Abstract
Objectives
This study sought to investigate associations of phosphate metabolism biomarkers with aortic valve calcification (AVC).
Background
Calcific aortic valve disease (CAVD) is a common progressive condition that involves inflammatory and calcification mediators. Currently there are no effective medical treatments, but mineral metabolism pathways may be important in the development and progression of disease.
Methods
We examined associations of phosphate metabolism biomarkers, including serum phosphate, urine phosphate, parathyroid hormone (PTH) and serum fibroblast growth factor (FGF)-23, with CT-assessed AVC at study baseline and in short-term follow-up in 6,814 participants of the Multi-Ethnic Study of Atherosclerosis (MESA).
Results
At baseline, AVC prevalence was 13.2%. Higher serum phosphate levels were associated with significantly greater AVC prevalence (relative risk 1.3 per 1mg/dL increment, 95% confidence incidence: 1.1 to 1.5, p < 0.001). Serum FGF-23, serum PTH, and urine phosphate were not associated with prevalent AVC. Average follow-up CT evaluation was 2.4 years (range 0.9–4.9 years) with an AVC incidence of 4.1%. Overall, phosphate metabolism biomarkers were not associated with incident AVC except in the top FGF-23 quartile.
Conclusions
Serum phosphate levels are significantly associated with AVC prevalence. Further study of phosphate metabolism as a modifiable risk factor for AVC is warranted.
Keywords: Phosphate, Aortic Valve, Calcification
Background
Calcific aortic valve disease (CAVD) is a common condition that affects more than a quarter of adults over the age of 65.(1) The presence of aortic valve calcification (AVC) is predictive of future cardiovascular events and motality,(2,3) and is associated with the severity of hemodynamic stenosis.(4,5) Currently there is no medical treatment for CAVD, and valve replacement remains the only therapy for advanced symptomatic disease.
CAVD is now well understood to be an active metabolic process involving mediators of both inflammation and calcification.(6) Histopathologic studies of early CAVD demonstrate a mixture of lipid deposition, inflammatory infiltrates, and calcification.(7) Despite evidence for an atherosclerotic-like process in CAVD development, studies have failed to show benefit of statin therapy among people with mild to moderate aortic stenosis.(8–10) Uncovering new pathways involved in the pathogenesis of CAVD is a critical next step toward developing effective therapeutic interventions.
Osteogenic pathways are likely important in the development and progression of CAVD,(11–14) and could represent novel, potentially-modifiable, treatment targets. Phosphate has been shown in vitro to activate osteogenic mediators in vascular smooth muscle cells.(15) We recently demonstrated a graded association of higher serum phosphate concentrations with aortic sclerosis among older adults in the Cardiovascular Health Study.(16) This intriguing result was based on qualitative measures of aortic sclerosis by echocardiography, assessment of serum phosphate levels as a single biomarker, and focus on mostly older, Caucasian individuals. Herein we expand our investigation of phosphate metabolism and CAVD by assessing quantitative measurements of aortic valve calcium by computed tomography (CT) and evaluating multiple markers of phosphate metabolism previously associated with cardiovascular outcomes, including fibroblast growth factor (FGF)-23, parathyroid hormone (PTH), and urine phosphate, in a large, multi-ethnic cohort.
Methods
Study Population
We evaluated participants from the Multi-Ethnic Study of Atherosclerosis (MESA), a prospective cohort study of 6,814 people who were free from clinical cardiovascular disease aged 45–84 years. Individuals were recruited between July 2000 and August 2002 from six U.S. communities (Baltimore, Maryland; Chicago, Illinois; Forsyth County, North Carolina; Los Angeles, California; New York, New York; and St. Paul, Minnesota) and were composed of four different ethnic groups (Black, Chinese, Hispanic and White). By design, the MESA recruited a final study population that was 38% White, 28% African American, 22% Hispanic, and 12% Asian, primarily of Chinese descent. A complete description of the study design has been published elsewhere.(17) Institutional review boards at each participating center approved the study, and all participants provided informed consent. Exclusions from participation in MESA included self-reporting of physician-diagnosed heart attack, angina or use of nitroglycerin, stroke, transient ischemic attack, heart failure, atrial fibrillation, or any history of previous cardiovascular procedures, including valve replacement.
Mineral Metabolism Measurements
Fasting morning blood and urine samples were obtained in all MESA participants at their baseline examination. Specimens were stored at −80°C at the University of Vermont Laboratory for Clinical Biochemistry using established methods from previous population cohort cardiovascular studies that maintain long-term stability of samples.(18) First use (no freeze thaw) samples were shipped on dry ice to the University of Washington, where all mineral metabolism measurements were performed. Samples were measured in random order and blinded to AVC status. Measurements were made in singlicate due to limited serum availability. Serum and urine phosphate concentrations were measured using a timed-rate colorimetry method on a Beckman-Coulter UniCel DxC instrument, with interassay coefficients of variation of 2.5% and 4.2%, respectively. Intact serum FGF-23 was measured using a sandwich immunoassay that detects the full length FGF-23 molecule (Kainos ELISA 96-well plate). The coefficient of variation for high and low control samples were 6.7% and 12.4%, respectively. Intact PTH was measured by automated immunoassay using a Beckman-Coulter UniCel DxI instrument (low and high coefficients of variation 6.1% and 3.4%, respectively).
Covariate Data
Demographics, medical history, smoking status and medication history were obtained using standardized questionnaires. Blood pressure (BP) was measured three times in a seated position using a Dinamap model Pro 100 automated oscilometric sphygmomanometer. The averages of the last two systolic and diastolic measurements were used for these analyses. Diabetes was defined as a fasting blood glucose level ≥ 126mg/dL or use of a diabetic medication. Estimated glomerular filtration rate (eGFR) was determined using cystatin C measurements according to the equation:(19) eGFR= 76.7 × (cystatin C)−1.19. Chronic kidney disease (CKD) was defined as eGFR < 60 ml/min/1.73m2. Serum 25-hydroxyvitamin D (25-OHD) concentrations were measured using liquid chromatogrphay tandem mass spectrometry. Urine albumin to creatinine ratio (ACR) was measured from single-voided fasting spot morning collections using nephelometry and the rate Jaffe reaction, respectively. Total and high-density lipoprotein (HDL) cholesterol were measured at a central lipid laboratory using a cholesterol oxidase method (Roche Diagnostics), the low-density lipoprotein (LDL) cholesterol was calculated using the Friedewald formula.(20)
Computed Tomography
ECG-triggered chest CT scans were performed on all participants during the baseline examination using either electron beam or multi-detector scanners and following standardized protocols. Descriptions of methodology, equipment, quality control, reproducibility and equivalency across scanner type have been previously published.(21–23) Follow-up CT scans were performed for a random half of the cohort between September 2002 and January 2004, and the other half between March 2004 and July 2005. Measurements of AVC scores were summated from individual lesions quantified using the method by Agatston et al.(24) Calcified lesions of the aortic annulus, aortic sinuses and proximal aorta were excluded from measurement. Absence of AVC was assigned a score of zero. All studies were evaluated at the MESA CT reading center (Harbor-UCLA Research and Education Institute) by a single blinded reader.
Statistical Analysis
Baseline characteristics stratified by presence of AVC were tabulated. Kendall tau rank correlation coefficients were calculated to examine relationships between different phosphate metabolism biomarkers. Prevalent AVC was defined as an AVC score > 0 at the baseline exam. Incident AVC was defined as having an AVC score > 0 on follow-up evaluation when AVC was absent (AVC score of 0) at baseline. Associations of prevalent and incident AVC with phosphate metabolism markers were examined continuously and categorically using a priori defined, nested, multivariable regression models. Flexible spline regression plots were used to assess the functional forms of relationships between exposures and the risk of AVC; no obvious departure from linearity was detected for any marker. Multivariable models included covariates selected prior to analyses based on the biologic plausibility that they may confound the associations of mineral metabolism markers with AVC. Relative risks for prevalent and incident AVC were estimated using a Poisson working model and inference was performed with robust variance estimates. The initial model contained demographic covariates including age, sex, ethnicity and study site. A second model included the demographic covariates, as well as other potential confounders including eFGR, systolic BP, diastolic BP, diabetes, smoking status, LDL cholesterol, HDL cholesterol, 25-OHD, calcium, and the log urine albumin to creatinine ratio. All models include scanner type, and incident and progression models adjusted for time between CT scans. Parallel models were created for the other phosphate metabolism exposure variables, FGF-23, PTH, and urine phosphate concentration. FGF-23 and urine phosphate concentrations were analyzed both continuously and as quartiles. PTH groups were dichotomized at a previously used cutpoint of 65 pg/mL. Extreme FGF-23 outlier values (top 0.25%) were excluded from the continuous analysis to avoid excessive influence in the models. The distribution of urine phosphate levels is skewed; thus, values were log transformed to yield more interpretable coefficients. Models of urine phosphate include the log of urine creatinine to account for spot urinary concentration. A final, mutually-adjusted model contained all covariates and the three phosphate axis exposure variables.
Participants with prevalent AVC were included in the severity and progression analyses. AVC progression was defined as the difference in AVC score between scans. AVC scores were log transformed due to a skewed distribution. Associations of phosphate axis variables with AVC severity were represented using geometric mean ratios from multivariable linear models with the same covariates as above. Interactions of phosphate axis exposures with predefined covariates, including age, ethnicity, sex, LDL, and CKD, were explored. The threshold for statistical significance was defined as p < 0.05.
Due to non-response and inadequate sample volume, approximately 5% of the study subjects were missing covariate data on one or more of the following variables: 25-OHD, cystatin C, smoking, and cholesterol. In regression analyses, these subjects' values were multiply imputed using chained equations.(25) The five analyses over the imputations were combined using Rubin's rules to account for the variability in the imputation procedure.(26) Missing covariate data accounted for less that 1% of the variation in the coefficient estimates of interest. All statistical analyses were performed using R version 2.13.
Results
Baseline characteristics
There were 6,814 participants in MESA with 2 individuals missing baseline AVC scores. Participants who had prevalent AVC were older, more likely to be male, and had more comorbidities than those who were free of AVC (Table 1). Phosphate metabolism biomarkers were adequately measurable in serum samples of 6,544 individuals. Serum phosphate levels were normally distributed with a mean of 3.67±0.52 mg/dL. The majority of phosphate levels were in the normal range; only 52 (0.8%) of participants had phosphate levels above 5 mg/dL. The respective mean FGF-23 and PTH levels were 39.8±14.2 pg/dL and 44.7±21.8 pg/mL, and the median urine phosphate level was 44.1 mg/dL (IQR: 24.9 to 67.7 mg/dL). Kidney function was predominately normal in the study sample (mean eGFR 98.3 ml/min/m2, CKD present in 5.4%). There was low correlation among the phosphate metabolism biomarkers (Table 2).
Table 1.
Baseline Participant Characteristics
| Aortic valve calcium | ||
|---|---|---|
| Characteristic | Absent (N=5,899) |
Present (N=913) |
| Age (years) | 60.86 ± 9.9 | 70.49 ± 8.1 |
| Male | 2663 (45.1) | 549 (60.1) |
| Ethnicity | ||
| Black | 1,660 (28.1) | 233 (25.5) |
| Chinese | 736 (12.5) | 67 (7.3) |
| Hispanic | 1,296 (22.0) | 200 (21.9) |
| White | 2,207 (37.4) | 413 (45.2) |
| Diabetes | 677 (11.5) | 181 (20.0) |
| Current smoking | 790 (13.4) | 96 (10.6) |
| Education | ||
| High school or less | 2,068 (35.2) | 392 (43.2) |
| High school to some college | 1,695 (28.8) | 241 (26.5) |
| College degree or higher | 2,118 (36.0) | 275 (30.3) |
| Body mass index (kg/m2) | 28.3 ± 5.6 | 28.49 ± 5.0 |
| Systolic blood pressure (mmHg) | 125.3 ± 21.1 | 135 ± 22.1 |
| Diastolic blood pressure (mmHg) | 71.9 ± 10.3 | 72.2 ± 10.1 |
| Hypertension medication | 2,030 (34.42) | 505(55.37) |
| Low-density lipoprotein cholesterol (mg/dL) | 117.0 ± 31.0 | 118.6 ± 34.4 |
| High-density lipoprotein cholesterol (mg/dL) | 51.3 ± 14.9 | 49.0 ± 14.2 |
| Lipid lowering medication | 864 (14.65) | 235(25.77) |
| Estimated glomerular filtration rate (ml/min/1.73m2) | 99.81 ± 25.8 | 86.33 ± 24.5 |
| Chronic kidney disease | 262(4.5) | 114(12.6) |
| Urine albumin to creatinine ratio | 25.3 ± 160.8 | 38.17 ± 222.8 |
| Mineral metabolism measurements | ||
| Serum 25-hydroxyvitamin D (ng/mL) | 25.2 ± 11.5 | 26.3 ± 12.0 |
| Serum calcium (mg/dL) | 9.7 ± 0.4 | 9.7 ± 0.4 |
| Serum phosphorus (mg/dL) | 3.7 ± 0.5 | 3.7 ± 0.5 |
| Urine phosphorus (mg/dL) | 51.2 ± 36.6 | 50.2 ± 30.8 |
| Serum fibroblast growth factor-23 (pg/mL) | 39.4 ± 14.1 | 42.4 ± 14.8 |
| Serum parathyroid hormone (pg/mL) | 44.3 ± 20.5 | 47.3 ± 28.7 |
Values are mean ± SD or n(%).
Table 2.
Correlation Coefficients Among Phosphorus Metabolism Biomarkers.
| Serum phosphorus | Urine phosphorus | FGF-23 | PTH | |
|---|---|---|---|---|
| Serum phosphorus | 1.00 | −0.03 | 0.06 | −0.15 |
| Urine phosphorus | −0.03 | 1.00 | 0.02 | 0.11 |
| FGF-23 | 0.06 | 0.02 | 1.00 | 0.03 |
| PTH | −0.15 | 0.11 | 0.03 | 1.00 |
FGF-23 = fibroblast growth factor-23; PTH = parathyroid hormone
Associations of phosphate metabolism markers with prevalent AVC
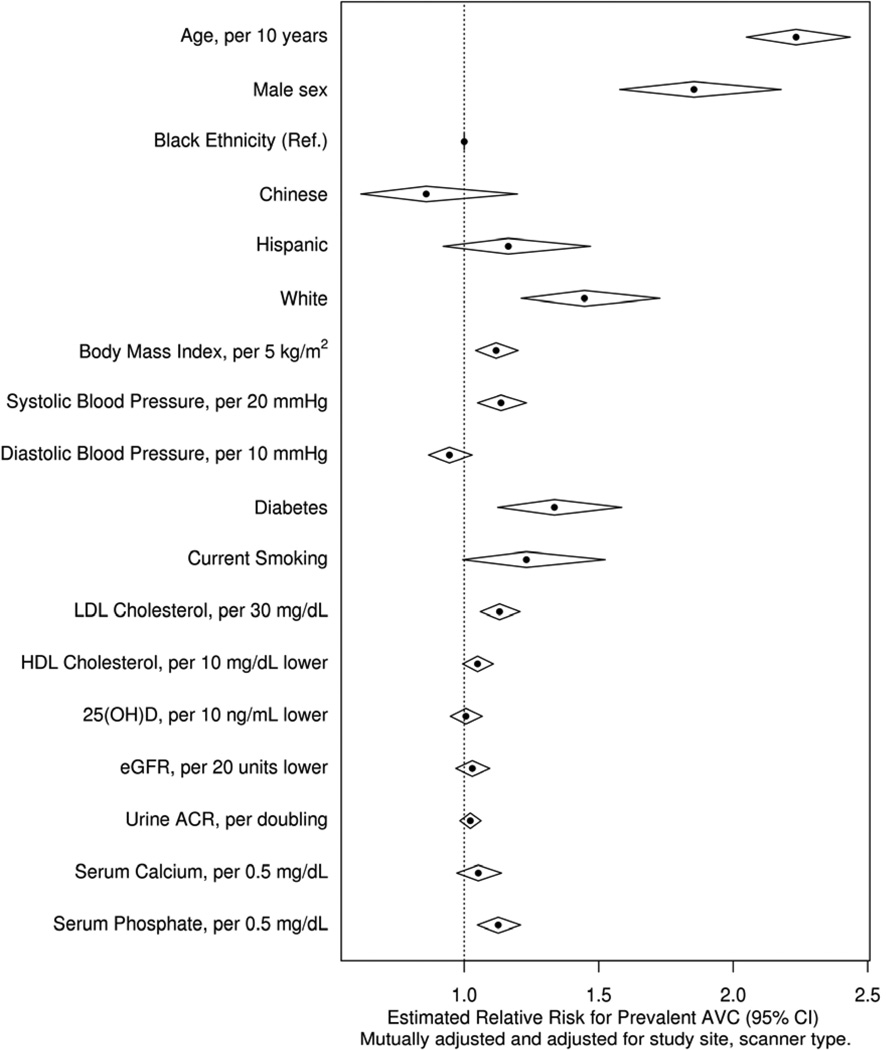
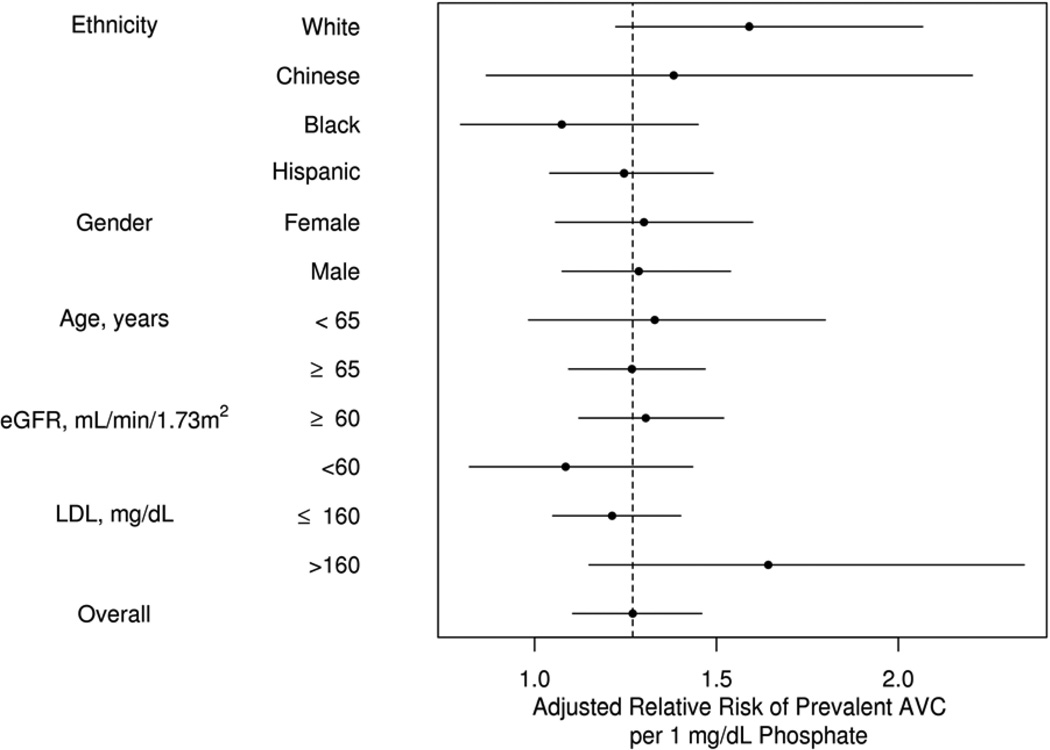
The prevalence of baseline AVC was 13.2%. There was a statistically significant association between higher serum phosphate concentrations and greater AVC prevalence in all models for the continuous and categorical evaluation of serum phosphate (Table 3). The association of serum phosphate with prevalent AVC was similar in magnitude to other previously identified modifiable risk factors, such as LDL cholesterol (Figure 1). Interactions were not statistically significant in the predetermined subgroups, including those with CKD (Figure 2). FGF-23, PTH, and urine phosphate concentrations were not associated with prevalent AVC in any of these models.
Table 3.
Associations of Phosphate Metabolism Markers with Prevalent Aortic Valve Calcium.
| Prevalent Cases N (%) |
Demographic adjusted* RR (95% CI) |
Fully adjusted† RR (95% CI) |
Adds mutual adjustment‡ RR (95% CI) |
P-value§ | |
|---|---|---|---|---|---|
| Serum phosphate (mg/dL) | |||||
| ≤3.0 | 103 (13.7%) | reference | |||
| 3.01 – 3.5 | 261 (13.3%) | 1.12 (0.91 to 1.38) | 1.08 (0.88 to 1.33) | 1.10 (0.89 to 1.36) | 0.36 |
| 3.51 – 4.0 | 294 (12.5%) | 1.16 (0.94 to 1.43) | 1.12 (0.91 to 1.38) | 1.14 (0.92 to 1.41) | 0.22 |
| >4.0 | 207 (14.2%) | 1.64 (1.30 to 2.06) | 1.50 (1.18 to 1.89) | 1.51 (1.19 to 1.92) | <0.001 |
| Linear per 1.0 mg/dL higher | 1.34 (1.18 to 1.53) | 1.27 (1.10 to 1.46) | 1.27 (1.10 to 1.47) | <0.001 | |
| Serum FGF-23 (pg/mL) | |||||
| ≤30.5 | 167 (10.2%) | reference | |||
| 30.5 – 37.7 | 184 (11.3%) | 0.98 (0.80 to 1.20) | 0.99 (0.81 to 1.20) | 0.97 (0.80 to 1.19) | 0.79 |
| 37.8 – 46.5 | 249 (15.2%) | 1.23 (1.02 to 1.48) | 1.17 (0.97 to 1.41) | 1.16 (0.97 to 1.40) | 0.11 |
| 46.5 – 590 | 262 (16%) | 1.19 (0.99 to 1.44) | 1.09 (0.90 to 1.32) | 1.07 (0.88 to 1.30) | 0.51 |
| Linear per 20 pg/mL higher | 1.11 (1.03 to 1.20) | 1.04 (0.96 to 1.13) | 1.03 (0.95 to 1.12) | 0.46 | |
| Urine phosphate (mg/dL)‖ | |||||
| ≤24.9 | 162 (11.3%) | reference | |||
| 24.9 – 44.1 | 245 (14.5%) | 1.10 (0.92 to 1.33) | 1.00 (0.81 to 1.25) | 1.01 (0.81 to 1.25) | 0.94 |
| 44.2 – 67.8 | 256 (15.2%) | 1.25 (1.04 to 1.50) | 1.07 (0.83 to 1.36) | 1.07 (0.84 to 1.37) | 0.57 |
| 67.9 – 398 | 205 (12.2%) | 1.11 (0.91 to 1.35) | 0.93 (0.7 to 1.24) | 0.93 (0.69 to 1.24) | 0.6 |
| Per doubling | 1.04 (0.98 to 1.11) | 0.96 (0.86 to 1.08) | 0.95 (0.85 to 1.06) | 0.37 | |
| Serum PTH (pg/mL) | |||||
| <65 | 743 (12.9%) | reference | |||
| ≥65 | 123 (15.8%) | 1.09 (0.92 to 1.30) | 0.96 (0.80 to 1.16) | 1.01 (0.83 to 1.22) | 0.95 |
| Linear per 10 pg/mL greater | 1.02 (0.99 to 1.04) | 0.99 (0.97 to 1.02) | 1.00 (0.97 to 1.03) | 0.81 |
Demographic adjusted models adjusted for age (in 10 year categories), gender, ethnicity, study site, and scanner type.
Addi6onally adjusts for body mass index, systolic blood pressure, diastolic blood pressure, diabetes, smoking, LDL-cholesterol, HDL-cholesterol, 25-hydroxyvitamin D, eGFR, log of albumin to creatinine ratio, and serum calcium.
Mutually adjusted models are fully adjusted models with additional adjustment for the other phosphate metabolism markers.
P-values are present for the complete mutually adjusted models
Models for urine phosphate additionally adjust for the log of urine creatinine.
CI = Confidence Interval; FGF = Fibroblast growth factor; PTH = Parathyroid Hormone; RR = Relative Risk
Figure 1. Risk of Aortic Valve Calcification by Demographics and Previously Studied Risk Factors.
Relative risk estimates demonstrating comparable effect size of serum phosphate and other modifiable risk factors. Diamond widths represent 95% confidence intervals. The magnitudes of effect for continuous risk factors are standardized to their respective standard deviation.
Figure 2. Risk of Aortic Valve Calcification in Predetermined Subgroups.
No statistically significant difference in adjusted relative risk for any predefined subgroup. Horizontal bars represent 95% confidence intervals.
Associations of phosphate markers with incident AVC
Mean follow-up time between CT scans was 2.4 years with a range of 0.9 to 4.9 years. Among the 5,145 participants who were free of baseline AVC and underwent CT scanning during follow-up, 211 (4.1%) developed incident AVC. There were no significant associations of serum phosphate, PTH or urine phosphate concentration with incident AVC (Table 4). There was also no association of serum FGF-23 concentrations with incident AVC when FGF-23 was analyzed as a continuous variable. In contrast, the highest FGF-23 quartile was associated with a greater incidence of AVC in fully adjusted models (RR: 1.52, 95% CI 1.01–2.28).
Table 4.
Associations of Phosphate Metabolism Markers with Incident Aortic Valve Calcium.
| Incident Cases N (%) |
Demographic adjusted* RR (95% CI) |
Fully adjusted† RR (95% CI) |
Adds mutual adjustment‡ RR (95% CI) |
P-value§ | |
|---|---|---|---|---|---|
| Serum phosphate (mg/dL) | |||||
| ≤3.0 | 31 (5.5%) | reference | |||
| 3.0 – 3.5 | 58 (3.9%) | 0.80 (0.53 to 1.22) | 0.79 (0.51 to 1.20) | 0.78 (0.51 to 1.19) | 0.25 |
| 3.51 – 4.0 | 67 (3.7%) | 0.82 (0.54 to 1.25) | 0.81 (0.52 to 1.25) | 0.79 (0.51 to 1.23) | 0.29 |
| >4.0 | 47 (4.3%) | 1.09 (0.68 to 1.74) | 1.11 (0.68 to 1.80) | 1.04 (0.64 to 1.69) | 0.87 |
| Linear per 1.0 mg/dL higher | 1.26 (0.92 to 1.73) | 1.25 (0.90 to 1.72) | 1.2 (0.87 to 1.64) | 0.27 | |
| Serum FGF-23 (pg/mL) | |||||
| ≤30.5 | 43 (3.4%) | reference | |||
| 30.5 – 37.7 | 45 (3.6%) | 0.99 (0.66 to 1.48) | 0.99 (0.66 to 1.48) | 1.00 (0.66 to 1.51) | 1 |
| 37.8 – 46.5 | 43 (3.5%) | 0.96 (0.63 to 1.45) | 0.94 (0.62 to 1.44) | 0.95 (0.62 to 1.46) | 0.81 |
| 46.5 – 590 | 72 (6.0%) | 1.52 (1.05 to 2.20) | 1.56 (1.05 to 2.33) | 1.52 (1.01 to 2.28) | 0.04 |
| Linear per 20 pg/mL higher | 1.16 (0.99 to 1.37) | 1.13 (0.94 to 1.35) | 1.10 (0.92 to 1.31) | 0.29 | |
| Urine phosphate (mg/dL)‖ | |||||
| ≤24.9 | 34 (3.1%) | reference | |||
| 24.9 – 44.1 | 57 (4.5%) | 1.38 (0.91 to 2.10) | 1.28 (0.78 to 2.10) | 1.28 (0.78 to 2.09) | 0.33 |
| 44.2 – 67.8 | 51 (4.2%) | 1.32 (0.86 to 2.02) | 1.15 (0.65 to 2.03) | 1.14 (0.65 to 2.01) | 0.64 |
| 67.9 – 398 | 65 (4.9%) | 1.61 (1.06 to 2.44) | 1.21 (0.65 to 2.24) | 1.18 (0.64 to 2.2) | 0.59 |
| Per doubling | 1.23 (1.08 to 1.41) | 1.18 (0.94 to 1.49) | 1.16 (0.92 to 1.46) | 0.2 | |
| Serum PTH (pg/mL) | |||||
| <65 | 179 (4.1%) | reference | |||
| ≥65 | 23 (4.2%) | 0.87 (0.56 to 1.33) | 0.84 (0.54 to 1.32) | 0.86 (0.54 to 1.36) | 0.51 |
| Linear per 10 pg/mL greater | 1.01 (0.94 to 1.09) | 1.01 (0.94 to 1.08) | 1.01 (0.95 to 1.08) | 0.75 |
Demographic adjusted models adjusted for age (in 10 year categories), gender, ethnicity, study site, and scanner type.
Addi6onally adjusts for body mass index, systolic blood pressure, diastolic blood pressure, diabetes, smoking, LDL-cholesterol, HDL-cholesterol, 25-hydroxyvitamin D, eGFR, log of albumin to creatinine ratio, and serum calcium.
Mutually adjusted models addi6onally adjust for the other phosphate metabolism markers.
P-values are present for the complete mutually adjusted models
Models for urine phosphate additionally adjust for the log of urine creatinine.
CI = Confidence Interval; FGF = Fibroblast growth factor; PTH = Parathyroid Hormone; RR = Relative Risk
Lack of associations of phosphate markers with AVC severity or progression
Among the 913 participants who had non-zero baseline AVC scores, the median AVC score was 58 (IQR 19–145). In this subgroup there was no relationship of any of the phosphate metabolism markers with AVC score severity or with progression of AVC score (Supplementary Table).
Discussion
In this large, multi-ethnic population that was free of clinical cardiovascular disease, higher serum phosphate levels were associated with a greater prevalence of AVC independent of demographics, atherosclerotic risk factors, kidney function, and other mineral metabolism markers. This result validates previous epidemiologic associations of serum phosphate with early CAVD in a larger, more diverse study population that includes gold-standard measurements of aortic valve calcium and adjustment for a comprehensive set of traditional risk factors for aortic valve disease.(16) Furthermore, the current study provides a more complete evaluation of phosphate metabolism by simultaneously evaluating FGF-23, PTH, and urinary phosphate concentrations, as related markers of phosphate metabolism.
The size of the association for serum phosphate concentrations is comparable to that of established modifiable risk factors such as cholesterol and hypertension. To date, studies using statins and angiotensin-converting enzyme inhibitors have not shown benefit at reducing progression in more advanced CAVD, when hemodynamic impairment has manifested. It has been postulated that risk factors governing disease progression in more advanced stages may differ from those involved in CAVD initiation.(27,28) This study did not find an association with phosphate in progression or severity of AVC. However, the population studied was a relatively young and cardiovascular disease-free cohort, and follow-up was relatively short for a disease process that develops over decades in later life. Future studies with longer follow-up are needed to further elucidate the relationship of mineral metabolism to disease progression.
In vitro studies have shown that osteogenic and apoptotic pathways are important for the development of AVC.(29,30) Transformation of aortic valvular interstitial cells into osteoblast-like phenotypes that mineralize valve tissue is likely a key component.(12,31,32) Uptake of phosphate via the Pit-1 receptor in multiple cell lines, including aortic valve interstitial cells (VIC), has been shown to upregulate bone-specific signaling cascades involving Cbfa1/Runx2 and bone morphogenic proteins.(15,33,34) Additionally, it has been demonstrated that phosphate uptake via Pit-1 induces apoptosis and mineralization of VICs via the mitochondrial pathway(35). The same authors found that explanted human aortic valves with CAVD show significantly higher Pit-1 expression near calcified nodules compared to control valves without CAVD. Finally, phosphate loading of aortic endothelial cells inhibits nitric oxide production through uncoupling of nitric oxide synthase, leading to increased levels of reactive oxygen species.(36) This dysregulation of antioxidant mechanisms may lead to upregulation of pro-osteogenic signaling cascades and the development of AVC.(37–39) Despite multiple mechanisms phosphate may have on mineralization of the aortic valve it remains unknown if serum phosphate levels are causative or only a biomarker for AVC. Future in vivo studies examining effects on AVC mechanisms by elevating or reducing serum phosphate concentrations within the normal range could be insightful. Moreover, the reasons for differences in serum phosphate levels in individuals without kidney impairment need to be elucidated.
To our knowledge, this is the first study examining the phosphate regulating hormone, FGF-23, with AVC in a population without kidney disease. A previous, unadjusted cross-sectional analysis in hemodialysis patients found that dialysis patients with prevalent AVC by echocardiography had, on average, higher FGF-23 values when compared to individuals without AVC.(40) In mouse models with moderate CKD, high-phosphate diets have been shown to increase FGF-23 levels and to lead to ectopic calcification without producing overt hyperphosphatemia.(41) It is possible that FGF-23 may be a marker of exposure to a chronic phosphate loading. However, this has not been confirmed in studies with normal kidney function, and mechanisms of phosphate effects on osteocyte production of FGF-23 remain unknown. The present study found an association of the highest quartile of FGF-23 levels with incident AVC. While there may be a threshold effect with FGF-23, it is possible that high FGF-23 identifies a sicker population with unmeasured confounders for which we did not adjust. We believe this result deserves further investigation in other populations, but should be interpreted cautiously here, given the lack of a dose-response relationship and the lack of association in the other AVC outcome measures.
We found no association of PTH or urinary phosphate concentration with any AVC outcome. A previous study reported higher age-adjusted 24-hour urinary phosphate levels, which reflect dietary phosphate intake, in patients with aortic stenosis as compared to those with aortic sclerosis. There is a strong linear correlation between spot urine phosphate measurements and 24-hour collections.(42) However, our urinary phosphate measurements exhibited wide variance, perhaps making associations more difficult to detect. Our null results for urine phosphorus could therefore be due to true lack of association or to misclassification of urine phosphorus excretion.
Our study has limitations. First, this is an observational study and remains subject to unmeasured confounders. The population studied was relatively healthy overall, and had a low prevalence of CAVD that was, on average, very mild. The lack of disease severity and short follow-up time limits the ability to detect associations with incident and progressive disease.
In conclusion, this study demonstrates that serum phosphate levels are independently associated with AVC prevalence in a healthy multi-ethnic population. Whether serum phosphate levels are a biomarker or directly implicated in AVC should be evaluated in future studies.
Supplementary Material
Highlights.
We investigated phosphate metabolism biomarkers with aortic valve calcification.
Serum phosphate levels are significantly associated with AVC prevalence.
Phosphate metabolism was not associated with AVC severity or progression.
Acknowledgements
The authors thank the other investigators, the staff, and the participants of the MESA study for their valuable contributions. A full list of participating MESA investigators and institutions can be found at http://www.mesa-nhlbi.org.
Funding:
This research was supported by R01 HL096875, R01 HL071739 and contracts N01-HC-95159 through N01-HC-95165 and N01-HC-95169 from the National Heart, Lung, and Blood Institute.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Disclosures: None
References
- 1.Otto C, Lind B, Kitzman D, Gersh B, Siscovick D. Association of aortic-valve sclerosis with cardiovascular mortality and morbidity in the elderly. N Engl J Med. 1999;341:142–147. doi: 10.1056/NEJM199907153410302. [DOI] [PubMed] [Google Scholar]
- 2.Owens DS, Budoff MJ, Katz R, et al. Aortic valve calcium independently predicts coronary and cardiovascular events in a primary prevention population. JACC Cardiovasc Imaging. 2012;5:619–625. doi: 10.1016/j.jcmg.2011.12.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Rosenhek R, Binder T, Porenta G, et al. Predictors of outcome in severe, asymptomatic aortic stenosis. N Engl J Med. 2000;343:611–617. doi: 10.1056/NEJM200008313430903. [DOI] [PubMed] [Google Scholar]
- 4.Cowell S, Newby D, Burton J, et al. Aortic valve calcification on computed tomography predicts the severity of aortic stenosis. Clin Radiol. 2003;58:712–716. doi: 10.1016/s0009-9260(03)00184-3. [DOI] [PubMed] [Google Scholar]
- 5.Cueff C, Serfaty JM, Cimadevilla C, et al. Measurement of aortic valve calcification using multislice computed tomography: correlation with haemodynamic severity of aortic stenosis and clinical implication for patients with low ejection fraction. Heart. 2011;97:721–726. doi: 10.1136/hrt.2010.198853. [DOI] [PubMed] [Google Scholar]
- 6.Rajamannan NM, Evans FJ, Aikawa E, et al. Calcific aortic valve disease: not simply a degenerative process: A review and agenda for research from the National Heart and Lung and Blood Institute Aortic Stenosis Working Group. Executive summary: Calcific aortic valve disease-2011 update. Circulation. 2011;124:1783–1791. doi: 10.1161/CIRCULATIONAHA.110.006767. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Otto C, Kuusisto J, Reichenbach D, Gown A, O'Brien K. Characterization of the early lesion of 'degenerative' valvular aortic stenosis. Histological and immunohistochemical studies. Circulation. 1994;90:844–853. doi: 10.1161/01.cir.90.2.844. [DOI] [PubMed] [Google Scholar]
- 8.Chan KL, Teo K, Dumesnil JG, Ni A, Tam J, Investigators A. Effect of Lipid lowering with rosuvastatin on progression of aortic stenosis: results of the aortic stenosis progression observation: measuring effects of rosuvastatin (ASTRONOMER) trial. Circulation. 2010;121:306–314. doi: 10.1161/CIRCULATIONAHA.109.900027. [DOI] [PubMed] [Google Scholar]
- 9.Rossebø A, Pedersen T, Boman K, et al. Intensive lipid lowering with simvastatin and ezetimibe in aortic stenosis. N Engl J Med. 2008;359:1343–1356. doi: 10.1056/NEJMoa0804602. [DOI] [PubMed] [Google Scholar]
- 10.Cowell S, Newby D, Prescott R, et al. A randomized trial of intensive lipid-lowering therapy in calcific aortic stenosis. N Engl J Med. 2005;352:2389–2397. doi: 10.1056/NEJMoa043876. [DOI] [PubMed] [Google Scholar]
- 11.Mohler Er, Adam L, McClelland P, Graham L, Hathaway D. Detection of osteopontin in calcified human aortic valves. Arterioscler Thromb Vasc Biol. 1997;17:547–552. doi: 10.1161/01.atv.17.3.547. [DOI] [PubMed] [Google Scholar]
- 12.Mohler Er, Chawla M, Chang A, et al. Identification and characterization of calcifying valve cells from human and canine aortic valves. J Heart Valve Dis. 1999;8:254–260. [PubMed] [Google Scholar]
- 13.Miller JD, Weiss RM, Heistad DD. Calcific aortic valve stenosis: methods, models, and mechanisms. Circ Res. 2011;108:1392–1412. doi: 10.1161/CIRCRESAHA.110.234138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.O'Brien K, Kuusisto J, Reichenbach D, et al. Osteopontin is expressed in human aortic valvular lesions. Circulation. 1995;92:2163–2168. doi: 10.1161/01.cir.92.8.2163. [DOI] [PubMed] [Google Scholar]
- 15.Jono S, McKee MD, Murry CE, et al. Phosphate regulation of vascular smooth muscle cell calcification. Circ Res. 2000;87:E10–E17. doi: 10.1161/01.res.87.7.e10. [DOI] [PubMed] [Google Scholar]
- 16.Linefsky JP, O'Brien KD, Katz R, et al. Association of serum phosphate levels with aortic valve sclerosis and annular calcification: the cardiovascular health study. J Am Coll Cardiol. 2011;58:291–297. doi: 10.1016/j.jacc.2010.11.073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Bild D, Bluemke D, Burke G, et al. Multi-ethnic study of atherosclerosis: objectives and design. Am J Epidemiol. 2002;156:871–881. doi: 10.1093/aje/kwf113. [DOI] [PubMed] [Google Scholar]
- 18.Cushman M, Cornell E, Howard P, Bovill E, Tracy R. Laboratory methods and quality assurance in the Cardiovascular Health Study. Clin Chem. 1995;41:264–270. [PubMed] [Google Scholar]
- 19.Stevens L, Coresh J, Schmid C, et al. Estimating GFR using serum cystatin C alone and in combination with serum creatinine: a pooled analysis of 3,418 individuals with CKD. Am J Kidney Dis. 2008;51:395–406. doi: 10.1053/j.ajkd.2007.11.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Friedewald WT, Levy RI, Fredrickson DS. Estimation of the concentration of low-density lipoprotein cholesterol in plasma, without use of the preparative ultracentrifuge. Clin Chem. 1972;18:499–502. [PubMed] [Google Scholar]
- 21.Budoff M, Mao S, Takasu J, Shavelle D, Zhao X, O'Brien K. Reproducibility of electron-beam CT measures of aortic valve calcification. Acad Radiol. 2002;9:1122–1127. doi: 10.1016/s1076-6332(03)80513-5. [DOI] [PubMed] [Google Scholar]
- 22.Budoff M, Takasu J, Katz R, et al. Reproducibility of CT measurements of aortic valve calcification, mitral annulus calcification, and aortic wall calcification in the multi-ethnic study of atherosclerosis. Acad Radiol. 2006;13:166–172. doi: 10.1016/j.acra.2005.09.090. [DOI] [PubMed] [Google Scholar]
- 23.Budoff M, Katz R, Wong N, et al. Effect of scanner type on the reproducibility of extracoronary measures of calcification: the multi-ethnic study of atherosclerosis. Acad Radiol. 2007;14:1043–1049. doi: 10.1016/j.acra.2007.05.021. [DOI] [PubMed] [Google Scholar]
- 24.Agatston A, Janowitz W, Hildner F, Zusmer N, Viamonte MJ, Detrano R. Quantification of coronary artery calcium using ultrafast computed tomography. J Am Coll Cardiol. 1990;15:827–832. doi: 10.1016/0735-1097(90)90282-t. [DOI] [PubMed] [Google Scholar]
- 25.van Buuren S, Groothuis-Oudshoorn K. MICE: Multivariate Imputation by Chained Equations in R. Journal of Statistical Software. 2011;43:1–67. [Google Scholar]
- 26.Rubin D. Multiple Imputation for Nonresponse in Surveys. New York: Wiley; 1987. [Google Scholar]
- 27.Owens DS, Otto CM. Is it time for a new paradigm in calcific aortic valve disease? JACC Cardiovasc Imaging. 2009;2:928–930. doi: 10.1016/j.jcmg.2009.02.016. [DOI] [PubMed] [Google Scholar]
- 28.Owens DS, Katz R, Takasu J, Kronmal R, Budoff MJ, O'Brien KD. Incidence and progression of aortic valve calcium in the Multi-ethnic Study of Atherosclerosis (MESA) Am J Cardiol. 2010;105:701–708. doi: 10.1016/j.amjcard.2009.10.071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Miller JD, Weiss RM, Serrano KM, et al. Evidence for active regulation of pro-osteogenic signaling in advanced aortic valve disease. Arterioscler Thromb Vasc Biol. 2010;30:2482–2486. doi: 10.1161/ATVBAHA.110.211029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Mohler Er, Gannon F, Reynolds C, Zimmerman R, Keane M, Kaplan F. Bone formation and inflammation in cardiac valves. Circulation. 2001;103:1522–1528. doi: 10.1161/01.cir.103.11.1522. [DOI] [PubMed] [Google Scholar]
- 31.Rajamannan N, Subramaniam M, Rickard D, et al. Human aortic valve calcification is associated with an osteoblast phenotype. Circulation. 2003;107:2181–2184. doi: 10.1161/01.CIR.0000070591.21548.69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.O'Brien K, Shavelle D, Caulfield M, et al. Association of angiotensin-converting enzyme with low-density lipoprotein in aortic valvular lesions and in human plasma. Circulation. 2002;106:2224–2230. doi: 10.1161/01.cir.0000035655.45453.d2. [DOI] [PubMed] [Google Scholar]
- 33.Babu AN, Meng X, Zou N, et al. Lipopolysaccharide stimulation of human aortic valve interstitial cells activates inflammation and osteogenesis. Ann Thorac Surg. 2008;86:71–76. doi: 10.1016/j.athoracsur.2008.03.008. [DOI] [PubMed] [Google Scholar]
- 34.Li X, Yang H, Giachelli C. Role of the sodium-dependent phosphate cotransporter, Pit-1, in vascular smooth muscle cell calcification. Circ Res. 2006;98:905–912. doi: 10.1161/01.RES.0000216409.20863.e7. [DOI] [PubMed] [Google Scholar]
- 35.El Husseini D, Boulanger MC, Fournier D, et al. High expression of the Pi-transporter SLC20A1/Pit1 in calcific aortic valve disease promotes mineralization through regulation of Akt-1. PLoS One. 2013;8:e53393. doi: 10.1371/journal.pone.0053393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Shuto E, Taketani Y, Tanaka R, et al. Dietary phosphorus acutely impairs endothelial function. J Am Soc Nephrol. 2009;20:1504–1512. doi: 10.1681/ASN.2008101106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Byon CH, Javed A, Dai Q, et al. Oxidative stress induces vascular calcification through modulation of the osteogenic transcription factor Runx2 by AKT signaling. J Biol Chem. 2008;283:15319–15327. doi: 10.1074/jbc.M800021200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Miller JD, Chu Y, Brooks RM, Richenbacher WE, Peña-Silva R, Heistad DD. Dysregulation of antioxidant mechanisms contributes to increased oxidative stress in calcific aortic valvular stenosis in humans. J Am Coll Cardiol. 2008;52:843–850. doi: 10.1016/j.jacc.2008.05.043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Liberman M, Bassi E, Martinatti MK, et al. Oxidant generation predominates around calcifying foci and enhances progression of aortic valve calcification. Arterioscler Thromb Vasc Biol. 2008;28:463–470. doi: 10.1161/ATVBAHA.107.156745. [DOI] [PubMed] [Google Scholar]
- 40.Kirkpantur A, Balci M, Gurbuz OA, et al. Serum fibroblast growth factor-23 (FGF-23) levels are independently associated with left ventricular mass and myocardial performance index in maintenance haemodialysis patients. Nephrol Dial Transplant. 2011;26:1346–1354. doi: 10.1093/ndt/gfq539. [DOI] [PubMed] [Google Scholar]
- 41.El-Abbadi M, Pai A, Leaf E, et al. Phosphate feeding induces arterial medial calcification in uremic mice: role of serum phosphorus, fibroblast growth factor-23, and osteopontin. Kidney Int. 2009;75:1297–1307. doi: 10.1038/ki.2009.83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Gökçe C, Gökçe O, Baydinç C, et al. Use of random urine samples to estimate total urinary calcium and phosphate excretion. Arch Intern Med. 1991;151:1587–1588. doi: 10.1001/archinte.1991.00400080083015. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.