Abstract
Due to their rapid and highly selective nature, bioorthogonal chemistry reactions are attracting a significant amount of recent interest in the radiopharmaceutical community. Over the last few years, reactions of this type have found tremendous utility in the construction of new radiopharmaceuticals and as a method of bioconjugation. Furthermore, reports are beginning to emerge in which these reactions are also being applied in vivo to facilitate a novel pretargeting strategy for the imaging and therapy of cancer. The successful implementation of such an approach could lead to dramatic improvements in image quality, therapeutic index, and reduced radiation dose to non-target organs and tissues. This review will focus on the potential of various bioorthogonal chemistry reactions to be used successfully in such an approach.
Keywords: Pretargeting, bioorthogonal chemistry, molecular imaging, PET, SPECT, click chemistry
Directly radiolabelled antibodies in imaging and therapy
The success of molecular imaging and therapy depends on the selective accumulation of the diagnostic probe or therapeutic agent at the site of interest. For imaging studies, this ability is required to effectively contrast the target site against surrounding tissues, organs and biological fluids, whilst in therapy it helps confine the toxic agent to the site(s) of disease and prevent off-target effects. In oncology, a multitude of targets have been identified corresponding to the various known hallmarks of cancer [1]. These include elevated rates of glucose metabolism [2], regions of hypoxia [3], changes in pH [4], and a plethora of cellular and chemical biomarkers [5]. This review will focus on the latter category and the development of novel imaging and therapeutic strategies towards such targets based on bioorthogonal chemistry.
Upon identifying a compelling target, several important considerations are required in order to develop the most effective agent. One of these is the selection of an appropriate targeting vector which will both localize at the site of interest and lead to the specific accumulation of the desired ‘payload’. When developing an agent against a particular biomarker it is important that the targeting vector has (i) high binding affinity for its intended target, (ii) high specificity, (iii) high signal-to-background ratio, (iv) high metabolic stability, and (v) low immunogenicity [6,7].
Since the beginning of the 20th century, antibodies have been considered as suitable vehicles for the delivery of imaging and therapeutic agents, mostly due to their high affinity and specificity [8-15]. Paul Ehrlich first conceived of antibody vectors as ‘magic bullets’ capable of delivering a payload of toxic agent to antigens associated with certain diseases, thus causing irreversible damage to sites of disease whilst sparing healthy tissue of the toxins deleterious effects [8]. Consequently, a vast array of epitopes (mostly at the cell surface) have been targeted by antibodies for non-invasive imaging and therapeutic applications.
The first examples of radiolabelled antibodies for cancer therapy emerged in the early 1950’s [16,17], although it took roughly two decades before their ability to target human tumour-associated antigens was demonstrated in cancer patients [18]. It was the development of hybridoma technology in 1975 that enabled the relatively facile generation of monoclonal (murine) antibodies (mAbs) in useful quantities [19] and, as a consequence, the number of studies in this area quickly increased. The use of murine antibodies in these applications was found to be problematic due to the provocation of a human anti-murine antibody response [20]. Therefore, significant efforts have been focused on the production of chimeric, humanized, and human monoclonal antibodies which are much less likely to invoke an immune reaction. Currently, the vast majority of approved mAbs are either chimeric or humanized [21].
Despite their attractive properties, antibodies have a few important shortcomings that have prevented their development into the magic bullets that Ehrlich had envisioned. One of the most critical barriers to achieving high tumour-to-blood (T/B) and tumour-to-muscle (T/M) ratios is the slow rate of clearance of antibodies from the blood and non-target tissues due to their high molecular weight [22,23]. This necessitates the use of radionuclides with correspondingly long radioactive half-lives (e.g. 89Zr; t 1/2 = 78.41 h [24]). Of course, the radiation dose to the patient increases as a function of exposure time and therefore there is a clear incentive to improve the pharmacokinetic properties of antibody constructs. One method of achieving this has involved a variety of lower molecular weight vectors which retain the essential antigen binding pharmacophore yet exhibit more rapid elimination. In decreasing order of size, these include minibodies, diabodies, single chain variable fragments (scFv), and single variable domain fragments (Fv), etc.
Pretargeted imaging and therapy: an alternative approach
In the mid-1980s, an alternative strategy referred to as pretargeted radioimmunotherapy (PRIT) was developed to circumvent the issues associated with the prolonged residence times of radiolabelled antibody constructs [25,26]. In essence, this approach involves the sequential administration of (i) a macromolecular (usually antibody-based) targeting vector and (ii) a low molecular weight radiolabelled effector species (Figure 1). Crucially, the radiolabelled species is administered following a predetermined lag period to allow the antibody sufficient time to accumulate at the target site and for any residual antibody to be cleared from the circulation.
Figure 1.
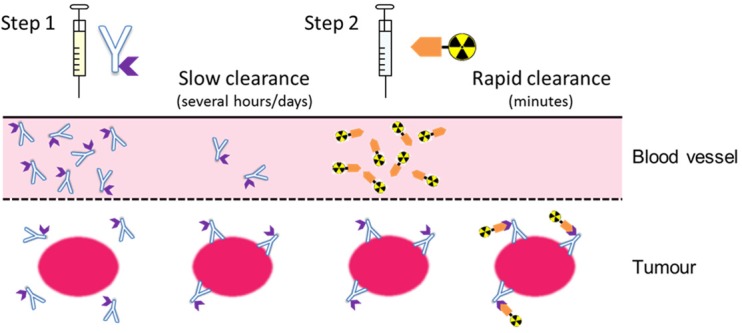
A simplified representation of a two-step pretargeting approach.
To ensure the two component parts bind strongly upon interaction at the site of interest, each must be suitably modified with complementary reactive species. In some cases, an additional ‘chaser’ species is administered prior to the radiolabelled effector, thus creating a ‘three-step’ approach. The purpose of this chaser species is to assist in the removal of any unbound antibody from the circulation. As a result, improved T/B ratios have been achieved [27].
The successful implementation of a pretargeting approach combines the high target specificity and affinity offered by antibody vectors with the superior pharmacokinetic properties of a low molecular weight compound. As a result, pretargeting strategies can lead to an improvement of imaging contrast at earlier time points following administration of the radiolabelled agent. Furthermore, this approach has been shown to decrease the overall radiation burden to non-target organs and tissues [28].
Whilst the concept of pretargeting has been known for several decades, the number of pretargeting systems has been limited to a few distinct classes. This is mostly due to the inherent difficulties of developing chemical reactions which proceed rapidly within living systems without also reacting with the vast array of chemical entities that are present often in vast molar excesses.
Conventional pretargeting systems
Bispecific antibodies and radiolabelled haptens
The original pretargeting concept is based on the use of bispecific antibodies which are capable of binding to both a target antigen and a radiolabelled hapten [11,29-33]. This approach was made possible when Reardan et al. developed monoclonal antibodies which were capable of binding radiometal chelates, specifically 111In complexes based on the hexadentate chelator ethylenediamine tetraacetic acid (EDTA) [25]. It was later found that an affinity enhancement effect could be achieved by tethering two of these haptens together via a short two amino acid linker [34]. This modification led to improved uptake and retention of the radiolabelled divalent hapten at the tumour site whilst still retaining the essential rapid clearance from the circulation and surrounding tissues.
A notable example of this approach involved an anti-CEA × anti-111In-benzyl-EDTA Fab’ × Fab’ bispecific mAb and an 111In-EDTA derivative (111In-EOTUBE) as the radiolabelled effector [35]. A clinical trial involving 14 patients with recurrent or metastatic adenocarcinoma of the colon revealed rapid clearance of the radiolabelled species from normal tissues while affording high T/M ratios [35].
Potential limitations of this approach include the practical complexities and high costs involved in the development of bispecific antibodies. Furthermore, a critical aspect of any pretargeted imaging approach is the affinity between the radiolabelled effector species and the antibody vector. Here, the binding interactions between radiolabelled haptens and bispecific antibodies are entirely non-covalent and binding constants greater than ~10-10 M are rarely achieved. In an effort to obtain greater binding constants, alternative systems offering much higher affinities such as the biotin-(strept)avidin interaction have been explored.
Biotin-(strept)avidin systems
Shortly after the development of bispecific antibodies for pretargeting, Hnatowich et al. reported an alternative strategy exploiting the extremely high binding affinity between biotin and (strept)avidin (Kd = 4×10-14 M) [36,37]. This approach has since been used in various forms which are discussed in depth in several comprehensive reviews [38-42]. The benefits of this approach were clearly demonstrated in a study by Axworthy et al. who compared the uptake of a 90Y-radiolabelled biotin in a tumour pretargeted with a streptavidin-modified mAb against a conventional directly radiolabelled antibody [43]. Promisingly, significantly higher T/B ratios were found using the pretargeting method.
Whilst this system shows clear promise, there are a number of limitations to this approach which require consideration. Perhaps most significant is the immunogenic response that occurs following administration of avidin/streptavidin foreign proteins. Another consideration is the presence of endogenous biotin (10-7-10-8 M) which could interfere with (strept)avidin pretargeting systems by saturating the biotin binding sites, as well as endogenous biotinidase which mediates the hydrolysis of radiolabelled biotin effector species. Lastly, more so than the other conventional pretargeting strategies discussed herein, it is often necessary to administer a chaser species to remove residual antibody from the circulation prior to the administration of the radiolabelled effector [44-49].
Complementary oligonucleotides
A comparatively more recent approach (also developed by Hnatowich and co-workers) relies on the high affinity interaction between complementary oligomers (such as DNA) [50-59]. Depending largely on the length and the base sequence of the complementary oligomeric chains, this chemical pairing can potentially lead to binding affinities that would rival, or even exceed, that of the biotin-(strept)avidin interaction. This approach can also potentially eliminate some of the inherent limitations of the biotin-(strept)avidin approach. For example, studies in which high doses of single strand DNAs have been repeatedly administered to patients have not revealed any significant immunogenic response or obvious toxicity [60]. Furthermore, unlike the biotin-(strept)avidin approach, the use of complementary oligomers would not be complicated or obstructed by the presence of competing endogenous species. It is important, however, that oligonucleotides are suitably modified to prevent their rapid degradation by nucleases [61]. The most successful oligomers from a pretargeting perspective have been those based on a morpholino backbone (MORFs). These agents have been used in conjunction with a variety of radionuclides for applications in imaging (99mTc [51-54,58], 111In [55,56]) and therapy (90Y [50], 188Re [57]).
Using bioorthogonal chemistry for pretargeted imaging of cancer
For a chemical reaction to be described as being truly bioorthogonal, it must result in the rapid formation of a covalent bond (even at low concentrations) whilst remaining completely selective against any other chemical species present within a living system. Given the abundance and variety of reactive functional groups within such a biologically and chemically complex environment, this reduces the number of possible reactions to a small selection [62-73]. In addition, it is important that at least one of the bioorthogonal species is small, and that both reacting components exert minimal toxicity [64].
Click chemistry reactions (as defined by Sharpless et al. in 2001 [74]) offer important advantages which give them the potential to translate well into an in vivo setting and all of the bioorthogonal reactions discussed in this review fall under this umbrella term.
The archetypal and most prominent click chemistry reaction evolved from work started by Rolf Huisgen in 1963 [75-77], although the development of the click chemistry concept itself did not arise until much later [74]. The Huisgen 1,3-dipolar cycloaddition involves the reaction between azide and alkyne starting materials yielding a triazole species (Figure 2). By itself, the reaction requires elevated temperatures or pressures and often results in a mixture of regioisomers (specifically, 1,4- and 1,5-substituted triazoles). However, in the early 2000s, the laboratories of both Sharpless and Meldal independently discovered that in the presence of a copper(I) catalyst the reaction proceeds at room temperature, at much higher rates (~106-fold), and yields only the 1,4-substituted regioisomer [78,79]. This click chemistry reaction is particularly attractive as both the alkyne and azide functional groups are easily incorporated into a diverse array of organic species and exhibit high stability under a variety of reaction conditions. As such, the Huisgen 1,3-dipolar cycloaddition has found tremendous utility in the construction of radiopharmaceuticals [80,81]. In particular, this click chemistry reaction has been used very effectively in the mild preparation of [18F]-radiolabelled peptides [82-84].
Figure 2.
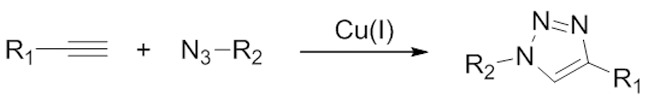
The Huisgen 1,3-dipolar cycloaddition is an extremely efficient click chemistry reaction, however the requirement of a toxic copper(I) catalyst to enhance the reaction rate has prevented its application in living systems.
Despite having many desirable qualities that would render the Huisgen 1,3-dipolar cycloaddition an attractive choice for a pretargeting strategy, the necessity of a copper(I) catalyst presents a major limitation due to its in vivo toxicity. As a result, the comparatively recent development of alternative click chemistry reactions which proceed without the presence of a copper(I) catalyst has attracted a significant amount of interest in the molecular imaging community [85]. The copper-free click chemistry reactions are instead usually driven either by the relief of steric strain (strain-promoted azide-alkyne cycloadditions, or SPAAC [63-68,72]), or by a so-called inverse electron-demand Diels-Alder mechanism [86] (Table 1).
Table 1.
Rates of reaction for a selection of bioorthogonal reactions
| Reaction type | Reacting species | k (x 10-3 M-1s-1)a | References |
|---|---|---|---|
| Staudinger (non-traceless) | Phosphine + azide | 0.83-3.8 | [88,89] |
| Staudinger (traceless) | Phosphine + azide | 0.12-7.70 | [89,90] |
| Strain-promoted cycloadditions | Cycloalkyne + azide | 0.9-4,000 | [93,99-106,108,110,119,132,170-174] |
| Cycloalkyne + diazo | 2.5-13,500 | [174] | |
| Cycloalkyne + nitrone | 1,660-58,800 | [105,115,116,136] | |
| IEDDA | Cyclopropene + tetrazine | 137-137,000 | [140] |
| Norbornene + tetrazine | 41-20,000 | [145,148,150,151,175] | |
| TCO + tetrazine | 3,100-380,000,000 | [152,166,175-177] |
The reported rates of each reaction are dependent on a number of experimental conditions, including the choice of solvent, pH, temperature, etc.
For a more precise comparison, refer to the individual references.
One of the first copper-free bioorthogonal reactions to be evaluated was the Staudinger ligation (Figure 3) [87]. This involves the reaction between azide and phosphine functional groups resulting in the formation of an amide bond. There are essentially two forms of this reaction: (i) a non-traceless version in which a phosphine oxide moiety remains attached to the final product, and (ii) a traceless version in which the phosphine oxide group is eliminated. This ligation and its precise mechanism [88] have been discussed in detail in several excellent review articles [89,90].
Figure 3.
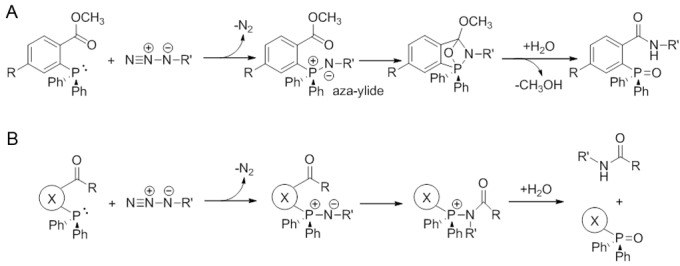
The non-traceless (A) and traceless (B) versions of the Staudinger ligation have been used for in vivo bioorthogonal chemistry reactions.
The Staudinger ligation is a reliable and highly selective reaction which has been used successfully for the modification of proteins [91], and the engineering of cell surfaces both in vitro [92,93] and in living animals [94]. It has also been used in combination with fluorophores in in vitro studies targeting azide groups present on the surface of live cells [95,96]. Furthermore, the traceless form of this reaction has also been used to synthesize heterobifunctional linkers to facilitate the construction of radiometal-containing imaging agents [97].
A study conducted by Vugts et al. in 2011 explored the possibility of using the Staudinger ligation to facilitate a pretargeted imaging strategy [98]. In this case, an anti-CD44v6 chimeric monoclonal antibody which had been modified with multiple azide functionalities was employed as a targeting vector. A series of phosphine-containing small molecules incorporating radionuclides for imaging (67/68Ga, 89Zr, 123I, and 177Lu) and therapeutic applications (177Lu) were then evaluated as secondary agents. Following the administration of a 67Ga-DFO-phosphine agent in non-tumour bearing mice, the presence of Staudinger products in the blood pool was monitored, however no evidence of any ligation was observed. Experiments in serum revealed that the phosphine species is prone to oxidation which renders it unable to undergo reaction with azide groups. Furthermore, the rate of the Staudinger ligation was found to be sub-optimal for in vivo bioorthogonal reactions, particularly considering the rapid clearance and elimination of the secondary phosphine agent.
Strain-promoted alkyne-azide (SPAAC) and alkyne-nitrone (SPANC) cycloadditions
In another effort to circumvent the use of a toxic Cu(I) catalyst required for traditional Huisgen-type click chemistry reactions, a related class of azide-alkyne [3 + 2] cycloadditions which are promoted by the relief of steric strain have gained prominence. Mostly, these reactions involve an alkyne moiety within a cyclic 8-membered (cyclooctyne [99]) system which causes the bond angles surrounding the two alkyne sp-hybridised carbon atoms to be severely constrained away from the ideal 180° (Figure 4).
Figure 4.
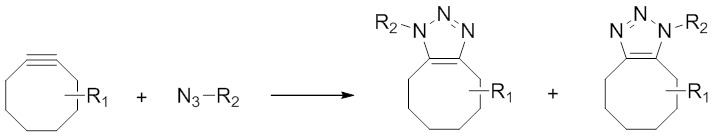
The use of highly strained cyclooctyne derivatives has circumvented the requirement of a toxic copper(I) catalyst and these reactions are therefore more compatible with living organisms compared to the traditional Huisgen 1,3-dipolar cycloaddition.
A host of cyclooctyne derivatives have been evaluated in terms of their ability to undergo copper-free click chemistry reactions. The purpose of these studies is often focused on enhancing the rate of the cycloaddition reaction by making structural modifications to the cyclooctyne species. For example, it has been found that introducing one or more electron-withdrawing substituents (e.g. fluorine [100]) in close proximity to the alkyne group leads to a substantial increase of rates of reaction. Other modifications, such as one [101] or two [102-104] adjacent aryl rings (e.g. dibenzocyclooctyne, DBCO), and bicyclic systems (e.g. bicyclononynes [105,106]) have also been found to improve reaction rates yet further. One cyclooctyne derivative ‘BARAC’ has achieved second order rates of reaction up to 0.96 M-1s-1 [104], although a recent study has found evidence that BARAC itself is prone to rearrangement yielding tetracyclic products [107].
Cyclooctyne derivatives can now be purchased from commercial suppliers and this increase in availability has led to the use of this technology in a wide variety of applications [93,105,108-123]. Notably, SPAAC reactions have been used in the preparation of fluorine-18 [124-130] and copper-64 [131] radiolabelled compounds for PET imaging applications.
Several studies have now demonstrated the ability of cyclooctyne derivatives to undergo bioorthogonal reactions with azide groups in vitro [119,121,132], in zebrafish [123], and in live mice [120]. In most of these examples, the azide groups are usually incorporated via metabolic glycoengineering.
Given their apparent high selectivity and promising rates of reaction in some cases, it is not surprising that SPAAC reactions have been considered as candidates to facilitate a pretargeting approach [130,133,134]. Recently, van den Bosch et al. reported an in depth study in which the reaction between a series of 177Lu-containing cyclooctyne derivatives and an azido-functionalised anti-CD20 mAb (Rituximab) was evaluated in non-tumour-bearing mice. In this case, the cyclooctyne species were derived from DIFO and DIBO as both had previously been shown to undergo rapid reactions with benzyl azide [63,116]. Preliminary in vitro studies in phosphate buffered saline and 50% mouse serum indicated sufficient stability within the expected circulation time of these low molecular weight probes. Mice were initially administered the azide-modified mAb and after a lag time of 5 minutes were subsequently injected with the relevant 177Lu-labelled cyclooctyne probe. Evidence of bioorthogonal reaction products was monitored in the blood pool and selected tissues. Unfortunately, this approach was unsuccessful as it was evident that the cyclooctyne probes did not have sufficiently high in vivo reactivity towards azides, particularly considering the rapid blood clearance of these agents. Furthermore, in some examples, binding to serum proteins was apparent which, whilst slightly lengthening the circulatory residence times, ultimately limited the availability of these secondary agents and therefore reduced the potential for in vivo cycloaddition reactions.
Lee et al. have also recently utilised SPAAC-based bioorthogonal chemistry for in vivo pretargeting using, in this case, fluorine-18 for PET imaging [134]. Here, rather than use an antibody as the primary targeting agent, the authors employed mesoporous silica nanoparticles (MSNs; 100-150 nm) which were expected to accumulate in tumours via the enhanced permeability and retention (EPR) effect. The MSNs were PEGylated and then modified with DBCO (~0.12 mmole of DBCO per gram of product, DBCO-PEG-MSN). These nanoparticles were administered intravenously to mice bearing U87 MG tumour xenografts and, after a lag time of 24 h, the secondary agent ([18F]fluoropentaethylene glycolic azide) was subsequently injected. Promisingly, PET-CT images acquired between 0.25-2 h p.i. revealed accumulation of radioactivity at the tumour site while control experiments performed without prior injection of the DBCO-PEG-MSNs exhibited substantially lower tumour uptake. This observation was supported by data obtained from ex vivo biodistribution experiments which showed an improved tumour-to-blood ratio using the pretargeting approach. These experiments provide a good indication that bioorthogonal chemistry reactions based on SPAAC can be used successfully to facilitate in vivo pretargeting for imaging and therapeutic applications.
A few studies focused on achieving faster rates of reaction have found that cyclooctyne derivatives also undergo rapid strain-promoted cycloaddition reactions with nitrone species (SPANC) [135]. The reaction between BARAC and one cyclic nitrone derivative yielded a second-order rate constant of 47.3 M-1s-1, representing a 47-fold increase compared to the equivalent reaction involving benzyl azide [136]. To the best of the authors knowledge this class of reactions has not yet been evaluated in a pretargeting strategy, although it holds clear promise particularly as cyclic nitrones can be readily conjugated to amine and carboxylic acid functional groups and have already been used successfully to functionalise extracellular targets on live cancer cells [115].
Inverse electron-demand Diels-Alder cycloadditions
In an opposite fashion to the traditional Diels-Alder reaction, inverse electron-demand Diels-Alder (IEDDA) cycloaddition reactions involve the use of an electron-rich dieneophile and an electron-deficient diene [86]. The reaction is formally accepted as a [4 + 2] cycloaddition, however it is still not clear whether it is a truly concerted mechanism. In the early 1990s, Sauer et al. demonstrated that the rates of reaction between electron deficient tetrazines and a variety of dienophiles were extremely fast [137], and consequently tetrazines have been commonly employed as dienes in these reactions.
From a bioorthogonal chemistry perspective, the two most prominent dienophiles which have so far been explored are norbornene and trans-cyclooctene (TCO) [138], although recently other species such as cyclopropene [139,140] and terminal alkenes [141] have also shown promise in this area.
The norbornene-tetrazine ligation was first reported in the 1980s [137,142] and has seen a resurgence of interest in recent years (Figure 5). Whilst this reaction leads to the formation of multiple isomers, it otherwise meets the criteria of a click chemistry reaction and is often placed in this category. In particular, this ligation has been shown to be very rapid, modular in scope, and high yielding, and has consequently been used very effectively in a variety of applications [143-148], including the preparation of various radiopharmaceutical agents. In 2011, Zeglis et al. demonstrated that this ligation can be applied as an effective tool for bioconjugation in their development of radiometallated antibody constructs [149]. Here, the authors synthesized both 64Cu-NOTA- and 89Zr-DFO-containing norbornene derivatives which were able to rapidly conjugate to a tetrazine-modified antibody under mild reaction conditions. In a more recent example, Knight et al. synthesised an [18F]-containing norbornene prosthetic group ([18F]NFB) which was used successfully to radiolabel a bombesin peptide-derivative, also under mild reaction conditions [150].
Figure 5.
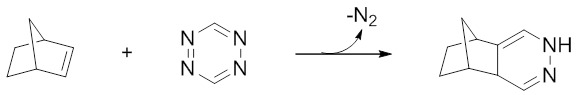
The norbornene-tetrazine ligation has been used successfully in the radiosynthesis of PET imaging agents based on peptide and antibody constructs. The rate of reaction between these species (and derivatives thereof) appears to be too slow to facilitate a pretargeting strategy.
This reaction has also been used by Devaraj et al. in in vitro pretargeting experiments involving SKBR3 human breast cancer cells [151]. In this case, norbornene-modified trastuzumab (Herceptin) was used to pretarget Her2/neu growth factor receptors at the cell surface. A near-infrared fluorescent VT68-tetrazine species was then used as a secondary labelling agent. Rapid and highly selective labelling was observed which highlights the utility of this bioorthogonal reaction.
In addition, experiments performed in whole mouse blood have further demonstrated the ability of this reaction to proceed efficiently even in complex biological environments [150]. This reaction has therefore been considered as a candidate to facilitate a pretargeted imaging strategy, however it should be noted that the rate of this reaction (~2 M-1s-1) appears to be sub-optimal for in vivo pretargeting applications.
The related TCO-tetrazine ligation proceeds via a similar inverse electron-demand Diels-Alder mechanism (Figure 6), although it is more rapid than the norbornene-tetrazine ligation by several orders of magnitude. Reaction rates of up to 3.8×105 M-1s-1 have been determined [152], making this among the most rapid bioorthogonal copper-free click chemistry reactions. As a result, the TCO-tetrazine ligation was quickly adopted by the radiopharmaceutical community and has been used in the preparation of radiolabelled small molecules [153] (including PARP inhibitors [154,155]), peptides (e.g. cyclic-RGD peptide antagonists of αvβ3 [156,157], Exendin-4 [158]), and proteins (e.g. VEGF [157]).
Figure 6.
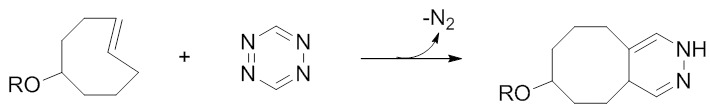
The TCO-tetrazine ligation has been the most successful bioorthogonal chemistry reaction for implementing a pretargeting strategy due to its extremely high rates of reaction.
There are now several reports in which the TCO-tetrazine ligation has been used to facilitate an in vitro pretargeting strategy [132,159-166]. In each of these examples, it is the TCO species which is coupled to the targeting vector, and a fluorescently-tagged tetrazine species is employed as the secondary agent. Epitopes at the cell surface [132,159,161,163,165] and in intracellular regions [160-164] have been successfully targeted using this strategy.
In their landmark study, Rossin et al. were first to demonstrate the utility of bioorthogonal chemistry as a pretargeting strategy in live mice bearing colon cancer xenografts [167]. In this example, the antigen TAG72 was selected as a model target due to its limited degree of internalisation (which would otherwise render it inaccessible) and its resistance to shedding. TAG72 also has clinical relevance as it is overexpressed in a variety of cancer cell lines. As a targeting vector, an anti-TAG72 mAb (CC49) was functionalised with an average of 7.4 TCO species using conventional NHS-based conjugation chemistry. A complementary dipyridyl tetrazine derivative was selected based on its high reactivity with TCO and good stability. This tetrazine species was modified with a DOTA chelating agent to facilitate radiolabelling with the SPECT radionuclide 111In (111In-1; Figure 7). Preliminary in vitro experiments conducted in PBS revealed that the bioorthogonal reaction between CC49-TCO and 111In-1 proceeded with a second order rate constant of 13,090±80 M-1s-1. In their in vivo experiments, a lag time of 1 day was used between the administration of the TCO-mAb construct and the radiolabelled tetrazine derivative. Promisingly, a rapid reaction between the two complementary bioorthogonal species occurred in vivo and SPECT/CT images acquired at 3 h p.i. revealed high contrast images of the tumour xenografts, yielding a T/M ratio of 13:1 (Figure 7). Importantly, only low levels of radioactivity were observed in the blood and non-target tissues (including the liver) at 24 h p.i. which were attributed to small amounts of TCO-CC49 which remained circulating in the blood pool.
Figure 7.
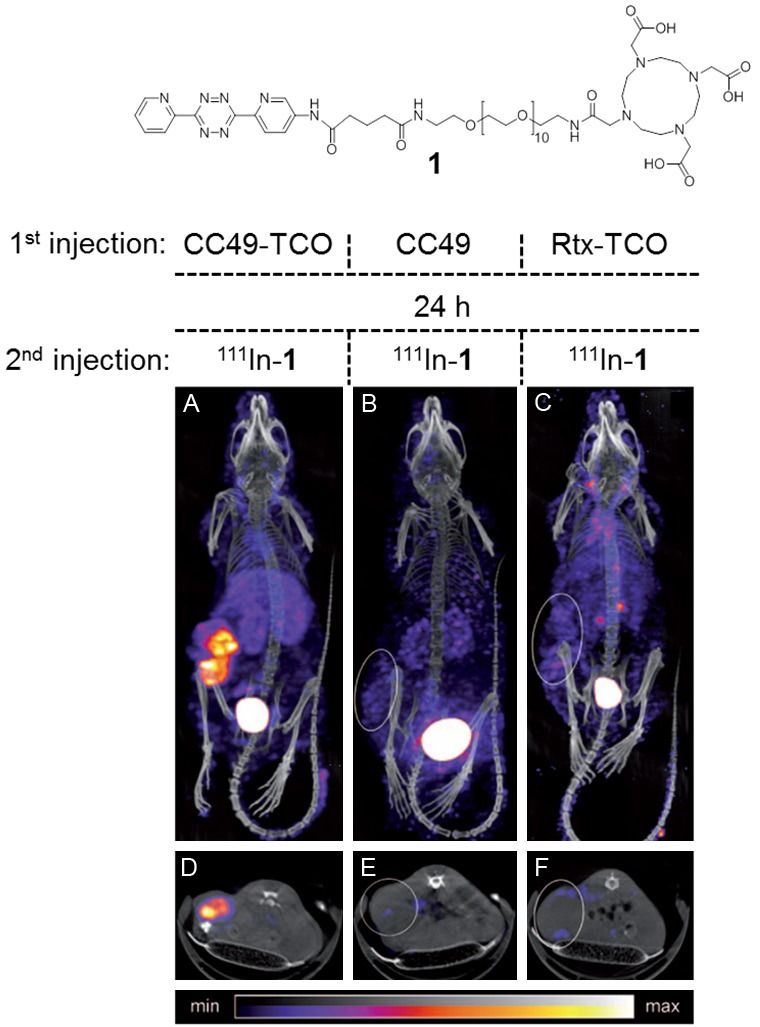
In the first example of in vivo pretargeting using the TCO-tetrazine ligation, Rossin et al. used the indium-111 complex of 1 (top) to obtain clearly distinguishable SPECT images of colon cancer xenografts. Tumour-bearing mice were injected with (A) CC49-TCO (100 µg) and after 24 h were subsequently injected with [111In]-1 (25 equiv. to CC49; 3.4 equiv. to TCO); (B) CC49 (100 µg) followed 24 h later with [111In]-1 (identical amount as in (A)); and (C) Rtx-TCO (100 µg) followed 24 h later by [111In]-1 (same amount as in (A)); (D-F) single transverse planar images intersecting the tumours in (A-C). Images acquired at 3 h p.i. Rossin R, Verkerk PR, van den Bosch SM, Vulders RCM, Verel I, Lub J, Robillard MS, In Vivo Chemistry for Pretargeted Tumor Imaging in Live Mice, Angew Chem Int Ed. 2010; 122: 3447-3450. Copyright Wiley-VCH Verlag GmbH & Co. KGaA. Reproduced with permission.
Shortly after, Devaraj et al. reported a study which was focused on further elucidating the parameters linked to the in vivo behaviour of the TCO-tetrazine ligation [168]. In this case, the secondary agents were based on dextran scaffolds (10 kDa and 40 kDa) modified with multiple tetrazine species. In non-tumour-bearing mice, the longer residence times of these high molecular weight constructs proved beneficial in achieving higher in vivo labelling efficiencies in the blood pool. This study also included pretargeting experiments aimed at imaging the A33 glycoprotein in LS174T tumour-bearing mice. The A33 glycoprotein was selected as a suitable target due to its high persistence at the cell surface and practical half-life (>2 days). A33 is also overexpressed in >95% of all human colorectal cancers, including the LS174T cell line, and therefore represents a target with high clinical relevance. The targeting vector in this case was an anti-A33 mAb modified with ~3 TCO moieties as well as a near-infrared VT680 fluorophore to enable the acquisition of complementary optical imaging data. For the acquisition of high contrast PET images, an [18F]-containing 10 kDa dextran construct ([18F]PMT10) containing multiple tetrazine species was administered as the secondary agent. In a similar manner to the previous study, a lag time of 24 h was used between the primary and secondary agent, and PET/CT images were acquired at 3 h p.i. of the radiolabelled probe. While T/M or T/B ratios were not reported, the A33 tumours were clearly contrasted against the surrounding tissues and had substantially higher uptake on comparison to control tumours which did not express the A33 antigen.
In another example, Zeglis et al. used the TCO-tetrazine ligation to successfully implement a pretargeted imaging strategy in mice bearing SW122 colorectal tumour xenografts (Figure 8) [28]. This group also used an anti-A33 mAb (this time modified with approximately 5 TCO moieties per mAb) and importantly found no significant detrimental effect on the overall immunoreactivity. Here, the secondary agent comprised of a tetrazine group modified with a NOTA chelator for labelling with the PET radiometal copper-64. In common with the two previous studies, a lag time of 24 h was used and, in this case, PET images were acquired at various time points between 2-18 h. This group compared their findings against the directly labelled construct, 64Cu-NOTA-A33, as well as the comparable 89Zr-labelled antibody (in which a DFO chelating agent was used). It was found that whilst the directly labelled antibody constructs resulted in higher overall tumour uptake at 12 h and 24 h p.i., the pretargeting approach yielded greater T/M ratios. Furthermore, it was also found that as a result of the faster clearance rate of the radiolabelled secondary agent, the overall radiation burden to off-target tissues and organs was substantially reduced.
Figure 8.
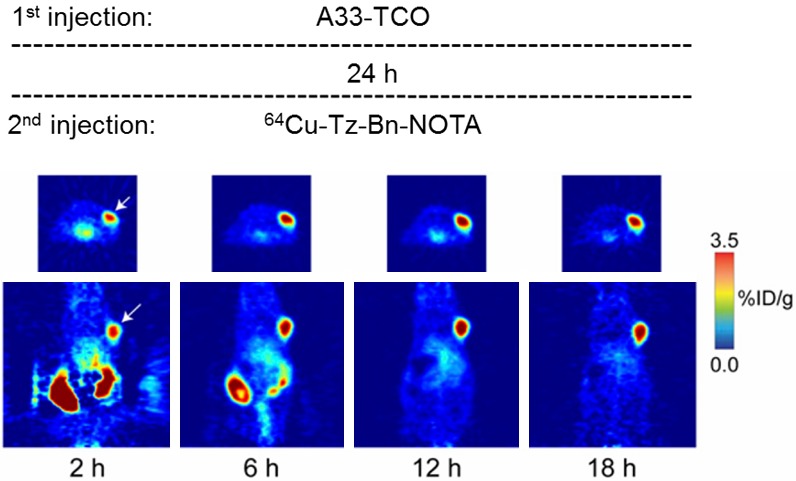
Zeglis et al. successfully employed the TCO-tetrazine ligation for in vivo pretargeted PET imaging of SW1222 tumour xenografts (white arrow; transverse [top] and coronal [bottom] images) in mice. A33-TCO (100 µg) was administered via tail vein injection and after a lag time of 24 h were then administered 64Cu-Tz-Bn-NOTA (10.2-12.0 MBq [275-325 µCi], 1.2-1.4 µg, for 2.5-2.8 Tz-to-A33 ratio). Reprinted by permission of SNMMI from: Zeglis BM, Sevak KK, Reiner T, et al. A Pretargeted PET Imaging Strategy Based on Bioorthogonal Diels-Alder Click Chemistry. J Nucl Med. 2013; 54(8): 1389-1396. Figure 4.
A major concern regarding the application of TCO in a pretargeting strategy has been its sub-optimal in vivo stability. Rossin et al. recently addressed this issue and were able to determine that protein-bound copper likely deactivates TCO by promoting its conversion to the comparatively unreactive cis-cyclooctene isomer (CCO) [169]. By shortening the distance between the TCO moiety and the lysine residue to which it binds, an increase in steric hindrance on the TCO tag was found to obstruct this deactivation. As a result, the in vivo stability of TCO in mice was greatly improved. In addition, the authors also reported significantly improved rates of reaction for TCO derivatives substituted in axial rather than equatorial positions with bulky linking groups (~10-fold higher). In vivo pretargeting experiments were performed (Figure 9) using a very similar experimental design compared to their previous study [167], however in this instance the more stable TCO-derivative allowed a longer three-day lag period prior to the addition of the 111In-labelled tetrazine secondary agent (111In-2; Figure 9). The extended lag period resulted in reduced background signal in the circulation and, promisingly, a similar degree of tumour uptake which substantiates the improved in vivo stability of this TCO species. Furthermore, after 3 days the radioactivity in the tumour was unchanged which indicates that the bioorthogonal reaction product is extremely stable in vivo.
Figure 9.
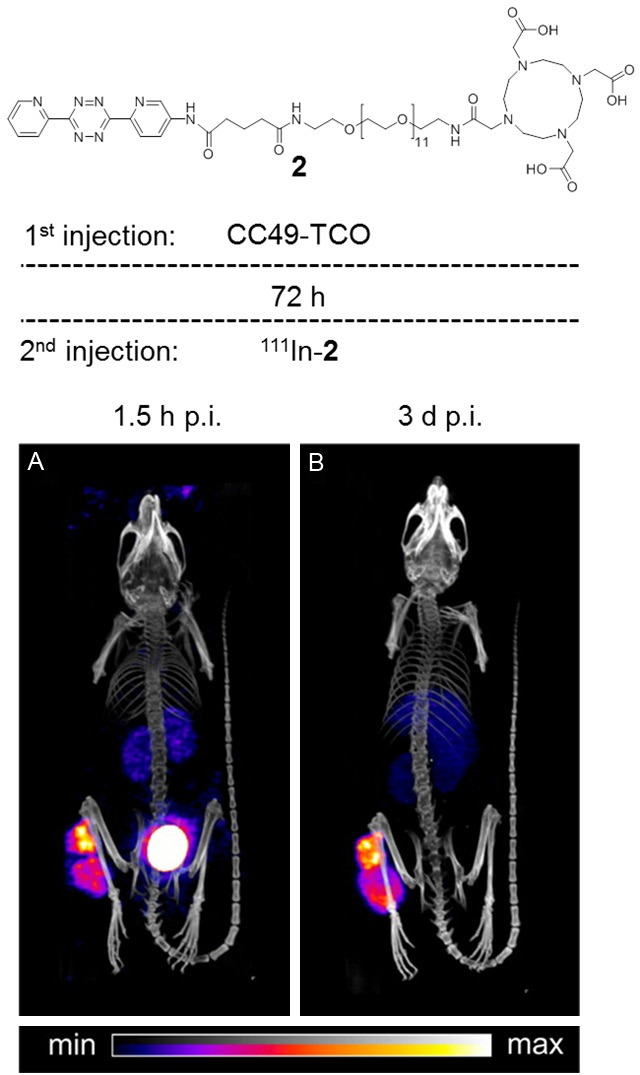
Rossin et al. significantly improved the in vivo stability and reactivity of the TCO-tetrazine ligation by increasing the steric hindrance of the TCO tag. An LS174T tumour-bearing mouse was administered CC49-TCO (100 µg) and after a lag-time of 72 h was then administered the 111In-labelled tetrazine secondary agent (2). SPECT/CT images were acquired 1.5 h (A) and 3 days (B) after injection of the tetrazine species. Adapted with permission from Rossin R, van den Bosch SM, ten Hoeve W, Carvelli M, Versteegen RM, Lub J, and Robillard MS, Highly Reactive trans-Cyclooctene Tags with Improved Stability for Diels-Alder Chemistry in Living Systems. Bioconjugate Chem. 2013; 24(7): 1210-1217. Copyright 2013 American Chemical Society.
Summary and future perspectives
Pretargeting is a more complex approach compared with the use of directly radiolabelled imaging or therapeutic agents and it will require careful optimisation in order to be successfully translated into a clinical setting. Ultimately, the incentive to undertake this costly endeavour will come from the demonstrable proof that this approach is superior to the use of directly radiolabelled macromolecules. To date, a small number of clinical trials involving conventional pretargeting strategies have shown such promise, despite suffering from a few inherent shortcomings.
Bioorthogonal chemistry has the potential to circumvent many of the limitations of its predecessors and has shown significant promise in a few pioneering preclinical studies. From a selection of potential candidates, only one bioorthogonal chemistry reaction has been used successfully to enable pretargeting of specific cancer biomarkers, namely the TCO-tetrazine ligation. However, as bioorthogonal reactions continue to be refined through innovative chemical design, this number is likely to increase, yielding chemical pairings with improved bioavailability, bioorthogonality, metabolic stability, and rates of reaction.
It is also worth noting that many of the most compelling biomarkers of cancer exist within the intracellular (and, indeed, intranuclear) compartments of tumour cells. This presents a significant challenge to the imaging community as these targets are much less accessible, particularly to the types of macromolecular targeting vectors that are involved in a pretargeting strategy. Therefore, research efforts should also be focussed on enhancing the cell permeability of these constructs. So far, only a handful of publications have addressed this.
In summary, the continual improvement of bioorthogonal chemistry reactions over recent years has facilitated an alternative pretargeting strategy which is demonstrating much promise for both imaging and therapy of cancer. This approach offers key advantages over more conventional strategies which will undoubtedly increase its potential for translation into a clinical setting.
Acknowledgements
The authors would like to thank Cancer Research UK for supporting this work.
Disclosure of conflict of interest
None.
References
- 1.Hanahan D, Weinberg Robert A. Hallmarks of Cancer: The Next Generation. Cell. 2011;144:646–674. doi: 10.1016/j.cell.2011.02.013. [DOI] [PubMed] [Google Scholar]
- 2.Gambhir SS, Czernin J, Schwimmer J, Silverman DHS, Coleman RE, Phelps ME. A Tabulated Summary of the FDG PET Literature. J Nucl Med. 2001;42:1S–93S. [PubMed] [Google Scholar]
- 3.Vavere AL, Lewis JS. Cu-ATSM: A radiopharmaceutical for the PET imaging of hypoxia. Dalton Trans. 2007:4893–4902. doi: 10.1039/b705989b. [DOI] [PubMed] [Google Scholar]
- 4.Warburg O. On the Origin of Cancer Cells. Science. 1956;123:309–314. doi: 10.1126/science.123.3191.309. [DOI] [PubMed] [Google Scholar]
- 5.Knight JC, Wuest FR. Nuclear (PET/SPECT) and optical imaging probes targeting the CXCR4 chemokine receptor. Med Chem Comm. 2012;3:1039–1053. [Google Scholar]
- 6.Chen K, Chen X. Design and Development of Molecular Imaging Probes. Curr Top Med Chem. 2010;10:1227–1236. doi: 10.2174/156802610791384225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Chen K, Conti PS. Target-specific delivery of peptide-based probes for PET imaging. Adv Drug Deliv Rev. 2010;62:1005–1022. doi: 10.1016/j.addr.2010.09.004. [DOI] [PubMed] [Google Scholar]
- 8.Strebhardt K, Ullrich A. Paul Ehrlich’s magic bullet concept: 100 years of progress. Nat Rev Cancer. 2008;8:473–480. doi: 10.1038/nrc2394. [DOI] [PubMed] [Google Scholar]
- 9.Wu AM, Olafsen T. Antibodies for molecular imaging of cancer. Cancer J. 2008;14:191–197. doi: 10.1097/PPO.0b013e31817b07ae. [DOI] [PubMed] [Google Scholar]
- 10.Knowles SM, Wu AM. Advances in Immuno–Positron Emission Tomography: Antibodies for Molecular Imaging in Oncology. J. Clin. Oncol. 2012;30:3884–3892. doi: 10.1200/JCO.2012.42.4887. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Sharkey RM, Rossi EA, Chang CH, Goldenberg DM. Improved Cancer Therapy and Molecular Imaging with Multivalent, Multispecific Antibodies. Cancer Biother Radiopharm. 2010;25:1–12. doi: 10.1089/cbr.2009.0690. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Boerman OC, Oyen WJG. Immuno-PET of Cancer: A Revival of Antibody Imaging. J Nucl Med. 2011;52:1171–1172. doi: 10.2967/jnumed.111.089771. [DOI] [PubMed] [Google Scholar]
- 13.Olafsen T, Wu AM. Antibody Vectors for Imaging. Semin Nucl Med. 2010;40:167–181. doi: 10.1053/j.semnuclmed.2009.12.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Wu AM. Antibodies and Antimatter: The Resurgence of Immuno-PET. J Nucl Med. 2009;50:2–5. doi: 10.2967/jnumed.108.056887. [DOI] [PubMed] [Google Scholar]
- 15.Goldenberg DM. Targeted Therapy of Cancer with Radiolabeled Antibodies. J Nucl Med. 2002;43:693–713. [PubMed] [Google Scholar]
- 16.Pressman D, Korngold L. The in vivo localization of anti-Wagner-osteogenic-sarcoma antibodies. Cancer. 1953;6:619–623. doi: 10.1002/1097-0142(195305)6:3<619::aid-cncr2820060319>3.0.co;2-y. [DOI] [PubMed] [Google Scholar]
- 17.Wissler RW, Barker PA, Flax MH, La Via MF, Talmage DW. A Study of the Preparation, Localization, and Effects of Antitumor Antibodies Labeled with I131. Cancer Res. 1956;16:761–773. [PubMed] [Google Scholar]
- 18.Goldenberg DM, DeLand F, Kim E, Bennett S, Primus FJ, van Nagell JR, Estes N, DeSimone P, Rayburn P. Use of Radio-Labeled Antibodies to Carcinoembryonic Antigen for the Detection and Localization of Diverse Cancers by External Photoscanning. New Engl J Med. 1978;298:1384–1388. doi: 10.1056/NEJM197806222982503. [DOI] [PubMed] [Google Scholar]
- 19.Kohler G, Milstein C. Continuous cultures of fused cells secreting antibody of predefined specificity. Nature. 1975;256:495–497. doi: 10.1038/256495a0. [DOI] [PubMed] [Google Scholar]
- 20.Tjandra JJ, Ramadi L, McKenzie IFC. Development of human anti-murine antibody (HAMA) response in patients. Immunol Cell Biol. 1990;68:367–376. doi: 10.1038/icb.1990.50. [DOI] [PubMed] [Google Scholar]
- 21.Chames P, Van Regenmortel M, Weiss E, Baty D. Therapeutic antibodies: successes, limitations and hopes for the future. Br J Pharmacol. 2009;157:220–233. doi: 10.1111/j.1476-5381.2009.00190.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Rosebrough SF, Grossman ZD, McAfee JG, Kudryk BJ, Subramanian G, Ritter-Hrncirik CA, Witanowski LS, Tillapaugh-Fay G, Urrutia E, Zapf-Longo C. Thrombus Imaging with Indium-111 and Iodine-131-Labeled Fibrin-Specific Monoclonal Antibody and Its F(ab′)2 and Fab Fragments. J Nucl Med. 1988;29:1212–1222. [PubMed] [Google Scholar]
- 23.Murray JL, Rosenblum MG, Lamki L, Glenn HJ, Krizan Z, Hersh EM, Plager CE, Bartholomew RM, Unger MW, Carlo DJ. Clinical Parameters Related to Optimal Tumor Localization of Indium-111-Labeled Mouse Antimelanoma Monoclonal Antibody ZME-018. J Nucl Med. 1987;28:25–33. [PubMed] [Google Scholar]
- 24.Deri MA, Zeglis BM, Francesconi LC, Lewis JS. PET imaging with 89Zr: From radiochemistry to the clinic. Nucl Med Biol. 2013;40:3–14. doi: 10.1016/j.nucmedbio.2012.08.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Reardan DT, Meares CF, Goodwin DA, McTigue M, David GS, Stone MR, Leung JP, Bartholomew RM, Frincke JM. Antibodies against metal chelates. Nature. 1985;316:265–268. doi: 10.1038/316265a0. [DOI] [PubMed] [Google Scholar]
- 26.Goodwin DA, Mears CF, McTigue M, David GS. Monoclonal antibody hapten radiopharmaceutical delivery. Nucl Med Commun. 1986;7:569–580. doi: 10.1097/00006231-198608000-00002. [DOI] [PubMed] [Google Scholar]
- 27.Goodwin DA, Meares CF, McCall MJ, McTigue M, Chaovapong W. Pre-Targeted Immunoscintigraphy of Murine Tumors with Indium-111-Labeled Bifunctional Haptens. J Nucl Med. 1988;29:226–234. [PubMed] [Google Scholar]
- 28.Zeglis BM, Sevak KK, Reiner T, Mohindra P, Carlin SD, Zanzonico P, Weissleder R, Lewis JS. A Pretargeted PET Imaging Strategy Based on Bioorthogonal Diels-Alder Click Chemistry. J Nucl Med. 2013;54:1389–1396. doi: 10.2967/jnumed.112.115840. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Goldenberg DM, Rossi EA, Sharkey RM, McBride WJ, Chang CH. Multifunctional Antibodies by the Dock-and-Lock Method for Improved Cancer Imaging and Therapy by Pretargeting. J Nucl Med. 2008;49:158–163. doi: 10.2967/jnumed.107.046185. [DOI] [PubMed] [Google Scholar]
- 30.Goldenberg DM, Chatal JF, Barbet J, Boerman O, Sharkey RM. Cancer imaging and therapy with bispecific antibody pretargeting. Update Cancer Ther. 2007;2:19–31. doi: 10.1016/j.uct.2007.04.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Sharkey RM, Karacay H, Goldenberg DM. Improving the treatment of non-Hodgkin lymphoma with antibody-targeted radionuclides. Cancer. 2010;116:1134–1145. doi: 10.1002/cncr.24802. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Sharkey RM, Rossi EA, McBride WJ, Chang CH, Goldenberg DM. Recombinant Bispecific Monoclonal Antibodies Prepared by the Dock-and-Lock Strategy for Pretargeted Radioimmunotherapy. Semin Nucl Med. 2010;40:190–203. doi: 10.1053/j.semnuclmed.2009.12.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Goldenberg DM, Chang CH, Rossi EA, McBride WJ, Sharkey RM. Pretargeted Molecular Imaging and Radioimmunotherapy. Theranostics. 2012;2:523–540. doi: 10.7150/thno.3582. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Le Doussal JM, Martin M, Gautherot E, Delaage M, Barbet J. In Vitro and In Vivo Targeting of Radiolabeled Monovalent and Divalent Haptens with Dual Specificity Monoclonal Antibody Conjugates: Enhanced Divalent Hapten Affinity for Cell-Bound Antibody Conjugate. J Nucl Med. 1989;30:1358–1366. [PubMed] [Google Scholar]
- 35.Stickney DR, Anderson LD, Slater JB, Ahlem CN, Kirk GA, Schweighardt SA, Frincke JM. Bifunctional Antibody: A Binary Radiopharmaceutical Delivery System for Imaging Colorectal Carcinoma. Cancer Res. 1991;51:6650–6655. [PubMed] [Google Scholar]
- 36.Hnatowich DJ, Virzi F, Rusckowski M. Investigations of Avidin and Biotin for Imaging Applications. J Nucl Med. 1987;28:1294–1302. [PubMed] [Google Scholar]
- 37.Green NM. Avidin and streptavidin. Methods Enzymol. 1990;184:51–67. doi: 10.1016/0076-6879(90)84259-j. [DOI] [PubMed] [Google Scholar]
- 38.Boerman OC, van Schaijk FG, Oyen WJG, Corstens FHM. Pretargeted Radioimmunotherapy of Cancer: Progress Step by Step. J Nucl Med. 2003;44:400–411. [PubMed] [Google Scholar]
- 39.Liu G, Hnatowich DJ. A Semiempirical Model of Tumor Pretargeting. Bioconjug Chem. 2008;19:2095–2104. doi: 10.1021/bc8002748. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Sharkey RM, Chang CH, Rossi EA, McBride WJ, Goldenberg DM. Pretargeting: taking an alternate route for localizing radionuclides. Tumour Biol. 2012;33:591–600. doi: 10.1007/s13277-012-0367-6. [DOI] [PubMed] [Google Scholar]
- 41.Sharkey RM, Goldenberg DM. Advances in Radioimmunotherapy in the Age of Molecular Engineering and Pretargeting. Cancer Invest. 2006;24:82–97. doi: 10.1080/07357900500449553. [DOI] [PubMed] [Google Scholar]
- 42.Goldenberg DM, Sharkey RM, Paganelli G, Barbet J, Chatal JF. Antibody Pretargeting Advances Cancer Radioimmunodetection and Radioimmunotherapy. J. Clin. Oncol. 2006;24:823–834. doi: 10.1200/JCO.2005.03.8471. [DOI] [PubMed] [Google Scholar]
- 43.Axworthy DB, Fritzberg AR, Hylarides MD, Mallett RW, Theodore LJ, Gustavson LM, Su FM, Beaumier PL, Reno JM. Preclinical Evaluation of An Anti-Tumor Monclonal Antibody/Streptavidin Conjugate for Pretargeted 90Y Radioimmunotherapy in A Mouse Xenograft Model. J Immunother. 1994;16:158. [Google Scholar]
- 44.Mohsin H, Jia F, Bryan JN, Sivaguru G, Cutler CS, Ketring AR, Miller WH, Simón J, Frank RK, Theodore LJ, Axworthy DB, Jurisson SS, Lewis MR. Comparison of Pretargeted and Conventional CC49 Radioimmunotherapy Using 149Pm, 166Ho, and 177Lu. Bioconjug Chem. 2011;22:2444–2452. doi: 10.1021/bc200258x. [DOI] [PubMed] [Google Scholar]
- 45.Pagel JM, Kenoyer AL, Bäck T, Hamlin DK, Wilbur DS, Fisher DR, Park SI, Frayo S, Axtman A, Orgun N, Orozco J, Shenoi J, Lin Y, Gopal AK, Green DJ, Appelbaum FR, Press OW. Anti-CD45 pretargeted radioimmunotherapy using bismuth-213: high rates of complete remission and long-term survival in a mouse myeloid leukemia xenograft model. Blood. 2011;118:703–711. doi: 10.1182/blood-2011-04-347039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Pagel JM, Matthews DC, Kenoyer A, Hamlin DK, Wilbur DS, Fisher DR, Gopal AK, Lin Y, Saganic L, Appelbaum FR, Press OW. Pretargeted Radioimmunotherapy Using Anti-CD45 Monoclonal Antibodies to Deliver Radiation to Murine Hematolymphoid Tissues and Human Myeloid Leukemia. Cancer Res. 2009;69:185–192. doi: 10.1158/0008-5472.CAN-08-2513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Pagel JM, Orgun N, Hamlin DK, Wilbur DS, Gooley TA, Gopal AK, Park SI, Green DJ, Lin Y, Press OW. A comparative analysis of conventional and pretargeted radioimmunotherapy of B-cell lymphomas by targeting CD20, CD22, and HLA-DR singly and in combinations. Blood. 2009;113:4903–4913. doi: 10.1182/blood-2008-11-187401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Shen S, Forero A, LoBuglio AF, Breitz H, Khazaeli MB, Fisher DR, Wang W, Meredith RF. Patient-Specific Dosimetry of Pretargeted Radioimmunotherapy Using CC49 Fusion Protein in Patients with Gastrointestinal Malignancies. J Nucl Med. 2005;46:642–651. [PubMed] [Google Scholar]
- 49.Förster GJ, Santos EB, Smith-Jones PM, Zanzonico P, Larson SM. Pretargeted Radioimmunotherapy with a Single-Chain Antibody/Streptavidin Construct and Radiolabeled DOTA-Biotin: Strategies for Reduction of the Renal Dose. J Nucl Med. 2006;47:140–149. [PubMed] [Google Scholar]
- 50.Liu G, Dou S, Liu Y, Wang Y, Rusckowski M, Hnatowich DJ. 90Y labeled Phosphorodiamidate Morpholino Oligomer for Pretargeting Radiotherapy. Bioconjug Chem. 2011;22:2539–2545. doi: 10.1021/bc200366t. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Liu G, Dou S, Cheng D, Leif J, Rusckowski M, Streeter PR, Shultz LD, Hnatowich DJ, Greiner DL. Human Islet Cell MORF/cMORF Pretargeting in a Xenogeneic Murine Transplant Model. Mol Pharm. 2011;8:767–773. doi: 10.1021/mp100382m. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.He J, Wang Y, Dou S, Liu X, Zhang S, Liu G, Hnatowich D. Affinity Enhancement Pretargeting: Synthesis and Testing of a 99mTc-Labeled Bivalent MORF. Mol Pharm. 2010;7:1118–1124. doi: 10.1021/mp9002909. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Liu G, Liu C, Zhang S, He J, Liu N, Gupta S, Rusckowski M, Hnatowich DJ. Investigations of 99mTc Morpholino Pretargeting in Mice. Nucl Med Commun. 2003;24:697–705. doi: 10.1097/00006231-200306000-00013. [DOI] [PubMed] [Google Scholar]
- 54.He J, Liu G, Gupta S, Zhang Y, Rusckowski M, Hnatowich DJ. Amplification Targeting: A Modified Pretargeting Approach with Potential for Signal Amplification-Proof of a Concept. J Nucl Med. 2004;45:1087–1095. [PubMed] [Google Scholar]
- 55.Liu G, Cheng D, Dou S, Chen X, Liang M, Pretorius PH, Rusckowski M, Hnatowich D. Replacing 99mTc with 111In Improves MORF/cMORF Pretargeting by Reducing Intestinal Accumulation. Mol Imaging Biol. 2009;11:303–307. doi: 10.1007/s11307-009-0209-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Liu G, Dou S, Rusckowski M, Hnatowich DJ. An experimental and theoretical evaluation of the influence of pretargeting antibody on the tumor accumulation of effector. Mol Cancer Ther. 2008;7:1025–1032. doi: 10.1158/1535-7163.MCT-07-2203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Liu G, Dou S, Baker S, Akalin A, Cheng D, Chen L, Rusckowski M, Hnatowich DJ. A preclinical 188Re tumor therapeutic investigation using MORF/cMORF pretargeting and an antiTAG-72 antibody CC49. Cancer Biol Ther. 2010;10:767–774. doi: 10.4161/cbt.10.8.12879. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Wang Y, Chang F, Zhang Y, Liu N, Liu G, Gupta S, Rusckowski M, Hnatowich DJ. Pretargeting with Amplification Using Polymeric Peptide Nucleic Acid. Bioconjug Chem. 2001;12:807–816. doi: 10.1021/bc0100307. [DOI] [PubMed] [Google Scholar]
- 59.He J, Rusckowski M, Wang Y, Dou S, Liu X, Zhang S, Liu G, Hnatowich D. Optical Pretargeting of Tumor with Fluorescent MORF Oligomers. Mol Imaging Biol. 2007;9:17–23. doi: 10.1007/s11307-006-0071-2. [DOI] [PubMed] [Google Scholar]
- 60.Crooke ST. Advances in Understanding the Pharmacological Properties of Antisense Oligonucleotides. In: August JT, editor. Advances in Pharmacology. Academic Press; 1997. pp. 1–49. [DOI] [PubMed] [Google Scholar]
- 61.Goodchild J. Conjugates of oligonucleotides and modified oligonucleotides: a review of their synthesis and properties. Bioconjug Chem. 1990;1:165–187. doi: 10.1021/bc00003a001. [DOI] [PubMed] [Google Scholar]
- 62.Boyce M, Bertozzi CR. Bringing chemistry to life. Nat Methods. 2011;8:638–642. doi: 10.1038/nmeth.1657. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Debets MF, van Berkel SS, Dommerholt J, Dirks AJ, Rutjes FPJT, van Delft FL. Bioconjugation with Strained Alkenes and Alkynes. Acc Chem Res. 2011;44:805–815. doi: 10.1021/ar200059z. [DOI] [PubMed] [Google Scholar]
- 64.Sletten EM, Bertozzi CR. From Mechanism to Mouse: A Tale of Two Bioorthogonal Reactions. Acc Chem Res. 2011;44:666–676. doi: 10.1021/ar200148z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Becer CR, Hoogenboom R, Schubert US. Click Chemistry beyond Metal-Catalyzed Cycloaddition. Angew Chem Int Ed Engl. 2009;48:4900–4908. doi: 10.1002/anie.200900755. [DOI] [PubMed] [Google Scholar]
- 66.Jewett JC, Bertozzi CR. Cu-free click cycloaddition reactions in chemical biology. Chem Soc Rev. 2010;39:1272–1279. doi: 10.1039/b901970g. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Baskin JM, Bertozzi CR. Bioorthogonal Click Chemistry: Covalent Labeling in Living Systems. QSAR Comb Sci. 2007;26:1211–1219. [Google Scholar]
- 68.Sletten EM, Bertozzi CR. Bioorthogonal Chemistry: Fishing for Selectivity in a Sea of Functionality. Angew Chem Int Ed Engl. 2009;48:6974–6998. doi: 10.1002/anie.200900942. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.van Berkel SS, van Delft FL. Metal-free bioconjugation reactions. Drug Discov Today Technol. 2013;10:e45–e51. doi: 10.1016/j.ddtec.2012.10.011. [DOI] [PubMed] [Google Scholar]
- 70.Devaraj NK. Tetrazine Bioorthogonal Reactions through the Development of New Synthetic Tools. Synlett. 2012;23:2147–2152. [Google Scholar]
- 71.Best MD. Click Chemistry and Bioorthogonal Reactions: Unprecedented Selectivity in the Labeling of Biological Molecules. Biochem. 2009;48:6571–6584. doi: 10.1021/bi9007726. [DOI] [PubMed] [Google Scholar]
- 72.Baskin JM, Bertozzi CR. Copper-free click chemistry: Bioorthogonal reagents for tagging azides. Aldrichimica Acta. 2010;43:15–23. [Google Scholar]
- 73.Debets MF, van Hest JCM, Rutjes FPJT. Bioorthogonal labelling of biomolecules: new functional handles and ligation methods. Org Biomol Chem. 2013;11:6439–6455. doi: 10.1039/c3ob41329b. [DOI] [PubMed] [Google Scholar]
- 74.Kolb HC, Finn MG, Sharpless KB. Click Chemistry: Diverse Chemical Function from a Few Good Reactions. Angew Chem Int Ed Engl. 2001;40:2004–2021. doi: 10.1002/1521-3773(20010601)40:11<2004::AID-ANIE2004>3.0.CO;2-5. [DOI] [PubMed] [Google Scholar]
- 75.Huisgen R. 1,3-Dipolar Cycloadditions. Past and Future. Angew Chem Int Ed. 1963;2:565–598. [Google Scholar]
- 76.Meldal M, Tornøe CW. Cu-Catalyzed Azide−Alkyne Cycloaddition. Chem Rev. 2008;108:2952–3015. doi: 10.1021/cr0783479. [DOI] [PubMed] [Google Scholar]
- 77.Huisgen R. Kinetics and Mechanism of 1,3-Dipolar Cycloadditions. Angew Chem Int Ed. 1963;2:633–645. [Google Scholar]
- 78.Rostovtsev VV, Green LG, Fokin VV, Sharpless KB. A Stepwise Huisgen Cycloaddition Process: Copper(I)-Catalyzed Regioselective “Ligation” of Azides and Terminal Alkynes. Angew Chem Int Ed Engl. 2002;41:2596–2599. doi: 10.1002/1521-3773(20020715)41:14<2596::AID-ANIE2596>3.0.CO;2-4. [DOI] [PubMed] [Google Scholar]
- 79.Tornøe CW, Christensen C, Meldal M. Peptidotriazoles on Solid Phase: [1,2,3] -Triazoles by Regiospecific Copper(I)-Catalyzed 1,3-Dipolar Cycloadditions of Terminal Alkynes to Azides. J Org Chem. 2002;67:3057–3064. doi: 10.1021/jo011148j. [DOI] [PubMed] [Google Scholar]
- 80.Nwe K, Brechbiel MW. Growing Applications of “Click Chemistry” for Bioconjugation in Contemporary Biomedical Research. Cancer Biother Radiopharm. 2009;24:289–302. doi: 10.1089/cbr.2008.0626. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Wängler C, Schirrmacher R, Bartenstein P, Wängler B. Click-chemistry reactions in radiopharmaceutical chemistry: fast & easy introduction of radiolabels into biomolecules for in vivo imaging. Curr Med Chem. 2010;17:1092–1116. doi: 10.2174/092986710790820615. [DOI] [PubMed] [Google Scholar]
- 82.Marik J, Sutcliffe JL. Click for PET: rapid preparation of [18F] fluoropeptides using CuI catalyzed 1,3-dipolar cycloaddition. Tetrahedron Lett. 2006;47:6681–6684. [Google Scholar]
- 83.Hausner SH, Marik J, Gagnon MKJ, Sutcliffe JL. In vivo positron emission tomography (PET) imaging with an αvβ6 specific peptide radiolabeled using 18F-”click” chemistry: Evaluation and comparison with the corresponding 4-[18F] fluorobenzoyl- and 2-[18F] fluoropropionyl- peptides. J Med Chem. 2008;51:5901–5904. doi: 10.1021/jm800608s. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Li ZB, Wu Z, Chen K, Chin FT, Chen X. Click Chemistry for 18F-Labeling of RGD Peptides and microPET Imaging of Tumor Integrin αvβ3 Expression. Bioconjug Chem. 2007;18:1987–1994. doi: 10.1021/bc700226v. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Carroll L, Evans HL, Aboagye EO, Spivey AC. Bioorthogonal chemistry for pre-targeted molecular imaging - progress and prospects. Org Biomol Chem. 2013;11:5772–5781. doi: 10.1039/c3ob40897c. [DOI] [PubMed] [Google Scholar]
- 86.Knall AC, Slugovc C. Inverse electron demand Diels-Alder (iEDDA)-initiated conjugation: a (high) potential click chemistry scheme. Chem Soc Rev. 2013;42:5131–5142. doi: 10.1039/c3cs60049a. [DOI] [PubMed] [Google Scholar]
- 87.Staudinger H, Meyer J. Ueber neue organische Phosphorverbindungen II. Phosphazine. Helv Chim Acta. 1919;2:619–635. [Google Scholar]
- 88.Lin FL, Hoyt HM, van Halbeek H, Bergman RG, Bertozzi CR. Mechanistic Investigation of the Staudinger Ligation. J Am Chem Soc. 2005;127:2686–2695. doi: 10.1021/ja044461m. [DOI] [PubMed] [Google Scholar]
- 89.van Berkel SS, van Eldijk MB, van Hest JCM. Staudinger Ligation as a Method for Bioconjugation. Angew Chem Int Ed Engl. 2011;50:8806–8827. doi: 10.1002/anie.201008102. [DOI] [PubMed] [Google Scholar]
- 90.Schilling CI, Jung N, Biskup M, Schepers U, Brase S. Bioconjugation via azide-Staudinger ligation: an overview. Chem Soc Rev. 2011;40:4840–4871. doi: 10.1039/c0cs00123f. [DOI] [PubMed] [Google Scholar]
- 91.Kiick KL, Saxon E, Tirrell DA, Bertozzi CR. Incorporation of azides into recombinant proteins for chemoselective modification by the Staudinger ligation. Proc Natl Acad Sci U S A. 2002;99:19–24. doi: 10.1073/pnas.012583299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Saxon E, Bertozzi CR. Cell Surface Engineering by a Modified Staudinger Reaction. Science. 2000;287:2007–2010. doi: 10.1126/science.287.5460.2007. [DOI] [PubMed] [Google Scholar]
- 93.Agard NJ, Baskin JM, Prescher JA, Lo A, Bertozzi CR. A Comparative Study of Bioorthogonal Reactions with Azides. ACS Chem Biol. 2006;1:644–648. doi: 10.1021/cb6003228. [DOI] [PubMed] [Google Scholar]
- 94.Prescher JA, Dube DH, Bertozzi CR. Chemical remodelling of cell surfaces in living animals. Nature. 2004;430:873–877. doi: 10.1038/nature02791. [DOI] [PubMed] [Google Scholar]
- 95.Chang PV, Prescher JA, Hangauer MJ, Bertozzi CR. Imaging Cell Surface Glycans with Bioorthogonal Chemical Reporters. J Am Chem Soc. 2007;129:8400–8401. doi: 10.1021/ja070238o. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Hangauer MJ, Bertozzi CR. A FRET-Based Fluorogenic Phosphine for Live-Cell Imaging with the Staudinger Ligation. Angew Chem Int Ed Engl. 2008;47:2394–2397. doi: 10.1002/anie.200704847. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Heldt JM, Kerzendörfer O, Mamat C, Starke F, Pietzsch HJ, Steinbach J. Synthesis of Short and Versatile Heterobifunctional Linkers for Conjugationof Bioactive Molecules with (Radio-)Labels. Synlett. 2013;24:0432–0436. [Google Scholar]
- 98.Vugts DJ, Vervoort A, Stigter-van Walsum M, Visser GWM, Robillard MS, Versteegen RM, Vulders RCM, Herscheid JDM, van Dongen GAMS. Synthesis of Phosphine and Antibody–Azide Probes for in Vivo Staudinger Ligation in a Pretargeted Imaging and Therapy Approach. Bioconjug Chem. 2011;22:2072–2081. doi: 10.1021/bc200298v. [DOI] [PubMed] [Google Scholar]
- 99.Stockmann H, Neves AA, Stairs S, Ireland-Zecchini H, Brindle KM, Leeper FJ. Development and evaluation of new cyclooctynes for cell surface glycan imaging in cancer cells. Chem Sci. 2011;2:932–936. doi: 10.1039/C0SC00631A. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Codelli JA, Baskin JM, Agard NJ, Bertozzi CR. Second-Generation Difluorinated Cyclooctynes for Copper-Free Click Chemistry. J Am Chem Soc. 2008;130:11486–11493. doi: 10.1021/ja803086r. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Varga BR, Kállay M, Hegyi K, Béni S, Kele P. A Non-Fluorinated Monobenzocyclooctyne for Rapid Copper-Free Click Reactions. Chem Eur J. 2012;18:822–828. doi: 10.1002/chem.201102329. [DOI] [PubMed] [Google Scholar]
- 102.Mbua NE, Guo J, Wolfert MA, Steet R, Boons GJ. Strain-Promoted Alkyne–Azide Cycloadditions (SPAAC) Reveal New Features of Glycoconjugate Biosynthesis. Chembiochem. 2011;12:1912–1921. doi: 10.1002/cbic.201100117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Ning X, Guo J, Wolfert MA, Boons GJ. Visualizing Metabolically Labeled Glycoconjugates of Living Cells by Copper-Free and Fast Huisgen Cycloadditions. Angew Chem Int Ed Engl. 2008;47:2253–2255. doi: 10.1002/anie.200705456. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Jewett JC, Sletten EM, Bertozzi CR. Rapid Cu-Free Click Chemistry with Readily Synthesized Biarylazacyclooctynones. J Am Chem Soc. 2010;132:3688–3690. doi: 10.1021/ja100014q. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Dommerholt J, Schmidt S, Temming R, Hendriks LJA, Rutjes FPJT, van Hest JCM, Lefeber DJ, Friedl P, van Delft FL. Readily Accessible Bicyclononynes for Bioorthogonal Labeling and Three-Dimensional Imaging of Living Cells. Angew Chem Int Ed Engl. 2010;49:9422–9425. doi: 10.1002/anie.201003761. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Gordon CG, Mackey JL, Jewett JC, Sletten EM, Houk KN, Bertozzi CR. Reactivity of Biarylazacyclooctynones in Copper-Free Click Chemistry. J Am Chem Soc. 2012;134:9199–9208. doi: 10.1021/ja3000936. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Chigrinova M, McKay CS, Beaulieu LPB, Udachin KA, Beauchemin AM, Pezacki JP. Rearrangements and addition reactions of biarylazacyclooctynones and the implications to copper-free click chemistry. Org Biomol Chem. 2013;11:3436–3441. doi: 10.1039/c3ob40683k. [DOI] [PubMed] [Google Scholar]
- 108.Agard NJ, Prescher JA, Bertozzi CR. A Strain-Promoted [3 + 2] Azide−Alkyne Cycloaddition for Covalent Modification of Biomolecules in Living Systems. J Am Chem Soc. 2004;126:15046–15047. doi: 10.1021/ja044996f. [DOI] [PubMed] [Google Scholar]
- 109.Bernardin A, Cazet Al, Guyon L, Delannoy P, Vinet Fo, Bonnaffé D, Texier I. Copper-Free Click Chemistry for Highly Luminescent Quantum Dot Conjugates: Application to in Vivo Metabolic Imaging. Bioconjug Chem. 2010;21:583–588. doi: 10.1021/bc900564w. [DOI] [PubMed] [Google Scholar]
- 110.Debets MF, van Berkel SS, Schoffelen S, Rutjes FPJT, van Hest JCM, van Delft FL. Aza-dibenzocyclooctynes for fast and efficient enzyme PEGylation via copper-free (3 + 2) cycloaddition. Chem Commun (Camb) 2010;46:97–99. doi: 10.1039/b917797c. [DOI] [PubMed] [Google Scholar]
- 111.van der Linden WA, Li N, Hoogendoorn S, Ruben M, Verdoes M, Guo J, Boons GJ, van der Marel GA, Florea BI, Overkleeft HS. Two-step bioorthogonal activity-based proteasome profiling using copper-free click reagents: A comparative study. Bioorg Med Chem. 2012;20:662–666. doi: 10.1016/j.bmc.2011.06.037. [DOI] [PubMed] [Google Scholar]
- 112.Reddington SC, Tippmann EM, Dafydd Jones D. Residue choice defines efficiency and influence of bioorthogonal protein modification via genetically encoded strain promoted Click chemistry. Chem Commun (Camb) 2012;48:8419–8421. doi: 10.1039/c2cc31887c. [DOI] [PubMed] [Google Scholar]
- 113.van Hest JCM, van Delft FL. Protein Modification by Strain-Promoted Alkyne–Azide Cycloaddition. Chembiochem. 2011;12:1309–1312. doi: 10.1002/cbic.201100206. [DOI] [PubMed] [Google Scholar]
- 114.Bostic HE, Smith MD, Poloukhtine AA, Popik VV, Best MD. Membrane labeling and immobilization via copper-free click chemistry. Chem Commun (Camb) 2012;48:1431–1433. doi: 10.1039/c1cc14415d. [DOI] [PubMed] [Google Scholar]
- 115.McKay CS, Blake JA, Cheng J, Danielson DC, Pezacki JP. Strain-promoted cycloadditions of cyclic nitrones with cyclooctynes for labeling human cancer cells. Chem Commun (Camb) 2011;47:10040–10042. doi: 10.1039/c1cc13808a. [DOI] [PubMed] [Google Scholar]
- 116.Ning X, Temming RP, Dommerholt J, Guo J, Ania DB, Debets MF, Wolfert MA, Boons GJ, van Delft FL. Protein Modification by Strain-Promoted Alkyne–Nitrone Cycloaddition. Angew Chem Int Ed Engl. 2010;49:3065–3068. doi: 10.1002/anie.201000408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Temming RP, Eggermont L, van Eldijk MB, van Hest JCM, van Delft FL. N-terminal dual protein functionalization by strain-promoted alkyne-nitrone cycloaddition. Org Biomol Chem. 2013;11:2772–2779. doi: 10.1039/c3ob00043e. [DOI] [PubMed] [Google Scholar]
- 118.Colombo M, Sommaruga S, Mazzucchelli S, Polito L, Verderio P, Galeffi P, Corsi F, Tortora P, Prosperi D. Site-Specific Conjugation of ScFvs Antibodies to Nanoparticles by Bioorthogonal Strain-Promoted Alkyne–Nitrone Cycloaddition. Angew Chem Int Ed Engl. 2012;51:496–499. doi: 10.1002/anie.201106775. [DOI] [PubMed] [Google Scholar]
- 119.Baskin JM, Prescher JA, Laughlin ST, Agard NJ, Chang PV, Miller IA, Lo A, Codelli JA, Bertozzi CR. Copper-free click chemistry for dynamic in vivo imaging. Proc Natl Acad Sci U S A. 2007;104:16793–16797. doi: 10.1073/pnas.0707090104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Chang PV, Prescher JA, Sletten EM, Baskin JM, Miller IA, Agard NJ, Lo A, Bertozzi CR. Copper-free click chemistry in living animals. Proc Natl Acad Sci U S A. 2010;107:1821–1826. doi: 10.1073/pnas.0911116107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Koo H, Lee S, Na JH, Kim SH, Hahn SK, Choi K, Kwon IC, Jeong SY, Kim K. Bioorthogonal Copper-Free Click Chemistry In Vivo for Tumor-Targeted Delivery of Nanoparticles. Angew Chem Int Ed Engl. 2012;51:11836–11840. doi: 10.1002/anie.201206703. [DOI] [PubMed] [Google Scholar]
- 122.Dehnert KW, Baskin JM, Laughlin ST, Beahm BJ, Naidu NN, Amacher SL, Bertozzi CR. Imaging the Sialome during Zebrafish Development with Copper-Free Click Chemistry. Chembiochem. 2012;13:353–357. doi: 10.1002/cbic.201100649. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Laughlin ST, Baskin JM, Amacher SL, Bertozzi CR. In Vivo Imaging of Membrane-Associated Glycans in Developing Zebrafish. Science. 2008;320:664–667. doi: 10.1126/science.1155106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Bouvet V, Wuest M, Wuest F. Copper-free click chemistry with the short-lived positron emitter fluorine-18. Org Biomol Chem. 2011;9:7393–7399. doi: 10.1039/c1ob06034a. [DOI] [PubMed] [Google Scholar]
- 125.Campbell-Verduyn LS, Mirfeizi L, Schoonen AK, Dierckx RA, Elsinga PH, Feringa BL. Strain-Promoted Copper-Free “Click” Chemistry for 18F Radiolabeling of Bombesin. Angew Chem Int Ed Engl. 2011;50:11117–11120. doi: 10.1002/anie.201105547. [DOI] [PubMed] [Google Scholar]
- 126.Sachin K, Jadhav VH, Kim EM, Kim HL, Lee SB, Jeong HJ, Lim ST, Sohn MH, Kim DW. F-18 Labeling Protocol of Peptides Based on Chemically Orthogonal Strain-Promoted Cycloaddition under Physiologically Friendly Reaction Conditions. Bioconjug Chem. 2012;23:1680–1686. doi: 10.1021/bc3002425. [DOI] [PubMed] [Google Scholar]
- 127.Hausner SH, Carpenter RD, Bauer N, Sutcliffe JL. Evaluation of an integrin αvβ6-specific peptide labeled with [18F] fluorine by copper-free, strain-promoted click chemistry. Nucl Med Biol. 2013;40:233–239. doi: 10.1016/j.nucmedbio.2012.10.007. [DOI] [PubMed] [Google Scholar]
- 128.Carpenter RD, Hausner SH, Sutcliffe JL. Copper-Free Click for PET: Rapid 1,3-Dipolar Cycloadditions with a Fluorine-18 Cyclooctyne. ACS Med Chem Lett. 2011;2:885–889. doi: 10.1021/ml200187j. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Arumugam S, Chin J, Schirrmacher R, Popik VV, Kostikov AP. [18F] Azadibenzocyclooctyne ([18F] ADIBO): A biocompatible radioactive labeling synthon for peptides using catalyst free [3 + 2] cycloaddition. Bioorg Med Chem Lett. 2011;21:6987–6991. doi: 10.1016/j.bmcl.2011.09.126. [DOI] [PubMed] [Google Scholar]
- 130.Evans HL, Slade RL, Carroll L, Smith G, Nguyen QD, Iddon L, Kamaly N, Stockmann H, Leeper FJ, Aboagye EO, Spivey AC. Copper-free click-a promising tool for pre-targeted PET imaging. Chem Commun (Camb) 2012;48:991–993. doi: 10.1039/c1cc16220a. [DOI] [PubMed] [Google Scholar]
- 131.Chen K, Wang X, Lin WY, Shen CKF, Yap LP, Hughes LD, Conti PS. Strain-Promoted Catalyst-Free Click Chemistry for Rapid Construction of 64Cu-Labeled PET Imaging Probes. ACS Med Chem Lett. 2012;3:1019–1023. doi: 10.1021/ml300236m. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Karver MR, Weissleder R, Hilderbrand SA. Bioorthogonal Reaction Pairs Enable Simultaneous, Selective, Multi-Target Imaging. Angew Chem Int Ed Engl. 2012;51:920–922. doi: 10.1002/anie.201104389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.van den Bosch SM, Rossin R, Renart Verkerk P, ten Hoeve W, Janssen HM, Lub J, Robillard MS. Evaluation of strained alkynes for Cu-free click reaction in live mice. Nucl Med Biol. 2013;40:415–423. doi: 10.1016/j.nucmedbio.2012.12.006. [DOI] [PubMed] [Google Scholar]
- 134.Lee SB, Kim HL, Jeong HJ, Lim ST, Sohn MH, Kim DW. Mesoporous Silica Nanoparticle Pretargeting for PET Imaging Based on a Rapid Bioorthogonal Reaction in a Living Body. Angew Chem Int Ed Engl. 2013;52:10549–10552. doi: 10.1002/anie.201304026. [DOI] [PubMed] [Google Scholar]
- 135.McKay CS, Moran J, Pezacki JP. Nitrones as dipoles for rapid strain-promoted 1,3-dipolar cycloadditions with cyclooctynes. Chem Commun (Camb) 2010;46:931–933. doi: 10.1039/b921630h. [DOI] [PubMed] [Google Scholar]
- 136.McKay CS, Chigrinova M, Blake JA, Pezacki JP. Kinetics studies of rapid strain-promoted [3 + 2] -cycloadditions of nitrones with biaryl-aza- cyclooctynone. Org Biomol Chem. 2012;10:3066–3070. doi: 10.1039/c2ob07165g. [DOI] [PubMed] [Google Scholar]
- 137.Thalhammer F, Wallfahrer U, Sauer J. Reaktivität einfacher offenkettiger und cyclischer dienophile bei Diels-Alder-reaktionen mit inversem elektronenbedarf. Tetrahedron Lett. 1990;31:6851–6854. [Google Scholar]
- 138.Selvaraj R, Fox JM. trans-Cyclooctene - a stable, voracious dienophile for bioorthogonal labeling. Curr Opin Chem Biol. 2013;17:753–60. doi: 10.1016/j.cbpa.2013.07.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Sauer J, Bäuerlein P, Ebenbeck W, Gousetis C, Sichert H, Troll T, Utz F, Wallfahrer U. [4 + 2] Cycloadditions of 1,2,4,5-Tetrazines and Cyclopropenes - Synthesis of 3,4-Diazanorcaradienes and Tetracyclic Aliphatic Azo Compounds. Eur J Org Chem. 2001;2001:2629–2638. [Google Scholar]
- 140.Yang J, Šečkutė J, Cole CM, Devaraj NK. Live-Cell Imaging of Cyclopropene Tags with Fluorogenic Tetrazine Cycloadditions. Angew Chem Int Ed Engl. 2012;51:7476–7479. doi: 10.1002/anie.201202122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Niederwieser A, Späte AK, Nguyen LD, Jüngst C, Reutter W, Wittmann V. Two-Color Glycan Labeling of Live Cells by a Combination of Diels-Alder and Click Chemistry. Angew Chem Int Ed Engl. 2013;52:4265–4268. doi: 10.1002/anie.201208991. [DOI] [PubMed] [Google Scholar]
- 142.Boger DL. Diels-Alder reactions of heterocyclic aza dienes. Scope and applications. Chem Rev. 1986;86:781–793. [Google Scholar]
- 143.Alge DL, Azagarsamy MA, Donohue DF, Anseth KS. Synthetically Tractable Click Hydrogels for Three-Dimensional Cell Culture Formed Using Tetrazine–Norbornene Chemistry. Biomacromolecules. 2013;14:949–953. doi: 10.1021/bm4000508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Plass T, Milles S, Koehler C, Szymański J, Mueller R, Wießler M, Schultz C, Lemke EA. Amino Acids for Diels-Alder Reactions in Living Cells. Angew Chem Int Ed Engl. 2012;51:4166–4170. doi: 10.1002/anie.201108231. [DOI] [PubMed] [Google Scholar]
- 145.Lang K, Davis L, Torres-Kolbus J, Chou C, Deiters A, Chin JW. Genetically encoded norbornene directs site-specific cellular protein labelling via a rapid bioorthogonal reaction. Nat Chem. 2012;4:298–304. doi: 10.1038/nchem.1250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Han HS, Devaraj NK, Lee J, Hilderbrand SA, Weissleder R, Bawendi MG. Development of a Bioorthogonal and Highly Efficient Conjugation Method for Quantum Dots Using Tetrazine−Norbornene Cycloaddition. J Am Chem Soc. 2010;132:7838–7839. doi: 10.1021/ja101677r. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.Hansell CF, Espeel P, Stamenović MM, Barker IA, Dove AP, Du Prez FE, O’Reilly RK. Additive-Free Clicking for Polymer Functionalization and Coupling by Tetrazine–Norbornene Chemistry. J Am Chem Soc. 2011;133:13828–13831. doi: 10.1021/ja203957h. [DOI] [PubMed] [Google Scholar]
- 148.Schoch J, Wiessler M, Jäschke A. Post-Synthetic Modification of DNA by Inverse-Electron-Demand Diels-Alder Reaction. J Am Chem Soc. 2010;132:8846–8847. doi: 10.1021/ja102871p. [DOI] [PubMed] [Google Scholar]
- 149.Zeglis BM, Mohindra P, Weissmann GI, Divilov V, Hilderbrand SA, Weissleder R, Lewis JS. Modular Strategy for the Construction of Radiometalated Antibodies for Positron Emission Tomography Based on Inverse Electron Demand Diels-Alder Click Chemistry. Bioconjug Chem. 2011;22:2048–2059. doi: 10.1021/bc200288d. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150.Knight JC, Richter S, Wuest M, Way JD, Wuest F. Synthesis and evaluation of an 18F-labelled norbornene derivative for copper-free click chemistry reactions. Org Biomol Chem. 2013;11:3817–3825. doi: 10.1039/c3ob40548f. [DOI] [PubMed] [Google Scholar]
- 151.Devaraj NK, Weissleder R, Hilderbrand SA. Tetrazine-Based Cycloadditions: Application to Pretargeted Live Cell Imaging. Bioconjug Chem. 2008;19:2297–2299. doi: 10.1021/bc8004446. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152.Schoch J, Staudt M, Samanta A, Wiessler M, Jäschke A. Site-Specific One-Pot Dual Labeling of DNA by Orthogonal Cycloaddition Chemistry. Bioconjug Chem. 2012;23:1382–1386. doi: 10.1021/bc300181n. [DOI] [PubMed] [Google Scholar]
- 153.Li Z, Cai H, Hassink M, Blackman ML, Brown RCD, Conti PS, Fox JM. Tetrazine-trans-cyclooctene ligation for the rapid construction of 18F labeled probes. Chem Commun (Camb) 2010;46:8043–8045. doi: 10.1039/c0cc03078c. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154.Keliher EJ, Reiner T, Turetsky A, Hilderbrand SA, Weissleder R. High-Yielding, Two-Step 18F Labeling Strategy for 18F-PARP1 Inhibitors. ChemMedChem. 2011;6:424–427. doi: 10.1002/cmdc.201000426. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 155.Reiner T, Lacy J, Keliher EJ, Yang KS, Ullal A, Kohler RH, Vinegoni C, Weissleder R. Imaging Therapeutic PARP Inhibition In Vivo through Bioorthogonally Developed Companion Imaging Agents. Neoplasia. 2012;14:169–177. doi: 10.1593/neo.12414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 156.Selvaraj R, Liu S, Hassink M, Huang CW, Yap LP, Park R, Fox JM, Li Z, Conti PS. Tetrazine-trans-cyclooctene ligation for the rapid construction of integrin αvβ3 targeted PET tracer based on a cyclic RGD peptide. Bioorg Med Chem Lett. 2011;21:5011–5014. doi: 10.1016/j.bmcl.2011.04.116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 157.Liu S, Hassink M, Selvaraj R, Yap LP, Park R, Wang H, Chen X, Fox JM, Li Z, Conti PS. Efficient 18F Labeling of Cysteine-Containing Peptides and Proteins Using Tetrazine–Trans-Cyclooctene Ligation. Mol Imaging. 2013;12:121–128. [PMC free article] [PubMed] [Google Scholar]
- 158.Wu Z, Liu S, Hassink M, Nair I, Park R, Li L, Todorov I, Fox JM, Li Z, Shively JE, Conti PS, Kandeel F. Development and Evaluation of 18F-TTCO-Cys40-Exendin-4: A PET Probe for Imaging Transplanted Islets. J Nucl Med. 2013;54:244–251. doi: 10.2967/jnumed.112.109694. [DOI] [PubMed] [Google Scholar]
- 159.Devaraj NK, Upadhyay R, Haun JB, Hilderbrand SA, Weissleder R. Fast and Sensitive Pretargeted Labeling of Cancer Cells through a Tetrazine/trans-Cyclooctene Cycloaddition. Angew Chem Int Ed Engl. 2009;48:7013–7016. doi: 10.1002/anie.200903233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 160.Yang KS, Budin G, Reiner T, Vinegoni C, Weissleder R. Bioorthogonal Imaging of Aurora Kinase A in Live Cells. Angew Chem Int Ed Engl. 2012;51:6598–6603. doi: 10.1002/anie.201200994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 161.Carlson JCT, Meimetis LG, Hilderbrand SA, Weissleder R. BODIPY-Tetrazine Derivatives as Superbright Bioorthogonal Turn-on Probes. Angew Chem Int Ed Engl. 2013;52:6917–6920. doi: 10.1002/anie.201301100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 162.Budin G, Yang KS, Reiner T, Weissleder R. Bioorthogonal Probes for Polo-like Kinase 1 Imaging and Quantification. Angew Chem Int Ed Engl. 2011;50:9378–9381. doi: 10.1002/anie.201103273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 163.Liu DS, Tangpeerachaikul A, Selvaraj R, Taylor MT, Fox JM, Ting AY. Diels-Alder Cycloaddition for Fluorophore Targeting to Specific Proteins inside Living Cells. J Am Chem Soc. 2011;134:792–795. doi: 10.1021/ja209325n. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 164.Reiner T, Earley S, Turetsky A, Weissleder R. Bioorthogonal Small-Molecule Ligands for PARP1 Imaging in Living Cells. Chembiochem. 2010;11:2374–2377. doi: 10.1002/cbic.201000477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 165.Devaraj NK, Hilderbrand S, Upadhyay R, Mazitschek R, Weissleder R. Bioorthogonal Turn-On Probes for Imaging Small Molecules inside Living Cells. Angew Chem Int Ed Engl. 2010;49:2869–2872. doi: 10.1002/anie.200906120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 166.Karver MR, Weissleder R, Hilderbrand SA. Synthesis and Evaluation of a Series of 1,2,4,5-Tetrazines for Bioorthogonal Conjugation. Bioconjug Chem. 2011;22:2263–2270. doi: 10.1021/bc200295y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 167.Rossin R, Renart Verkerk P, van den Bosch SM, Vulders RCM, Verel I, Lub J, Robillard MS. In Vivo Chemistry for Pretargeted Tumor Imaging in Live Mice. Angew Chem Int Ed Engl. 2010;122:3447–3450. doi: 10.1002/anie.200906294. [DOI] [PubMed] [Google Scholar]
- 168.Devaraj NK, Thurber GM, Keliher EJ, Marinelli B, Weissleder R. Reactive polymer enables efficient in vivo bioorthogonal chemistry. Proc Natl Acad Sci U S A. 2012;109:4762–4767. doi: 10.1073/pnas.1113466109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 169.Rossin R, van den Bosch SM, ten Hoeve W, Carvelli M, Versteegen RM, Lub J, Robillard MS. Highly Reactive trans-Cyclooctene Tags with Improved Stability for Diels-Alder Chemistry in Living Systems. Bioconjug Chem. 2013;24:1210–1217. doi: 10.1021/bc400153y. [DOI] [PubMed] [Google Scholar]
- 170.Poloukhtine AA, Mbua NE, Wolfert MA, Boons GJ, Popik VV. Selective Labeling of Living Cells by a Photo-Triggered Click Reaction. J Am Chem Soc. 2009;131:15769–15776. doi: 10.1021/ja9054096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 171.Sletten EM, Bertozzi CR. A Hydrophilic Azacyclooctyne for Cu-Free Click Chemistry. Org Lett. 2008;10:3097–3099. doi: 10.1021/ol801141k. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 172.de Almeida G, Sletten EM, Nakamura H, Palaniappan KK, Bertozzi CR. Thiacycloalkynes for Copper-Free Click Chemistry. Angew Chem Int Ed Engl. 2012;51:2443–2447. doi: 10.1002/anie.201106325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 173.Kii I, Shiraishi A, Hiramatsu T, Matsushita T, Uekusa H, Yoshida S, Yamamoto M, Kudo A, Hagiwara M, Hosoya T. Strain-promoted double-click reaction for chemical modification of azido-biomolecules. Org Biomol Chem. 2010;8:4051–4055. doi: 10.1039/c0ob00003e. [DOI] [PubMed] [Google Scholar]
- 174.McGrath NA, Raines RT. Diazo compounds as highly tunable reactants in 1,3-dipolar cycloaddition reactions with cycloalkynes. Chem Sci. 2012;3:3237–3240. doi: 10.1039/C2SC20806G. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 175.Lang K, Davis L, Wallace S, Mahesh M, Cox DJ, Blackman ML, Fox JM, Chin JW. Genetic Encoding of Bicyclononynes and trans-Cyclooctenes for Site-Specific Protein Labeling in Vitro and in Live Mammalian Cells via Rapid Fluorogenic Diels-Alder Reactions. J Am Chem Soc. 2012;134:10317–10320. doi: 10.1021/ja302832g. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 176.Blackman ML, Royzen M, Fox JM. Tetrazine Ligation: Fast Bioconjugation Based on Inverse-Electron-Demand Diels-Alder Reactivity. J Am Chem Soc. 2008;130:13518–13519. doi: 10.1021/ja8053805. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 177.Taylor MT, Blackman ML, Dmitrenko O, Fox JM. Design and Synthesis of Highly Reactive Dienophiles for the Tetrazine–trans-Cyclooctene Ligation. J Am Chem Soc. 2011;133:9646–9649. doi: 10.1021/ja201844c. [DOI] [PMC free article] [PubMed] [Google Scholar]


