Abstract
Purpose
In contrast to EEG, which has guidelines for interpretation and a plethora of textbooks, the full range of activity seen in Magnetoencephalography (MEG) has not been fleshed out. Currently, magnetoencephalographers apply criteria for EEG waveforms to MEG signals based on an assumption that MEG activity should have morphology that is similar to EEG. The purpose of this paper is to show the characteristic MEG profile of Positive Occipital Sharp Transients of Sleep (POSTS)
Method
Simultaneous MEG-EEG recordings of two cases are shown.
Result
In the both cases, the morphological features of POSTS in MEG vary, and sometimes mimic epileptic spikes.
Conclusion
This report raises a caution that a normal variant may have an even more epileptic appearance on MEG than on EEG. Employing the simultaneously recorded EEG to avoid misinterpretation of spikey-looking POSTS in MEG is a natural and prudent practice.
Keywords: EEG, Magnetoencephalography, Positive Occipital Sharp Transients of Sleep, Epileptic Spikes
Introduction
For EEG evaluation of patients with epilepsy, the sine qua non of genuine epileptiform activity is sharply contoured or spikey activity which stands out from the background (Chatrian et al, 1974). Although interpretation of EEG is empirically based, there is a long history of cataloging and characterizing EEG waveforms as normal or abnormal, such as the definitions by the International Federation of Clinical Neurophysiology (IFCN) in 1974 (Chatrian et al, 1974), and the various atlases which are too numerous to list. EEG reading is based on criteria from such guidelines, but no similar classification of benign variants and other Magnetoencephalography (MEG) waveforms has been assembled. Determination of whether a given discharge is abnormal or not can be based on our experience with EEG (Iwasaki et al., 2005), but the direct application of criteria which have been created from EEG may not always be straightforward. In this report two cases demonstrate that the morphological appearance of Positive Occipital Sharp Transients of Sleep (POSTS) in MEG is variable and sometimes mimics epileptic spikes.
Case presentations
Case 1
A 34-year-old right-handed male began to have seizures from the age of 2. His perinatal and development history was not remarkable. Family history was also unremarkable, although he had history of febrile seizures at the age of 2 years. The seizures started with an aura followed by automotor seizure-like fumbling with his clothes or lip-smacking without consciousness with loss of conciousness. They typically lasted 10–15 seconds. Postictally, he was tired and confused. Seizures occurred 1–2 per week, despite trials of multiple antiepileptic medications. EEG monitoring showed sharp waves in the right temporal region and an ictal event was also confirmed with rhythmical theta starting from right temporal region. The MRI suggested hippocampal sclerosis. Fluorodeoxyglucose positron emission tomography (FDG-PET) showed hypometabolism involving right temporal region. He underwent a right temporal lobectomy at the age of 30. Pathology of the resected tissue was hippocampal sclerosis. He was seizure-free 4 years, but then relapsed. Post-operative seizure semiology consisted of lip- smacking with staring. Video-EEG monitoring to characterize the seizures after the relapse showed sharp waves in right temporal region again, and ictal activity was also confirmed with rhythmical theta involving the right hemisphere, maximally in the fronto-temporal region. Because scalp EEG is difficult to localize after resective neurosurgical procedures, a MEG with simultaneous EEG was performed.
Case 2
A left-handed female developed seizures at the age of 23, which were well controlled for five years by antiepileptic medication. Seizures reemerged at age 28, and at age 37 she was referred because of intractability. Her perinatal, developmental, family and other past medical history was unremarkable. The seizures started either with the left arm tingling sensation or with abdominal aura followed by mouth automatism. They typically lasted 60–90 seconds, and occurred 2–3 per week. EEG monitoring showed intermittent rhythmic slow in the right temporal region and ictal activity was also confirmed to be associated with a low voltage fast activity followed by rhythmical theta in right temporal region. FDG-PET showed mild hypometabolism involving right anterior and lateral temporal region. Because the MRI was normal, simultaneous MEG- EEG recording was performed.
Methods
Simultaneous magnetic and electrical brain data were recorded with a whole-head MEG system of 204 planar gradiometers in magnetically shielded room with simultaneous scalp EEG according to the international 10–20 system supplemented by additional anterior temporal leads. Both EEG and MEG signals were sampled at 1000 Hz, band-pass filtered between 0.1 and 330 Hz on acquisition and at 0.5 to 70 Hz during interpretation. We picked up MEG activities whose EEG correlate was concordant with POSTS defined as follows: positive sharp deflections in the theta range occurring during drowsiness, maximal in the occipital region. Source localization was carried out at the peak of the corresponding MEG signal using a single dipole model in a spherical head model by using incorporated source modeling software (Neuromag, Helsinki, Finland).
Results
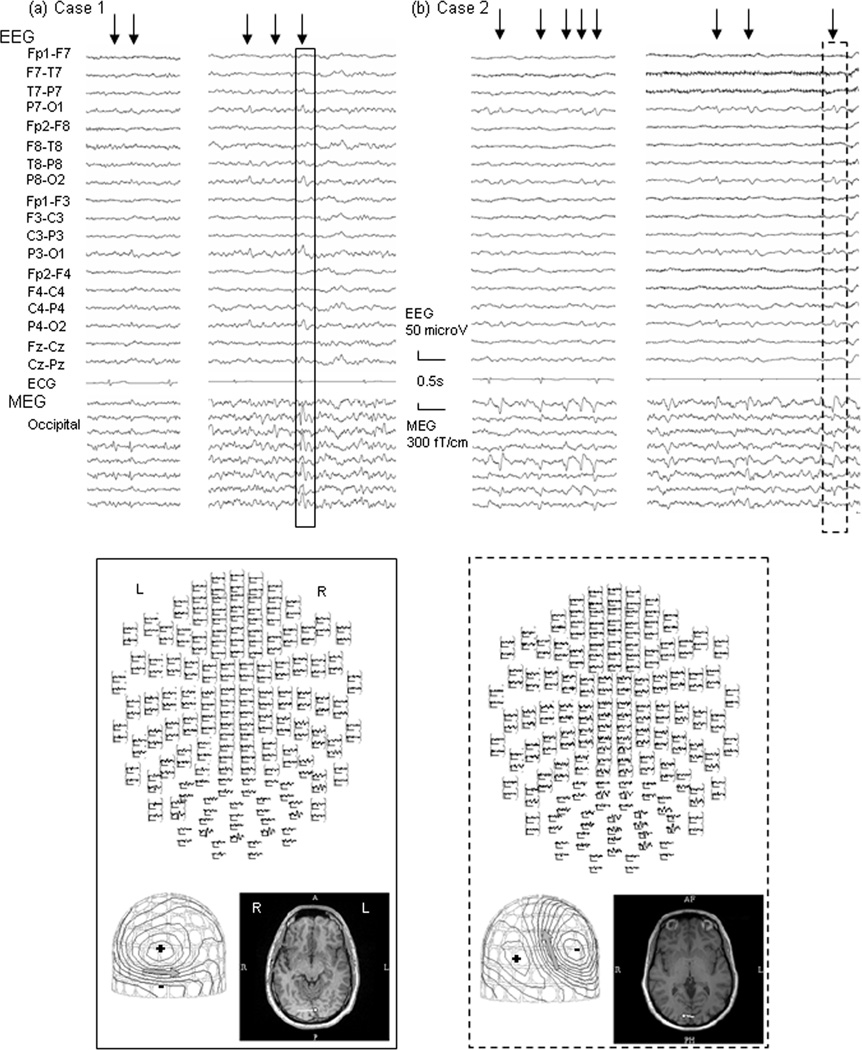
Fig. 1 shows representative electrical/magnetic feature of POSTS of cases 1 and 2, respectively. The MEG signals which are correlated with what are clearly POSTS in EEG show some variability in both morphology and amplitude. In both patients, the larger discharges are apparent on EEG, but the smaller ones, though clear on MEG, are sometimes imperceptible on EEG. The morphology of the POSTS seen in the MEG channels is sometimes epileptiform in appearance, with an initial spike or sharp wave followed by a slow wave. The topographical distribution, however, is similar for both activities, benign- or epileptiform-appearing. Note the identical estimation of the dipole location in the mesial part of the left or right occipital lobe.
Figure 1.
Top Panel: EEG and Magnetoencephalography (MEG) waveforms of Positive Occipital Sharp Transients of Sleep (POSTS) of Cases 1 (a) and 2 (b).
The signals enclosed by the solid and broken lines are representative POSTS of cases 1 and 2, respectively, whose dipole analysis results are shown in the lower panels. Arrows indicate EEG-defined POSTS. Note that MEG correlates of EEG-defined POSTS show variability in morphology, some of them mimicking epileptic spikes; with initial deflections that are spikey, associated with after-going slow wave activity.
Bottom Panel: A top view image showing distribution of magnetic activity of POSTS (upper), MEG contour map (left lower), and dipole source locations on the patient’s individual MRI (right lower). MEG contour maps: Arrows on the contour maps reflect the estimated dipoles projected to the sensor surface. The solid line indicates the efflux of the magnetic field from the head surface, and the broken line indicates influx of the magnetic field into the head. MRI: The circle and bar on the MRI scans indicate the dipole location and orientation, respectively. Note that, unlike the variability of morphology, their distribution are similar between activities and patients; mainly involving bilateral occipital region and their dipoles are estimated on mid to mesial part of either left or right occipital lobe.
Discussion
This report shows the variable morphology of POSTS in MEG for the first time, and raises the possibility that a normal variant could be misinterpreted as epileptic activity in MEG.
These two cases reveal several characteristics of POSTS in MEG. Firstly, as mentioned above, the morphology and amplitude vary within a single individual --- sometimes mimicking epileptic activity. Reassuringly, the spatial distribution of the more worrisome discharges is identical to the location of the activity that clearly represents POSTS, i.e. bi-occipital. It is likely that MEG’s better sensitivity to activity arising from fissures rather than gyri is responsible for many of the differences in manifestations (Ebersole & Ebersole, 2010). The frequency of occurrence of POSTS in MEG may therefore be considerably different than in EEG.
By definition, all of the occipital transients analyzed in this study were associated with discharges that were convincingly demonstrated to be POSTS on the simultaneously recorded EEG. Additionally, there was no evidence in either case that the patient had genuine epileptic activity coming from the posterior head regions. The difficulties pointed out by these two cases support the requirement for simultaneous EEG recordings in all MEG studies, as recommended by the American Clinical Magnetoencephalography Society guidelines (Bagić et al, 2011).
The danger of misinterpretation of benign variants as indicative of epilepsy is well known in electroencephalography (Benbadis & Tatum, 2003). Because the relative frequency of epileptiform-appearing transients in MEG has not been studied, and since the morphology of wickets, small sharp spikes, etc has not been fully characterized in MEG, caution is advised and the development of further guidelines for MEG is anxiously awaited.
Acknowledgments
This work was supported in part by the National Institutes of Health under grants DP2-OD006469, R01-EB009048, R01-NS074980, and by the Epilepsy Center of the Cleveland Clinic Neurological Institute.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Conflict of Interest
No financial interest is involved in the publication of this manuscript.
References
- Bagić AI, Knowlton RC, Rose DF, Ebersole JS. ACMEGS Clinical Practice Guideline (CPG) Committee. American Clinical Magnetoencephalography Society Clinical Practice Guideline 1: recording and analysis of spontaneous cerebral activity. J Clin Neurophysiol. 2011;28:348–354. doi: 10.1097/WNP.0b013e3182272fed. [DOI] [PubMed] [Google Scholar]
- Benbadis SR, Tatum WO. Overintepretation of EEGs and misdiagnosis of epilepsy. J Clin Neurophysiol. 2003;20:42–44. doi: 10.1097/00004691-200302000-00005. [DOI] [PubMed] [Google Scholar]
- Chatrian GE, Bergamini L, Dondey M, Klass DW, Lennox-Buchthal M, Petersen I. A glossary of terms most commonly used by clinical electroencephlographers. Electroenceph Clin Neurophysiol. 1974;37:538–548. doi: 10.1016/0013-4694(74)90099-6. [DOI] [PubMed] [Google Scholar]
- Ebersole JS, Ebersole SM. Combining MEG and EEG source modeling in epilepsy evaluations. J Clin Neurophysiol. 2010;27:360–371. doi: 10.1097/WNP.0b013e318201ffc4. [DOI] [PubMed] [Google Scholar]
- Iwasaki M, Pestana E, Burgess RC, Lüders HO, Shamoto H, Nakasato N. Detection of epileptiform activity by human interpreters: blinded comparison between electroencephalography and magnetoencephalography. Epilepsia. 2005;46:59–68. doi: 10.1111/j.0013-9580.2005.21104.x. [DOI] [PubMed] [Google Scholar]