Abstract
In the first brain relay of the olfactory system, odors are encoded by combinations of glomeruli, but it is not known how glomerular signals are ultimately integrated. In Drosophila, the majority of glomerular projections target the lateral horn. Here we show that lateral horn neurons (LHNs) receive input from sparse and stereotyped combinations of glomeruli that are co-activated by odors, and certain combinations of glomeruli are over-represented. One morphological LHN type is broadly tuned and sums input from multiple glomeruli. These neurons have a broader dynamic range than their individual glomerular inputs do. By contrast, a second morphological type is narrowly tuned and receives prominent odor-selective inhibition via both direct and indirect pathways. We show this wiring scheme confers increased selectivity. The biased stereotyped connectivity of the lateral horn contrasts with the probabilistic wiring of the mushroom body, reflecting the distinct roles of these regions in innate versus learned behaviors.
Most individual odor stimuli activate multiple odorant receptors, and thus multiple types of olfactory receptor neurons. All the olfactory receptor neurons that express the same odorant receptor project to the same glomerulus in the brain, and so most individual stimuli are encoded by the combined activity of multiple glomeruli1-3. As a consequence, higher-order neurons must combine signals across glomeruli in order to extract information about olfactory features of the environment. To understand higher olfactory processing, it is therefore fundamental to understand how signals from different glomeruli are combined.
In Drosophila melanogaster and other insects, there are two higher order olfactory brain regions – the lateral horn and the mushroom body. The lateral horn has been proposed to be analogous to the vertebrate amygdala, which receives a major olfactory projection from the olfactory bulb4-6. The lateral horn likely holds the key to many olfactory behaviors in Drosophila. In particular, it is sufficient to mediate behavioral responses to odors that do not involve learned associations3, 7, 8, and it receives the majority of glomerular projections9, 10.
Nevertheless, little is known about how odors are encoded in the lateral horn, or how lateral horn neurons might integrate information across glomeruli. Individual olfactory glomeruli are known to send projections to stereotyped subregions of the lateral horn11-13, but we do not know whether connectivity is stereotyped at the level of individual cells. A recent study described a cluster of lateral horn neurons in Drosophila which receives input from a single glomerulus, and which is devoted to the processing of pheromones14. This raises the possibility that each lateral horn neuron is dedicated to a single glomerulus. At the other extreme, a study in locusts found that lateral horn neurons were broadly tuned to odors, and on this basis suggested that individual neurons receive input from massive numbers of glomeruli15. A theoretical study proposed a third alternative: lateral horn neurons might add and subtract sparse, weighted inputs from co-activated glomeruli4. The latter study also suggests that some combinations of glomeruli should be overrepresented – namely, glomeruli whose sum or difference represents a behaviorally useful computation.
By comparison, more is known about the connectivity of the mushroom body, which has been proposed to be analogous to the vertebrate piriform cortex4-6. Individual mushroom body neurons combine input from sparse sets of glomeruli16, 17. These connections are regionally biased13, 18, and glomeruli having similar odor tuning tend to wire together19. However, connectivity appears to be probabilistic, and the pattern of glomerular inputs to the mushroom body seems to be different in different individuals11-13, 16, 20. This is consistent with the conclusion that the mushroom body is involved in learned olfactory behaviors but not innate behaviors. Given the different roles of the mushroom body and lateral horn in olfactory behaviors, we might predict that these brain regions receive different patterns of connectivity from olfactory glomeruli and perform different sorts of computations on those glomerular inputs.
In this study, we investigated connectivity and olfactory coding in the Drosophila lateral horn. Our results suggest a conceptual framework for understanding how this region integrates input from different glomeruli. Our results also show that there are distinctive differences in connectivity in the Drosophila higher order brain regions mediating innate versus learned olfactory behaviors.
RESULTS
Two morphological types of lateral horn projection neurons
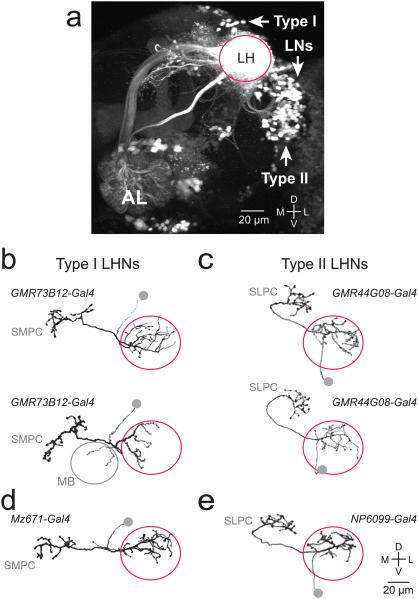
To visualize all lateral horn neurons, we expressed photoactivatable GFP (PA-GFP) pan-neuronally and used 2-photon excitation microscopy to activate PA-GFP throughout the lateral horn neuropil. As a consequence, activated PA-GFP labels most neurons that have neurites in the lateral horn. We observed several large clusters of labeled somata. One cluster was dorsomedial to the lateral horn neuropil, and one cluster was ventrolateral (Fig. 1a). Both clusters are connected to major neurite tracts that enter and exit the horn at distinctive locations. We define these as type I and type II neurons, respectively. Together, these clusters comprise a substantial fraction of all labeled somata, although they do not encompass all morphological types18.
Figure 1. Morphological types of lateral horn projection neurons.
a, Z-projection of a coronal 2-photon stack through a portion of the brain. PA-GFP is expressed pan-neuronally (under the control of n-synaptobrevin-Gal4) and photo-converted throughout the lateral horn (LH) neuropil. Magenta circle marks the boundary of the lateral horn. Arrows mark the three clusters of somata described in this study: type I, type II, and lateral horn local neurons (LNs; see below). The antennal lobe (AL) is weakly labeled because PN axons are photoconverted. Dorsal is up, lateral is right. Similar results were obtained in a total of 4 experiments.
b-c, Morphologies traced from biocytin-filled single neurons, where recorded neurons expressed GFP under the control of the indicated Gal4 lines. Somata were detached when the pipette was removed and so are symbolized by gray circles. Type I neurons have dendrites in the lateral horn and project to the superior medial protocerebrum (SMPC). Type II neurons have dendrites in the lateral horn and project to the superior lateral protocerebrum (SLPC). Two examples are shown for each type. Morphologies similar to the examples shown here were observed in all neurons of a given type (n = 8 for each). In type I fills we noted minor variations across cells, including a small projection to the mushroom body (MB) calyx in two cases.
d-e, The morphologies of Mz671 neurons (a subtype of type I) and NP6099 neurons (a subtype of type II) were all essentially identical to the examples shown here (n=7 and 10).
Through a visual screen of ~7000 Gal4 enhancer trap lines21 we obtained two lines that label a large fraction of type I neurons, along with two lines that label a large fraction of type II neurons. We used these lines to drive GFP expression and we biocytin-filled a sample of GFP+ neurons using in vivo whole-cell patch clamp recordings. These fills revealed that type I neurons all innervate the superior medial protocerebrum, although they differ in their fine morphological structure. The same was true of type II neurons and the superior lateral protocerebrum (Fig. 1b,c; see also Methods).
Two enhancer trap lines have been identified previously which label small numbers of neurons having these morphologies. Specifically, Mz671-Gal4 labels three type I neurons on each side of the brain, and NP6099-Gal4 labels three type II neurons13, 18. We used single-cell biocytin fills to confirm these morphologies (Fig. 1d,e). These lines provide genetic access to small, genetically-defined subtypes of neurons belonging to each major type.
Odor selectivity in type I and type II neurons
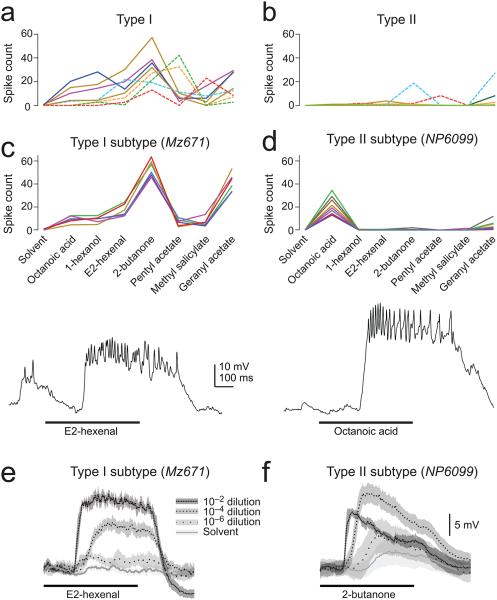
We next surveyed the odor responses of the type I and type II populations, using the Gal4 lines that label large numbers of neurons within each type to drive expression in these neurons with GFP. We made in vivo whole-cell patch clamp recordings from a sample of GFP+ neurons within each line. We used a test panel of chemically diverse odors in these experiments in order to coarsely sample odor space. Because these Gal4 lines label many cells, we expect the labeled cells to exhibit diverse odor preferences. Indeed, within each morphological type, we found that different neurons had different preferred odors (Fig.2a,b). Interestingly, we found a systematic and significant difference between the odor tuning of type I and type II neurons: the former were broadly tuned, whereas the latter were more selective.
Figure 2. Odor selectivity in type I and type II neurons.
a, Odor selectivity in a population of type I neurons. Spikes are counted over a duration of 1 s starting at the odor onset command. Each data point is the trial-averaged response to one odor in one experiment, with a line connecting all the responses from the same experiment (n=4 from GMR48F03-Gal4 [solid] and 4 from GMR73B12-Gal4 [dashed]). All odors are 10−2 dilutions in paraffin oil (solvent), except where noted. Note the relatively broad tuning of type I neurons.
b, Same for a population of type II neurons (n=4 from GMR44G08-Gal4 [solid] and 4 from GMR12H12-Gal4 [dashed]; note that some neurons did not spike in response to any odor, but all showed subthreshold responses). Tuning is significantly narrower in type II neurons as compared to type I neurons (unpaired two-tailed t-test comparing lifetime sparseness [see Methods], P<0.01).
c, Within a subtype of type I neurons (labeled by Mz671-Gal4), odor selectivity is stereotyped (n=6). The trace shows a typical in vivo whole-cell current clamp recording from one of these neurons. In all figures, a thick horizontal line indicates the 500-ms period when the odor valve was open.
d, Same as panel c but for the subtype of type II neurons labeled by NP6099-Gal4 (n=8). The trace shows a typical recording from one of these neurons. Tuning is significantly narrower in NP6099 neurons as compared to Mz671 neurons (unpaired two-tailed t-test, P<10−7).
e, Responses to an odor concentration series. Traces are averaged across trials and neurons (± s.e.m. across neurons). Responses are steady over time and grow monotonically with concentration.
f, Same as panel e for NP6099 neurons (n=8). Responses are more transient and are suppressed at high concentrations, suggesting the recruitment of inhibition.
We then focused specifically on the small numbers of type I and type II neurons defined by Mz671-Gal4 and NP6099-Gal4. We found that all three of the Mz671 neurons showed stereotyped odor responses, both within brains and across brains. Like most type I neurons, they were broadly tuned (Fig. 2c).
Similarly, NP6099 neurons also showed stereotyped odor responses, both within and across brains. Like most type II neurons, they were narrowly tuned (Fig. 2d). Thus, the morphologies of both Mz671 neurons and NP6099 neurons accurately predicted their tuning breadth.
In addition, we noticed differences in the concentration tuning of these neurons. The responses of Mz671 neurons grew monotonically over a large dynamic range of concentrations. By contrast, the responses of NP6099 neurons were suppressed by high concentrations, and also became more transient at high concentrations (Fig. 2e,f). This suggests that some of the odor responses of NP6099 neurons might be suppressed by inhibition.
In sum, these results show that type I and type II neurons differ systematically in the breadth of their odor tuning. The Mz671 neurons and the NP6099 neurons are exemplars of each type. In order to understand the connectivity which underlies the odor responses of lateral horn neurons of each type, we focus in the rest of this study on the Mz671 and NP6099 neurons.
Connectivity from glomeruli to type I neurons
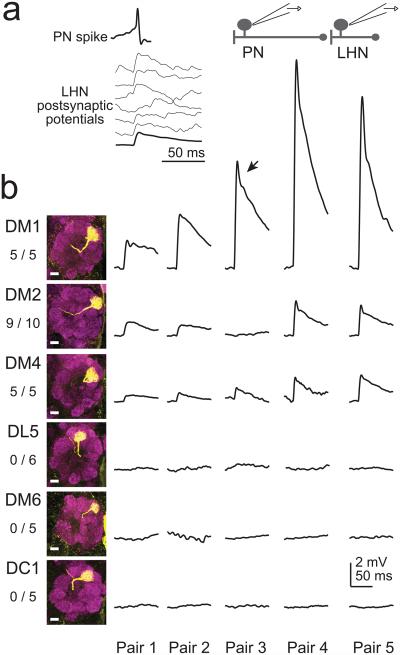
We next screened for connections from antennal lobe projection neurons (PNs) onto lateral horn neurons, using paired in vivo whole cell patch clamp recordings, and focusing initially on the Mz671 population. In every experiment we targeted one electrode to one Mz671 neuron and one randomly-selected PN,depolarizing each PN with direct current injection so that it fired one spike per trial. In most cases the cells were not connected. In a small minority of pairs, we observed excitatory postsynaptic potentials with short and consistent latencies (<1.5 ms), indicative of monosynaptic connections (Fig. 3a). In these cases, we filled the PN with biocytin for post hoc identification.
Figure 3. Paired recordings identify convergent glomerular inputs.
a, An example paired recording from a GFP+ Mz671 neuron and one of its presynaptic PNs. Top trace is a single PN spike evoked by direct current injection. Bottom traces are postsynaptic membrane potentials in individual trials (thicker line is the trial-averaged response). Vertical scale for postsynaptic responses is the same as panel b.
b, Results from multiple experiments of this type. In these experiments, both the pre- and postsynaptic neuron were GFP+. A single row shows trial-averaged postsynaptic responses from five paired recordings, each in a different brain. Shown at left are z-projections of confocal stacks through the antennal lobe displaying the dendritic tufts of biocytin-filled PNs (scale bars 10 μm), together with the total number of connections observed and total number of pairs recorded. DM1 consistently evoked larger responses than either DM2 or DM4 (1-way ANOVA followed by post hoc unpaired two-tailed t-tests, P<0.05 for both comparisons). Note that some responses have a transient peak (arrow) which likely reflects a contribution of voltage-gated postsynaptic conductances. Data are not shown for the following glomeruli, none of which were connected: VA4 (0/1), VC1 (0/3), VC2 (0/3), VL2A (0/1), VM2 (0/2), VM7 (0/2).
In this screen, we performed 120 separate paired recordings and obtained five connected pairs. All the PNs in these pairs innervated one of three glomeruli (DM2, DM4, VA7l). This connection rate implies that the Mz671 neurons receive input from only a handful of glomeruli – nominally four or fewer glomeruli if we were to assume unbiased sampling (see Discussion).
To ask whether connections are stereotyped, we performed paired recordings where antennal lobe PNs were labeled with GFP rather than randomly selected. We selectively targeted PNs in twelve glomeruli: two that emerged from our screen (DM2 and DM4), plus 10 others (DC1, DL5, DM1, DM6, VA4, VC1, VC2, VL2A, VM2, VM7). All PNs recorded in this data set were filled with biocytin to confirm their identity.
These experiments showed that connectivity from PNs to Mz671 neurons was invariant. Three glomeruli were always or almost always connected (DM1, DM2, DM4), and the other 10 glomeruli were never connected (Fig. 3b and legend). Notably, a stereotyped synaptic weight was associated with each connected glomerulus: DM1 consistently evoked larger synaptic responses than either DM2 or DM4.
Summing excitatory input from multiple glomeruli
Three glomeruli that provide input to Mz671 neurons (DM1, DM2, and DM4) are co-activated by many fruity-smelling organic acetates22, 23. Therefore, some salient olfactory stimuli (such as fruits) might co-activate these glomeruli. To determine how signals from co-activated glomeruli are integrated in these LHNs, we labeled one Mz671 neuron and two of its presynaptic PNs with GFP, and we made simultaneous triple in vivo recordings from these neurons. The two PNs were depolarized with current injection so that they fired trains of spikes, either individually or together (Fig.4a).
Figure 4. Summing excitatory input from multiple glomeruli.
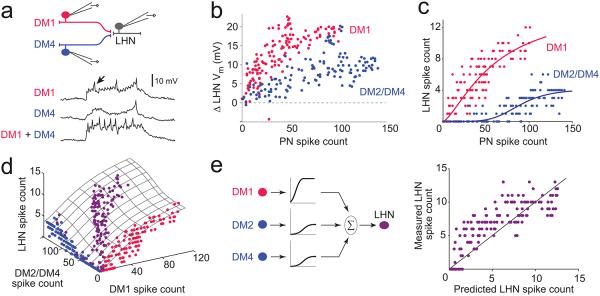
a, A Mz671 neuron and two of its presynaptic PNs are labeled with GFP and are recorded simultaneously. In interleaved trials, the PNs are driven to fire either alone or together (using 500-ms steps of depolarizing current injected through the patch pipettes), and the responses of the LHN are recorded. The postsynaptic membrane potential is shown for three trials (arrow indicates a spike). The saw-tooth fluctuations (visible especially when DM1 is spiking) are reflect large unitary postsynaptic potentials which time-lock to individual PN spikes. Voltage traces shown are 1 s in duration.
b, Relationship between PN spike count and LHN membrane potential, where the two PNs are each stimulated in separate trials. Magenta points are from trials where DM1 PNs were stimulated and blue points are from trials where either DM2 or DM4 PNs were stimulated, depending on the experiment (3 experiments in total). DM2 and DM4 connections had similar strength, and so we pooled data from these glomeruli.
c, Relationship between PN spike count and LHN spike count, where the two PNs are each stimulated in separate trials. Fits are sigmoid functions.
d, Data from all trials, including trials where the two PNs were stimulated separately (magenta and blue), and trials where they were stimulated simultaneously (purple). Hatched surface is a fit to the model.
e, Left, model schematic. Spike rates from each glomerulus are passed through an input-specific saturating nonlinearity, and then summed to generate the LHN firing rate. The input-specific nonlinearities are first fit to single-PN data, and then these same nonlinearities are used to generate the prediction for trials where both PNs were stimulated simultaneously. Right, measured LHN spike counts versus the spike counts predicted by the model. Each point represents a different trial where both PNs were stimulated simultaneously.
These experiments revealed that postsynaptic spiking could be driven by a single PN. When PNs spiked at high rates, either individually or simultaneously, the postsynaptic response followed a saturating sigmoid function (Fig.4b,c). Because input from different PNs saturated at different levels, the mechanism of saturation likely resides at the synapse, not the process of spike generation in the postsynaptic neuron.
In trials where both PNs were stimulated, the postsynaptic response was accurately predicted by summing the responses to each input alone (Fig. 4d,e). The prediction was generated by fitting sigmoid functions to the trials where single PNs were stimulated individually, and then simply summing the predicted postsynaptic responses to each PN input. This model provided a reasonably good fit to the data (R2 =0.69; Fig. 4e; see also Methods). However, the model did systematically underestimate postsynaptic responses to relatively weak presynaptic inputs. In these cases, one input was often too weak to elicit postsynaptic spikes when stimulated alone, but was strong enough to modestly increase postsynaptic spike rates when co-activated with the second input. Thus, the inputs to the Mz671 neurons sum in a fairly linear manner, although they elicit modestly supra-linear postsynaptic responses at weak presynaptic firing rates.
These triple recordings show that a single glomerulus can be sufficient to drive spikes in an LHN, and that recruitment of additional presynaptic glomeruli causes LHN responses to increase further. Based on these results, we would predict that LHN odor responses can be driven by odor-evoked spiking in a single presynaptic glomerulus. We would also predict that LHN odor responses should increase as additional glomeruli are recruited by an odor stimulus. Summing over glomeruli in this manner could allow LHNs to be sensitive to a broader range of stimuli than any single one of their input glomeruli.
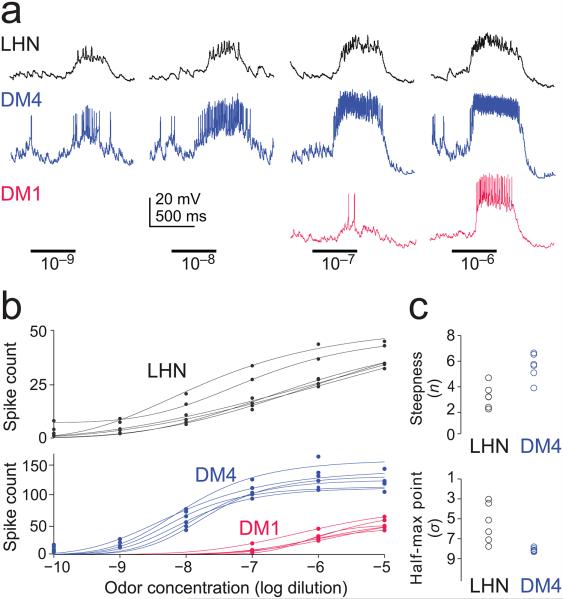
To test these predictions directly, we recorded the odor responses of Mz671 neurons, DM4 PNs, and DM1 PNs. We focused on one odor (methyl acetate) diluted over a large concentration range. We chose this odor because it activates both DM4 PNs and DM1 PNs, but it activates them at different concentrations; also, this odor is selective for DM4 at low concentrations (see Methods). As such, this odor allows us to test the specific predictions emerging from our triple recordings. As expected, we found that the Mz671 neurons were recruited by low concentrations of this odor that are selective for glomerulus DM4 (Fig. 5a,b). This confirms that odor responses in a single presynaptic glomerulus are sufficient to drive these neurons. When odor concentration was increased, DM4 PNs were saturated and DM1 PNs were recruited. As expected, LHN responses were sensitive to the recruitment of DM1 PNs: their responses continued to grow although input from DM4 was no longer growing (Fig. 5a,b). This result confirms that odor responses in the Mz671 neurons increase as additional glomeruli are recruited by an odor.
Figure 5. Lateral horn neuron odor responses are sensitive to single glomeruli.
a, Typical in vivo whole-cell recordings from a Mz671 neuron, a DM1 PN, and a DM4 PN (recorded separately). The odor is methyl acetate. Low concentrations recruit DM4 but not DM1. High concentrations saturate DM4 and begin recruiting DM1. LHN responses increase over the entire concentration range.
c, The fitted parameters which describe the shape of the curve (n and σ) are significantly different for DM4 PNs and LHNs (P<0.01 for both n and σ, unpaired two-tailed t-tests). Parameter n measures the steepness of the curve and σ is the concentration which produces a half-maximal LHN response (in units of −log dilution). The discrepancy between spike counts shown in panel b and Fig. 4 is addressed in Supplementary Fig. 1.
Note that the Mz671 neurons encode a broader range of concentrations as compared to their individual presynaptic PNs. We quantified this by measuring the steepness of the concentration-response functions; this analysis showed that the LHN responses are significantly less steep (Fig. 5c). This finding arises from the fact that the PNs in question are sensitive to different concentration ranges of the same odor. As a result, summing the two PN responses produces a broader dynamic range in the postsynaptic LHNs. Note that PN concentration-response functions are steeper than those of LHNs over a relatively limited portion of the odor concentration range. In addition, the trial-to-trial reliability of PN responses is similar to that of LHN responses, for matched odor-evoked firing rates (data not shown). Thus, PNs are more informative about concentration over a narrow range, but LHNs carry information about a broader range.
Connectivity from glomeruli to type II neurons
Next, we investigated connectivity onto the NP6099 neurons (type II). Overall, type II neurons are more narrowly tuned than type I (Fig. 2), suggesting they receive excitation from fewer glomeruli. Indeed, we found zero connections in 82 paired recordings from randomly-selected PNs and NP6099 neurons. As there are only 49 glomeruli in total, this connection rate raises the possibility that the NP6099 neurons receive PN input from only one glomerulus.
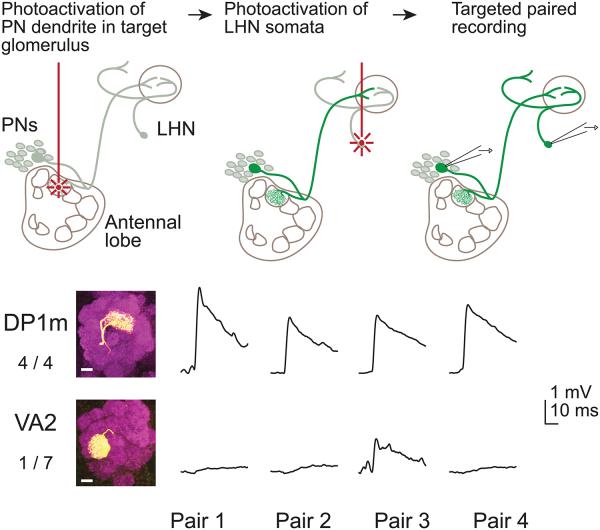
A previous study predicted that the NP6099 neurons receive direct input from glomerulus DP1m and glomerulus VA2, because their dendrites overlap with the projections from these glomeruli13. Because there is no available Gal4 line selective for the PNs in either of these glomeruli, we used an alternative approach to target these PNs for paired recordings (Fig. 6). Namely, we expressed PA-GFP under the control of a Gal4 line expressed in many PNs, and we photoactivated specifically in the glomerulus of interest. This allowed us to target our electrodes selectively to PNs in either DP1m or VA2. We filled each recorded PN to confirm its identity post hoc.
Figure 6. Paired recordings identify excitation from one glomerulus.
Top, paired in vivo recordings guided by PA-GFP. PA-GFP was expressed in many PNs (under the control of GH146-Gal4) and in NP6099 neurons. We photoactivated in a single glomerulus (either DP1m or VA2) to label the corresponding PN soma. We next we photoactivated the NP6099 neuron somata, and then simultaneously recorded from a PN and a LHN. Bottom, results from multiple experiments of this type (see Fig. 3b legend). Four of four DP1m pairs showed a connection. Only one of seven VA2 pairs showed a connection and this response was unusually weak.
Using this approach, we performed four paired recordings with DP1m PNs and NP6099 neurons. In all four cases, we found a connection. By contrast, we observed no connection with glomerulus VA2 in six of seven experiments, although there was a weak connection in one experiment (Fig. 6). This example suggests that there are small variations in the wiring of connections from PNs onto LHNs, perhaps due to developmental errors (another example is the missing connection from a DM2 PN in Fig. 3b). Nonetheless, the overall conclusion from these recordings is that connectivity is highly stereotyped. In particular, it is notable that the VA2 PNs do not form connections with the NP6099 neurons, although their axons and dendrites exhibit considerable overlap13.
Gating of feedforward excitation in type II neurons
Identifying presynaptic antennal lobe PNs for the NP6099 neurons allowed us to compare the odor tuning of these synaptically connected PNs and LHNs. We found that every test odor that activated the LHNs also activated the DP1m PNs (Fig. 7a,b). This is consistent with the idea that the NP6099 neurons receive most or all of their excitation from DP1m. In further support of this idea, we found that the input from DP1m is strong enough to account for the size of the excitatory odor responses in the LHN. Specifically, in paired recordings, direct current injection into DP1m PNs elicited a LHN response that matched the strongest odor-evoked response to the same odor-evoked PN firing rate (Fig. 7a). Thus, DP1m may be the only glomerulus that provides direct excitatory input to the NP6099 neurons.
Figure 7. Odor-selective inhibition gates excitation from glomerulus DP1m.
a, Odor responses of NP6099 neurons plotted against responses of DP1m PNs. Each point is an odor stimulus (mean ± s.e.m. computed across experiments, n=4 - 13 PNs and 4 - 17 LHNs per point, odors shown in Supplementary Fig. 2). The lower panel shows data from a separate set of experiments (paired recordings from DP1m PNs and NP6099 neurons) where the PN was directly depolarized to fire trains of spikes via current injection through the patch pipette (n=2 pairs, pooled trials binned by PN spike rate, averaged within a bin, and fit with an exponential). These paired recordings show that synaptic excitation from DP1m is strong enough to account for the strongest odor responses of NP6099 neurons (see gray fitted function in top panel reproduced from lower panel).
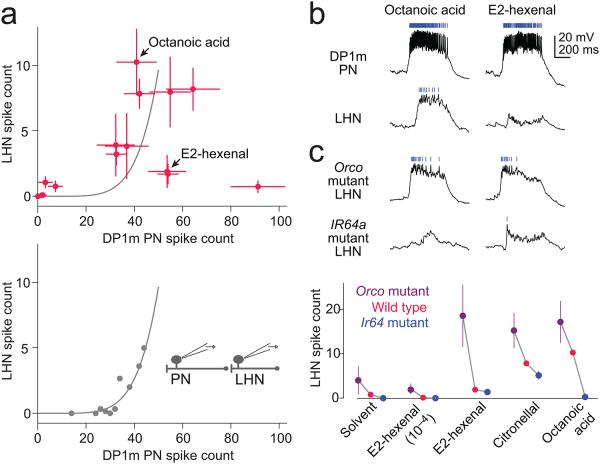
b, Odor responses of DP1m PNs and NP6099 neurons to octanoic acid and E2-hexenal (both 10−2). Rasters above each trace show spikes. Although these two stimuli elicit similarly strong responses in DP1m PNs, the postsynaptic response to E2-hexenal is selectively suppressed.
c, Both responses are disinhibited by the Orco mutation (which also eliminates the difference between the responses) and attenuated by the Ir64a mutation. The plot below quantifies responses to several stimuli in wild type flies, Orco mutants, and Ir64a mutants (± s.e.m.). For both genotypes, the effect across all odors was significant (n = 4 - 6 experiments per odor for each mutant and 9 - 17 experiments per odor for wild type, 2-way ANOVA, P<0.005 for both comparisons).
Interestingly, some odors elicited a robust response in DP1m PNs but little or no postsynaptic spiking in the NP6099 neurons. This suggests that excitatory input from DP1m is gated by strong, odor-selective inhibition from co-activated glomeruli. For example, whereas both octanoic acid and E2-hexenal elicit a robust PN response, only octanoic acid elicits a response in the LHNs (Fig. 7a,b). This suggests there is a glomerulus which is activated by E2-hexenal but not octanoic acid, and inhibition from this glomerulus gates the excitation arising from DP1m. Because inhibition is tuned, it likely arises from a small number of glomeruli, rather than the summed activity of many glomeruli (Supplementary Fig. 2).
To further explore the origins of inhibition, we recorded from these same LHNs in Orco mutants. The Orco gene encodes a co-receptor which is expressed by most olfactory receptor neurons24. Many olfactory receptor neurons are Orco-positive, and in these neurons, Orco is absolutely required for olfactory transduction24. However, DP1m olfactory receptor neurons do not require Orco for normal transduction25. We found that the Orco mutation disinhibited odor responses in the NP6099 neurons (Fig. 7c). This result indicates that one or more Orco-positive glomeruli are the source of inhibition in these LHNs.
Conversely, odor responses in NP6099 neurons were reduced by a mutation in Ir64a. The Ir64a gene encodes an odorant receptor which is expressed by DP1m olfactory receptor neurons and is necessary for their normal function26. This mutation does not completely abolish odor responses in the DP1m olfactory receptor neurons [G. Suh, personal communication], and so the residual odor responses we observe in the mutant are compatible with the conclusion that DP1m is the only source of direct excitation to the NP6099 neurons. Together, these two mutations indicate that odor-specific inhibition from Orco-positive glomeruli gates excitation from glomerulus DP1m.
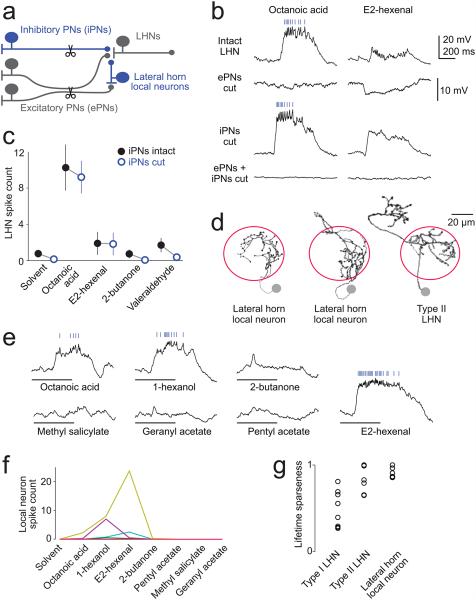
What inhibitory neurons relay odor-specific inhibition to these LHNs? Likely candidates are the GABAergic inhibitory antennal lobe PNs (iPNs)10, 27, 28. These PNs project through an axon tract which is separate from the tract carrying the axons of excitatory PNs (ePNs), so we could use two-photon laser-transection to selectively cut the axons of ePNs, leaving iPN axons intact (Fig. 8a). This manipulation abolished excitation in the NP6099 neurons, revealing pure inhibition (Fig. 8b). This residual inhibition likely originated from iPNs, because it disappeared when we cut both the iPN axon tract and the ePN axon tract (Fig. 8b). However, this residual inhibition was weak, and when we cut the axons of iPNs rather than the axons of ePNs, we observed little to no disinhibition (Fig.8b,c), especially as compared to the effects of the Orco mutation (Fig. 7c).
Figure 8. Circuit origin of inhibition.
a, Two inhibitory circuits: one via GABAergic inhibitory PNs (iPNs) in the antennal lobe, the other via GABAergic local neurons in the lateral horn. The axon tracts of iPNs and excitatory PNs (ePNs) are segregated and so can be cut selectively.
b, Odor responses of DP1m PNs and NP6099 neurons (top trace is reproduced from Fig. 7b). Cutting ePN axons eliminates excitation and reveals inhibition (cells were held at a depolarized potential to better show inhibition; note different vertical scale in this row; similar results in 9 experiments). However cutting iPN axons does not completely abolish inhibition (compare with the Orco mutation, Fig. 7c). Cutting both tracts eliminates all odor responses.
c, Odor responses of NP6099 neurons in experiments were iPNs were intact (data from Fig. 7) or cut (n=6, ± s.e.m.). There is no significant effect on spike counts (2-way ANOVA, P=0.51), although subthreshold responses were often partly disinhibited.
d, Sample morphologies of lateral horn local neurons, traced from biocytin fills. These neurons are immunopositive for GABA (Supplementary Figure 3). Similar fills were obtained for a total of 7 cells. A few of these neurons have local arbors that extend beyond the boundaries of the lateral horn (e.g., the second example here). The morphology of a type II LHN is shown for comparison (reproduced from Fig. 1).
e, Odor responses of a GABAergic lateral horn local neuron. Scale same as b.
f, Odor selectivity of a population of lateral horn local neurons. Spikes are counted over a duration of 1 s starting at the odor onset command. Each line connects spike counts from the same neuron (n=7; some cells do not spike in response to any odors but all displayed subthreshold responses).
g, Lifetime sparseness of odor-evoked spike rates in type I and II neurons (same cells as Fig. 2a,b) and lateral horn LNs (n=5 cells, same as panel f, except that here the cells that did not spike in response to either current injection or odor are omitted). Each symbol represents a different experiment. A sparseness of 1 is maximally selective, 0 is nonselective. One LN and one type II neuron spiked in response to current injection but not odor and were assigned a sparseness of 1. Note that the spiking responses of lateral horn LNs are highly selective.
Together, these results imply a second source of inhibition, in addition to the inhibition arising from iPNs. The logical candidates are GABAergic neurons in the lateral horn itself (Fig. 8a). Therefore, we performed a GABA immunostain and found a cluster of GABAergic somata adjacent to the lateral horn (Supplementary Fig.3). We identified a Gal4 line which labels these neurons, and we patched and filled individual labeled cells from this line. This confirmed that these neurons have purely local arbors (Fig. 8d). Interestingly, we observed that all the neurons we recorded from had narrow odor tuning (Fig. 8e-g). Thus, these local neurons are well-positioned to provide odor-specific inhibition.
In sum, these results provide evidence that inhibition arises from two sources. Some inhibition arises from a direct GABAergic projection from the antennal lobe, and additional inhibition arises from a local GABAergic circuit within the lateral horn. Although inhibition is much more prominent in type II neurons, it can occasionally be seen in type I neurons as well (Supplementary Fig.4).
DISCUSSION
Glomerular connections to lateral horn neurons
In order to understand higher olfactory processing, it is fundamentally important to know how many glomeruli provide input to a typical higher-order neuron. It is also important to know whether these connections are stereotyped, whether different glomerular inputs are associated with different synaptic weights, and whether some combinations of glomeruli occur preferentially. In this study, we used paired recordings to map the connectivity of representative lateral horn neuron projection neurons, and to address these questions. Because we found that connectivity is stereotyped, we could use large numbers of paired recordings to build a cumulative picture of the connectivity of these neurons.
First, our random samples of PN-LHN pairs allow us to estimate the number of input glomeruli for each LHN type. For our representative type I neurons (the Mz671 neurons), we performed 120 paired recordings with random PNs and found 5 connections. Given 49 glomeruli29, binomial statistics would indicate with ~95% confidence that there are at most four connected glomeruli. Indeed, we identified four inputs for these neurons (DM1, DM2, DM4, VA7l). This calculation assumes that there are equal numbers of PNs in all glomeruli. In total there are ~150 PNs30, which would predict three PNs per glomerulus, but it is known that some glomeruli contain more than three (e.g., glomerulus DA131) and some contain only one32. If a glomerulus contained only one PN, then we would be more likely to miss it, and indeed glomerulus DM1 was a near miss: it contains one PN32, and it did not turn up in our random screen; we identified it only as a result of our targeted paired recordings. Thus, four glomeruli might be an underestimate. Nonetheless, it seems likely that each type I neuron receives input from fewer than ten glomeruli.
For our representative type II neurons (the NP6099 neurons), we performed 82 paired recordings with random PNs and found zero connections. Binomial statistics would indicate that there are at most two connected glomeruli, with the same caveats as above. However, for these neurons, there is independent evidence arguing that DP1m is the only excitatory input. Specifically, all the odors that activate these neurons also activate DP1m PNs, and the firing rates of DP1m PNs are sufficient to predict the strongest odor responses in these neurons. It will be interesting to learn if all type II neurons receive excitatory input from a single glomerulus. It is notable that VA2 PNs do not connect to these neurons, despite substantial axon-dendrite overlap13. These results raise the question of how a lateral horn neuron reliably forms a connection with one axon but avoids forming a connection with another axon, in a case where the two axons are overlapping.
An notable conclusion of our study is that some glomerular combinations are substantially over-represented in the lateral horn. Consider the fact that there are three Mz671 neurons per lateral horn, but only several hundred lateral horn neurons in total (based on cell counts in experiments where we expressed PA-GFP pan-neuronally and photoactivated in a large volume of the lateral horn; Fig. 1a). We identified 4 glomeruli connected to Mz671 neurons. Given 49 glomeruli in total29, there are >200,000 possible combinations of four glomeruli. This is far larger than the total number of neurons in the lateral horn. Moreover, the particular glomerular combination sampled by the Mz671 neurons occurs not once, but at least three times in every lateral horn. Therefore, the space of possible glomerular combinations is sampled non-randomly.
Our paired recordings also show that different glomerular inputs to an LHN can be associated with non-uniform and stereotyped synaptic weights. This has been proposed previously as a way to render LHNs more selective for a particular olfactory feature4. This too indicates a high level of precision in the development of this circuit.
In many of these respects, our results show that the lateral horn differs radically from the mushroom body, the other third-order olfactory region in insects. In the mushroom body, the pattern of glomerular inputs to the mushroom body appears to be different in different individuals11-13, 16, 20. And although there are regional biases in connections from glomeruli to the mushroom body18, 33, and glomeruli with similar tuning tend to wire together19, connectivity in the mushroom body nonetheless seems to be probabilistic rather than deterministic. This contrasts with the highly stereotyped wiring we found in the lateral horn. Disrupting the mushroom body impairs learned but not unlearned olfactory discriminations, implying that the lateral horn is sufficient for innate olfactory behavior7, 8. Thus, the mushroom body and lateral horn serve different behavioral functions, and our results demonstrate that they also sample differently from olfactory glomeruli.
It should be noted that the stereotypy we observe may be entirely specified by the genetic inheritance of these organisms, but this need not be the case. All the flies we used in our experiments were raised in a similar environment. Future studies will be needed to know whether there is any experience-dependent element in these connections or their weights.
Odor coding and computations in lateral horn neurons
Our results demonstrate that different types of lateral horn neurons carry out distinct computations on the information they receive from olfactory glomeruli. Type I neurons are broadly tuned to odors, and the Mz671 neurons are typical of type I in this respect. Consonant with this, we found that the Mz671 neurons neurons pool excitation from a handful of co-activated glomeruli, and input from even a single glomerulus can be sufficient to drive postsynaptic spiking. For this reason, we might expect these LHNs to be more broadly tuned to odors than PNs are. Broad odor tuning to a group of related chemicals might be a useful way to link a large region of chemical space (e.g., odors associated with fruit) with an innate behavioral program (e.g., feeding).
In addition, we observed that the Mz671 neurons have a broader dynamic range for concentration encoding, as compared to their presynaptic PNs. Drosophila can generalize across different concentrations of the same odor, and this behavioral performance requires integrating activity across multiple glomeruli that are co-activated by some odors, but with different sensitivities to those odors34, 35. Whereas each individual glomerulus can only encode concentration over roughly two orders of magnitude, summing several glomeruli having different dynamic ranges can yield a broader range of sensitivity34. This is precisely what the Mz671 neurons do. Thus, type I LHNs might play a role in concentration generalization.
In contrast to type I, type II neurons are narrowly tuned, and the NP6099 neurons are typical of type II neurons in this respect. Again, consonant with their narrow tuning, we showed that the NP6099 neurons combine excitation from one (or a few) glomeruli with tuned inhibition from co-activated glomeruli, yielding greater selectivity. This represents a computation which is distinct from that performed by the Mz671 neurons. On theoretical grounds, combining excitation and inhibition from co-activated glomeruli has been proposed previously as a way to generate selectivity4. Behavioral data shows that Drosophila can perform fine discriminations among odor stimuli with different chemical compositions34, 36. Neurons with high selectivity might be a useful way to link specific odor stimuli with behavioral programs.
In other sensory systems, a neuron’s receptive field can be described as a set of positive and negative weights over stimulus space37 or neural space38. Here we show this framework can be extended to higher-order olfactory receptive fields, which are in essence a set of positive and negative weights over olfactory glomeruli. Each glomerulus corresponds to an odorant receptor, and each receptor is selective for a molecular feature1. Thus, higher-order olfactory receptive fields represent weighted sums of molecular features. In other sensory systems, receptive field structures are non-random, insofar as they have a strong tendency to sample from overlapping regions of stimulus space, reflecting the statistical regularities of the environment39. Analogous to this, we have described neurons which sample from glomeruli with overlapping chemical tuning, and we have shown that sampling is highly non-random. It will be interesting to investigate how the computations which occur in the lateral horn might relate to the statistical distribution of odors in the environment, as well as their ecological relevance to the organism.
Online Methods
Fly stocks
Flies were raised in intermediate-density cultures on conventional cornmeal agar medium supplemented with rehydrated potato flakes (Carolina Biological Supply) under a 12hr light / 12hr dark cycle at 25°C. All experiments were performed on adult female flies within the first two days post-eclosion. The genotypes used, by figure, are listed in Supplementary Table 1. Supplementary Table 1 also cites previous publications for most of the mutants and transgenic strains in this study, as well as additional detail about unpublished Gal4 lines.
Electrophysiology
In vivo whole cell patch clamp recordings were performed as previously described40. Generally one neuron was recorded per brain. The internal patch pipette solution contained (in mM): potassium aspartate 140, HEPES 10, MgATP 4, Na3GTP 0.5, EGTA 1, KCl 1, biocytin hydrazide 13 (pH = 7.3, osmolarity adjusted to ~ 268 mOsm). The external saline contained (in mM): NaCl 103, KCl 3, N-tris(hydroxymethyl) methyl-2-aminoethane-sulfonic acid 5, trehalose 8, glucose 10, NaHCO3 26, NaH2PO4 1, CaCl2 1.5, and MgCl2 4. Osmolarity was adjusted to 270–273 mOsm. The saline was bubbled with 95% O2/5% CO2 and reached a final pH = 7.3. Recordings were obtained with an Axopatch 200B model amplifier with a CV-203BU headstage and acquired with custom written IgorPro or Matlab routines. Recorded voltages and currents were low-pass filtered at 5 kHz prior to digitization at 10 kHz. Patch pipettes were made from borosilicate glass (Sutter, 1.5 mm o.d., 0.86 mm i.d.) and were fire polished using a microforge (Narishige). For some lateral horn neuron recordings, the patch pipette was pressure-polished to reduce resistance as described previously41. The estimated final pipette tip opening was submicron in diameter, with pipette resistance between 10-15 MΩ. In “randomly-targeted” PN recordings, PNs were not labeled with a visible marker, but were identified based on their cell body location and characteristic intrinsic properties42. In these “randomly-targeted” PN recordings, we made an effort to sample PN somata in both the anterodorsal cluster and the lateral cluster, and also to sample both large and small somata, but these recordings are nonetheless probably somewhat biased toward large somata in the anterodorsal cluster. In some paired recordings we removed one or both antennae to allow for easier access to the antennal lobe. Recordings from DP1m PNs and VA2 PNs were performed using PA-GFP to target our recording electrode to the PN soma (see below). For the paired recording experiments from NP6099 LHNs and DP1m PNs or VA2 PNs, in order to gain the necessary optical and mechanical access to the antennal lobe and lateral horn, we removed the brain from the head capsule and pinned it in a Sylgard-coated dish.
Odor delivery
An air stream (2.005 L/min) was passed through activated carbon and directed at the fly through a carrier tube (6.3 mm inner diameter) and positioned 15 mm from the fly. The fly was positioned to face away from the carrier tube. 5 mL/min of this air stream (the “odor stream”) was diverted from the carrier and directed by a 3-way solenoid valve into the headspace of a clean 1-mL vial (National Scientific, C4011-5W) containing 200 μL of a solution of odor in paraffin oil, or else an identical empty vial. The solenoid normally directed the odor stream to the empty vial, and switched airflow into the odor vial upon receiving a command. After passing through either vial the odor stream joined the carrier stream again. Odor dilutions refer to the dilution factor by volume of odor in solvent. Odor pulses were 500 ms in duration with an inter-pulse interval of 40 sec. Because the odor stream flow rate is relatively low, we reduced the distance the stream has to travel from the solenoid to the vials (9 cm) and the from the vials back into the carrier stream (1 cm). Odor dilutions in paraffin oil were prepared fresh daily, and vials were used for only one experiment before they were discarded. Paraffin oil was stripped of low molecular weight volatiles by storing under negative pressure, generally for at least several days prior to use. Based on previous work, we know that methyl acetate is relatively selective for DM4 olfactory receptor neurons at low concentrations43, which is relevant to the design of the experiments in Fig. 5. This previous study used a slightly different olfactometer, but in calibration experiments, we verified that the olfactometer we used in this study delivered if anything somewhat more dilute stimuli than those delivered by the previous study at the same nominal dilution.
2-photon laser scanning microscopy, PA-GFP photoactivation, and laser transection
Photoactivation of PA-GFP44, 45 was performed for anatomical investigation in the lateral horn (Fig. 1) and to target PNs for whole cell recordings in cases where no specific Gal4 line was available (Fig. 6). We used a custom built 2-photon laser scanning microscope running ScanImage acquisition software46. For both anatomy and targeting, we used a procedure similar to that described previously14, 45. Briefly, the neuropils of interest were identified using the resting fluorescence of PA-GFP at the imaging wavelength (925 nm). After defining volumes of interest based on these background images, PA-GFP was photo-converted by imaging through the volume with 710 nm light. In each photoactivation block, we moved through the z-depth of the volume of interest with 0.25 μm steps, imaging each z-frame 3 times. We adjusted laser power on an experiment-by-experiment basis. After PA-GFP is photoactivated in the neuropil (i.e., axons and dendrites), it diffuses into the somata of the corresponding neurons.
For the anatomical experiments in Fig. 1a, we photoactivated in a large portion of the dorsal lateral horn, taking care not to directly photoactivate any cell bodies. We performed three photoactivation blocks separated by 5 minute inter-block intervals. Based on several experiments like that shown in Fig. 1a, we conservatively estimate that there are at least one dozen type I and two dozen type II neurons on each side of the brain.
For the physiology experiments in Fig. 6, we located the desired glomerulus and photoactivated in a volume of several μm3 that was entirely circumscribed by that glomerulus. In order to confirm that the recorded PN did indeed arborize in the correct glomerulus, we filled every recorded PN with biocytin, visualized it using a fluorescent streptavidin conjugate, and inspected it post hoc, using nc82 antibody to label glomerular compartments (see below). In these experiments, we performed only one photoactivation block (rather than three) in order to avoid damaging brain tissue before the recording.
For laser transection experiments shown in Fig. 8a-c, we labeled the inner antenno-cerebral tract (iACT) and middle antenno-cerebral tract (mACT) by expressing GFP under the control of GH146-Gal4. The iACT contains the axons of excitatory PNs, and the mACT contains the axons of inhibitory PNs. The iACT was transected between the mushroom body calyx and the lateral horn. The mACT was transected where it appears from beneath the mushroom body peduncle before it enters the lateral horn. For both transection experiments we defined volumes of interest that completely circumscribed the tract we aimed to cut. Volumes were approximately 5-10 μm on each side. In order to transect the tract, we scanned through the depth of these volumes once or twice in 0.5-μm steps for approximately 0.5 s dwell time per frame. Laser power at the back aperture of the objective was 50-80 mW at 800 nm, the transection wavelength. The volume, imaging duration, and laser power was adjusted on an experiment-by-experiment basis so as to achieve a visible cavitation bubble that encompassed the axon tract.
Immunohistochemistry and anatomy
In order to ascertain or confirm the glomerular identity of recorded PNs, we filled them with biocytin and visualized the fills with fluorescent conjugated streptavidin. This was done in two situations. First, every time we recorded from a GFP-labeled PN, we filled it to confirm its putative identity. Second, in random recordings from unlabeled PNs, we filled PNs that turned out to be connected to simultaneously-recorded LHNs. To identify glomeruli, the glomerular neuropil was visualized using fluorescence immunohistochemistry with nc82 antibody (Developmental Studies Hybridoma Bank, nc82-s, 1:50 dilution).
In some experiments, the identity of the recorded LHNs was confirmed in a similar manner. The morphology of single dye-filled LHNs was compared with an atlas of brain neuropil divisions (www.virtualflybrain.org) in order to determine the region(s) where LHN axons arborized. Type I neurons arborized in the superior medial protocerebrum, as noted previously18, but their arbors also extended into the superior intermediate protocerebrum and the crepine. Type II neurons arborized in the superior lateral protocerebrum, as noted previously18.
The protocol for processing these fills has been described previously42. To reconstruct neuronal morphology from biocytin fills, we hand-traced the skeletonized morphology using the Simple Neurite Tracer plugin in Fiji (http://fiji.sc), using the Fill Out command to automatically generate a 3D volume, which we subsequently converted to a z-projection. Triple immunofluorescence against GABA (Sigma A2052), CD8, and nc82 (Supplementary Fig. 3) was performed essentially as previously described40 except that a different anti-CD8 antibody was used (Invitrogen MCD0800, 1:50) and the anti-nc82 antibody was used at a dilution of 1:50.
Analysis
Spike detection
Spikes were detected using custom written Matlab routines. A two-threshold routine was used to detect the events in the voltage trace that were both the fastest to rise and also the fastest to decay (Supplementary Fig. 5). The first threshold was initially used to detect positive peaks in the second time derivative of the voltage trace in order to pick out the fastest rising events. Next, a threshold was used to detect negative peaks in the first time derivative of the voltage trace in the time window [−0.3 ms,+12 ms] around the second derivative threshold crossing. Both thresholds were set manually and independently for each recording, in order to accurately capture the spikes identified by visual inspection. Automated spike detection with this routine was robust at spontaneous and lower odor-evoked firing rates. However at the higher firing rates produced by type I LHNs, action potential size became very small and post hoc visual inspection became necessary to correct errors. In order to ensure that our results in Fig. 5 were not affected by experimenter intervention in spike detection, we blinded the spike detector to the stimulus concentration. In roughly a quarter of the cells we recorded from in our recordings of odor responses, spike sorting could not be performed reliably. Those recordings were excluded from analysis.
Odor response metrics
In cases where we measure odor-evoked spike counts, we counted spikes over a 1-s window starting at the odor onset command. The odor valve remained open for 500 ms. Due to the construction of our olfactometer, there was a ~150-ms delay from when the odor onset command is sent by the acquisition computer to the solenoid to the time odorant reaches the fly, as determined using a fast photoionization detector at the fly’s location (miniPID, Aurora Scientific). As both LHN types showed relatively low levels of spontaneous spiking, we simply counted spikes over a 1 sec window starting at the odor onset command, in order to ensure that all odor-evoked spiking was captured. Lifetime sparseness of odor-evoked spike count data was computed as described previously47, except that the baseline firing rate (which was always close to zero) was not subtracted from odor-evoked firing rates.
Spike-triggered averages of postsynaptic voltage
The existence of a monosynaptic connection between a projection neuron and the lateral horn neuron was assessed using spike-triggered averaging of the lateral horn neuron membrane potential, triggered on single PN spikes. On each trial, we injected a brief (30-100 ms) step of depolarizing current into the PN via the patch pipette to elicit a single action potential. We obtained between 16 and 250 trials for each paired recording. We aligned the postsynaptic voltage trace to the time of the peak of the presynaptic action potential, defined as time t=0. We averaged over the time window t=−20 ms to t=80 ms and defined the average voltage in the window −20 ms to 0 ms as the baseline. An EPSP was said to occur if in the time window t=0 ms to t=5 ms the spike-triggered average crossed a threshold of +5 standard deviations computed over the 20 ms baseline period. In most experiments we triggered only single action potentials in PNs, but in 4 experiments we also included data from trials where up to three PN spikes were evoked (using a 100-ms step of current injection in the PN). This could potentially bias EPSP amplitudes; however these four experiments revealed no connection. EPSP amplitude was measured as the baseline-subtracted peak depolarization in the time window t=0 to t=20 ms. EPSP latency was defined as the time of extrapolated zero-crossing of linear fits to the 20-80% rising phase of the spike-triggered average.
Triplet recordings
In Fig. 4b, we measured changes in LHN membrane potential (Vm) while presynaptic PNs were stimulated with a 500-ms step of depolarizing current. For this measurement, Vm was first low-pass filtered to remove spikes. We then computed average Vm over a steady-state period during the stimulus (100 – 500 ms after stimulus onset) and we subtracted the average Vm over a baseline period preceding the stimulus.
Modeling
In Fig. 4c, we fit LHN spike counts with the following equations:
PN1 and PN2 are spike counts associated with the first and second PNs, respectively. LHNPN1 and LHNPN2 are spike counts of the LHN on trials where only one PN was active. The equations define input-specific sigmoid nonlinearities, where the fitted parameters [Rmax1, Rmax2] are the amplitudes of the sigmoids and can be interpreted as the weights associated with the two inputs. The fitted parameter σ is the semi-saturation constant (i.e., the level of PN input at which the LHN response is half-maximal). The fitted exponential parameter n represents the shape and steepness of the sigmoid.
In Fig. 4d-e, we fit the 3-dimensional transformation from presynaptic spike counts to postsynaptic spike counts with the surface defined by the following equation:
where the parameters were fixed at the values obtained above from fitting the transformation for individual PN inputs. In other words, we predicted the postsynaptic spiking response to combined activation of two inputs simply as the sum of the responses to each input alone. This model yielded a reasonable prediction of the actual spike counts obtained (R2=0.69). Much of the residual variance represents measurement uncertainty (i.e., trial-to-trial or cell-to-cell variation), and so cannot be accounted for by any model of this kind.
We also fit the same data set with an alternative model that incorporates a third term:
where the third term represents a multiplicative interaction between the two PN inputs, and C is the coefficient of the interaction term. Again the parameters for LHNPN1 and LHNPN2 were fixed at the values obtained from fits to individual PN input transformations. This model did not improve fit quality (R2 = 0.69). Furthermore, the fitted coefficient C of the interaction term was two orders of magnitude smaller than Rmax1 and Rmax2. In other words, there was a minimal contribution from the multiplicative term. This indicates the data is adequately explained as a sum over PN inputs, and including a cooperative interaction between the two PN inputs provides no additional explanatory power.
Supplementary Material
ACKNOWLEDGEMENTS
We are grateful to Gerhardt Technau for the gift of Mz671-Gal4, Julie Simpson for c315a-Gal4, Gerald Rubin and Barret Pfeiffer for pJFRC7-20XUAS-IVS-mCD8::GFP(attp40), and Sandeep Robert Datta for UAS-C3PAGFP and UAS-SPA-GFP. We thank members of the Wilson laboratory for comments on the manuscript. This work was supported by a research project grant from the National Institutes of Health (R01DC008174). R.I.W. is an HHMI Investigator. M.F. was supported in part by an HHMI International Student Research Fellowship.
Footnotes
AUTHOR CONTRIBUTIONS
M.F. and R.I.W. designed the experiments. M.F. performed the experiments and analyzed the data. M.F. and R.I.W. wrote the manuscript.
References
- 1.Bargmann CI. Comparative chemosensation from receptors to ecology. Nature. 2006;444:295–301. doi: 10.1038/nature05402. [DOI] [PubMed] [Google Scholar]
- 2.Vosshall LB, Stocker RF. Molecular architecture of smell and taste in Drosophila. Annu. Rev. Neurosci. 2007;30:505–533. doi: 10.1146/annurev.neuro.30.051606.094306. [DOI] [PubMed] [Google Scholar]
- 3.Masse NY, Turner GC, Jefferis GS. Olfactory information processing in Drosophila. Curr. Biol. 2009;19:R700–713. doi: 10.1016/j.cub.2009.06.026. [DOI] [PubMed] [Google Scholar]
- 4.Luo SX, Axel R, Abbott LF. Generating sparse and selective third-order responses in the olfactory system of the fly. Proc. Natl. Acad. Sci. U. S. A. 2010;107:10713–10718. doi: 10.1073/pnas.1005635107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Miyamichi K, et al. Cortical representations of olfactory input by trans-synaptic tracing. Nature. 2011;472:191–196. doi: 10.1038/nature09714. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Sosulski DL, Bloom ML, Cutforth T, Axel R, Datta SR. Distinct representations of olfactory information in different cortical centres. Nature. 2011;472:213–216. doi: 10.1038/nature09868. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.de Belle JS, Heisenberg M. Associative odor learning in Drosophila abolished by chemical ablation of mushroom bodies. Science. 1994;263:692–695. doi: 10.1126/science.8303280. [DOI] [PubMed] [Google Scholar]
- 8.Connolly JB, et al. Associative learning disrupted by impaired Gs signaling in Drosophila mushroom bodies. Science. 1996;274:2104–2107. doi: 10.1126/science.274.5295.2104. [DOI] [PubMed] [Google Scholar]
- 9.Stocker RF. The organization of the chemosensory system in Drosophila melanogaster: a review. Cell Tissue Res. 1994;275:3–26. doi: 10.1007/BF00305372. [DOI] [PubMed] [Google Scholar]
- 10.Tanaka NK, Endo K, Ito K. Organization of antennal lobe-associated neurons in adult Drosophila melanogaster brain. J. Comp. Neurol. 2012;520:4067–4130. doi: 10.1002/cne.23142. [DOI] [PubMed] [Google Scholar]
- 11.Marin EC, Jefferis GS, Komiyama T, Zhu H, Luo L. Representation of the glomerular olfactory map in the Drosophila brain. Cell. 2002;109:243–255. doi: 10.1016/s0092-8674(02)00700-6. [DOI] [PubMed] [Google Scholar]
- 12.Wong AM, Wang JW, Axel R. Spatial representation of the glomerular map in the Drosophila protocerebrum. Cell. 2002;109:229–241. doi: 10.1016/s0092-8674(02)00707-9. [DOI] [PubMed] [Google Scholar]
- 13.Jefferis GS, et al. Comprehensive maps of Drosophila higher olfactory centers: spatially segregated fruit and pheromone representation. Cell. 2007;128:1187–1203. doi: 10.1016/j.cell.2007.01.040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Ruta V, et al. A dimorphic pheromone circuit in Drosophila from sensory input to descending output. Nature. 2010;468:686–690. doi: 10.1038/nature09554. [DOI] [PubMed] [Google Scholar]
- 15.Gupta N, Stopfer M. Functional analysis of a higher olfactory center, the lateral horn. J. Neurosci. 2012;32:8138–8148. doi: 10.1523/JNEUROSCI.1066-12.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Masuda-Nakagawa LM, Tanaka NK, O'Kane CJ. Stereotypic and random patterns of connectivity in the larval mushroom body calyx of Drosophila. Proc. Natl. Acad. Sci. U. S. A. 2005;102:19027–19032. doi: 10.1073/pnas.0509643102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Caron SJ, Ruta V, Abbott LF, Axel R. Random convergence of olfactory inputs in the Drosophila mushroom body. Nature. 2013;497:113–117. doi: 10.1038/nature12063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Tanaka NK, Awasaki T, Shimada T, Ito K. Integration of chemosensory pathways in the Drosophila second-order olfactory centers. Curr. Biol. 2004;14:449–457. doi: 10.1016/j.cub.2004.03.006. [DOI] [PubMed] [Google Scholar]
- 19.Gruntman E, Turner GC. Integration of the olfactory code across dendritic claws of single mushroom body neurons. Nat. Neurosci. 2013 doi: 10.1038/nn.3547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Murthy M, Fiete I, Laurent G. Testing odor response stereotypy in the Drosophila mushroom body. Neuron. 2008;59:1009–1023. doi: 10.1016/j.neuron.2008.07.040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Pfeiffer BD, et al. Tools for neuroanatomy and neurogenetics in Drosophila. Proc. Natl. Acad. Sci. U. S. A. 2008;105:9715–9720. doi: 10.1073/pnas.0803697105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.de Bruyne M, Foster K, Carlson JR. Odor coding in the Drosophila antenna. Neuron. 2001;30:537–552. doi: 10.1016/s0896-6273(01)00289-6. [DOI] [PubMed] [Google Scholar]
- 23.Hallem EA, Carlson JR. Coding of odors by a receptor repertoire. Cell. 2006;125:143–160. doi: 10.1016/j.cell.2006.01.050. [DOI] [PubMed] [Google Scholar]
- 24.Larsson MC, et al. Or83b encodes a broadly expressed odorant receptor essential for Drosophila olfaction. Neuron. 2004;43:703–714. doi: 10.1016/j.neuron.2004.08.019. [DOI] [PubMed] [Google Scholar]
- 25.Silbering AF, et al. Complementary function and integrated wiring of the evolutionarily distinct Drosophila olfactory subsystems. J. Neurosci. 2011;31:13357–13375. doi: 10.1523/JNEUROSCI.2360-11.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Ai M, et al. Acid sensing by the Drosophila olfactory system. Nature. 2010;468:691–695. doi: 10.1038/nature09537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Liang L, et al. GABAergic projection neurons route selective olfactory inputs to specific higher-order neurons. Neuron. 2013;79:917–931. doi: 10.1016/j.neuron.2013.06.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Parnas M, Lin AC, Huetteroth W, Miesenbock G. Odor discrimination in Drosophila: from neural population codes to behavior. Neuron. 2013;79:932–944. doi: 10.1016/j.neuron.2013.08.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Couto A, Alenius M, Dickson BJ. Molecular, anatomical, and functional organization of the Drosophila olfactory system. Curr. Biol. 2005;15:1535–1547. doi: 10.1016/j.cub.2005.07.034. [DOI] [PubMed] [Google Scholar]
- 30.Jefferis GS, Marin EC, Stocker RF, Luo L. Target neuron prespecification in the olfactory map of Drosophila. Nature. 2001;414:204–208. doi: 10.1038/35102574. [DOI] [PubMed] [Google Scholar]
- 31.Jefferis GS, et al. Developmental origin of wiring specificity in the olfactory system of Drosophila. Development. 2004;131:117–130. doi: 10.1242/dev.00896. [DOI] [PubMed] [Google Scholar]
- 32.Yaksi E, Wilson RI. Electrical coupling between olfactory glomeruli. Neuron. 2010;67:1034–1047. doi: 10.1016/j.neuron.2010.08.041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Lin HH, Lai JS, Chin AL, Chen YC, Chiang AS. A map of olfactory representation in the Drosophila mushroom body. Cell. 2007;128:1205–1217. doi: 10.1016/j.cell.2007.03.006. [DOI] [PubMed] [Google Scholar]
- 34.Kreher SA, Mathew D, Kim J, Carlson JR. Translation of sensory input into behavioral output via an olfactory system. Neuron. 2008;59:110–124. doi: 10.1016/j.neuron.2008.06.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Asahina K, Louis M, Piccinotti S, Vosshall LB. A circuit supporting concentration-invariant odor perception in Drosophila. J. Biol. 2009;8:9. doi: 10.1186/jbiol108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Borst A. Computation of olfactory signals in Drosophila melanogaster. J. Comp. Physiol. [A] 1983;152:373–383. [Google Scholar]
- 37.Dayan P, Abbott LF. Theoretical Neuroscience. MIT Press; Cambridge, MA: 2001. [Google Scholar]
- 38.Rust NC, Mante V, Simoncelli EP, Movshon JA. How MT cells analyze the motion of visual patterns. Nat. Neurosci. 2006;9:1421–1431. doi: 10.1038/nn1786. [DOI] [PubMed] [Google Scholar]
- 39.Simoncelli EP. Vision and the statistics of the visual environment. Curr. Opin. Neurobiol. 2003;13:144–149. doi: 10.1016/s0959-4388(03)00047-3. [DOI] [PubMed] [Google Scholar]
- 40.Wilson RI, Laurent G. Role of GABAergic inhibition in shaping odor-evoked spatiotemporal patterns in the Drosophila antennal lobe. J. Neurosci. 2005;25:9069–9079. doi: 10.1523/JNEUROSCI.2070-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Goodman MB, Lockery SR. Pressure polishing: a method for re-shaping patch pipettes during fire polishing. J. Neurosci. Methods. 2000;100:13–15. doi: 10.1016/s0165-0270(00)00224-7. [DOI] [PubMed] [Google Scholar]
- 42.Wilson RI, Turner GC, Laurent G. Transformation of olfactory representations in the Drosophila antennal lobe. Science. 2004;303:366–370. doi: 10.1126/science.1090782. [DOI] [PubMed] [Google Scholar]
- 43.Olsen SR, Bhandawat V, Wilson RI. Divisive normalization in olfactory population codes. Neuron. 2010;66:287–299. doi: 10.1016/j.neuron.2010.04.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Patterson GH, Lippincott-Schwartz J. A photoactivatable GFP for selective photolabeling of proteins and cells. Science. 2002;297:1873–1877. doi: 10.1126/science.1074952. [DOI] [PubMed] [Google Scholar]
- 45.Datta SR, et al. The Drosophila pheromone cVA activates a sexually dimorphic neural circuit. Nature. 2008;452:473–477. doi: 10.1038/nature06808. [DOI] [PubMed] [Google Scholar]
- 46.Pologruto TA, Sabatini BL, Svoboda K. ScanImage: flexible software for operating laser scanning microscopes. Biomed. Eng. Online. 2003;2:13. doi: 10.1186/1475-925X-2-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Perez-Orive J, et al. Oscillations and sparsening of odor representations in the mushroom body. Science. 2002;297:359–365. doi: 10.1126/science.1070502. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.