Abstract
This review is based in part on a roundtable discussion session: “Physiological roles for heterotypic/heteromeric channels” at the 2013 International Gap Junction Conference (IGJC 2013) in Charleston, South Carolina. It is well recognized that multiple connexins can specifically co-assemble to form mixed gap junction channels with unique properties as a means to regulate intercellular communication. Compatibility determinants for both heteromeric and heterotypic gap junction channel formation have been identified and associated with specific connexin amino acid motifs. Hetero-oligomerization is also a regulated process; differences in connexin quality control and monomer stability are likely to play integral roles to control interactions between compatible connexins. Gap junctions in oligodendrocyte:astrocyte communication and in the cardiovascular system have emerged as key systems where heterotypic and heteromeric channels have unique physiologic roles. There are several methodologies to study heteromeric and heterotypic channels that are best applied to either heterologous expression systems, native tissues or both. There remains a need to use and develop different experimental approaches in order to understand the prevalence and roles for mixed gap junction channels in human physiology.
1. Introduction
Proteins known as connexins form gap junction channels that provide a direct connection and enabling the exchange of small molecules between adjacent cells. Different connexins form channels with different permeability and gating characteristics that dictate the type of intercellular communication they mediate. Moreover, different connexins are subject to different classes of posttranslational modification, such as phosphorylation, which further regulate gap junctional communication.
As an added level of complexity, gap junction channels can be formed containing more than one connexin isoforms [1, 2]. This allows formation of channels with unique gating and permeability that would not be otherwise attainable with channels composed of a single connexin isoform. Not all connexins are compatible to interact, which enables specific networks of interconnected cells to be formed and independently regulated.
There are now considerable data demonstrating which connexins are compatible to form a mixed gap junction channels and which cannot. Most of the evidence in support of the potential for connexins to interact has come from using transfected connexin-null cell models expressing one or more exogenous connexins. While this does provide useful information, observations obtained using expressed transgenes need to be interpreted in the context of native tissue systems. This requires taking into account tissue specific connexin expression, tissue architecture, molecular composition of cell-cell interfaces, and regulation via signal transduction pathways. In this review we summarize the current state of the art of how connexins interact and discuss implications for this in regulating tissue function.
2. Molecular Basis for Connexin Compatibility
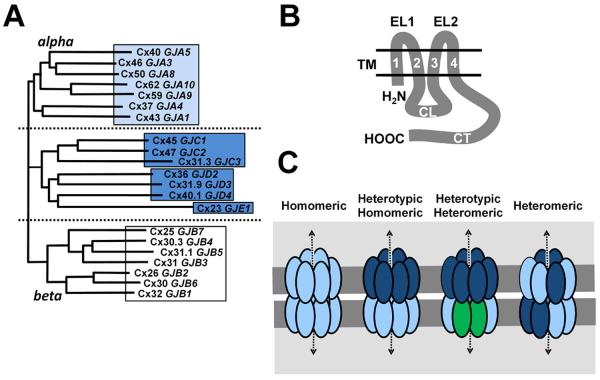
Connexins are multipass transmembrane proteins with both the N- and C-termini oriented towards the cytosol (Figure 1). There are 21 human connexin genes that are translated into functional proteins. By amino acid sequence homology connexins form three clusters, alpha connexins, beta connexins and a third cluster with intermediate homology composed of gamma, delta and epsilon connexins [3-5].
Figure 1. Structure and interactions between human connexins.
A. Shown is a dendrogram arranged using amino acid homology [5], where human connexin protein names were used (connexin; Cx) and gene names are in italics. B. Line diagram of a generic connexin, showing both the N-terminus (NH2) and C-terminus (CT) oriented towards the cytoplasm. Other protein elements include the two Extracellular Loop (EL) domains, four Transmembrane (TM ) domains, Cytoplasmic Loop (CL) domain. C. Diagram of different classes of channels, including homomeric, heterotypic and heteromeric.
Twelve connexins interact in order to form a complete gap junction channel; six connexins in the plasma membrane of one cell oligomerize and dock with compatible hexamers on an adjacent cell [2, 6]. Hexamers that act as bona fide plasma membrane channels without docking are called hemichannels. Gap junction channels composed of a single type of connexin protein are homomeric; heteromeric channels contain two or more different types of connexins (Figure 1). Heterotypic channels are formed by a hexamer on one cell docked to a hexamer with different connexin composition on the other. Heterotypic channels are most typically formed from two homomeric hexamers (Figure 1), however, they can also consist of a homomeric and heteromeric hexamer or two heteromeric hexamers. Based largely on sequence homology, connexin structure determination and the analysis of connexin interactions in model systems, there is a considerable amount known about the molecular determinants that regulate connexin compatibility.
2.1 Heteromeric Compatibility
The amino acid homology dendrogram in Figure 1 provides a reasonable guide to heteromeric compatibility among connexins [5, 7]. Heteromeric compatibility of alpha vs beta connexins correlates well with a signature amino acid motif localized at the interface region where the cytosolic intracellular loop (CL) domain transitions into the third transmembrane domain (TM3) (Table 1; Figure 2). For most alpha connexins, this motif contains a conserved arginine or lysine residue (which we have referred to as R type connexins) [7]. By contrast, beta connexins contain a di-tryptophan (“WW”) motif (W type connexins) [7, 8].
Table 1.
Motifs which regulate heteromeric compatibility
| R type | |
| Cx43 | 151 – LLRTY – 155 |
| Cx46 | 145 – LLRTY – 149 |
| Cx50 | 147 – LLRTY – 151 |
| Cx45 | 173 – LMKIY – 177 |
| Cx47 | 209 – LMRVY – 213 |
| Cx36 | 195 – ISRFY – 199 |
| Cx31.9 | 132 – ARRCY – 136 |
| W type | |
| Cx26 | 132 – LWWTY – 136 |
| Cx30 | 132 – LWWTY – 136 |
| Cx30.3 | 127 – LWWTY – 131 |
| Cx31 | 127 – LWWTY – 131 |
| Cx32 | 131 – LWWTY – 135 |
| Other | |
| Cx37 | 151 – LMGTY – 155 |
| Cx40 | 149 – LLNTY – 153 |
Figure 2. Heteromeric and heterotypic compatibility.
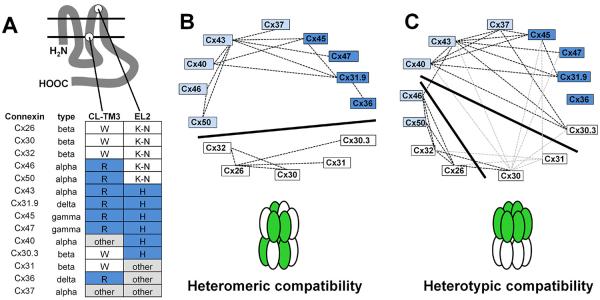
A. Location of heteromeric and heterotypic compatibility motifs. Shown are selected connexins, gene homology group, heteromeric specificity motif in the transition between the cytoplasmic loop (CL) and third transmembrane (TM3) domains and heterotypic specificity motif in the second extracellular loop (EL2) domain. B. Heteromeric interactions indicated by the wheel diagram where connexins known to form heteromeric channels are connected by dashed lines. The solid dividing line separates beta connexins (white) from other connexins to indicate a lack of heteromeric compatibility. C. Heterotypic interactions indicated by the wheel diagram where connexins known to form heteromeric channels are connected by dashed lines. There are conflicting data regarding heterotypic Cx30 and Cx31 containing channels, this is indicated by the gray dashed lines (see text). The solid dividing lines show three putative compatibility groups which do not align with overall amino acid homology groups. Based on [1, 18, 20, 34, 35, 38, 39, 65-67, 89, 90, 92, 97, 101, 106-112, 113].
Control of hetero-oligomerization by R and W motifs is most likely indirect. Based on the high resolution structure of Cx26, the WW motif is localized to the cytosol-membrane interface of the cytoplasmic leaflet and does not directly mediate interprotein connexin-connexin interactions [7, 9]. Instead, several broadly conserved amino acids in TM2 and TM4 near the extracellular aspect of these domains form salt bridges or hydrogen bonds to stabilize hexamers [9]. Moreover, mutations in amino acids directly involved in connexin-connexin interactions are associated with human disease [10, 11]. Thus, roles for R and W type motifs in regulating hetero-oligomerization are indirect and most likely due to control of the initiation of oligomerization as olpposed to hexamer stabilization.
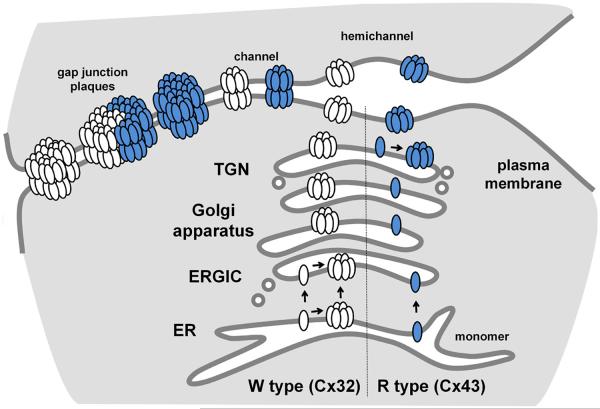
In fact, R type and W type connexins oligomerize via different pathways, which, in turn, play a key role in preventing heteromer formation between them. The most thoroughly studied R type connexin is Cx43. In contrast to most oligomeric transmembrane channel proteins, Cx43 is stabilized as monomers in the endoplasmic reticulum (ER) and only oligomerizes after transport to the trans Golgi network (TGN) (Figure 3) [7, 12-14]. Part of this pathway is regulated by a quality control protein, ERp29, which binds to the second extracellular loop domain and stabilizes a conformation favoring monomeric Cx43 in all cell types tested thus far [13]. However, arginine 153 at the CL to TM3 interface is also required to maintain monomeric Cx43 in the ER (Table 1) [14]. Moreover, substituting tryptophan for arginine at position 153 destabilizes Cx43, resulting in premature oligomerization and misfolding. It is likely that the substitution of a bulky hydrophobic amino acid for a charged amino acid alters the positioning of TM3 relative to the plane of the membrane, which is needed to maintain stable monomers. This change in amino acid may also impair ERp29 binding, which would further destabilize a monomeric conformation. Cx46, another R type alpha connexin, has also been shown to oligomerize in the TGN [15]. It is likely that other R type connexins follow a comparable TGN oligomerization pathway but this has not been directly demonstrated. In particular, whether connexins other than Cx43 interact with ERp29 or a comparable chaperone remains to be determined.
Figure 3. Connexin oligomerization pathways.
Connexins are co-translationally inserted into the ER membrane. Depending on the connexin subtype, oligomerization can occur either in the ERGIC (W type, Cx32, white) or the TGN (R type, Cx43, shaded). The potential for connexin oligomerization in the ER, driven by high levels of connexin expression, is also shown for Cx32. Hemichannels are subsequently transported to the plasma membrane, where they can function as channels or pair with hemichannels on adjacent cells to form complete intercellular channels. Channels at the plasma membrane further assemble into semi-crystalline arrays known as gap junction plaques, which can contain from tens to thousands of channels. Homogenous plaques are composed of either a single connexin or heteromeric connexins (not shown). Heterogeneous plaques contain regions enriched for different connexins. Adapted from [2], with permission.
By contrast, most evidence suggests that Cx26 and Cx32, which are W type beta connexins, oligomerize in the ER [13, 14]. Both the WW motif and the inability to bind to ERp29 promote Cx32 oligomerization in the ER. However, at low levels of expression, Cx26 and Cx32 do not oligomerize when transport out of the ER is inhibited with brefeldin A [16]. Clearly, stabilizing monomeric connexins in the ER is a saturable process, since Cx43 overexpression can force premature oligomerization [17]. Thus, it is important to consider expression level when interpreting results using transfected cell systems. A conservative interpretation of the current literature is that oligomerization of W type connexins occurs at a lower concentration than R type connexins. This difference in oligomerization capacity between W type and R type connexins provides a mechanistic basis for heteromeric compatibility, since pre-formed hexamers will prevent incorporation of connexins that are more stably maintained as monomers irrespective of the compartment where they are assembled (i.e. ER and/or TGN).
2.2 Regulation of Hetero-oligomerization
In addition to the R type and W type connexins, several connexins do not conform to either category based on amino acid sequence homology and are categorized as “other”(Figure 2; Table 1) [7]. Two of these are alpha connexins, Cx37 and Cx40, are particularly relevant to cardiovascular physiology. Cx40 has a leucine-asparagine (LN) motif instead of the leucine-arginine (LR) motif present in Cx43. Despite this motif difference, Cx40 monomers are stable in the ER, suggesting that the asparagine residue has sufficient charge at the CL to TM3 interface to behave comparably to an R type connexin [7]. By contrast, Cx37 completely lacks the LR motif and instead has a methionine-glycine (MG) motif. This motif has significant consequences for Cx37 oligomerization [7]. In HeLa cells transfected with Cx37, half of Cx37 oligomerizes in the ER while the remainder is stabilized as monomers. Moreover, substituting an MG motif for the LR motif on a Cx43 backbone has a similar effect, further implicating the MG motif in destabilizing connexin monomers in the ER.
It is known that both Cx37 and Cx40 have the ability to hetero-oligomerize with Cx43 [18, 19]. Interestingly, Cx37 and Cx40 differ in dose response of heteromer formation with Cx43 based on single channel conductance measurements using an inducible expression system [20]. In the case of Cx37, channels with intermediate/low conductance are more prevalent at high levels of Cx37 expression than at low levels of Cx37 expression, where there are more channels with high conductance, more similar to homomeric Cx37. By contrast, Cx40 at a low level of expression shows more intermediate conductance channels than at high Cx40 expression.
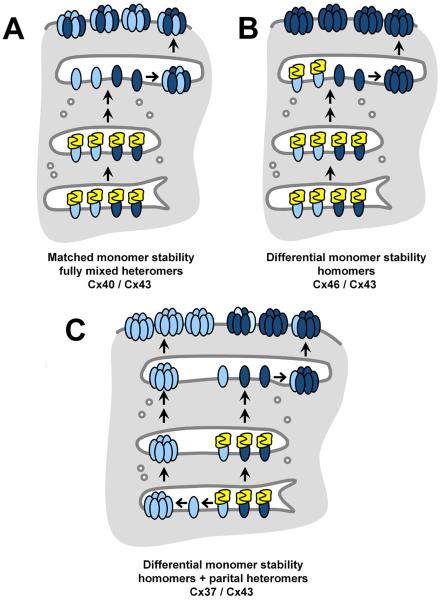
Since vascular gap junction composition is variable and tends to show less co-localization of Cx37 with Cx40 and Cx43 in situ, hetero-oligomerization between Cx37, Cx40 and Cx43 is highly likely to be regulated rather than stochastic [21-24]. These data on monomer stability, along with the differential monomer stability of Cx37 and Cx40 suggest a potential model for regulated hetero-oligomerization (Figure 4). For Cx40 and Cx43, monomer stability is comparable (Figure 4A), and so at a matched level of expression, heteromeric Cx40+Cx43 should be the predominant type of channel. However, when Cx40 expression increases, this may saturate the capacity of Cx43 to hetero-oligomerize, resulting in more homomeric Cx40. Cx37 has the reverse situation (Figure 4C). In this case, low levels of Cx37 would be prone to oligomerization in the ER, preventing hetero-oligomerization with Cx43. Higher levels of Cx37 are expected to increase the Cx37 monomer pool, which may increase the potential to hetero-oligomerize with Cx43.
Figure 4. Hypothetical monomer stability model for regulated hetero-oligomerization.
Different connexins are depicted by different colored ovals, yellow represents quality control chaperones which stabilize monomeric connexins (e.g. ERp29 [13]) A. A cell expressing connexins with matched monomer stability would have oligomerization occur in the same intracellular compartment and be expected to form fully heteromeric channels. A pair of connexins with matched monomer stability are Cx40 (light) and Cx43 (dark) [7]. B. A cell expressing connexins where one connexin has greater monomer stability than another would have restricted hetero-oligomerization, leading to monomeric channels. A pair of connexins with this type of mismatched monomer stability are Cx46 (light) and Cx43 (dark) [15]. C. A cell expressing connexins where one connexin has less monomer stability than another would have reduced hetero-oligomerization as a result of prior hexamer formation. A pair of connexins with this type of mismatched monomer stability are Cx37 (light) and Cx43 (dark) [7]. In either case B or C, expression and function of the connexin quality control pathway can potentially act as a “rheostat” to regulate hetero-oligomerization by a direct effect on relative monomer stability.
Regulated hetero-oligomerization can also occur when connexin monomers are highly stabilized. For example, in some cells co-expressing Cx43 and Cx46, Cx46 is retained as monomers in the TGN while Cx43 assembles into homomeric channels (Figure 4B) [15, 25]. Differential hetero-oligomerization occurs in several cell types, including osteoblasts and lung alveolar epithelial cells [15, 25, 26]. Moreover, differential Cx43+Cx46 hetero-oligomerization is dependent on alveolar epithelial cell phenotype. The best example of this is in alveolar epithelial cell differentiation, where type II cells restrict Cx43+Cx46 hetero-oligomerization until they differentiate into type I cells, where Cx43 and Cx46 hetero-oligoemrize [25, 26]. The functional role for this change in oligomerization is not known at present.
A likely candidate to regulate hetero-oligomerization between these different alpha connexins is ERp29 [13]. Whether this is the case will require demonstrating that ERp29 interacts with other connexins beyond Cx43. However, we propose that ERp29 or other quality control chaperones act as a “rheostat” to alter connexin assembly. In this model, changes in expression, activity or target affinity either promote or inhibit oligomerization. Organelle microenvironment, such as changes in lumen pH, calcium and/or membrane lipid composition could also influence connexin oligomerization. In particular, controlling whether connexins oligomerize in the same or different intracellular compartments provides a potential mechanism for regulated hetero-oligomerization. Testing this in native systems or in disease states where quality control is impaired is anticipated to provide evidence to determine whether this is the case.
2.3 Heterotypic compatibility
Hemichannel docking is controlled by motifs in the connexin extracellular loop domains [27]. Based on the high resolution structure of Cx26 channels there are several key hydrogen bonds that need to form for proper hemichannel docking to produce a complete gap junction channel (Figure 5) [9, 28, 29]. As is the case with heteromeric compatibility, there are compatible and incompatible combinations of heterotypic hexamers. However, heterotypic compatibility does not parallel heteromeric compatibility (Figure 2) [27].
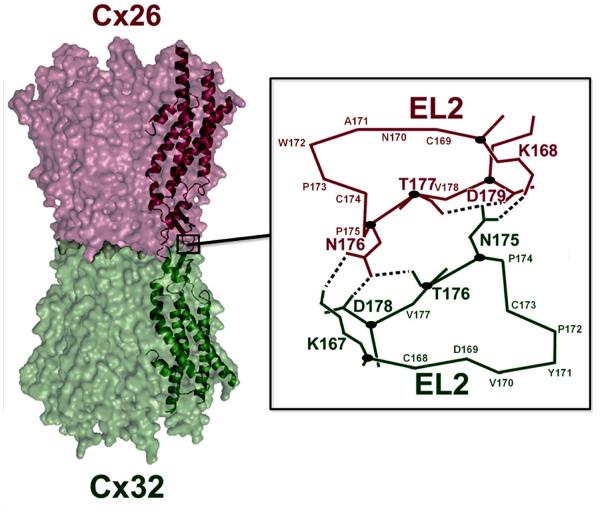
Figure 5. Structural homology model of a heterotypic Cx26/Cx32 gap junction channel.
One pair of end-to-end docked Cx26 and Cx32 subunits is highlighted as ribbon and line diagrams overlaid on the structural model. Six hydrogen bonds at the EL2-EL2 docking interface are predicted by the homology model (inset). Residues involved in the spatial positioning of the interface are labeled and the central carbon atoms of the residues involved in hydrogen bonding are represented by a dark oval. Hydrogen-bonds shown are between Cx32 N175 and Cx26 K168, T177 and D179 and between Cx26 N176 and Cx32 K167, T176 and D178. Adapted from [28] with permission.
Instead, there are two different prevailing heterotypic compatibility groups, Group 1 and Group 2 that can be categorized by compatibility motifs in the second extracellular loop (EL2) domain (Table 2) [28]. By contrast, the significant level of amino acid homology in the first extracellular loop domain (EL1) indicates this domain may be less critical for heterotypic specificity than the EL2 domain [28].
Table 2.
Motifs which regulate heterotypic compatibility
| Group 1 – K-N type | |
| Consensus -Φ(K/R)CxxxPCPNxVDCΩΨS- | |
| Cx26 | 167 – VKCNAWPCPNTVDCFVS – 183 |
| Cx30 | 167 – LKCGIDPCPNLVDCFIS – 183 |
| Cx32 | 166 – VKCDVYPCPNTVDCFVS – 182 |
| Cx46 | 179 – YRCDRWPCPNTVDCFIS – 195 |
| Cx50 | 181 – YRCSRWPCPNVVDCFVS – 197 |
| Group 1 – Other | |
| Cx31 | 162 – VQCANVAPCPNIVDCYIA – 179 |
| Group 2 – H type | |
| Consensus -ΦxCxxxPCPHxVDCΩΨS- | |
| Cx30.3 | 162 – VACSVEPCPHTVDCYIS – 178 |
| Cx31.9 | 164 – FACAGPPCPHTVDCFVS – 182 |
| Cx43 | 185 – YTCKRDPCPHQVDCFLS – 201 |
| Cx45 | 207 – YVCSRLPCPHKIDCFIS – 223 |
| Cx47 | 240 – FPCSRQPCPHVVDCFVS – 256 |
| Cx40 | 183 – HVCRRSPCPHPVNCYVS – 199 |
| Group 2 – Other | |
| Cx37 | 185 – FVCQRAPCPYLVDCFVS – 201 |
| Cx36 | 229 – YECNRYPCIKEVECYVS – 245 |
Shown are motifs in the second extracellular loop (EL2) domain that help confer heterotypic specificity. Group 1 and Group 2 designations are from [28]. The consensus amino acid sequences for K-N and H type EL2 domains are also shown, where Φ (refers to hydrophobic residues, Ω refers to aromatic residues, and Ψ refers to amino acids with large aliphatic side chains [105]. Cx31, Cx36 and Cx37 do not fit well in either the K-N or H type categories. Note that aside from a few key residues (underlined, bold), the K-N and H type consensus sequences are comparable. See also Figure 2.
The consensus EL2 motif for both Group 1 and Group 2 connexins is very similar (Table 2). For Group 1 connexins, the consensus EL2 sequence is Φ(K/R)CxxxPCPNxVDCΩΨS (Table 2). The signature lysine/arginine (K/R) and asparagine (N) residues are particularly critical as specificity determinants for Group 1 heterotypic interactions. The Group 2 consensus sequence, ΦxCxxxPCPNxVHCΩΨS, differs from Group 1 connexins in that the initial K/R residue is not conserved and has a histidine (H) instead of the asparagine found in the Group 1 connexins. Despite such close similarity in sequence, functional data demonstrate that a few key amino acids have a profound effect on heterotypic compatibility (below).
Some connexins do not match well with either consensus motif. Most dramatically, Cx31 has an additional amino acid insertion in this EL2 motif that is not present in other connexins, which alters the spacing between the conserved cysteine residues (Table 2). Human Cx31 is heterotypically compatible with several Group 1 and Group 2 connexins, despite the observation that mouse Cx31 is not, suggesting that this difference in EL2 has functional ramifications [30, 31]. Cx36 and Cx37 also do not conform well with either EL2 consensus sequence. Cx36 and Cx37 have neither the H nor N signature residue. Cx40 has an H residue at position 192, suggesting that it belongs in Group 2, although it lacks the bulky hydrophobic amino acid (Φ) at position 183 that is present in both consensus motifs.
Given these differences, we propose to refer to heterotypic compatibility motifs as either a K-N type motif, an H type motif or “other” (Table 2). Connexin classification based on CL-TM3 and EL2 motifs is shown for selected connexins in Figure 2. Note that there are connexins with many different combinations of these motifs. Interestingly, Cx37 falls into the “other” category for both the CL-TM3 and EL2 motif, underscoring the unique regulation of this connexin likely critical for its physiologic function.
The high resolution structure of Cx26 has enabled the molecular basis for heterotypic Cx26:Cx32 interactions to be examined in detail [9, 28]. Heterotypic compatibility between Cx26 and Cx32 is mediated by conserved positively charged residues (K, R and N) that interact along with a critical aspartate (D) to form hydrogen bonds to stabilize channel docking (Figure 5). On the other hand, the conserved histidine (H) residue in H type connexins does not form a comparably stable complex with the K-N type EL2 motifs. This was elegantly shown by demonstrating that mutant forms of Cx32 (Cx32N175H and Cx32N175D) that were unable to homotypically interact with wild type Cx32 were fully rescued by compensatory mutations in Cx26 (Cx26K168V and Cx26D179N respectively) [28]. Critically, the Cx32N175D mutation, which is unable to form gap junction channels, causes Charcot-Marie-Tooth disease, underscoring the importance of proper hemichannel docking in this and other connexin-related diseases [32].
In addition to beta connexins, the two alpha connexins, Cx46 and Cx50 are also K-N type connexins. Moreover, a beta connexin, Cx30.3, is an H type connexin (Figure 2; Table 2). This raises the potential for a heteromeric hexamer containing both K-N type and H type connexins (e.g. Cx46 and Cx43) to dock with a hexamer containing either another K-N type connexin. This would require precise alignment of compatible connexins in hemichannel docking and so innate incompatibility seems a more likely outcome to dominate and prevent a heterotypic interaction. However, heterotypic compatibility seems to be remarkably flexible, which may permit unusual docking interactions. For instance, Cx46 has been demonstrated to be heterotypically compatible with both K-N type connexins (Cx26, Cx32, Cx50) and an H-type connexin (Cx43) [33]. Cx32N175D also can partially interact with wild type Cx26 [28], consistent with the concept of plasticity in heterotypic compatibility.
Also there are conflicting data in the literature as to whether human Cx30 is heterotypically compatible with H type connexins, e.g. Cx43 and Cx47 (see below) [34, 35]. Interestingly, the more variable part of the signature K-N EL2 domain for human Cx30 lacks the aromatic residue at position 171 that is present in other K-N type connexins. Instead, Cx30 has an aspartate residue (D), which is also found in Cx43 at position 190 (Table 2). Thus, differences in Cx30 underscore the more general concept that heterotypic compatibility is influenced by structural elements that are not directly involved in inter-loop hydrogen bonding. Examining the compatibility profile of targeted point mutations will help determine whether this is the case.
3. Detecting and assessing the function of heteromeric and heterotypic channels
3.1 Use of expression systems
One approach to determine whether connexins interact is to use connexin-null cells that are transfected to heterologously express one or more connexins. Mammalian connexin-null cell models that have been used for this purpose include BHK, HeLa, N2A, SK Hep1, or RIN cells (e.g. [8, 16, 18, 31, 36-38]). In addition to connexin null cells, it can also be useful to transfect connexins into cell lines expressing a single connexin for the purposes of evaluating hetero-oligomerization. These mammalian expression systems are amenable to biochemical, morphological and functional analysis. Xenopus oocytes, in which endogenous Cx38 is knocked down using antisense oligonucleotides, provide a system that is especially amenable to double, whole-cell, voltage clamp measurement of gap junction properties, given their size [39, 40]. In mammalian cells it is possible to assess the properties of gap junctions as well as their comprising channels [41].
The potential for high levels of connexin expression is a particular concern with the use of heterologous expression systems since this can yield false positives, e.g. by over-representing events such as formation of channels that might occur but at extremely low, sub-physiologic levels. Accumulation of misfolded connexins could also have an impact on channel function by inducing cell stress responses. Relative connexin stoichiometry is also difficult to control, especially when relying strictly on immunoblots, which can provide quantitative insight on expression levels but not definitive composition of assembled channels. Analysis of cells with radiolabeled connexins coupled with immunoprecipitation and standards of known specific activity is one way to more quantitatively define heteromer stoichiometry [42-44]. Inducible expression systems are another approach that enables gap junction channels of differing stoichiometry to be formed and analyzed in the same cell background (e.g. [8, 20]).
Cells expressing multiple connexins enable heteromeric compatibility to be measured whereas two populations of cells expressing different connexin isoforms can be co-cultured to assess heterotypic compatibility. Each of these systems allows connexin composition to be manipulated and is particularly useful for analysis of the impact of mutations and chimeras on connexin-connexin interactions.
The relative simplicity of transfected cell systems is a strength of the approach, since analysis is performed using a single cell type expressing one vs. two connexins. This is also a pitfall of the approach though, because it is difficult to match the stoichiometry and context of connexin expression in a native tissue using a heterologous system.
3.2 Morphologic criteria for heteromeric and heterotypic interactions
Immunofluorescence microscopy can provide some clues as to whether connexins may be compatible, based on whether two different connexins co-localize or not. Of course, antibody specificity and lack of cross-reactivity are critical for the success of any immunodetection technique [23, 45]. However, even conventional imaging techniques and confocal microscopy are subject to the Rayleigh limit of ~250 - 300 nm resolution in the x-y plane, which is well above the diameter of a gap junction channel (~20 nm) [9]. Thus, immunofluorescence co-localization could reflect a mixture of homomeric/homotypic channels within the same gap junction plaque and not necessarily reflect heteromerization (e.g. Figure 3). On the other hand, if a particular cell-cell interface is known to be composed of two cells expressing different connexins, then co-localization would support heterotypic compatibility.
Getting beyond the resolution limit of fluorescence microscopy is essential for definitive morphological analysis of connexin hetero-oligomerization. Photoactivatable molecules (FlAsH, ReAsH) have been used to tag engineered connexins and offer the ability to translate fluorescence imaging to the EM level of resolution [46, 47]. However this approach is not well suited to the simultaneous imaging of two different connexin isoforms in the same sample.
Fluorescence Resonance Energy Transfer (FRET) microscopy is another approach that has been used to measure protein-protein interactions within 10 nm [48, 49]. Of relevance to connexins, FRET microscopy was used to demonstrate that Cx26, Cx30 and Cx31 form heteromeric channels in transfected keratinocytes [50]. It is surprising that FRET microscopy has not been more widely used to measure hetero-oligomerization between other connexins in other cell systems. Also, while useful for transfected model systems, FRET requires fluorescently tagged proteins and cannot be used to image heteromers in native tissues.
In the last several years there have been advances in the field of super-resolution fluorescence microscopy, which extends the limit of resolution to 20-50 nm making the localization of small protein complexes possible. These include Photoactivation Localization Microscopy (PALM) [51] and Stochastic Optical Reconstruction Microscopy (STORM) [52]. STORM relies on photo-switchable fluorescent antibodies that differ from conventional fluorescent antibodies in that they are double labeled. Imaging in STORM uses an activation/deactivation cycle to temporally separate probes that are otherwise unresolvable. This next generation technology enables studying in situ the fine structure of subcellular components including the plasma membrane, cytoskeleton, focal adhesions and tight junctions [53-56]. It is likely to have comparable utility in studying the fine structure of gap junction plaques as well, although this level of resolution is still too coarse to definitively assign two different connexins to the same channel, although refinements to superresolution microscopy may enable detection of heteromeric channels. Nonetheless, super-resolution microscopy presents a novel way to approach questions related to the trafficking and assembly of connexins into channels, as well as more generally applicable to characterizing gap junction plaque composition.
The Duolink Proximity Ligation Assay (PLA) is another approach which can increase the ability to detect protein-protein co-localization to within ~40 nm [57]. The PLA assay is based on using two different primary antibodies coupled to oligonucleotides that anneal and can be ligated when in close proximity to form a circular DNA molecule. This DNA is then amplified by rolling circle DNA synthesis and detected using fluorescent oligonucleotides. Thus, the fluorescence in and of itself is the result of an amplified signal that is created at the expense of resolution. However, the inherent resolution of the PLA technique lies in the proximity of the two antibodies, which enable the DNA to circularize and anneal; this essentially provides a yes or no answer as to whether two proteins are within 40 nm, again larger than the diameter of a gap junction channel. Nonetheless, this approach was successfully used to characterize complexes of ZO-1 and voltage gated sodium channels with gap junction plaques [58, 59].
Transmission and freeze fracture EM enable high resolution imaging and, when combined with immunogold labeling, can assign specific connexins to highly localized areas of gap junction plaques. Freeze fracture is particularly informative, since the gap junction particles are readily identifiable and can be aligned to immunogold labeling [60-63]. The utility of the freeze fracture approach was further extended through the development of matched double-replica freeze fracture replica immunolabeling (DR-FRIL) [45]. In this approach, both sides of the fracture plane are retrieved and paired, followed by double immunolabeling with antibodies followed by secondary antibodies conjugated to differently sized gold particles [45]. While technically challenging, this approach enables connexins on both sides of a plaque to be identified to high resolution and in a native tissue. For instance, DR-FRIL was used to demonstrate that in the retina, Cx36 and Cx45 are in close apposition in the same gap junction plaque [45]. However, heterotypic Cx36+Cx45 was not detected, suggesting that they were unlikely to form heterotypic channels, as was previously thought to be the case.
3.3 Biochemical criteria
Gap junction hemichannels are relatively stable structures and can be isolated under defined solubilization conditions. This enables co-immunopurification to be used as a criterion for hetero-oligomerization. This has been used with both native and tagged connexins to demonstrate a direct interaction [26, 36, 38, 61, 64-68]. This approach is best applied to transfected cell models, where the two connexins to be tested are at comparable and high levels of expression. Co-immunopurification has also been used in native cell and tissue systems to demonstrate hetero-oligomerization [26, 61, 69, 70]. One pitfall of co-immunoprecipitation is that while heteromeric channel formation can be detected, it is difficult to determine the extent of hetero-oligomerization. In other words, heteromer detection does not necessarily mean that the majority of gap junction channels are in fact heteromeric.
Several studies have demonstrated that connexin channels partition into cholesterol enriched membrane microdomains (also known as lipid rafts) and associate with caveolins, which is important for connexin trafficking from the TGN to the plasma membrane [71-74]. Locke et. al [72] extended this analysis to measure differential partitioning of Cx26 homomers, Cx32 homomers and Cx26+Cx32 heteromers into detergent resistant membrane microdomains. Critical keys to the success of this approach were to use multiple different detergents and detergent concentrations coupled with sucrose gradient flotation centrifugation. In TX-100 and NP-40 solubilized samples, homomeric Cx32 and heteromeric Cx26+Cx32 co-migrated with a lipid marker for microdomains, GM1. By contrast Cx26 was not present in the microdomain fractions in TX-100 solubilized samples [72]. Thus, these different classes of hexamers have different biophysical properties. This has implications for gap junction plaque formation, since it suggests that different classes of channels will likely self-partition into distinct regions of a plaque [75].
Measuring the dominant negative effects of connexins on trafficking and delivery is another approach that can be used to identify potential hetero-oligomerization compatibility in intact cell models since oligomerization is required for transport to the plasma membrane. This can be done using connexins tagged with different retention and/or localization motifs that, when co-expressed with untagged connexins inhibit delivery to the plasma membrane [26, 76, 77]. Connexins associated with human diseases can also be analyzed using this approach, either by trapping wild type connexins inside cells or by having retention of the mutant connexin relieved by co-expression with wild type connexins [50, 78]. This approach has particular relevance to human disease by either impairing gap junction formation or by forming channels with impaired function. However, it is important to consider that interactions of mutant connexins with wild type connexins do not necessarily reflect how wild type heteromers form and are instead relevant to a human disease state. For example, several Cx26 mutations result in an aberrant interaction with Cx43 to cause palmoplantar keratoderma associated with hearing impairment that does not reflect the inherent incompatibility of Cx26 and Cx43 [79].
3.4 Functional criteria
Formation of functional gap junction channels is the gold standard for determining whether connexins interact or not. The predominant methods for measuring intercellular communication include measuring intercellular transfer of fluorescent dyes of differing size and charge and electrophysiological approaches, including analysis of gap junction conductance, channel gating and single channel conductance measurements [27, 80]. While both of these approaches have utility, they do not necessarily correlate well with the permeability of connexin channels to endogenous substrates, which is the physiological role for gap junctional communication. It is feasible, but difficult, to measure transfer of actual, biologically relevant molecules through gap junction channels, in part because of the large number of molecules that may be permeants, and the unknown relevance of those molecules to specific cell functions. Metabolic capture of metabolites [81], molecule transfer through reconstituted channels [82] and indirect assays for metabolite transfer [83-85] have all been used to measure intercellular transfer of cytosolic biomolecules [80]. These methods are best suited to measure intercellular communication in model cell systems; a major challenge will be to develop similar approaches that can be used in intact tissues.
3.5 Heterotypic coupling
Nonetheless, measurements of dye transfer do have some utility in determining whether connexins interact to form functional channels or not, provided that the size, shape and charge of the probes used are taken into consideration. Dye transfer also has been successfully used with intact brain tissue slices and correlated with immunohistological identification of connexins and cell types to measure communication between cells in the central nervous system (e.g. [86-88]). Thus, at face value, it would appear that heterotypic interactions would be easy to measure using co-cultures of cells expressing different connexins [31].
However, a particularly conspicuous example where measuring heterotypic coupling has proven problematic involves Cx30. Initial studies examined N2A and HeLa cells stably transfected with untagged connexin cDNA and then transiently transfected with vectors encoding a marker protein (either EGFP or DsRed). This method enabled identification of live cells expressing different connexins, effectively labeling transiently transfected cells that interact with the stably transfected cells expressing a single connexin [89]. In this study Cx30 formed functional heterotypic channels with Cx32 but not Cx43 or Cx47 as measured by net junctional conductance. By contrast, a subsequent study using the same Cx30 cDNA construct engineered into lentiviral expression vectors and then used to transduce HeLa cells or primary Cx43-null astrocytes showed heterotypic dye transfer between Cx30 and Cx32, Cx43 and Cx47 [35]. One possibility is that heterotypic compatibility may be context sensitive, e.g. Cx30 expressed by HeLa cells may be more heterotypically promiscuous than Cx30 expressed by N2A cells or in a native context of natural oligodendrocyte:astrocyte (O:A) junctions [34, 35, 45]. In particular, connexin knockout mice were used to show that O:A junctions in Cx32 deficient mice lack Cx30 but retain Cx43 and Cx47 [90] whereas, mice lacking astrocytic Cx43 show specific disruption of Cx47 [91]. These data reveal likely heterotypic compatibility in the native tissue and suggest a model where heterotypic docking stabilizes connexin localization to gap junctions through channel formation. Tissue specific connexin targeting and quality control are also likely to be involved in this process.
Taken together these data are most consistent with the majority of gap junction channels at O:A junctions being Cx32+Cx30 and Cx47+Cx43 heterotypic channels. Furthermore, in contrast to mice harboring a single connexin knockout that retains some O:A coupling, Cx30+Cx43 double knockout mice lack detectable O:A gap junctional communication consistent with two parallel heterotypic communication pathways [87, 92-95]. As with any mouse knockout model, connexin deficiency can have pleiotropic effects beyond the effects on specific, compatible connexins. For instance, Cx47 deficient mice lacked Cx30 and Cx32 at O:A junctions in addition to Cx43, due to downstream disruption of MUPP-1 and ZO-1, which are required junctional components involved in gap junction plaque organization [87, 96].
The physiological roles for heterotypic coupling have not been fully elucidated. However, several heterotypic channels have asymmetric voltage gating profiles [97]. This means that heterotypic channels can be modulated to enable a directional pathway for intercellular signaling that is not achievable for homomeric or fully heteromeric channels.
3.6 Heteromeric coupling
Heteromeric coupling is more challenging to measure than heterotypic coupling since, by necessity, it involves detecting a change in the character of intercellular communication rather than the presence or absence of cell coupling. Differences in permeability and channel gating have been used as criteria to demonstrate that functional heteromeric channels have formed [1]. For example, Cx26+Cx32 heteromers have enhanced permeability to cGMP vs cAMP, whereas Cx32 has comparable permeability to both cyclic nucleotides, suggesting fine tuning of metabolic signaling can be mediated by heteromeric channels [70]. Changes in the distribution of single channel conductances as a result of co-expression of compatible connexins also provide strong evidence in support of heteromeric channel formation [20] as well as coupling functional data with biochemical evidence of an interaction between two connexins [19].
Changes to cardiac conduction examined in the context of connexin knockout and knockin mice demonstrate the complexity of connexin interactions affecting gap junctional communication in a physiological context, subject to the caveats mentioned above (reviewed in [98]). For example, Cx40 −/− mice show cardiac arrhythmias and significant impairment of AV node conduction [99, 100]. Cx40+Cx43 heteromers are particularly critical in atrial conduction, where Cx40 and Cx43 are expressed at comparable levels as opposed to the ventricle where Cx43 is the predominant connexin [101]. Spermine sensitivity is a particularly useful diagnostic of whether Cx40 is mediating conduction, since Cx40 channels are sensitive to spermine inhibition, while Cx43 channels are not [66].
Aside from being a useful tool to dissect out roles for Cx40 in regulating conduction in the heart, spermine block relates directly to interactions between Cx40 and Cx43 in heteromeric channels. In particular, the N-terminal domain of Cx43 is homologous to spermine; cell permeable peptides corresponding to this Cx43 domain specifically interact with the N-terminus of Cx40 to inhibit Cx40 channels [18]. This raises the intriguing hypothesis that Cx40 and Cx43 hetero-oligomerize, but that a large fraction of these channels may be closed under baseline conditions. However, the C terminal and cytoplasmic domains of Cx40 and Cx43 also interact and regulate chemical gating of heteromeric Cx40+Cx43 channels [102]. Roles for these other domains in regulating how the N-terminal domain of Cx43 regulates Cx40+Cx43 remain to be determined. These findings underscore the need for additional structural information beyond homomeric Cx26 in order to understand how hetero-oligomerization affects gap junction channel function.
4. Conclusion and Perspectives
Given the high resolution structure of Cx26 and our current knowledge about motifs that regulate connexin compatibility, understanding the molecular basis for connexin interactions at a deeper level seems close at hand. This effort would benefit from more direct structural data, particularly for other connexins beyond Cx26. Crystals and high resolution data of heterotypic connexin channels might be feasible to generate. However, given the potential for a binomial distribution of combinations of heteromeric channels [1], crystalizing and solving structures for these directly will be exceptionally difficult. Homology modeling and directed mutations, analogous to the approaches used to study heterotypic Cx26+Cx32 interactions is likely to yield useful information on heteromeric compatibility determinants and permissive combinations of connexin stoichiometry in heteromeric channels. In addition to identifying compatibility motifs, defining structural elements that uniquely regulate heteromeric and heterotypic channel function have only begun to be analyzed, particularly when considering how the cytosolic aspects of different connexins can interact and influence channel behavior.
There is also a need to better understand heterotypic interactions, particularly given some of the inconsistencies in the literature related to heterotypic connexin compatibility. There is a particular need to transition from transfected cell models to a more native context to better define physiologic roles for heterotypic channels. Cell coupling in the CNS seems to be the most tractable native context to study heterotypic connexin interactions [94, 95]. There is a need to expand the scope of these studies to other tissues, especially the cardiovascular system but kidney and lung are other organs that are also likely to have physiologically relevant heterotypic channels [103, 104].
Studying heterotypic and heteromeric gap junction channels in situ presents a particular challenge, especially when trying to consider native molecules that use these channels for intercellular communication. Reporter assays seem to be a logical, although indirect approach. Using a series of well calibrated fluorescent tracers may prove useful to better characterize the charge, shape and size thresholds for different channels in situ, provided they can be administered and traced in intact tissues. Advances in whole animal imaging would be needed to more precisely measure intercellular transfer in situ. However, immunolocalization of connexins is already starting to benefit from new high resolution techniques at both the light and EM level and is particularly amenable to analysis of intact tissues as well. Combining these approaches with analysis of properly constructed and analyzed transgenic mouse models should help further our understanding of how multiple connexins assemble and interact to regulate intercellular communication in a physiologic context.
Supplementary Material
Acknowledgements
We thank Barbara Schlingmann for critical reading of the manuscript. This work was supported by the Emory Alcohol and Lung Biology Center through P50-AA013757 (MK), by R01-HL116958 (MK), T32-AA013528 (SAM) and R01-HL058732 (JMB).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Cottrell GT, Burt JM. Functional consequences of heterogeneous gap junction channel formation and its influence in health and disease. Biochim Biophys Acta. 2005;1711:126–41. doi: 10.1016/j.bbamem.2004.11.013. [DOI] [PubMed] [Google Scholar]
- 2.Koval M. Pathways and control of connexin oligomerization. Trends Cell Biol. 2006;16:159–66. doi: 10.1016/j.tcb.2006.01.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Abascal F, Zardoya R. Evolutionary analyses of gap junction protein families. Biochim Biophys Acta. 2013;1828:4–14. doi: 10.1016/j.bbamem.2012.02.007. [DOI] [PubMed] [Google Scholar]
- 4.Sohl G, Willecke K. Gap junctions and the connexin protein family. Cardiovasc Res. 2004;62:228–32. doi: 10.1016/j.cardiores.2003.11.013. [DOI] [PubMed] [Google Scholar]
- 5.Beyer EC, Berthoud VM. The Family of Connexin Genes. In: Harris AL, Locke D, editors. Connexins: A Guide. Humana Press; New York, NY: 2009. pp. 3–26. [Google Scholar]
- 6.Laird DW. Life cycle of connexins in health and disease. Biochem J. 2006;394:527–43. doi: 10.1042/BJ20051922. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Smith TD, Mohankumar A, Minogue PJ, Beyer EC, Berthoud VM, Koval M. Cytoplasmic amino acids within the membrane interface region influence connexin oligomerization. J Membr Biol. 2012;245:221–30. doi: 10.1007/s00232-012-9443-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Lagree V, Brunschwig K, Lopez P, Gilula NB, Richard G, Falk MM. Specific amino-acid residues in the N-terminus and TM3 implicated in channel function and oligomerization compatibility of connexin43. J Cell Sci. 2003;116:3189–201. doi: 10.1242/jcs.00604. [DOI] [PubMed] [Google Scholar]
- 9.Maeda S, Nakagawa S, Suga M, Yamashita E, Oshima A, Fujiyoshi Y, Tsukihara T. Structure of the connexin 26 gap junction channel at 3.5 A resolution. Nature. 2009;458:597–602. doi: 10.1038/nature07869. [DOI] [PubMed] [Google Scholar]
- 10.Laird DW. Syndromic and non-syndromic disease-linked Cx43 mutations. FEBS Lett. 2014 doi: 10.1016/j.febslet.2013.12.022. [DOI] [PubMed] [Google Scholar]
- 11.Xu J, Nicholson BJ. The role of connexins in ear and skin physiology - functional insights from disease-associated mutations. Biochim Biophys Acta. 2013;1828:167–78. doi: 10.1016/j.bbamem.2012.06.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Musil LS, Goodenough DA. Multisubunit assembly of an integral plasma membrane channel protein, gap junction connexin43, occurs after exit from the ER. Cell. 1993;74:1065–1077. doi: 10.1016/0092-8674(93)90728-9. [DOI] [PubMed] [Google Scholar]
- 13.Das S, Smith TD, Das Sarma J, Ritzenthaler JD, Maza J, Kaplan BE, Cunningham LA, Suaud L, Hubbard MJ, Rubenstein RC, Koval M. ERp29 restricts connexin43 oligomerization in the endoplasmic reticulum. Mol Biol Cell. 2009;20:2593–2604. doi: 10.1091/mbc.E08-07-0790. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Maza J, Das Sarma J, Koval M. Defining a minimal motif required to prevent connexin oligomerization in the endoplasmic reticulum. J Biol Chem. 2005;280:21115–21. doi: 10.1074/jbc.M412612200. [DOI] [PubMed] [Google Scholar]
- 15.Koval M, Harley JE, Hick E, Steinberg TH. Connexin46 is retained as monomers in a trans-Golgi compartment of osteoblastic cells. J Cell Biol. 1997;137:847–857. doi: 10.1083/jcb.137.4.847. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Gemel J, Valiunas V, Brink PR, Beyer EC. Connexin43 and connexin26 form gap junctions, but not heteromeric channels in co-expressing cells. J Cell Sci. 2004;117:2469–80. doi: 10.1242/jcs.01084. [DOI] [PubMed] [Google Scholar]
- 17.Das Sarma J, Das S, Koval M. Regulation of connexin43 oligomerization is saturable. Cell Commun Adhes. 2005;12:237–47. doi: 10.1080/15419060500511875. [DOI] [PubMed] [Google Scholar]
- 18.Beyer EC, Lin X, Veenstra RD. Interfering amino terminal peptides and functional implications for heteromeric gap junction formation. Front Pharmacol. 2013;4:67. doi: 10.3389/fphar.2013.00067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.He DS, Jiang JX, Taffet SM, Burt JM. Formation of heteromeric gap junction channels by connexins 40 and 43 in vascular smooth muscle cells. Proc Natl Acad Sci U S A. 1999;96:6495–500. doi: 10.1073/pnas.96.11.6495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Gemel J, Nelson TK, Burt JM, Beyer EC. Inducible coexpression of connexin37 or connexin40 with connexin43 selectively affects intercellular molecular transfer. J Membr Biol. 2012;245:231–41. doi: 10.1007/s00232-012-9444-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Gabriels JE, Paul DL. Connexin43 is highly localized to sites of disturbed flow in rat aortic endothelium but connexin37 and connexin40 are more uniformly distributed. Circ Res. 1998;83:636–43. doi: 10.1161/01.res.83.6.636. [DOI] [PubMed] [Google Scholar]
- 22.Isakson BE, Best AK, Duling BR. Incidence of protein on actin bridges between endothelium and smooth muscle in arterioles demonstrates heterogeneous connexin expression and phosphorylation. Am J Physiol Heart Circ Physiol. 2008;294:H2898–904. doi: 10.1152/ajpheart.91488.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Severs NJ, Rothery S, Dupont E, Coppen SR, Yeh HI, Ko YS, Matsushita T, Kaba R, Halliday D. Immunocytochemical analysis of connexin expression in the healthy and diseased cardiovascular system. Microsc Res Tech. 2001;52:301–22. doi: 10.1002/1097-0029(20010201)52:3<301::AID-JEMT1015>3.0.CO;2-Q. [DOI] [PubMed] [Google Scholar]
- 24.Inai T, Shibata Y. Heterogeneous expression of endothelial connexin (Cx) 37, Cx40, and Cx43 in rat large veins. Anat Sci Int. 2009;84:237–45. doi: 10.1007/s12565-009-0029-y. [DOI] [PubMed] [Google Scholar]
- 25.Abraham V, Chou ML, DeBolt KM, Koval M. Phenotypic control of gap junctional communication by cultured alveolar epithelial cells. Am J Physiol. 1999;276:L825–34. doi: 10.1152/ajplung.1999.276.5.L825. [DOI] [PubMed] [Google Scholar]
- 26.Das Sarma J, Meyer RA, Wang F, Abraham V, Lo CW, Koval M. Multimeric connexin interactions prior to the trans-Golgi network. J Cell Sci. 2001;114:4013–24. doi: 10.1242/jcs.114.22.4013. [DOI] [PubMed] [Google Scholar]
- 27.Harris AL. Emerging issues of connexin channels: biophysics fills the gap. Q Rev Biophys. 2001;34:325–472. doi: 10.1017/s0033583501003705. [DOI] [PubMed] [Google Scholar]
- 28.Gong XQ, Nakagawa S, Tsukihara T, Bai D. A mechanism of gap junction docking revealed by functional rescue of a human-disease-linked connexin mutant. J Cell Sci. 2013;126:3113–20. doi: 10.1242/jcs.123430. [DOI] [PubMed] [Google Scholar]
- 29.Nakagawa S, Gong XQ, Maeda S, Dong Y, Misumi Y, Tsukihara T, Bai D. Asparagine 175 of connexin32 is a critical residue for docking and forming functional heterotypic gap junction channels with connexin26. J Biol Chem. 2011;286:19672–81. doi: 10.1074/jbc.M110.204958. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Abrams CK, Freidin MM, Verselis VK, Bargiello TA, Kelsell DP, Richard G, Bennett MV, Bukauskas FF. Properties of human connexin 31, which is implicated in hereditary dermatological disease and deafness. Proc Natl Acad Sci U S A. 2006;103:5213–8. doi: 10.1073/pnas.0511091103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Elfgang C, Eckert R, Lichtenberg-Frate H, Butterweck A, Traub O, Klein RA, Hulser DF, Willecke K. Specific permeability and selective formation of gap junction channels in connexin-transfected HeLa cells. J Cell Biol. 1995;129:805–817. doi: 10.1083/jcb.129.3.805. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Bai D, Wang AH. Extracellular domains play different roles in gap junction formation and docking compatibility. Biochem J. 2014;458:1–10. doi: 10.1042/BJ20131162. [DOI] [PubMed] [Google Scholar]
- 33.White TW, Paul DL, Goodenough DA, Bruzzone R. Functional analysis of selective interactions among rodent connexins. Mol Biol Cell. 1995;6:459–470. doi: 10.1091/mbc.6.4.459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Orthmann-Murphy JL, Abrams CK, Scherer SS. Gap junctions couple astrocytes and oligodendrocytes. J Mol Neurosci. 2008;35:101–16. doi: 10.1007/s12031-007-9027-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Magnotti LM, Goodenough DA, Paul DL. Functional heterotypic interactions between astrocyte and oligodendrocyte connexins. Glia. 2011;59:26–34. doi: 10.1002/glia.21073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Martinez AD, Maripillan J, Acuna R, Minogue PJ, Berthoud VM, Beyer EC. Different domains are critical for oligomerization compatibility of different connexins. Biochem J. 2011;436:35–43. doi: 10.1042/BJ20110008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Kanaporis G, Brink PR, Valiunas V. Gap junction permeability: selectivity for anionic and cationic probes. Am J Physiol Cell Physiol. 2011;300:C600–9. doi: 10.1152/ajpcell.00316.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Yum SW, Zhang J, Valiunas V, Kanaporis G, Brink PR, White TW, Scherer SS. Human connexin26 and connexin30 form functional heteromeric and heterotypic channels. Am J Physiol Cell Physiol. 2007;293:C1032–48. doi: 10.1152/ajpcell.00011.2007. [DOI] [PubMed] [Google Scholar]
- 39.Tong JJ, Sohn BC, Lam A, Walters DE, Vertel BM, Ebihara L. Properties of two cataract-associated mutations located in the NH2 terminus of connexin 46. Am J Physiol Cell Physiol. 2013;304:C823–32. doi: 10.1152/ajpcell.00344.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Ambrosi C, Walker AE, Depriest AD, Cone AC, Lu C, Badger J, Skerrett IM, Sosinsky GE. Analysis of trafficking, stability and function of human connexin 26 gap junction channels with deafness-causing mutations in the fourth transmembrane helix. PLoS One. 2013;8:e70916. doi: 10.1371/journal.pone.0070916. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Harris AL. Connexin specificity of second messenger permeation: real numbers at last. J Gen Physiol. 2008;131:287–92. doi: 10.1085/jgp.200809998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Cottrell GT, Wu Y, Burt JM. Cx40 and Cx43 expression ratio influences heteromeric/ heterotypic gap junction channel properties. Am J Physiol Cell Physiol. 2002;282:C1469–82. doi: 10.1152/ajpcell.00484.2001. [DOI] [PubMed] [Google Scholar]
- 43.Burt JM, Fletcher AM, Steele TD, Wu Y, Cottrell GT, Kurjiaka DT. Alteration of Cx43:Cx40 expression ratio in A7r5 cells. Am J Physiol Cell Physiol. 2001;280:C500–8. doi: 10.1152/ajpcell.2001.280.3.C500. [DOI] [PubMed] [Google Scholar]
- 44.Heyman NS, Kurjiaka DT, Ek Vitorin JF, Burt JM. Regulation of gap junctional charge selectivity in cells coexpressing connexin 40 and connexin 43. Am J Physiol Heart Circ Physiol. 2009;297:H450–9. doi: 10.1152/ajpheart.00287.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Rash JE, Kamasawa N, Davidson KG, Yasumura T, Pereda AE, Nagy JI. Connexin composition in apposed gap junction hemiplaques revealed by matched double-replica freeze-fracture replica immunogold labeling. J Membr Biol. 2012;245:333–44. doi: 10.1007/s00232-012-9454-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Lichtenstein A, Gaietta GM, Deerinck TJ, Crum J, Sosinsky GE, Beyer EC, Berthoud VM. The cytoplasmic accumulations of the cataract-associated mutant, Connexin50P88S, are long-lived and form in the endoplasmic reticulum. Exp Eye Res. 2009;88:600–9. doi: 10.1016/j.exer.2008.11.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Gaietta G, Deerinck TJ, Adams SR, Bouwer J, Tour O, Laird DW, Sosinsky GE, Tsien RY, Ellisman MH. Multicolor and electron microscopic imaging of connexin trafficking. Science. 2002;296:503–7. doi: 10.1126/science.1068793. [DOI] [PubMed] [Google Scholar]
- 48.Kenworthy AK. Imaging protein-protein interactions using fluorescence resonance energy transfer microscopy. Methods. 2001;24:289–96. doi: 10.1006/meth.2001.1189. [DOI] [PubMed] [Google Scholar]
- 49.Kenworthy AK, Edidin M. Distribution of a glycosylphosphatidylinositol-anchored protein at the apical surface of MDCK cells examined at a resolution of <100 A using imaging fluorescence resonance energy transfer. J Cell Biol. 1998;142:69–84. doi: 10.1083/jcb.142.1.69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Di WL, Gu Y, Common JE, Aasen T, O’Toole EA, Kelsell DP, Zicha D. Connexin interaction patterns in keratinocytes revealed morphologically and by FRET analysis. J Cell Sci. 2005;118:1505–14. doi: 10.1242/jcs.01733. [DOI] [PubMed] [Google Scholar]
- 51.Betzig E, Patterson GH, Sougrat R, Lindwasser OW, Olenych S, Bonifacino JS, Davidson MW, Lippincott-Schwartz J, Hess HF. Imaging intracellular fluorescent proteins at nanometer resolution. Science. 2006;313:1642–5. doi: 10.1126/science.1127344. [DOI] [PubMed] [Google Scholar]
- 52.Bates M, Huang B, Dempsey GT, Zhuang X. Multicolor super-resolution imaging with photo-switchable fluorescent probes. Science. 2007;317:1749–53. doi: 10.1126/science.1146598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Shroff H, Galbraith CG, Galbraith JA, White H, Gillette J, Olenych S, Davidson MW, Betzig E. Dual-color superresolution imaging of genetically expressed probes within individual adhesion complexes. Proc Natl Acad Sci U S A. 2007;104:20308–13. doi: 10.1073/pnas.0710517105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Hsu CJ, Baumgart T. Spatial association of signaling proteins and F-actin effects on cluster assembly analyzed via photoactivation localization microscopy in T cells. PLoS One. 2011;6:e23586. doi: 10.1371/journal.pone.0023586. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Sengupta P, Jovanovic-Talisman T, Skoko D, Renz M, Veatch SL, Lippincott-Schwartz J. Probing protein heterogeneity in the plasma membrane using PALM and pair correlation analysis. Nat Methods. 2011;8:969–75. doi: 10.1038/nmeth.1704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Kaufmann R, Piontek J, Grull F, Kirchgessner M, Rossa J, Wolburg H, Blasig IE, Cremer C. Visualization and quantitative analysis of reconstituted tight junctions using localization microscopy. PLoS One. 2012;7:e31128. doi: 10.1371/journal.pone.0031128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Soderberg O, Gullberg M, Jarvius M, Ridderstrale K, Leuchowius KJ, Jarvius J, Wester K, Hydbring P, Bahram F, Larsson LG, Landegren U. Direct observation of individual endogenous protein complexes in situ by proximity ligation. Nat Methods. 2006;3:995–1000. doi: 10.1038/nmeth947. [DOI] [PubMed] [Google Scholar]
- 58.Rhett JM, Ongstad EL, Jourdan J, Gourdie RG. Cx43 associates with Na(v)1.5 in the cardiomyocyte perinexus. J Membr Biol. 2012;245:411–22. doi: 10.1007/s00232-012-9465-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Rhett JM, Jourdan J, Gourdie RG. Connexin 43 connexon to gap junction transition is regulated by zonula occludens-1. Mol Biol Cell. 2011;22:1516–28. doi: 10.1091/mbc.E10-06-0548. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Rash JE, Yasumura T, Dudek FE, Nagy JI. Cell-specific expression of connexins and evidence of restricted gap junctional coupling between glial cells and between neurons. J Neurosci. 2001;21:1983–2000. doi: 10.1523/JNEUROSCI.21-06-01983.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Bao M, Kanter EM, Huang RY, Maxeiner S, Frank M, Zhang Y, Schuessler RB, Smith TW, Townsend RR, Rohrs HW, Berthoud VM, Willecke K, Laing JG, Yamada KA. Residual Cx45 and its relationship to Cx43 in murine ventricular myocardium. Channels (Austin) 2011;5:489–99. doi: 10.4161/chan.5.6.18523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Tenbroek E, Arneson M, Jarvis L, Louis C. The distribution of the fiber cell intrinsic membrane proteins MP20 and connexin46 in the bovine lens. J Cell Sci. 1992;103:245–257. doi: 10.1242/jcs.103.1.245. [DOI] [PubMed] [Google Scholar]
- 63.Yeh HI, Rothery S, Dupont E, Coppen SR, Severs NJ. Individual gap junction plaques contain multiple connexins in arterial endothelium. Circ Res. 1998;83:1248–63. doi: 10.1161/01.res.83.12.1248. [DOI] [PubMed] [Google Scholar]
- 64.Beyer EC, Gemel J, Martinez A, Berthoud VM, Valiunas V, Moreno AP, Brink PR. Heteromeric mixing of connexins: compatibility of partners and functional consequences. Cell Commun Adhes. 2001;8:199–204. doi: 10.3109/15419060109080723. [DOI] [PubMed] [Google Scholar]
- 65.Gemel J, Lin X, Collins R, Veenstra RD, Beyer EC. Cx30.2 can form heteromeric gap junction channels with other cardiac connexins. Biochem Biophys Res Commun. 2008;369:388–94. doi: 10.1016/j.bbrc.2008.02.040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Gemel J, Lin X, Veenstra RD, Beyer EC. N-terminal residues in Cx43 and Cx40 determine physiological properties of gap junction channels, but do not influence heteromeric assembly with each other or with Cx26. J Cell Sci. 2006;119:2258–68. doi: 10.1242/jcs.02953. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Sun J, Ahmad S, Chen S, Tang W, Zhang Y, Chen P, Lin X. Cochlear gap junctions coassembled from Cx26 and 30 show faster intercellular Ca2+ signaling than homomeric counterparts. Am J Physiol Cell Physiol. 2005;288:C613–23. doi: 10.1152/ajpcell.00341.2004. [DOI] [PubMed] [Google Scholar]
- 68.Ahmad S, Diez JA, George CH, Evans WH. Synthesis and assembly of connexins in vitro into homomeric and heteromeric functional gap junction hemichannels. Biochem J. 1999;339:247–253. [PMC free article] [PubMed] [Google Scholar]
- 69.Jiang JX, Goodenough DA. Heteromeric connexons in lens gap junction channels. Proc Nat Acad Sci USA. 1996;93:1287–1291. doi: 10.1073/pnas.93.3.1287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Bevans CG, Kordel M, Rhee SK, Harris AL. Isoform composition of connexin channels determines selectivity among second messengers and uncharged molecules. J Biol Chem. 1998;273:2808–2816. doi: 10.1074/jbc.273.5.2808. [DOI] [PubMed] [Google Scholar]
- 71.Langlois S, Cowan KN, Shao Q, Cowan BJ, Laird DW. Caveolin-1 and -2 interact with connexin43 and regulate gap junctional intercellular communication in keratinocytes. Mol Biol Cell. 2008;19:912–28. doi: 10.1091/mbc.E07-06-0596. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Locke D, Liu J, Harris AL. Lipid rafts prepared by different methods contain different connexin channels, but gap junctions are not lipid rafts. Biochemistry. 2005;44:13027–42. doi: 10.1021/bi050495a. [DOI] [PubMed] [Google Scholar]
- 73.Schubert AL, Schubert W, Spray DC, Lisanti MP. Connexin family members target to lipid raft domains and interact with caveolin-1. Biochemistry. 2002;41:5754–64. doi: 10.1021/bi0121656. [DOI] [PubMed] [Google Scholar]
- 74.Langlois S, Cowan KN, Shao Q, Cowan BJ, Laird DW. The tumor-suppressive function of Connexin43 in keratinocytes is mediated in part via interaction with caveolin-1. Cancer Res. 2010;70:4222–32. doi: 10.1158/0008-5472.CAN-09-3281. [DOI] [PubMed] [Google Scholar]
- 75.Falk MM. Connexin-specific distribution within gap junctions revealed in living cells. J Cell Sci. 2000;113:4109–20. doi: 10.1242/jcs.113.22.4109. [DOI] [PubMed] [Google Scholar]
- 76.Das Sarma J, Wang F, Koval M. Targeted gap junction protein constructs reveal connexin-specific differences in oligomerization. J Biol Chem. 2002;277:20911–20918. doi: 10.1074/jbc.M111498200. [DOI] [PubMed] [Google Scholar]
- 77.Yazdani N, Firme CP, 3rd, Macagno ER, Baker MW. Expression of a dominant negative mutant innexin in identified neurons and glial cells reveals selective interactions among gap junctional proteins. Dev Neurobiol. 2013;73:571–86. doi: 10.1002/dneu.22082. [DOI] [PubMed] [Google Scholar]
- 78.Su CC, Li SY, Su MC, Chen WC, Yang JJ. Mutation R184Q of connexin 26 in hearing loss patients has a dominant-negative effect on connexin 26 and connexin 30. Eur J Hum Genet. 2010;18:1061–4. doi: 10.1038/ejhg.2010.50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Rouan F, White TW, Brown N, Taylor AM, Lucke TW, Paul DL, Munro CS, Uitto J, Hodgins MB, Richard G. trans-dominant inhibition of connexin-43 by mutant connexin-26: implications for dominant connexin disorders affecting epidermal differentiation. J Cell Sci. 2001;114:2105–13. doi: 10.1242/jcs.114.11.2105. [DOI] [PubMed] [Google Scholar]
- 80.Harris AL. Connexin channel permeability to cytoplasmic molecules. Prog Biophys Mol Biol. 2007;94:120–43. doi: 10.1016/j.pbiomolbio.2007.03.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Goldberg G, Lampe PD, Nicholson BJ. Selective transfer of endogenous metabolites through gap junctions composed of different connexins. Nat Cell Biol. 1999;1:457–459. doi: 10.1038/15693. [DOI] [PubMed] [Google Scholar]
- 82.Ayad WA, Locke D, Koreen IV, Harris AL. Heteromeric, but not homomeric, connexin channels are selectively permeable to inositol phosphates. JBiolChem. 2006 doi: 10.1074/jbc.M600136200. [DOI] [PubMed] [Google Scholar]
- 83.Kanaporis G, Mese G, Valiuniene L, White TW, Brink PR, Valiunas V. Gap junction channels exhibit connexin-specific permeability to cyclic nucleotides. J Gen Physiol. 2008;131:293–305. doi: 10.1085/jgp.200709934. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Niessen H, Harz H, Bedner P, Kramer K, Willecke K. Selective permeability of different connexin channels to the second messenger inositol 1,4,5-trisphosphate. J Cell Sci. 2000;113(Pt 8):1365–72. doi: 10.1242/jcs.113.8.1365. [DOI] [PubMed] [Google Scholar]
- 85.Boitano S, Dirksen ER, Sanderson MJ. Intercellular propagation of calcium waves mediated by inositol trisphosphate. Science. 1992;258:292–5. doi: 10.1126/science.1411526. [DOI] [PubMed] [Google Scholar]
- 86.Tress O, Maglione M, May D, Pivneva T, Richter N, Seyfarth J, Binder S, Zlomuzica A, Seifert G, Theis M, Dere E, Kettenmann H, Willecke K. Panglial gap junctional communication is essential for maintenance of myelin in the CNS. J Neurosci. 2012;32:7499–518. doi: 10.1523/JNEUROSCI.0392-12.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Maglione M, Tress O, Haas B, Karram K, Trotter J, Willecke K, Kettenmann H. Oligodendrocytes in mouse corpus callosum are coupled via gap junction channels formed by connexin47 and connexin32. Glia. 2010;58:1104–17. doi: 10.1002/glia.20991. [DOI] [PubMed] [Google Scholar]
- 88.Gosejacob D, Dublin P, Bedner P, Huttmann K, Zhang J, Tress O, Willecke K, Pfrieger F, Steinhauser C, Theis M. Role of astroglial connexin30 in hippocampal gap junction coupling. Glia. 2011;59:511–9. doi: 10.1002/glia.21120. [DOI] [PubMed] [Google Scholar]
- 89.Orthmann-Murphy JL, Freidin M, Fischer E, Scherer SS, Abrams CK. Two distinct heterotypic channels mediate gap junction coupling between astrocyte and oligodendrocyte connexins. J Neurosci. 2007;27:13949–57. doi: 10.1523/JNEUROSCI.3395-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Nagy JI, Ionescu AV, Lynn BD, Rash JE. Coupling of astrocyte connexins Cx26, Cx30, Cx43 to oligodendrocyte Cx29, Cx32, Cx47: Implications from normal and connexin32 knockout mice. Glia. 2003;44:205–18. doi: 10.1002/glia.10278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.May D, Tress O, Seifert G, Willecke K. Connexin47 protein phosphorylation and stability in oligodendrocytes depend on expression of Connexin43 protein in astrocytes. J Neurosci. 2013;33:7985–96. doi: 10.1523/JNEUROSCI.5874-12.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Wasseff SK, Scherer SS. Cx32 and Cx47 mediate oligodendrocyte:astrocyte and oligodendrocyte:oligodendrocyte gap junction coupling. Neurobiol Dis. 2011;42:506–13. doi: 10.1016/j.nbd.2011.03.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Lynn BD, Tress O, May D, Willecke K, Nagy JI. Ablation of connexin30 in transgenic mice alters expression patterns of connexin26 and connexin32 in glial cells and leptomeninges. Eur J Neurosci. 2011;34:1783–93. doi: 10.1111/j.1460-9568.2011.07900.x. [DOI] [PubMed] [Google Scholar]
- 94.Nualart-Marti A, Solsona C, Fields RD. Gap junction communication in myelinating glia. Biochim Biophys Acta. 2013;1828:69–78. doi: 10.1016/j.bbamem.2012.01.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Cotrina ML, Nedergaard M. Brain connexins in demyelinating diseases: therapeutic potential of glial targets. Brain Res. 2012;1487:61–8. doi: 10.1016/j.brainres.2012.07.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Li X, Penes M, Odermatt B, Willecke K, Nagy JI. Ablation of Cx47 in transgenic mice leads to the loss of MUPP1, ZONAB and multiple connexins at oligodendrocyte-astrocyte gap junctions. Eur J Neurosci. 2008;28:1503–17. doi: 10.1111/j.1460-9568.2008.06431.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Palacios-Prado N, Bukauskas FF. Heterotypic gap junction channels as voltage-sensitive valves for intercellular signaling. Proc Natl Acad Sci U S A. 2009;106:14855–60. doi: 10.1073/pnas.0901923106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Verheule S, Kaese S. Connexin diversity in the heart: insights from transgenic mouse models. Front Pharmacol. 2013;4:81. doi: 10.3389/fphar.2013.00081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Kirchhoff S, Nelles E, Hagendorff A, Kruger O, Traub O, Willecke K. Reduced cardiac conduction velocity and predisposition to arrhythmias in connexin40-deficient mice. Curr Biol. 1998;8:299–302. doi: 10.1016/s0960-9822(98)70114-9. [DOI] [PubMed] [Google Scholar]
- 100.Simon AM, Goodenough DA, Paul DL. Mice lacking connexin40 have cardiac conduction abnormalities characteristic of atrioventricular block and bundle branch block. Curr Biol. 1998;8:295–8. doi: 10.1016/s0960-9822(98)70113-7. [DOI] [PubMed] [Google Scholar]
- 101.Lin X, Gemel J, Glass A, Zemlin CW, Beyer EC, Veenstra RD. Connexin40 and connexin43 determine gating properties of atrial gap junction channels. J Mol Cell Cardiol. 2010;48:238–45. doi: 10.1016/j.yjmcc.2009.05.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Bouvier D, Spagnol G, Chenavas S, Kieken F, Vitrac H, Brownell S, Kellezi A, Forge V, Sorgen PL. Characterization of the structure and intermolecular interactions between the connexin40 and connexin43 carboxyl-terminal and cytoplasmic loop domains. J Biol Chem. 2009;284:34257–71. doi: 10.1074/jbc.M109.039594. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Koval M. Sharing signals: connecting lung epithelial cells with gap junction channels. Am J Physiol Lung Cell Mol Physiol. 2002;283:L875–93. doi: 10.1152/ajplung.00078.2002. [DOI] [PubMed] [Google Scholar]
- 104.Hanner F, Sorensen CM, Holstein-Rathlou NH, Peti-Peterdi J. Connexins and the kidney. Am J Physiol Regul Integr Comp Physiol. 2010;298:R1143–55. doi: 10.1152/ajpregu.00808.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Aasland R, Abrams C, Ampe C, Ball LJ, Bedford MT, Cesareni G, Gimona M, Hurley JH, Jarchau T, Lehto VP, Lemmon MA, Linding R, Mayer BJ, Nagai M, Sudol M, Walter U, Winder SJ. Normalization of nomenclature for peptide motifs as ligands of modular protein domains. FEBS Lett. 2002;513:141–4. doi: 10.1016/s0014-5793(01)03295-1. [DOI] [PubMed] [Google Scholar]
- 106.Manthey D, Banach K, Desplantez T, Lee CG, Kozak CA, Traub O, Weingart R, Willecke K. Intracellular domains of mouse connexin26 and -30 affect diffusional and electrical properties of gap junction channels. J Membr Biol. 2001;181:137–48. doi: 10.1007/s00232-001-0017-1. [DOI] [PubMed] [Google Scholar]
- 107.Ahn M, Lee J, Gustafsson A, Enriquez A, Lancaster E, Sul JY, Haydon PG, Paul DL, Huang Y, Abrams CK, Scherer SS. Cx29 and Cx32, two connexins expressed by myelinating glia, do not interact and are functionally distinct. J Neurosci Res. 2008;86:992–1006. doi: 10.1002/jnr.21561. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Rackauskas M, Kreuzberg MM, Pranevicius M, Willecke K, Verselis VK, Bukauskas FF. Gating properties of heterotypic gap junction channels formed of connexins 40, 43, and 45. Biophys J. 2007;92:1952–65. doi: 10.1529/biophysj.106.099358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Rackauskas M, Verselis VK, Bukauskas FF. Permeability of homotypic and heterotypic gap junction channels formed of cardiac connexins mCx30.2, Cx40, Cx43, and Cx45. Am J Physiol Heart Circ Physiol. 2007;293:H1729–36. doi: 10.1152/ajpheart.00234.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Locke D, Jamieson S, Stein T, Liu J, Hodgins MB, Harris AL, Gusterson B. Nature of Cx30-containing channels in the adult mouse mammary gland. Cell Tissue Res. 2007;328:97–107. doi: 10.1007/s00441-006-0301-6. [DOI] [PubMed] [Google Scholar]
- 111.Magnotti LM, Goodenough DA, Paul DL. Deletion of oligodendrocyte Cx32 and astrocyte Cx43 causes white matter vacuolation, astrocyte loss and early mortality. Glia. 2011;59:1064–74. doi: 10.1002/glia.21179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Kreuzberg MM, Schrickel JW, Ghanem A, Kim JS, Degen J, Janssen-Bienhold U, Lewalter T, Tiemann K, Willecke K. Connexin30.2 containing gap junction channels decelerate impulse propagation through the atrioventricular node. Proc Natl Acad Sci U S A. 2006;103:5959–64. doi: 10.1073/pnas.0508512103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Kreuzberg MM, Willecke K, Bukauskas FF. Connexin-mediated cardiac impulse propagation: connexin 30.2 slows atrioventricular conduction in mouse heart. Trends Cardiovasc Med. 2006;16:266–72. doi: 10.1016/j.tcm.2006.05.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.