Abstract
Fresh cadaveric human tissue is a valuable resource that is used to address important clinical questions. However, it is unknown how post-mortem time impacts skeletal muscle mechanical and biochemical properties. We simulated morgue conditions in rabbits and tested the passive mechanical properties of muscle bundles, and the degradation of myosin heavy chain, collagen, and titin at specific intervals up to 7 days post-mortem. While a great deal of inter-specimen variability was observed, it was independent of post-mortem time. Passive mechanics, myosin heavy chain, and collagen content were all unaffected while the titin protein degraded up to 80% over 7 days postmortem. These data indicate that fresh cadaveric tissue may be used for passive mechanical testing and that certain biochemical properties are unchanged up to 7 days after death.
Keywords: muscle, titin, collagen, myosin heavy chain
Introduction
Procuring human tissue for mechanical and biochemical research has become increasingly important in order to answer clinically relevant questions. Restrictions in the use of primates in research have made human tissue even more valuable. Typically, human muscle tissue is obtained during a surgical procedure or through cadaveric donation programs. It is particularly difficult to obtain “normal” human muscle since muscle obtained in a surgical setting is often compromised due to disuse or pathology. Often, muscles that may be of interest are not physically accessible during surgery. Cadavers provide a valuable alternative source of human muscle tissue, but if the cadaver is fixed, the tissue is unusable for many biomechanical and biochemical tests. Fresh cadaver tissue thus often represents the best option, but it is unknown how the mechanical and biochemical properties of skeletal muscle are altered with time postmortem. The purpose of this study was to measure changes in passive mechanics of skeletal muscle bundles, titin degradation, myosin heavy chain composition, and hydroxyproline content over 7 days post-mortem using rabbit muscle under simulated morgue conditions.
Methods
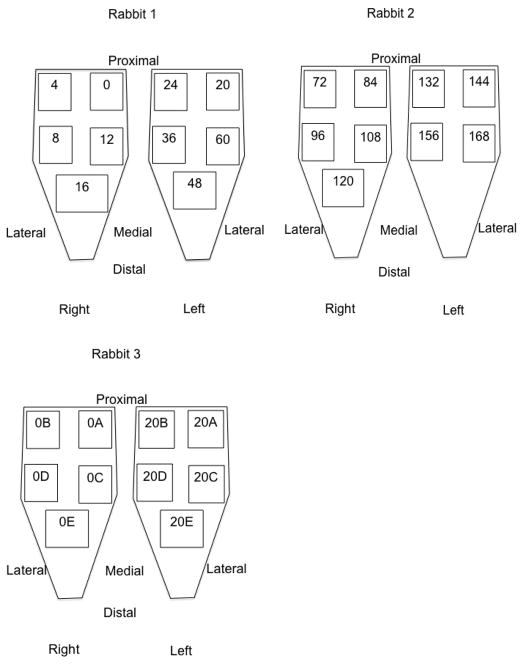
Three New Zealand White rabbits (Oryctolagus cuniculus) were anesthetized with a subcutaneous injection of a ketamine-xylazine cocktail (50 and 5 mg/kg body mass, respectively). Animals were euthanized with pentobarbital (Euthasol; Virbac AH, Fort Worth, TX) and then stored at a constant temperature of −4 degrees Celsius for the remainder of the study. Biopsies (approximately 2cm × 1cm × 0.5cm) were obtained from the tibialis anterior muscle of each rabbit (N=6) immediately post-mortem and then every 4 hours for the first 24 hours and then every 12 hours up to 7 days post-mortem. Tissue was placed immediately into a glycerinated storage solution and refrigerated at −20 degrees Celsius until further testing (Friden and Lieber, 2003). Additional biopsies were obtained at the initial time point and at 20 hours post-mortem to specifically test for proposed rigor effects. Unilateral tibialis anterior muscle was biopsied sequentially before samples were taken from the contralateral leg (Figure 1) in order to minimize the effects of skin incision and tissue exposure. Muscle was covered by the overlying skin as much as possible between biopsies in order to minimize tissue drying.
Figure 1.
Schematic representation of sampling procedures. Numbers indicate hours post-mortem.
Passive Mechanics
Passive mechanics of muscle bundles was performed using previously reported methods (Fridén and Lieber, 2003). Briefly, biopsies were removed from storage solution and placed in relaxing solution. Muscle bundles (approximately 10 muscle fibers per bundle) were dissected from the biopsy (N=3 bundles per biopsy) and secured in a custom apparatus using 10-0 monofilament nylon suture on one end to a force transducer and on the other end to a titanium wire rigidly attached to a rotational bearing. Sarcomere length was measured by laser diffraction (Lieber et al. 1984). The bundle was brought to slack length and bundle dimensions were measured with a cross-hair reticule mounted on a dissecting microscope and micromanipulators. The bundle was deformed to strains of approximately 0.25 μm sarcomere−1 at 100 fiber lengths s−1. Each stretch was held for 3 min during which stress relaxation was measured (Fridén and Lieber, 2003). Force was converted to stress by dividing force by the baseline cross-sectional area determined assuming a cylindrical sample with an average diameter determined from three separate points along the bundle. Tangent stiffness of the nonlinear fit to the stress-sarcomere length relationship (in units of kPa/μm) at sarcomere length 3.5μm is reported.
Titin Degradation
Titin molecular mass was determined from muscle bundles tested for passive mechanics using SDS-VAGE as previously described (Warren et al., 2003). Relative mobility and intensity of each band was quantified using a GS-800 Calibrated Densitometer and Quantity One 1-D Analysis software. Relative mobility of protein on the gel is linearly related to the log of its molecular mass; this relationship was used to calculate molecular mass of titin based on its positions relative to rat cardiac and human soleus titin standards. As titin degrades, T1 and T2 bands appear, with the T2 band representing a degradation product (Ali et. al, 2010). Thus, titin results are expressed in units of %T1 where 100% represents no degradation.
Collagen Content
Hydroxyproline content was used to determine collagen percentage using a modification of a previously validated protocol (Edwards and O’Brien 1980). A small tissue sample (2 mg) was taken from each muscle biopsy and hydrolyzed in 6N HCl at 110°C for 24 hours. Samples were then pipetted with standards into 96 well plates and were incubated with a chloramine T solution for 20 minutes at room temperature, followed by the addition of a p-dimethylaminobenzaldehyde solution and incubated at 60°C for 30 minutes. Hydroxyproline concentration was determined based on the extinction coefficient of hydroxyproline at 550 nm and normalized to the mass of the original tissue sample. Values are reported as μg collagen/mg tissue.
Myosin Heavy Chain Composition
Myosin heavy chain bands were identified and quantified with densitometry as previously described (Talmadge and Roy, 1993). The progression of the bands was compared and identified based on their relative molecular weight to that of a human protein standard prepared from a normal semitendinosus biopsy that shows all three human MHC bands (types IIa, IIx and I). Values are presented as percent IIA and percent IIX.
Statistical Analysis
A two-way analysis of variance (ANOVA) was used to determine the effect of leg and biopsy location (laterality). Linear regression was used to determine if slopes were different from 0 for muscle bundle tangent stiffness, titin degradation, collagen content, and myosin heavy chain content over time.
Results and Discussion
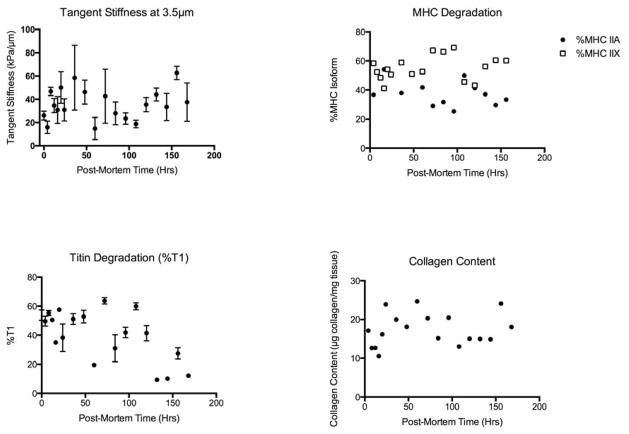
Prior to analysis of the time data, we first determined that there was no systematic difference in measures between rabbits or due to the biopsy location within the muscle (proximal, distal, medial, lateral portions of the muscle). While muscle bundle mechanics were variable (average standard deviation of 17 kPa/μm), this variability was independent of post-mortem time (Figure 2). Collagen content and myosin heavy chain content variability was also independent of post-mortem time (Figure 2). Titin degradation was significantly affected by post-mortem time (P<0.005) where it was observed that titin degraded by almost 80% by 7 days post-mortem (Figure 2).
Figure 2.
Summary data of degradation of mechanical and biochemical properties over post-mortem time. Presented as mean value with SEM.
Summary
Based on these data, we showed that muscle bundle passive mechanical properties are unaffected by post-mortem time of up to 7 days. Collagen content and myosin heavy chain proteins are also unaffected by post-mortem time of up to 7 days. Titin, as expected, was the protein most affected by post-mortem time with almost 80% protein degradation occurring by day 7. The significant decay in titin at very early time points is consistent with other reports in the literature showing a decline in titin in the first 24 hours post-mortem with preservation of myosin proteins (Bandman and Zdanis 1988). Interestingly, passive bundle mechanics were not affected by the marked titin degradation providing further evidence that bundle mechanics are dominated by the mechanical properties of the extracellular matrix rather than fiber properties (Meyer and Lieber, 2011). The loss of titin would have certainly had a large impact had the samples been tested at the myofibrillar level.
Muscle bundle mechanics were variable, but this variability was independent of postmortem time and there was no difference in tangent stiffness among rabbits and different locations of the muscle biopsy within the tibialis anterior muscle. The average standard deviation of tangent stiffness measures was 17 kPa/μm, which is consistent with previous data reported in human tissue (Regev et. al 2011). The variability should be considered in the design of future studies when determining sample size and the ability to resolve differences between study groups.
This study has limitations that should be considered. First, the biopsy locations were not selected at random, but were taken sequentially from an individual muscle before taking biopsies from the next muscle. This was necessary in order to keep muscle tissue from drying and affecting testing. Despite the lack of randomization, we found no differences in measures between biopsies taken from different locations or from different animals. A second limitation is that we tested samples from a single muscle in rabbits. There is no evidence to suggest that post-mortem time would have a greater impact on other muscles in the body than it does on the tibialis anterior, so we believe this is a reasonable representative muscle. Also, we are assuming that rabbit muscle is completely representative of mammalian muscle in general and humans in particular.
This report provides support that muscle tissue obtained from fresh cadavers may be used for passive mechanical and biochemical testing for up to 7 days, with the exception of titin which degrades more quickly than collagen and myosin heavy chain proteins. This information may allow investigators access to muscle tissue that might have been impossible to obtain via surgery or unable to be tested due to fixation.
Acknowledgments
This work was supported in part by NIH Grant R24 HD 50837 and by the Department of Veterans Affairs, Veterans Health Administration, Office of Research and Development, Senior Research Career Scientist Award.
Footnotes
Conflict of Interest Statement:
The authors have no financial or personal conflicts of interest to disclose.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Ali MA, Cho WJ, Hudson B, Kasiri Z, Granzier H, Schulz R. Titin is a target of metalloproteinase-2: implications in myocardial ischemia/reperfusion injury. Circulation. 2010;122:2039–2047. doi: 10.1161/CIRCULATIONAHA.109.930222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bandman E, Zdanis D. An immunological method to assess protein degradation in post-mortem muscle. Meat Science. 1988;22:1–19. doi: 10.1016/0309-1740(88)90023-X. [DOI] [PubMed] [Google Scholar]
- Edwards CA, O’Brien WD. Modified assay for determination of hydroxyproline in a tissue hydrolyzate. Clinica Chimica Acta. 1980;104:161–167. doi: 10.1016/0009-8981(80)90192-8. [DOI] [PubMed] [Google Scholar]
- Friden J, Lieber RL. Spastic muscle cells are shorter and stiffer than normal cells. Muscle Nerve. 2003;27:157–164. doi: 10.1002/mus.10247. [DOI] [PubMed] [Google Scholar]
- Lieber RL, Yeh Y, Baskin RJ. Sarcomere length determination using laser diffraction. Effect of beam and fiber diameter. Biophysical Journal. 1984;45:1007–1016. doi: 10.1016/S0006-3495(84)84246-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meyer GA, Lieber RL. Elucidation of extracellular matrix mechanics from muscle fibers and fiber bundles. Journal of Biomechanics. 2011;44:771–773. doi: 10.1016/j.jbiomech.2010.10.044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Regev GJ, Kim CW, Tomiya A, Lee YP, Ghofrani H, Garfin SR, Lieber RL, Ward SR. Psoas muscle architectural design, in vivo sarcomere length range, and passive tensile properties support its role as a lumbar spine stabilizer. Spine. 2011;36:E1666–1674. doi: 10.1097/BRS.0b013e31821847b3. [DOI] [PubMed] [Google Scholar]
- Talmadge RJ, Roy RR. Electrophoretic separation of rat skeletal muscle myosin heave-chain isoforms. Journal of Applied Physiology. 1993;75:2337–2340. doi: 10.1152/jappl.1993.75.5.2337. [DOI] [PubMed] [Google Scholar]
- Warren CM, Krzesinski PR, Greaser ML. Vertical agarose gel electrophoresis and electroblotting of high-molecular-weight proteins. Electrophoresis. 2003;24:1695–1702. doi: 10.1002/elps.200305392. [DOI] [PubMed] [Google Scholar]