Abstract
Background
Catch-up growth may predispose to obesity and metabolic sequelae. We sought to examine the trajectory and correlates of growth and catch-up among extremely low birth weight (ELBW, <1 kg) adolescents.
Methods
Cohort study of 148 neurologically normal ELBW children and 115 normal birth weight (NBW) controls born 1992 through 1995. Longitudinal measures of gender-specific growth of ELBW children from birth, and growth and measures of obesity of ELBW and NBW children at 14 years.
Results
Following neonatal growth failure, ELBW children had accelerated growth, but at 8 years they still had lower weight and height z scores than NBW children. By 14 years ELBW boys had caught up in growth to their NBW controls but ELBW girls remained significantly smaller. ELBW children however did not differ from their controls in measures of obesity. In hierarchical multiple regression analyses only maternal BMI and weight gain during infancy and childhood predicted the ELBW children’s 14-year weight z scores, BMI z scores and abdominal circumference. Perinatal risk factors including intrauterine growth only predicted growth up to 20 months.
Conclusion
Maternal BMI and rate of growth, rather than perinatal factors, predict 14-year obesity among neurologically normal ELBW adolescents.
Preterm infants have traditionally suffered from neonatal growth failure due to inadequate nutrition and chronic complications of prematurity. The majority catch up in growth, although their growth attainment may be less than that of NBW children (1,2). The implications of this catch-up growth for long term cardiovascular and metabolic health have been the subject of interest for many years (3,4), but have become more critical since childhood obesity became epidemic (5).
We recently reported on the increase in rates of obesity between ages 8 and 14 years among ELBW children born 1992–1995 (6). In the current report, we sought to examine the children’s gender specific trajectory and correlates of growth and catch-up from birth. We hypothesized that by age 14 years, the ELBW children would catch up to NBW controls in weight, height, and clinical measures of obesity, and that the predictors of growth would include socioenvironmental, perinatal and neonatal risk factors.
RESULTS
Descriptors of ELBW and NBW Children
ELBW boys and girls did not differ significantly from their respective NBW peers in maternal sociodemographic factors (SES) with the exception that mothers of ELBW girls were older and had a higher mean family income than mothers of NBW girls. ELBW girls reported less physical activity than NBW girls. Age of puberty was similar between groups (Table 1). Within the ELBW population, bronchopulmonary dysplasia (BPD, oxygen dependence at 36 weeks) and sepsis were the most common neonatal complications. ELBW boys had higher rates of BPD and postnatal steroid therapy than girls.
Table 1.
MATERNAL DEMOGRAPHIC FACTORS, PERINATAL DATA AND 14-YEAR OUTCOMES
| Boys | Girls | |||
|---|---|---|---|---|
| ELBW | NBW | ELBW | NBW | |
| n=52 | n=42 | n=96 | n=73 | |
| Caregiver Demographic Dataa | ||||
| Age (years ± SD)b | 41.3 ± 6 | 40.3 ± 7 | 43.1 ± 6* | 40.6 ± 6* |
| Married | 25 (48%) | 26 (62%) | 36 (38%) | 37 (51%) |
| Education | ||||
| <High school | 5 (10%) | 3 (7%) | 6 (6%) | 9 (12%) |
| High schoolc | 16 (31%) | 9 (21%) | 28 (29%) | 13 (18%) |
| >High school | 31 (60%) | 30 (71%) | 62 (65%) | 51 (70%) |
| Race | ||||
| White | 22 (42%) | 15 (36%) | 33 (34%) | 24 (33%) |
| Black | 30 (58%) | 27 (64%) | 63 (66%) | 49 (67%) |
| Family income (mean dollars)d | $43,485 | $43,861 | $44,728 | $38,056* |
| Perinatal and Birth Data | ||||
| Preeclampsia | 8 (15%) | NA | 17 (18%) | NA |
| Smoking | 2 (4%) | NA | 4 (7%) | NA |
| Antenatal steroid therapy | 15 (42%) | NA | 21 (30%) | NA |
| Birth weight (gm ± SD) | 825 ± 119 | 3323 ± 597† | 813 ± 124 | 3238 ± 411† |
| Gestational age (wk ± SD) | 26.5 ± 2 | ≥37 | 26.5 ± 2 | ≥37 |
| Small for gestational age | ||||
| < −2SD | 7(13%) | NA | 18 (19%) | NA |
| <10th percentile | 16 (31%) | NA | 37 (39%) | NA |
| Multiple birth | 7 (13%) | 0 | 19 (20%) | 0 |
| Neonatal Risk Factors | ||||
| Necrotizing enterocolitis | 2 (4%) | NA | 5 (5%) | NA |
| Septicemiae | 24 (46%) | NA | 42 (44%) | NA |
| Cerebral abnormalityf | 11 (21%) | NA | 15 (16%) | NA |
| Bronchopulmonary dysplasia | 26 (50%) | NA | 30 (31%) | NA |
| Total complications (mean ± SD) | 1.12 ± 1 | NA | 0.96 ± 1 | NA |
| Postnatal steroid therapy | 33 (63%) | NA | 44 (46%) | NA |
| Hyperalimenation (days) | 32.8 ± 25 | NA | 28.8 ± 18 | NA |
| 14-Year Outcomes | ||||
| Age at study (years ± SD) | 14.6 ± 0.6 | 14.6 ± 0.7 | 14.8 ± 0.6 | 14.9 ± 0.7 |
| Age of puberty (years ± SD) | 12.0 ± 1.3 | 11.8 ± 1.3 | 11.8 ± 1.4 | 12.0 ± 1.2 |
| Physical activity (mean ± SD)g | 3.0 ± 1.0 | 3.3 ± 0.9 | 2.5 ± 1.0 | 2.9 ± 0.9** |
ELBW, extremely low birth weight; NBW, normal birth weight; NA, not available or applicable
p<0.05;
p<0.01;
p<0.001.
Unless otherwise stated, refers to primary caregiver
Biologic mothers only: 40 ELBW and 36 NBW mothers of boys and 76 ELBW and 65 NBW mothers of girls.
Includes General Education Diploma (GED)
Mean of median family income per US $1000, according to the 2000 census tract neighborhood in which the families lived.
Positive blood culture
Cerebral ultrasound grade III-IV hemorrhage, periventricular leukomalacia and/or ventricular dilatation at discharge
Physical activity, subdomain mean score13
Longitudinal Changes in Growth
The mean weight z scores, i.e. standard deviation scores (ZWT) of ELBW children at birth was −0.72 for boys and −0.96 for girls. Due to neonatal growth failure, these decreased by 40 weeks to −1.97 among boys and −2.02 among girls, and then increased to +0.07 and +0.14 respectively by age 14 years. Mean birth length or height z scores similarly decreased by 40 weeks and then increased to 14 years (Figure 1 and Supplemental Table S1 (online)). Among the ELBW children, catch-up growth (≥0.67 SD) occurred between all periods of study. Between ages 8 and 14 years, the increases in growth as measured by an increase in mean weight and height z scores per month or by catch-up growth, were significantly greater for ELBW boys than for NBW boys, whereas this was not evident among girls (Table 2). The growth of the children with measures from their biologic mothers was similar to that of the children of mothers who did not have growth measures (data not shown).
Figure 1.
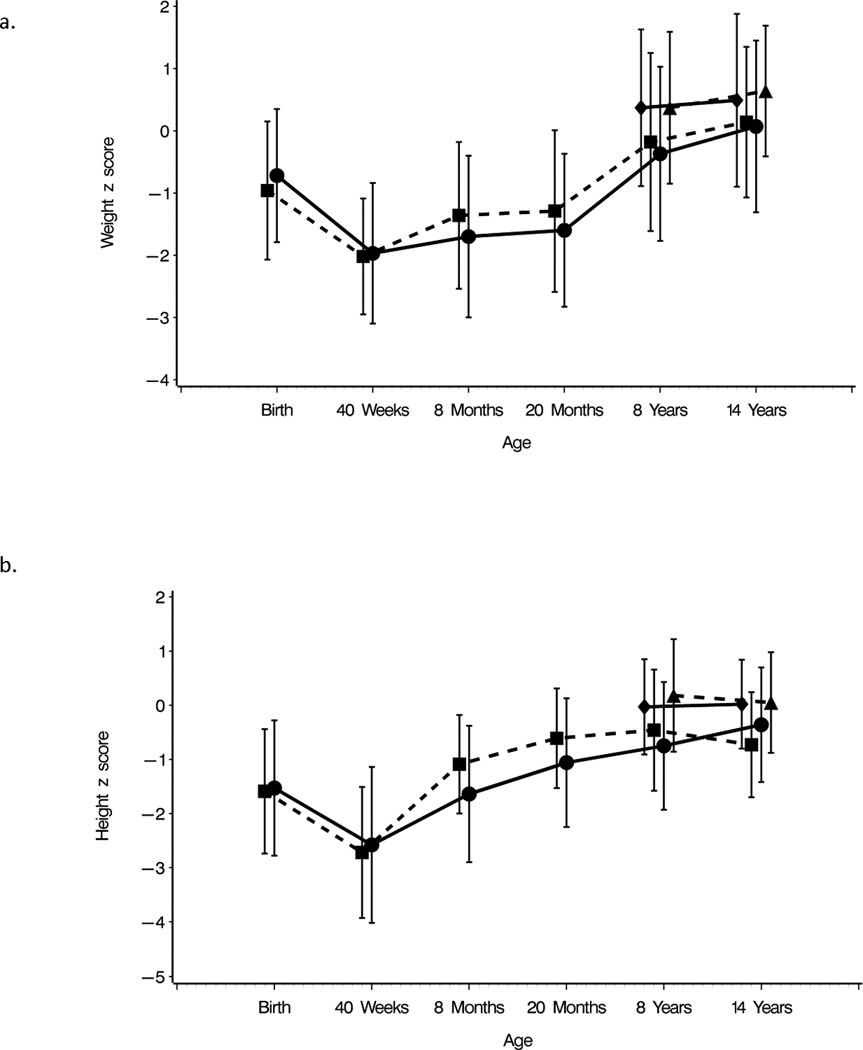
Mean weight and height z scores ± SD of extremely low birth weight (ELBW) boys and girls at birth, 40 weeks (term), 8 and 20 months, and 8 and 14 years, and mean weight and height z scores ±SD of normal birth weight (NBW) boys and girls at ages 8 and 14 years. Weight measures are indicated in Panel a and height measures in Panel b. Measures of ELBW boys are indicated by dots and solid lines and measures of ELBW girls are indicated by squares and dashed lines. Measures of NBW boys are indicated by diamonds and solid lines and measures of NBW girls are indicated by triangles and dashed lines.
Table 2.
CHANGES IN WEIGHT AND HEIGHT Z SCORES BETWEEN TIME PERIODS OF STUDY
| EXTREMELY LOW BIRTH WEIGHT | NORMAL BIRTH WEIGHT |
|||||
|---|---|---|---|---|---|---|
| CHANGES IN Z SCORES |
Birth to 40 weeksa |
40 weeks to 8 monthsb |
8 to 20 monthsc |
20 months to 8 yearsd |
8 to 14 yearse | 8 to 14 years |
| BOYS, n=52 | n=41 | |||||
| Z-WEIGHT | ||||||
| ≥0.67 | 1 (2%) | 21 (47%) | 7(16%) | 29 (60%) | 15 (29%)* | 5(12%)* |
| Per month | −0.390±0.339 | 0.030±0.224 | 0.015±0.065 | 0.015±0.065 | 0.006±0.009* | 0.002±0.011* |
| Z-LENGTH/HEIGHT | ||||||
| ≥ 0.67 | 6 (13%) | 23 (56%) | 15 (37%) | 16 (33%) | 18 (35%)** | 2 (5%)** |
| Per month | −0.336±0.576 | 0.132±0.179 | 0.054±0.124 | 0.005±0.013 | 0.005±0.008** | 0.000±0.008** |
| GIRLS, n=96 | n=72 | |||||
| Z-WEIGHT | ||||||
| ≥0.67 | 7 (7%) | 44 (49%) | 18 (20%) | 55 (60%) | 32 (33%) | 21 (29%) |
| Per month | −0.333±0.356 | 0.076±0.129 | 0.005±0.069 | 0.013±0.015 | 0.004±0.011 | 0.004±0.012 |
| Z-LENGTH/HEIGHT | ||||||
| ≥0.67 | 8 (10%) | 65 (83%) | 36 (43%) | 22 (25%) | 8 (8%) | 7 (10%) |
| Per month | −0.391±0.520 | 0.195±0.129 | 0.048±0.067 | 0.001±0.012 | −0.004±0.011 | −0.002±0.011 |
p<0.05
p<0.01
Numbers of missing children: Weight, n= a7, b9, c4, d1, e0. Length, n=a12,b18, c7, d1.
Eight- and Fourteen-Year Growth of ELBW and NBW Children
Among both ELBW and NBW children the height z score was lower than ZWT at both 8 and 14 years (Table 3). At 8 years, ELBW boys and girls had significantly lower mean weight and height z scores than their NBW peers. At 14 years, although ELBW boys still had a lower weight and height than NBW boys, the differences were not significant. ELBW girls, however, remained significantly smaller than their peers. Nine of 15 girls who were overweight at 8 years became obese by 14 years with rates of obesity increasing from 12% to 21% (p=0.049) compared to an increase of 18% to 19% among the NBW children. The mean body mass index (BMI, wt/ht2) z scores and rates of obesity did not differ significantly between ELBW and NBW boys or girls at 8 or 14 years nor did the mean 14-year abdominal circumference.
Table 3.
COMPARISON OF GROWTH AND MEASURES OF OBESITY BETWEEN ELBW AND NBW CHILDREN AT 8 AND 14 YEARS
| 8 YEARS | Boys | Girls | ||||
|---|---|---|---|---|---|---|
| ELBW | NBW | Mean Differencea or Odds Ratio (95% CI)b |
ELBW | NBW | Mean Differencea or Odds Ratio (95% CI)b |
|
| n=51 | n=41 | n=96 | n=73 | |||
| Weight | ||||||
| Mean (kg ± SD) | 28.1 ±1 0.8 | 33.1 ± 10.4 | −4.8(−9.4,−0.3)* | 29.3 ± 9.0 | 35.2 ± 11.2 | −5.7(−2.6, −8.7)† |
| Z score ± SD | −0.37 ± 1.4 | 0.37 ± 1.26 | −0.74(−1.31, −0.17)* | −0.18 ± 1.43 | 0.37 ± 1.22 | −0.52(−0.10, −0.93)** |
| Less than −2 SD | 5 (10%) | 1 (2%) | 4.5(0.5,41.2) | 8 (8%) | 0 (0%) | NA |
| Height | ||||||
| Mean (cm ± SD) | 126.6 ± 8.8 | 133.4 ± 8.0 | −6.8(−3.2, −10.4)† | 128.7 ± 8.3 | 136.2 ± 8.3 | −7.3(−4.7, −9.9)† |
| Z score ± SD | −0.75 ± 1.18 | −0.03 ± 088 | −0.72(−1.17, −0.27)** | −0.46±1.12 | 0.18 ± 1.04 | −0.62(−0.30, −0.96)† |
| Less than −2 SD | 7 (14%) | 1 (2%) | 6.0(0.7,51.4) | 7 (7%) | 0 (0%) | NA |
| Measures of Obesity | ||||||
| BMI, mean (kg/m2 ± SD) | 17.1 ± 4.2 | 18.3 ± 4.2 | −1.1(−2.9,0.7) | 17.4 ± 4.0 | 18.6 ± 4.2 | −1.2(−2.4,0.08) |
| Z score ± SD | 0.07 ± 1.1 | 0.4 ± 1.2 | −0.36(−0.86,0.13) | 0.05 ± 1.4 | 0.42 ± 1.1 | −0.36(−0.03,0.75) |
| Underweight (<5th %) | 2 (4%) | 2 (5%) | 0.8(0.1,6.2) | 12 (13%) | 2 (3%) | 4.8(1.0,22.5)* |
| Normal weight (5th–84th %) | 40 (78%) | 25 (61%) | 2.4(0.9,6.0) | 57 (60%) | 45 (62%) | 0.9(0.5,1.7) |
| Overweight (85th–94th %) | 3 (6%) | 8 (20%) | 0.3(0.1,1.1) | 15 (16%) | 13 (18%) | 0.9(0.4,2.0) |
| Obese (≥95th %) | 6 (12%) | 6 (15%) | 0.7(0.2,2.5) | 11 (12%) | 13 (18%) | 0.6(0.5,1.7) |
| 14 YEARS | BOYS | GIRLS | ||||
|---|---|---|---|---|---|---|
| ELBW | NBW | Mean Differencea or Odds Ratio (95% CI)b |
ELBW | NBW | Mean Differencea or Odds Ratio (95% CI)b |
|
| n=52 | n=42 | n=96 | n=73 | |||
| Weight | ||||||
| Mean (kg ± SD) | 58.5 ± 21.0 | 63.0 ± 18.0 | −4.6(−12.7,3.6) | 56.6 ± 15.1 | 63.2 ± 19.3 | −6.2(−11.4,−0.9)* |
| Z score ± SD | 0.07 ± 1.38 | 0.49 ± 139 | −0.43(−1.01,0.15) | 0.14 ± 1.21 | 0.64 ± 1.05 | −0.47(−0.12, −0.83)** |
| Less than −2 SD | 2 (4%) | 2 (5%) | 0.80(0.1,6.0) | 3 (3%) | 0 (0%) | NA |
| Height | ||||||
| Mean (cm ± SD) | 164.0 ± 9.4 | 167.1±7.6 | −3.1(−6.7,0.5) | 156.8 ± 6.2 | 161.9 ± 6.1 | −5.1(−3.2, −7.0)† |
| Z score ± SD | −0.36 ± 1.06 | 0.02 ± 0.82 | −0.38(−0.78,0.02) | −0.73 ± 0.97 | 0.05 ± 0.9 | −0.78(−1.08, −0.48)† |
| Less than −2 SD | 3 (6%) | 1 (2%) | 2.7(0.3,29.0) | 9 (9%) | 0 (0%) | NA |
| Measures of Obesity | ||||||
| BMI, mean (kg/m2 ± SD) | 21.4 ± 6.1 | 22.4 ± 5.5 | −0.9(−3.4,1.5) | 22.9 ± 5.6 | 24.0 ± 6.8 | −0.9(−2.8,1.0) |
| Z score ± SD | 0.17 ± 1.1 | 0.40 ± 1.4 | −0.24(−0.76,0.28) | 0.48 ± 1.1 | 0.65 ± 1.0 | −0.15(−0.47,0.18) |
| Underweight (<5th %) | 2 (4%) | 2 (5%) | 0.8(0.1,6.0) | 3 (3%) | 1 (1%) | 2.4(0.2,24.3) |
| Normal weight (5th–84th %) | 39 (75%) | 26 (62%) | 1.8(0.7,4.4) | 67 (70%) | 46 (63%) | 1.3(0.7,2.5) |
| Overweight (85th–94th %) | 3 (6%) | 5 (12%) | 0.5(0.1,2.1) | 6 (6%) | 12 (16%) | 0.3(0.1,0.96)* |
| Obese (≥95th %) | 8 (15%) | 9 (21%) | 0.7(0.2,1.9) | 20 (21%) | 14 (19%) | 1.2(0.5,2.6) |
| Abdominal Circumference (cm±SD) | 74.7 ± 15.1 | 75.0 ± 12.7 | −0.45 (−6.3,5.4) | 73.5 ± 13.6 | 75.3 ± 14.9 | −1.5(−5.9,2.9) |
ELBW, extremely low birth weight; NBW, normal birth weight
Postnatal age of NBW children at 8 years was 9.0 ± 0.9 for boys and 9.4 ± 0.8 for girls and at 14 years was 14.6 ± 0.7 for boys and 14.9 ± 0.7 for girls.
Extremely low birth weight minus normal birth weight
Adjusted difference in means or adjusted Odds Ratio, when adjusting for race and socioeconomic status.
<0.05;
<0.01;
<0.001
NA, not applicable as no NBW children had measures less than −2 SD
Longitudinal Correlates of Growth
The univariate perinatal and neonatal correlates of the weight and BMI z scores were significant up to 8 years, the majority only up to 20 months. They included preeclampsia, birth weight z score, small for gestational age (SGA) status, gestational age, total number of neonatal complications, and duration of hyperalimentation (Supplemental Tables S2 and S3 (online)). The predictors of the height z scores were similar (data not shown).
In the hierarchical multiple regression analyses (Table 4), birth weight z score was positively, and duration of hyperalimentation was negatively associated with ZWT at 40 weeks. Birth weight z score, gestational age, and change in ZWT from 40 weeks to 8 months’ conceptual age (CA), along with maternal BMI and the interaction of maternal BMI and gender, were associated with ZWT at 8 months. Maternal BMI was positively associated with 8-month ZWT in girls (β = 0.048 (95% CI, 0.018 to 0.078), p = 0.002), but not in boys (β = 0.004 (95% CI, −0.024 to 0.031), p = 0.80). This relationship also held at other ages as illustrated in a plot of ZWT vs. maternal BMI by gender at age 14 years (Figure 2). Results of the regressions of ZWT at 20 months were similar to those at 8 months, except that gestational age was no longer a significant predictor. In the regression of ZWT at 8 years, maternal BMI along with changes in the child’s ZWT from 40 weeks to 8 months, and from 8 months to 8 years were all significant predictors. In addition, changes in ZWT during all three prior periods were significantly associated with ZWT at 14 years (all p’s <0.001). After adjusting for variables included in the final model, neither age of puberty, nor physical activity were significant predictors (data not shown). Hierarchical multiple regressions of the adolescent BMI z score and of abdominal circumference at 14 years were very similar to results for the 14-year ZWT (Table 5). Only 13 ELBW children of biologic mothers were obese at 14 years, precluding multivariable logistic regression modeling of obesity.
Table 4.
MULTIPLE REGRESSION ANALYSIS OF PREDICTORS OF Z-WEIGHT FROM 40 WEEKS TO 14 YEARS (N=94)a
| Independent Variables | 40 Weeks | 8 Months | 20 Months | 8 Years | 14 Years |
|---|---|---|---|---|---|
| Beta (95% CI) | Beta (95% CI) | Beta (95% CI) | Beta (95% CI) | Beta (95% CI) | |
| Demographics, Maternal BMI | |||||
| Race (Black) | 0.004 (−0.406,0.414) | −0.180 (−0.626,0.265) | −0.421 (−0.981,0.139) | 0.014 (−0.377,0.406) | 0.006 (−0.367,0.379) |
| Gender (male) | −0.084 (−0.449,0.282) | −0.558 (−1.051, −0.066)* | −0.448 (−1.074,0.179) | −0.208 (−0.554,0.139) | −0.136 (−0.470,0.20) |
| z-SES | −0.027 (−0.253,0.199) | 0.032 (−0.172,0.236) | 0.046 (−0.218,0.310) | 0.013 (−0.207,0.234) | 0.033 (−0.177,0.244) |
| Maternal BMIb | 0.011 (−0.012,0.033) | 0.048 (0.018,0.078)** | 0.060 (0.021,0.010)** | 0.056 (0.023,0.088)† | 0.065 (0.034,0.096)† |
| GenderaMaternal BMI | --- | −0.044 (−0.085, −0.004)* | −0.050 (−0.103,0.003) | −0.040 (−0.083,0.004) | −0.049 (−0.091, −0.008)* |
| GenderaRace | --- | 0.429 (−0.238,1.096) | 0.594 (−0.245,1.433) | --- | --- |
| Birth and Perinatal Factors | |||||
| Z-birth weight | 0.388 (0.240,0.536)† | 0.501 (0.325,0.676)† | 0.310 (0.137,0.483)* | --- | --- |
| Gestational age | --- | 0.120 (0.009,0.232)* | --- | --- | --- |
| Preeclampsia | --- | --- | --- | --- | --- |
| Hyperalimentationc | −0.016 (−0.023, −0.008)† | --- | --- | --- | --- |
| Neonatal risk factorsd | --- | --- | --- | --- | --- |
| Change in ZWT from birth to 40 weeks | --- | --- | --- | --- | --- |
| Intermediate Growth (change in weight z score) | |||||
| 40 weeks to 8 months | 0.595 (0.474,0.717)† | 0.655 (0.497,0.813)* | 0.565(0.436,0.694)† | 0.523 (0.397,0.649)† | |
| 8 months to 8 years | 0.732 (0.608,0.857)† | 0.622 (0.484,0.760)† | |||
| 8 years to 14 years | 0.652 (0.430,0.874)† | ||||
| R-square | 0.3911 | 0.6799 | 0.5647 | 0.7390 | 0.6857 |
Biologic mothers and their ELBW children;
p<0.05;
p<0.01;
p<0.001;
Maternal BMI was centered at its overall mean;
Duration (days);
Total number of neonatal risk factors.
Figure 2.
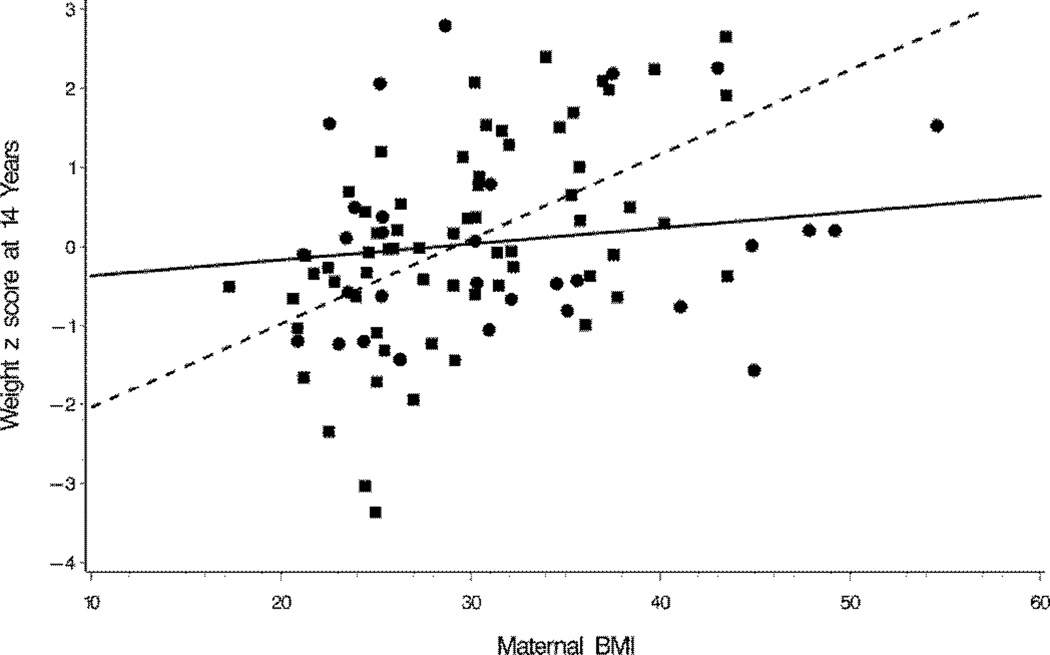
Plots of weight z scores of ELBW boys and girls at 14 years vs. maternal BMI by gender illustrating the interactive effect of gender and maternal BMI on the weight z score. Estimated regression coefficients of maternal BMI on weight z score were 0.1068 (95 CI, 0.0633 to 0.1503) for girls and 0.0202 (−0.0279 to 0.0682) for boys.
TABLE 5.
PREDICTORS OF BMI Z SCORE AND WAIST CIRCUMFERENCE AT 14 YEARS (N=95)a
| Independent Variables | BMI Z SCORE Beta (95% CI) |
Waist Circumference Beta (95% CI) |
|---|---|---|
| Demographics, Maternal BMI | ||
| Race (Black) | −0.024 (−0.332, 0.283) | −3.525 (−8.045, 0.995) |
| Gender (male) | −0.395 (−0.674, −0.115)** | −2.266 (−6.368, 1.837) |
| ZSES | 0.013 (−0.159, 0.185) | −1.217 (−3.748, 1.314) |
| Maternal b | 0.055 (0.028, 0.081)† | 0.754 (0.368, 1.140)† |
| Gendera Maternal BMI | −0.045 (−0.078, −0.011)** | −0.775 (−1.264, −0.286)** |
| Birth and Perinatal Factors | ||
| Z-birth weight | --- | --- |
| Gestational age | --- | --- |
| Preeclampsia | --- | --- |
| Hyperalimentation | --- | --- |
| Total neonate risk factors | --- | --- |
| Change Z-WT from birth to 40 weeks | --- | --- |
| Intermediate Growth (Change in WT z score) | ||
| 40 weeks to 8 months | 0.411 (0.305, 0.517)† | 4.245 (2.692, 5.798)† |
| 8 months to 8 years | 0.537 (0.424, 0.649)† | 5.934 (4.284, 7.585)† |
| 8 years to 14 years | 0.616 (0.433, 0.799)† | 6.286 (3.597, 8.974)† |
| R-square | 0.6931 | 0.5692 |
p<0.01
p<0.001
Biologic mothers and their ELBW children
Maternal BMI was centered at its overall mean
The findings were similar when SGA, defined either as weight < −2SD or <10th percentile for gestational age, was used in place of ZWT at birth, with the exception that gestational age was no longer a significant predictor (Supplemental Tables S4–S7 (online)).
DISCUSSION
The results of this longitudinal study reveal that neurologically normal ELBW children demonstrated accelerated growth following neonatal growth failure. Although their weight and height z scores were still lower than those of NBW controls at age 8 years, by 14 years these did not differ significantly among boys, whereas ELBW girls continued to have significantly lower weight and height z scores than their NBW peers. Both ELBW and NBW children had higher weight than height z scores. The rates of obesity, mean BMI z scores and 14-year abdominal circumference of the ELBW children, although lower, did not differ significantly from those of their NBW peers. Multivariable analyses revealed that neither intrauterine growth, as measured by birth weight z score or SGA status, nor neonatal risk factors were associated with the child’s gain in weight after 20 months’ CA. The only factors that predicted 14-year measures of obesity were the child’s weight gain during each of the periods studied and maternal BMI which affected girls more than boys.
The only study of the adolescent growth of preterm children born in the 1990s pertains to 11-year old <26-week gestation Swedish children who remained smaller than controls, but similar to our findings, did not differ in BMI (7). It is interesting that the obesity epidemic in the 1990s has been associated with an increase in the rates of obesity of both preterm and NBW children as compared to their rates of obesity reported in the 1980s (2,5,8).
Studies of predictors of growth among preterm children have pertained mainly to infancy and childhood. Correlates of reduced growth have included male gender, lower birth weight and gestation, SGA birth weight, and various neonatal complications (9–14). Similar to our results, Ehrenkranz found the duration of hyperalimentation to be inversely associated with growth, as sicker infants need hyperalimentation for longer periods (14). Smoking during pregnancy and postnatal steroid therapy were not significant predictors of growth in our univariate analyses possibly due to the fact that the effects of postnatal steroids on growth may be transient (9), and that maternal smoking affects fetal growth only in term born children (15). These factors were thus not included in our multivariable models. SES and ethnicity, known correlates of growth, were not significant predictors in our population. Self-reported physical activity was also not predictive, possibly related to the lesser physical activity of ELBW children (6).
Significant associations between parent and child growth have been reported in normal (16,17) and preterm populations (1,2,7,18,19), and similar to our findings, to be greater in girls than boys (17). Multiple factors contribute to effect of parental growth including genetic, hormonal, and shared environmental and psychosocial characteristics.
Our finding that weight gain throughout infancy and childhood is associated with 14-year measures of obesity is in agreement with the literature that there is no specific critical period of child growth that predicts later obesity (20) or its cardiovascular and metabolic sequelae (21–23). Although the ELBW children attained similar rates of obesity to their peers, their catch-up growth is of concern especially among girls whose rates of obesity increased significantly between 8 and 14 years, a finding also reported by Saigal (2). Among preterm children, rapid growth is associated with insulin sensitivity and may be a risk factor for type 2 diabetes and cardiovascular risk (24,25).
This is the first report of the sequential correlates of growth of preterm children born in the 1990s. Strengths of the study include its longitudinal design, relatively good follow-up rate, and the many risk factors considered. The rates of obesity of our NBW children and their mothers were representative of national data (5,26). We also acknowledge several limitations. Our results may have been influenced by the lower follow-up rate of ELBW boys and that participant boys had less BPD, thus lower neonatal risk, than non-participant boys. Difficulty in stretching immature sick infants may have influenced the initial length z scores, which were much lower than those of weight at birth and 40 weeks. Had we included the neurologically abnormal children, neonatal risk factors such as postnatal steroid therapy and periventricular hemorrhage, which predispose to the development of cerebral palsy, may have been predictive of the growth outcomes. Multiple births were included in the study as they did not influence longitudinal growth which was similar to that of singletons (data not presented). A further consideration is that early postnatal nutrition was less than currently advised (27), and that we lack detailed information on neonatal nutrition including breast milk. However, current modes of neonatal nutrition have not eliminated childhood growth failure, and although beneficial for brain growth (28), may contribute to future metabolic risk (29). Maternal pre-pregnancy weight and paternal weight were also lacking, although the mother’s current weight should reflect her pre-pregnancy weight and maternal effects on obesity are greater than paternal effects (17). The CDC norms are not representative of our urban, predominantly minority population, but we had a sociodemographically similar control population for comparison. We lacked measures of body composition and metabolic markers, but BMI, the measure we used, is associated with elevated body fat (30), and abdominal circumference is a proxy measure of abdominal fat mass (31).
The accelerated catch-up growth of the ELBW children and its potential associated cardiovascular and metabolic risk (32) is concerning as it may add to their high rates of chronic problems and further increase health care utilization (6). Possible intervention strategies include attempts to decrease maternal obesity and optimize diet to promote catch-up in height without promoting overweight. The latter may be very difficult as the lower height than weight z scores that we have documented reflect the notion that growth in weight may end in overshooting and obesity, whereas growth in height may be limited by a “self-stat” mechanism (33). Physical activities should also be encouraged despite the respiratory and subtle neurologic difficulties of ELBW children.
METHODS
Extremely Low Birth Weight Group
The birth cohort of 161 boys and 183 girls was admitted to Rainbow Babies and Children’s Hospital, Cleveland, Ohio between 1992 and 1995. Thirteen children were excluded because of congenital conditions (6). Of the remaining children, 101 (65%) boys and 137 (78%) girls survived, of whom 70 (69%) boys and 111 (81%) girls were followed to age 14 years. Sixteen boys and 15 girls with cerebral palsy were excluded because of the known poor growth of neurologically abnormal children and 2 boys did not have 14-year growth measures. The study population thus included 52 boys and 96 girls who had 14-year growth measures. They did not differ from the non-participant birth cohort with the exception that fewer boys participated (51% boys vs 70% girls, p<0.01) and that the participant boys had lower rates of BPD and a shorter neonatal hospitalization.
Normal Birth Weight Children
Sixty-five NBW boys and 111 NBW girls, of the same sex, race, school and age within 3 months, were recruited at 8 years, of whom 42 boys (65%) and 73 girls (65%) were followed to 14 years, all of whom had growth measures. They did not differ in SES from the non-participants with the exception that more mothers of boys were married.
Biologic Mothers
Biologic mothers represented 116 (78%) caregivers of ELBW children and 101 (88%) caregivers of NBW children of whom 105 (91%) and 96 (83%) of mothers respectively had growth measures. Mothers of the ELBW children with growth measures were significantly younger than those who did not have growth measures, but did not differ in SES, perinatal, or their children’s neonatal risk factors. The mothers of ELBW children did not differ in weight, height or BMI from those of NBW children (Supplemental Table S8 (online)) and were also representative of national data for women age 40–59 (26).
Neonatal Care and Measures of Outcome
Neonatal care was according to practice during the 1990s. The majority of infants received parenteral nutrition (hyperalimentation) of <3 mg/kg of protein per day. Sociodemographic, perinatal, and neonatal data were documented at neonatal hospital discharge. Weight and length were measured at birth and then at 40 weeks (term date as estimated from the last menstrual period and pregnancy ultrasound, when available); at 8 and 20 months’ CA; and at 8 and 14 years’ postnatal age. The children were measured according to standard procedures. The children were weighed unclothed but lightly clothed at 14 years. To correct for this clothing, we subtracted 1.0 kg for boys and 0.5 kg for girls. Length was measured supine with a tape measure at birth; with an infantometer at 40 weeks, 8 and 20 months’ CA; and with a stadiometer after removing shoes at 8 and 20 years (Harpenden, Holtain, Crymych, UK). Maternal weight and height were similarly measured. The children’s abdominal circumference, a proxy for visceral fat, was measured at 14 years according to the NHANES procedure (34).
Weight z scores were computed at birth and 40 weeks using standards which exclude infants delivered for maternal and fetal indications, many complicated with intrauterine growth failure (35). Length z scores at birth were computed according to Usher (36). At 8 and 20 months’ CA and at 8 and 14 years, weight and height z scores were computed from the Center for Disease Control and Prevention (CDC) growth data (37). The CDC BMI norms (z scores) are only available from age 24 months. We could thus only calculate the BMI z scores of the ELBW cohort at 8 and 14 years. BMI was thus computed at 8 and 14 years and obesity defined as BMI ≥95th percentile. Catch-up growth was defined as an increase in weight or height z score (SD) of >0.67 (i.e., crossing of percentiles) (38). Additional 14-year measures included the adolescent self-report of physical activity during the last 4 weeks (39) and pubertal development (40).
The study was approved by the Institutional Review Board of University Hospitals Case Medical Center, Cleveland, Ohio. Written consent was obtained from parents and assent from children.
Data analysis
Within the ELBW cohort, we examined gender specific growth parameters at each age, changes in z scores between each age studied, and rates of catch-up growth. The 8- and 14-year growth measures of the ELBW and NBW children were compared using two sample t-tests after adjusting for race and z-SES.
Correlates of growth were considered only for children of biologic mothers with growth measures (16). Pearson correlation coefficients calculated at each age studied included maternal education, race, and z-SES, defined as a composite of the sample z score for maternal education and family income (6). Perinatal data included a history of smoking during pregnancy, preeclampsia, antenatal steroid therapy, birth weight z score, gestational age, SGA, considered both as birth weight < −2SD and as <the 10th percentile for gestational age, and multiple birth. Neonatal risk factors included the rates of BPD, sepsis (positive blood culture), severe cerebral ultrasound abnormality, necrotizing enterocolitis, the total number of these neonatal complications, the duration of parenteral nutrition (hyperalimentation), duration of hospitalization, and postnatal steroid therapy. Maternal growth correlates considered included weight, height, and BMI.
A hierarchical multiple regression approach was used to examine risk factors related to ZWT at 40 weeks, 8 and 20 months’ corrected age and 8 and 14 years post natal age. ZWT, rather than BMI, was used for these longitudinal analyses as the CDC norms for BMI are only available from age 24 months (37). In the first and all stages, sociodemographic factors (z-SES, race, gender) and maternal BMI were forced into the models. In the first stage, interactions of gender with the other factors were tested. In the second stage, factors forced in or found to be statistically significant (p<0.05) in the first stage were included and perinatal factors associated with intrauterine and/or postnatal growth and the change in weight z score from birth to 40 weeks were then examined using stepwise regression, retaining those factors significant at p<0.05. Interactions of birth and perinatal factors with gender were also examined in stage 2 and included if found significant (p<0.05). The third stage, carried out only when examining ZWT at 8 and 14 years, included terms retained in stages 1 and 2, and examined changes in ZWT from birth to 40 weeks, 40 weeks to 8 months, 8 months to 8 years, and 8 to 14 years (when examining ZWT at 14 years) using stepwise regression. Interactions of predictors found significant in stage 3 with gender were also examined. The maternal BMI*gender interaction was significant in modeling ZWT at 8 and 20 months and 14 years, and bordered on significance in modeling these scores at 8 years (p=0.07); hence, this term was also included in the final 8-year model. Age of puberty and physical activity were each examined by testing whether they added significantly to the final model. A similar approach was used at 14 years to examine predictors of the child’s BMI z score, rates of obesity and abdominal circumference. In addition, in separate analyses we examined the effect of SGA birth rather than ZWT on the longitudinal growth. All the analyses included only subjects with no missing covariates and growth measured at all-time points (n=94 ZWT, n=95 for BMI z score).
Supplementary Material
ACKNOWLEDGEMENTS
Dr. Hack supervised the study, had full access to all of the data in the study, and takes responsibility for the integrity of the data and the accuracy of the data analysis.
Drs. Hack, Taylor, and Schluchter developed the study concept and design.
Drs. Hack and Andreias acquired the data.
Drs. Hack, Schluchter, Taylor, Cuttler, Andreias, and Ms. Margevicius participated in the analysis, including statistical analysis, and interpretation of the data.
Dr. Hack drafted the first version of the manuscript and all the co-authors participated in the critical revision of the manuscript and approve its submission for publication.
We thank Kathy Winter who coordinated the project and participated in the interview of the parents; Ellen Durand MA and Heather Marcinick MA, research assistants, who tested the children, administered the questionnaires and measured the subjects; Bonnie Tarantino BA who provided clerical assistance; and Bonnie Siner RN who provided editorial assistance.
Statement of Financial Support:
This study was supported by grants R01 HD 39756, M01 RR000, and ULI RR024989 from the U.S. National Institutes of Health (Bethesda, MD).
Footnotes
DISCLOSURES: None of the authors have any conflicts of interest.
Category of Study: Population Study
REFERENCES
- 1.Hack M, Schluchter M, Cartar L, Rahman M, Cuttler L, Borawski E. Growth of very low birth weight infants to age 20 years. Pediatrics. 2003;112:e30–e38. doi: 10.1542/peds.112.1.e30. [DOI] [PubMed] [Google Scholar]
- 2.Saigal S, Stoskopf B, Streiner D, Paneth N, Pinelli J, Boyle M. Growth trajectories of extremely low birth weight infants from birth to young adulthood: A longitudinal, population-based study. Pediatr Res. 2006;60:751–758. doi: 10.1203/01.pdr.0000246201.93662.8e. [DOI] [PubMed] [Google Scholar]
- 3.Lucas A. Long-term programming effects of early nutrition - Implications for the preterm infant. J Perinat. 2005;25:S2–S6. doi: 10.1038/sj.jp.7211308. [DOI] [PubMed] [Google Scholar]
- 4.Hales CN, Ozanne SE. The dangerous road of catch-up growth. J Physiol. 2003;547:5–10. doi: 10.1113/jphysiol.2002.024406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Ogden CL, Carroll MD, Kit BK, Flegal KM. Prevalence of obesity and trends in body mass index among US children and adolescents, 1999–2010. JAMA. 2012;307:483–490. doi: 10.1001/jama.2012.40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Hack M, Schluchter M, Andreias L, et al. Change in prevalence of chronic conditions between childhood and adolescence among extremely low-birth-weight children. JAMA. 2011;306:394–401. doi: 10.1001/jama.2011.1025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Farooqi A, Hagglof B, Sedin G, Gothefors L, Serenius F. Growth in 10- to 12-year-old children born at 23 to 25 weeks’ gestation in the 1990’s: a Swedish national prospective follow-up study. Pediatrics. 2006;118:e1452–e1465. doi: 10.1542/peds.2006-1069. [DOI] [PubMed] [Google Scholar]
- 8.Ogden CL, Fryar CD, Carroll MD, Flegal KM. Mean body weight, height, and body mass index, United states 1960–2002. Adv Data. 347:1–17. [PubMed] [Google Scholar]
- 9.Bracewell MA, Hennessy EM, Wolke D, Marlow N. The EPICure study: growth and blood pressure at 6 years of age following extremely preterm birth. Arch Dis Child Fetal Neonatal Ed. 2008;93:F108–F114. doi: 10.1136/adc.2007.118596. [DOI] [PubMed] [Google Scholar]
- 10.Dusick AM, Poindexter BB, Ehrenkranz RA, Lemons JA. Growth failure in the preterm infant: can we catch up? Semin Perinatol. 2003;27:302–310. doi: 10.1016/s0146-0005(03)00044-2. [DOI] [PubMed] [Google Scholar]
- 11.Clark RH, Thomas P, Peabody J. Extrauterine growth restriction remains a serious problem in prematurely born neonates. Pediatrics. 2003;111:986–990. doi: 10.1542/peds.111.5.986. [DOI] [PubMed] [Google Scholar]
- 12.Rijken M, Wit JM, Le Cessie S, Veen S. The effect of perinatal risk factors on growth in very preterm infants at 2 years of age. The Leiden follow-up project on prematurity. Early Hum Dev. 2007;83:527–534. doi: 10.1016/j.earlhumdev.2006.10.002. [DOI] [PubMed] [Google Scholar]
- 13.Cole CR, Hansen NI, Higgins RD, Ziegler TR, Stoll BJ for the Eunice Kennedy Shriver NICHD Neonatal Research Network. Very low birth weight preterm infants with surgical short gut syndrome: incidence, morbidity and mortality, and growth outcomes at 18–22 months. Pediatrics. 2008;122:e573–e582. doi: 10.1542/peds.2007-3449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Ehrenkranz RA, Younes N, Lemons JA, et al. Longitudinal growth of hospitalized very low birth weight infants. Pediatrics. 1999;104:280–289. doi: 10.1542/peds.104.2.280. [DOI] [PubMed] [Google Scholar]
- 15.Kayemba-Kay’s S, Ribrault A, Burguet A, et al. Maternal smoking during pregnancy and fetal growth. Effects in preterm infants of gestational age less than 33 weeks. Swiss Med Wkly. 2010;140:w13139. doi: 10.4414/smw.2010.13139. [DOI] [PubMed] [Google Scholar]
- 16.Whitaker RC, Wright JA, Pepe MS, Seidel KD, Dietz WH. Predicting obesity in young adulthood from childhood and parental obesity. N Eng J Med. 1997;337:869–873. doi: 10.1056/NEJM199709253371301. [DOI] [PubMed] [Google Scholar]
- 17.Hesketh K, Wake M, Waters E, Carlin J, Crawford D. Stability of body mass index in Australian children: a prospective cohort study across the middle childhood years. Public Health Nutr. 2003;7:303–309. doi: 10.1079/phn2003537. [DOI] [PubMed] [Google Scholar]
- 18.Casey PH, Bradley RH, Whiteside-Mansell L, Barrett K, Gossett JM, Simpson PM. Evolution of obesity in a low birth weight cohort. J Perinatol. 2012;32:91–96. doi: 10.1038/jp.2011.75. [DOI] [PubMed] [Google Scholar]
- 19.Doyle LW, Faber B, Callanan C, Ford GW, Davis NM. Extremely low birth weight and body size in early adulthood. Arch Dis Child. 2004;89:347–350. doi: 10.1136/adc.2002.025924. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Parsons TJ, Power C, Manor O. Fetal and early life growth and body mass index from birth to early adulthood in 1958 British cohort: longitudinal study. BMJ. 2001;323:1331–1335. doi: 10.1136/bmj.323.7325.1331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Barker DJ, Osmond C, Forsen TJ, Kajantie E, Eriksson JG. Trajectories of growth among children who have coronary events as adults. N Engl J Med. 2005;353:1802–1809. doi: 10.1056/NEJMoa044160. [DOI] [PubMed] [Google Scholar]
- 22.Bhargava SK, Sachdev HS, Fall CH, et al. Relation of serial changes in childhood body-mass index to impaired glucose tolerance in young adulthood. N Engl J Med. 2004;350:865–875. doi: 10.1056/NEJMoa035698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Forsen T, Eriksson J, Tuomilehto J, Reunanen A, Osmond C, Barker D. The fetal and childhood growth of persons who develop type 2 diabetes. Ann Intern Med. 2000;133:176–182. doi: 10.7326/0003-4819-133-3-200008010-00008. [DOI] [PubMed] [Google Scholar]
- 24.Fewtrell MS, Doherty C, Cole TJ, Stafford M, Hales CN, Lucas A. Effects of size at birth, gestational age and early growth in preterm infants on glucose and insulin concentrations at 9–12 years. Diabetologia. 2000;43:714–717. doi: 10.1007/s001250051368. [DOI] [PubMed] [Google Scholar]
- 25.Rotteveel J, van Weissenbruch MM, Twisk JWR, Delemarre-Van de Waal HA. Infant and childhood growth patterns, insulin sensitivity, and blood pressure in prematurely born young adults. Pediatrics. 2008;122:313–321. doi: 10.1542/peds.2007-2012. [DOI] [PubMed] [Google Scholar]
- 26.Flegal KM, Carroll MD, Kit BK, Ogden CL. Prevalence of obesity and trends in the distribution of body mass index among US adults, 1999–2010. JAMA. 2012;307:491–497. doi: 10.1001/jama.2012.39. [DOI] [PubMed] [Google Scholar]
- 27.Hay WW. Strategies for feeding the preterm infant. Neonatology. 2008;94:245–254. doi: 10.1159/000151643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Poindexter BB, Langer JC, Dusick AM, Ehrenkranz RA. Early provision of parenteral amino acids in extremely low birth weight infants: relation to growth and neurodevelopmental outcome. J Pediatr. 2006;148:300–305. doi: 10.1016/j.jpeds.2005.10.038. [DOI] [PubMed] [Google Scholar]
- 29.Wiedmeier JE, Joss-Moore LA, Lane RH, Neu J. Early postnatal nutrition and programming of the preterm neonate. Nutr Rev. 2011;69:76–82. doi: 10.1111/j.1753-4887.2010.00370.x. [DOI] [PubMed] [Google Scholar]
- 30.Freedman DS, Wang J, Thornton JC, et al. Classification of body fatness by body mass index-for-age categories among children. Arch Pediatr Adolesc Med. 2009;163:805–811. doi: 10.1001/archpediatrics.2009.104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Klein S, Allison DB, Heymsfield SB, et al. Waist circumference and cardiometabolic risk: a consensus statement from Shaping America’s Health: Association for Weight Management and Obesity Prevention; NAASO, the Obesity Society; the American Society for Nutrition; the American Diabetes Association. Am J Clin Nutr. 2007;85:1197–1202. doi: 10.1093/ajcn/85.5.1197. [DOI] [PubMed] [Google Scholar]
- 32.Hovi P, Sture A, Eriksson JG, et al. Glucose regulation in young adults with very low birth weight. N Engl J Med. 2007;356:2053–2063. doi: 10.1056/NEJMoa067187. [DOI] [PubMed] [Google Scholar]
- 33.Kayemba-Kay’s S, Hindmarsh PC. Catch-up growth: an overview. Pediatr Endocrinol Rev (PER) 2006;3:365–378. [PubMed] [Google Scholar]
- 34.Li C, Ford ES, Mokdad AH, Cook S. Recent trends in waist circumference and waist-height ratio among US children and adolescents. Pediatrics. 2006;118:e1390–e1398. doi: 10.1542/peds.2006-1062. [DOI] [PubMed] [Google Scholar]
- 35.Yudkin PL, Aboualfa M, Eyre JA, Redman CW, Wilkinson AR. New birth weight and head circumference centiles for gestational ages 24 to 42 weeks. Early Human Dev. 1987;15:45–52. doi: 10.1016/0378-3782(87)90099-5. [DOI] [PubMed] [Google Scholar]
- 36.Usher R, McLean F. Intrauterine growth of live-born Caucasian infants at sea level: standards obtained from measurements in 7 dimensions of infants born between 25 and 44 weeks of gestation. Pediatrics. 1969;74:901–910. doi: 10.1016/s0022-3476(69)80224-6. [DOI] [PubMed] [Google Scholar]
- 37.Kuczmarski RJ, Ogden CL, Grummer-Strawn LM, et al. CDC growth charts: United States. Adv Data. 2000;314:1–27. [PubMed] [Google Scholar]
- 38.Ong KK, Ahmed ML, Emmett PM, Preece MA, Dunger DB. Association between postnatal catch-up growth and obesity in childhood: prospective cohort study. BMJ. 2000;320:967–971. doi: 10.1136/bmj.320.7240.967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Starfield B, Riley AW, Green BF, et al. The Adolescent Child Health and Wellness Profile. A population-based measure of health. Med Care. 1995;33:553–566. doi: 10.1097/00005650-199505000-00008. [DOI] [PubMed] [Google Scholar]
- 40.Taylor SJ, Whincup PH, Hindmarsh PC, Lampe F, Odoki K, Cook DG. Performance of a new pubertal self-assessment questionnaire: a preliminary study. Paediatr Perinatol Epidemiol. 2001;15:88–94. doi: 10.1046/j.1365-3016.2001.00317.x. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


