jeong KS, Song HJ, Lee JJ, Seo JH, Kim GC, Lee HJ, Kim HJ, Ahn JH, Park SJ, Kim SH, Kwon YJ, Chang Y. Altered white matter microstructural integrity revealed by voxel-wise analysis of diffusion tensor imaging in welders with manganese exposure. Neurotoxicology. 2011;32:100–9. doi: 10.1016/j.neuro.2010.11.004. [DOI] [PubMed] [Google Scholar]
- Kim Y, Kim JM, Kim JW, Yoo CI, Lee CR, Lee JH, Kim HK, Yang SO, Chung HK, Lee DS, Jeon B. Dopamine transporter density is decreased in parkinsonian patients with a history of manganese exposure: What does it mean? Mov. Disord. 2002;17:568–75. doi: 10.1002/mds.10089. [DOI] [PubMed] [Google Scholar]
- Kim Y, Kim JW, Ito K, Lim HS, Cheong HK, Kim JY, Shin YC, Kim KS, Moon Y. Idiopathic parkinsonism with superimposed manganese exposure: utility of positron emission tomography. Neurotoxicology. 1999a;20:249–52. [PubMed] [Google Scholar]
- Kim Y, Kim KS, Yang JS, Park IJ, Kim E, Jin Y, Kwon KR, Chang KH, Kim JW, Park SH, Lim HS, Cheong HK, Shin YC, Park J, Moon Y. Increase in signal intensities on T1-weighted magnetic resonance images in asymptomatic manganese-exposed workers. Neurotoxicology. 1999b;20:901–7. [PubMed] [Google Scholar]
- Koller WC, Lyons KE, Truly W. Effect of levodopa treatment for parkinsonism in welders: A double-blind study. Neurology. 2004;62:730–3. doi: 10.1212/01.wnl.0000113726.34734.15. [DOI] [PubMed] [Google Scholar]
- Korczynski RE. Occupational health concerns in the welding industry. Appl. Occup. Environ. Hyg. 2000;15:936–45. doi: 10.1080/104732200750051175. [DOI] [PubMed] [Google Scholar]
- Krieger S, Jauss M, Jansen O, Stiehl A, Sauer P, Geissler M, Theilmann L, Krieger D. MRI findings in chronic hepatic encephalopathy depend on portosystemic shunt: results of a controlled prospective clinical investigation. J. Hepatol. 1997;27:121–6. doi: 10.1016/s0168-8278(97)80290-5. [DOI] [PubMed] [Google Scholar]
- Laakso A, Vilkman H, Bergman J, Haaparanta M, Solin O, Syvalahti E, Salokangas RK, Hietala J. Sex differences in striatal presynaptic dopamine synthesis capacity in healthy subjects. Biol. Psychiatry. 2002;52:759–63. doi: 10.1016/s0006-3223(02)01369-0. [DOI] [PubMed] [Google Scholar]
- Lu CS, Huang CC, Chu NS, Calne DB. Levodopa failure in chronic manganism. Neurology. 1994;44:1600–2. doi: 10.1212/wnl.44.9.1600. [DOI] [PubMed] [Google Scholar]
- Lucking CB, Durr A, Bonifati V, Vaughan J, De Michele G, Gasser T, Harhangi BS, Meco G, Denefle P, Wood NW, Agid Y, Brice A. Association between early-onset Parkinson’s disease and mutations in the parkin gene. French Parkinson’s Disease Genetics Study Group. N. Engl. J. Med. 2000;342:1560–7. doi: 10.1056/NEJM200005253422103. [DOI] [PubMed] [Google Scholar]
- Martin WRW, Perlmutter JS. Assessment of fetal tissue transplantation in Parkinson’s disease: does PET play a role? Neurology. 1994;44:1777–80. doi: 10.1212/wnl.44.10.1777. [DOI] [PubMed] [Google Scholar]
- Meeker JD, Susi P, Flynn MR. Manganese and welding fume exposure and control in construction. Journal of Occupational and Environmental Hygiene. 2007;4:943–51. doi: 10.1080/15459620701718867. [DOI] [PubMed] [Google Scholar]
- Myers JE, teWaterNaude J, Fourie M, Zogoe HB, Naik I, Theodorou P, Tassel H, Daya A, Thompson ML. Nervous system effects of occupational manganese exposure on South African manganese mineworkers. Neurotoxicology. 2003;24:649–56. doi: 10.1016/S0161-813X(03)00035-4. [DOI] [PubMed] [Google Scholar]
- Nandhagopal R, Mak E, Schulzer M, McKenzie J, McCormick S, Sossi V, Ruth TJ, Strongosky A, Farrer MJ, Wszolek ZK, Stoessl AJ. Progression of dopaminergic dysfunction in a LRRK2 kindred: a multitracer PET study. Neurology. 2008;71:1790–5. doi: 10.1212/01.wnl.0000335973.66333.58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nelson G, Criswell SR, Zhang J, Murray J, Racette BA. Research capacity development in South African manganese mines to bridge exposure and neuropathologic outcomes. Neurotoxicology. 2012;33:683–6. doi: 10.1016/j.neuro.2012.01.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nelson K, Golnick J, Korn T, Angle C. Manganese Encephalopathy: Utility of early magnetic-resonance-imaging. British Journal of Industrial Medicine. 1993;50:510–3. doi: 10.1136/oem.50.6.510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Olanow CW. Manganese-induced parkinsonism and Parkinson’s disease. Ann N. Y. Acad. Sci. 2004;1012:209–23. doi: 10.1196/annals.1306.018. [DOI] [PubMed] [Google Scholar]
- Park RM, Schulte PA, Bowman JD, Walker JT, Bondy SC, Yost MG, Touchstone JA, Dosemeci M. Potential occupational risks for neurodegenerative diseases. Am. J. Ind. Med. 2005;48:63–77. doi: 10.1002/ajim.20178. [DOI] [PubMed] [Google Scholar]
- Parkinson Study Group The safety and tolerability of a mixed lineage kinase inhibitor (CEP-1347) in PD. Neurology. 2004;62:330–2. doi: 10.1212/01.wnl.0000103882.56507.20. [DOI] [PubMed] [Google Scholar]
- Parkinson’s Study Group DATATOP: a multicenter clinical trial in early Parkinson’s disease. Arch. Neurol. 1989;46:1052–60. doi: 10.1001/archneur.1989.00520460028009. [DOI] [PubMed] [Google Scholar]
- Perl DP, Olanow CW. The neuropathology of manganese-induced Parkinsonism. J. Neuropathol. Exp. Neurol. 2007;66:675–82. doi: 10.1097/nen.0b013e31812503cf. [DOI] [PubMed] [Google Scholar]
- Pujol A, Pujol J, Graus F, Rimola A, Peri J, Mercader JM, Garcia-Pagan JC, Bosch J, Rodes J, Tolosa E. Hyperintense globus pallidus on T1-weighted MRI in cirrhotic patients is associated with severity of liver failure. Neurology. 1993;43:65–9. doi: 10.1212/wnl.43.1_part_1.65. [DOI] [PubMed] [Google Scholar]
- Racette BA, Criswell SR, Lundin JI, Hobson A, Seixas N, Kotzbauer PT, Evanoff BA, Perlmutter JS, Zhang J, Sheppard L, Checkoway H. Increased risk of parkinsonism associated with welding exposure. Neurotoxicology. 2012;33:1356–61. doi: 10.1016/j.neuro.2012.08.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Racette BA, McGee-Minnich L, Moerlein SM, Mink JW, Videen TO, Perlmutter JS. Welding-related parkinsonism: clinical features, treatment, and pathophysiology. Neurology. 2001;56:8–13. doi: 10.1212/wnl.56.1.8. [DOI] [PubMed] [Google Scholar]
- Racette BA, Tabbal SD, Jennings D, Good L, Perlmutter JS, Evanoff B. Prevalence of parkinsonism and relationship to exposure in a large sample of Alabama welders. Neurology. 2005;64:230–5. doi: 10.1212/01.WNL.0000149511.19487.44. [DOI] [PubMed] [Google Scholar]
- Rappaport SM, Weaver M, Taylor D, Kupper L, Susi P. Application of mixed models to assess exposures monitored by construction workers during hot processes. Ann. Occup. Hyg. 1999;43:457–69. [PubMed] [Google Scholar]
- Rodier J. Manganese poisoning in Moroccan miners. Br. J. Ind. Med. 1955a;12:21–35. doi: 10.1136/oem.12.1.21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sadek AH, Rauch R, Schulz PE. Parkinsonism due to Manganism in a Welder. International Journal of Toxicology. 2003;22:393–401. doi: 10.1177/109158180302200511. [DOI] [PubMed] [Google Scholar]
- Sanotsky Y, Lesyk R, Fedoryshyn L, Komnatska I, Matviyenko Y, Fahn S. Manganic encephalopathy due to “ephedrone” abuse. Mov. Disord. 2007;22:1337–43. doi: 10.1002/mds.21378. [DOI] [PubMed] [Google Scholar]
- Schoenberg BS, Anderson DW, Haerer AF. Prevalence of Parkinson’s disease in the biracial population of Copiah County, Mississippi. Neurology. 1985;35:841–5. doi: 10.1212/wnl.35.6.841. [DOI] [PubMed] [Google Scholar]
- Selikhova M, Fedoryshyn L, Matviyenko Y, Komnatska I, Kyrylchuk M, Krolicki L, Friedman A, Taylor A, Jager HR, Lees A, Sanotsky Y. Parkinsonism and dystonia caused by the illicit use of ephedrone--a longitudinal study. Mov. Disord. 2008;23:2224–31. doi: 10.1002/mds.22290. [DOI] [PubMed] [Google Scholar]
- Shinotoh H, Snow BJ, Chu NS, Huang CC, Lu CS, Lee C, Takahashi H, Calne DB. Presynaptic and postsynaptic striatal dopaminergic function in patients with manganese intoxication: a positron emission tomography study. Neurology. 1997;48:1053–6. doi: 10.1212/wnl.48.4.1053. [DOI] [PubMed] [Google Scholar]
- Shinotoh H, Vingerhoets FJ, Schulzer M, Snow BJ. The presymptomatic period in a patient with idiopathic parkinsonism. Parkinsonism. Relat Disord. 1996;2:127–30. doi: 10.1016/1353-8020(96)00010-7. [DOI] [PubMed] [Google Scholar]
- Sikk K, Taba P, Haldre S, Bergquist J, Nyholm D, Askmark H, Danfors T, Sorensen J, Thurfjell L, Raininko R, Eriksson R, Flink R, Farnstrand C, Aquilonius SM. Clinical, neuroimaging and neurophysiological features in addicts with manganese-ephedrone exposure. Acta Neurol Scand. 2010;121:237–43. doi: 10.1111/j.1600-0404.2009.01189.x. [DOI] [PubMed] [Google Scholar]
- Spahr L, Butterworth RF, Fontaine S, Bui L, Therrien G, Milette PC, Lebrun LH, Zayed J, Leblanc A, Pomier-Layrargues G. Increased blood manganese in cirrhotic patients: relationship to pallidal magnetic resonance signal hyperintensity and neurological symptoms. Hepatology. 1996;24:1116–20. doi: 10.1002/hep.510240523. [DOI] [PubMed] [Google Scholar]
- Stepens A, Stagg CJ, Platkajis A, Boudrias MH, Johansen-Berg H, Donaghy M. White matter abnormalities in methcathinone abusers with an extrapyramidal syndrome. Brain. 2010;133:3676–84. doi: 10.1093/brain/awq281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Susi P, Goldberg M, Barnes P, Stafford E. The use of a task-based exposure assessment model (T-BEAM) for assessment of metal fume exposures during welding and thermal cutting. Appl. Occup. Environ. Hyg. 2000;15:26–38. doi: 10.1080/104732200301827. [DOI] [PubMed] [Google Scholar]
- Tanaka S, Lieben J. Manganese poisoning and exposure in Pennsylvania. Arch. Environ. Health. 1969;19:674–84. doi: 10.1080/00039896.1969.10666909. [DOI] [PubMed] [Google Scholar]
- Wang JD, Huang CC, Hwang YH, Chiang JR, Lin JM, Chen JS. Manganese induced parkinsonism: an outbreak due to an unrepaired ventilation control system in a ferromanganese smelter. Br. J Ind. Med. 1989;46:856–9. doi: 10.1136/oem.46.12.856. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Willis AW, Evanoff BA, Lian M, Galarza A, Wegrzyn A, Schootman M, Racette BA. Metal emissions and urban incident Parkinson disease: a community health study of medicare beneficiaries by using geographic information systems. Am. J. Epidemiol. 2010;172:1357–63. doi: 10.1093/aje/kwq303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Willis AW, Schootman M, Kung N, Evanoff BA, Perlmutter JS, Racette BA. Predictors of Survival in Patients With Parkinson Disease. Arch. Neurol. 2012;69:601–7. doi: 10.1001/archneurol.2011.2370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wolters EC, Huang CC, Clark C, Peppard RF, Okada J, Chu NS, Adam MJ, Ruth TJ, Li D, Calne DB. Positron emission tomography in manganese intoxication. Ann. Neurol. 1989;26:647–51. doi: 10.1002/ana.410260510. [DOI] [PubMed] [Google Scholar]
- Wright WA, Evanoff BA, Lian M, Criswell SR, Racette BA. Geographic and ethnic variation in Parkinson disease: a population-based study of US Medicare beneficiaries. Neuroepidemiology. 2010;34:143–51. doi: 10.1159/000275491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamada M, Ohno S, Okayasu I, Okeda R, Hatakeyama S, Watanabe H, Ushio K, Tsukagoshi H. Chronic manganese poisoning: a neuropathological study with determination of manganese distribution in the brain. Acta Neuropathol. (Berl) 1986;70:273–8. doi: 10.1007/BF00686083. [DOI] [PubMed] [Google Scholar]