Abstract
Post-implant device thrombosis remains a life-threatening complication and limitation of continuous-flow ventricular assist devices (VADs). Utilizing advanced CFD simulations, we successfully depicted various flow patterns, recirculation zones and stagnant platelet trajectories which promote thrombus formation and observed that they matched actual thrombus formation patterns observed in Thoratec© HeartMate II™ VADs explanted from patients with pump thrombosis. Previously, these small eddies could not be captured by either DPIV or CFD due to insufficient resolution. Our study successfully demonstrated the potential capability of advanced CFD to be adopted for device optimization, leading to enhanced safety and efficacy of VADs for long-term destination therapy.
Keywords: Thrombosis, CFD, HeartMate II, VAD
Introduction
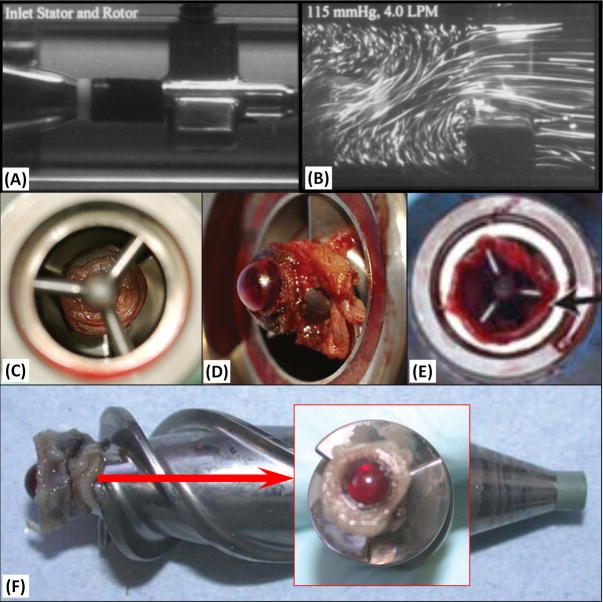
The utilization of ventricular assist devices (VADs) as a means of stabilizing congestive heart failure (CHF) patients as a bridge-to-transplant has increased dramatically over the past few years.1 The HeartMate II™ (HMII, Thoratec Corp., Pleasanton, CA) VAD is currently the most widely implanted VAD, with more than 10,000 implants worldwide.2 With the increasing numbers of CHF patients and the growing experience gained with extended use of this device, the FDA recently approved the HMII for destination therapy.3 Rotary VADs offer the advantages of smaller dimension and simpler structures as compared to pulsatile VADs; however the continuous, high speed, rotating blood flow patterns generated are a potential risk factor for adverse events, including thrombus formation, thromboembolic complications and device malfunction. Pump thrombosis is one of the main causes for device malfunction, and patients are exposed to the risk of sudden death or the risks involved in complex device replacement surgery.4,5 In recent years various cases of pump thrombosis in patients implanted with the HMII were reported in the clinical literatures, despite the anticoagulation regimens mandated for device recipients. The incidence reported was approximately 6%, with some cases being fatal.6,7 Specifically, since the FDA has approved the HMII for bridge-to-transplant and for destination therapy in 2008 and 2010, respectively, the incidence of pump thrombosis has grown steadily.4-7 In most of these cases, thrombus formation was observed at the flow-straightener and the rear hub bearing (between the flow-straightener and the impeller of the device). Typical cases are shown in explanted devices (Figure 2 (C)8, (D)9, (E)10 & (F)11).
Figure 2. Clinical Observations of Thrombus Formation in HMII.
(A & B) Previous DPIV results along the flow-straightener of HMII: (A) The geometry of HMII flow straightener. (B) The velocity flow field along the flow straightener obtained with DPIV.12 Thrombus formation was reported at the regions of the rear hub (i.e. the bearing between the flow-straightener and the impeller) and the entry of the impeller blades very similar to those observed in our study (C8, D9 & F11). The thrombus extended along the flow-straightener blades as has been reported clinically (E).10
Thrombus formation in blood recirculating devices is highly correlated to irregular flow patterns formed within the device. Various methods such as digital particle image velocimetry (DPIV)12 and computational fluid dynamic (CFD)13 had been employed for visualizing or predicting the streamlines of blood flow through these devices. Thrombus formation arises from the combined effect of elevated shear stress levels and recirculating flow patterns in specific regions within a device. Advanced CFD methodology which was developed by our group and refined over the years combined with recently developed algorithms tuned for capturing thrombus formation patterns enable us to predict whether platelets may be driven beyond their activation threshold and identify potential thrombus formation regions. Briefly, this advanced CFD approach offers the ability to compute the stress levels that blood constituents (e.g. RBCs or platelets) are exposed to while flowing through these pathological flow patterns, and estimate the thrombogenicity which may lead to thrombus formation within the device. This is achieved by computing the trajectories of multiple particles and the dynamic stresses they are exposed to within the device flow field. A detailed description of the methodology – applied to the optimization of a VAD, appears in our recent PLoS One publication.14 In the present study, we utilized advanced CFD simulations to predict the stress exposure and flow trajectories of platelets flowing through the HMII VAD and leading to observed thrombus formation locations within the device.
Methods
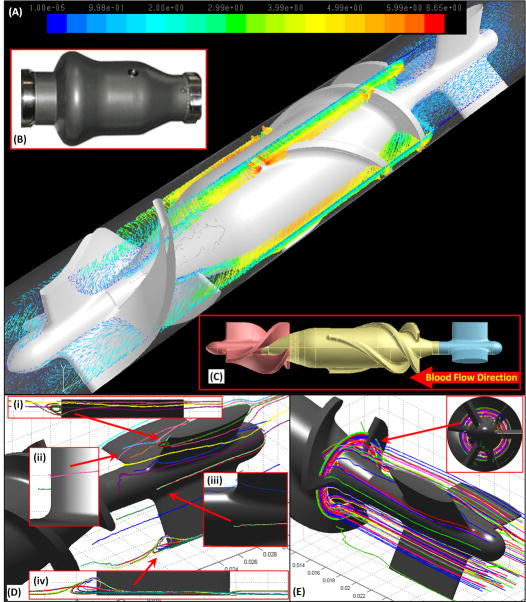
The Fluent CFD solver (Ansys Fluent Inc., Lebanon, NH) was utilized for conducting highly resolved mesh numerical simulations of multi-phase FSI (Fluid Structure Interaction)URANS (Unsteady Reynolds Averaged Navier-Stokes) blood flow using the two equation k-ω turbulence model.14 Blood was modeled as a two-phase Newtonian fluid with viscosity of 0.0035 kg/m-s and density of 1,081 kg/m3, with platelets assumed as neutrally buoyant solid spherical particles (ø = 3 μm; density: 998.2 kg/m3). The HMII VAD components (Figure 1B) include the inlet flow-straightener, rear hub (the bearing connecting the stationary flow-straightener and the impeller), impeller, front hub (the bearing connecting the impeller and the stationary diffuser), and the diffuser. In order to simulate the clinical operating condition of the HMII, the impeller spins at 10,000 rpm generating a corresponding cardiac output of 4 L/min. Sliding mesh was employed for the impeller, and the mass flow inlet and pressure outlet were applied as the inlet and outlet boundary conditions, respectively. After conducting mesh independence studies, the highly resolved mesh consisted of 17 ×106 tetrahedral volumetric elements, and an optimized time step of 7 ×10-5 s was carefully selected.14 Approx. 30,000 platelets were seeded and released from a plane located upstream of the VAD. An in-house code was developed to compute and analyze these platelet trajectories with the aim to track the stagnant or entrapped trajectories and the recirculation zones. Approx.90% of the simulated platelets had a residence time of less than 0.184 sec while flowing through the device.
Figure 1. Numerical Prediction of Thrombus Formation Patterns in HMII.
(A) Freeze frame depiction of the velocity vector field of the HMII simulation (4 L/min of cardiac output with the impeller speed of 10,000 rpm). Exterior (B) and interior (C) features of HMII: flow-straightener, impeller and diffuser (blue, yellow and pink, accordingly). (D & E) Stagnant platelet trajectories are observed along the posterior side of the flow-straightener and the entry of the impeller blades is characterized by stagnant flow patterns; (D: inset i, ii & iii) recirculation zones were observed at the downstream of the flow straightener blades spanning approximately 1-2 mm. (D: inset iv) Platelet trajectories indicate a stagnation zone at the rear hub, and (E) Entrapped circular flow patterns at the entry towards the impeller blades.
Results
Several platelet trajectories depicted the formation of well-defined recirculation zones that spanned approx. 1-2 mm across at the downstream of the flow-straightener blades (Figure 1C; insets i & iv), while other platelet trajectories formed stagnant flow patterns, attaching to the rear hub (Figure 1D; inset ii & iii). Some platelet trajectories were observed to form entrapped circular patterns at the entry of the impeller blades, closely following the rotational motion of impeller (Figure 1E).
These flow patterns correspond geographically to the sites of the thrombus formation that have been directly observed clinically and in analysis of malfunctioning explanted HMII devices (e.g. between rear hub region and entry of impeller blades, Figure 2C8, D9 & F11, and the flow-straightener blades, Figure 2E10). Previous PDIV12 and CFD13 studies of HMII lacked the resolution to capture these small eddies and the stagnant trajectories at the rear hub (Figure 2A & B)12.
Discussion and Conclusion
Pump thrombosis has been reported in approx. 6% of continuous flow VAD recipients regardless of the mandatory long-term anticoagulation/antiplatelet therapy utilized.7 The present study demonstrates that flow patterns and ensuing platelet trajectories (a time history depicting the trajectory/pathline) are strongly associated with the thrombus formation patterns observed in explanted VADs. The number of simulated platelets(approx. 30,000 platelets released from single surface)was chosen to correspond to a typical physiological platelet count(i.e. 150,000 platelets/μL) for a single passage flowing through the device. Although the platelets which ended up in the stagnant and entrapped trajectories and the recirculation zones were only a small fraction of the platelet population, those represent a single passage through the VAD. As these VADs generate continuous flow, repeated passages through the same platelet trajectories may eventually accumulate at these regions and promote thrombus formation.
Our study clearly indicates that flow-induced device thrombogenicity resulting from certain geometric device characteristics may promote thrombus formation, leading to device malfunction. We have previously performed an optimization study in a similar VAD, in which the thrombogenic potential of the device was reduced by an order of magnitude.14 With the progressive rise of LVAD implantation for destination therapy, device optimization could be achieved by utilizing advanced numerical approaches to eliminate the undesired flow patterns that may lead to thrombus formation in a specific device such as those found in the HMII. Utilizing this suggested strategy will further enhance the safety and efficacy of VADs for long-term destination therapy.
Acknowledgments
This was funded by NIH NIBIB Quantum Award Implementation Phase II, 1U01EB012487-0, DB.
Footnotes
Disclosure Statement:
This paper is not under consideration elsewhere.
None of the paper’s contents have been previously published.
All authors have read and approved the manuscript.
None of the authors has a conflict of interest to declare.
References
- 1.Kirklin JK, et al. Second INTERMACS annual report: more than 1,000 primary left ventricular assist device implants. J Heart Lung Transplant. 2010;29:1–10. doi: 10.1016/j.healun.2009.10.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Yamakawa M, et al. Destination therapy: the new gold standard treatment for heart failure patients with left ventricular assist devices. Gen Thorac Cardiovasc Surg. 2013;61:111–117. doi: 10.1007/s11748-012-0181-5. [DOI] [PubMed] [Google Scholar]
- 3.Slaughter MS. Long-term continuous flow left ventricular assist device support and end-organ function: prospects for destination therapy. J Card Surg. 2010;25:490–494. doi: 10.1111/j.1540-8191.2010.01075.x. [DOI] [PubMed] [Google Scholar]
- 4.Kirklin JK, et al. The Fourth INTERMACS Annual Report 4,000 implants and counting. J Heart Lung Transplant. 2012;31:117–126. doi: 10.1016/j.healun.2011.12.001. [DOI] [PubMed] [Google Scholar]
- 5.Slaughter MS, et al. Advanced heart failure treated with continuous-flow left ventricular assist device. N Engl J Med. 2009;361:2241–2251. doi: 10.1056/NEJMoa0909938. [DOI] [PubMed] [Google Scholar]
- 6.Pagani FD, et al. Extended mechanical circulatory support with a continuous-flow rotary left ventricular assist device. J Am Coll Cardiol. 2009;54:312–321. doi: 10.1016/j.jacc.2009.03.055. [DOI] [PubMed] [Google Scholar]
- 7.Park SJ, et al. Outcomes in advanced heart failure patients with left ventricular assist devices for destination therapy. Circ Heart Fail. 2012;5:241–248. doi: 10.1161/CIRCHEARTFAILURE.111.963991. [DOI] [PubMed] [Google Scholar]
- 8.Meyer AL, et al. Thrombus formation in a HeartMate II left ventricular assist device. The Journal of thoracic and cardiovascular surgery. 2008;135:203–204. doi: 10.1016/j.jtcvs.2007.08.048. [DOI] [PubMed] [Google Scholar]
- 9.Mokadam NA, Andrus S, Ungerleider A. Thrombus formation in a HeartMate II. Eur J Cardiothorac Surg. 2011;39:414. doi: 10.1016/j.ejcts.2010.06.015. [DOI] [PubMed] [Google Scholar]
- 10.Najib MQ, Wong RK, Pierce CN, DeValeria PA, Chaliki HP. An unusual presentation of left ventricular assist device thrombus. Eur Heart J Cardiovasc Imaging. 2012;13:532. doi: 10.1093/ehjci/jes011. [DOI] [PubMed] [Google Scholar]
- 11.Capoccia M, Bowles CT, Sabashnikov A, Simon A. Recurrent Early Thrombus Formation in HeartMate II Left Ventricular Assist Device. Journal of Investigative Medicine High Impact Case Reports. 2013;1:3. doi: 10.1177/2324709613490676. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Griffith BP, et al. HeartMate II left ventricular assist system: from concept to first clinical use. Ann Thorac Surg. 2001;71:S116–120. doi: 10.1016/s0003-4975(00)02639-4. discussion S114-116. [DOI] [PubMed] [Google Scholar]
- 13.Burgreen GW, Antaki JF, Griffith BP. A design improvement strategy for axial blood pumps using computational fluid dynamics. ASAIO J. 1996;42:M354–360. doi: 10.1097/00002480-199609000-00010. [DOI] [PubMed] [Google Scholar]
- 14.Girdhar G, et al. Device thrombogenicity emulation: a novel method for optimizing mechanical circulatory support device thromboresistance. PLoS One. 2012;7:e32463. doi: 10.1371/journal.pone.0032463. [DOI] [PMC free article] [PubMed] [Google Scholar]