Abstract
To optimize imaging of cells in three dimensional culture we studied confocal backscattering, Second Harmonic Generation (SHG) and autofluorescence as source of contrast in extracellular matrix (ECM) mimics and evaluated the attenuation as well as bleaching of endogenous cellular fluorescence signals. All common ECM mimics exhibit contrast observable with confocal reflectance microscopy. SHG imaging on collagen I based hydrogels provides high contrast and good optical penetration depth. Agarose is a useful embedding medium because it allows for large optical penetration and exhibits minimal autofluorescence while still providing good reflectance to detect voids in the embedding medium. We labeled breast cancer cells’ outline with DsRed2 and nucleus with eGFP. DsRed2 can be excited with confocal imaging at 568nm, and with two photon excitation (TPE) in the red and longer NIR. eGFP was excited at 488nm for confocal and in the NIR for TPE. While there is small difference in the bleaching rate for eGFP between confocal and TPE we observed significant difference for DsRed2 where bleaching is strongest during TPE in the red wavelengths and smallest during confocal imaging. After a few hundred microns depth in a collagen I hydrogel, TPE fluorescence becomes twice as strong compared to confocal imaging.
Keywords: Extra Cellular Matrix Mimics, Confocal Microscopy, Two-photon microscopy, Photobleaching, DsRed2, eGFP
Introduction
Three dimensional culture is important to study the interaction of cells and cell clusters with the extracellular matrix (ECM) in a realistic environment. Growing cells on a two dimensional culture dish results in an artificial sheet of cells. ECM mimics provide a native three dimensional microenvironment enabling studies of proliferation and migration of individual cells as well as cell collectives. For example collagen type I hydrogels embedded with fibrosarcoma and breast cancer cells were used to study proteolysis and adhesions associated cell structures during cancer cell migration(Wolf & Friedl, 2009). Sprouting of adipose tissue derived microvessels was studied by embedding them in collagen I gels and incubating over several days (Hoying, et al., 1996). Those studies desire non-destructive visualization techniques compatible with time series imaging and long term culture. Micron to submicron resolution is needed in order to detect cell protrusion enriched with proteolytic enzymes or adhesion and contraction associated biomolecules. Laser scanning microscopy is an ideal approach given that exposure is optimized for minimal photochemical breakdown and cytotoxicity. Reflectance confocal microscopy can identify micron and submicron size structures due to scattering that is based on a mismatch of refractive index between the scattering structure and the embedding medium resulting in good contrast from fibrillar structures(Friedl, 2004; Roeder, et al., 2002). Similarly the repetitive structural nature of fibrillar collagen gives rise to frequency doubled oscillations, known as second harmonic generation (SHG) (Mohler, et al., 2003). With the use of ultra-short pulsed lasers, instantaneous power densities required to elicit this otherwise weak process can be achieved up to several hundred microns in tissue and millimeters in collagen hydrogels. Endogenous fluorescence contrast is preferred when identifying cells in living three dimensional cultures compared to histochemical labeling. Either cellular auto fluorescence due to emission of enzymatic co-factors NADH and flavins or expression of genetically engineered fluorescent proteins are most commonly observed in cell migration and proliferation studies (Bjorkoy, et al., 2005; Condeelis & Segall, 2003; Hadjantonakis & Papaioannou, 2004; Heikal, et al., 2000; Kim, et al., 2009; Wu, et al., 2005). Scattering limits the ability to focus light in turbid media and because scattering is inversely dependent on wavelength, two photon absorption enables greater penetration depth when compared with traditional confocal excitation and emission collection. However, similar to second harmonic generation, the optical cross section for the two photon absorption process is small, therefore requiring high instantaneous power densities to observe. Often dual color labeling is required to label cell outline and nucleus separately. One can also detect cellular activities such as increased expression of membrane bound metalloproteinase (MT1-MMP) with a chimera of MT1 and the green fluorescent protein (Cao, et al., 2005) and the cell outline with another fluorescent protein. One choice for dual color labeling is the simultaneous recording of green (i.e. eGFP) and red (i.e. DsRed2) fluorescent proteins which is studied here.
Excitation of the green fluorescent protein has been well established for two photon microscopy with the Titanium Sapphire (TiSaph) laser. The largest two photon cross section is at 920nm and does not vary substantially between the wild type and the photo chemically enhanced version (Blab, et al., 2001). Previous studies have shown that GFP is relatively resistant to photobleaching for both confocal and two photon excitation (TPE) (Patterson, et al., 2001; Tsien, 1998). TPE of DsRed2 occurs most efficiently at 1120nm (Andresen, et al., 2009; Drobizhev, et al., 2009) however excitation is also possible below 800nm with a peak possibly at 700nm. Excitation at 1120nm cannot be achieved with a TiSaph laser alone and requires for example the combination of a TiSaph laser with an optical parametric oscillator. When exciting DsRed2 with a TiSaph laser alone, the choice of wavelength is often limited by the power available at the sample from the optical system. Both GFP and RFP can be efficiently excited in confocal microscopy with an Argon Ion laser (488nm) and an Argon Krypton laser (568nm) respectively. Due to the complexity of the TiSaph laser system, confocal laser scanning microscopy is usually more cost effective in imaging fluorescent proteins than two photon microscopy however excitation in the NIR has demonstrated significant advantages in imaging depth (Andresen, et al., 2009; Durr, et al., 2011; Kobat, et al., 2009). In this paper we discuss tradeoffs between two photon and single photon excitation for DsRed2 and eGFP especially in terms of photobleaching and fluorescence intensity ratio as a function of depth. We found that although TPE of DsRed2 yields higher optical penetration at red TiSaph wavelengths, the resulting photobleaching is stronger compared to confocal imaging. As published by others (Andresen, et al., 2009), TPE with an TiSaph and OPO in the IR reduces that problem, but our extrapolation concludes that photobleaching of DsRed2 remains lowest with confocal microscopy.
When imaging multi colored cells in ECM mimics several factors determine the choice of imaging modality and configuration. For imaging of the ECM mimics small diameter fibers will need to be visualized. Ideally the ECM of choice does not exhibit strong scattering or absorption so that an imaging depth of several hundred micrometers can be achieved. Several components of the ECM exhibit autofluorescence (i.e. collagen and elastin) and most ECM mimics are porous and exhibit condensed materials that scatter light. Here we study confocal backscattering, SHG and autofluorescence as source of contrast in ECM mimics and evaluate the signal intensity and its attenuation with depth in commonly used and commercially available matrix gels. Because of its high resolution and high contrast, confocal reflectance microscopy is a common method used to visualize fibrous structures (Friedl, 2004; Hartmann, et al., 2006). SHG exhibits exquisite contrast and high resolution as well as deep penetration when imaging fibrilar collagen (Friedl, 2004; Mohler, et al., 2003). In addition several ECM proteins exhibit auto fluorescence with fluorescence of elastin being the strongest and collagen’s auto fluorescence depending on its extent and type of cross linking (Mycek & Pogue, 2003).
Images of ECM mimics should exhibit high contrast; however contrast mechanisms that produce a strong signal are also responsible for background and signal attenuation. For example the confocal reflectance signal depends on the scattering cross section and anisotropy of the ECM fibers and the more light is scattered backwards the stronger the signal. However the attenuation with imaging depth and background also becomes stronger with increased scattering, essentially limiting imaging depth due to a lack of contrast and not a lack of signal. Similar to confocal fluorescence imaging, in TPEF deep tissue imaging there is out of focus background collected which limits the contrast (Durr, et al., 2011; Leray & Mertz, 2006). During two photon imaging in turbid media the Point Spread Function (PSF) broadens with increased imaging depth (Niesner, et al., 2007). Therefore besides a decrease of contrast due to attenuation and background there is additional reduction of resolution. In this paper we do not attempt to quantify contrast and resolution with imaging depth but we compared signal decay with depth as an exponential decay parameter which we call optical penetration.
The gels we attempted to study were based on the following materials;
Agarose which is based on a polysaccharide, that consists of alternating D-galactose and 3,6-anhydro-L-galactose units, and as hydrogel forms has porous structure (average pore size depends on concentration and type of agarose, but is typically 100 to 300nm (Griess, et al., 1989)) which can provide a lattice for cells. Agarose by itself lacks the ability to enhance cell attachment, which to some extent restricts its application (Lin, et al., 2005), however it is widely used as an embedding medium and for example serves as three-dimensional scaffold in neural engineering (Cao, et al., 2009). Collagen I is found in most tissue’s extracellular matrix and serves both for adhesion and proteolysis studies. Because of its abundance it is usually derived from dermis, tendon or bones. Some of its applications include the study of tumor cell invasion and migration (Leavesley, et al., 1993; Seppa, et al., 1982; Seppa, 1988) and fibrillogenesis (Gelman, et al., 1979). Fibrin gels are prepared from fibrinogen and thrombin. The benefit of using fibrin as compared to collagen gels is that cells are more likely to remodel the fibrin matrix and replace it with their own ECM (Black, et al., 2009). Because of its highly extensible, soft nature and controllable degradation (by the use of aprotinin), fibrin is an important ECM component and involved in hemostasis, wound healing, and cancer (Atala & Lanza, 2001; Jockenhoevel, et al., 2001). Gelatin is a denatured form of collagen I. It has properties comparable with collagen I gels and is more cost effective (Ratanavaraporn, 2006). But it has relatively low antigenicity compared to collagen (Lien, et al., 2009). Gelatin-based composite scaffolds have been used for tissue engineering applications such as cartilage (Huang, et al., 2005). PuraMatrix (3DM, Inc., Cambridge MA) is based on a synthetic peptide and developed to create a defined 3D microenvironment for a variety of cell culture experiments (Ma & Elisseeff, 2005). PuraMatrix has better hydration and nutrient diffusion than natural reconstituted ECM gels. PuraMatrix enables a consistent and defined ECM microenvironment. Some of its applications include tissue regeneration, cell therapies and drug delivery system. Matrigel (BD Biosciences, California) is a mixture of various extracellular matrix proteins to more closely resemble natural ECM. It contains 56% Laminin, 31% Collagen IV and of 8% Entactin. Matrigel is effective for the attachment and differentiation of both normal and transformed anchorage-dependent epithelial and other cell types but is more expensive than agarose or collagen I gels. PEGDA (poly ethylene glycol diacrylate) (Glycosan Biosystems Inc., Salt Lake City, Utah) is a synthetic polymer that can form a hydrogel in the presence of a photoinitiator and UV light. ECM proteins and/or growth factors can be incorporated into PEGDA hydrogels and mechanical properties of the hydrogels can be controlled to better mimic the 3D environment of human tissue (Baird, et al., 2008). Because of UV curing requirements we did not study PEGDA hydrogels in detail. OptiMaix (Matricel GmbH, Herzogenrath, Germany) is a three dimensionally engineered collagen scaffold that contains parallel collagen lined channels of 20–40 micron diameter. Through a temperature gradient freezing process ice crystals are formed in a low concentration collagen dispersion, forming a porous structure ideal to culture cells within a collagen sponge [Kroehne 2008]. This ECM mimic is mentioned here similarly to the PEGDA hydrogel and was not studied further.
In our studies highest optical penetration depth and weakest autofluorescence was obtained with agarose. Fibrin exhibited a high contrast but had low optical penetration.
Materials and Methods
Microscopy
Imaging of DsRed2, eGFP and ECM mimics was performed with a single beam intravital microscope (TrimScope, LaVision BioTec, Bielefeld, Germany). Our configuration of the TrimScope includes a confocal unit making the system capable of simultaneous two-photon excitation, second harmonic, and confocal imaging. Two-photon processes are recorded through non-descanning reverse detection using a triple port (PMT 3,4&5 in Fig. 1a). Gallium Arsenide (H7422A-40, Hamamatsu, Hamamatsu City, Japan) and Bialkali sensors (H6780-01 and H6780- 20, Hamamatsu) can be modularly attached to this port. Confocal fluorescence and reflectance imaging was implemented to allow excitation in the green and detection in the red (PMT 10 in Fig. 1b, H6780-20, Hamamatsu). In this study confocal fluorescence of DsRed2 and reflectance of ECM mimics were recorded through the confocal unit (Fig. 1b) while fluorescence of eGFP, DsRed2 and SHG of ECM mimics were recorded with the two photon unit (Fig. 1a).
Figure 1.
a. Schematic diagram of the two-photon unit of the microscope. After expanding the beam in a telescope to match the back aperture of the microscope objective the laser beam passes the scan unit, is focused by the scan lens on an intermediate image plane and re-collimated by the field lens to match the infinity corrected microscope optics. Fluorescence and SHG is collected by three PMTs in the microscope’s non-descanned detection port. Emission and SHG wavelengths are selected by appropriate bandpass and dichroic filters.
b. Schematic diagram of the confocal unit of the microscope. The confocal illumination laser enters the unit through a fiberoptic port, is collimated and passes polarization optics which converts linear polarization to circular polarization. After reflecting on a dichroic mirror the beam path is merged with the two-photon system by two beam steering mirrors. Reflected light is separated from the illumination light through a polarizing beam splitter cube and imaged on the PMT through a confocal pinhole. Fluorescence passes the dichroic mirror and is imaged similarly on a PMT.
The confocal unit uses linear polarized light from an ArKr laser at 568nm (643-PERYB-A01, Melles Griot). Laser light is coupled to the unit with a fiberoptic cable (Kineflex-P-3-S-488…640-0.7-FCP-P2, Pointsource, Hamble, UK) and collimated inside the unit. The collimated beam passes a polarizing beam splitter cube (PBS) a quarter wave plate (QWP) which converts the linearly polarized light to circular polarization. When reflected from the sample the circularly polarized beam flips the rotation and by passing back through the QWP is reflected by the PBS to the detection module. This approach allows simultaneous fluorescence detection through a dichroic mirror without the use of a partial beam splitter cube. In our experiments, light in the reflectance detection channel had to be attenuated by an absorptive neutral density filter (NE510B/10%, Thorlabs) in order to avoid saturation of the sensor at laser intensities needed for fluorescence detection.
The two photon unit uses a Ti:Saph light source (Chameleon Ultra2, Coherent, UK) that is coupled to the scanner unit which is composed of a triple lens telescope that allows adjusting the beam diameter to the exit pupil of the objective lens, a fixed prism based chirp compensator keeping the pulse width down to 120fs in the sample, and an x/y scanner allowing up to 2000 lines to be scanned per second for a 500 micron field of view.. The laser intensity is adjusted with an electro-optical modulator (EOM 350-80, Conoptics, USA).
Lateral and axial confocal pinhole alignment was accomplished with 15 micron diameter microspheres (Focal Check, Invitrogen) suspended in agarose. Imaging through the center of a bead resulted in a ring and the fluorescence intensity of that ring was maximized and adjusted to be uniform. The confocal beam was co-aligned with the Ti:Saph beam by passing both beams through the system with scan, field and objective lenses (Fig. 1a) removed and assuring overlap at two points two meters apart. Further fine adjustment was conducted with the confocal beam steering mirrors by overlaying images of multi color calibration standards.
The microscope objective lens was a 20× water dipping lens with a 2 mm working distance, Numerical Aperture (NA) of 0.95 and a 17mm back aperture diameter (XLUMPLFL, Olympus). By adjusting the telescope, the Ti:Saph beam was set to approximately 18mm. The confocal laser beam diameter was 16 mm and not adjustable.
TrimScope microscope performance was evaluated by measuring axial and lateral FWHM of the point spread function (PSF) using Molecular Probes’ PS-Speck™ microscope point source kit (P7220) and custom gold spheres embedded in agarose gel. Fluorescent spheres had a diameter of 0.175 ± 0.005 µm and gold particles were approximately 30nm. Results are listed in table 1. The theoretical diffraction limited resolution of the optical system is approximately 0.4 micron (r0=0.61λ/NA, NA = 0.9) (Born & Wolf, 1999). Using confocal fluorescence imaging the theoretical radial resolution of our system is 0.29 micron (FWHMr0=0.61λexc/NA/sqrt(1+β2), with β= λexc/λdet=0.9, NA = 0.9) (Muller, 2006). The theoretical axial resolution of our confocal fluorescence system with an infinitesimal small pinhole is 1.38 micron (FWHMz=2nλexc/NA2/sqrt(1+β2) (Muller, 2006), and with a pinhole diameter of 20 micron 4.33 micron (FWHMzlargepinhole=7.4na2/λ/M2, n=1.33, a=10, M=20) (Corle & Kino, 1996).
Table 1.
Axial and lateral FWHM of the point spread function (PSF) for our TrimScope microscope.
| PSF Table | FWHMlateral | FWHMaxial | Reference |
|---|---|---|---|
| TPEF 0.2mu bead | 0.5x 0.4y | 2.4z | 0.35xy / 1.47z (Niesner 2007) |
| CR 0.03mu bead | 0.42x 0.37y | 2.8z | |
| CF 0.2mu bead | 0.5x 0.45y | 3z | 0.29xy / 4.33z (Corle & Kino 1996, Mueller 2006) |
Confocal lateral fluorescence resolution was worse than expected as the magnification of our system was 20 and the pinhole diameter 20 micron resulting in an aperture at the sample of 1 micron which is approximately 2 Airy units. In addition, our resolution target was 0.2 micron in diameter adding to the theoretical FWHM. Confocal reflectance FWHM was 0.1 micron smaller than confocal fluorescence FWHM as our target was 7 times smaller. Because confocal reflectance imaging is a coherent imaging technique, its PSF is not a multiplication of the illumination and detection PSF as it is in confocal fluorescence imaging and one can not directly compare the two. Our TPEF estimations were close to the measurements by Niesner et al (Niesner, et al., 2007) who used a similar optical setup, however our resolution beads were twice in size and PSF was estimated with FWHM and not with a three dimensional fit. Our PSF estimations showed an asymmetry whereas the FWHM in fast scanning axis was closer to theoretical value compared to slow axis. However this difference does not affect the results presented here.
As the confocal unit of the system did not have a configuration to excite eGFP, experiments comparing confocal and TPEF of eGFP were conducted with at Zeiss Laser Scanning Microscope (LSM 510, objective lens: 40× NA:1.0 W.I. W Plan-Apochromat) using the same detector but having the pinhole fully opened for TPEF measurements and set to 2 Airy units for confocal measurements. Fluorescence was collected through a band-pass filter (500–550nm) and a dual wavelength beam splitter was used to pass both NIR and Argon laser (HFT KP 700/488).
ECM Mimics Preparation
A series of hydrogels, including Agarose, Collagen I, Fibrin, Gelatin, Matrigel and PuraMatrix, were prepared in chambers approximately 20×20×2 mm on a microscope slide and covered with a No. 0 cover glass. Agarose (Sigma) was prepared by making a 2% solution in PBS, melting in a microwave, then allowing to cool on the slide chamber. Collagen I (rat tail, BD Biosciences) was prepared on ice by making a 3mg/ml final concentration using 250ul/ml of a 4× culture medium, appropriate volume of collagen I, adding water to a final volume of 1ml, and adjusting the pH with a small volume of 1M NaOH. Collagen was added to the slide chamber, and placed at 37°C to polymerize for 20 minutes. Fibrin was prepared by mixing equal parts of a 3 mg/ml stock of fibrinogen (Sigma) with a 2U/ml solution of thrombin (Sigma). Gelatin (Sigma) was prepared as a 5% solution in PBS, melted in a microwave, and allowed to cool on the slide chamber. Matrigel (BD Biosciences) was thawed at 4 degrees C, added at full strength to the slide chamber, and allowed to polymerize at 37°C for 20 minutes. PuraMatrix (BD Biosciences) was prepared following manufacturer’s protocol. PuraMatrix was diluted 1:1 with water, a 50ml drop added to slide chamber, and PBS was gently added around the edge of the drop. The slide was placed in a 37°C incubator, and PBS was changed three times to equilibrate pH of the PuraMatrix gel.
Dual Color Cells
MCF-7 breast cancer cells were co-transfected with RFP and a histone tagged GFP fusion protein. Cells were transfected using FuGene-6 (Roche) and selected with Zeocin and blasticidin. The RFP vector was made using DsRed2 (Clontech) that was cloned into pcDNA3.1/Zeo(+) (Invitrogen) at BamHI and NotI restriction sites. The nuclear targeted GFP was achieved using the pBOS-H2BGFP vector (BD Pharmingen). For imaging of 3D cultures, the dual color cells were embedded in collagen gels at a concentration of approximately 1–5 million cells/ml.
ECM Mimics Imaging
ECM mimics were imaged using confocal reflectance at 568nm and SHG at laser wavelength 780nm. Auto fluorescence was assumed to occur primarily with UV excitation and emission in the blue, therefore we choose excitation wavelength 780nm. For SHG detection we used bandpass filter FF01-377/50 (Semrock) and dichroic mirror Di01-R405-25×36 (Chroma), for autofluorescence we used bandpass filter HQ450/100M-2p-25 (Chroma) and dichroic mirror 505dcxr (Chroma).
ECM mimics were imaged at two different settings to obtain one data set optimized for 400µm depth and the other for 80µm. Background images with laser blocked were recorded and subtracted from each image. Average intensity of the whole image was calculated for all images after subtracting sensor background. Image intensities were corrected by the power which was recorded for each imaging configuration at the focus of the objective lens.. Intensity depends linearly with power for confocal microscopy and quadratically with TPEF; therefore the image intensities were adjusted correspondingly.
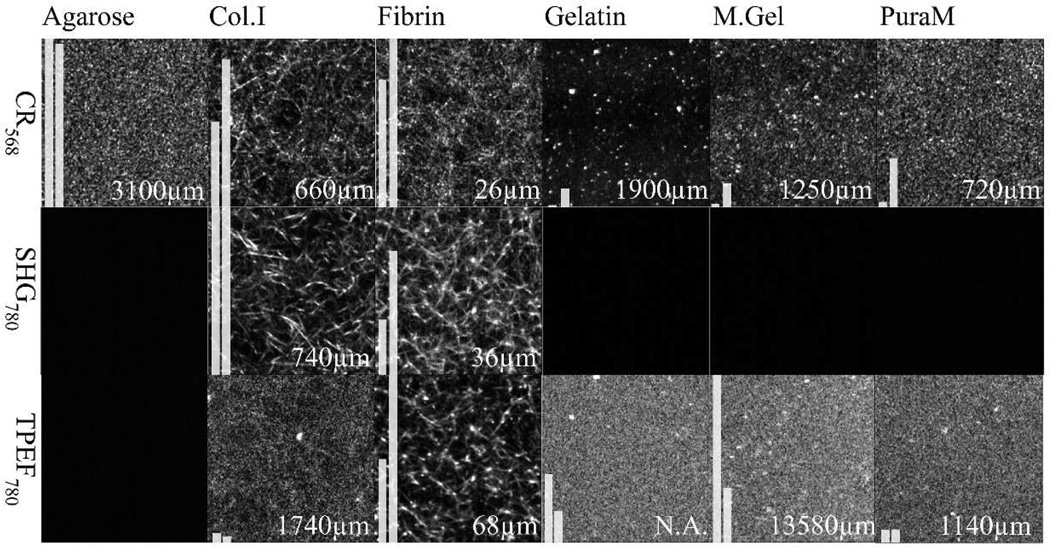
To quantify image quality metrics, the average intensity and contrast factor which is defined as the difference of highest 10% and lowest 10% of average intensities in an image were calculated and illustrated with two bars on the images in Fig. 2. Bars were normalized according to the bar with maximum intensity, for each imaging approach (represented in the same row in Fig. 2) separately. For display purpose, images were filtered and contrast adjusted (median 2×2, 1% high/low eliminated).With the 400µm depth scan data, average intensity versus depth curve was fit to an exponential decay function:
| (1) |
where μ is the attenuation coefficient and z is the depth. The attenuation coefficient is primarily a scattering coefficient as absorption is significantly lower but it is also affected by scattering anisotropy and NA of the objective lens (Jacques & Gareau, 2006). From this exponential function, depth values where intensity decays to 1/e (37 %) were computed for comparisons (optical penetration).
Figure 2.
Summary of ECM Mimic imaging. All images are 50×50 micron square in size and imaged with a precision of 474×474 pixel2. The first row illustrates confocal reflectance images of gels at 568nm illumination. Second and third rows show the second harmonic generation (SHG) and two photon excited fluorescence (TPEF) images of the gels (laser at 780nm). The first bar in each image illustrates average intensity and the second bar illustrates image contrast factor of images. Numbers on the images indicates depth where intensity decays to 1/e (37%, optical penetration). Images below an intensity threshold are shown black. For display purpose, all images were filtered and contrast adjusted (median 2×2, 1% high/low eliminated) while calculations were performed on images having only sensor background subtracted.
DsRed2 Imaging
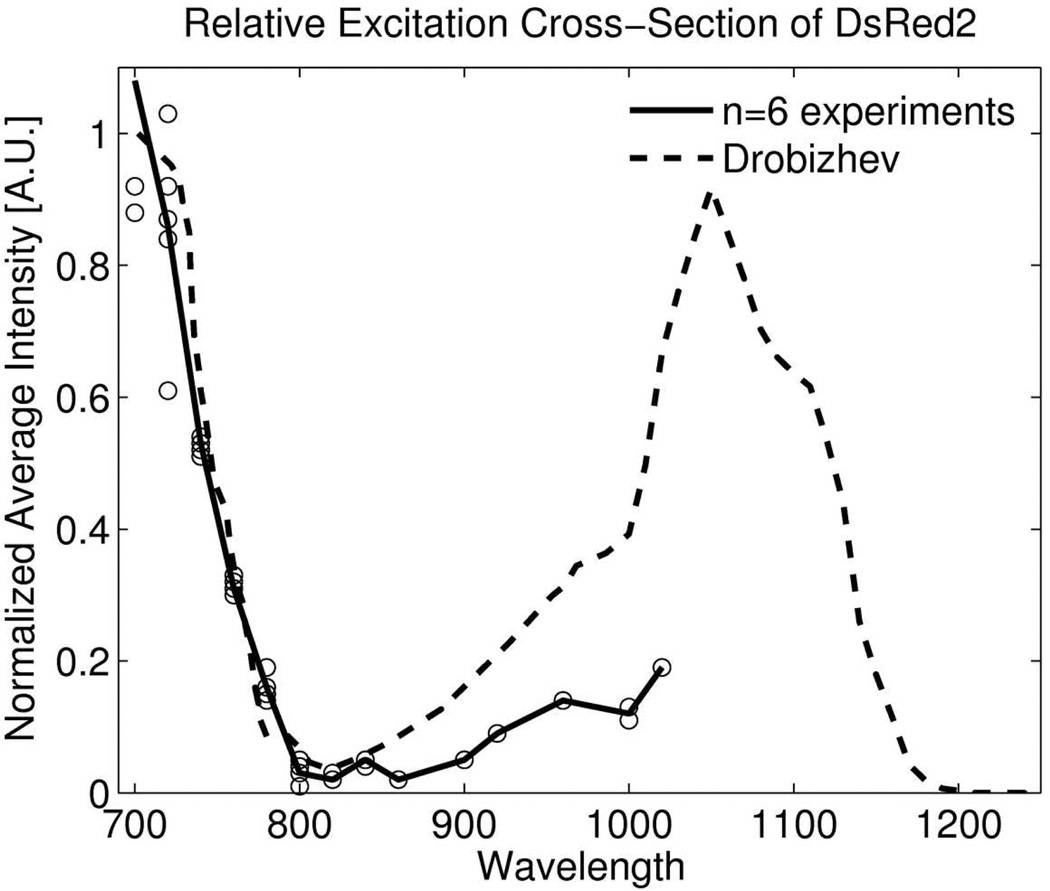
DsRed2 was imaged of dual color cells embedded in collagen I gels, to compare penetration depth and photobleaching with the TrimScope confocal fluorescence unit at an excitation wavelength of 568nm and TrimScope two photon unit at excitation wavelengths 720 nm and 760nm. Before starting these experiments, we performed an excitation wavelength scan as shown in Fig. 3 between wavelengths 700nm to 1020nm (step size 20nm) at a laser power of 20mW and found that excitation efficiency compares well with other data (Drobizhev, et al., 2009).
Figure 3.
Relative excitation cross-section of DsRed2. An excitation wavelength scan was performed using TPEF microscopy on DsRed2. Our results are consistent with literature in the lower wavelength range.
For DsRed2 detection in the confocal port, band-pass filter FF01-630/92 (Semrock) was placed in front of confocal fluorescence PMT (PMT 10 Fig. 1b) and for detection of TPEF the same filter was placed in front of the last PMT of the non-descan port (PMT 5, Fig. 1a).
Penetration depth experiments of DsRed2 were performed over 400µm depth at a step size of 4µm. Average intensity ratio of confocal fluorescence vs. TPEF as a function of penetration depth were calculated by applying the same circular region of interest around a cell or group of cells in the confocal image as well as the TPEF image and obtaining the mean intensity within the ROI. ROI selection was repeated throughout the 3D data stack, and for each cell or group of cells. The intensity data points for confocal and TPEF were divided which resulted in a ratio as function of depth. The ratio data was fit to an exponential decay function (see Eq.1) whereas the exponential constant is equivalent to the difference in the attenuation coefficient between the two imaging modalities.
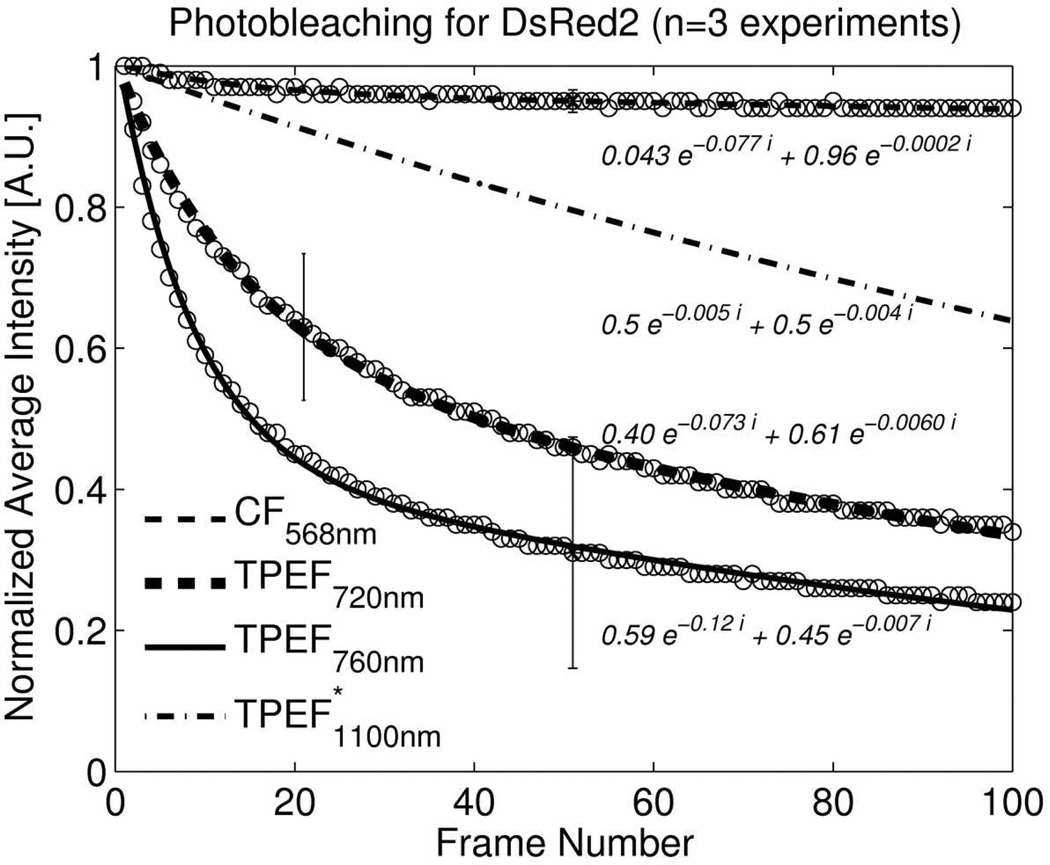
Photobleaching of DsRed2 was measured as a decrease in emission intensity during consecutive scanning for confocal (568nm, 9.2µW at sample) and two photon imaging (720nm, 22mW, 760nm, 44mW). These power settings resulted in same average intensity on the same type and make of PMT sensor (H6780-20) at the same gain setting. Losses in the optical path to the confocal sensor were estimated to be 20% larger compared to the TPEF detection. Data at 1100nm was taken from Andresen et al (Andresen, et al., 2009) and scaled so that their data at 760nm matched our data at 760nm. The samples were subjected to 100 consecutive scans at same depth location. For illustration, emission intensities were normalized according to the intensities obtained in the first frame. All curves were fitted to two-exponential functions as previously reported by Moissoglu, et al.(Moissoglu, et al., 2006):
| (2) |
where A, α, B, β are positive numbers and allow for in plane bleaching and diffusion of unbleached molecules into the imaging plane.
To evaluate the effect of the beam diameter entering the objective lens on photobleaching, a reduced beam diameter of 13mm was compared to the regular beam diameter of 18mm.. Total laser power between the two beam diameters was adjusted to be the same at the focus. Intensity decay data was fit to Eq.2.
Furthermore, to investigate the effect of out-of-plane photobleaching, a three dimensional stack of images was recorded over 40µm depth with a step size of 1µm, 40 times and the difference between confocal and TPEF imaging was analyzed.
eGFP Imaging
To compare confocal and TPEF of eGFP, we used an LSM-510 (Zeiss Confocal Microscope) and selected for confocal fluorescence imaging 488nm excitation (102 µW) and TPEF an excitation wavelength of 920nm (22mW). The same PMT in the descanned beam path was used for both experiments; however the pinhole was fully opened for TPEF imaging.
Imaging of eGFP in dual color cells embedded in collagen gels was performed over 476µm depth with a step size of 4µm. Cells appeared at approximately 120µm depth in our sample. Same methods as described with DsRed2 were used to obtain comparable curves. As both intensity decay curves and photobleaching curves are normalized, exponential intensity decay data do not depend where in the image stack cells are observed. Since the collagen density and polymerization conditions were kept constant, attenuation in our samples was similar for all samples..
Combined Confocal and TPEF Imaging of Dual Color Cells
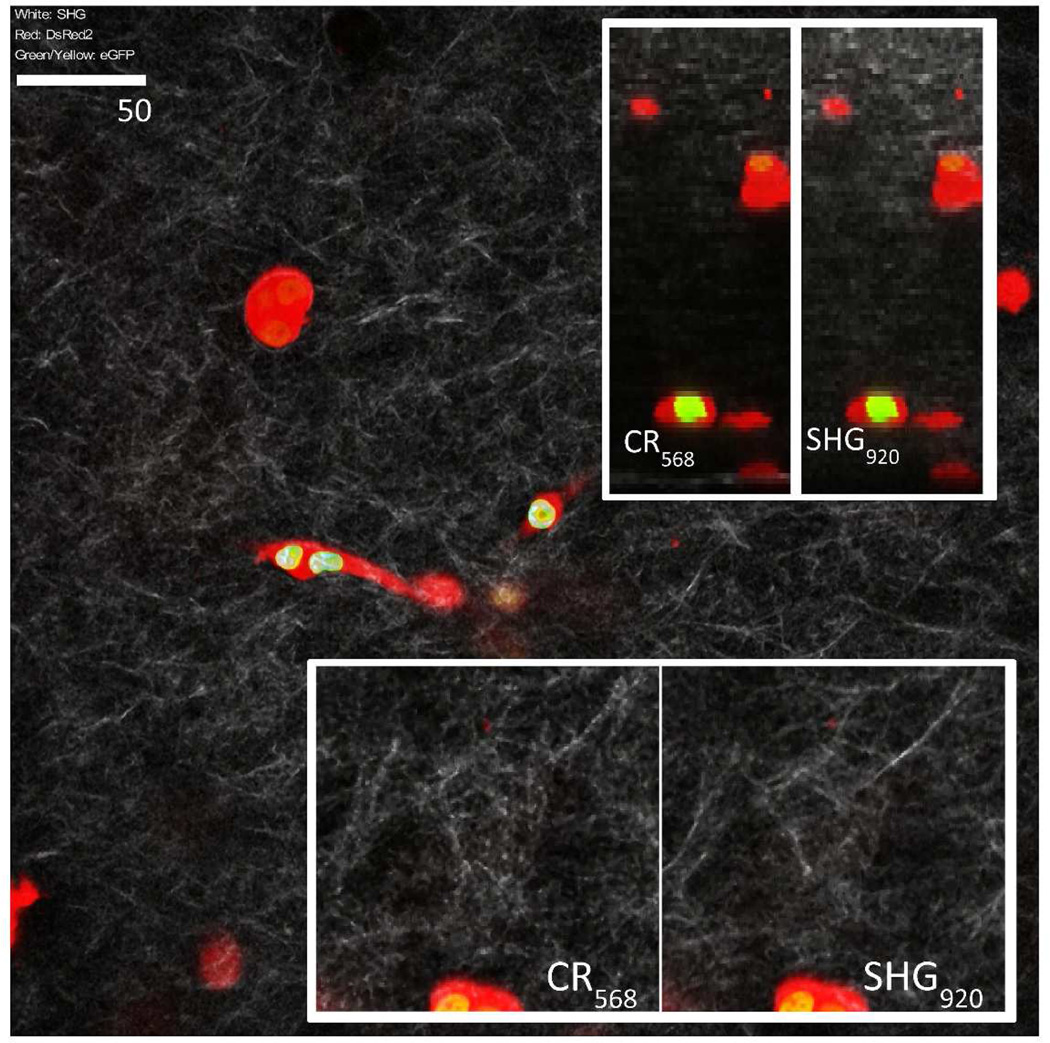
For illustration of a combined confocal and TPEF imaging system, dual color cells were embedded in collagen I gels and imaged using both confocal reflectance, SHG and TPEF microscopy (Fig. 4). Filters and dichroic mirrors for eGFP and SHG detection at 920nm were bandpass filters FF01-525/50 (Semrock) and FF02-460/80 (Chroma) with a dichroic mirror 490dcxr (Chroma) to reflect SHG) and FF568-Di01 (Semrock) to reflect eGFP onto EGFP PMT (PMT4, Fig. 1a) and SHG PMT (PMT3, Fig. 1a). Confocal PMTs were configured as described earlier to record DsRed2 and confocal reflectance. It should be noted that SHG signal obtained in the ECM mimic imaging is stronger at 780nm compared to 920nm because SHG intensity increases significantly with shorter wavelengths (Zipfel, et al., 2003).
Figure 4.
3D culture of dual colored cells in collagen ECM mimic. Nucleus exhibits eGPF fluorescence at 920nm excitation and cells were outlined with confocal DsRed2 imaging at 568nm excitation. Image stacks were filtered and contrast adjusted (median 3×3). Image pairs illustrate the difference between confocal reflectance and SHG imaging. Images at the top right illustrate cross sections of the image stacks down to a depth of 180 microns. Each frame is 400×400 micron square in size and imaged with a precision of 1021×1021 pixel2. Inserts at the bottom are a closer view (2×) to illustrate the contrast difference in confocal reflectance and SHG imaging of collagen fibers.
Results
ECM mimic imaging
As can be seen in Fig. 2, all gels exhibited structures observable by confocal reflectance microscopy. SHG was observed only in Collagen and Fibrin and auto fluorescence was negligible in agarose. Highest intensity and contrast with confocal reflectance microscopy were obtained for agarose, collagen and fibrin gels. Agarose exhibits small structures whereas only collagen and fibrin gels have fibrillar structures on a scale observed with the investigated microscopy approaches. Best optical penetration was observed with agarose reaching 3mm and least in fibrin (100 microns) making fibrin gels difficult to investigate at larger depths. With Matrigel and gelatin optical penetration of more than one mm can be reached however image contrast of the gel is weak. Collagen gels optical penetration reaches the mm range and like with all mimics, non-linear imaging techniques extend the obervation range. Collagen SHG exhibited strong contrast as well as finest details compared to other imaging modalities. Highest autofluorescence was observed for Matrigel and Fibrin whereas Fibrin had highest contrast. Some fluorescent structures can be observed in collagen gels as well.
Dual color cell imaging with confocal and TPEF microscopy
Results from combined confocal and TPEF imaging is illustrated in Fig. 4. DsRed2 and reflectance imaging was performed with the confocal unit whereas eGFP and SHG imaging with non-linear technique at 920nm illumination. Dual colored cells can be easily observed at larger depths while attenuation of confocal reflectance and SHG can be observed. SHG at 920nm illumination exhibits finer details compared to confocal reflectance imaging and attenuation of confocal reflectance is stronger than SHG as expected from data in Fig. 2.
Deep sample imaging with confocal and TPEF microscopy
DsRed2 and eGFP were imaged to compare the performance of confocal fluorescence (568nm, 488nm) versus TPEF (760nm, 920nm) in terms of average intensity ratio as a function of penetration depth. Fig. 5a illustrates the DsRed2 ratio and Fig. 5b the eGFP ratio. TPEF has an intensity advantage because of lower attenuation of the excitation beam compared to confocal fluorescence, hence the curves in Fig. 5 are decreasing. DsRed2 was imaged at 760nm and 568nm excitation and eGFP at 488nm and 920nm. We observed double the intensity at a depth of approximately 320 and 180 micron for imaging of DsRed2 and eGPF respectively.
Figure 5.
a: Intensity ratios of confocal fluorescence vs. two-photon excited fluorescence for DsRed2 as a function of penetration depth. Attenuation difference is 21cm−1 giving TPEF an intensity advantage of ~2 at a depth of 320 microns.
b: Intensity ratios of confocal fluorescence vs. two-photon excited fluorescence for eGFP as a function of penetration depth (only cells 100 micron below the sample surface were analyzed). Data was obtained on an LSM510 with 40× NA:1.0 WI objective lens. TPEF signal advantage is approximately twice that of DsRed2.
In plane photo bleaching
Two-photon microscopy is known to have reduced photobleaching in out of focus regions (Denk, et al., 1990). But photobleaching is more rapid under two-photon excitation compared with one-photon excitation (Patterson & Piston, 2000). Our results for DsRed2 and eGFP imaging are illustrated in Fig. 6a and 6b respectively. DsRed2 photobleaching under TPEF is significantly increased compared to confocal imaging. Photobleaching decreased in the order of 760nm, 720nm and 1100nm excitation wavelength (Fig. 6a). Photobleaching with confocal microscopy was at least 15 times lower after 100 repeated frames. We found that eGFP is more resistant to photobleaching with TPE and difference between TPEF and confocal microscopy was small (Fig. 6b).
Figure 6.
a: Photobleaching of DsRed2 measured as decrease in emission intensity during consecutive scanning for confocal (568nm, 9.2µW at sample) and two photon imaging (720nm, 22mW, 760nm 44mW). Settings resulted in same average intensity on same type of PMT sensor. Data at 1100nm was taken from Andresen et al(Andresen, et al., 2009) and scaled so that our and their data at 760nm matched. The samples were subjected to 100 consecutive scans. For illustration, emission intensities were normalized according to the intensities obtained in the first frame. All curves were fitted to two-exponential functions.
b: Photobleaching of eGFP for confocal (488nm, 102 µW) and two photon imaging (920nm, 22mW). Data was obtained on LSM510, 40× NA:1.0 WI.
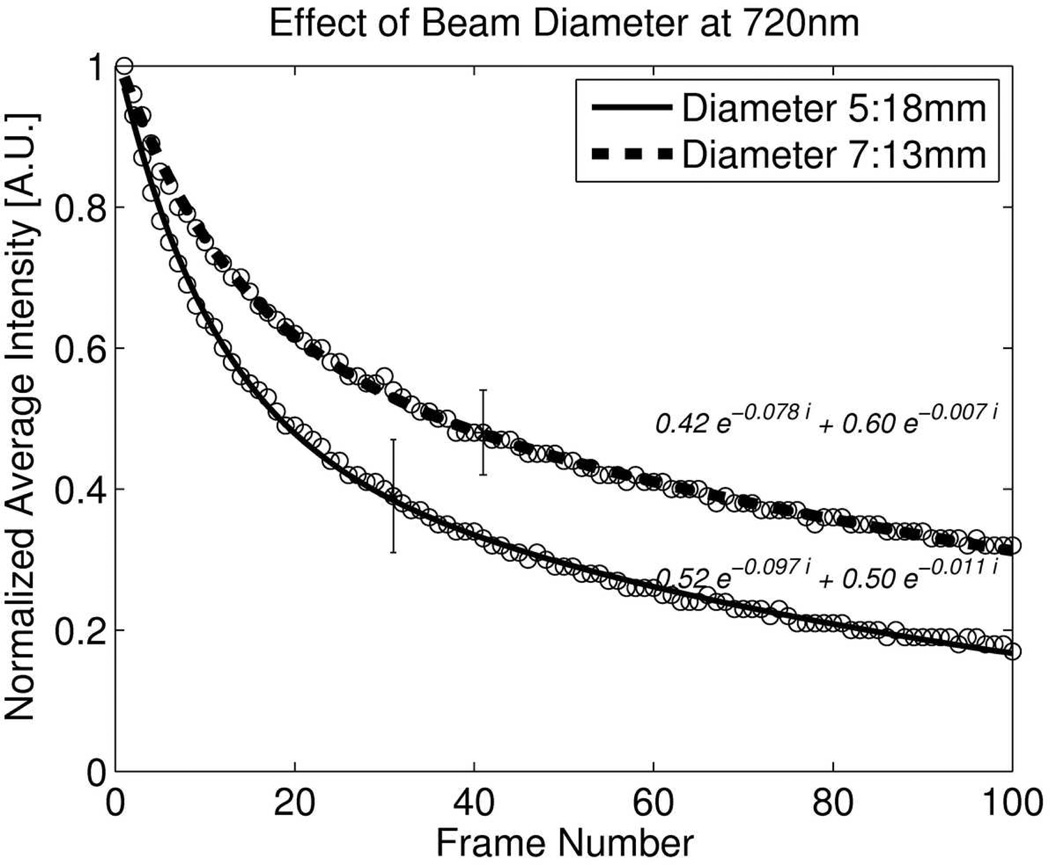
c: Effect of two different beam diameters on DsRed2 bleaching at 720nm.
Enlarging the pinhole in confocal microscopy can reduce photobleaching while maintaining the signal level at cost of axial resolution. Similarly reducing the beam diameter with TPEF increases the sampling volume. Fig. 6c illustrates the effect of reducing the beam by 30% which reduced bleaching by 45% after 100 repeated frames for DsRed2 imaging. However overall improvement is small compared to confocal imaging.
Out of plane photobleaching
Fig. 7 illustrates in plane and out of plane photobleaching of DsRed2 when imaging a stack of 41 images axially separated by one micron 40 times. Imaging the same plane 40 times will result in an intensity decrease of 5% for confocal and 50% in TPEF imaging. If a stack of 41 images is measured with TPEF 40 times there is negligible difference in photobleaching at the top of the stack and the bottom of the stack as photobleaching is limited to the focus of the beam. However in confocal imaging the intensity at the top of the stack is reduced to 80% and at the bottom the 90% which illustrates bleaching occurs along the excitation beam and not just in the focus. Out of plane photobleaching is as expected higher at the top as there is attenuation of the excitation beam with increasing imaging depth. The additional out of plane photobleaching rate is however lower than the in plane bleaching because if the rates were the same the signal would have been reduced to 65% where as it was only reduced to 80% in our example. In order for the out of plane photobleaching during confocal imaging to add up to similar bleaching levels as with TPEF imaging, very large stacks would need to be measured for DsRed2.
Figure 7.
Comparison of in plane and out of plane photobleaching. Out of plane photo bleaching is measured on a stack of 41 images separated by 1 micron. First bars of the two groups indicate intensity decrease after 40 consecutively measured frames at a fixed depth location (in plane) and second bar illustrates intensity decrease for the hypothetical case of maximum out of plane bleaching when the bleaching rate of the neighboring planes would be the same as the one in the currently imaged planed which in this example is equivalent to 40×41 exposures of a single plane. Third and fourth bars indicate intensities at the bottom and top of stacks after 40 consecutive stack measurements. Contrary to TPE, OPE has out of focal plane photobleaching and bleaching at the top of the stack is increased.
Conclusions
All investigated hydrogels exhibited label free contrast, making it possible to identify voids in the gels. Our observations show that agarose is useful as an embedding medium for optical investigation of samples because it allows for large optical penetration and exhibits minimal autofluorescence while still providing good reflectance contrast. This is most likely due to relatively small scattering cross section and anisotropic scattering occurring primarily in forward and backward direction. This means that scattering and attenuation occurs at a lower rate but if it occurs it is likely to scatter back onto the detector. Matrigel and Fibrin are not preferable when imaging weak fluorophores because of their high autofluorescence intensity compared to collagen and agarose. Fibrin also exhibits high signal attenuation limiting imaging depth. For label free imaging of cellular interactions with ECM, collagen I based hydrogels are ideal because they not only provide a native microenvironment but they also provide good contrast for confocal reflectance and SHG imaging and have a good optical penetration.
For dual color imaging of cells in native 3D environment, we found that there is a signal advantage of a factor of two after an imaging depth of 180 microns for eGPF and 320 microns for DsRed2. Because scattering is decreasing with increased wavelength and water absorption is not relevant in 2PEF microscopy between 350 and 1300nm (Kobat, et al., 2009), one can expect the advantage of TPEF imaging to be largest with increasing difference in confocal versus TPEF excitation wavelength. For TPEF of eGFP this difference is approximately 400nm and for DsRed2 200nm explaining why a doubling of signal intensity is already seen at 180 microns depth for eGFP and is less pronounced for DsRed2 imaging. It should however be mentioned that optical penetration is not the only factor limiting imaging depth as a build up of background and increase in PSF size with larger imaging depth leads to decrease in contrast and poorer resolution.
We report that TPE of DsRed2 creates significant photobleaching compared to confocal imaging but others have shown that bleaching can be reduced when extending wavelength up to 1120nm with an OPO (Andresen, et al., 2009). Our calculations however show that TPE induced photo bleaching of DsRed2 will remain significantly higher than with confocal imaging. Others (Patterson & Piston, 2000) have hypothesized that beside two photon absorption, three and two photon processes might be involved simultaneously and based on the differences in bleaching rates we observed it appears that three photon absorption in the UV-C increases those rates. Three photon absorption of eGFP is not in the UV-C which could explain in part the disadvantage when imaging DsRed2 at 760 or 720nm.. We found that photo bleaching rate is lower at 720nm compared to 760nm and although photo bleaching might not directly lead to photo toxicity, it is interesting to note that the UV action spectrum for skin is 25% lower at 240nm (three photon absorption at 720nm) compared to 253nm (760nm) (International Commission on Non-ionizing Radiation Protection, 2010).
As expected out of plane bleaching with TPEF imaging is minimal and additional out of plane bleaching occurs with confocal imaging. Because of the increased photo bleaching rate for TPE of DsRed2 we conclude however that very large stacks would need to be measured until confocal imaging of DsRed2 approaches similar bleaching as with TPE. 3D imaging of eGFP is ideally conducted with TPE because of its improved penetration depth and minimal increase of photobleaching compared to confocal imaging.
Acknowledgements
The authors wish to thank the Turkish government’s Higher Education Council for supporting this project with a scholarship. The majority of work was conducted on an NIH sponsored shared instrument (S10RR023737). The frame work to investigate novel 3D imaging techniques was provided by NIH R01HL077683. The authors which to thank the engineers and scientists at Lavision Biotec for creating a modular intravital microscope and sharing their experience in designing and maintaining state of the art microscopy equipment.
References
- Andresen V, Alexander S, Heupel WM, Hirschberg M, Hoffman RM, Friedl P. Infrared multiphoton microscopy: subcellular-resolved deep tissue imaging. Curr Opin Biotechnol. 2009;20(1):54–62. doi: 10.1016/j.copbio.2009.02.008. [DOI] [PubMed] [Google Scholar]
- Atala A, Lanza RP. Methods of tissue engineering. San Diego, CA: Academic Press; 2001. [Google Scholar]
- Baird IS, Yau AY, Mann BK. Mammalian cell-seeded hydrogel microarrays printed via dip-pin technology. Biotechniques. 2008;44(2):249–256. doi: 10.2144/000112683. [DOI] [PubMed] [Google Scholar]
- Bjorkoy G, Lamark T, Brech A, Outzen H, Perander M, Overvatn A, Stenmark H, Johansen T. p62/SQSTM1 forms protein aggregates degraded by autophagy and has a protective effect on huntingtin-induced cell death. J Cell Biol. 2005;171(4):603–614. doi: 10.1083/jcb.200507002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blab GA, Lommerse PHM, Cognet L, Harms GS, Schmidt T. Two-photon excitation action cross-sections of the autofluorescent proteins. Chemical Physics Letters. 2001;350(1–2):71–77. [Google Scholar]
- Black LD, 3RD, Meyers JD, Weinbaum JS, Shvelidze YA, Tranquillo RT. Cell-induced alignment augments twitch force in fibrin gel-based engineered myocardium via gap junction modification. Tissue Eng Part A. 2009;15(10):3099–3108. doi: 10.1089/ten.tea.2008.0502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Born M, Wolf E. Principles of optics : electromagnetic theory of propagation, interference and diffraction of light. Cambridge ; New York: Cambridge University Press; 1999. [Google Scholar]
- Cao J, Chiarelli C, Kozarekar P, Adler HL. Membrane type 1-matrix metalloproteinase promotes human prostate cancer invasion and metastasis. Thromb Haemost. 2005;93(4):770–778. doi: 10.1160/TH04-08-0555. [DOI] [PubMed] [Google Scholar]
- Cao Z, Gilbert RJ, He W. Simple agarose-chitosan gel composite system for enhanced neuronal growth in three dimensions. Biomacromolecules. 2009;10(10):2954–2959. doi: 10.1021/bm900670n. [DOI] [PubMed] [Google Scholar]
- Condeelis J, Segall JE. Intravital imaging of cell movement in tumours. Nat Rev Cancer. 2003;3(12):921–930. doi: 10.1038/nrc1231. [DOI] [PubMed] [Google Scholar]
- Corle TR, Kino GS. Confocal scanning optical microscopy and related imaging systems. San Diego: Academic Press; 1996. [Google Scholar]
- Denk W, Strickler JH, Webb WW. Two-photon laser scanning fluorescence microscopy. Science. 1990;248(4951):73–76. doi: 10.1126/science.2321027. [DOI] [PubMed] [Google Scholar]
- Drobizhev M, Tillo S, Makarov NS, Hughes TE, Rebane A. Absolute two-photon absorption spectra and two-photon brightness of orange and red fluorescent proteins. J Phys Chem B. 2009;113(4):855–859. doi: 10.1021/jp8087379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Durr NJ, Weisspfennig CT, Holfeld BA, Ben-Yakar A. Maximum imaging depth of two-photon autofluorescence microscopy in epithelial tissues. J Biomed Opt. 2011;16(2) doi: 10.1117/1.3548646. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Friedl P. Dynamic imaging of cellular interactions with extracellular matrix. Histochem Cell Biol. 2004;122(3):183–190. doi: 10.1007/s00418-004-0682-0. [DOI] [PubMed] [Google Scholar]
- Gelman RA, Poppke DC, Piez KA. Collagen fibril formation in vitro. The role of the nonhelical terminal regions. J Biol Chem. 1979;254(22):11741–11745. [PubMed] [Google Scholar]
- Griess GA, Moreno ET, Easom RA, Serwer P. The sieving of spheres during agarose gel electrophoresis: quantitation and modeling. Biopolymers. 1989;28(8):1475–1484. doi: 10.1002/bip.360280811. [DOI] [PubMed] [Google Scholar]
- Hadjantonakis AK, Papaioannou VE. Dynamic in vivo imaging and cell tracking using a histone fluorescent protein fusion in mice. BMC Biotechnol. 2004;4:33. doi: 10.1186/1472-6750-4-33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hartmann A, Boukamp P, Friedl P. Confocal reflection imaging of 3D fibrin polymers. Blood Cells Mol Dis. 2006;36(2):191–193. doi: 10.1016/j.bcmd.2005.12.033. [DOI] [PubMed] [Google Scholar]
- Heikal AA, Hess ST, Baird GS, Tsien RY, Webb WW. Molecular spectroscopy and dynamics of intrinsically fluorescent proteins: coral red (dsRed) and yellow (Citrine) Proc Natl Acad Sci U S A. 2000;97(22):11996–12001. doi: 10.1073/pnas.97.22.11996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoying JB, Boswell CA, Williams SK. Angiogenic potential of microvessel fragments established in three-dimensional collagen gels. In Vitro Cell Dev Biol Anim. 1996;32(7):409–419. doi: 10.1007/BF02723003. [DOI] [PubMed] [Google Scholar]
- Huang Y, Onyeri S, Siewe M, Moshfeghian A, Madihally SV. In vitro characterization of chitosan-gelatin scaffolds for tissue engineering. Biomaterials. 2005;26(36):7616–7627. doi: 10.1016/j.biomaterials.2005.05.036. [DOI] [PubMed] [Google Scholar]
- International Commission on Non-ionizing Radiation Protection. ICNIRP statement - Protection of workers againts ultraviolet radiation. Health Physics. 2010;99(1):66–87. doi: 10.1097/HP.0b013e3181d85908. [DOI] [PubMed] [Google Scholar]
- Jacques SL, Gareau DS. Confocal microscopy to measure tissue optical properties. Saratov Fall Meeting 2005: Optical Technologies in Biophysics and Medicine VII. :61630X–61635. SPIE. [Google Scholar]
- Jockenhoevel S, Zund G, Hoerstrup SP, Chalabi K, Sachweh JS, Demircan L, Messmer BJ, Turina M. Fibrin gel -- advantages of a new scaffold in cardiovascular tissue engineering. Eur J Cardiothorac Surg. 2001;19(4):424–430. doi: 10.1016/s1010-7940(01)00624-8. [DOI] [PubMed] [Google Scholar]
- Kim Y, Comte I, Szabo G, Hockberger P, Szele FG. Adult mouse subventricular zone stem and progenitor cells are sessile and epidermal growth factor receptor negatively regulates neuroblast migration. PLoS One. 2009;4(12):e8122. doi: 10.1371/journal.pone.0008122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kobat D, Durst ME, Nishimura N, Wong AW, Schaffer CB, Xu C. Deep tissue multiphoton microscopy using longer wavelength excitation. Opt Express. 2009;17(16):13354–13364. doi: 10.1364/oe.17.013354. [DOI] [PubMed] [Google Scholar]
- Leavesley DI, Schwartz MA, Rosenfeld M, Cheresh DA. Integrin beta 1- and beta 3-mediated endothelial cell migration is triggered through distinct signaling mechanisms. J Cell Biol. 1993;121(1):163–170. doi: 10.1083/jcb.121.1.163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leray A, Mertz J. Rejection of two-photon fluorescence background in thick tissue by differential aberration imaging. Opt Express. 2006;14(22):10565–10573. doi: 10.1364/oe.14.010565. [DOI] [PubMed] [Google Scholar]
- Lien SM, Ko LY, Huang TJ. Effect of pore size on ECM secretion and cell growth in gelatin scaffold for articular cartilage tissue engineering. Acta Biomater. 2009;5(2):670–679. doi: 10.1016/j.actbio.2008.09.020. [DOI] [PubMed] [Google Scholar]
- Lin PW, Wu CC, Chen CH, Ho HO, Chen YC, Sheu MT. Characterization of cortical neuron outgrowth in two- and three-dimensional culture systems. J Biomed Mater Res B Appl Biomater. 2005;75(1):146–157. doi: 10.1002/jbm.b.30276. [DOI] [PubMed] [Google Scholar]
- Ma PX, Elisseeff JH. Scaffolding in tissue engineering. Boca Raton: Taylor&Francis; 2005. [Google Scholar]
- Mohler W, Millard AC, Campagnola PJ. Second harmonic generation imaging of endogenous structural proteins. Methods. 2003;29(1):97–109. doi: 10.1016/s1046-2023(02)00292-x. [DOI] [PubMed] [Google Scholar]
- Moissoglu K, Slepchenko BM, Meller N, Horwitz AF, Schwartz MA. In vivo dynamics of Rac-membrane interactions. Mol Biol Cell. 2006;17(6):2770–2779. doi: 10.1091/mbc.E06-01-0005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Muller M. Introduction to confocal fluorescence microscopy. Bellingham, Wash.: SPIE Press; 2006. [Google Scholar]
- Mycek M-A, Pogue BW. Handbook of biomedical fluorescence. New York: Marcel Dekker; 2003. [Google Scholar]
- Niesner R, Andresen V, Neumann J, Spiecker H, Gunzer M. The power of single and multibeam two-photon microscopy for high-resolution and high-speed deep tissue and intravital imaging. Biophys J. 2007;93(7):2519–2529. doi: 10.1529/biophysj.106.102459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Patterson G, Day RN, Piston D. Fluorescent protein spectra. J Cell Sci. 2001;114(5):837–838. doi: 10.1242/jcs.114.5.837. [DOI] [PubMed] [Google Scholar]
- Patterson GH, Piston DW. Photobleaching in two-photon excitation microscopy. Biophys J. 2000;78(4):2159–2162. doi: 10.1016/S0006-3495(00)76762-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ratanavaraporn JEA. Comparison of Gelatin and Collagen Scaffolds for Fibroblast Cell Culture. Journal of Metals, Materials and Minerals. 2006;Vol.16(No.1):31–36. [Google Scholar]
- Roeder BA, Kokini K, Sturgis JE, Robinson JP, Voytik-Harbin SL. Tensile mechanical properties of three-dimensional type I collagen extracellular matrices with varied microstructure. J Biomech Eng. 2002;124(2):214–222. doi: 10.1115/1.1449904. [DOI] [PubMed] [Google Scholar]
- Seppa H, Grotendorst G, Seppa S, Schiffmann E, Martin GR. Platelet-derived growth factor in chemotactic for fibroblasts. J Cell Biol. 1982;92(2):584–588. doi: 10.1083/jcb.92.2.584. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seppa L. Effects of a sodium fluoride solution and a varnish with different fluoride concentrations on enamel remineralization in vitro. Scand J Dent Res. 1988;96(4):304–309. doi: 10.1111/j.1600-0722.1988.tb01560.x. [DOI] [PubMed] [Google Scholar]
- Tsien RY. The green fluorescent protein. Annu Rev Biochem. 1998;67:509–544. doi: 10.1146/annurev.biochem.67.1.509. [DOI] [PubMed] [Google Scholar]
- Wolf K, Friedl P. Mapping proteolytic cancer cell-extracellular matrix interfaces. Clin Exp Metastasis. 2009;26(4):289–298. doi: 10.1007/s10585-008-9190-2. [DOI] [PubMed] [Google Scholar]
- Wu RF, Xu YC, Ma Z, Nwariaku FE, Sarosi GA, Jr, Terada LS. Subcellular targeting of oxidants during endothelial cell migration. J Cell Biol. 2005;171(5):893–904. doi: 10.1083/jcb.200507004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zipfel WR, Williams RM, Christie R, Nikitin AY, Hyman BT, Webb WW. Live tissue intrinsic emission microscopy using multiphoton-excited native fluorescence and second harmonic generation. Proc Natl Acad Sci U S A. 2003;100(12):7075–7080. doi: 10.1073/pnas.0832308100. [DOI] [PMC free article] [PubMed] [Google Scholar]