Abstract
The safety, pharmacokinetics, biodistribution and radiation dosimetry of 111In-DTPA-hEGF, an Auger electron-emitting radiopharmaceutical, were evaluated in a first-in-human trial. Dose escalation was performed in patients with EGFR-positive metastatic breast cancer who had received ≥2 prior courses of systemic treatment. 111In-DTPA-hEGF (0.25 mg) was administered once intravenously (i.v.). Blood was collected for biochemistry/hematology testing and pharmacokinetic and immunogenicity analyses at selected times post injection (p.i.). Whole body planar images were acquired at 1, 4-6, 24 and 72 h p.i. and SPECT images at 24 and/or 72 h p.i. Macrodosimetry (MIRD) for the whole body and organs was estimated using OLINDA. Correlative radiological imaging was obtained at baseline, 1 and 3 months and then 6 monthly. Toxicity was scored using Common Terminology Criteria for Adverse Events (CTCAE)v2.0. Sixteen patients, median age 47 yr (range, 35-59), received 111In-DTPA-hEGF as follows: 357-434 MBq (7), 754-805 MBq (3), 1,241-1,527 MBq (3) and 2,030-2,290 MBq (3). Fifteen were evaluable for toxicity. The commonest adverse events (AE) were flushing, chills, nausea, and vomiting occurring during or immediately p.i. One patient experienced Grade 3 thrombocytopenia (attributed to bone marrow infiltration by cancer). There were no other Grade 3 or 4 AEs. Maximum tolerated dose was not reached. Clear accumulation of radiopharmaceutical in at least one known site of disease was observed in 47% of patients. 111In-DTPA-hEGF was cleared biexponentially from the blood with α-phase T½ of 0.16 ± 0.03 h and β-phase T½ of 9.41 ± 1.93 h. 111In-DTPA-hEGF was not immunogenic. The mean radiation dose estimates in mGy/MBq for whole body, liver, kidneys, spleen and thyroid were 0.08, 0.86, 0.74, 0.37 and 0.30, respectively. No objective antitumor responses were observed at the doses studied. In summary, administered amounts of up to 2,290 MBq (0.25 mg) of 111In-DTPA-hEGF were well tolerated as a single i.v. injection.
Keywords: Auger electron, 111In-DTPA-hEGF, breast cancer, Phase I trial
Introduction
The epidermal growth factor receptor (EGFR) is a cell surface signaling glycoprotein that contains extracellular EGF-binding, transmembrane and cytoplasmic domains. It has tyrosine kinase activity and undergoes autophosphorylation following ligand binding. Upregulation of EGFR has been demonstrated in many human cancers including breast carcinoma [1]. Since overexpression of EGFR plays a role in proliferation and is associated with poor prognosis, it is an attractive therapeutic target [2]. Interventions exploiting overexpression of EGFR include antibodies that block ligand-binding and tyrosine kinase inhibitors that interfere with receptor autophosphorylation and mitogenic signaling [3]. These EGFR-targeted agents are of limited efficacy in breast cancer although their role in triple negative breast cancer, in which EGFR is frequently overexpressed, is being evaluated [1]. We investigated a strategy for EGFR-targeting which, unlike small molecule and antibody inhibitors, does not rely on inhibition of receptor activation, but uses EGF conjugated to 111In (111In-DTPA-hEGF) to exploit the EGFR as a means of transporting radioactivity into cancer cells [4]. 111In emits nanometer-range Auger and micrometer-range conversion electrons, which are densely ionizing, making it an attractive alternative to beta-emitting isotopes for targeted radiotherapy. On binding the EGFR, 111In-DTPA-hEGF is internalized and delivered to the cell nucleus where it leads to DNA damage and cell death. Normal cells, with modest or absent EGFR expression, do not internalize or internalize less 111In-DTPA-hEGF and are therefore relatively resistant to its cytotoxic effect [5]. The use of EGF labeled with 131I to target tumors was reported previously [6]. However, efficient internalization of radioiodinated peptides results in loss of radiolabel from tumor cells due to deiodination. In contrast, intracellular retention of 111In is prolonged, a significant advantage of this radionuclide for imaging and Auger electron radiotherapy [7].
111In-DTPA-hEGF was cytotoxic in MDA-MB-468 human breast cancer cells that overexpress EGFR in vitro and inhibited the growth of MDA-MB-468 tumor xenografts implanted subcutaneously (s.c.) into athymic mice [5,8]. Mice received a total of 27.7, 55.5 or 92.5 MBq 111In-DTPA-hEGF split over 5 weekly doses. Significant anti-tumor effects were observed even in the lowest dose group at 7 weeks after treatment [8].
To assess the toxicity of 111In-DTPA-hEGF, female BALB/c mice received 111In-DTPA-hEGF (44.4 MBq; equivalent to 8.5 GBq in humans) by intravenous (i.v.) injection [8]. After 15 days no significant changes, relative to controls, were observed in hematological parameters (RBC, WBC, platelet counts and hemoglobin), biochemical parameters (plasma alanine transaminase [ALT] and creatinine [Cr]) or in body weight. Histopathological assessment of 19 tissues revealed no significant abnormalities. To assess the toxicity of repeat dosing athymic mice bearing MDA-MB-468 xenografts received five injections of 111In-DTPA-hEGF (18.5 MBq) at weekly intervals. No significant differences in ALT or Cr and no pathological changes in the liver, kidneys or heart were observed 7 weeks after treatment. There was a small decrease in WBC and platelet counts in treated mice but these remained within the normal range.
111In-DTPA-hEGF was formulated for human administration [9]. Patients with EGFR-positive metastatic breast cancer received 111In-DTPA-hEGF i.v. on a single occasion. The administered amount was escalated in groups of 3-7 patients. The aims of the trial were to investigate the pharmacokinetics, toxicity profile and biodistribution of 111In-DTPA-hEGF in this first-in-human study.
Materials and methods
Study participants and design
Study participants attending the Princess Margaret Hospital and Odette Cancer Centre, Toronto, were recruited after giving written informed consent. Separate consent was obtained for pre-screening of archived tumor samples for EGFR expression by immunohistochemistry. Study participants aged 18-80 years with Eastern Cooperative Oncology Group (ECOG) performance status of 0-2 were eligible. Histologic diagnosis of invasive breast carcinoma with positive immunostaining for EGFR and radiologically assessable disease were required. Serum bilirubin, Cr, leukocyte and platelet counts and hemoglobin were required to be normal. Alkaline phosphotase (ALP) and hepatic transaminases were required to be less than twice the upper limit of normal. Study participants had received at least two chemotherapy/hormonal regimens for metastatic disease and were excluded if they had received chemotherapy up to four weeks previously. This study was approved by the Research Ethics Board at University Health Network (UHN), Toronto (reference: 02-0788-C) and received Clinical Trial Application (CTA) approval by Health Canada (CTA number 085648).
An up-and-down dose-finding design, with study participants treated in cohorts of at least three, was used. Each participant received a single administration of 111In-DTPA-hEGF. The radioactivity levels administered were 370, 740, 1,480 and 2,220 MBq (6.2-37 MBq/kg, assuming an average female weight of 60 kg). The starting level of 111In (370 MBq) was selected as it is close to the maximum dose of 111In-pentetreotide used for imaging neuroendocrine tumors (300 MBq). This amount of 111In was therefore known to be safe. The highest amount of radioactivity (2,220 MBq, 37.0 MBq/kg) corresponded to the equivalent maximum dose of radioactivity safely administered by i.v. injection to female white New Zealand rabbits (i.e. 42.5 MBq/kg) in pre-clinical toxicology studies [4]. The mass amount of hEGF that was administered was fixed at 0.25 mg per administration. These dose limits were restricted by the Health Canada approval of the CTA since 111In-DTPA-hEGF had not previously been administered to humans.
Vital signs were recorded at baseline and at 5, 15 and 30 min and 1, 2, 4-6, 24, 48 and 72 h post-injection (p.i.). Hematological and biochemical analyses were performed at baseline, at 1 week and 1, 2, 3 and 6 months and then 6 monthly p.i. Adverse events (AE) were recorded at 10 min, 1 h, 7 days and 1, 2 and 3 months p.i. and every 3-6 months thereafter. Toxicity was graded using the Common Toxicity Criteria for AEs version 2.0 (CTCAEv2.0; National Cancer Institute, Bethesda, MD) [10]. Dose limiting toxicity (DLT) was defined as Grade 3 or 4 hematologic or non-hematologic toxicity. Blood was collected at baseline, 5, 15, 30 minutes and 1, 2, 4-6, 24, 48 and 72 h p.i. for pharmacokinetic analysis. Whole-body planar images were acquired at 1, 4-6, 24 and 72 h p.i. and SPECT images at 24 h and in some cases at 72 h. Macrodosimetry (Medical Internal Radiation Dose [MIRD]) estimates were calculated for the whole body and selected organs using the Organ Level INternal Dose Assessment/EXponential Modeling code (OLINDA/EXM) [11]. CT scans were performed within 4 weeks before treatment and at 4 and 12 weeks p.i. and 6 monthly thereafter for Response Evaluation Criteria In Solid Tumors (RECIST) assessment [12].
Tumor EGFR expression
Archived primary tumors of potential study subjects were screened for EGFR-positivity. Histologic sections (5 μm) were prepared from formalin-fixed, paraffin-embedded tumor blocks. Immunohistochemical staining was performed using anti-EGFR monoclonal antibody (Clone 31G7, Zymed Laboratories) at 1:100 dilution. All slides were reviewed by a pathologist (N.M). A tumor was considered EGFR-positive if staining of any intensity of cytoplasm or cell membrane was observed in invasive carcinoma [13].
111In-DTPA-hEGF injection
111In-DTPA-hEGF was prepared from a kit manufactured at the University Health Network radiopharmacy [9]. Each kit contained DTPA-hEGF (0.25 mg) in 1.0 mL sodium acetate buffer (1 M, pH 6.0). 111In-DTPA-hEGF was prepared by adding 370-2,220 MBq of 111In chloride (MDS-Nordion, Kanata, ON) and incubating at room temperature for 30 min. The final radiopharmaceutical was obtained by diluting 111In-DTPA-hEGF with Sodium Chloride Injection USP and filtration through a 0.22 μm Millex-GV (EMD Millipore, Billerica, MA) filter. Radiochemical purity was assayed using instant thin layer-silica gel (ITLC-SG) chromatography as previously described [9] and >90% was required for clinical use.
Imaging and radiation dosimetry
Imaging was performed using a dual-detector gamma camera fitted with medium energy, general purpose collimators (Cobra II® Series Auto-Gamma System Model 5003, Packard Instrument Company, Meriden, CT, USA). Two 20% windows, to include the gamma photon peaks of 111In (173 and 247 keV) were used. Whole-body planar images were acquired at 1, 4-6, 24 and 72 h p.i. SPECT images were acquired to evaluate radiopharmaceutical uptake at known sites of disease. SPECT was performed at 24 and 72 h for the first 5 patients to determine the optimal time point for imaging and performed for other study participants at 24 h only. Interpretation of 111In-DTPA-hEGF scintigraphy was performed in the presence of CT images (showing sites of suspected tumors). 111In-DTPA-hEGF scintigraphy was recorded as positive by study radiologists (M.F., I.C.) when radiopharmaceutical uptake was greater in at least one metastatic site (based on correlative CT scans) compared to the background for the corresponding region.
Radioactivity in the syringe containing 111In-DTPA-hEGF was measured in a dose calibrator (Capintec CRC25R, Ramsay, NJ) and, following injection, the syringe was re-measured. The difference between the two measurements was the administered amount of 111In. Conjugate view whole-body images were analyzed to obtain the counts in regions-of-interest (ROIs) defining normal organs. The geometric mean counts per minute in the ROIs from anterior and posterior views were calculated and corrected for tissue attenuation using an attenuation map. The program used to extract the volume and count data was ADAC PegasysTMX, Version 4.2 (Philips Healthcare, Best, Netherlands). The attenuation map was calculated by obtaining an anterior image of the patient with a homogeneous 57Co flood source (370 MBq) suspended beneath the couch. Attenuation for the 57Co flood was converted to the expected attenuation for 111In using a previously developed calibration curve. The ratio of counts obtained in each pixel with and without the patient on the bed was used to derive tissue attenuation correction factors. Pixel counts, once corrected for attenuation and scatter, were converted to radioactivity values (kBq) using a conversion factor based on imaging a point source of 111In. The radioactivity (kBq) in organs at each imaging time point was plotted versus time p.i. using Prism® Version 4.0 software (Graphpad, San Diego, CA). The area under the curve (AUC) from time zero to the final time point (kBq × h) was calculated. The mean residence time for 111In (τ, h) in each organ was calculated by dividing the total AUC (kBq × h) by the injected radioactivity (kBq). Radiation dosimetry estimates were calculated using the OLINDA/EXM program.
Pharmacokinetics
Blood was collected at baseline, 5, 15, 30 minutes and 1, 2, 4-6, 24, 48 and 72 h p.i. A 1.0 mL sample of whole blood and plasma at each time was dispensed in duplicate into gamma counting tubes and the contained radioactivity, along with a standard of the radiopharmaceutical, was measured in a gamma scintillation counter (Cobra II® Series Auto-Gamma® Counting System, Packard Instrument Co., Meriden, CT, USA). Counts were converted to kBq/mL and blood or plasma radioactivity plotted versus time using Prism® Version 4.0. The curve was fitted to a one- or two-compartment pharmacokinetic model by Scientist Ver. 2.0 (Micromath, St. Louis, MO). Pharmacokinetic parameters (t½α, t½β, V1, Vss, CL) were calculated. For participants receiving the highest administered amount (2,220 MBq), samples at 24, 48 and 72 h p.i. only were available. A 24-h urine collection starting immediately p.i. was obtained. The percentage of the injected radioactivity eliminated in the urine in 24 h was calculated.
Immunogenicity
Testing for anti-DTPA-hEGF antibodies was performed by ELISA on serum samples obtained before administration of 111In-DTPA-hEGF and at 2, 4 and 12 weeks p.i. Wells of a microplate were coated for 1 h at 37°C with 50 μL of DTPA-hEGF (10 μg/mL in 50 mM sodium bicarbonate buffer, pH 7.5) and rinsed with 0.5% (v/v) Tween-80 in Phosphate-Buffered Saline, pH 7.4 (Tween-80/PBS). Serum samples (200 μL) (diluted 1:100 to 1:10,000 in Tween-80/PBS) were added. Following incubation for 1 h at 37°C, wells were rinsed with Tween-80/PBS, then incubated for 1 h at 37°C with 200 μL of goat anti-human IgG (Fc-specific) antibodies conjugated to horseradish peroxidase (1:15,000 dilution). Following rinsing with Tween-80/PBS, 200 μL of o-phenylenediamine dihydrochloride solution and H2O2 were added and allowed to react for 30 mins. The color produced was measured in a ELx800 multi-well plate reader (Bio Tek Instruments Inc., Winooski, VT, USA) at 450 nm with subtraction of background at 690 nm (Abs450-690).
Statistical analysis
Descriptive statistics were used to summarize patient characteristics, immunogenicity, dosimetry and biodistribution. All reported P-values were 2-sided and P≤0.05 was considered significant. SASv9.1 software (SAS Institute, Cary, NC) was used.
Results
Study participants
Sixteen women (median age 47 yr, range 35-59) with metastatic EGFR-positive breast cancer received 111In-DTPA-hEGF (Table 1). All had received between 2 and 10 prior regimens of systemic treatment for metastatic disease (median, 4.4). The number of study participants that had previously received a regimen containing an anthracycline, taxol or trastuzumab (Herceptin) was 15, 12 and 10, respectively. No patient received prior EGFR-targeted therapy. ECOG performance status at trial entry was 0, 1 or 2 in 9, 5 and 2 patients, respectively. Seven patients were recruited to the first cohort and three patients to each subsequent cohort.
Table 1.
Patients
| Patient No. | Age (yr) | No. of prior courses of systemic therapy | Known sites of metastases§ | Dose of 111In-DTPA-hEGF (MBq)‡ | |||
|---|---|---|---|---|---|---|---|
|
|
|||||||
| Chemotherapy | Hormone therapy | ER* | PR† | ||||
| 1 | 44 | 4 | 0 | N | N | Li, Lu, B, Br | 408 |
| 2 | 42 | 9 | 1 | P | P | Li, Lu, B | 382 |
| 3 | 51 | 4 | 0 | N | N | CW, LN | 406 |
| 4 | 44 | 6 | 0 | N | N | B | 406 |
| 5 | 50 | 5 | 0 | N | N | Lu | 434 |
| 6 | 59 | 6 | 3 | P | UK | Li, Lu, B | 378 |
| 7 | 51 | 3 | 5 | P | P | B | 357 |
| 8 | 58 | 4 | 0 | N | N | LN | 805 |
| 9 | 48 | 4 | 4 | N | P | B, LN | 790 |
| 10 | 46 | 6 | 2 | P | P | SC | 754 |
| 11 | 52 | 3 | 1 | N | N | Lu, LN | 1,527 |
| 12 | 56 | 4 | 0 | N | N | Lu, B | 1,408 |
| 13 | 43 | 2 | 0 | N | N | SC | 1,241 |
| 14 | 35 | 3 | 0 | N | N | Lu, B | 2,030 |
| 15 | 46 | 6 | 1 | N | N | Sk, LN, Lu | 2,290 |
| 16 | 46 | 2 | 1 | N | P | Lu, SC | 2,195 |
ER, Estrogen receptor status of primary tumour.
PR, Progesterone receptor status of primary tumour.
N, negative; P, positive; UK, unknown.
Li, liver; Lu, lung; B, bone; Br, brain; CW, chest wall, LN, lymph nodes; SC, subcutaneous; Sk, skin.
The mass of DTPA-hEGF was 0.25 mg for all radioactivity doses.
Toxicity and antitumor activity
One study participant in the first cohort died within 6 weeks of treatment with 111In-DTPA-hEGF from progressive disease (PD) and was unevaluable for toxicity. An additional patient was enrolled. Another member of the first cohort developed Grade 3 thrombocytopenia. She had extensive bone marrow involvement by cancer and thrombocytopenia was thought unlikely to be related to 111In-DTPA-hEGF (CTCAEv2.0 attribution standards) [10]. However, this event was classed as a DLT and the first cohort was expanded by 3 subjects. A summary of AEs is shown (Table 2). The commonest AEs were flushing, chills, nausea and vomiting that occurred transitorily during and after 111In-DTPA-hEGF administration. No non-hematological Grade 3 or 4 AEs occurred. Two patients experienced Grade 2 hypotension. Hepatic transaminases were elevated (Grade 1) in 2 patients at up to 180 days p.i. This hepatic dysfunction was observed at the time of PD. Serum Cr was normal for all patients throughout the trial. One patient experienced Grade 1 anemia. None experienced a fall in neutrophil count below the normal range. There was no clear relationship between the severity of toxicity and the amount of radioactivity administered. For example, the patients who experienced Grade 2 hypotension were members of the first and second cohorts. There were 4 episodes of Grade 2 vomiting and these were experienced by one patient from each of the cohorts.
Table 2.
Adverse Events and Laboratory Toxicities of All Grades*
| Number of patients | ||||
|---|---|---|---|---|
|
|
||||
| Toxicity | Grade 1 | Grade 2 | All | |
|
| ||||
| No | % | |||
| Elevated AST or ALT† | 2 | − | 2 | 13 |
| Anemia | 1 | − | 1 | 7 |
| Hypotension | 1 | 2 | 3 | 20 |
| Nausea | 6 | 6 | 12 | 80 |
| Vomiting | 4 | 4 | 8 | 53 |
| Heartburn | 1 | − | 1 | 7 |
| Flatulence | − | 1 | 1 | 7 |
| Dysphagia | 1 | − | 1 | 7 |
| Dry Mouth | 4 | 4 | 8 | 53 |
| Taste disturbance | 5 | − | 5 | 33 |
| Flushing | 14 | − | 14 | 93 |
| Chills | 8 | 2 | 10 | 67 |
| Fatigue | 6 | 1 | 7 | 47 |
| Diaphoresis | 1 | − | 1 | 7 |
| Fever | − | 1 | 1 | 7 |
| Headache | 4 | 3 | 7 | 47 |
| Pain | 6 | 1 | 7 | 47 |
| Dry eye | − | 2 | 2 | 13 |
| Pruritus | 3 | − | 3 | 20 |
| Paraesthesia | 5 | − | 5 | 33 |
| Tremor | − | 1 | 1 | 7 |
| Dizziness | 5 | 3 | 8 | 47 |
| Vision-flashing | 1 | − | 1 | 7 |
This table includes adverse events that were considered to be ‘possibly’, ‘probably’ or ‘definitely’ related to 111In-DTPA-hEGF (according to NCI-CTCv2 attribution standards).
Common Terminology Criteria for Adverse Events (CTCAE) v2 was used to score toxicity [10].
AST or ALT: Aspartate aminotransferase or Alanine aminotransferase.
Stable disease (SD) was observed in 4 (27%) patients (two from dose level one and one each from dose levels 2 and 3). The mean duration of SD was 5.8 months. No objective responses (i.e. complete [CR] or partial response [PR]) were observed in 15 evaluable patients determined using RECIST criteria. Tumor response was a secondary aim in this first-in-human study of 111In-DTPA-hEGF. The primary aims of the study were to determine the pharmacokinetics, toxicity profile and biodistribution of 111In-DTPA-hEGF.
Imaging, radiation dosimetry, pharmacokinetics and immunogenicity
There was accumulation of radioactivity in at least one known site of disease in either the whole-body planar or SPECT images in 7 of 15 evaluable patients (47%) and slight uptake in at least one known site of disease in a further 3 patients (20%). 111In-DTPA-hEGF positive lesions were observed in lung, bone, subcutaneous/ skin and lymph node metastases. Sensitivity for liver metastases was poor, possibly because of high hepatic background, particularly at early time points. The number of study participants with clearly positive scans per number of evaluable patients in cohorts 1, 2, 3, and 4 were 3/6, 0/3, 2/3 and 2/3, respectively. Thus, there was no clear relationship between the amount of injected radioactivity and visualization of tumor uptake of 111In-DTPA-hEGF. Representative SPECT images showing tumor accumulation, with correlative CT images are shown (Figures 1 and 2).
Figure 1.
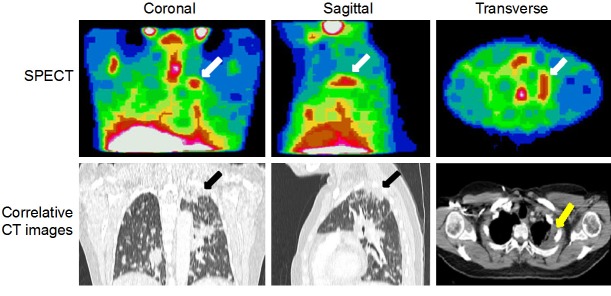
Representative images from Patient 11 showing tumor accumulation of 111In-DTPA-hEGF with correlative CT images. Coronal, sagittal and transverse SPECT images at 24 h p.i. A tumor deposit present in the left lung apex (arrows) shows 111In uptake.
Figure 2.
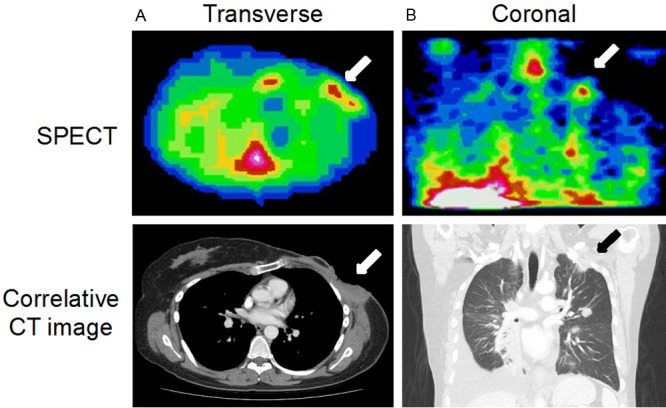
Representative images from two patients showing tumor accumulation of 111In-DTPA-hEGF with correlative CT images. A: Transverse SPECT image (Patient 13) acquired 24 h p.i. showing accumulation of 111In in an area of chest wall disease (arrow). B: Shows a coronal SPECT image (Patient 15), acquired 24 h p.i. demonstrating accumulation of radioactivity in a left infraclavicular fossa and lung apex tumor deposit.
Normal tissue accumulation of 111In-DTPA-hEGF was mainly in the liver (30-40%), kidneys (10-15%), spleen (1.5-2%) and thyroid (<1%). Mean organ dose estimates are shown in Table 3. For the highest radioactivity level administered (2,200 MBq) the radiation absorbed doses for whole body, liver, kidneys, spleen and thyroid were 0.18, 1.91, 1.62, 0.82, 0.67 Gy, respectively. 111In-DTPA-hEGF was cleared biexponentially from the blood with an α-phase T½ of 0.16 ± 0.03 h and β-phase T½ of 9.41 ± 1.93 h. A representative elimination curve is shown (Figure 3). Pharmacokinetic parameters for 111In-DTPA-hEGF are shown in Table 4. The 24 h urinary excretion of radioactivity was 16.05 ± 2.45% (mean ± SEM). Although the risk of antibody formation against human EGF was predicted to be low, it was considered prudent to test for it, as the addition of a chelator (DTPA) could alter immunogenicity. However, there was no evidence of immunogenicity since the logarithm of the titer for binding of serum samples to DTPA-hEGF in a microELISA plate and detected with goat anti-human IgG secondary antibodies did not increase (up to 3 months p.i.). These values did not significantly differ from unity and were 0.97 ± 0.08, 1.08 ± 0.12 and 1.09 ± 0.13 for <1, 1 and 3 months p.i.
Table 3.
Mean Organ Radiation Absorbed Dose Estimates
| Organ | Mean Dose ± SD (mGy/MBq) |
|---|---|
| Adrenals | 0.20 ± 0.01 |
| Brain | 0.02 ± <0.01 |
| Breasts | 0.04 ± <0.01 |
| Gallbladder | 0.27 ± 0.02 |
| Heart | 0.10 ± 0.01 |
| Kidneys | 0.74 ± 0.14 |
| Liver | 0.86 ± 0.10 |
| Lower large intestine | 0.05 ± 0.01 |
| Lungs | 0.09 ± 0.01 |
| Muscle | 0.05 ± <0.01 |
| Bone | 0.10 ± 0.01 |
| Ovaries | 0.05 ± <0.01 |
| Pancreas | 0.18 ± 0.01 |
| Bone marrow | 0.06 ± <0.01 |
| Skin | 0.03 ± <0.01 |
| Small intestine | 0.08 ± 0.01 |
| Spleen | 0.37 ± 0.10 |
| Stomach | 0.10 ± 0.01 |
| Thymus | 0.05 ± <0.01 |
| Thyroid | 0.30 ± 0.15 |
| Upper large intestine | 0.10 ± 0.01 |
| Urinary bladder | 0.17 ± 0.06 |
| Uterus | 0.06 ± 0.01 |
| Total body | 0.08 ± 0.01 |
Figure 3.
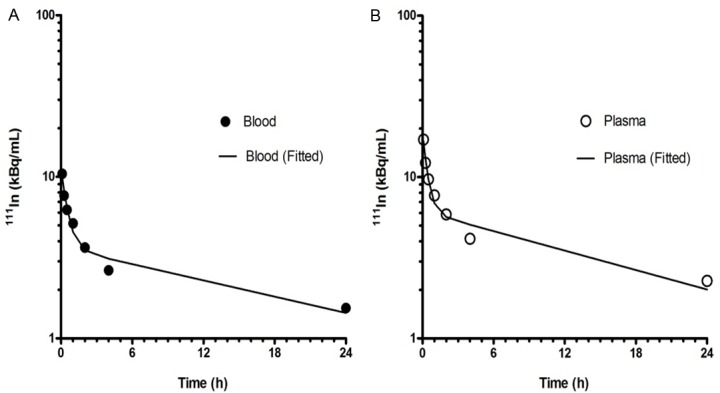
Curves showing representative biphasic elimination of radioactivity from (A) blood and (B) plasma following administration of 111In-DTPA-hEGF (Patient 8). In this patient the T½α and T½β in the blood were 0.35 and 17.9 h, respectively while the T½α and T½β in the plasma were 0.28 and 14.9 h, respectively.
Table 4.
Pharmacokinetic Parameters for Elimination of 111In-DTPA-hEGF
| Blood | Plasma | |
|---|---|---|
|
|
||
| Parameter | Mean ± SEM | |
| V1 (L/kg) | 0.46 ± 0.11 | 0.49 ± 0.12 |
| Vss (L/kg) | 2.64 ± 0.73 | 5.68 ± 3.81 |
| CL (L/h/kg) | 0.27 ± 0.06 | 0.21 ± 0.06 |
| T½α (h) | 0.16 ± 0.03 | 0.16 ± 0.03 |
| T½β (h) | 9.41 ± 1.93 | 12.19 ± 3.70 |
| k21 (h-1) | 0.90 ± 0.16 | 1.77 ± 0.44 |
| k10 (h-1) | 1.91 ± 0.62 | 3.06 ± 1.05 |
| k12 (h-1) | 4.62 ± 1.24 | 6.04 ± 2.11 |
V1: volume of distribution; Vss: volume of distribution at steady-state; CL: systemic clearance; T½α: alpha-phase half-life; T½β: beta phase half-life; k21: microconstant for transfer from compartment 2 to 1; k10: microconstant for elimination from compartment 1; k12: microconstant for transfer from compartment 1 to 2.
Discussion
There is growing recognition that molecularly-targeted radiopharmaceuticals that incorporate Auger electron-emitting radionuclides can provide a precise means of delivering lethal radiation doses to cancer cells. However, to be effective, Auger electron-emitters should accumulate specifically in the nucleus [14]. Strategies for achieving this include linkage of cell-penetrating peptides, nuclear localizing sequences (NLS) or both [15], to radiolabeled carrier molecules in order to enhance nuclear uptake or the use of radiolabeled triplex-forming oligonucleotides that deliver radiation to specific genes [16]. Another approach is to take advantage of a peptide ligand that accumulates in the nucleus. This is the principle behind the design of 111In-DTPA-hEGF, which binds the EGFR. The receptor-ligand complex, through a NLS in the juxtamembrane region of EGFR, translocates to the nucleus, coming into close proximity with DNA [5].
Although Auger electron radiation represents an attractive alternative to higher energy β-particle therapy, this approach has only been tested in a small number of clinical studies to date. 111In-DTPA-octreotide (111In-pentetreotide) was tested in a Phase I trial of 50 patients with neuroendocrine tumors who received 20-160 GBq [17]. Of 40 evaluable patients, 20 experienced SD or minor response (25-50% tumor volume reduction). Bone marrow toxicity was common and usually mild although 3 patients, who received >100 GBq, developed myelodysplastic syndrome or leukemia. In another trial, 26 patients with somatostatin receptor-positive malignancies received two doses of 111In-pentetreotide of 6.6 GBq each [18]; PR was observed in two patients. These results indicate that objective tumor responses are unusual with 111In-labeled somatosatin analogues. This may be because the uptake of 111In-DTPA-octreotide into cell nuclei appears to be modest [19]. Auger electron-emitting radiopharmaceuticals such as 111In-DTPA-hEGF, that are specifically designed to accumulate in the nucleus, have the potential to be effective due to the 30-fold higher radiation absorbed dose delivered to the nucleus from 111In in the nucleus compared to the cytoplasm [20]. In a case report of a patient with advanced pancreatic cancer with meningeal metastases a combination of intrathecal 5-iodo-2’-deoxuyridine (125IUdR) plus methotrexate resulted in a fall in CA 19-9 and clinical improvement [21]. Thus the current report of the first-in-human trial of 111In-DTPA-hEGF contributes new knowledge to an under-researched area, particularly the feasibility of targeted Auger electron therapy of tumors in patients.
In pre-clinical studies, mice received a total of 27.7, 55.5 or 92.5 MBq 111In-DTPA-hEGF divided over 5 weekly doses. Significant anti-tumor effects were observed at 7 weeks post-treatment even in the lowest dose group [8]. These preclinical studies established proof-of-principle for the treatment of EGFR-amplified tumors with 111In-DTPA-hEGF. As the current study was a first-in-human trial of an investigational agent, the lowest dose of 111In-DTPA-hEGF selected (370 MBq) was guided by the clinical experience with 111In-pentetreotide used for diagnostic imaging which is known to be safe. The highest dose administered was selected on the basis that this fell within in the range safely administered to rabbits and mice in pre-clinical toxicology studies [4]. In addition, this dose range was restricted by Health Canada approval of the CTA.
Consistent with pre-clinical toxicology studies, 111In-DTPA-hEGF did not cause perturbation of hematological, renal or hepatic function [4]. Myelosuppression is often a DLT of β-emitting radiopharmaceuticals [22]. In contrast the estimated radiation absorbed dose to the bone marrow after 111In-DTPA-hEGF was low (0.06 mGy/MBq) and no bone marrow toxicity was observed. One possible explanation for this is the low EGFR expression of haemopoietic lineages, which would limit internalization and cytotoxicity of 111In-DTPA-hEGF [23]. EGFR is expressed to moderate levels in renal and hepatic cells but no adverse effect of 111In-DTPA-hEGF on these organs was observed [24]. The mean radiation absorbed dose to kidneys and liver was 0.74 and 0.86 mGy/MBq, respectively. Thus, following the highest administered dose of 111In-DTPA-hEGF (2,200 MBq), the radiation absorbed doses to the kidneys and liver were 1.62 and 1.91 Gy, respectively. Doses of 23 Gy to the kidney and 30 Gy to the liver delivered using external beam radiation therapy would be expected to cause significant toxicity in approximately 5% of individuals [25]. The radiation absorbed dose to these organs following treatment with 111In-DTPA-hEGF, therefore suggests dose escalation would be possible. However, the organ and whole body doses (Table 3) were calculated using a macrodosimetry method and do not account for cellular and subcellular distribution of the radionuclide which could result in dose heterogeneities, making the probability of toxicity difficult to predict [26]. The lack of renal toxicity observed in patients who received 111In-DTPA-hEGF is in contrast to several other radiopeptides, such as 90Y- or 177Lu-DOTATOC [27]. Radiation toxicity depends on microdosimetry and particularly the radiation dose deposited in the glomeruli which are radiosensitive. 111In-pentetreotide resulted in heterogeneous dose distribution with low dose deposition in the glomeruli [26]. Even following high-activity 111In-pentetreotide therapy (with cumulative administered amounts as high as 36.6 Gy), renal toxicity was not observed [28]. It is possible that 111In-DTPA-hEGF has a similar dose distribution accounting for its lack of renal toxicity.
The amount of DTPA-hEGF administered was 0.25 mg (approximately 5 μg/kg) and was much lower than the equivalent maximum amount administered to mice (1,500 mg/kg) in toxicology studies [4]. An observed side-effect following 111In-DTPA-hEGF was transitory flushing and 2 patients experienced Grade 2 hypotension. Hypotension was observed previously in primates receiving EGF, suggesting that the vasodilatory effect of 111In-DTPA-hEGF is due to the EGF moiety. For example, monkeys given 3 or 300 μg/kg EGF as a 20 min infusion experienced a fall in blood pressure of up to 20% and 32%, respectively [29]. In humans 131I -labeled EGF (0.3 mg) was administered to patients with lung cancer [6]. Some patients, who received up to 2.7 mg of co-infused non-labeled EGF (giving a total EGF dose of 3 mg), experienced AEs similar to those observed with 111In-DTPA-hEGF: nausea, vomiting and chills. The dose of EGF used in the current trial was selected to allow consistently high radiolabeling efficiency (>90%) and to avoid the need for post-labeling purification at high amounts of 111In. 111In-DTPA-hEGF exhibited biexponential clearance, with α-phase T½ of 0.16 ± 0.03 h and β-phase T½ of 9.41 ± 1.93 h (Table 4). This is consistent with results for 131I -labeled-EGF in lung cancer patients, in whom α-phase T1/2 was 0.84 ± 0.40 h and the β-phase T1/2 was 15.2 ± 1.4 h following 0.3 mg EGF [6].
The moderately high expression level of EGFR in hepatocytes could result in ligand accumulation in the liver, limiting the amount available for tumor uptake. Others have sought to overcome liver uptake of radiolabeled anti-EGFR antibodies by increasing the mass dose [30]. However this strategy was not feasible in the current study due to the vasodilatory effect of EGF noted at a mass dose of 0.25 mg. Thus the biological activity of native EGF is a limitation. However modified forms of the peptide that have EGFR blocking activity have recently been reported [31]. It is possible that these will avoid some adverse biological effects of the native peptide and be useful for targeted radiotherapy.
Antitumor response was not a defined endpoint in this Phase I trial. However, tumor size was evaluated using RECIST. There were no objective responses. The lack of objective response to 111In-DTPA-hEGF could be due to low or heterogenous expression of EGFR in the tumors of some study participants, as it has been shown that 111In-DTPA-hEGF is only cytotoxic in cells with ≥1.3 × 105 EGFR [32]. In many studies of EGFR immunohistochemistry, tumors are regarded as positive if any level of staining is observed [13]. This approach was taken in the current study, so patients whose tumors exhibited only modest EGFR expression were eligible. It is possible that in cases with low or heterogenous EGFR expression, there was insufficient accumulation of 111In-DTPA-hEGF to produce an antitumor effect. In the future it would be prudent to select for 111In-DTPA-hEGF treatment only patients whose tumors are highly EGFR-amplified. Concordance between EGFR in primary breast cancers and their metastases has been reported [33]. Expression of EGFR has been observed to be higher in metastases compared to corresponding primary tumors more frequently than the reverse [34]. Using the EGFR protein expression level of the primary tumor as the basis for selection for treatment with 111In-DTPA-hEGF therefore seems reasonable based on current evidence.
Maximum tolerated dose (MTD) was not reached in this trial. To increase the administered amount of radioactivity above 2,200 MBq would have necessitated increasing the mass amount of hEGF above 0.25 mg. This was considered undesirable because flushing, nausea/vomiting and hypotension were thought to be attributable to EGF. However, imaging showed that tumor localization was achieved and since there were no serious adverse effects, it is reasonable to consider administering higher doses of radioactivity through multiple administrations of doses that were found to be safe. Therefore, a decision was made to stop this trial and plan to increase the administered amount of 111In-DTPA-hEGF, through repeat dosing, in a future trial. Anti-DTPA-hEGF antibodies were not detected, therefore evaluation of 111In-DTPA-hEGF in a multi-dose schedule is unlikely to result in an immunological response and should be feasible.
Conclusion
111In-DTPA-hEGF is safe when given as a single dose up to 2,300 MBq (0.25 mg). It is cleared bioexponentially from the blood and excreted predominantly in the urine. Normal organs which accumulate 111In-DTPA-hEGF include the liver and kidneys, but mean radiation absorbed dose estimates were within accepted tolerance. 111In-DTPA-hEGF localized in EGFR-positive lesions in many patients even when a broad definition of EGFR positivity was used. There was no evidence of significant treatment-associated toxicity at the doses studied during median follow-up of 8.3 months. The safety profile, favourable radiation dosimetric data and lack of immunogenicity indicate that further dose escalation of 111In-DTPA-hEGF is feasible.
Acknowledgements
This research was supported by the Susan G. Komen Breast Cancer Foundation (Grant BCTR0100840) and Cancer Research-UK. The authors thank colleagues at the Princess Margaret Hospital and Odette Cancer Centre, Toronto, who assisted with recruitment to this trial, the patients who participated in the trial and Amit Oza for advice regarding trial design and conduct.
Disclosure of conflict of interest
The authors declare that they have no conflicts of interest.
References
- 1.Dawson SJ, Provenzano E, Caldas C. Triple negative breast cancers: clinical and prognostic implications. Eur J Cancer. 2009;45:27–40. doi: 10.1016/S0959-8049(09)70013-9. [DOI] [PubMed] [Google Scholar]
- 2.Hynes NE, MacDonald G. ErbB receptors and signaling pathways in cancer. Curr Opin Cell Biol. 2009;21:177–184. doi: 10.1016/j.ceb.2008.12.010. [DOI] [PubMed] [Google Scholar]
- 3.Klein S, Levitzki A. Targeting the EGFR and the PKB pathway in cancer. Curr Opin Cell Biol. 2009;21:185–193. doi: 10.1016/j.ceb.2008.12.006. [DOI] [PubMed] [Google Scholar]
- 4.Chen P, Cameron R, Wang J, Vallis KA, Reilly RM. Antitumor effects and normal tissue toxicity of 111In-labeledepidermal growth factor administered to athymic mice bearing epidermal growth factor receptor-positive human breast cancer xenografts. J Nucl Med. 2003;44:1469–1478. [PubMed] [Google Scholar]
- 5.Reilly RM, Kiarash R, Cameron RG, Porlier N, Sandhu J, Hill RP, Vallis K, Hendler A, Gariepy J. 111In-labeledEGF is selectively radiotoxic to human breast cancer cells overexpressing EGFR. J Nucl Med. 2000;41:429–438. [PubMed] [Google Scholar]
- 6.Cuartero-Plaza A, Martinez-Miralles E, Rosell R, Vadell-Nadal C, Farre M, Real FX. Radiolocalization of squamous lung carcinoma with 131I-labeled epidermal growth factor. Clin Cancer Res. 1996;2:13–20. [PubMed] [Google Scholar]
- 7.Duncan JR, Welch MJ. Intracellular metabolism of indium-111-DTPA-labeled receptor targeted proteins. J Nucl Med. 1993;34:1728–1738. [PubMed] [Google Scholar]
- 8.Reilly RM, Chen P, Wang J, Scollard D, Cameron R, Vallis KA. Preclinical pharmacokinetic, biodistribution, toxicology, and dosimetry studies of 111In-DTPA-human epidermal growth factor: an Auger electron-emitting radiotherapeutic agent for epidermal growth factor receptor-positive breast cancer. J Nucl Med. 2006;47:1023–1031. [PubMed] [Google Scholar]
- 9.Reilly RM, Scollard DA, Wang J, Mondal H, Chen P, Henderson LA, Bowen BM, Vallis KA. A kit formulated under good manufacturing practices for labeling human epidermal growth factor with 111In for radiotherapeutic applications. J Nucl Med. 2004;45:701–708. [PubMed] [Google Scholar]
- 10.National Institutes of Health. Common Terminology Criteria for Adverse Events. version 2.0 1999. [Google Scholar]
- 11.Stabin MG, Siegel JA. Physical models and dose factors for use in internal dose assessment. Health Phys. 2003;85:294–310. doi: 10.1097/00004032-200309000-00006. [DOI] [PubMed] [Google Scholar]
- 12.Therasse P, Arbuck SG, Eisenhauer EA, Wanders J, Kaplan RS, Rubinstein L, Verweij J, Van Glabbeke M, van Oosterom AT, Christian MC, Gwyther SG. New guidelines to evaluate the response to treatment in solid tumors. European Organization for Research and Treatment of Cancer, National Cancer Institute of the United States, National Cancer Institute of Canada. J Natl Cancer Inst. 2000;92:205–216. doi: 10.1093/jnci/92.3.205. [DOI] [PubMed] [Google Scholar]
- 13.Cheang MC, Voduc D, Bajdik C, Leung S, McKinney S, Chia SK, Perou CM, Nielsen TO. Basal-like breast cancer defined by five biomarkers has superior prognostic value than triple-negative phenotype. Clin Cancer Res. 2008;14:1368–1376. doi: 10.1158/1078-0432.CCR-07-1658. [DOI] [PubMed] [Google Scholar]
- 14.Reilly RM, Kassis AI. Targeted Auger electron radiotherapy of malignancies. In: Reilly RM, editor. Monoclonal antibody and peptide-targeted radiotherapy of cancer. NJ: John Wiley & Sons Inc.; 2010. pp. 289–348. [Google Scholar]
- 15.Costantini DL, Chan C, Cai Z, Vallis KA, Reilly RM. 111In-labeled trastuzumab (Herceptin) modified with nuclear localization sequences (NLS): an Auger electron-emitting radiotherapeutic agent for HER2/neu-amplified breast cancer. J Nucl Med. 2007;48:1357–1368. doi: 10.2967/jnumed.106.037937. [DOI] [PubMed] [Google Scholar]
- 16.Panyutin IG, Sedelnikova OA, Karamychev VN, Neumann RD. Antigene radiotherapy: targeted radiodamage with 125I-labeled triplex-forming oligonucleotides. Ann N Y Acad Sci. 2003;1002:134–140. doi: 10.1196/annals.1281.012. [DOI] [PubMed] [Google Scholar]
- 17.Valkema R, De Jong M, Bakker WH, Breeman WA, Kooij PP, Lugtenburg PJ, De Jong FH, Christiansen A, Kam BL, De Herder WW, Stridsberg M, Lindemans J, Ensing G, Krenning EP. Phase I study of peptide receptor radionuclide therapy with [In-DTPA] octreotide: the Rotterdam experience. Semin Nucl Med. 2002;32:110–122. doi: 10.1053/snuc/2002.31025. [DOI] [PubMed] [Google Scholar]
- 18.Anthony LB, Woltering EA, Espenan GD, Cronin MD, Maloney TJ, McCarthy KE. Indium-111-pentetreotide prolongs survival in gastroenteropancreatic malignancies. Semin Nucl Med. 2002;32:123–132. doi: 10.1053/snuc.2002.31769. [DOI] [PubMed] [Google Scholar]
- 19.Janson ET, Westlin JE, Ohrvall U, Oberg K, Lukinius A. Nuclear localization of 111In after intravenous injection of [111In-DTPA-D-Phe1] -octreotide in patients with neuroendocrine tumors. J Nucl Med. 2000;41:1514–1518. [PubMed] [Google Scholar]
- 20.Goddu SM, Howell RW, Rao DV. Cellular dosimetry: absorbed fractions for monoenergetic electron and alpha particle sources and S-values for radionuclides uniformly distributed in different cell compartments. J Nucl Med. 1994;35:303–316. [PubMed] [Google Scholar]
- 21.Rebischung C, Hoffmann D, Stefani L, Desruet MD, Wang K, Adelstein SJ, Artignan X, Vincent F, Gauchez AS, Zhang H, Fagret D, Vuillez J, Kassis AI, Balosso J. First human treatment of resistant neoplastic meningitis by intrathecal administration of MTX plus 125IUdR. Int J Radiat Biol. 2008;84:1123–1129. doi: 10.1080/09553000802395535. [DOI] [PubMed] [Google Scholar]
- 22.Vriesendorp HM, Quadri SM, Andersson BS, Dicke KA. Hematologic side effects of radiolabeled immunoglobulin therapy. Exp Hematol. 1996;24:1183–1190. [PubMed] [Google Scholar]
- 23.Real FX, Rettig WJ, Chesa PG, Melamed MR, Old LJ, Mendelsohn J. Expression of epidermal growth factor receptor in human cultured cells and tissues: relationship to cell lineage and stage of differentiation. Cancer Res. 1986;46:4726–4731. [PubMed] [Google Scholar]
- 24.Damjanov I, Mildner B, Knowles BB. Immunohistochemical localization of the epidermal growth factor receptor in normal human tissues. Lab Invest. 1986;55:588–592. [PubMed] [Google Scholar]
- 25.Emami B, Lyman J, Brown A, Cola L, Goitein M, Munzenrider JE, Shank B, Solin LJ, Wesson M. Tolerance of normal tissue to therapeutic irradiation. Int J Radiat Oncol Biol Phys. 1991;21:109–122. doi: 10.1016/0360-3016(91)90171-y. [DOI] [PubMed] [Google Scholar]
- 26.Konijnenberg M, Melis M, Valkema R, Krenning E, de Jong M. Radiation dose distribution in human kidneys by octreotides in peptide receptor radionuclide therapy. J Nucl Med. 2007;48:134–142. [PubMed] [Google Scholar]
- 27.Valkema R, Pauwels SA, Kvols LK, Kwekkeboom DJ, Jamar F, de Jong M, Barone R, Walrand S, Kooij PP, Bakker WH, Lasher J, Krenning EP. Long-term follow-up of renal function after peptide receptor radiation therapy with 90Y-DOTA(0),Tyr(3)-octreotide and 177Lu-DOTA(0), Tyr(3)-octreotate. J Nucl Med. 2005;46:83S–91S. [PubMed] [Google Scholar]
- 28.Buscombe JR, Caplin ME, Hilson AJ. Long-term efficacy of high-activity 111In-pentetreotide therapy in patients with disseminated neuroendocrine tumors. J Nucl Med. 2003;44:1–6. [PubMed] [Google Scholar]
- 29.Keiser JA, Ryan MJ. Hemodynamic effects of epidermal growth factor in conscious rats and monkeys. Proc Natl Acad Sci U S A. 1996;93:4957–4961. doi: 10.1073/pnas.93.10.4957. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Divgi CR, Welt S, Kris M, Real FX, Yeh SD, Gralla R, Merchant B, Schweighart S, Unger M, Larson SM, Mendelsohn J. Phase I and imaging trial of indium 111-labeled anti-epidermal growth factor receptor monoclonal antibody 225 in patients with squamous cell lung carcinoma. J Natl Cancer Inst. 1991;83:97–104. doi: 10.1093/jnci/83.2.97. [DOI] [PubMed] [Google Scholar]
- 31.Panosa C, Tebar F, Ferrer-Batalle M, Fonge H, Seno M, Reilly RM, Massaguer A, De Llorens R. Development of an epidermal growth factor derivative with EGFR blocking activity. PLoS One. 2013;8:e69325. doi: 10.1371/journal.pone.0069325. doi:10.1371/journal.pone.0069325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Cai Z, Chen Z, Bailey KE, Scollard DA, Reilly RM, Vallis KA. Relationship between induction of phosphorylated H2AX and survival in breast cancer cells exposed to 111In-DTPA-hEGF. J Nucl Med. 2008;49:1353–1361. doi: 10.2967/jnumed.108.051805. [DOI] [PubMed] [Google Scholar]
- 33.van Agthoven T, Timmermans M, Dorssers LC, Henzen-Logmans SC. Expression of estrogen, progesterone and epidermal growth factor receptors in primary and metastatic breast cancer. Int J Cancer. 1995;63:790–793. doi: 10.1002/ijc.2910630607. [DOI] [PubMed] [Google Scholar]
- 34.Wu JM, Fackler MJ, Halushka MK, Molavi DW, Taylor ME, Teo WW, Griffin C, Fetting J, Davidson NE, De Marzo AM, Hicks JL, Chitale D, Ladanyi M, Sukumar S, Argani P. Heterogeneity of breast cancer metastases: comparison of therapeutic target expression and promoter methylation between primary tumors and their multifocal metastases. Clin Cancer Res. 2008;14:1938–1946. doi: 10.1158/1078-0432.CCR-07-4082. [DOI] [PMC free article] [PubMed] [Google Scholar]


