Abstract
We present two pediatric patients with an uncommon electrophysiological seizure propagation pattern. Both had dialeptic seizure as the main or only symptom. Case 1 had a small mass in the left medial temporal structures; Case 2 had no lesion in MRI. In both, EEG showed not only left temporal spikes but also bilaterally synchronous 3Hz spike-and-wave complexes (SWC) from onset and unusual secondarily generalized 3 Hz SWC patterns arising from the left temporal region. Case 1 was seizure-free following mass resection; focal or generalized epileptiform EEG abnormalities were no longer present. In Case 2, magnetoencephalography localized the spikes to the left superior and mid-temporal gyrus, which ictal SPECT suggested was the origin of onset. These cases illustrate the close relationship between focal epileptic area and 3 Hz SWC and suggest that the focal area can trigger 3 Hz SWC. The therapeutic strategy may need to be altered in such patients.
Keywords: 3 Hz spike-and-wave complex, Focal epileptic activity, Magnetoencephalography
Introduction
Absence epilepsy is an idiopathic epilepsy characterized by dialeptic seizures associated with bilateral synchronous and symmetric spike-and-wave discharges at 3 Hz as defined by the International League Against Epilepsy [1]. The pathophysiology of absence epilepsy with generalized discharges has been debated in terms of whether the discharges originate from the thalamus or cortex. In recent studies, the cortical region has been considered to be the primary generator of this epilepsy [2]. In epilepsies other than absence epilepsy, generalized epileptic activity has been suggested to be closely related to focal epileptic activity [3–5].
We present two pediatric patients with intractable epilepsy in which dialeptic seizure was the main or only symptom. Notably, both patients showed not only focal epileptic spikes but also 3 Hz generalized spike-and-wave complexes, which we concluded were generated from a focal area based on the preoperative investigation or postoperative freedom from seizures.
Case reports
Case 1
A 10-year-old boy, without a past or family history of epilepsy, began to have seizures starting at age 6 years. His seizure semiology consisted of either dialeptic seizures or an abdominal aura evolving to automotor seizures; the frequency was about 5 per day while he was receiving oxcarbazepine. His perinatal course, postnatal development, and family history were not remarkable. His presurgical EEG showed various patterns of epileptic activity including focal spikes in the left temporal area and secondarily generalized 3 Hz spike-and-wave complexes (Fig. 1). Magnetic resonance imaging (MRI) revealed a small (approximately 2×2 cm) mass in the left medial temporal structures (Fig. 2A). At age 7, he underwent resection of the left temporal lobe tumor (Fig. 2B), and its histological profile was compatible with a diagnosis of ganglioglioma. Postoperatively, he was seizure-free and follow-up electroencephalography (EEG) repeatedly showed no interictal spikes (follow-up, 29 months).
Fig. 1.
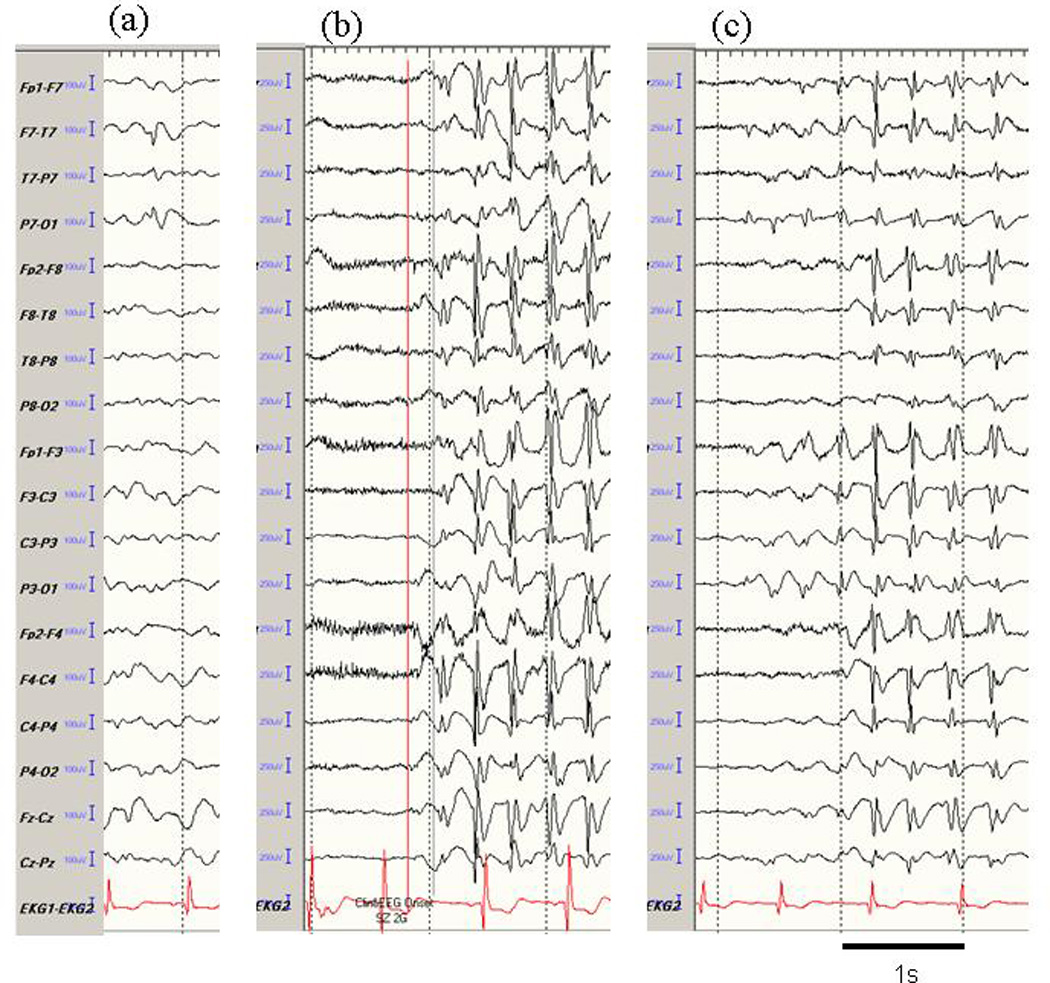
Representative EEG activity in Case 1. (a) single spike in the left temporal area, (b) synchronous generalized 3 Hz spike-and-wave complexes from seizure onset, and (c) secondarily generalized 3 Hz spike-and-wave complexes. After undergoing resection of a tumor in the left temporal lobe, the patient was seizure-free, and the epileptic activities shown here also disappeared completely.
Fig. 2.
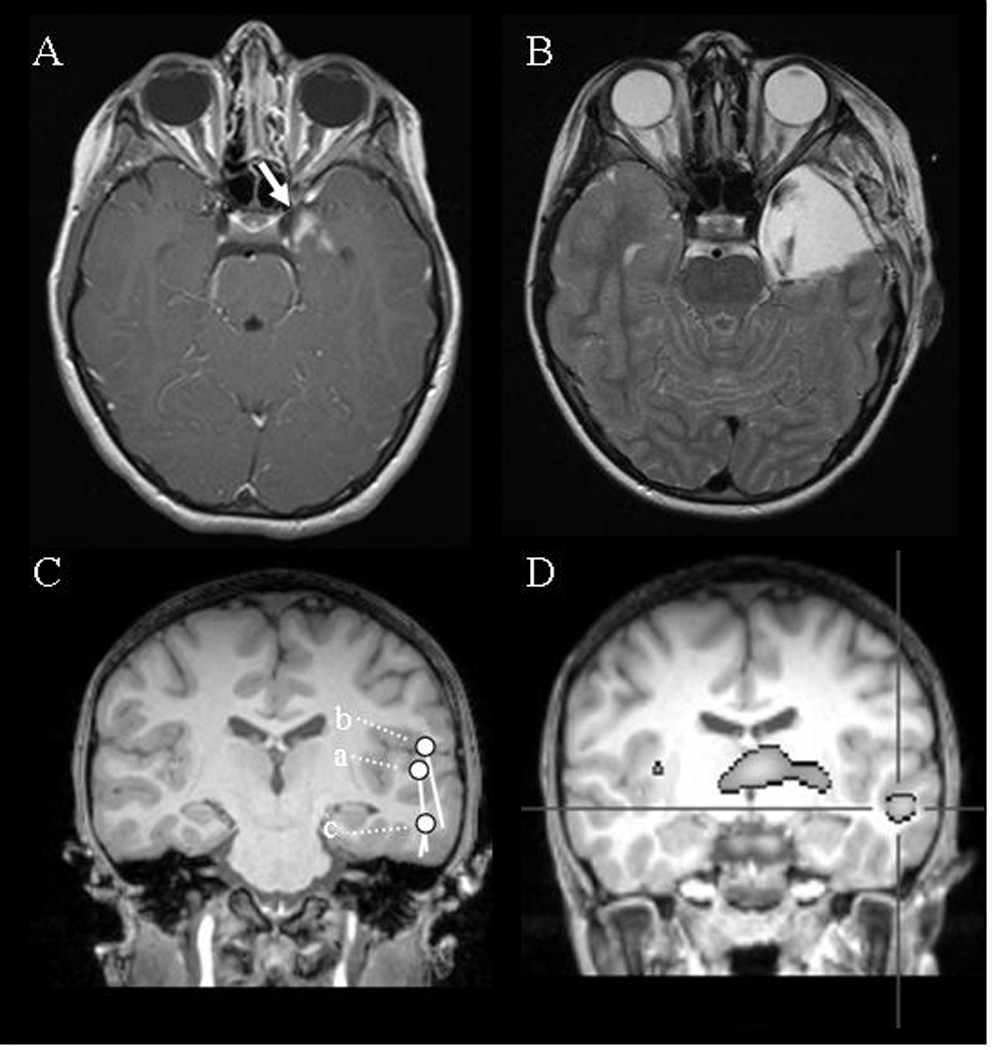
(A) Case 1: Presurgical MRI of Case 1 showing a lesion in left temporal lobe, approximately 2×2 cm, which enhances with gadolinium (white arrow). (B) Postoperative MRI of Case 1 showing complete surgical resection of the brain tumor. (C) Case 2: Superimposed spike dipole on a coronal image of patient’s individual MRI. Note that the spikes, shown in Fig. 3 as (a), (b), and (c), are estimated to come from left temporal area. (D) Case 2: Subtraction ictal SPECT with 99m-Tc ethyl cysteinate dimmer. Note that the hyperperfusion (defined as over 2 with a Z score) occurs in the left superior to middle temporal gyrus.
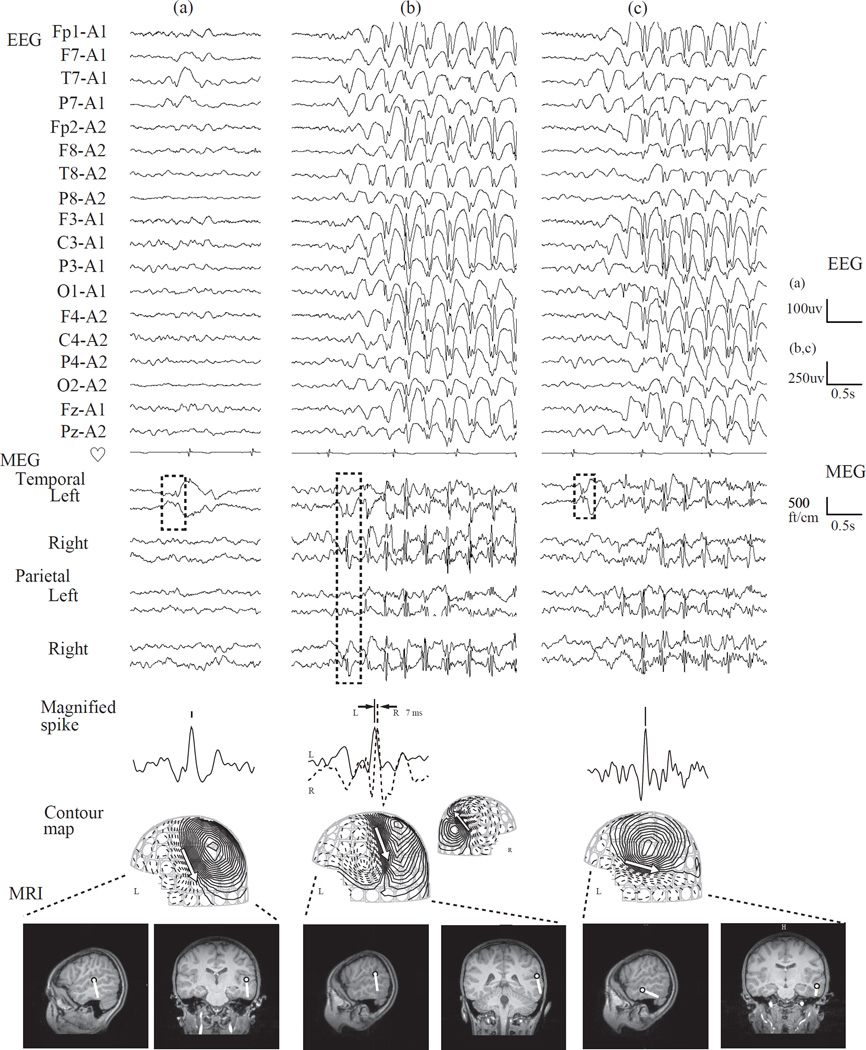
Case 2
A 9-year-old girl had seizures that started at the age of 5 years and became intractable by the age of 7. She had frequent dialeptic seizures, ranging from 5 to 100 per day in spite of multiple antiepileptic medications, such as lamotrigine and levetiracetam. Clobazam, ethosuximide, valproic acid, and zonisamide had to be stopped due to their side effects. Her perinatal course was not remarkable other than her mother having had meningitis at about 5 to 7 months gestation. Her father had epilepsy late in life. She was alert and had no remarkable mental problems. Simultaneous video and EEG monitoring revealed that she lost consciousness without motor symptoms when the EEG showed generalized 3 Hz spike-and-wave discharges lasting 7 to 12 seconds. She underwent a simultaneous EEG and magnetoencephalography (MEG) evaluation. The precise methodology is described elsewhere [6]. As in Case 1, various epileptic activities were present (Fig. 3): (a) a left temporal spike that was more prominent on MEG than EEG, (b) bilaterally synchronous 3 Hz spike-and-wave complexes occurring from seizure onset onward, and (c) secondarily generalized 3 Hz spike-and-wave complexes. The time latency of the bilateral spikes was 7 ms (Fig. 3b). MEG spike dipoles of these activities were estimated in the left temporal area (Fig. 2C). Her MRI was normal, but an ictal single-photon emission computed tomography (SPECT) with 99m-Tc ethyl cysteinate dimmer showed areas of hyperperfusion in the left superior and middle temporal gyrus, implying this was the area of seizure origin (Fig. 2D). Although it was possible that the left temporal area was involved in seizure generation, the therapeutic strategy team concluded that she should be treated medically rather than surgically at that time because the seizure origin was near the Broca’s area.
Fig. 3.
From top, EEG and MEG waveforms, magnified spike waveform, contour map of MEG, and dipoles on individual MRI of representative epileptic discharges are shown for Case 2. (a) single spike in the left temporal area, (b) synchronous generalized 3 Hz spike-and-wave complex from seizure onset, and (c) secondarily generalized 3 Hz spike-and-wave complex. MEG: The analyzed portions of the MEG waveform are indicated by the broken box. Note that MEG tends to show spikes with greater clarity than EEG. The time latency between the left and right epileptic discharges during generalized spikes, seen in panel b, is 7 ms. Contour maps: Arrows on the contour maps indicate the estimated dipoles. The solid line indicates magnetic field efflux, and the broken line indicates magnetic field influx from the brain surface. MRI: The circle and bar shown on the MRI scans indicate the dipole location and orientation, respectively.
Discussion
In Case 1, seizure remission and the absence of interictal spikes after surgical removal of the left temporal brain tumor strongly support the idea that a focal lesion could account for both the focal spikes and generalized 3 Hz spike-and-wave complexes. Based on the similar electrophysiological profile in Case 2, the underlying mechanisms in both patients are likely to be similar. Some investigators have reported that a focal lesion might be related to generalized EEG abnormalities, mainly slow spike-and-wave complexes [3]. However, the relationship between focal spikes and generalized 3 Hz spike-and-wave complexes in cases that clinically appear to be absence epilepsy remains to be determined.
Concerning the secondarily generalized 3 Hz spike-and-wave complexes, to the best of our knowledge, a similar electrophysiological finding has been reported only in three other patients, all of whom had Gastaut-type childhood occipital lobe epilepsy [7]. As none of these patients underwent surgical intervention, it cannot be stated with certainty whether the focal spikes and the peculiar generalized 3 Hz spike-and-wave complexes were related. The investigators also concluded that whether the Gastaut-type occipital lobe epilepsy and the peculiar seizure generation were related or coincidental findings could not be determined. In Case 1, the disappearance the unusual evolving seizure after surgery clearly indicates a close relationship between focal epileptic activity and generalized 3 Hz spike-and-wave.
The precise mechanisms underlying the various epileptic activities observed in our cases remain unknown because invasive recording was not performed. Interestingly, some have suggested that “primary” generalized epilepsy in which the EEG can be described as having 3 Hz spike-and-wave complexes is the expression of a cortical abnormality [4]. Other than 3 Hz spike-and-wave complexes, some authors have proposed that in subset of cases, generalized epileptic spikes could originate from focal epileptic regions, based the disappearance of seizures and spikes after epilepsy surgery [3,5]. Meeren et al. suggested that in cases of what appears to be primary generalized epilepsy, a “focus” might exist for generation of seizures. They speculated that generalized spike-and-wave complexes could be generated from mutual excitation of the corticocortical and thalamocortical networks triggered by focal epileptic activity [2]. Considering the fact that during seizures, electrical activity is abnormally accelerated in the thalamocortical network regardless of whether the thalamus is intact [8] or not [9,10], possibly our patients’ pathophysiology is a reciprocal excitation of the cortex and thalamus that is triggered by a left temporal focus.
Conventionally, epilepsy is understood as a disease continuum, with one pole representing pure focal epilepsy and the other pure generalized epilepsy. The present cases are in part similar to absence epilepsy and are at a midpoint on the disease continuum, namely case 1 is similar to those epilepsies with focal onset and both focal (automatism) and generalized spike (dialeptic) manifestation, and case 2 is those with focal onset and only generalized spike (dialeptic) manifestation. We believe that the acknowledgment of possible relationship between focal epileptic activity and 3 Hz generalized spike-and-wave complex seen in our cases is important. Although conventional therapeutic strategy for patients showing 3 Hz generalized spike-and-wave complex, typically absence epilepsy, would not consider epilepsy surgery, this report showed epilepsy surgery might be an option in subset of cases whose symptoms are clinically similar to absence epilepsy. Unfortunately, we could not definitively identify the factor regulating this peculiar seizure propagation in our cases. Further study, especially basic studies in animal models, may allow us to investigate this idea.
MEG showed potential in evaluating the epileptic focus in Case 2, because the slow wave and obscure spikes seen on interictal recordings were clearer on MEG than on EEG (Fig. 3a). We believe that this difference may derive not only from the spike origin, (the fissural cortex, where MEG is sensitive), but also from the high spatial resolution of MEG, which allows it to better differentiates epileptic activity from slow background activity [10]. Spike dipoles in MEG, perpendicular to the fissure cortex, indicated that spike origin was on the left temporal lobe (Fig. 2C), the center of which ictal SPECT showed to be hyperperfused (Fig. 2D). The high temporal resolution of MEG also clearly showed the time latency of bilateral spikes. Based on a recent study revealing that the corpus callosum has an important role in seizure activity propagation [12,13], seizures originating from left temporal lobe might propagate through the corpus callosum, midline structures, or both.
Case 2 does not provide definitive evidence that the left temporal lobe was the source for seizure generation, which is a limitation. However, we have no doubt that the epileptic focus was in the left temporal lobe, based on the concordance of results from the different assessment modalities, as well as the electrophysiological similarities to Case 1.
Focal spikes are not uncommon in typical absence seizures, likely representing primitive forms of generalized spike-wave discharges. Likewise, many 3-Hz spike wave discharges will have a subtle lateralized onset, as illustrated in fig. 1b and fig. 3b. These findings do not necessarily indicate a focal pathological process in involved in the generalized discharges. The prominent lateralizing signs as seen in fig. 1c and fig. 3c, however, have not been reported in the literature, and may shed light on the relationship between focal epileptic activity and generalized 3 Hz spike-and-wave complexes. We believe that the findings shown here would provide an important clue to understand unknown mechanism of absence epilepsy and related condition.
Acknowledgments
The authors thank Dr. Cassandra Talerico for her editorial contribution.
References
- 1.Commission on Classification and Terminology of the International League Against Epilepsy. Proposal for revised classification of epilepsies and epileptic syndromes. Epilepsia. 1989;30:389–399. doi: 10.1111/j.1528-1157.1989.tb05316.x. [DOI] [PubMed] [Google Scholar]
- 2.Meeren H, van Luijtelaar G, Lopes da Silva F, Coenen A. Evolving concepts on the pathophysiology of absence seizures: the cortical focus theory. Arch Neurol. 2005;62:371–376. doi: 10.1001/archneur.62.3.371. [DOI] [PubMed] [Google Scholar]
- 3.Gupta A, Chirla A, Wyllie E, Lachhwani DK, Kotagal P, Bingaman WE. Pediatric epilepsy surgery in focal lesions and generalized electroencephalogram abnormalities. Pediatr Neurol. 2007;37:8–15. doi: 10.1016/j.pediatrneurol.2007.03.004. [DOI] [PubMed] [Google Scholar]
- 4.Luders H, Lesser RP, Dinner DS, Morris HH., 3rd Generalized epilepsies: a review. Cleve Clin Q. 1984;51:205–226. doi: 10.3949/ccjm.51.2.205. [DOI] [PubMed] [Google Scholar]
- 5.Wyllie E, Lachhwani DK, Gupta A, Chirla A, Cosmo G, Worley S, et al. Successful surgery for epilepsy due to early brain lesions despite generalized EEG findings. Neurology. 2007;69:389–397. doi: 10.1212/01.wnl.0000266386.55715.3f. [DOI] [PubMed] [Google Scholar]
- 6.Salayev KA, Nakasato N, Ishitobi M, Shamoto H, Kanno A, Iinuma K. Spike orientation may predict epileptogenic side across cerebral sulci containing the estimated equivalent dipole. Clin. Neurophysiol. 2006;117:1836–1843. doi: 10.1016/j.clinph.2006.05.003. [DOI] [PubMed] [Google Scholar]
- 7.Caraballo RH, Cersosimo RO, Fejerman N. Late-onset, "Gastaut type", childhood occipital epilepsy: an unusual evolution. Epileptic Disord. 2005;7:341–346. [PubMed] [Google Scholar]
- 8.Guerrini R, Genton P, Bureau M, Parmeggiani A, Salas-Puig X, Santucci M, et al. Multilobar polymicrogyria, intractable drop attack seizures, and sleep-related electrical status epilepticus. Neurology. 1998;51:504–512. doi: 10.1212/wnl.51.2.504. [DOI] [PubMed] [Google Scholar]
- 9.Battaglia D, Veggiotti P, Lettori D, Tamburrini G, Tartaglione T, Graziano A, et al. Functional hemispherectomy in children with epilepsy and CSWS due to unilateral early brain injury including thalamus: sudden recovery of CSWS. Epilepsy Res. 2009;87:290–298. doi: 10.1016/j.eplepsyres.2009.09.014. [DOI] [PubMed] [Google Scholar]
- 10.Tezer FI, Saygi S. Unilateral thalamic lesions and generalized or lateralized spike wave discharges. Epilepsy Res. 2009;86:228–231. doi: 10.1016/j.eplepsyres.2009.05.017. [DOI] [PubMed] [Google Scholar]
- 11.Nakasato N. Introduction to clinical examination of magnetoencephalography (MEG) Igaku kensa. 2006;55:1083–1091. [Google Scholar]
- 12.Ono T, Matsuo A, Baba H, Ono K. Is a cortical spike discharge "transferred" to the contralateral cortex via the corpus callosum?: An intraoperative observation of electrocorticogram and callosal compound action potentials. Epilepsia. 2002;43:1536–1542. doi: 10.1046/j.1528-1157.2002.13402.x. [DOI] [PubMed] [Google Scholar]
- 13.Salayev KA, Nakasato N, Ishitobi M, Shamoto H, Kanno A, Tominaga T, et al. Evaluation of interhemispheric time difference by magnetoencephalography before and after total callosotomy. Two case reports. Neurol Med Chir (Tokyo) 2006;46:136–142. doi: 10.2176/nmc.46.136. [DOI] [PubMed] [Google Scholar]