Abstract
Positron emission tomography/magnetic resonance imaging (PET/MRI) leverages the high soft-tissue contrast and the functional sequences of MR with the molecular information of PET in one single, hybrid imaging technology. This technology, which was recently introduced into the clinical arena in a few medical centers worldwide, provides information about tumor biology and microenvironment. Studies on indirect PET/MRI (use of positron emission tomography/computed tomography (PET/CT) images software fused with MRI images) have already generated interesting preliminary data to pave the ground for potential applications of PET/MRI. These initial data convey that PET/MRI is promising in neuro-oncology and head & neck cancer applications as well as neoplasms in the abdomen and pelvis. The pediatric and young adult oncology population requiring frequent follow-up studies as well as pregnant woman might benefit from PET/MRI due to its lower ionizing radiation dose. The indication and planning of therapeutic interventions and specifically radiation therapy in individual patients could be and to a certain extent are already facilitated by performing PET/MRI. The objective of this article is to discuss potential clinical oncology indications of PET/MRI.
Keywords: PET/MRI, oncologic imaging, attenuation correction, ovarian cancer
Introduction
The concept of hybrid imaging holds a promising value in modern-day medical care, overcoming the existing boundaries between morphological, functional and molecular information in current diagnostic imaging [1]. Positron emission tomography/computed tomography (PET/CT) has gained widespread use in oncologic imaging by combining the metabolic information of positron emission tomography (PET) with the anatomic detail of computed tomography (CT) [2-4].
The establishment and success of PET/CT in clinical practice stimulated the need and research of further hybrid medical technologies including PET/MRI to overcome inherent limitations, mostly in soft tissue resolution. Initially, PET and magnetic resonance (MR) data were fused retrospectively with software, mainly in the brain [5,6].
Recently, the first PET/MRI scanners for use in humans were introduced in the clinical arena. PET/MRI as a new hybrid imaging technology has the potential to repeat the success of PET/CT, particularly for oncologic indications [7], which might be superiorly addressed with magnetic resonance imaging (MRI) in anatomical regions where high soft-tissue contrast is required [8-11].
Technical aspects of PET/MRI
PET/MRI system designs on the market and technical highlights
PET/MRI poses technical challenges, such as compatibility between PET components and MR magnetic field. To date, the following systems with different designs are commercially available: The Philips Ingenuity TF PET/MR (Philips Healthcare, Andover, MA), the Biograph mMR (Siemens Healthcare, Erlangen, Germany) and the Discovery PET/CT 690 + Discovery MR 750 (GE Healthcare, Waukesha, WI). The Ingenuity TF PET/MR features the use of time-of-flight (TOF) PET technology and the full capability of a 3 Tesla MR with parallel RF transmission in a sequential design with two gantries, positioned at each end of a common patient table in a single room. In the Biograph mMR, the PET detectors are fully integrated into the 3 Tesla MR system with one single gantry. For this purpose, hardware modifications were necessary and an avalanche photodiode-based technology was developed. This system design enables simultaneous acquisition of PET and MR data [1,12,13]. GE has chosen the “trimodality solution”, comprising a PET/CT scanner and a 3 Tesla MRI system in two adjacent rooms with the patient transferred from one scanner to the other using a detachable table operating as a shuttle. This design has the advantage to operate the system with CT-based attenuation correction.
MR-based attenuation correction
One of the technical challenges for the success of PET/MRI imaging is to provide a reliable MR-based technique for tissue attenuation correction (MRAC) [14,15], as two of the available technical solutions of PET/MR scanners do not include transmission of CT scans [15,16]. Attenuation correction is mandatory for quantitative assessments with standardized uptake values (SUVs) or radiotracer kinetics. SUVs are routinely used in clinical settings for tissue characterization and in treatment follow-up of pathologic lesions.
Current approaches in MRAC are based on tissue classification with help of dedicated MR imaging sequences followed by an algorithm of anatomic segmentation to allow for assignment of tissue specific linear attenuation coefficients to each segmented tissue/organ. Several approaches to MRAC have been proposed: The three segment model accounts for air, soft tissue and lung [17,18] and the four segment model accounting for air, soft tissue, fat and lung [19]. The models are based on T1-weighted multi-station spoiled gradient echo or T1-weighted 2-point mDixon sequences, respectively. Beyond these two clinically validated MRAC approaches, various other regimens have also been explored by several research groups including a combination of Dixon with ultra-short echo time (UTE) sequences [20]. Furthermore atlas-based methods are available [24-27].
All available methods of MRAC are currently subject to ongoing research in comparison to transmission based scans [28-30]. A significant challenge is that the MR signal is based on proton precession which depends on factors completely different than those which impact attenuation of photons. This entails discrepancies in tracer uptake quantification for certain areas of the body. Especially attenuation of anatomic areas close to or within bone are underestimated with MRAC [31,32]. In addition, the signal characteristics of the spine and the respiratory motion to the diaphragm and liver occasionally generate errors in segmentation of the lung resulting in inappropriate assignment of attenuation correction factors limiting the ability of quantification [33,34].
Despite these discrepancies between the two hybrid modalities in some areas, the preliminary experience in the direct comparison of PET/MR and PET/CT yields sufficient correlation between the two techniques to perform routine scans [31,33,35-37].
As in the early days of transition from PET to PET/CT, readers are currently advised to review the attenuation correction (AC) maps for failure of the software based segmentation and review the non attenuated scans in order to ascertain the proper functioning of these algorithms.
Clinical applications in oncologic imaging
Pediatric patients and pregnant women
For pediatric oncology applications PET/MRI has potential to reduce overall radiation exposure to the patient. In tumors with need for repetitive follow up studies PET/CT can lead to a significant radiation burden. If with PET/MR the radiation dose from CT omitted, the actual radiation exposure is limited to the radiation dose from the PET component only which is substantially minor in comparison to the radiation dose from CT [16]. A recently published study in pediatric patients shows a triple risk of leukemia after a cumulative CT dose of 50 milligray (mGy) and a nearly triple risk of brain tumors after a cumulative CT dose of 60 mGy. This study emphasized the need to reduce the CT dose in this vulnerable population to the lowest dose possible and to try to establish alternative diagnostic procedures without ionizing radiation [38]. PET/MRI has the potential to represent this diagnostic alternative solution. A recent study evaluating co-registration of PET and MRI datasets for staging and re-staging of pediatric cancers yielded very promising results [39].
Not only pediatric patients but also the pregnant population may benefit from PET/MRI. For certain cancers during pregnancy an imaging modality including PET can be crucial for further treatment decisions. As MRI is not associated with any radiation burden a PET/MRI exam should be preferred vs. PET/CT.
Neurologic and head & neck applications
To date, several feasibility studies have shown promising results for neuroradiology PET/MRI applications. One of the early feasibility studies investigated PET/MRI in 10 patients with different types of brain tumors. Despite streak artifacts, the authors could demonstrate diagnostic image quality in patients with intracranial masses undergoing PET/MRI. This investigation also revealed that 11C-methionine or 68Ga-DOTATOC radiopharmaceuticals could be reliably used in PET imaging of intracranial tumors simultaneously with MRI [40]. In another study of the same group the simultaneous PET/MRI prototype system proved feasibility in the head and upper neck areas in 11 patients with head & neck cancer. Increased detailed resolution and improved image contrast could be found in the PET images of the PET/MRI system in comparison to the standard PET/CT without negatively impacting the MR component in the PET/MRI hybrid imaging modality. The metabolic ratios showed excellent agreement when comparing the PET components of PET/CT with PET/MRI. But the authors also found limitations like streak artifacts and the PET component’s limited axial field of view, precluding visualization of tumors below the angle of the mandible and precluding full staging of cervical nodes [41]. Since then, significant improvements have been made to the imaging device. Eiber et al. were able to demonstrate that performing an PET/MRI protocol integrating the Dixon sequence for AC purposes in head & neck squamous cell cancer led to similar diagnostic capabilities when compared to PET/CT [42]. The experience with 50 patients having different neurological diseases was recently published using the simultaneous brain PET/MRI system [43]. The authors not only acquired morphological MR sequences, but also introduced functional MR information in their protocol like diffusion tensor imaging, arterial spin labeling (ASL) and proton-spectroscopy. Diagnostic MR image quality along with functional and molecular information was proved to be useful justifying the neuroradiology application of PET/MRI in further trials [43].
The strongest evidence for a clinical indication of PET/MRI exists in the head & neck cancer population. Due to frequent distant metastases the whole body approach of the novel hybrid imaging technology is of significant advantage for distant metastases staging (M-staging). For local staging the high spatial and contrast resolution of MRI can delineate the tumor extent and lymph node involvement from surrounding normal tissue in the complex head and neck anatomical region. This may lead to a superior primary tumor staging (T-staging) and regional lymph node staging (N-staging). Furthermore PET/MRI can be useful for radiation therapy and presurgical treatment planning in head and neck cancer patients.
Chest
Lung cancer is one of the common clinically established indications for 2-deoxy-2[F-18]fluoro-D-glucose (FDG)-PET. Diagnosis, staging, and restaging of lung cancer are among the most extensively studied applications of FDG-PET [44,45]. With the exception of bronchioloalveolar cell cancer and carcinoid, lung neoplasms are generally very FDG-avid. The integration of CT and PET information has improved correlation of functional and morphologic characteristics. The staging of nodal and distant metastatic sites is the major strength of combined PET/CT [46,47]. However, T-staging can be difficult, especially in cases of well-differentiated tumors, infiltration of the surrounding tissue, and post obstructive pulmonary parenchyma changes. A recent study could show comparable results between PET/CT and PET/MRI in terms of pulmonary nodule detection when using a 3-dimensional Dixon-based dual-echo gradient-echo sequence [48]. A strong Spearman correlation was found between the tumor-to-liver ratios with PET/CT and those with PET/MRI imaging. The staging results showed a high concordance between PET/CT and PET/MRI in most of the cases. In one patient, infiltration of the mediastinal pleura could not be excluded at PET/CT, whereas MR imaging showed an intact mediastinal fat stripe adjacent to the tumor. These initial results point out that PET/MRI imaging could be a promising tool in the staging of superior sulcus tumors because of the combination of high spatial resolution MR imaging for the involvement of the brachial plexus and spine and molecular information based on PET results regarding metastatic status [49].
Abdomen and pelvis
Abdominal and pelvic applications for PET/MRI are numerous. For example, MR has shown a superior sensitivity for the detection of focal liver lesions, especially when <1 cm in size. PET can provide information on their potentially neoplastic nature, thus making PET/MRI an excellent method to screen for metastases (Figure 1) or monitor embolization theraoy for liver lesions [50-52]. Liver screening in colorectal cancer reduces patient mortality by 25%. Outcome is improved when liver metastases are treated surgically at the time of initial primary diagnosis or during early follow up [53]. PET/MR could help in the comprehensive staging including improving liver assessment.
Figure 1.
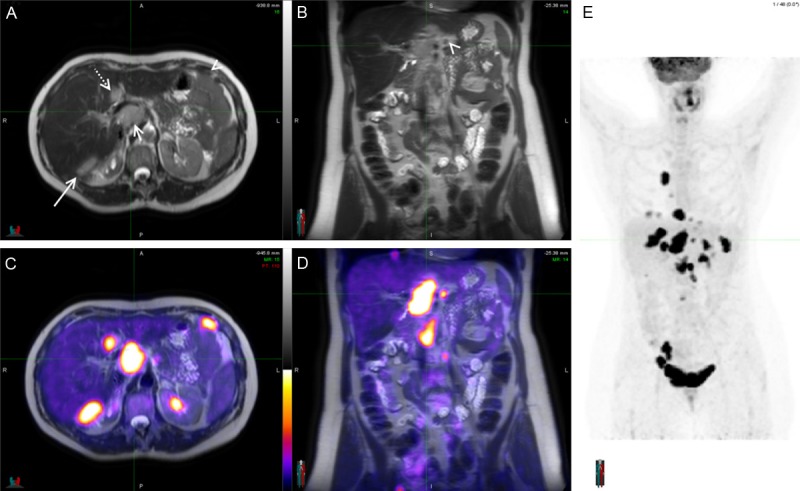
Staging PET/MRI scan of a 56-year-old woman with known ovarian cancer. Axial and coronal T2 weighted images (A and B) show multiple intermediate to high signal lesions abutting the liver posteriorly (long arrow), occupying the porta hepatis (short arrow) and seeding the peritoneum (arrowheads). A round, well defined lesion with similar characteristics is also seen in segment IV of the liver (dotted arrow). On PET/MRI images (C and D) the lesions previously described, and others not so apparent, are revealed by high FDG uptake confirming their malignant nature. MIP of the whole body (E) shows multiple lesions both in the chest and abdomen.
Other oncologic diseases in the abdomen are likely to benefit from combined PET and MRI. MRI with its superior tissue resolution is already helpful in the morphologic assessment of pancreatic, biliary and upper gastrointestinal neoplasms. The add-on of functional MR sequences such as diffusion-weighted imaging (DWI) has improved the tissue information on a cellular level specifically in tumors with increased heterogeneity, cystic areas and necrosis or after treatment when fibrosis and scar have to be distinguished from vital tumor tissue.
Based on our own initial experience, PET/MRI has already demonstrated to be helpful in individual cases in the post treatment setting of pancreatic cancer, when significant post surgical changes are present and disease recurrence is suspected on standard follow up with contrast enhanced CT [54].
Pelvic oncologic diseases is another highly promising field for PET/MRI. not only due to its higher tissue resolution but also because the pelvic cavity is surrounded by bone, which makes MR superior to CT with its inherent various bone artifacts. Gynecologic and prostate cancer are malignancies, where MR has proven utmost diagnostic importance and where efforts will be directed to evaluate PET/MRI and its capabilities [55]. It is accepted knowledge that MRI is already a powerful tool for the local staging of cervical, ovarian and endometrial cancer given its superb soft tissue resolution. Its implementation in clinical practice, however, suffers from availability and relatively high costs. Adding the strengths of PET in staging nodal and distant metastatic disease to the strengths of MRI in local staging, however, may make the use of the hybrid modality PET/MRI attractive as a tool for comprehensive assessment of disease within a single examination. The potential for more accurate staging and more cost effective imaging remains to be explored.
Ovarian cancer (Figures 1 and 2) for example is still a disease where no imaging method has been able to accurately stage patients, usually under-staging them, because of difficulties in peritoneal dissemination detection. Both PET/CT and MRI [56-58], more specifically DWI and diffusion-weighted whole-body imaging with background body signal suppression (DWIBS), have been studied towards the goal of properly staging these patients and have demonstrated usefulness in detecting recurrent disease. However, from the daily clinical practice, it can be envisioned, that the synergy of both and combining the diagnostic power of two individually strong modalities may exceed the current performance.
Figure 2.
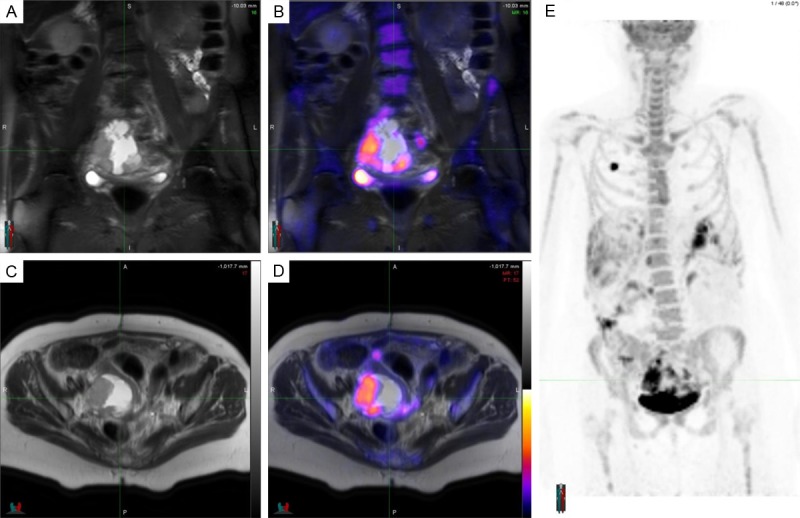
Re-staging PET/MRI scan of a 56-year-old woman with ovarian cancer. Coronal T2 fat saturated weighted image (A) and axial T2 weighted image (B) demonstrate a large heterogeneous lesion with cystic and solid components in the right adnexal area suspicious for malignancy. PET/MRI fusion images (C and D) show FDG uptake of the solid component confirming malignant suspicion. Maximum intensity projection (MIP) whole body image (E) shows diffuse spread throughout the abdomen and pelvis. The round FDG positive pseudolesion in the right lung corresponds to the chemotherapy infusion port.
With regard to prostate cancer, MR imaging combining anatomic (T2) and DWI has shown to have a role in persistently elevated prostate-specific antigen and non-diagnostic trans-rectal ultrasound biopsies, decreasing the number of repetitive biopsies necessary in high risk patients. Furthermore MRI demonstrated high specificity and accuracy in detecting recurrence of this disease entity when using dynamic contrast enhanced sequences [59].
The value of FDG PET for local tumor detection is limited, although FDG-PET may still be useful for detection of distant metastasis [60].
Newly developed dedicated tracers such as 11C acetate and 11C choline which are sensitive to prostatic tissue are promising to improve the overall results in imaging prostate cancer and its metastases [60]. Added value of 11C choline PET is somehow accepted in the setting of biochemical recurrence of prostate cancer as multiple studies have shown [61-64] but is more debated in localizing primary cancer. Nodal staging seems to benefit from 11C choline PET. Combining the two strong modalities MRI and PET in one approach justifies the hope for complementary information and improved results. Preliminary study with 11C-Choline PET/MRI has already proven feasibility [65] and has demonstrated this complementary effect of PET and multiparametric information from MRI [66].
Finally, another pelvic pathology that might highly benefit to be imaged with PET/MR is rectal cancer. While MRI can aid in appropriate treatment selection by accurately determining the depth of extramural invasion, its moderate reported accuracy rates (71-91%) for detecting node-positive disease, which is generally an indication for preoperative chemoradiation, makes MRI fall short. The main reason of this lack of accuracy is that size criterion alone is not sufficient for the diagnosis of lymph node metastasis, because 94% of the involved nodes will be as small as 5 mm [67]. On the other hand, PET/CT has been shown to alter therapy in almost one-third of patients with advanced primary rectal cancer [68] mainly because of its higher sensitivity for lymph node and extraperitoneal metastatic disease [69] and has proven helpful in determining recurrence when other imaging modalities fail [70]. Based on our institutional experience rectal cancer might become one of the indications of PET/MRI. Due to the superior soft-tissue and contrast resolution MRI is improving T-staging. As previously discussed it has its limitations with N-staging, however in a PET/MRI system the additional metabolic information from the PET component may increase the accuracy of N staging. The diagnostic accuracy for extrahepatic metastatic disease with PET/CT is high and MRI is sensitive for the detection of liver lesions. To this end PET/MRI could be of significant benefit in rectal cancer patients with regard to staging, follow-up for treatment response evaluation and re-staging as a one stop solution.
Role for therapy procedure planning
The usefulness of PET in the evaluation of treatment response in oncologic patients has made major progress with regard to the management of patients. FDG-PET has demonstrated efficacy for early therapy assessment in multiple oncological applications [71-73]. Quantification of the PET tracer using SUVs is of value when assessing treatment response. Although SUVs may show significant differences between systems, it is generally reproducible and useful when using the same scanner as it was shown in PET/CT scanner systems [74]. Nevertheless it should be considered that proper SUV quantification can only be achieved when a reliable MRAC technique is applied.
MRI has proven to be useful in imaging cancer and it is superior to CT in T-staging of many malignant processes such as in brain and breast neoplasms, among others. It offers high soft-tissue contrast allowing excellent anatomical delineation [75]. Sequences like DWI [76,77], dynamic contrast enhanced MRI, and other perfusion MR imaging techniques (ASL and blood-oxygenation level dependent imaging) as well as MR spectroscopy may provide important information such as tissue composition, tissue vascularization or different physiologic processes beyond anatomic imaging [78-80].
Therefore, the potential use of combining PET and MR in one machine opens exciting possibilities to monitor therapy response including molecular targeted cancer therapies.
Many cancers are aimed to be of interest when assessing treatment response with PET/MRI. The metabolic information offered by the PET component can be quantified. Novel MRI sequences such as DWI and dynamic contrast enhanced MRI go beyond the evaluation of volume and tumor size offering biological information on tissue composition and perfusion. Breast cancer might become one potential application of PET/MRI. Regarding FDG-PET, many authors have suggested its use in the evaluation of therapy response [81]. Ueda et al. demonstrated that FDG-PET was able to detect treatment response after only one to two cycles of chemotherapy. Early reduction in SUVs after one cycle of neoadjuvant chemotherapy measured by sequential FDG-PET/CT is an independent predictor of pathological response of primary breast cancer [82]. PET/MRI might be able to identify responders versus non-responders early in the treatment course which is of utmost importance in this patient population.
Another future application of PET/MRI can be found in the evaluation of therapy response in soft-tissue sarcomas. The superb soft-tissue and contrast resolution offered by MRI in accordance with the advance sequences DWI and dynamic contrast enhanced MRI might provide essential information when assessing response to cytotoxic drugs or other types of chemotherapy [83]. These studies analyzing PET/CT and MRI as separate modalities show that the hybdrid technology PET/MRI may improve treatment response evaluation in soft-tissue sarcoma patients.
Conclusion
PET/MRI is a promising new imaging modality, which has started to enter the clinical arena. PET/MRI couples the MR strengths of superior soft-tissue contrast compared to CT and sophisticated sequences to characterize the microenvironment of the neoplasm with PET’s molecular and metabolic information of tumor biology. In PET/CT, PET is the main partner whereas in PET/MRI it is of importance to bring the MR component to at least an equal level in order to use this hybrid imaging modality effectively in clinical settings. In the initial patient studies PET/MRI performed favorably for head and neck cancer. For this tumor entity a high spatial resolution at the primary site is important due to the complex head and neck anatomy. A whole body approach can help to detect metastases and secondary malignancies in patients with head and neck cancer. Another future indication of PET/MRI could be pelvic malignancies, in particular rectal cancer. Furthermore PET/MRI may provide a valuable tool for the assessment of treatment response in soft-tissue sarcoma by uniting metabolic information offered by PET with DWI as functional MR sequence. Further studies for different oncologic applications are warranted in larger patient cohorts in order to assess the value of PET/MRI for diagnosis, staging, follow up and therapy assessment of neoplastic diseases.
Disclosure of conflict of interest
Funding to the institution provided by research grants from the State of Ohio (Ohio Third Frontier Grant) and from Philips Healthcare.
References
- 1.Mansi L, Ciarmiello A, Cuccurullo V. PET/MRI and the revolution of the third eye. Eur J Nucl Med Mol Imaging. 2012;39:1519–1524. doi: 10.1007/s00259-012-2185-x. [DOI] [PubMed] [Google Scholar]
- 2.Fletcher JW, Djulbegovic B, Soares HP, Siegel BA, Lowe VJ, Lyman GH, Coleman RE, Wahl R, Paschold JC, Avril N, Einhorn LH, Suh WW, Samson D, Delbeke D, Gorman M, Shields AF. Recommendations on the Use of 18F-FDG PET in Oncology. J Nucl Med. 2008;49:480–508. doi: 10.2967/jnumed.107.047787. [DOI] [PubMed] [Google Scholar]
- 3.Ben-Haim S, Ell P. 18F-FDG PET and PET/CT in the evaluation of cancer treatment response. J Nucl Med. 2009;50:88–99. doi: 10.2967/jnumed.108.054205. [DOI] [PubMed] [Google Scholar]
- 4.Schöder H, Larson SM, Yeung HWD. PET/CT in oncology: integration into clinical management of lymphoma, melanoma, and gastrointestinal malignancies. J Nucl Med. 2004;45(Suppl 1):72S–81S. [PubMed] [Google Scholar]
- 5.Zaidi H, Montandon ML, Alavi A. The clinical role of fusion imaging using PET, CT, and MR imaging. Magn Reson Imaging Clin N Am. 2010;18:133–149. doi: 10.1016/j.mric.2009.09.010. [DOI] [PubMed] [Google Scholar]
- 6.Slomka PJ. Software approach to merging molecular with anatomic information. J Nucl Med. 2004;45(Suppl 1):36S–45S. [PubMed] [Google Scholar]
- 7.Drzezga A, Souvatzoglou M, Eiber M, Beer AJ, Fürst S, Martinez-Möller A, Nekolla SG, Ziegler S, Ganter C, Rummeny EJ, Schwaiger M. First Clinical Experience with Integrated Whole-Body PET/MR: Comparison to PET/CT in Patients with Oncologic Diagnoses. J Nucl Med. 2012;53:845–855. doi: 10.2967/jnumed.111.098608. [DOI] [PubMed] [Google Scholar]
- 8.Antoch G, Bockisch A. Combined PET/MRI: a new dimension in whole-body oncology imaging? Eur J Nucl Med Mol Imaging. 2009;36(Suppl 1):S113–20. doi: 10.1007/s00259-008-0951-6. [DOI] [PubMed] [Google Scholar]
- 9.Loeffelbein DJ, Souvatzoglou M, Wankerl V, Martinez-Möller A, Dinges J, Schwaiger M, Beer AJ. PET-MRI fusion in head-and-neck oncology: current status and implications for hybrid PET/MRI. J Oral Maxillofac Surg. 2012;70:473–483. doi: 10.1016/j.joms.2011.02.120. [DOI] [PubMed] [Google Scholar]
- 10.Schlemmer HP, Pichler BJ, Krieg R, Heiss WD. An integrated MR/PET system: prospective applications. Abdom Imaging. 2009;34:668–674. doi: 10.1007/s00261-008-9450-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Schulthess von GK, Schlemmer HPW. A look ahead: PET/MR versus PET/CT. Eur J Nucl Med Mol Imaging. 2009;36(Suppl 1):S3–9. doi: 10.1007/s00259-008-0940-9. [DOI] [PubMed] [Google Scholar]
- 12.Yankeelov TE, Peterson TE, Abramson RG, Garcia-Izquierdo D, Arlinghaus LR, Li X, Atuegwu NC, Catana C, Manning HC, Fayad ZA, Gore JC. Simultaneous PET-MRI in oncology: a solution looking for a problem? Magn Reson Imaging. 2012;30:1342–1356. doi: 10.1016/j.mri.2012.06.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Herzog H, Van Den Hoff J. Combined PET/MR systems: an overview and comparison of currently available options. Q J Nucl Med Mol Imaging. 2012;56:247–267. [PubMed] [Google Scholar]
- 14.Zaidi H. Is MR-guided attenuation correction a viable option for dual-modality PET/MR imaging? Radiology. 2007;244:639–642. doi: 10.1148/radiol.2443070092. [DOI] [PubMed] [Google Scholar]
- 15.Zaidi H, Ojha N, Morich M, Griesmer J, Hu Z, Maniawski P, Ratib O, Izquierdo-Garcia D, Fayad ZA, Shao L. Design and performance evaluation of a whole-body Ingenuity TF PET-MRI system. Phys Med Biol. 2011;56:3091–3106. doi: 10.1088/0031-9155/56/10/013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Delso G, Fürst S, Jakoby B, Ladebeck R, Ganter C, Nekolla SG, Schwaiger M, Ziegler SI. Performance measurements of the Siemens mMR integrated whole-body PET/MR scanner. J Nucl Med. 2011;52:1914–1922. doi: 10.2967/jnumed.111.092726. [DOI] [PubMed] [Google Scholar]
- 17.Schulz V, Torres-Espallardo I, Renisch S, Hu Z, Ojha N, Börnert P, Perkuhn M, Niendorf T, Schäfer WM, Brockmann H, Krohn T, Buhl A, Günther RW, Mottaghy FM, Krombach GA. Automatic, three-segment, MR-based attenuation correction for whole-body PET/MR data. Eur J Nucl Med Mol Imaging. 2011;38:138–152. doi: 10.1007/s00259-010-1603-1. [DOI] [PubMed] [Google Scholar]
- 18.Kalemis A, Delattre BMA, Heinzer S. Sequential whole-body PET/MR scanner: concept, clinical use, and optimisation after two years in the clinic. The manufacturer’s perspective. MAGMA. 2013;26:5–23. doi: 10.1007/s10334-012-0330-y. [DOI] [PubMed] [Google Scholar]
- 19.Martinez-Möller A, Souvatzoglou M, Delso G, Bundschuh RA, Chefd’hotel C, Ziegler SI, Navab N, Schwaiger M, Nekolla SG. Tissue classification as a potential approach for attenuation correction in whole-body PET/MRI: evaluation with PET/CT data. J Nucl Med. 2009;50:520–526. doi: 10.2967/jnumed.108.054726. [DOI] [PubMed] [Google Scholar]
- 20.Berker Y, Franke J, Salomon A, Palmowski M, Donker HCW, Temur Y, Mottaghy FM, Kuhl C, Izquierdo-Garcia D, Fayad ZA, Kiessling F, Schulz V. MRI-based attenuation correction for hybrid PET/MRI systems: a 4-class tissue segmentation technique using a combined ultrashort-echo-time/Dixon MRI sequence. J Nucl Med. 2012;53:796–804. doi: 10.2967/jnumed.111.092577. [DOI] [PubMed] [Google Scholar]
- 21.Keereman V, Fierens Y, Broux T, De Deene Y, Lonneux M, Vandenberghe S. MRI-based attenuation correction for PET/MRI using ultrashort echo time sequences. J Nucl Med. 2010;51:812–818. doi: 10.2967/jnumed.109.065425. [DOI] [PubMed] [Google Scholar]
- 22.Johansson A, Karlsson M, Nyholm T. CT substitute derived from MRI sequences with ultrashort echo time. Med Phys. 2011;38:2708–2714. doi: 10.1118/1.3578928. [DOI] [PubMed] [Google Scholar]
- 23.Larsson A, Johansson A, Axelsson J, Nyholm T, Asklund T, Riklund K, Karlsson M. Evaluation of an attenuation correction method for PET/MR imaging of the head based on substitute CT images. MAGMA. 2013;26:127–136. doi: 10.1007/s10334-012-0339-2. [DOI] [PubMed] [Google Scholar]
- 24.Montandon ML, Zaidi H. Atlas-guided non-uniform attenuation correction in cerebral 3D PET imaging. Neuroimage. 2005;25:278–286. doi: 10.1016/j.neuroimage.2004.11.021. [DOI] [PubMed] [Google Scholar]
- 25.Hofmann M, Steinke F, Scheel V, Charpiat G, Farquhar J, Aschoff P, Brady M, Schölkopf B, Pichler BJ. MRI-based attenuation correction for PET/MRI: a novel approach combining pattern recognition and atlas registration. J Nucl Med. 2008;49:1875–1883. doi: 10.2967/jnumed.107.049353. [DOI] [PubMed] [Google Scholar]
- 26.Zaidi H, Montandon ML, Slosman DO. Magnetic resonance imaging-guided attenuation and scatter corrections in three-dimensional brain positron emission tomography. Med Phys. 2003;30:937–948. doi: 10.1118/1.1569270. [DOI] [PubMed] [Google Scholar]
- 27.Hofmann M, Bezrukov I, Mantlik F, Aschoff P, Steinke F, Beyer T, Pichler BJ, Schölkopf B. MRI-Based Attenuation Correction for Whole-Body PET/MRI: Quantitative Evaluation of Segmentation- and Atlas-Based Methods. J Nucl Med. 2011;52:1392–1399. doi: 10.2967/jnumed.110.078949. [DOI] [PubMed] [Google Scholar]
- 28.Keereman V, Mollet P, Berker Y, Schulz V, Vandenberghe S. Challenges and current methods for attenuation correction in PET/MR. MAGMA. 2013;26:81–98. doi: 10.1007/s10334-012-0334-7. [DOI] [PubMed] [Google Scholar]
- 29.Wagenknecht G, Kaiser HJ, Mottaghy FM, Herzog H. MRI for attenuation correction in PET: methods and challenges. MAGMA. 2013;26:99–113. doi: 10.1007/s10334-012-0353-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Hofmann M, Pichler B, Schölkopf B, Beyer T. Towards quantitative PET/MRI: a review of MR-based attenuation correction techniques. Eur J Nucl Med Mol Imaging. 2009;36(Suppl 1):S93–104. doi: 10.1007/s00259-008-1007-7. [DOI] [PubMed] [Google Scholar]
- 31.Kim JH, Lee JS, Song IC, Lee DS. Comparison of segmentation-based attenuation correction methods for PET/MRI: evaluation of bone and liver standardized uptake value with oncologic PET/CT data. J Nucl Med. 2012;53:1878–1882. doi: 10.2967/jnumed.112.104109. [DOI] [PubMed] [Google Scholar]
- 32.Samarin A, Burger C, Wollenweber SD, Crook DW, Burger IA, Schmid DT, Schulthess GK, Kuhn FP. PET/MR imaging of bone lesions – implications for PET quantification from imperfect attenuation correction. Eur J Nucl Med Mol Imaging. 2012;39:1154–1160. doi: 10.1007/s00259-012-2113-0. [DOI] [PubMed] [Google Scholar]
- 33.Schramm G, Langner J, Hofheinz F, Petr J, Beuthien-Baumann B, Platzek I, Steinbach J, Kotzerke J, van den Hoff J. Quantitative accuracy of attenuation correction in the Philips Ingenuity TF whole-body PET/MR system: a direct comparison with transmission-based attenuation correction. MAGMA. 2013;26:115–126. doi: 10.1007/s10334-012-0328-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Keereman V, Holen RV, Mollet P, Vandenberghe S. The effect of errors in segmented attenuation maps on PET quantification. Med Phys. 2011;38:6010–6019. doi: 10.1118/1.3651640. [DOI] [PubMed] [Google Scholar]
- 35.Martinez-Möller A, Nekolla SG. Attenuation correction for PET/MR: problems, novel approaches and practical solutions. Z Med Phys. 2012;22:299–310. doi: 10.1016/j.zemedi.2012.08.003. [DOI] [PubMed] [Google Scholar]
- 36.Kuhn FP, Crook DW, Mader CE, Appenzeller P, Schulthess von GK, Schmid DT. Discrimination and anatomical mapping of PET-positive lesions: comparison of CT attenuation-corrected PET images with coregistered MR and CT images in the abdomen. Eur J Nucl Med Mol Imaging. 2013;40:44–51. doi: 10.1007/s00259-012-2236-3. [DOI] [PubMed] [Google Scholar]
- 37.Eiber M, Martinez-Möller A, Souvatzoglou M, Holzapfel K, Pickhard A, Löffelbein D, Santi I, Rummeny EJ, Ziegler S, Schwaiger M, Nekolla SG, Beer AJ. Value of a Dixon-based MR/PET attenuation correction sequence for the localization and evaluation of PET-positive lesions. Eur J Nucl Med Mol Imaging. 2011;38:1691–1701. doi: 10.1007/s00259-011-1842-9. [DOI] [PubMed] [Google Scholar]
- 38.Pearce MS, Salotti JA, Little MP, McHugh K, Lee C, Kim KP, Howe NL, Ronckers CM, Rajaraman P, Sir Craft AW, Parker L, Berrington de González A. Radiation exposure from CT scans in childhood and subsequent risk of leukaemia and brain tumours: a retrospective cohort study. Lancet. 2012;380:499–505. doi: 10.1016/S0140-6736(12)60815-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Pfluger T, Melzer HI, Mueller WP, Coppenrath E, Bartenstein P, Albert MH, Schmid I. Diagnostic value of combined (18)F-FDG PET/MRI for staging and restaging in paediatric oncology. Eur J Nucl Med Mol Imaging. 2012;39:1745–1755. doi: 10.1007/s00259-012-2228-3. [DOI] [PubMed] [Google Scholar]
- 40.Boss A, Bisdas S, Kolb A, Hofmann M, Ernemann U, Claussen CD, Pfannenberg C, Pichler BJ, Reimold M, Stegger L. Hybrid PET/MRI of intracranial masses: initial experiences and comparison to PET/CT. J Nucl Med. 2010;51:1198–1205. doi: 10.2967/jnumed.110.074773. [DOI] [PubMed] [Google Scholar]
- 41.Boss A, Stegger L, Bisdas S, Kolb A, Schwenzer N, Pfister M, Claussen CD, Pichler BJ, Pfannenberg C. Feasibility of simultaneous PET/MR imaging in the head and upper neck area. Eur Radiol. 2011;21:1439–1446. doi: 10.1007/s00330-011-2072-z. [DOI] [PubMed] [Google Scholar]
- 42.Eiber M, Souvatzoglou M, Pickhard A, Loeffelbein DJ, Knopf A, Holzapfel K, Martinez-Möller A, Nekolla SG, Scherer EQ, Schwaiger M, Rummeny EJ, Beer AJ. Simulation of a MR-PET protocol for staging of head-and-neck cancer including Dixon MR for attenuation correction. Eur J Radiol. 2012;81:2658–2665. doi: 10.1016/j.ejrad.2011.10.005. [DOI] [PubMed] [Google Scholar]
- 43.Schwenzer NF, Stegger L, Bisdas S, Schraml C, Kolb A, Boss A, Müller M, Reimold M, Ernemann U, Claussen CD, Pfannenberg C, Schmidt H. Simultaneous PET/MR imaging in a human brain PET/MR system in 50 patients--current state of image quality. Eur J Radiol. 2012;81:3472–3478. doi: 10.1016/j.ejrad.2011.12.027. [DOI] [PubMed] [Google Scholar]
- 44.Ahuja V, Coleman RE, Herndon J, Patz EF. The prognostic significance of fluorodeoxyglucose positron emission tomography imaging for patients with nonsmall cell lung carcinoma. Cancer. 1998;83:918–924. [PubMed] [Google Scholar]
- 45.Boiselle PM, Ernst A, Karp DD. Lung cancer detection in the 21st century: potential contributions and challenges of emerging technologies. AJR Am J Roentgenol. 2000;175:1215–1221. doi: 10.2214/ajr.175.5.1751215. [DOI] [PubMed] [Google Scholar]
- 46.Dizendorf EV, Baumert BG, Schulthess von GK, Lütolf UM, Steinert HC. Impact of whole-body 18F-FDG PET on staging and managing patients for radiation therapy. J Nucl Med. 2003;44:24–29. [PubMed] [Google Scholar]
- 47.Hicks RJ, Kalff V, MacManus MP, Ware RE, Hogg A, McKenzie AF, Matthews JP, Ball DL. (18)F-FDG PET provides high-impact and powerful prognostic stratification in staging newly diagnosed non-small cell lung cancer. J Nucl Med. 2001;42:1596–1604. [PubMed] [Google Scholar]
- 48.Stolzmann P, Veit-Haibach P, Chuck N, Rossi C, Frauenfelder T, Alkadhi H, Schulthess von G, Boss A. Detection rate, location, and size of pulmonary nodules in trimodality PET/CT-MR: comparison of low-dose CT and Dixon-based MR imaging. Invest Radiol. 2013;48:241–246. doi: 10.1097/RLI.0b013e31826f2de9. [DOI] [PubMed] [Google Scholar]
- 49.Schwenzer NF, Schraml C, Müller M, Brendle C, Sauter A, Spengler W, Pfannenberg AC, Claussen CD, Schmidt H. Pulmonary lesion assessment: comparison of whole-body hybrid MR/PET and PET/CT imaging--pilot study. Radiology. 2012;264:551–558. doi: 10.1148/radiol.12111942. [DOI] [PubMed] [Google Scholar]
- 50.Mainenti PP, Mancini M, Mainolfi C, Camera L, Maurea S, Manchia A, Tanga M, Persico F, Addeo P, D‘Antonio D, Speranza A, Bucci L, Persico G, Pace L, Salvatore M. Detection of colo-rectal liver metastases: prospective comparison of contrast enhanced US, multidetector CT, PET/CT, and 1.5 Tesla MR with extracellular and reticulo-endothelial cell specific contrast agents. Abdom Imaging. 2010;35:511–521. doi: 10.1007/s00261-009-9555-2. [DOI] [PubMed] [Google Scholar]
- 51.Wissmeyer M, Heinzer S, Majno P, Buchegger F, Zaidi H, Garibotto V, Viallon M, Becker CD, Ratib O, Terraz S. 90Y Time-of-flight PET/MR on a hybrid scanner following liver radioembolisation (SIRT) Eur J Nucl Med Mol Imaging. 2011;38:1744–1745. doi: 10.1007/s00259-011-1792-2. [DOI] [PubMed] [Google Scholar]
- 52.Muhi A, Ichikawa T, Motosugi U, Sou H, Nakajima H, Sano K, Sano M, Kato S, Kitamura T, Fatima Z, Fukushima K, Iino H, Mori Y, Fujii H, Araki T. Diagnosis of colorectal hepatic metastases: Comparison of contrast-enhanced CT, contrast-enhanced US, superparamagnetic iron oxide-enhanced MRI, and gadoxetic acid-enhanced MRI. J Magn Reson Imaging. 2011;34:326–335. doi: 10.1002/jmri.22613. [DOI] [PubMed] [Google Scholar]
- 53.Desch CE, Benson AB, Somerfield MR, Flynn PJ, Krause C, Loprinzi CL, Minsky BD, Pfister DG, Virgo KS, Petrelli NJ Oncology American ociety of Clinical. Colorectal cancer surveillance: 2005 update of an American Society of Clinical Oncology practice guideline. J. Clin. Oncol. 2005;23:8512–8519. doi: 10.1200/JCO.2005.04.0063. [DOI] [PubMed] [Google Scholar]
- 54.Vercher-Conejero JL, Paspulati RM, Kohan A, Rubbert C, Partovi S, Ros P, Faulhaber P, Herrmann KA. Imaging Pancreatic Pathology with PET/MRI: A Pictorial Essay. Annual Meeting of the Radiological Society of North America. 2013 [Google Scholar]
- 55.Vargas MI, Becker M, Garibotto V, Heinzer S, Loubeyre P, Gariani J, Lovblad K, Vallée JP, Ratib O. Approaches for the optimization of MR protocols in clinical hybrid PET/MRI studies. MAGMA. 2013;26:57–69. doi: 10.1007/s10334-012-0340-9. [DOI] [PubMed] [Google Scholar]
- 56.Colombo N, Peiretti M, Parma G, Lapresa M, Mancari R, Carinelli S, Sessa C, Castiglione M ESMO Guidelines Working Group. Newly diagnosed and relapsed epithelial ovarian carcinoma: ESMO Clinical Practice Guidelines for diagnosis, treatment and follow-up. Ann Oncol. 2010;21(Suppl 5):v23–30. doi: 10.1093/annonc/mdq244. [DOI] [PubMed] [Google Scholar]
- 57.De Iaco P, Musto A, Orazi L, Zamagni C, Rosati M, Allegri V, Cacciari N, Al-Nahhas A, Rubello D, Venturoli S, Fanti S. FDG-PET/CT in advanced ovarian cancer staging: value and pitfalls in detecting lesions in different abdominal and pelvic quadrants compared with laparoscopy. Eur J Radiol. 2011;80:e98–103. doi: 10.1016/j.ejrad.2010.07.013. [DOI] [PubMed] [Google Scholar]
- 58.Booth SJ, Turnbull LW, Poole DR, Richmond I. The accurate staging of ovarian cancer using 3T magnetic resonance imaging - a realistic option. BJOG. 2008;115:894–901. doi: 10.1111/j.1471-0528.2008.01716.x. [DOI] [PubMed] [Google Scholar]
- 59.Talab SS, Preston MA, Elmi A, Tabatabaei S. Prostate cancer imaging: what the urologist wants to know. Radiol Clin North Am. 2012;50:1015–1041. doi: 10.1016/j.rcl.2012.08.004. [DOI] [PubMed] [Google Scholar]
- 60.Hricak H, Choyke PL, Eberhardt SC, Leibel SA, Scardino PT. Imaging prostate cancer: a multidisciplinary perspective. Radiology. 2007;243:28–53. doi: 10.1148/radiol.2431030580. [DOI] [PubMed] [Google Scholar]
- 61.Mamede M, Ceci F, Castellucci P, Schiavina R, Fuccio C, Nanni C, Brunocilla E, Fantini L, Costa S, Ferretti A, Colletti PM, Rubello D, Fanti S. The role of 11C-choline PET imaging in the early detection of recurrence in surgically treated prostate cancer patients with very low PSA level. Clin Nucl Med. 2013;38:e342–5. doi: 10.1097/RLU.0b013e31829af913. [DOI] [PubMed] [Google Scholar]
- 62.Rinnab L, Mottaghy FM, Simon J, Volkmer BG, de Petriconi R, Hautmann RE, Wittbrodt M, Egghart G, Moeller P, Blumstein N, Reske S, Kuefer R. [11C] Choline PET/CT for targeted salvage lymph node dissection in patients with biochemical recurrence after primary curative therapy for prostate cancer. Preliminary results of a prospective study. Urol Int. 2008;81:191–197. doi: 10.1159/000144059. [DOI] [PubMed] [Google Scholar]
- 63.Contractor K, Challapalli A, Barwick T, Winkler M, Hellawell G, Hazell S, Tomasi G, Al-Nahhas A, Mapelli P, Kenny LM, Tadrous P, Coombes RC, Aboagye EO, Mangar S. Use of [11C] choline PET-CT as a noninvasive method for detecting pelvic lymph node status from prostate cancer and relationship with choline kinase expression. Clin Cancer Res. 2011;17:7673–7683. doi: 10.1158/1078-0432.CCR-11-2048. [DOI] [PubMed] [Google Scholar]
- 64.Schwarzenböck S, Souvatzoglou M, Krause BJ. Choline PET and PET/CT in Primary Diagnosis and Staging of Prostate Cancer. Theranostics. 2012;2:318–330. doi: 10.7150/thno.4008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Wetter A, Lipponer C, Nensa F, Beiderwellen K, Olbricht T, Rübben H, Bockisch A, Schlosser T, Heusner TA, Lauenstein TC. Simultaneous 18F choline positron emission tomography/magnetic resonance imaging of the prostate: initial results. Invest Radiol. 2013;48:256–262. doi: 10.1097/RLI.0b013e318282c654. [DOI] [PubMed] [Google Scholar]
- 66.Wetter A, Lipponer C, Nensa F, Heusch P, Rübben H, Altenbernd JC, Schlosser T, Bockisch A, Poppel T, Lauenstein T, Nagarajah J. Evaluation of the PET component of simultaneous [(18)F] choline PET/MRI in prostate cancer: comparison with [(18)F] choline PET/CT. Eur J Nucl Med Mol Imaging. 2014;41:79–88. doi: 10.1007/s00259-013-2560-2. [DOI] [PubMed] [Google Scholar]
- 67.Gowdra Halappa V, Corona Villalobos CP, Bonekamp S, Gearhart SL, Efron J, Herman J, Kamel IR. Rectal imaging: part 1, High-resolution MRI of carcinoma of the rectum at 3 T. AJR Am J Roentgenol. 2012;199:W35–42. doi: 10.2214/AJR.11.8134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Dewhurst C, Rosen MP, Blake MA, Baker ME, Cash BD, Fidler JL, Greene FL, Hindman NM, Jones B, Katz DS, Lalani T, Miller FH, Small WC, Sudakoff GS, Tulchinsky M, Yaghmai V, Yee J. ACR Appropriateness Criteria(®) Pretreatment Staging of Colorectal Cancer. J Am Coll Radiol. 2012;9:775–781. doi: 10.1016/j.jacr.2012.07.025. [DOI] [PubMed] [Google Scholar]
- 69.Gearhart SL, Frassica D, Rosen R, Choti M, Schulick R, Wahl R. Improved staging with pretreatment positron emission tomography/computed tomography in low rectal cancer. Ann Surg Oncol. 2006;13:397–404. doi: 10.1245/ASO.2006.04.042. [DOI] [PubMed] [Google Scholar]
- 70.Samee A, Selvasekar CR. Current trends in staging rectal cancer. World J Gastroenterol. 2011;17:828–834. doi: 10.3748/wjg.v17.i7.828. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Avril NE, Weber WA. Monitoring response to treatment in patients utilizing PET. Radiol Clin North Am. 2005;43:189–204. doi: 10.1016/j.rcl.2004.09.006. [DOI] [PubMed] [Google Scholar]
- 72.Therasse P, Arbuck SG, Eisenhauer EA, Wanders J, Kaplan RS, Rubinstein L, Verweij J, Van Glabbeke M, van Oosterom AT, Christian MC, Gwyther SG. New guidelines to evaluate the response to treatment in solid tumors. European Organization for Research and Treatment of Cancer, National Cancer Institute of the United States, National Cancer Institute of Canada. J Natl Cancer Inst. 2000;92:205–216. doi: 10.1093/jnci/92.3.205. [DOI] [PubMed] [Google Scholar]
- 73.Avril N. GLUT1 expression in tissue and (18)F-FDG uptake. J Nucl Med. 2004;45:930–932. [PubMed] [Google Scholar]
- 74.Boellaard R. Need for standardization of 18F-FDG PET/CT for treatment response assessments. J Nucl Med. 2011;52(Suppl 2):93S–100S. doi: 10.2967/jnumed.110.085662. [DOI] [PubMed] [Google Scholar]
- 75.Dhermain FG, Hau P, Lanfermann H, Jacobs AH, van den Bent MJ. Advanced MRI and PET imaging for assessment of treatment response in patients with gliomas. Lancet Neurol. 2010;9:906–920. doi: 10.1016/S1474-4422(10)70181-2. [DOI] [PubMed] [Google Scholar]
- 76.Thoeny HC, Ross BD. Predicting and monitoring cancer treatment response with diffusion-weighted MRI. J Magn Reson Imaging. 2010;32:2–16. doi: 10.1002/jmri.22167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Bains LJ, Zweifel M, Thoeny HC. Therapy response with diffusion MRI: an update. Cancer Imaging. 2012;12:395–402. doi: 10.1102/1470-7330.2012.9047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Fellah S, Girard N, Chinot O, Cozzone PJ, Callot V. Early evaluation of tumoral response to antiangiogenic therapy by arterial spin labeling perfusion magnetic resonance imaging and susceptibility weighted imaging in a patient with recurrent glioblastoma receiving bevacizumab. J. Clin. Oncol. 2011;29:e308–11. doi: 10.1200/JCO.2010.32.6082. [DOI] [PubMed] [Google Scholar]
- 79.Bezabeh T, Odlum O, Nason R, Kerr P, Sutherland D, Patel R, Smith ICP. Prediction of treatment response in head and neck cancer by magnetic resonance spectroscopy. AJNR Am J Neuroradiol. 2005;26:2108–2113. [PMC free article] [PubMed] [Google Scholar]
- 80.Neuner I, Kaffanke JB, Langen KJ, Kops ER, Tellmann L, Stoffels G, Weirich C, Filss C, Scheins J, Herzog H, Shah NJ. Multimodal imaging utilising integrated MR-PET for human brain tumour assessment. Eur Radiol. 2012;22:2568–2580. doi: 10.1007/s00330-012-2543-x. [DOI] [PubMed] [Google Scholar]
- 81.Avril N, Sassen S, Roylance R. Response to therapy in breast cancer. J Nucl Med. 2009;50(Suppl 1):55S–63S. doi: 10.2967/jnumed.108.057240. [DOI] [PubMed] [Google Scholar]
- 82.Ueda S, Tsuda H, Saeki T, Osaki A, Shigekawa T, Ishida J, Tamura K, Abe Y, Omata J, Moriya T, Fukatsu K, Yamamoto J. Early reduction in standardized uptake value after one cycle of neoadjuvant chemotherapy measured by sequential FDG PET/CT is an independent predictor of pathological response of primary breast cancer. Breast J. 2010;16:660–662. doi: 10.1111/j.1524-4741.2010.01011.x. [DOI] [PubMed] [Google Scholar]
- 83.Dudeck O, Zeile M, Pink D, Pech M, Tunn PU, Reichardt P, Ludwig WD, Hamm B. Diffusion-weighted magnetic resonance imaging allows monitoring of anticancer treatment effects in patients with soft-tissue sarcomas. J Magn Reson Imaging. 2008;27:1109–1113. doi: 10.1002/jmri.21358. [DOI] [PubMed] [Google Scholar]
- 84.Eugene T, Corradini N, Carlier T, Dupas B, Leux C, Bodet-Milin C. 18F-FDG-PET/CT in initial staging and assessment of early response to chemotherapy of pediatric rhabdomyosarcomas. Nucl Med Commun. 2012;33:1089–95. doi: 10.1097/MNM.0b013e328356741f. [DOI] [PubMed] [Google Scholar]


