Abstract
Background
Cognitive impairment is a recognized consequence of heart failure; however, there are no neuropsychological batteries with documented psychometric data in the chronic heart failure population.
Aims
To document the psychometric properties of a brief neuropsychological battery in a chronic heart failure sample.
Methods
The Repeatable Battery for the Assessment of Neuropsychological Status, Trail Making Test Part A and Part B, and letter fluency was administered to a sample of individuals with chronic heart failure.
Results
Eighty individuals with stable heart failure participated in this study. Individuals with chronic heart failure scored significantly lower than expected age and education adjusted norms in the domains of attention (p < 0.001), memory (p < 0.001), language (p < 0.001), executive function (p < 0.001), and psychomotor speed (p = 0.02). Scores on the tests of memory and executive function correlated to functional status (r = 0.28, p = 0.02 and r = 0.29, p = 0.03, respectively). Acceptable convergent validity and test–retest reliability were documented for this battery.
Conclusion
The neuropsychological battery had adequate reliability and validity in individuals with chronic heart failure.
Keywords: Heart failure, neuropsychological assessment, cognitive impairment, psychometrics
Introduction
Intact cognitive function is paramount to an individual’s overall ability to follow complex medical regimens, recognize worsening symptoms and avoid frequent hospitalizations. Approximately 28% to 58% of individuals with heart failure (HF) suffer from impairment of one or more cognitive domains (i.e. attention, working memory, learning, delayed memory, executive function, and psychomotor speed)1,2. The neurological processes that occur in these domains allow an individual to perceive and store information (attention, working memory and learning) and then recall and respond to subsequent environmental stimuli (delayed memory, executive function and psychomotor speed). For example, individuals with HF are taught to monitor daily weights and increase diuretic dosage or contact their medical provider if a weight gain above a prescribed threshold or increased shortness of breath occurs.
Cognitive impairment to any one of the above listed domains can complicate self-management and lead to undesired outcomes for individuals with HF. This hypothesis is borne out as one considers that science has made great strides in understanding the etiology and treatment of HF, yet, individuals with HF still experience one of the highest 90-day re-admission rates of any chronic illness, with inability to follow complex regimens and recognition of worsening symptoms cited as main reasons for re-admission3–6. While the etiology of cognitive impairment in HF remains unidentified, interventions are needed to compensate for the cognitive impairment that is present. However, before interventions can be designed and tested, several scientific gaps need to be addressed. Documentation of psychometric data is a major gap that must be addressed in order to assure sound methodology for future research of cognitive function in HF. Based on the limitations of the current literature, the following factors influenced choice of neuropsychological battery. First, the battery assesses multiple domains (i.e. attention, immediate memory, delayed memory, learning, executive function and psychomotor speed). Early studies used only measures of global cognitive function such as the Mini-Mental Status Exam (MMSE)7–10 that have a limited ability to identify subtle impairment in specific domains that are more commonly experienced by individuals with chronic HF. Second, the battery is brief, because fatigue is a common complaint in patients with HF and can affect neuropsychological test scores. Previous studies that utilized comprehensive neuropsychological batteries documented that as many as onethird of the participants were unable to complete various tests because of fatigue11–12. Therefore, after the brief yet comprehensive neuropsychological battery was constructed, our group documented feasibility of the battery in a sample of patients with chronic HF13. The purpose of this study was to document the psychometric properties of the battery in a sample of individuals with chronic HF.
The specific aims were to:
Compare scores for the Repeatable Battery for the Assessment of Neuropsychological Status (RBANS), Trail Making Test, and letter fluency to various clinical characteristics and published age and education adjusted norms.
Document concurrent validity for the RBANS, Trail Making Test, and letter fluency with the Instrumental Activities of Daily Living (IADL) questionnaire by comparing the total score from the IADL Questionnaire with individual neuropsychological test scores.
Document convergent validity of the 5 RBANS Indices, Trail Making Test, and letter fluency.
Estimate test–retest reliability for the RBANS, Trail Making Test, and letter fluency.
Methods
Sample
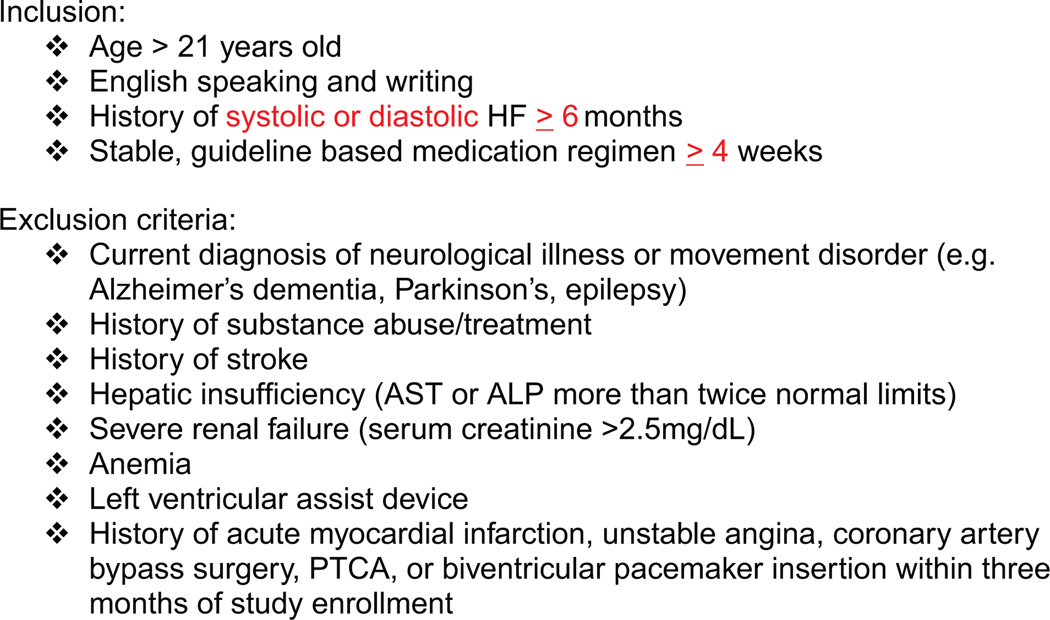
Following Institutional Review Board approval, a convenience sample of stable community dwelling NYHA class I–IV individuals with chronic HF was recruited from a large Midwestern HF Clinic. Individuals with systolic or diastolic dysfunction were recruited based on the inclusion/exclusion criteria listed in Fig. 1.
Figure 1.
Inclusion/exclusion criteria.
Procedures
Prior to study implementation, the primary investigator (L.C.B.) was trained in administration and scoring of the neuropsychological instruments. Participants provided informed consent for the study, and then completed the neuropsychological battery in a private examination room within the clinic. Following the neuropsychological testing, participants completed questionnaires related to various clinical characteristics (e.g. activity and functional status). Finally, participants were interviewed for demographic data and chart review was completed for collection of clinical data.
Participants who lived less than 20 min from the clinic were scheduled for repeat neuropsychological testing approximately one week following the initial assessment in order to obtain test–retest reliability data. In the literature test–retest reliability was documented within a broad range (1-day to over 2-years)14–20. Decisions related to test–retest timing rely on factors such as population stability. Given the relative instability of the HF population, a goal of seven days between testing sessions was set to avoid possible confounders. If testing sessions occurred to closely together there was an increased risk of practice effects on several of the tests in the battery (e.g. Trail Making Test, letter fluency) and if the testing sessions were too distant, there was an increased risk of changes in disease/symptom status (i.e. instability). Stable weight (changes of ≤2 pounds over the course of one week) was used as a proxy measure for stability of HF, because it is unknown whether cognitive function differs between individuals with stable HF versus acutely decompensated HF. Participants were allowed to complete test–retest measures at intervals longer than seven days if stability of HF was documented. Several participants had vacations scheduled and therefore could not be back in clinic within the seven day goal time period.
Instruments
The instruments have been described in detail previously13, so only a brief description is provided, along with pertinent psychometric data. The Repeatable Battery for the Assessment of Neuropsychological Status (RBANS) was used to measure several of the cognitive domains (attention, immediate and delayed memory). The test contains 12 subsets (list learning, story memory, figure copy, line orientation, picture naming, semantic fluency, digit span, coding, list recall, list recognition, story memory, and figure recall) that contribute to 5 index (i.e. domain) scores (immediate memory, visual/spatial construction, language, attention, and delayed memory) and a general global score. For this study the Duff et al. normative sample was used. In the Duff normative sample, the means for all five RBANS indices and general global score are 100 (SD = 15)21. The RBANS alternate form (B) was administered during the test–retest data collection in order to minimize practice effects. The RBANS has been validated against other, more lengthy batteries, such as the Wechsler Adult Intelligence Scale — Revised (WAIS-R), with index and total score correlations ranging between 0.62 and 0.78 in a sample of 127 adults18. Unfortunately, no psychometric data for the RBANS were identified within the HF literature.
Trail Making Test Parts A and B were used as measures of psychomotor speed (Part A) and executive function (Part B). Trail Making Test Part A and B have age and education adjusted normative data from a wide range of clinical populations, including cardiovascular disease17,20. In terms of validity as compared to other measures, Trail Making Test Part B has been measured against the Wisconsin Card Sort Test (WCST) in patients treated in Veteran’s Administration clinics, and was found to be a more sensitive measure of cognitive flexibility and ability to maintain set22. For this study we used the published normative sample data from Tombaugh et al. in which the normative sample mean scale score for Trail Making Test Part A was 9.8 (SD = 2.8) and 9.9 (SD = 2.9) for Trail Making Test Part B20.
Letter fluency (D words in 1 min) was also used as a measure of executive function. Letter fluency has been validated against neuroimaging in multiple clinical populations (including Alzheimer’s dementia, Parkinson’s disease and multiple sclerosis) and was associated with frontal lobe structures vital to executive function tasks16. The normative sample means for letter fluency were used with permission from the UCSF Memory and Aging Center. The mean raw scores were delineated by age groups (in years): ≤59, mean = 18 (SD = 3.5); 60–69, mean = 16.3 (SD = 6.0); 70–79, mean = 15.9 (SD = 4.6); ≥80, mean = 14 (SD = 4.6)23.
In order to identify cognitive impairment, z-scores were calculated. RBANS Index and Total Scale scores, Trail Making Test Part A and Part B scores, and letter fluency raw scores were converted to Z-scores. A Z-score of ≤1.50 was used as the cut-off for cognitive impairment per accepted neuropsychological guidelines17.
Data analysis
SPSS for Windows Version 17 was used for data analyses. Descriptive statistics were completed for demographic variables and for all neuropsychological test scores. A single-sample t-test was performed to compare the mean of the chronic HF population to the mean of a published normative sample for each instrument. Because individuals with chronic HF as a group are hypothesized to have experienced some decline in cognitive function, one-tailed tests were used across the set of tests. To explore the effects of possible confounders, Pearson correlation coefficients were calculated between individual test scores and age, previous education, and disease severity indicators (LVEF, NYHA class). Each test score from the neuropsychological battery was correlated with the total score on the IADL instrument; then, the five RBANS indices, Trail Making Test and letter fluency were correlated in order to document similarities (convergence) among the tests. In order to estimate test– retest reliability, Intraclass correlation coefficients with 95% confidence intervals around the point estimates for each subscale, index, or test for the scores were calculated from the two administrations of the test battery (referred to as test–retest correlation throughout Results and Discussion sections). All statistical tests were conducted using α = 0.05.
Results
One hundred and two potential participants were approached to participate in the study. Fifteen declined participation because of time constraints (e.g. conflicts with scheduled transportation to/from the clinic that was provided by a family member or friend). Three declined participation stating that they were ‘too tired’ or ‘just not feeling up to testing’ on that day. Finally, four participants were excluded because of history of stroke or other neurological condition. The final sample consisted of 80 community dwelling NYHA class I–IV individuals with chronic HF. The sample was primarily Caucasian (99%), with a mean age of 72 ± 12 years. Duration of HF diagnosis ranged from 6 to 380 months (mean 87 ± 76). Participants were on stable doses of beta-blockers, angiotensin converting enzymes, angiotensin II receptor blockers, diuretics, and cholesterol lowering medications unless contraindicated. Other commonly prescribed medications included blood thinners, vasodilators, and digitalis preparations. Remaining demographic data for the sample are listed in Table 1.
Table 1.
Sample demographics (N = 80)
| Variable | n | % |
|---|---|---|
| Gender | ||
| Male | 51 | 64 |
| Female | 29 | 36 |
| Age | ||
| < 50 | 4 | 5 |
| 50–59 | 8 | 10 |
| 60–69 | 15 | 19 |
| 70–79 | 33 | 41 |
| 80–89 | 19 | 24 |
| > 90 | 1 | 1 |
| Years of education | ||
| < 12 | 9 | 11 |
| ≥12 | 71 | 89 |
| Etiology | ||
| Systolic | 47 | 59 |
| Diastolic | 33 | 41 |
| LVEF | ||
| ≤40 | 51 | 64 |
| > 41 | 29 | 36 |
| NYHA Class | ||
| I | 2 | 3 |
| II | 34 | 42 |
| III | 43 | 54 |
| IV | 1 | 1 |
| # Medications | ||
| ≤5 | 2 | 3 |
| 6–10 | 37 | 46 |
| 11–15 | 34 | 42 |
| ≥16 | 7 | 9 |
Aim 1 — normative comparisons
The scores for all RBANS indices, RBANS Total Scale, Trail Making Test Part A and Part B and letter fluency were normally distributed with minimal skewness (range 0.41–(−)0.93) and kurtosis (range 1.72–(−)0.75). The mean values of this sample were significantly lower than the normative sample means in tests of immediate memory (p < 0.001), language (p < 0.001), attention (p < 0.001), delayed memory (p < 0.001), executive function (p < 0.001) and psychomotor speed (p = 0.028) (see Table 2).
Table 2.
Mean neuropsychological test scores of the heart failure sample
| Test | n | Range of scale scores | Mean (standard deviation) |
t(df) | p |
|---|---|---|---|---|---|
| RBANS Immediate Memory Index | 80 | 53–120 | 88.50 (16.11) | −6.386 (79) | < 0.001** |
| RBANS Visual Constructional Index | 80 | 64–132 | 100.13 (16.67) | 0.067 (79) | 0.947 |
| RBANS Language Index | 80 | 67–123 | 94.11 (10.62) | −4.961 (79) | < 0.001* |
| RBANS Attention Index | 80 | 23–135 | 89.45 (18.23) | −5.177 (79) | < 0.001* |
| RBANS Delayed Memory Index | 80 | 45–122 | 94.20 (15.43) | −3.363 (79) | < 0.001* |
| RBANS Total Scale | 80 | 56–128 | 94.11 (14.12) | −5.631 (79) | < 0.001* |
| Letter Fluencya (≤59) | 12 | 5–20 | 10.27 (4.63) | −5.538 (10) | < 0.001* |
| Letter fluencya (60–69) | 15 | 3–18 | 8.67 (5.18) | −5.710 (14) | < 0.001* |
| Letter fluencya (70–79) | 33 | 1–21 | 10.00 (4.56) | −7.429 (32) | < 0.001* |
| Letter fluencya (≥80) | 20 | 2–20 | 9.00 (4.57) | −4.898 (19) | < 0.001* |
| Trail Making A | 79 | 2–18 | 9.09 (2.83) | −2.238 (78) | 0.028* |
| Trail Making B | 79 | 2–17 | 9.72 (3.21) | −0.495 (78) | 0.622 |
Letter fluency values represent raw scores.
Significant at p < 0.05.
While clinical characteristics, such as sleep quality, daytime sleepiness, physical activity, and depressive symptoms were not associated with scores on the neuropsychological tests, other demographics were significantly associated with various neuropsychological test scores. New York Heart Association Class was significantly negatively correlated with measures of psychomotor speed (r = −0.43, p < 0.001), executive function (Trail Making Test Part B) (r = −0.27, p = 0.018), attention (r = −0.24, p = 0.036) and global cognition (RBANS Total Scale Score) (r = −0.23, p = 0.037). Previous education was significantly correlated with measures of psychomotor speed (r = 0.29, p = 0.009), executive function (letter fluency and Trail Making Test Part B) r = 0.43, p < 0.001 and r = 0.40, p < 0.001, respectively), and global cognition (RBANS Total Scale Score) (r = 0.22, p = 0.048).
Aim 2 — concurrent validity
The Lawton Instrumental Activities of Daily Living questionnaire was significantly correlated with the RBANS Immediate Memory Index (r = 0.28, p = 0.02), RBANS Total Scale Score (r = 0.29, p = 0.02), letter fluency (r = 0.26, p = 0.03), Trail Making Test Part A (r = 0.35, p = 0.00) and Trail Making Test Part B (r = 0.27, p = 0.03). See Table 3.
Table 3.
Significant (p ≤ 0.05) Pearson correlation coefficients among neuropsychological and Lawton IADS functional test scores (N = 80)
| IM | VC | Lan | Atten | DM | Total | Letter | TMTA | TMTB | IADL | |
|---|---|---|---|---|---|---|---|---|---|---|
| IM | – | |||||||||
| VC | 0.23 | – | ||||||||
| Lan | 0.26 | – | ||||||||
| Atten | 0.38 | 0.33 | 0.23 | – | ||||||
| DM | 0.67 | 0.34 | 0.33 | 0.31 | – | |||||
| Total | 0.78 | 0.64 | 0.48 | 0.65 | 0.76 | – | ||||
| Letter | 0.28 | 0.29 | 0.36 | 0.34 | – | |||||
| TMTA | 0.31 | 0.24 | 0.30 | 0.51 | 0.37 | 0.53 | 0.42 | – | ||
| TMTB | 0.38 | 0.36 | 0.32 | 0.51 | 0.41 | 0.61 | 0.42 | 0.70 | – | |
| IADL | 0.28 | 0.29 | 0.26 | 0.35 | 0.27 | – |
KEY: IM = RBANS Immediate Memory Index; VC = RBANS Visual/Constructional Index; Lan = RBANS Language Index; Atten = RBANS Attention Index; DM = RBANS Delayed Memory Index; Total = RBANS Total Scale Index; Letter = letter fluency test; TMTA = Trail Making Part A Test; TMTB = Trail Making Test Part B; IADL = Lawton Instrumental Activities of Daily Living Questionnaire Score.
Aim 3 — convergent validity
For the most part, the RBANS indices, Trail Making Test Part A and Part B, and letter fluency were moderately correlated to each other. The magnitudes of these correlations indicate that the tests are measuring related, but different domains of cognition. The highest correlations were observed between the RBANS Total Scale Score and RBANS Immediate and Delayed Memory Indices (r = 0.78, p < 0.001 and r = 0.76, p < 0.001, respectively) (see Table 3).
Aim 4 — test–retest reliability
A subsample of 21 participants was eligible to retest with the neuropsychological battery. The average time between testing session was 12 (SD = 6.9; mode = 7) days. Table 4 illustrates the test–retest correlations for this neuropsychological battery. The test–retest correlations for the RBANS indices (i.e. visual/spatial/constructional, language, attention, immediate and delayed memory and the Total Scale Score) ranged from 0.39 to 0.84. The test–retest correlations for letter fluency and Trail Making Test Part A and B in this sample ranged from 0.79 to 0.84. Table 4 illustrates the 95% confidence intervals around the test–retest correlations as well as the calculated Chronbach’s alpha for each test in the neuropsychological battery.
Table 4.
Test–retest psychometric values (N = 21)
| Test | Intraclass correlation coefficient |
p-value | 95% Confidence interval | Chronbach’s alpha |
|---|---|---|---|---|
| RBANS Immediate Memory Index | 0.39 | 0.037* | −0.04–0.70 | 0.56 |
| RBANS Visual/Constructional Index | 0.71 | < 0.001* | 0.41–0.87 | 0.83 |
| RBANS Language Index | 0.61 | < 0.001* | 0.25–0.82 | 0.76 |
| RBANS Attention Index | 0.73 | < 0.001* | 0.45–0.88 | 0.85 |
| RBANS Delayed Memory Index | 0.62 | < 0.001* | 0.27–0.83 | 0.77 |
| RBANS Total Scale | 0.84 | < 0.001* | 0.64–0.93 | 0.91 |
| Letter fluency | 0.84 | < 0.001* | 0.65–0.93 | 0.92 |
| Trail Making A | 0.79 | < 0.001* | 0.55–0.91 | 0.89 |
| Trail Making B | 0.81 | < 0.001* | 0.58–0.92 | 0.89 |
Significant at p < 0.05.
Discussion
This study is the first to document the psychometric properties of a neuropsychological battery that is appropriate for use in individuals with chronic HF. Reliability and validity measures documented by this study support continued use of this battery in the HF population. Similar to our previous feasibility findings, the mean time of administration for the neuropsychological battery in this sample of individuals with HF was 45 min (all participants required less than 60 min). Impairment in attention, immediate memory, delayed memory, executive function and psychomotor speed were prevalent in this sample. These findings are congruent with previous findings from the HF literature11,12,24,25.
Two measures of executive function were utilized for this study (i.e. Trail Making Test Part B and letter fluency) due to the complexity of this domain and the lack of sensitivity of Trail Making Test Part B in previous literature12,26–28. Research in other clinical populations has successfully used letter fluency as a measure of executive function (e.g. Alzheimer’s dementia, and Mild Cognitive Impairment)29,30. Findings from this study provide preliminary support for the use of letter fluency tasks as a measure of executive function in the HF population. However, given the dearth of literature related to executive function testing in the HF population, replication of these findings is warranted.
Only a limited number of studies have examined the immediate memory and visual/constructional domains. Similar to studies by Wolfe et al. and Suavé et al., this study documented impairment in immediate memory. The literature related to visual/constructional testing is even scarcer. Only two studies have examined the visual/constructional domain, with mixed results31,32. Findings from this study document that the visual/constructional domain is preserved in patients with HF. Given the lack of evidence and conflicting results, further evaluation of immediate memory and visual/constructional skills is necessary as science continues to tease out which cognitive domains are impaired and preserved within the chronic HF population.
Neuropsychological instruments may be influenced by age, education, and other clinical factors such as disease severity. In this sample of individuals with chronic HF, disease severity (NYHA class) had significant relationships with measures of psychomotor speed (TMT A), attention (RBANS Attention Index), executive function (TMT B) and global cognition (RBANS Total Scale Score). Other studies have found similar relationships between neuropsychological test scores and disease severity11,12,24,33. Future studies should further explore the influences of age, education and disease severity on cognitive impairment in the chronic HF population.
Instrumental activities of daily living, such as those measured by the Lawton Instrumental Activities of Daily Living questionnaire, require intact cognitive processing that goes beyond the physical function necessary to complete activities of daily living (e.g. bathing, toileting). Commonly in the neuropsychological literature, researchers document concurrent validity of scores against measures of instrumental activities of daily living. In elderly chronic illness populations such as dementia and Huntington’s disease, declines in cognitive function significantly correlate to decreased scores of the Lawton questionnaire16,34–36. This study is one of the first to document the relationship between cognitive impairment and activities integral to independent living. Therefore, while correlations achieved in the current study were all moderate (0.27–0.35), the results do document concurrent validity between the RBANS indices, RBANS Total Score, Trail Making Test Part A and Part B, and letter fluency and functional measures such as Lawton’s Instrumental Activities of Daily Living questionnaire. However, since concurrent and ecological validity are an integral part of population specific instrument validation, further study, utilizing Lawton’s Instrumental Activities of Daily Living questionnaire, as well as other objective functional measures is necessary as researchers continue to advance the science related to cognitive function in the chronic HF population.
In general, moderate correlations were documented between the RBANS indices, Trail Making Test Part A and Part B, and letter fluency (r = 0.15–r = 0.38). The highest correlation (r = 0.67) was between the RBANS Immediate and Delayed Memory Indices. This is an expected finding as both tests are designed to measure the domain of memory. The findings from this study compare to findings from the original RBANS normative sample18,37. Further, the RBANS Attention Index was significantly correlated to other measures of attention in this study (i.e. Trail Making Test Part A and Part B; r = 0.50, r = 0.51, respectively) and letter fluency was significantly correlated to the other measure of executive function (i.e. Trail Making Test Part B, r = 0.61). Overall, these findings suggest that this group of neuropsychological tests is measuring distinct aspects or domains of cognitive function with evidence for convergent validity.
Previous RBANS normative samples have reported moderate to strong test–retest correlations of 0.46–0.8214,15,18. The RBANS test–retest results from this study are congruent with normative literature. Previous literature reports test–retest correlation coefficients of 0.67 to 0.94 for Trail Making Test Part A and Part B, with the higher coefficients coming from clinical populations such as cardiac disease and vascular disorders19,38. Letter fluency test–retest correlations in other clinical populations, including Alzheimer’s dementia, stroke, and multiple sclerosis have ranged from 0.69 to 0.85, with length between testing sessions ranging from one day to one year19,39. The test–retest correlations for this study are congruent with previous literature and suggest that Trail Making Test Part A and Part B and letter fluency are stable measures of cognitive function in the chronic HF population.
Our findings support the use of the neuropsychological battery tested here in future research studies. Further research is warranted to examine the trajectory of cognitive impairment across HF disease duration in order to better understand etiology and progression. Validity evidence provided by the use of neuroimaging is also warranted in future studies. New neuroimaging techniques may increase understanding related to areas of the brain that are affected by the chronic HF disease process, as well as the etiology of cognitive impairment, guiding compensatory interventions for cognitively impaired individuals.
While this battery shows promise for use in HF research, more studies are needed to analyze individual tests to explore optimal screening instruments for use in the clinical setting. Because the RBANS requires specific neuropsychological training related to administration and scoring and results must be interpreted by a licensed or certified neuropsychologist, making it impractical for use in most clinic settings. So, while this study provides support for use of the entire battery for research purposes, further studies are needed to explore whether specific tests or sub-tests provide adequate sensitivity to detect the presence of cognitive impairment in HF clinic populations.
Finally, the current findings support the use of age and education adjusted normative data as an acceptable alternative to age and education matched controls in the chronic HF population. Given the difficulty and expense of recruiting age and education matched controls for studies of cognitive impairment in HF, this finding is important.
Limitations
The findings from this study add to the current literature but are limited by several factors. Convenience sampling was used, which limits generalizability of the study’s findings. The test–retest sample size was small, limiting power of the analyses. Medications such as beta-blockers and angiotensin converting enzyme inhibitors may affect scores on neuropsychological tests; however, they were not controlled for during this study. The sample was from a single practice where the majority of participants were on similar medication regimens, however, future studies would benefit from analysis of specific medication classes and their relationship to neuropsychological test scores. No control group was utilized, therefore, no comparisons could be made between the age and education adjusted normative sample data used in this study versus age and education matched normative data. Finally, a self-report measure of functional status was used to provide concurrent validity evidence, and participants may not be able to accurately estimate or self-report their functional status.
Acknowledgements
The corresponding author would like to acknowledge the University of San Francisco’s T32 (2T32NR007088-15) post-doctoral fellowship, Dr. Kathleen Dracup for her guidance and editorial expertise, and the expert staff at Bryan LGH Heart Institute for their assistance and support of this project.
Funding
There are no funding sources to acknowledge for this project.
References
- 1.Pressler SJ. Cognitive functioning and chronic heart failure: a review of the literature (2002–July 2007) J Cardiovasc Nurs. 2008;23(3):239–249. doi: 10.1097/01.JCN.0000305096.09710.ec. [DOI] [PubMed] [Google Scholar]
- 2.Vogels RLC, Scheltens P, Schroeder-Tanka JM, Weinstein HC. Cognitive impairment in heart failure: a systematic review of the literature. Eur J Heart Fail. 2007;9:440–449. doi: 10.1016/j.ejheart.2006.11.001. [DOI] [PubMed] [Google Scholar]
- 3.Bennett SJ, Pressler ML, Hays L, Firestine LA, Huster GA. Psychosocial variables and hospitalization in persons with chronic heart failure. Prog Cardiovasc Nurs. 1997;12(4):4–11. [PubMed] [Google Scholar]
- 4.Happ MB, Naylor MD, Roe-Prior P. Factors contributing to rehospitalization of elderly patients with heart failure. J Cardiovasc Nurs. 1997;11(4):75–84. doi: 10.1097/00005082-199707000-00008. [DOI] [PubMed] [Google Scholar]
- 5.Naylor MD, Stephens C, Bowles KH, Bixby MB. Cognitively impaired older adults: from hospital to home. Am J Nurs. 2005;105(2):52–61. doi: 10.1097/00000446-200502000-00028. [DOI] [PubMed] [Google Scholar]
- 6.Riegel B, Weaver TE. Poor sleep and impaired self-care: towards a comprehensive model linking sleep, cognition, and heart failure outcomes. Eur J Cardiovasc Nurs. 2009;8:337–344. doi: 10.1016/j.ejcnurse.2009.06.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Cacciatore F, Abete P, Ferrara N, Calabrese C, Napoli C, Maggi S, et al. Congestive heart failure and cognitive impairment in an older population. J Am Geriatr Soc. 1998;46:1343–1348. doi: 10.1111/j.1532-5415.1998.tb05999.x. [DOI] [PubMed] [Google Scholar]
- 8.Rengo F, Acanfora D, Trojano L, Scognamiglio P, Ciaburri F, Ceriello A, et al. Congestive heart failure and cognitive impairment in the elderly. Arch Gerontol Geriatr. 1995;20(1):63–68. doi: 10.1016/0167-4943(94)00607-9. [DOI] [PubMed] [Google Scholar]
- 9.Shapiro PA, Levin HR, Oz MC. Left ventricular assist devices psychosocial burden and implications for heart transplant programs. Gen Hosp Psychiatry. 1996;18(Supplement 6):30–35. doi: 10.1016/s0163-8343(96)00076-x. [DOI] [PubMed] [Google Scholar]
- 10.Zuccala G, Marzetti E, Cesari M, Monaco MR, Antonica L, Cocchi A, et al. Correlates of cognitive impairment among patients with heart failure: results of a multicenter survey. Am J Med. 2005;118:496–502. doi: 10.1016/j.amjmed.2005.01.030. [DOI] [PubMed] [Google Scholar]
- 11.Incalzi RA, Trojano L, Acanfora D, Crisci C, Tarantino F, Abete P, et al. Verbal memory impairment in congestive heart failure. J Clin Exp Neuropsychol. 2003;25(1):14–23. doi: 10.1076/jcen.25.1.14.13635. [DOI] [PubMed] [Google Scholar]
- 12.Vogels RLC, Oosterman JM, van Harten B, Scheltens P, van der Flier WM, Schroeder-Tanka JM, et al. Profile of cognitive impairment in chronic heart failure. J Am Geriatr Soc. 2007;55:1764–1770. doi: 10.1111/j.1532-5415.2007.01395.x. [DOI] [PubMed] [Google Scholar]
- 13.Bauer LC, Pozehl BJ. Measurement of cognitive function in chronic heart failure: a feasibility study. Applied Nursing Research; in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Duff K, et al. Test–retest stability and practice effects of the RBANS in a community dwelling elderly sample. J Clin Exp Neuropsychol. 2005;27(5):565–575. doi: 10.1080/13803390490918363. [DOI] [PubMed] [Google Scholar]
- 15.Duff K, et al. Normative and retest data on the RBANS cortical/subcortical index in older adults. J Clin Exp Neuropsychol. 2007;29(8):854–859. doi: 10.1080/13803390601147629. [DOI] [PubMed] [Google Scholar]
- 16.Lezak MD. Neuropsychological assessment. 3rd ed. New York: Oxford University Press; 2004. [Google Scholar]
- 17.Mitrushina MN, Boone KB, Razani J, D’Elia LF. Handbook of normative data for neuropsychological assessment. 2nd ed. New York: Oxford University Press; 2005. [Google Scholar]
- 18.Randolph C. RBANS: Repeatable Battery for the Assessment of Neuropsychological Status. San Antonio: The Psychological Corporation; 1998. [Google Scholar]
- 19.Strauss E, Sherman EMS, Spreen O. A compendium of neuropsychological tests — administration, norms and commentary ed. Third edition. Oxford: University Press; 2006. [Google Scholar]
- 20.Tombaugh TN. Trail Making Test A and B: normative data stratified by age and education. Arch Clin Neuropsychol. 2004;19:203–214. doi: 10.1016/S0887-6177(03)00039-8. [DOI] [PubMed] [Google Scholar]
- 21.Duff K, Patton D, Schoenberg MR, Mold J, Scott JG, Adams RL. Age- and education-corrected independent normative data for the RBANS in a community dwelling elderly sample. Clin Neuropsychol. 2003;17(3):351–366. doi: 10.1076/clin.17.3.351.18082. [DOI] [PubMed] [Google Scholar]
- 22.Kortte CB, Horner MD, Windham WK. The Trail Making Test, Part B: cognitive flexibility or ability to maintain set? Appl Neuropsychol. 2002;9:106–109. doi: 10.1207/S15324826AN0902_5. [DOI] [PubMed] [Google Scholar]
- 23.Kramer JH, Jurik J, Sha SJ, Rankin KP, Rosen HJ, Johnson JK, et al. Distinctive neuropsychological patterns in frontotemporal dementia, semantic dementia, and Alzheimer disease. Cogn Behav Neurol. 2003;16(4):211–218. doi: 10.1097/00146965-200312000-00002. [DOI] [PubMed] [Google Scholar]
- 24.Sauvé MJ, Lewis WR, Blankenbiller M, Rickabaugh B, Pressler SJ. Cognitive impairments in chronic heart failure: a case controlled study. J Card Fail. 2009;15(1):1–10. doi: 10.1016/j.cardfail.2008.08.007. [DOI] [PubMed] [Google Scholar]
- 25.Trojano L, Incalzi RA, Acanfora D, Picone C, Mecocci P, Rengo F. Cognitive impairment: key feature of congestive heart failure in the elderly. J Neurol. 2003;250:1456–1463. doi: 10.1007/s00415-003-0249-3. [DOI] [PubMed] [Google Scholar]
- 26.Bornstein RA, Starling RC, Myerowitz P, Haas GJ. Neuropsychological function in patients with end-stage heart failure before and after cardiac transplantation. Acta Neurol Scand. 1995;91:260–265. doi: 10.1111/j.1600-0404.1995.tb07001.x. [DOI] [PubMed] [Google Scholar]
- 27.Putzke JD, Williams MA, Daniel FJ, Foley BA, Kirklin JK, Boll TJ. Neuropsychological functioning among heart transplant candidates: a case control study. J Clincal Experimental Neuropsychology. 2000;22(1):95–103. doi: 10.1076/1380-3395(200002)22:1;1-8;FT095. [DOI] [PubMed] [Google Scholar]
- 28.Roman DD, Kubo SH, Ormaza S, Francis GS, Bank AJ, Shumway SJ. Memory improvement following cardiac transplantation. J Clinical Experimental Psychology. 1997;19:692–697. doi: 10.1080/01688639708403754. [DOI] [PubMed] [Google Scholar]
- 29.Chamberlain SR, Balckwell AD, Nathan PJ, Hammond G, Robbins TW, Hodges JR, et al. Differential cognitive deterioration in dementia: a two year longitudinal study. Journal of Alzheimers Disease. 2011 doi: 10.3233/JAD-2010-100450. [DOI] [PubMed] [Google Scholar]
- 30.Clark LJ, Gatz M, Zheng L, Chen YL, McCleary C, Mack WJ. Longitudinal verbal fluency in normal aging, preclinical, and prevalent Alzheimer’s disease. American J Alzheimers Disease Other Dementias. 2009;24(6):461–468. doi: 10.1177/1533317509345154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Hoth K, Poppas A, Moser DJ, Paul RH, Cohen RA. Cardiac dysfunction and cognition in older adults with heart failure. Cogn Behav Neurol. 2008;21(2):65–72. doi: 10.1097/WNN.0b013e3181799dc8. [DOI] [PubMed] [Google Scholar]
- 32.Wolfe R, Worrall-Carter L, Foster K, Keks N, Howe V. Assessment of cognitive function in heart failure patients. Eur J Cardiovasc Nurs. 2006;5(2):158–164. doi: 10.1016/j.ejcnurse.2005.10.005. [DOI] [PubMed] [Google Scholar]
- 33.Gorkin L, Norvell NK, Rosen RC, Charles E, Shumaker SA, McIntyre KM, et al. Assessment of quality of life as observed from the baseline data of the studies of left ventricular dysfunction (SOLVD) trial quality of life substudy. Am J Cardiol. 1993;71:1069–1073. doi: 10.1016/0002-9149(93)90575-w. [DOI] [PubMed] [Google Scholar]
- 34.Cahn-Weiner DA, Boyle PA, Malloy PF. Tests of executive function predict instrumental activities of daily living in community-dwelling older individuals. Appl Neuropsychol. 2002;9:187–191. doi: 10.1207/S15324826AN0903_8. [DOI] [PubMed] [Google Scholar]
- 35.Freilich BM, Hyer LA. Relation of the repeatable battery for assessment of neuropsychological status to measures of daily functioning in dementia. Psychol Rep. 2007;101:119–129. doi: 10.2466/pr0.101.1.119-129. [DOI] [PubMed] [Google Scholar]
- 36.Tierney MC, Charles J, Jaglai S, Snow WG, Szalai JP, Spizziri F, et al. Identification of those at greatest risk of harm among cognitively impaired people who live alone. Aging Neuropsychology Cognition. 2001;8:182–191. [Google Scholar]
- 37.Randolph C, Tierney MC, Mohr E, Chase TN. The Repeatable Battery for the Assessment of Neuropsychological Status (RBANS): preliminary clinical validity. J Clinical Experimental Neuropsychology. 1998;20:310–319. doi: 10.1076/jcen.20.3.310.823. [DOI] [PubMed] [Google Scholar]
- 38.Hom J, Reitan RM. Generalized cognitive function after stroke. J Experimental Neuropsychology. 1990;12:644–654. doi: 10.1080/01688639008401008. [DOI] [PubMed] [Google Scholar]
- 39.Vlaar AMM, Wade DT. Verbal fluency assessment of patients with multiple sclerosis: test–retest and inter-observer reliability. Clin Rehabil. 2003;17(7):756. doi: 10.1191/0269215503cr674oa. [DOI] [PubMed] [Google Scholar]