Abstract
Objective
To characterize the population pharmacokinetics (PK) of oral baclofen and assess impact of patient-specific covariates in children with cerebral palsy (CP) in order to support its clinical use.
Subjects design
Children (2-17 years of age) with CP received a dose of titrated oral baclofen from 2.5 mg 3 times a day to a maximum tolerated dose of up to 20 mg 4 times a day. PK sampling followed titration of 10-12 weeks. Serial R- and S-baclofen plasma concentrations were measured for up to 16 hours in 49 subjects. Population PK modeling was performed using NONMEM 7.1 (ICON PLC; Ellicott City, Maryland).
Results
R- and S-baclofen showed identical concentration-time profiles. Both baclofen enantiomers exhibited linear and dose/kg-proportional PK, and no sex differences were observed. Average baclofen terminal half-life was 4.5 hours. A 2-compartment PK model with linear elimination and transit absorption steps adequately described concentration-time profiles of both baclofen enantiomers. The mean population estimate of apparent clearance/F was 0.273 L/h/kg with 33.4% inter-individual variability (IIV), and the apparent volume of distribution (Vss/F) was 1.16 L/kg with 43.9% IIV. Delayed absorption was expressed by a mean transit time of 0.389 hours with 83.7% IIV. Body weight, a possible genetic factor, and age were determinants of apparent clearance in these children.
Conclusion
The PK of oral baclofen exhibited dose-proportionality and were adequately described by a 2-compartment model. Our population PK findings suggest that baclofen dosage can be based on body weight (2 mg/kg per day) and the current baclofen dose escalation strategy is appropriate in the treatment of children with CP older than 2 years of age.
Baclofen is one of the skeletal muscle relaxants for the treatment of spasticity of cerebral palsy (CP) in adults. It is an agonist for gamma-aminobutyric acid B receptors on both pre- and post-synaptic neurons in spinal cord and brain.1 Baclofen binding to gamma-aminobutyric acid receptors may reduce release of excitatory neurotransmitters in pre-synaptic neurons and stimulate inhibitory neuronal signals in the post-synaptic neurons.2 Oral baclofen has been used to treat spasticity in children for more than 3 decades,3 although clinical results do not show consistent effectiveness.3,4 Careful dose titration is recommended until symptomatic effect is attained and possible side effects are minimized.
Baclofen is rapidly and extensively absorbed following oral administration, with bioavailability of 70%-85%. It is primarily eliminated by renal excretion in unchanged form (renal clearance: 10-17 L/h). Large inter-individual variability (IIV) (over 90%) has been observed in both oral absorption and elimination processes.5,6 Safety and efficacy of oral baclofen are well documented for adults in the drug package insert. However, there is little information on the pharmacokinetics (PK) properties of baclofen in children. Establishment of safe and effective dosing strategies for children with CP is needed.
This study investigated the PK properties of baclofen in children with CP and sought subject-specific properties that may influence PK profiles and clinical responses among pediatric patients. The concentration-time profiles of baclofen in these children were obtained after dose titration. The PK properties of both R- and S-baclofen were characterized using population modeling methods.
Methods
Subjects with spastic CP (n = 61; age 2-17 years) were enrolled after attainment of institutional review board approval at 9 sites as part of the National Institute of Child Health and Human Development-2005-13-2 protocol. Enrollment was monitored to ensure approximately even representation of sex and age groups. Eligible subjects had a Gross Motor Function Classification Scale level of II-V, leg hypertonia/spasticity defined as an Ashworth Scale score of at least 2, and a Tardieu score of 2 (spastic catch) in at least 1 knee (flexors and/or extensors). Eligibility criteria precluded subjects with active intrathecal baclofen pump within the past 6 months, use of baclofen and other tone-modifying agents within the past 4 months, non-medically prescribed drug abuse, alcohol/tobacco use, and use of enzyme inducers or drugs known to alter renal function. Subjects were also excluded if they had severe gastroesophageal reflux disease (GERD) including prior biopsy or endoscopy proven esophagitis or a history of vomiting more than 3 times per week, delayed gastric emptying, malnutrition, renal or liver disease, severe respiratory or cardiac disease, and uncontrolled seizures.
This was an open-label, ascending dose study of oral baclofen in pediatric subjects with spastic CP. The range of doses and escalation strategies used for this study were based on current clinical practices and preliminary data. Subjects under age 6 years did not receive dosages above 10 mg 3 times a day (TID). Otherwise, the maximum dosage was 20 mg 4 times a day (QID). Three formulations were used: 2 mg/mL liquid solution (UPM Pharmaceuticals, Baltimore, Maryland), 5 mg tablets (Novartis Pharmaceuticals, Basel, Switzerland), and 10 mg tablets (Qualitest Pharmaceuticals, Huntsville, Alabama). The first 15 subjects started the study on the liquid preparation, but only 2 were still on liquid baclofen at the time of the PK visit. Eight (of 15) switched to the tablets during dose escalation and provided PK samples.
All subjects started on oral baclofen at 2.5 mg TID and received an increased dose every 2 weeks until they reached either the maximum tolerated dose or the maximum study dosage. The first dose was given at approximately 7 a.m. The TID and QID regimens involved unequal dosing intervals of 7/7/10 and 5/5/5/9 hours. Doses were given at least 30 minutes before or at least 2 hours after a meal. The 2-week interval between each dose escalation was chosen to reduce the risk for side effects and to allow transient side effects to resolve. Subjects who could not tolerate at least 5 mg TID were discontinued from the study with a baclofen tapering process.
One pre-dose and 13 post-dose blood samples were collected serially over 16 hours after the second daily dose during a PK visit that occurred at the maximum tolerated dose or maximum dose limit (80 mg/d). The subsequent scheduled doses were not given until the PK sampling period was completed. R- and S-baclofen were measured in plasma, using a validated tandem mass spectrometry method (Appendix; available at www.jpeds.com).
PK Modeling Analysis
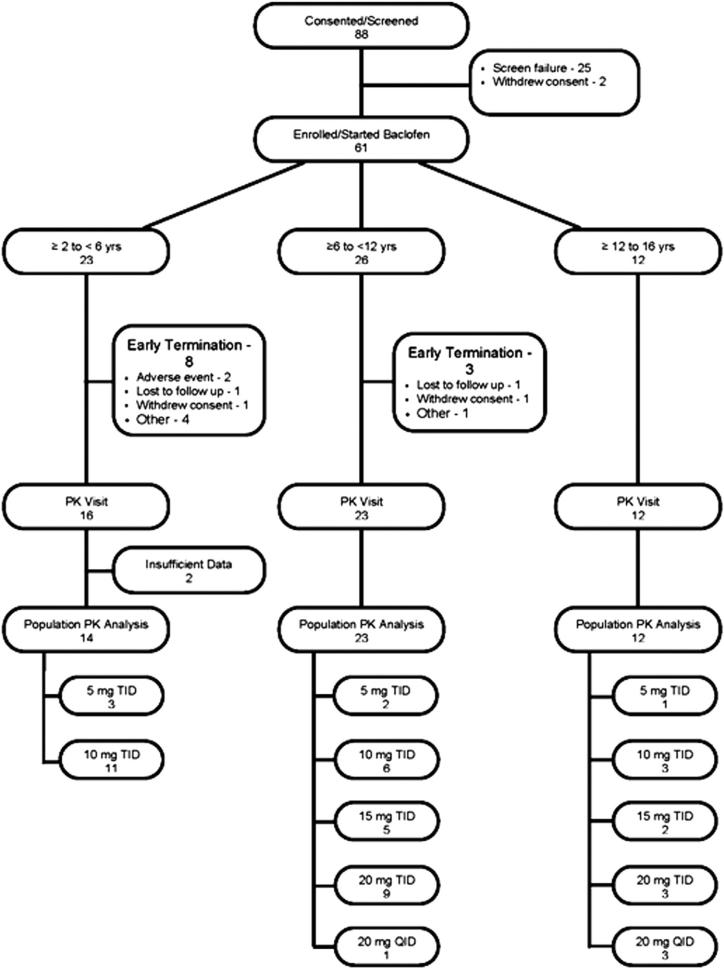
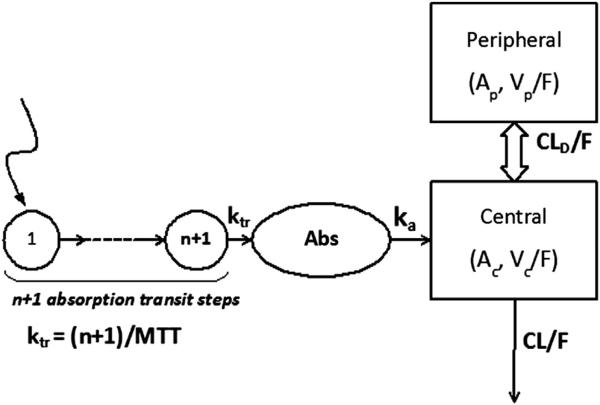
Figure 1 (available at www.jpeds.com) depicts the subject array and disposition during the baclofen dosing period and PK analysis process. The PK of oral baclofen was analyzed with a mixed-effects population model, incorporating intra-individual variability and IIV. A 2-compartment model with first-order absorption and elimination was used as the base model (Figure 2; available at www.jpeds.com) after evaluation of various PK model candidates based on the NONMEM objective functions and weighted residual plots. The absorption process was modeled by chained transit steps that describe delayed absorption during multiple dosing7,8 with an assumption that absorption is completed within each dosing interval. IIV, represented by Eta, was estimated using an exponential variability model for clearance (CL), the volume of the central compartment (Vc), the absorption rate into plasma (ka), mean transit time (MTT), and number of absorption transit steps (n). Log-normal distribution was assumed for the PK variables. The unidentified residual variability was modeled with a combination of additive and proportional errors. An empirical Bayes estimate of Eta for each PK variable was obtained from the base model. Covariate analysis was then performed by visually exploring the graphs (Eta-plots) of IIV versus patient-specific characteristics, including body weight in kg (WTKG), age, gestational age (GAGE), birth body weight, sex, race, serum creatinine concentration, creatinine clearance (CLCR), bladder or kidney function, gastric motility/gastroesophageal reflux status, and disease severity status. A preliminary pharmacogenomics (PG) analysis was performed to explore the impact of genetic variation on PK variables. Potential covariates were subsequently evaluated using a model fitting procedure. The details of structural model equations, variability models, covariate assessment, and exploratory PG analysis are presented in the Appendix.
Figure 1.
Subject screening and disposition chart.
Figure 2.
Schematic model structure of baclofen PK. A 2-compartment model was constructed with a first-order absorption rate constant (ka) and first-order clearance. The delayed absorption process was described by a number (n) of chained transit steps with a first-order transit rate constant (ktr). Ac and Ap are the amounts of drug in central (Vc) and peripheral (Vp) compartments, CLD represents distributional clearance between the two compartments, and F denotes bioavailability.
Model Assessment
It has been reported that R-baclofen is pharmacologically active, although both baclofen enantiomers can bind to the same receptor on neurons.9,10 In this study, the PK data of R-baclofen was of primary interest, and the data for S-baclofen was used secondarily for model assessment and to derive the final model estimates.
Verification of the developed model from R-baclofen was performed using the S-baclofen plasma concentration dataset. The R-baclofen model predictions were compared with observed values from the S-baclofen data set at corresponding time points. Goodness-of-fit plots were also generated, including observed versus predicted values at individual and population levels and weighted residuals versus predictions. The values of Eta and Eps shrinkage were calculated for indication of goodness-of-fit plots reliability.
Subsequently, model variables were re-estimated with a combined dataset from R- and S-baclofen. A standardized visual predictive check (SVPC)11 was implemented for final evaluation (Appendix) because a continuous covariate (WTKG) and categorical covariates (60 mg/d group: total daily dose [TDOS] 60 and 80 mg/day group: TDOS 80) were included in the final model. If the model structure and variable estimation are adequate, the percentiles (Pi, j) should be uniformly distributed between 0 and 1. Nonparametric bootstrap (BS) analysis was also performed to assess the accuracy and robustness of the final multivariable model variable estimation, using the combined R- and S-baclofen data. A for each variable was constructed using the BS results from all fitted models.
Results
Forty-nine subjects had at least one post-dose PK blood sample and were included in the population pharmacokinetics (PopPK) analysis. The first 15 enrolled subjects initiated the study with the liquid baclofen preparation. Two of these subjects (about 4%) finished the PK study while still on liquid baclofen. Another six (of the first 15 subjects) were switched from the liquid to the tablet formulation at least 2 weeks before the PK visit. The median age of the subjects was 8.3 years with a range of 2.2-17.7 years (Table I; available at www.jpeds.com). Only subjects older than 6 years of age received baclofen doses of 15 mg TID (45 mg daily dose) or above. Most subjects (over 66.7%) were prematurely born, and the median GAGE was 30 weeks. The serum creatinine concentrations ranged from 0.2-0.9 mg/dL (normal). Over one-half (57%) of subjects were ambulatory, and 33% of subjects showed impaired bladder control. Many exhibited gastroesophageal problems. The medical history revealed that 36 (73%) participants included in the PK analysis had at least one present symptom of oral motor dysfunction, and 36 (73%) also had at least one present symptom of gastric motility disorder or GERD.
Table 1.
Demographic characteristics of subjects included in the PK analysis
| Subject Covariate | Median (range) |
|---|---|
| Body weight (kg) | 22.2 (11.2-84.8) |
| Age (yr) | 8.3 (2.2-17.7) |
| GAGE (wk) | 30.0 (23-41) |
| Serum creatinine (mg/dL) | 0.4 (0.2-0.9) |
| CLCR (mL/min) | 96.7 (47.9-191) |
| Subject Covariate | n (%) |
|---|---|
| Sex | |
| Male | 27 (55) |
| Female | 22 (45) |
| Race* | |
| White or Causasian | 38 (78) |
| Black or African American | 9 (18) |
| Multiracial | 1 (2) |
| Gross Motor Function Status† | |
| Ambulatory | 28 (57) |
| Nonambulatory | 21 (43) |
| Kidney or bladder dysfunction | |
| Abnormal VCUG | |
| Absent | 26 (53) |
| Present | 2 (4) |
| NA/ND | 21 (43%) |
| Abnormal renal or bladder ultrasound | |
| Absent | 27 (55) |
| Present | 1 (2) |
| NA/ND | 21 (43) |
| Impaired bladder control | |
| Absent | 33 (67) |
| Present | 16 (33) |
| GERD/Gastrointestinal motility disorder | |
| Vomiting not associated with known gastrointestinal infection | |
| Absent | 20 (41) |
| Present | 29 (59) |
| Gastric acid/stomach contents in mouth | |
| Absent | 40 (82) |
| Present | 9 (18) |
| Constipation | |
| Absent | 41 (84) |
| Present | 8 (16) |
VCUG, voiding cystourethrography; NA/ND, not available or note done and represents testing results that were unavailable or tests that were not performed.
One subject has no reported race but ethnicity was reported as Hispanic or Latino.
Determined from the Gross Motor Function Classification System.
PK Data
A total of 1200 concentration-time points of R- and S-baclofen are included in the PopPK analysis and depicted with stratification of daily dose in Figure 3 (available at www.jpeds.com). The scheduled PK sampling plan covered the majority of the time range of baclofen absorption and elimination. Many subjects showed delayed absorption, followed by typical multi-exponential declines after reaching the concentration peaks. None of the baclofen concentrations were below the assay limit of quantification.
Figure 3.
The measured R- and S-baclofen concentrations versus time after the last dose for all subjects. The data were stratified according to Daily Dose, TDOSs.
Exploratory Analysis
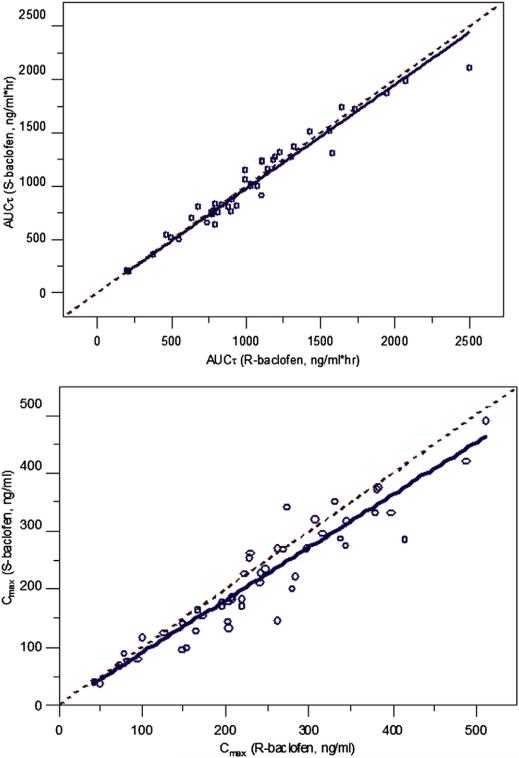
The terminal half-life (t1/2), time to maximum concentration (Tmax), dose-normalized Cmax, and dose-normalized area under the curve within the dosing interval (AUCt) of R- or S-baclofen were obtained for each subject using noncompart-mental analysis (NCA). There were no differences in the NCA PK variables between R- and S-baclofen (Table II; available at www.jpeds.com). The AUCt and Cmax of R-baclofen and S-baclofen were linearly correlated with each other with a slope close to unity (AUCt, slope = 0.99; Cmax, slope = 0.90) (Figure 4; available at www.jpeds.com). This indicated that both enantiomers of baclofen share the same PK characteristics. The AUCt of both R- and S-baclofen linearly increased with body weight-normalized daily dose (R-baclofen, rR = 0.82; S-baclofen, rS = 0.79; Figure 5), which indicates linear PK for both enantiomers. No difference between sexes was evident. Thus, linear elimination of baclofen was assumed in the following PK modeling analysis.
Table II.
Noncompartmental PK parameters for R- and S-baclofen
| R-baclofen (n = 43) |
S-baclofen (n = 43) |
||||
|---|---|---|---|---|---|
| Description | Mean (SD) | Range | Mean (SD) | Range | |
| Cmax/dose (ng/mL/mg) | Dose-normalized maximum concentration | 6.25 (2.60) | 1.61-13.4 | 5.63 (2.16) | 1.30-11.1 |
| Tmax (h) | Time to reach maximum concentration | 2.08 (1.05) | 0.5-7.0 | 2.86 (1.04) | 1.0-7.0 |
| AUCτ/dose (ng·h/mL/mg) | Dose-normalized AUCτ | 25.4 (10.7) | 6.96-62.3 | 25.3 (10.1) | 6.73-54.8 |
| t1/2 (h) | Terminal half-life (t1/2) | 4.48 (1.67) | 2.03-12.0 | 4.32 (1.75) | 2.27-13.5 |
Figure 4.
Correlation plots of AUCt and Cmax for R- versus S-baclofen. Open circles are AUCt or Cmax values; solid lines are linear regression line for correlation; dashed lines are identity lines.
Figure 5.
The relationships of observed AUCt versus TDOS per kg body weight for R- (top) and S-(bottom) baclofen. Solid lines are regression lines.
Population PK Analysis
Structural Model
The R-baclofen plasma concentration datafrom all 49 subjectswere included for model development. Various PK model structures were tested with different combinations of absorption and elimination processes. The PK of R-baclofen was best described by a 2-compartment model with linear elimination in which sequential absorption transit steps were incorporated to describe the delayed absorption process. The optimal number of absorption transit steps was estimated as a model variable. IIV of the associated PK variables was evaluated in stepwise manner by assessing reductions in NONMEM objective function values. Figure 6 depicts representative individual model fittings. Model prediction successfully captured the observed baclofen concentrations in all dose groups during the entire sample time period.
Figure 6.
Model fitting plots for representative individuals in different dose groups.
Covariate Model
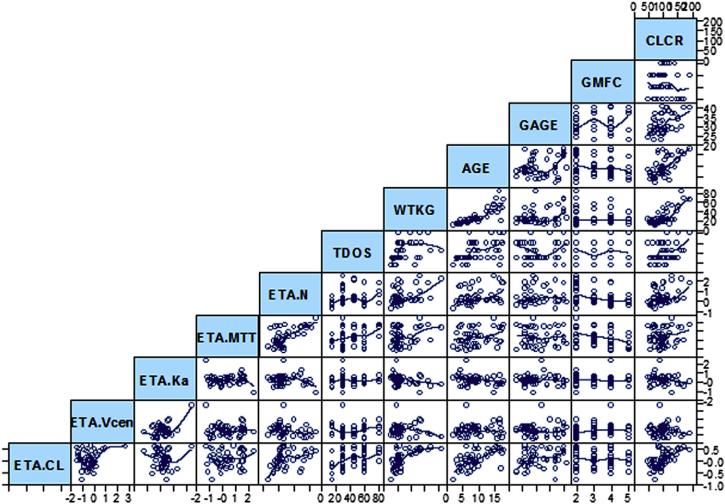
The Eta-plots (Figure 7; available at www.jpeds.com) were generated to assess possible covariates effects on PK variables, with the implementation of the Loess curve-fitting method.12 Nearly linear correlations were observed among WTKG, age, and CLCR. Each of the 3 patient properties showed possible correlations with CL, central volume (Vc), and absorption rate constant (ka), but not with others. The model fitting with forward-selection and backward-elimination procedures examined variables including body weight, age, GAGE, and the other factors. Body weight had significant impacts on CL/F and V/F according to:
| (1) |
| (2) |
| (3) |
| (4) |
The power coefficient of body weight (0.529) differed from the conventional allometric factor (0.75). This is in agreement with studies showing that pediatric body weight exponents for CL often differ from 0.75.13-15 CLCR and age did not have significant effects on CL/F, nor on V/F, after incorporating WTKG with the estimated allometric factor in the model.
Figure 7.
Correlation matrix plots for IIV (Eta) of Bayesian parameter estimations (CL, Vc, ka, MTT, and N) versus possible covariates WTKG, AGE in years, GAGE in weeks, TDOS in mg, disease severity (GMFC), and CLCR. Solid lines are the Loess regression lines.
The add-on exploratory PG analysis found that one single-nucleotide polymorphism (SNP) in ABCC9, a member of the ABC superfamily of adenosine 5’-triphosphate-binding transporter proteins, was significantly associated with CL/WTKG. Three subjects heterozygous for the ABCC9 rs11046232 AT genotype exhibited markedly higher CL than the others with the homozygous reference TT genotype (CL/F = 0.51 ± 0.05 for the AT genotype vs 0.25 ± 0.07 L/h/kg for the TT genotype, adjusted P = .0001), and this difference persisted when the CL values were corrected for the power coefficient of weight (0.529) as described above (Figures 3 and 8). However, the subsequent model fitting process found no significant impact of this SNP variation on CL after incorporating the WTKG covariate, probably owing to the small number with the AT genotype. Moderate age-dependence for CL/WTKG was observed in subjects with the TT homozygous genotype (r2 = 0.459; Figure 8), but it was obviated with the model-estimated allometric scaling factor on WTKG. In addition, the MTT for absorption differed in the dose groups.
Figure 8.
Relationship between R-baclofen clearance and age. A, Apparent clearance corrected for body weight in kg (CL/WTKG). B, Clearance corrected for the power coefficient of weight (CL/BWT0.529). Open symbols are the subjects with ABCC9 rs11046232 heterozygous AT genotypes and solid symbols are subjects with TT homozygous genotypes. The linear regression line depicts subjects with TT homozygous genotypes (r2 = 0.459, panel A; r2 = 0.001, panel B).
Multivariable Model
The NONMEM objective function was significantly reduced by incorporating covariate effects of body weight and daily dose groups (TDOS 60 and TDOS 80). The IIV of CL/F, Vc/F, ka, or MTT was also reduced because the covariate model partially explained the origins of this variability. There was consistency between observed and predicted values for individuals and the entire group (Figure 9, A; available at www.jpeds.com). No pattern in the corrected weighted residuals versus time-after-last-dose or population prediction plots was observed, indicating no model misspecification. The model also performed reasonably well in describing variability in the time course of baclofen concentrations (Figure 9, B). Overall, this model with the indicated covariates appropriately describes the PK in these subjects. A summary of model variables and statistics is provided in Table III.
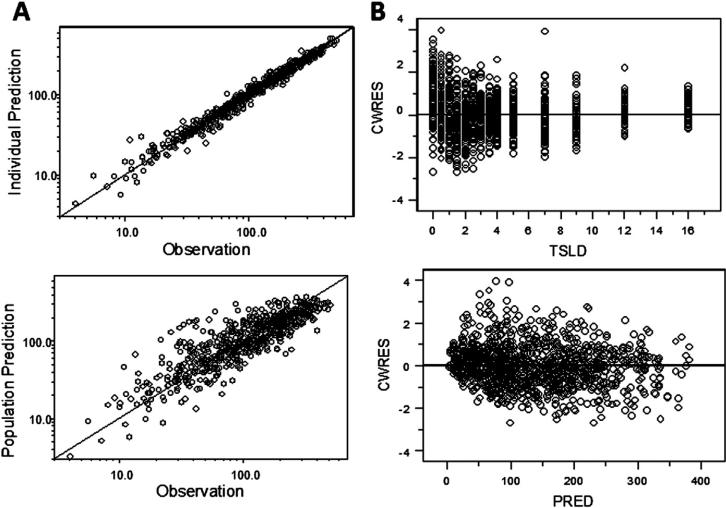
Figure 9.
A, Goodness-of-fit plots for R-baclofen PK data, individual model predictions (top) and population model predictions (bottom) versus observed data points. Solid lines are identity lines. B, Conditional weighted residuals (CWRES) versus time after last dose (top) or model predictions (bottom; PRED, population prediction). Solid lines are reference lines (y = 0).
Table III.
Parameter estimates for R- and S-baclofen PK in the final multivariable model with bootstrap results
| Population mean |
IIV |
||||||
|---|---|---|---|---|---|---|---|
| Parameter | Symbol | Estimate (CV%) | BS mean | BS CI* | Estimate (CV%) | BS mean | BS CI* |
| Final multivariable model | |||||||
| CL/F (L/h) | θ 1 | 5.98 (4.35) | 5.81 | 5.03-6.69 | 0.334 (19.3) | 0.317 | 0.190-0.380 |
| VC/F (L) | θ 2 | 14.3 (2.23) | 14.87 | 11.6-20.1 | 0.439 (43.1) | 0.430 | 0.307-0.507 |
| CLD/F (L/h) | θ 3 | 3.56 (2.25) | 3.55 | 2.65-4.74 | |||
| Vp/F (L) | θ 4 | 8.94 (0.113) | 8.89 | 7.01-11.2 | |||
| ka (1/h) | θ 5 | 0.823 (2.29) | 0.810 | 0.600-1.15 | 0.879 (46.5) | 0.843 | 0.616-0.936 |
| MTT (h) | θ 6 | 0.410 (1.86) | 0.389 | 0.275-0.549 | 0.837 (38.6) | 0.888 | 0.761-1.10 |
| n | θ 7 | 2.43 (1.26) | 2.49 | 1.78-3.87 | 1.01 (53.4) | 1.18 | 1.01-1.73 |
| Covariate model | |||||||
| WTKG ~ CL/F | θ 8 | 0.529 (25.0) | 0.437 | 0.158-0.712 | |||
| TDOS ~ MTT (If 60 mg/d) | θ 8 | 0.0656 (40.64) | 0.0875 | –0.0745-0.311 | |||
| TDOS ~ MTT (If 80 mg/d) | 010 | 0.522 (50.75) | 0.797 | 0.173-2.05 | |||
| Residual variability model | |||||||
| Proportional | ε 1 | 0.131 (5.89) | 0.140 | 0.125-0.159 | |||
| Additive | ε 2 | 7.612 (6.93) | 5.78 | 2.39-7.61 | |||
CV%, coefficient of variation %.
Nonparametric BS results from 1000 resampling data sets. BS CI, 95% CI.
Model Validation and Final Variable Estimation
The PK data of S-baclofen were used secondarily for verification. The individual and population PK model predictions based on R-baclofen were comparable with the observed S-baclofen concentrations at corresponding time points. The goodness-of-fit plots suggested that random variability of the S-baclofen concentration data can also be well described by the developed PK model based on R-baclofen. The results indicated that the multivariable PK model adequately describes both R- and S-baclofen PK profiles with good precision.
Both R- and S-baclofen PK datasets were combined and the multivariable model was fitted again for finalizing the variable estimation. Table III lists the final estimates of population PK variables, along with the BS mean and 95% CIs for all variable estimates. Most model variables were estimated with high precision (coefficient of variation % #25%). Large IIV for ka, MTT, and n were found. The Eta-shrinkage estimates for IIV for CL/F, Vc/F, ka, MTT, and n were 15.1%, 24.7%, 24.9%, 4.40%, and 26.9%, respectively. The model expandable polystyrene shrinkage estimate is 9.97%. No model misspecification was suggested.
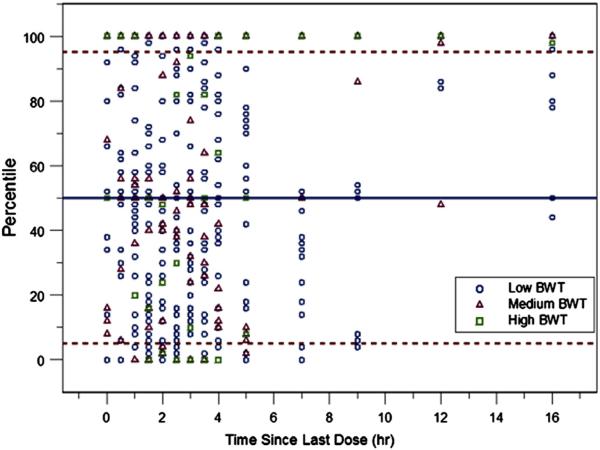
The final population variable estimates were close to the BS mean values and within the 95% CIs in the BS analysis, indicating robustness of the developed model and accuracy of the variable estimates. In addition, SVPC was implemented with the combined dataset (Figure 10; available at www.jpeds.com). A reasonable number of points fell out of the 5th and 95th percentiles with an even distribution of Pi,j for all subjects observed. Overall, the final model was deemed adequate in describing both the central tendencies and variability in observed drug concentrations.
Figure 10.
SVPC plots for combined R- and S-baclofen PK data. Solid lines are model predicted medians; dashed lines are 5th (upper) and 95th (lower) percentiles.
Discussion
This study utilizes a population modeling approach to characterize the PK of oral baclofen, identify patient-specific covariates, and assess influence of growth and maturation on baclofen PK in children (2-16 years old) with CP in order to provide support for clinical application of baclofen. Additionally, similarity of PK characteristics of both baclofen enantiomers was investigated.
Baclofen is a racemic compound that contains a mixture of R- and S-enantiomers. In many cases, pairs of optical enantiomers differ in one or more PK processes and in their pharmacological effects due to stereoselectivity, interactions between optical isomers, and/or interconversion.16-18 Stereo-selectivity is generally a concern for chiral drugs that are subjected to transporter-mediated uptake or efflux, and absorption of one enantiomer may be favored by selective binding to a specific transporter. Both baclofen enantiomers bind to amino acid carriers in the small intestine,19 but no stereoselection in carrier-mediated transport and absorption was evident in this study. Both enantiomers showed very similar Cmax, Tmax, and t1/2 values. Tissue and plasma binding often differ considerably for enantiomers. Both baclofen enantiomers have relatively low protein binding (fraction unbound = 65%-70%), and a small portion (15%) of oral doses undergoes deamination in the liver, which likely reduces stereoselectivity in these processes. Although optical enantiomers may behave differentially in glomerular filtration and passive reabsorption, the renal clearance of both R- and S-baclofen was reported to be similar and dependent on CLCR in dogs.20 Consistent with this observation, the entire concentration-time profiles of both enantiomers were essentially identical.
The PK of baclofen was previously investigated in adults. Wuis et al6 examined oral racemic baclofen PK in 4 healthy subjects, and reported an absorption rate constant of 0.932 ± 0.788 h–1, total CL of 16.5 ± 4.46 L/h, and central V of 33.9 ± 18.5 L. Shellenberger et al5 reported similar PK variable of oral baclofen in 15 healthy volunteers. A multiple-dose PK study of racemic baclofen found that PK variables in neurologic adult patients with spastic CP were similar to healthy volunteers, but with large variability.20 Highly variable PK variable values of R-baclofen were reported in a single-dose PK study of eight children (age 2-12 y) with non-neurologic disease (CL: 0.175-0.586 L/h/kg; V: 0.653-7.141 L/kg).21,22 Our study analyzed oral R- and S-baclofen PK data from 49 pediatric subjects with CP. The apparent clearance (CL/F) of 0.270 L/h/kg and the total apparent volume (Vss/F) of 1.05 L/kg in our children with CP are in agreement with previous studies. The estimated MTT (0.410 hour) is comparable with the reported Tlag of 0.59 ± 0.28 hours.22 Both variables describe the time gap between oral dosing and the initiation of absorption.
The PopPK modeling analysis found over 80% IIV for absorption related variables (ka, MTT, and n). The variability in oral absorption is considered to be a consequence of impairments associated with CP. More than 90% of children with CP have clinically significant chronic gastrointestinal symptoms, mostly due to disorders of gastrointestinal motility.23 Over 70% of children with CP have symptoms of GERD, most of whom have abnormal pH-monitoring and/or esophagitis23-25 Impaired oral motor control, drooling, and swallowing problems occur in 30%-60% of children with CP, and delayed gastric emptying and regular vomiting are also commonly observed.25-27 These symptoms can affect drug retention in the stomach and transit time through the gastrointestinal tract. Our study found that the time of absorption delay varied among the subjects. The population PK model successfully described the central tendency of this delayed baclofen absorption and estimated the IIV of oral absorption in children with CP. In spite of the absorption delay, there was good consistency in Cmax and AUCt values among the subjects.
Currently, clinical pediatric dosing guidelines for baclofen, including dose ranges, dosing schedules, dose escalation strategies, and anticipated side effects are extrapolated from adult data. The dose of baclofen is not usually determined by body weight. Instead, a gradual dosing escalation strategy is commonly applied to find best clinical response in absence of intolerable side effects, which results in wide variability in dosing regimens among clinicians who treat children with CP. Our study finding indicates that body weight is a major contributing covariate to baclofen clearance. The results revealed that the influence of maturation on baclofen PK variables can be well elaborated by changes in body weight with the allometric scaling factor of 0.529 because age and CLCR are both correlated with body weight in these children. Extrapolation of baclofen doses (2 mg/kg per day) from adults based on body weight may be suitable in treatment of CP for children older than 2 years of age. The moderate age-dependent CL/WTKG may further suggest need for a larger dose/kg in the younger children when a linear WTKG-based extrapolation is used. Similar recommendations of body weight-based dose extrapolation have been reported in previous studies.22,28 Alternatively, an allometric coefficient of 0.67 is equivalent to dosing on a square meter body surface area basis as is sometimes done in children. Interestingly, the add-on exploratory PG analysis found that subjects with heterozygous AT genotype in ABCC9 rs11046232 had relatively higher CL than those with homozygous TT genotypes, but further model fitting testing showed limited contribution of this genetic variation to PK variables.
ABCC9 is a member of the gene superfamily that includes drug transporters, such as ABCB1 (P-glycoprotein) and the multidrug-resistant protein transporters (ABCC1-ABCC5), and shares both the transmembrane and nucleotide-binding domains characteristic of these proteins. However, ABCC9 does not appear to have any transporter function nor has it been implicated as a factor in drug elimination.29 Rather, ABCC9 appears to play a role in regulating muscle-specific adenosine 5’-triphosphate-sensitive potassium currents. It does so by forming an octameric complex consisting of 4 ABCC9 subunits and 4 subunits of an inward rectifying potassium channel (Kir6.1 or Kir6.2). Also known as the sulfonylurea receptor-2 (SUR2), ABCC9/SUR2 and Kir6.2 complexes generally predominate in skeletal muscle with ABCC9/SUR2 conferring drug sensitivity to adenosine 5’-triphosphate-sensitive potassium channel activity.30 There are no data regarding baclofen interactions with ABCC9, and the observed association between ABCC9 genotype and baclofen CL requires evaluation in future prospective studies to determine its value in predicting greater than anticipated drug clearance and the subsequent risk of reduced response to treatment.
Careful dose escalation should be performed for each patient to avoid severe adverse effects. In addition, to address the concern of significant neurogenic bladder pathology in patients with CP,31,32 we examined the impact of abnormal bladder function, but did not find significant alterations of baclofen PK variables in these subjects.
In conclusion, the time courses of baclofen concentrations following a range of oral doses in children with CP were well-characterized with a 2-compartment PK model with linear elimination and a delayed absorption process. Body weight and age were identified as contributing to the clearance and drug exposure for this patient population with preliminary findings of higher clearances in a genetic subgroup. Baclofen PK was largely proportional to dose/kg, making dose adjustments uncomplicated by PK issues. This finding suggests that baclofen dose amounts can be determined based on either body weight (2 mg/kg per day) or body surface area and the current baclofen dose escalation strategy is appropriate in the treatment of CP for children older than 2 years of age. Additional findings from this clinical trial will describe the efficacy and adverse effects of baclofen in this subject population in relation to the observed drug exposures. n
Acknowledgments
The authors thank all co-investigators and staff who worked on this project for their assistance at all investigational sites, including Pediatric Clinical Research Unit, St. Louis Children's Hospital and Washington University School of Medicine, St. Louis, MO; Seattle Children's Pediatric Clinical Research Center, Seattle, WA; Clinical Research Unit of Ann & Robert H. Lurie Children's Hospital, Chicago, IL; General Clinical Research Center, Kluge Children's Rehabilitation Center and Research Institute, Charlottesville, VA; Pediatric Clinical Research Units, Johns Hopkins Institute for Clinical and Translational Research, Baltimore, MD; Pediatric Pharmacology Research Unit, Baylor College of Medicine/Texas Children's Hospital, Houston, TX; Clinical Translational Research Center, Cincinnati Children's Hospital Medical Center, Cincinnati, OH; Pediatric Clinical Research Unit, Children's Mercy Hospital, Kansas City, MO; Clinical Research Unit, Upstate Medical University, Syracuse, NY.
The authors specially thank Ms Freda Branch for her extraordinary amount of work throughout the study, Ms Suzette Mis for her assistance with managing the preliminary data, and Mr Deo Garlock for his assistance with coordinating PK sample delivery and communications with Covance for all the sites throughout the study.
Supported by the Eunice Kennedy Shriver Institute of Child Health and Human Development.
Glossary
- AUCt
Area under the curve within the dosing interval
- BS
Bootstrap
- CL
Clearance
- CLCR
Creatinine clearance
- CP
Cerebral palsy
- GAGE
Gestational age
- GERD
Gastroesophageal reflux disease
- IIV
Inter-individual variability
- MTT
Mean transit time
- NCA
Noncompartmental analysis
- PG
Pharmacogenomics
- PK
Pharmacokinetics
- PopPK
Population pharmacokinetics
- SNP
Single-nucleotide polymorphism
- SVPC
Standardized visual predictive check
- QID
4 times a day
- TID
3 times a day
- TDOS
Total daily dose
- WTKG
Body weight in kg
Appendix
Methods
Assay for Racemic Baclofen
Baclofen is a racemic compound and the tablet formulation contains R- and S-baclofen. Both enantiomers were measured in plasma samples by COVANCE Lab Inc (Madison, Wisconsin). The R- and S-enantiomers of baclofen in samples and internal standard (Sigma-Aldrich, St. Louis, Missouri) were extracted by protein precipitation, and then derivatized with Marfey's reagent (Sigma-Aldrich) into their corresponding diastereomers. The latter were analyzed using liquid chromatography connected with tandem mass spectrometry detection. The standard curve range was 1 to 500 ng/mL for each baclofen enantiomer in human plasma.
PG
Whole blood or saliva from the study subjects was subjected to DNA isolation using the QIAamp DNA Blood Mini Kit (Qiagen, Valencia, California) in the Developmental Pharmacology and Experimental Therapeutics Laboratory in the Division of Clinical Pharmacology and Therapeutic Innovation at Children's Mercy Hospitals and Clinics (Kansas City, Missouri). Quality was assessed by agarose gel electrophoresis, and DNA concentration was determined spectrophotometrically with a NanoDrop instrument at the time of isolation. The DNA samples subsequently were genotyped for SNPs at the Centre for Molecular Medicine and Therapeutics, University of British Columbia (Vancouver, British Columbia, Canada) using a customized Illumina GoldenGate SNP genotyping assay (Illumina, San Diego, California). The genetic variations of 307 key genes involved in drug absorption, distribution, metabolism, and excretion were tested. Clearance for each subject was obtained from the PopPK model estimation. A univariate analysis was then conducted to determine the association between WTKG-normalized CL/F and each SNP. Continuous outcome variables were analyzed by a general linear model to compare the means of these variables by genotype. Multiple tests were adjusted by false discovery rate. Tests with an adjusted P-value (false discovery rate) less than .05 were considered statistically significant.
NCA
Subjects were excluded in the NCA if their blood sampling ceased within 7 or 5 hours post dose for the TID or QID dosing group, respectively. AUCt for R- and S-baclofen over 7 or 5 hours after dosing were calculated at steady-state for subjects with TID or QID dosing schedules using the trapezoidal rule. The Cmax and AUCt were then normalized by the TDOS for comparison. The terminal half-life (t1/2), time to maximum concentration (Tmax), dose-normalized Cmax, and dose-normalized AUCt of R- or S-baclofen were summarized for the study subjects.
PK Model Structure
A two-compartment model with first-order elimination was used to describe baclofen PK profiles in children, and chained transit steps were incorporated for the delayed absorption. The rate of change of baclofen in the absorption compartment (Aabs) and initial condition are
| (1) |
where ktr (h–1) is the transit rate constant expressed by (n+1)/MTT. The (n+1) is the number of absorption transit steps and MTT is the mean transit time (hour) for drug reaching the absorption compartment, and ka (h–1) is the first-order absorption rate constant for baclofen. The Γ(n) is the gamma function used to extend the factorial expression n! to non-integer numbers. The Stirling approximation was applied to numerically compute n!. Time after dose (TAD) was obtained by subtracting dosing time from the actual treatment time.
The rates of change in amounts of drug for the central (Ac) and peripheral (Ap) compartments are
| (2) |
| (3) |
where CL (L/h) is the apparent clearance, CLD (L/h) is the distribution clearance, and Vc (L) and Vp (L) are apparent volumes of central and peripheral compartments.
Covariate Assessment in PK Modeling Analysis
The IIV for each variable was obtained from the base two-compartment model without covariates. Covariate analysis was performed firstly by visually exploring the graphs of IIV of PK variable estimates versus patient properties (Eta-plot) to identify their potential contribution, namely WTKG, age, GAGE, birth body weight, sex, race, serum creatinine concentration, CLCR, bladder or kidney function, gastric motility/gastroesophageal reflux status, and disease severity status (Figure 7; available at www.jpeds.com). The Loess curve-fitting method was implemented to assess possible covariates effects on PK variables.
The WTKG values were standardized to the median body weight in the study subjects. The preselected covariates were then evaluated in model fitting in a forward stepwise fashion, with one introduced each time of model fitting evaluation. Inclusion of a specific covariate in the final model should result in a significant reduction in the NONMEM objective function by at least 3.84 (α = 0.05, 1 degree change in freedom, χ2 distribution). The resulting multi-variable model was finally refined with a recursive backward-elimination procedure in a one-by-one stepwise fashion. Removal of a covariate was accepted if the run results in the non-significant change in the objective function value (no more than 10.83, a = 0.001, 1 degree change in freedom, χ2 distribution).
In the final model, the effects of standardized WTKG on CL, Vc and Vp was incorporated as Eq. 1-3. The differences in MTT for various dose groups were identified and described empirically as Equation 4.
| (1) |
| (2) |
| (3) |
| (4) |
where CLi/F and CLpop/F are the individual and population estimates of apparent clearance, WTKGmedian is the median value of subject body weights, Vc,i/F and Vc,pop/F are individual and population estimates of apparent central volumes, Vp,i/F and Vp,pop/F are individual and population estimates of apparent peripheral volumes, and MTTi and MTTpop are the individual and population estimates of mean transit times.
Model Validation—SVPC
One thousand data sets were simulated using the final model, based upon the combined datasets. The percentile of the jth observation for the ith subject was calculated by
where δij,n = 1 if yij,n > y‘ij,n (otherwise, δij,n = 0), yij is the jth observation in the ith subject, and the y‘ij,n is the nth simulated value corresponding to yij. The median and the nonparametric 90% prediction interval (5%-95% percentiles) were calculated, and overlaid with the observed data.
Software
Noncompartment analysis utilized WinNonlin 5.3 (Phar-sight Corporation, Mountain View, California). The first-order conditional estimation with Interaction (FOCE_I) in NONMEM 7.1 (Icon Development Solutions, Ellicott City, Maryland) was used with Intel Fortran compiler 9.1 for model fitting and simulations. All plots were generated in S-plus (Insightful Corporation, Seattle, Washington). Statistical percentiles were calculated in SVPC and BS analysis, using Pearl-speaks-NONMEM (PsN) tool, SAS version 9.1 and JMP 10 (SAS Institute Inc, Cary, North Carolina).
Results
Goodness-of-Fit for Multivariable Model
Figure 9, A (available at www.jpeds.com) shows model-predicted versus observed concentrations at individual and population levels. The points gather evenly along the identity line for individuals and the entire population, which indicates that the predicted values match the observations very well. Figure 9, B depicts conditional weighted residual (CWRES) error plots from the model fitting. The CWRES points are evenly distributed around the zero lines with no pattern. It indicates random variability is reasonably described with the model structure.
SVPC
The model was assessed using SVPC with the combined dataset (Figure 10; available at www.jpeds.com). The body weight effect and dose differences were taken into account because observed concentrations were compared with simulations from the same model template of particular subjects. Individuals with different WTKG were categorized and well distributed between 0 and 1 in the SVPC plot. A reasonable number of points fell out of the 5th and 95th percentiles, which indicated a good model performance in describing both the central tendencies and diversity characteristics in observed drug concentrations.
Footnotes
The authors declare no conflicts of interest.
References
- 1.Albright AL. Baclofen in the treatment of cerebral palsy. J Child Neurol. 1996;11:77–83. doi: 10.1177/088307389601100202. [DOI] [PubMed] [Google Scholar]
- 2.Gracies JM, Nance P, Elovic E, McGuire J, Simpson DM. Traditional pharmacological treatments for spasticity. Part II: General and regional treatments. Muscle Nerve Suppl. 1997;6:S92–120. [PubMed] [Google Scholar]
- 3.Milla PJ, Jackson ADM. A controlled trial of baclofen in children with cerebral palsy. J Int Med Res. 1977;5:398–404. doi: 10.1177/030006057300100203. [DOI] [PubMed] [Google Scholar]
- 4.Delgado MR, Hirtz D, Asien M, Ashwal S, Fehlings DL, McLaughlin J, et al. Practice parameter: pharmacological treatment of spasticity in children and adolescents with cerebral palsy (an evidence-based review). Neurology. 2010;74:336–43. doi: 10.1212/WNL.0b013e3181cbcd2f. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Shellenberger MK, Groves L, Shah J, Novack GD. A controlled pharmacokinetic evaluation of tizanidine and baclofen at steady state. Drug Metab Dispos. 1999;27:201–4. [PubMed] [Google Scholar]
- 6.Wuis EW, Dirks MJ, Termond EF, Vree TB, Van der Kleijn E. Plasma and urinary excretion kinetics of oral baclofen in healthy subjects. Eur J Clin Pharmacol. 1989;37:181–4. doi: 10.1007/BF00558228. [DOI] [PubMed] [Google Scholar]
- 7.Savic RM, Jonker DM, Kerbusch T, Karlsson MO. Implementation of a transit compartment model for describing drug absorption in pharmacokinetic studies. J Pharmacokinet Pharmacodyn. 2007;34:711–26. doi: 10.1007/s10928-007-9066-0. [DOI] [PubMed] [Google Scholar]
- 8.Wilkins JJ, Savic RM, Karlsson MO, Langdon G, McIlleron H, Pillai G, et al. Population pharmacokinetics of rifampin in pulmonary tuberculosis patients, including a semimechanistic model to describe variable absorption. Antimicrob Agents Chemother. 2008;52:2138–48. doi: 10.1128/AAC.00461-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Olpe HR, Demieville H, Baltzer V, Bencze WL, Koella WP, Wolf P, et al. The biological activity of d- and l-baclofen (Lioresal). Eur J Pharmacol. 1978;52:133–6. doi: 10.1016/0014-2999(78)90032-8. [DOI] [PubMed] [Google Scholar]
- 10.Hill DR, Bowery NG. 3H-baclofen and 3H-GABA bind to bicuculline-insensitive GABA B sites in rat brain. Nature. 1981;290:149–52. doi: 10.1038/290149a0. [DOI] [PubMed] [Google Scholar]
- 11.Wang DD, Zhang S. Standardized visual predictive check vs visual predictive check for model evaluation. J Clin Pharmacol. 2012;52:39–54. doi: 10.1177/0091270010390040. [DOI] [PubMed] [Google Scholar]
- 12.Cleveland WS, Devlin SJ. Locally weighted regression—an approach to regression-analysis by local fitting. JASA. 1988;83:596–610. [Google Scholar]
- 13.Knibbe CA, Krekels EH, van den Anker JN, DeJongh J, Santen GW, van Dijk M, et al. Morphine glucuronidation in preterm neonates, infants, and children younger than 3 years. Clin Pharmacokinet. 2009;48:371–85. doi: 10.2165/00003088-200948060-00003. [DOI] [PubMed] [Google Scholar]
- 14.Mahmood I. Prediction of drug clearance in children: impact of allometric exponents, body weight, and age. Ther Drug Monit. 2007;29:271–8. doi: 10.1097/FTD.0b013e318042d3c4. [DOI] [PubMed] [Google Scholar]
- 15.Mahmood I. Application of fixed exponent 0.75 to the prediction of human drug clearance: an inaccurate and misleading concept. Drug Metabol Drug Interact. 2009;24:57–81. doi: 10.1515/dmdi.2009.24.1.57. [DOI] [PubMed] [Google Scholar]
- 16.Tucker GT, Lennard MS. Enantiomer specific pharmacokinetics. Pharmacol Ther. 1990;45:309–29. doi: 10.1016/0163-7258(90)90069-e. [DOI] [PubMed] [Google Scholar]
- 17.Jenner P, Testa B. The influence of stereochemical factors on drug disposition. Drug Metab Rev. 1973;2:117–84. doi: 10.3109/03602537409030008. [DOI] [PubMed] [Google Scholar]
- 18.Levy RH, Boddy AV. Stereoselectivity in pharmacokinetics: a general theory. Pharm Res. 1991;8:551–6. doi: 10.1023/a:1015884102663. [DOI] [PubMed] [Google Scholar]
- 19.Cercos-Fortea T, Polache A, Nacher A, Cejudo-Ferragud E, Casabo VG, Merino M. Influence of leucine on intestinal baclofen absorption as a model compound of neutral alpha-aminoacids. Biopharm Drug Dispos. 1995;16:563–77. doi: 10.1002/bdd.2510160705. [DOI] [PubMed] [Google Scholar]
- 20.Wuis EW, Dirks MJ, Termond EF, Vree TB, Van der Kleijn E. Comparison of the pharmacokinetics of intravenously administered rac-baclofen and its (−)-(R)- and (+)-(S)-enantiomers in dogs. Int J Clin Pharmacol Res. 1989;9:239–46. [PubMed] [Google Scholar]
- 21.Wuis EW, Dirks MJ, Vree TB, Van der Kleijn E. Pharmacokinetics of baclofen in spastic patients receiving multiple oral doses. Pharm Weekbl Sci. 1990;12:71–4. doi: 10.1007/BF01970149. [DOI] [PubMed] [Google Scholar]
- 22.Wiersma HE, van Boxtel CJ, Butter JJ, van Aalderen WM, Omari T, Benninga MA. Pharmacokinetics of a single oral dose of baclofen in pediatric patients with gastroesophageal reflux disease. Ther Drug Monit. 2003;25:93–8. doi: 10.1097/00007691-200302000-00014. [DOI] [PubMed] [Google Scholar]
- 23.Del Giudice E, Staiano A, Capano G, Romano A, Florimonte L, Miele E, et al. Gastrointestinal manifestations in children with cerebral palsy. Brain Dev. 1999;21:307–11. doi: 10.1016/s0387-7604(99)00025-x. [DOI] [PubMed] [Google Scholar]
- 24.Campanozzi A, Capano G, Miele E, Romano A, Scuccimarra G, Del Giudice E, et al. Impact of malnutrition on gastrointestinal disorders and gross motor abilities in children with cerebral palsy. Brain Dev. 2007;29:25–9. doi: 10.1016/j.braindev.2006.05.008. [DOI] [PubMed] [Google Scholar]
- 25.Fung EB, Samson-Fang L, Stallings VA, Conaway M, Liptak G, Henderson RC, et al. Feeding dysfunction is associated with poor growth and health status in children with cerebral palsy. J Am Diet Assoc. 2002;102:361–73. doi: 10.1016/s0002-8223(02)90084-2. [DOI] [PubMed] [Google Scholar]
- 26.Ravelli AM, Milla PJ. Vomiting and gastroesophageal motor activity in children with disorders of the central nervous system. J Pediatr Gastroenterol Nutr. 1998;26:56–63. doi: 10.1097/00005176-199801000-00010. [DOI] [PubMed] [Google Scholar]
- 27.Spiroglou K, Xinias I, Karatzas N, Karatza E, Arsos G, Panteliadis C. Gastric emptying in children with cerebral palsy and gastroesophageal reflux. Pediatr Neurol. 2004;31:177–82. doi: 10.1016/j.pediatrneurol.2004.02.007. [DOI] [PubMed] [Google Scholar]
- 28.Anderson BJ, Allegaert K, Holford NH. Population clinical pharmacology of children: modeling covariate effects. Eur J Pediatr. 2006;165:819–29. doi: 10.1007/s00431-006-0189-x. [DOI] [PubMed] [Google Scholar]
- 29.Zhou S-F, Wang L-L, Di YM, Xue CC, Duan W, Li CG, et al. Substrates and inhibitors of human multidrug resistance associated proteins and the implications in drug development. Curr Med Chem. 2008;15:1981–2039. doi: 10.2174/092986708785132870. [DOI] [PubMed] [Google Scholar]
- 30.Flagg TP, Enkvetchakul D, Koster JC, Nichols CG. Muscle KATP channels: recent insights to energy sensing and myoprotection. Physiol Rev. 2010;90:799–829. doi: 10.1152/physrev.00027.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Houle AM, Vernet O, Jednak R, Pippi Salle JL, Farmer JP. Bladder function before and after selective dorsal rhizotomy in children with cerebral palsy. J Urol. 1998;160:1088–91. doi: 10.1097/00005392-199809020-00032. [DOI] [PubMed] [Google Scholar]
- 32.Karaman MI, Kaya C, Caskurlu T, Guney S, Ergenekon E. Urodynamic findings in children with cerebral palsy. Int J Urol. 2005;12:717–20. doi: 10.1111/j.1442-2042.2005.01120.x. [DOI] [PubMed] [Google Scholar]