Abstract
Haloperidol induced catalepsy was determined using the classic bar test and a new MED Associates Catalepsy Test Chamber instrument. The dose that produced an adverse effect in 50% of rats (AED50) for haloperidol was calculated using the instrument data as 0.29 mg/kg. Hand scoring of the video recordings gave AED50 values of 0.30 and 0.31 mg/kg, both well within the 95% CL of the instrument data. Clozapine was also evaluated and catalepsy was not detected up to 40 mg/kg. No significant difference was found between the instrument and hand scoring data. The instrument was useful for testing haloperidol and clozapine, relieving much of the tedium and variability experienced without its use. It was especially valuable at measuring shorter time periods, where the researcher cannot react as quickly. Finally, olanzapine was also evaluated. However, clenched forepaws and hind paws prevented the use of the instrument alone at higher doses. A backup stopwatch was used for the bar test in these cases. Some of the advantages and limitations are discussed.
Results are also compared to the crossed-legs position (CLP) test for all three antipsychotics. While haloperidol gave similar results at all concentrations tested, clozapine deviated significantly at the highest dose (40 mg/kg) displaying catalepsy in the CLP test but not in the bar test. Olanzapine displayed catalepsy in rats significantly different from vehicle at 40 mg/kg in both the bar and CLP tests. However, the CLP test may be more suited to compounds with gripping problems which prevent the consistent grasping of the bar.
Overall, the instrument was found to be a useful aid in conducting the bar test for catalepsy. The CLP test was found to complement the bar test under certain conditions and could provide additional data that might be missed by the bar test for compounds producing grasping problems.
Keywords: Catalepsy instrument, Bar test for catalepsy, Extrapyramidal side effects, Antipsychotic side effects, Haloperidol, Clozapine, Olanzapine, Crossed-leg position (CLP) test for catalepsy
1. Introduction
Results from Phase I of the Clinical Antipsychotic Trials of Intervention Effectiveness (CATIE) study indicated that 74% of schizophrenic patients discontinued the use of antipsychotic drugs within 18 months due to medication efficacy problems, intolerable side effects, or the patient’s decision (Lieberman et al., 2005). Extrapyramidal side effects (EPS) accounted for 8 % and patient decision (patient independently chose to stop treatment) accounted for 30 % of the discontinuation of low doses of the representative typical antipsychotic drug perphenazine in this study. Consequently, the development of new antipsychotics with reduced or no EPS has been a major driving force in drug development to improve patient compliance in the taking of antipsychotic medications (Aberg et al., 2010; Meltzer et al., 2003; Wadenberg, 1996).
To affect that end, the rodent catalepsy test has been developed to predict EPS in patients using antipsychotic medications and is an important component of the drug discovery process (Hoffman and Donovan, 1995). Neuroleptics producing severe EPS in patients have been found to also produce potent catalepsy in rats (Seeger et al., 1995). Most definitions of catalepsy in rodents refer to placing the animal in an unusual position and observing the time the animal remains in this position (Castagne et al., 2009; Sanberg et al., 1988; Wadenberg, 1996). For a given set of experimental and apparatus variables, the longer the rat stays in the position, the more cataleptic the response and hence the prediction of more pronounced EPS in patients taking the antipsychotic.
One of the most widely used catalepsy tests is the bar test (Ellenbroek, 1993; Sanberg et al., 1988). Typically, in this test, the forepaws of the rat are placed on a bar elevated to 10 cm for example, with the hind paws remaining on the floor. The time until descent to the floor or some other change in posture is then recorded. Unfortunately, as pointed out by Sanberg et al, 1988 and others (Ferre et al., 1990; Sanberg et al., 1996), this simple sounding test is anything but simple, with numerous variations having been used throughout the literature, making comparison of data from different laboratories difficult, especially if all the details are not defined in the article. Some of the variables include number of forepaws on the ground to define catalepsy time, height of bar, diameter of bar, shape of bar (round, rectangular, twisted wire), material used for bar (smooth steel, wood, wire), weight of animal, species of animal, cut-off times for recording catalepsy, reporting seconds in cataleptic position or converting seconds to a score, repeated testing versus one time testing, researcher interpretation of postural change, and researcher reaction time, fatigue or inattention. Automation would help simplify and standardize some of these variables.
Various prototypes for automating the measurement of catalepsy using the bar test have been described in the literature, including a timer activated by the rat with no computer storage of data (Moss et al., 1981), using an animal infrared beam activity monitoring system to record vertical motion to detect when one paw was removed from the bar (Sanberg et al., 1988) and a homemade electronic system consisting of a wooden chamber with metal floor and metal bar, Reduced Instruction Set Computer (RISC) microcontroller, Electrically Erasable Programmable Read Only Memory (EEPROM), and other electronic components interfaced to a personal computer (Alvarez-Cervera et al., 2005). Our lab has routinely utilized the bar test and the crossed legs position (CLP) test in evaluating the potential of synthetic antipsychotic agents to induce catalepsy (Bricker et al., 2012; Ablordeppey et al, 2008, Lyles-Eggleston et al. 2004, Sikazwe et al. 2003). In an attempt to overcome some of the challenges related to the use of the bar test in evaluating the potential of synthetic agents to induce catalepsy in rats, we selected a commercially available instrument to use in our work. The purpose of this study is two-fold: first, to evaluate the relatively new, commercially available, electronic, complete system for catalepsy in rodents using the bar test, and secondly, to compare the bar test to the CLP test so as to ascertain their effectiveness in identifying the potential of standard drugs (haloperidol, clozapine, and olanzapine) to induce catalepsy in rats.
2. Materials and methods
2.1. Animals
All experiments were carried out on male Sprague-Dawley rats (120-200 g), (5.5-7 weeks old) from Harlan Laboratories, Inc., with no prior drug experience. Animals were housed in the Florida A & M University Animal Care Facility which is fully AAALAC accredited, and operates with a 12 h light/dark cycle and controlled temperature (24 ± 2 °C). The rats were given free access to food and water and at least 5 days to adjust before the start of each experiment. Rats were then fasted the night before each experiment. All experimental procedures were performed in accordance with protocols approved by the Florida A & M University Institutional Animal Care and Use Committee.
2.2. Drugs and chemicals
Haloperidol and clozapine were purchased from Sigma and Olanzapine was purchased from AK Scientific in free base form and were dissolved in filtered (0.22μ) 1% lactic acid vehicle for all the animal studies. The lactic acid was from Fisher Scientific (ACROS) and was ACS grade. Water used to make solutions was HPLC grade. Doses are reported as the free base and were given in a volume of 10 mL/kg by intraperitoneal (ip) injection.
2.3. Instrument description
As first partially reported by us in Bricker et al., 2012, a new instrument, the Catalepsy Test Chamber (Med Associates, Inc., St. Albans, VT) shown in figure 1, was used to assist in performing the classic catalepsy bar test with rats and is further evaluated in this work. Forelimbs were placed on a 1.3 cm diameter (4 diameters available) horizontal cylindrical metal bar at a comfortable standing height of 10 cm (8 heights available) and hind limbs were placed on the stainless steel floor. The instrument measures the time that contact is maintained between the floor and the bar and collects the data using computer software. Contact time in seconds was recorded up to a maximum of 30 seconds. The instrument automatically began collecting data as soon as a complete electrical circuit was made. A video-recorder was available to record each session. Two researchers per compound independently analyzed the video recordings at a later date for comparison to the instrument data. Four chambers were used although up to 8 chambers may be connected per input card.
Figure 1.
Med Associates Catalepsy Test Chamber system. Four white test chambers with bars are shown in the foreground. Computer used for data collection and associated hardware (center blue module on left only) are shown in the background. Other units are for other test systems.
2.4. Behavioral procedures
2.4.1. Bar test for catalepsy
Sixty minutes after injection with haloperidol, clozapine, olanzapine, or vehicle, the rats were evaluated in the bar test for 30 seconds followed by the CLP test (see 2.4.2.) for 30 seconds, followed by the righting test (see 2.4.3.) then returned to their home cage. The 60 minute time point provides a peak cataleptic response for haloperidol as indicated by DeRyck et al., 1982 and was also used by Prinssen et al., 1998; and Bardin et al., 2007 for Haloperidol, Clozapine, and Olanzapine and was therefore chosen for this study. The evaluation was repeated at 3 and 6 min from the start of the first trial and the mean time in seconds for contact with the bar and floor (described in section 2.3) of the three trials was reported for each rat. When the mean time (seconds) for seven rats per dose is significantly different from vehicle (see section 2.5), catalepsy is indicated unless otherwise noted. (Depoortere et al., 2007; Kleven et al., 2005; Prinssen et al., 1998).
For these tests, each rat was placed on the bar by gently holding the tail and lowering the forepaws until the rat could grasp the bar, then lowering the hind paws until they and the tail touched the floor. If good electrical contact was made, the instrument would then begin collecting data. If contact failed due to positioning problems, a stopwatch was started to record the time in this position. Alternatively, the program provides an option to restart the trial over, but we chose to use a stopwatch for convenience and to avoid having to re-position the rat in case any learning effect might take place. All sessions were also video-recorded for later evaluation and comparison to the instrument data.
2.4.2. Crossed-legs position (CLP) test for catalepsy
Rats were placed on the stainless steel floor of the catalepsy test chamber, abdomen towards the floor, and the hind paws brought forward and the front paws backwards so that the ipsilateral hind paws could hold onto the top of the front paws, and the time the rat stayed in this position recorded up to 30 seconds (Depoortere et al., 2007, Kleven et al., 1996). Mean time in seconds from 3 trials was reported for each rat, then the mean of 7 rats per dose compared to vehicle using one-way ANOVA as described in section 2.5.
2.4.3. Righting test
Rats were placed gently on their back and observed immediately following the bar and CLP tests. If the rat did not stay in this position, and flipped over without assistance it was scored as “righted” (McCreary et al. 2007; Reeve et al., 1992).
2.5. Statistical analyses
All statistical analyses were performed using Prism 5.04, GraphPad Software Inc. One-way ANOVA with Bonferroni’s post tests was used to compare mean seconds of time on the bar or in the CLP position for vehicle to doses of antipsychotic. The differences between groups with p < 0.05 were considered significant. All error bars are standard error of the mean (SEM).
3. Results
3.1. Catalepsy tests for haloperidol
3.1.1. Bar Test, measured by instrument (Fig. 2A)
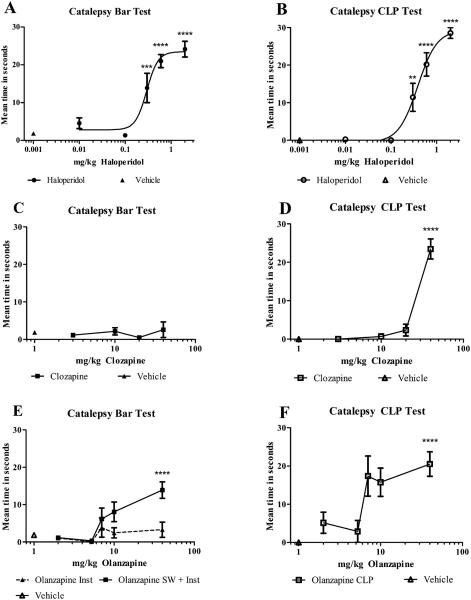
Figure 2 A-F.
Meantime in seconds in the bar test measured using the instrument (Inst) (graphs A, C, E), instrument plus stopwatch (SW + Inst) (graph E, solid line), or in the CLP test measured with a stopwatch (graphs B, D, and F). Graph A, haloperidol bar instrument, AED50 = 0.29 mg/kg. Graph B, haloperidol CLP, AED50 = 0.38 mg/kg. Graph C, clozapine bar instrument, no significant catalepsy. Graph D, clozapine CLP, significant catalepsy at 40 mg/kg. Graph E, olanzapine stopwatch + instrument (SW + Inst.), significant catalepsy at 40 mg/kg. Graph E, Inst. only, gripping problems prevented accurate detection of time on the bar. Dashed line shown only to illustrate problem. Graph F, olanzapine CLP, significant catalepsy at 40 mg/kg. n=7 rats/dose. Compared to vehicle, **, ***, and **** indicate p < 0.01, 0.001, and 0.0001 respectively.
An adverse effect dose-response curve (figure 2A) was obtained for haloperidol when using the instrument to detect and record the time in seconds in the catalepsy bar test as described in section 2.4.1. An AED50 = 0.29 mg/kg (95% CL = 0.22 to 0.40 mg/kg) was calculated using the bar catalepsy instrument data. No loss of righting was noted.
3.1.2. CLP Test, measured by stopwatch (Fig. 2B)
An adverse effect dose-response curve was obtained for haloperidol when using a stopwatch to detect and record the time in the CLP test for catalepsy (figure 2B) as described in section 2.4.2. An AED50 = 0.38 mg/kg (95% CL = 0.27 to 0.56 mg/kg) was calculated using the CLP data. No loss of righting was noted.
3.2. Catalepsy tests for clozapine
3.2.1. Bar Test, measured by instrument (Fig. 2C)
No catalepsy was detected by the bar test (figure 2C), (1-way ANOVA, Bonferroni post tests, p > 0.05), when using the instrument to detect and record the time as described in section 2.4.1. Doses up to 40 mg/kg clozapine were tested. No loss of righting reflex was noted.
3.2.2. CLP Test, measured by stopwatch (Fig. 2D)
No catalepsy was detected by the CLP test up to 20 mg/kg clozapine. However, at 40 mg/kg clozapine, time in CLP, which was deemed significant was observed (figure 2D), (1-way ANOVA, Bonferroni post tests, p < 0.0001), when using a stopwatch to detect and record the time in the CLP test for catalepsy as described in section 2.4.2. No loss of righting reflex was noted.
3.3. Catalepsy tests for olanzapine
3.1.1. Bar Test, measured by instrument plus stopwatch or instrument alone (Fig. 2E)
An adverse effect dose-response graph (figure 2E, solid line) was obtained for olanzapine when using the instrument + stopwatch to detect and record the time in seconds in the catalepsy bar test as described in section 2.4.1. Time on bar which was deemed significant (p < 0.0001) was detected at 40 mg/kg. At this dose however, the animals clenched their fore- and hind paws and thus, they could not grip the bar so as to make effective contact and hence the instrument alone could not accurately detect time on the bar at these doses. In these cases, the back-up stop watch was used to obtain the time on the bar. This is illustrated by the dashed line in figure 2E, showing loss of contact or no contact before time on the bar was over. No loss of righting was noted.
3.1.2. CLP Test, measured by stopwatch (Fig. 2F)
An adverse effect dose-response graph was obtained for olanzapine when using a stopwatch to detect and record the time in the CLP test for catalepsy (figure 2F) as described in section 2.4.2. Time in CLP, which was deemed significant (p < 0.0001) was detected at 40 mg/kg. No loss of righting was noted.
3.3. Comparison of data from instrument and researchers
3.3.1. Bar Test results for haloperidol (Figs. 3A and 3C)
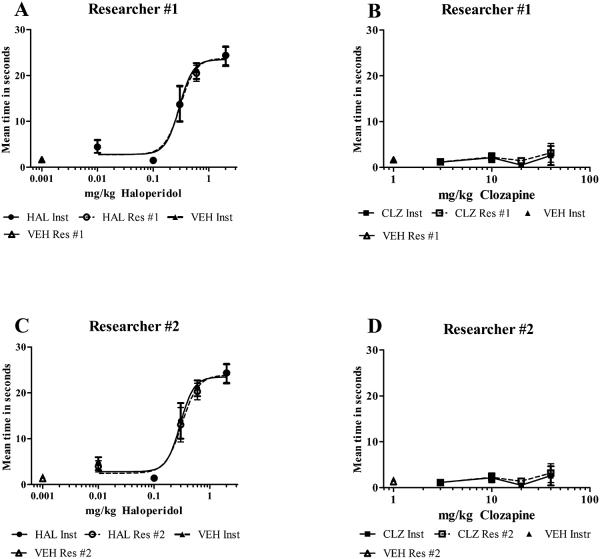
Figure 3 A-D.
Comparison of time in seconds measured by the instrument (filled circles, squares, and triangle) to hand scoring of video playback (open circles, squares, and triangle). No significant difference between the instrument and either researcher #1 (graphs A and B) or #2 (graphs C and D) at any dose, t-test, p > 0.05. (n=7 rats/dose).
Researcher #1 analyzed a video recording of the haloperidol animal experiments in fig 2A and an AED50 of 0.30 mg/kg (95% CL = 0.22 to 0.42 mg/kg) was calculated. This compares well with the instrument data and falls within the 95% confidence interval for the instrument data (AED50 = 0.29 mg/kg, 95% CL = 0.22 to 0.40 mg/kg). Also, no significant difference was noted between the instrument and researcher #1 at any dose, t-test, p > 0.05. Fig 3A is a plot of both data curves.
Researcher #2 also analyzed a video recording of the haloperidol animal experiments in fig 2A and an AED50 of 0.31 mg/kg (95% CL = 0.22 to 0.43 mg/kg) was calculated. This compares well with the instrument data and falls within the 95% confidence interval for the instrument data (AED50 = 0.29 mg/kg, 95% CL = 0.22 to 0.40 mg/kg). Also, no significant difference was noted between the instrument and researcher #2 at any dose, t-test, p > 0.05. Fig 3C is a plot of both data curves.
3.3.2. Bar Test results for clozapine (Fig. 3B and 3D)
Researcher #1 analyzed a video recording of the clozapine animal experiments in figure 2C and a plot of both instrument and researcher #1 data is shown in figure 3B. No significant difference was noted between the instrument and researcher #1 at any dose, t-test, p > 0.05.
Researcher #2 analyzed a video recording of the clozapine animal experiments in figure 2C and a plot of both instrument and researcher #2 data is shown in figure 3D. No significant difference was noted between the instrument and researcher #2 at any dose, t-test, p > 0.05.
3.3.3. Correlation of time (seconds) for the bar test between using the instrument and reading the video data for haloperidol (Figs. 4A and 4B)
Figure 4 A-B.
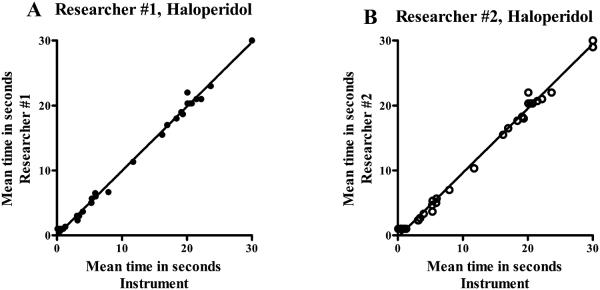
Correlation between data obtained using the instrument and data obtained from researcher observations of video recordings for haloperidol using the bar test. The times (seconds) for 35 data pairs for graphs A and B, were evaluated. Correlation coefficients r = 0.9985 (graph A) and r = 0.9975 (graph B) were found, with a 2-tailed probability p < 0.0001 for all graphs (A, B).
A correlation coefficient was calculated between time (seconds) measured by the instrument and time (seconds) measured by researcher #1 for the bar test for haloperidol, and the data plotted in figure 4A. A correlation coefficient of r = 0.9985 was found, 2-tailed probability p < 0.0001.
A correlation coefficient was calculated between time (seconds) measured by the instrument and time (seconds) measured by researcher #2 for the bar test for haloperidol, and the data plotted in figure 4B. A correlation coefficient of r = 0.9975 was found, 2-tailed probability p < 0.0001.
4. Discussion
The semi-automated bar catalepsy system provides several advantages over manual bar testing. One of the most striking advantages is a fixed definition of “change in posture” to define the end of the recording of “time on the bar”. By instrumental design, the recording of seconds on the bar ends when a complete break in electrical contact between the floor and the bar occurs. This is the same for all researchers using the instrument. In contrast, as noted by Sanberg et al, 1996, with the manual test, many definitions of “change in posture” have been used in the literature, causing problems with the comparison of data. These include: the first movement of any paw, even if it was returned to the bar; complete removal of one paw from the bar with no return; complete removal of both paws from the bar with no return; or even a change in head position, all of which affect “time on the bar”.
Another advantage noted was the automatic recording of the time in seconds without the need for human intervention. This provided several positive outcomes, including more accurate data for shorter times where human reaction time may play a role, a backup on the computer of the observation data, and little or no errors in time on the bar from human distraction. These distractions may include tending to another animal or stopwatch while monitoring a group of rats at the same time, or researcher fatigue.
A distinct advantage for us was the commercial availability of the instrument, backed by a reputable company specializing in animal behavioral testing, with one year of software maintenance and support. The use of a commercial chamber provides yet another advantage in that the chamber construction and materials are the same for all researchers, eliminating some of the variability due to bar and box materials used in different chambers. The chamber can also be placed in a cage washer after removal of the modular detector.
Some problems noted with the version of the instrument tested in this article with our protocol were: an initial software issue with data retrieval; an occasional failure (haloperidol and clozapine) to continue to record time in seconds due to an early start and then early break in contact during positioning of the rat on the bar; and a rare failure to record the time due to the rat’s claws preventing electrical contact with the bar, the floor, or both. In addition, severe clenching of the rat’s paws during catalepsy testing of olanzapine at higher doses, and some lower doses prevented accurate detection of time on the bar in these cases. These issues and their outcomes are further discussed herein.
Positioning contact problems resulting in a premature end to the recording of the time occurred in 8 out of 126 trials for haloperidol, 1 out of 84 trials for clozapine, and 0 out of 21 trials for vehicle. Most cases were due to a regripping or failure to grip properly by the rat during initial placement on the bar. Two instances were due to curled toes not making contact through the toenails, hindering positioning. In addition, there was one instance of the rat being supported by its tail on the floor, forepaws on the bar, but holding its hindpaws up. This did not provide electrical contact, so counting was not begun. Since a stopwatch was used as a backup, times were still recorded for all rats that experienced positioning problems. However, for purposes of this comparison, these data were eliminated because no usable instrument data was recorded. For the higher doses of clozapine (20 mg/kg and 40 mg/kg) curled or clenched paws were observed for the animals. After testing as in section 2.4.1, attempts were made to see if curled paws were hindering gripping the bar. Placement of the paws by hand did not result in an increase in time spent holding onto the bar except in 2 out of 19 trials for 40 mg/kg clozapine only.The increases in times were 29 and 30 seconds for these two trials, suggesting that gripping problems may play a role in bar test detection of catalepsy.Some rats at 40 mg/kg clozapine tended to fall off the bar, executing a less controlled movement to descend than vehicle or haloperidol treated rats. Olanzapine presented a special case due to severely clenched paws which prevented initial electrical contact to be made or maintained throughout the trial, which resulted in positioning contact problems in 41 out of 105 trials. This observation may constitute other side effects related to off-target binding of individual antipsychotic drugs. Additional observations included the rat holding both hind paws together, preventing a proper stance at the bar.
The instrument used an automatic electronic start to define the beginning of the time measurement. The advantage of a manual start, after all positioning problems are over is noted. However, for the shorter times observed for vehicle and low dose points on a curve, there were no positioning errors and the rats were able to grip normally, unlike at the higher doses of the antipsychotics tested. At these lower doses a manual start introduces a small error in seconds. We preferred to use the electronic automatic start on the instrument with the manual start stopwatch as a backup, since most of the data for haloperidol and clozapine were unaffected by positioning problems. As noted earlier, a stopwatch was used when contact problems occurred.
As shown in figure 2A, instrument data only, no stopwatch data, an adverse effect dose-response curve was generated from the bar test as recorded automatically by the instrument at the selected doses of haloperidol. An AED50 of 0.29 mg/kg haloperidol was calculated using non-linear regression. Other researchers have also consistently found that haloperidol produces catalepsy, even though manual methods and different definitions of change in posture and cut off times have been used (Prinssen et al., 2002; Sanberg et al., 1988). Literature values for AED50 for haloperidol range from 0.3 mg/kg to 0.6 mg/kg (Bardin et al., 2007; Kaftka and Corbett, 1996; Natesan et al., 2008) with most researchers reporting near maximal catalepsy seconds at 1 - 2 mg/kg (Sanberg et al., 1988). A second test of catalepsy, the CLP test, was also performed immediately after the bar test, to see how it compared with the instrument bar test data for the typical antipsychotic haloperidol. As seen in figure 2B, the haloperidol acute injections again produced an adverse effect dose-response curve, with an AED50 of 0.38 mg/kg, which is within the 95% CL produced by the instrument bar test. The rats passed the righting reflex test, and so these effects were attributed to catalepsy and not sedation.
The atypical antipsychotic clozapine was also tested to see how the instrument performed when catalepsy was not expected to be present. As shown in figure 2C, catalepsy was not detected by the instrument up to 40 mg/kg clozapine in the bar test. This is consistent with other reports in the literature (Hoffman and Donovan, 1995; Kruzich and See, 2000). However, results with the CLP test for catalepsy were significantly different for the highest dose of clozapine tested. In the CLP test, catalepsy was detected at 40 mg/kg clozapine as shown in figure 2D. Bardin et al., 2007, also reported observing catalepsy deemed significant at 40 mg/kg with a much higher response for clozapine when using the CLP test compared to the bar test. Since all the rats tested with 40 mg/kg clozapine righted themselves during the righting test with no latency, and had open eyes, this observation could not be attributed solely to sedation (McCreary et al., 2007; Zhao and Ming, 2009). It can be argued that curled toes and paws at the highest doses of clozapine may have prevented some of the rats from gripping the bar well. This could have interfered with the bar test, and so catalepsy was not detected as well by the bar method. The CLP test was not dependent on gripping. Since the rats were unable to initiate a change in an externally imposed posture (Sanberg et al., 1996; Sanberg et al., 1988) for the CLP test at 40 mg/kg, catalepsy was inferred and judged to be significant by one-way ANOVA with Bonferroni post tests, p < 0.0001. Indeed, the characteristic and unusual CLP posture was maintained and visually looked the same as when the highest doses of haloperidol were evaluated. Bardin et al., 2007, imposed an upper dose limit of sc 40 mg/kg clozapine in their catalepsy studies to avoid interfering with levels of sedation and ataxia.
Olanzapine caused the forepaws and hind paws to curl under or clench, especially at higher doses, preventing normal contact of the pads with the metal bar and floor. For this reason, reliable electrical contact could not be made and so time on the bar could not be measured properly in 41 out of 105 trials. However, in these cases a backup stopwatch was used. These data are presented in figure 2E, with the solid line indicating the use of the instrument plus the use of the stopwatch for contact problem trials. Time on the bar deemed as catalepsy and found to be significant, p< 0.0001, was detected at 40 mg/kg. A similar graph was reported by Bardin et al., 2007 for bar catalepsy. The dashed line in figure 2E indicates the instrumental data only, and is presented to illustrate the lack of detection of total seconds on the bar by the instrument alone when gripping problems occurred. For compounds with gripping problems, a backup stopwatch is required. Results for olanzapine for the CLP test are presented in fig 2F. An adverse effect dose-response was again observed, with catalepsy deemed significant at 40 mg/kg olanzapine, p<0.0001.
The CLP test was included in this study to see how the standard antipsychotics performed in the CLP test versus the semi-automated bar test. The CLP test has been reported to be sensitive to the anticataleptic effects of 5-HT1A partial agonists (Kleven et al., 2005; Bardin et al., 2007). Since some of the newer antipsychotic drugs have been designed to have partial agonism at the 5-HT1A receptor, it is of interest to compare the two tests. The CLP and semi-automated catalepsy bar test results in this study are in line with the CLP and manual bar test results of Bardin et al., 2007. Time in CLP (seconds) were slightly higher than the time on the bar for haloperidol in both, the current work and the report by Bardin et al., 2007. In the case of clozapine, time in CLP demonstrated an extreme increase over the time on the bar (seconds) at 40 mg/kg. These further confirm the validity of the semi-automated bar test described herein when compared to the manual bar test relative to the CLP test for catalepsy. The olanzapine CLP seconds were also higher than the bar test seconds (instrument + stopwatch), also in agreement with test data from Bardin et al., 2007. The CLP test is recommended as an additional catalepsy test to help identify catalepsy in cases where gripping the bar may be a problem.
Comparisons were made between the bar data collected by the instrument, and the data collected independently by two researchers from video recordings of the same experiments. Dose-response curves were generated and a visual comparison shown in figures 3A and 3C for haloperidol. The instrument produced nearly identical curves to those produced by the researchers. AED50 for haloperidol induced catalepsy was calculated as 0.29 mg/kg (95% CL = 0.22 to 0.40 mg/kg) for the instrument, 0.30 mg/kg (95% CL = 0.22 to 0.42 mg/kg) for researcher #1, and 0.31 mg/kg (95% CL = 0.22 to 0.43 mg/kg) for researcher #2. The AED50 values produced by the two researchers fell well within the 95% confidence limits produced by the instrument data. In addition, each haloperidol dose point generated by the instrument was compared to the corresponding dose point generated by the researchers using the t-test. No significant difference was noted between the instrument and the researchers at any dose.
The bar test data for clozapine generated by the instrument or researchers were likewise compared in the graphs in figures 3B and 3D and again were very similar. No catalepsy was detected by either the instrument or the researchers. The times in seconds for corresponding doses were compared using the t-test, and no significant differences were found between the instrument and either researcher.
A final set of comparisons were made between the instrument data and that generated by the researchers by plotting time in seconds for the instrument versus researcher #1 or researcher #2 observations for haloperidol (figure 4A and 4B). Correlation coefficients for the instrument versus researcher #1 or #2 were 0.9985 and 0.9975 respectively for haloperidol. The results of these experiments further confirm that data collected using the instrument will be equivalent to data collected by researchers using video recordings. Contact problems will necessitate the additional use of a stopwatch.
Recommendations for a future version of this instrument would be to improve the software so that data collected over several trials are automatically displayed at the end of the session without the need for the researcher to issue additional commands, or to call up another program to display the data file with trial number and catalepsy time labels.
While there is still room for further automation, the use of a semi-automated bar test for catalepsy determination as described herein will help standardize or improve several variables including: a) change in posture defined as a complete break in contact between the bar and the floor of the catalepsy chamber; b) bar construction (round, smooth, metal); c) human reaction time, fatigue, or inattention not being a factor; d) recording of seconds on the bar ( instrument default condition) and not converting to a score. In addition, for rats between 120 - 200g, selecting a bar height of 10 cm, bar diameter 1.3 cm, and reporting the average seconds of 3 trials as mean time on the bar have produced useful data for the authors and are suggested for use in a standardized method. As noted in the instrument description, bar height and diameter are selectable, providing appropriate dimensions for smaller animals like mice, and for larger rats. The authors chose a cut-off time of 30 seconds following the example of Kleven et al., 2005, as a compromise between speed and information gained, producing dose-response curves that can indicate cataleptogenic properties of the test drug. This is especially useful for testing newer antipsychotics that produce little or no catalepsy response, for which a longer cut-off time serves little if any useful purpose.
The present device has several advantages and improvements over previous prototypes. As far as we know, it is the first commercially available bar test system based on electrical contact. It can be purchased, saving the researcher the time and expertise needed to construct the chambers and electrical components and to write the software needed for computer interfacing. The chambers are not made of wood and are designed to be placed in an automated washer, unlike the homemade system described in Alvarez-Cervera et al., 2005. It has software and interfaces for computer storage of data and convenient setting of several parameters (cut-off times, number of trials), unlike the system in Moss et al., 1981. It directly detects the removal of both forepaws from the bar, a clear indication that the cataleptic bar posture has ended, versus the latency to first vertical movement of one paw detected by the infrared beam activity system of Sanberg et al., 1988. This is of occasional value when the rat has one paw slip off the bar but still maintains a cataleptic hold on the bar with the remaining paw, in which case, timing should continue as the rat is still experiencing the cataleptic condition.
Overall, the semi-automated bar catalepsy instrument under review has performed well, and is a big improvement over homemade boxes and stopwatches. The data is consistent between researchers and the change in posture well defined and not subject to interpretation by each researcher. Occasional contact problems require the use of a stopwatch or other means to record the time for those few trials. For compounds like olanzapine with gripping problems, the use of a back-up stopwatch is necessary in those cases. The CLP test is also recommended to provide additional data that might be missed by the bar test for compounds producing grasping problems. Since the main objective is to record the times subjects remained on the bar, the use of the instrument, with a stopwatch as a backup, produced consistency and was deemed acceptable, labor-saving, and more observant than a manual, non-instrumental bar test method for catalepsy alone.
Highlights.
A new semi-automated instrument for evaluating the potential of a drug to induce catalepsy.
Both typical and atypical antipsychotics were evaluated and produced appropriate results.
The instrument was validated by comparing instrument and human evaluation data.
The bar test results were compared to the crossed-legs position test for catalepsy.
The new instrument was found to be very helpful for catalepsy determination.
Acknowledgements
This work was supported by Grants # 5SC1GM088451-01-04 and a Title III Grant to Florida A&M University. The work was also supported in part by the Pharmaceutical Research Center NIH/NCRR 1C06-RR12512-01 grant. No conflict of interest exits. The objective evaluation of this instrument was solely the idea of the authors, to benefit the behavioral pharmacology community. No funding or financial benefit of any kind was received from the manufacturer before or during the study.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Åberg K, Adkins DE, Bukszár J, Webb BT, Caroff SN, Miller DD, Sebat J, Stroup S, Fanous AH, Vladimirov VI, McClay JL, Lieberman JA, Sullivan PF, van den Oord EJCG. Genomewide association study of movement-related adverse antipsychotic effects. Biol Psychiatry. 2010;67:279–82. doi: 10.1016/j.biopsych.2009.08.036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ablordeppey SY, Altundas R, Bricker B, Zhu XY, Suresh Kumar EVK, Jackson T, Khan A, Roth BL. Identification of A Butyrophenone Analog as a Potential Atypical Antipsychotic Agent: 4-[4-(4-Chlorophenyl)-1,4-diazepan-1-yl]-1-(4-fluorophenyl)butan-1-one. Bioorg Med Chem. 2008;16:7291–7301. doi: 10.1016/j.bmc.2008.06.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alvarez-Cervera FJ, Villanueva-Toledo J, Moo-Puc RE, Heredia-Lopez FJ, Alvarez-Cervera M, Pineda JC, Gongora-Alfaro JL. A novel automated rat catalepsy bar test system based on a RISC microcontroller. J Neuroscience Methods. 2005;146:76–83. doi: 10.1016/j.jneumeth.2005.01.018. [DOI] [PubMed] [Google Scholar]
- Bricker B, Jackson T, Boateng B, Zhu XY, Ablordeppey SY. Evaluation of the behavioral and pharmacokinetic profile of SYA013, a homopiperazine analog of haloperidol in rats. Pharmacol Biochem Behav. 2012;102:294–301. doi: 10.1016/j.pbb.2012.05.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bardin L, Auclair A, Kleven MS, Prinssen EPM, Koek W, Newman-Tancredi A, Depoortere R. Pharmacological profiles in rats of novel antipsychotics with combined dopamine D2/serotonin 5-HT1A activity: comparison with typical and atypical conventional antipsychotics. Behav Pharmacol. 2007;18:103–18. doi: 10.1097/FBP.0b013e3280ae6c96. [DOI] [PubMed] [Google Scholar]
- Castagne V, Moser PC, Porsolt RD. Preclinical behavioral models for predicting antipsychotic activity. Advances in Pharmacology. 2009;57:381–418. doi: 10.1016/S1054-3589(08)57010-4. [DOI] [PubMed] [Google Scholar]
- Depoortere R, Bardin L, Auclair AL, Kleven MS, Prinssen E, Colpaert F, Vacher B, Newman-Tancredi A. F15063, a compound with D2/D3 antagonist, 5-HT1A agonist and D4 partial agonist properties: (II) Activity in models of positive symptoms of schizophrenia. Br J Pharmacol. 2007;151:253–65. doi: 10.1038/sj.bjp.0707159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Ryck M, Hruska RE, Silbergeld EK. Estrogen and haloperidol-induced versus handling-related catalepsy in male rats. Pharmacol Biochem Behav. 1982;17:1027–35. doi: 10.1016/0091-3057(82)90489-0. [DOI] [PubMed] [Google Scholar]
- Ferre S, Guix T, Prat G, Jane F, Casas M. Is experimental catalepsy properly measured? Pharmacol Biochem Behav. 1990;35:753–57. doi: 10.1016/0091-3057(90)90354-k. [DOI] [PubMed] [Google Scholar]
- Ellenbroek BA. Treatment of schizophrenia: A clinical and preclinical evaluation of neuroleptic drugs. Pharmacol Ther. 1993;57:1–78. doi: 10.1016/0163-7258(93)90036-d. [DOI] [PubMed] [Google Scholar]
- Hoffman DC, Donovan H. Catalepsy as a rodent model for detecting antipsychotic drugs with extrapyramidal side effect liability. Psychopharmacology. 1995;120:128–33. doi: 10.1007/BF02246184. [DOI] [PubMed] [Google Scholar]
- Kafka SH, Corbett R. Selective adenosine A2A receptor/dopamine D2 receptor interactions in animal models of schizophrenia. Eur J Pharmacol. 1996;295:147–54. doi: 10.1016/0014-2999(95)00668-0. [DOI] [PubMed] [Google Scholar]
- Kleven MS, Barret-Grevoz C, Slot LB, Newman-Tancredi A. Novel antipsychotic agents with 5-HT1A agonist properties: Role of 5-HT1A receptor activation in attenuation of catalepsy induction in rats. Neuropharmacology. 2005;49:135–43. doi: 10.1016/j.neuropharm.2005.02.005. [DOI] [PubMed] [Google Scholar]
- Kleven M, Prinssen EPM, Koek W. Role of 5-HT1A receptors in the ability of mixed 5-HT1A receptor agonist/dopamine D2 receptor antagonists to inhibit methylphenidate-induced behaviors in rats. Eur J Pharmacol. 1996;313:25–34. doi: 10.1016/0014-2999(96)00498-0. [DOI] [PubMed] [Google Scholar]
- Kruzich PJ, See RE. An evaluation of the role of 5-HT2 receptor antagonism during subchronic antipsychotic drug administration in rats. Brain Res. 2000;875:35–43. doi: 10.1016/s0006-8993(00)02574-9. [DOI] [PubMed] [Google Scholar]
- Lieberman JA, Stroup TS, McEvoy JP, Swartz MS, Rosenheck RA, Perkins DO, Keefe RS, Davis SM, Davis CE, Lebowitz BD, Severe J, Hsiao JK. Clinical Antipsychotic Trials of Intervention Effectiveness (CATIE) Investigators. Effectiveness of antipsychotic drugs in patients with chronic schizophrenia. N Engl J Med. 2005;353:1209–23. doi: 10.1056/NEJMoa051688. [DOI] [PubMed] [Google Scholar]
- Lyles-Eggleston M, Altundas R, Xia J, Sikazwe DMN, Fan P, Yang Q, Li S, Zhang W, Zhu X, Schmidt AW, Vanase-Frawley M, Shrihkande M, Villalobos A, Borne RF, Ablordeppey SY. Design, Synthesis, and Evaluation of Metabolism-Based Analogues of Haloperidol Incapable of Forming MPP+-like Species. J Med Chem. 2004;47:497–508. doi: 10.1021/jm0301033. [DOI] [PubMed] [Google Scholar]
- McCreary AC, Glennon JC, Ashby CR, Jr, Meltzer HY, Li Z, Reinders JH, Hesselink MB, Long SK, Herremans AH, Stuivenberg HV, Feenstra RW, Kruse CG. SLV313 (1-(2,3-dihydrobenzo[1,4]-dioxin-5-yl)-4-[5-(4-fluoro-phenyl)-pyridin-3-ylmethyl]-piperazinemonohydrochloride): A Novel Dopamine D2 Receptor Antagonist and 5-HT1A Receptor Agonist Potential Antipsychotic Drug. Neuropsychopharmacology. 2007;32:78–94. doi: 10.1038/sj.npp.1301098. [DOI] [PubMed] [Google Scholar]
- Meltzer HY, Li Z, Kaneda Y, Ichikawa J. Serotonin receptors: their key role in drugs to treat schizophrenia. Prog Neuro-Psychopharmacology and Biological Psychiatry. 2003;27(7):1159–72. doi: 10.1016/j.pnpbp.2003.09.010. [DOI] [PubMed] [Google Scholar]
- Moss DE, McMaster SB, Rogers J. Tetrahydrocannabinol potentiates reserpine-induced hypokinesia. Pharmacol Biochem Behav. 1981;15:779–83. doi: 10.1016/0091-3057(81)90022-8. [DOI] [PubMed] [Google Scholar]
- Natesan S, Reckless GE, Barlow KBL, Nobrega JN, Kapur S. Amisulpride the ‘atypical’ antipsychotic – Comparison to haloperidol, risperidone and clozapine. Schizophrenia Res. 2008;105:224–35. doi: 10.1016/j.schres.2008.07.005. [DOI] [PubMed] [Google Scholar]
- Prinssen EPM, Kleven MS, Koek W. The cataleptogenic effects of the neuroleptic nemonapride are attenuated by its 5-HT1A receptor agonist properties. Eur J Pharmacol. 1998;356:189–92. doi: 10.1016/s0014-2999(98)00536-6. [DOI] [PubMed] [Google Scholar]
- Prinssen EPM, Colpaert FC, Koek W. 5-HT1A receptor activation and anti-cataleptic effects: high-efficacy agonists maximally inhibit haloperidol-induced catalepsy. Eur J Pharmacol. 2002;453:217–221. doi: 10.1016/s0014-2999(02)02430-5. [DOI] [PubMed] [Google Scholar]
- Reeve B, Dingwall B, Darlington CL, Scott SJ, Sansom AJ, Smith PF. Simple Device for Quantifying Drug Effects on the Righting Reflex. Pharmacol Biochem Behav. 1992;42:183–85. doi: 10.1016/0091-3057(92)90464-q. [DOI] [PubMed] [Google Scholar]
- Sanberg PR, Bunsey MD, Giordano M, Norman AB. The catalepsy test: Its ups and downs. Behav Neurosci. 1988;102:748–59. doi: 10.1037//0735-7044.102.5.748. [DOI] [PubMed] [Google Scholar]
- Sanberg PR, Martinez R, Shytle RD, Cahill DW. The catalepsy test: Is a standardized method possible? In: Sanberg PR, Ossenkopp K-P, Kavaliers M, editors. Motor activity and movement disorders. Humana Press; Totowa, New Jersey: 1996. pp. 197–211. [Google Scholar]
- Seeger TF, Seymour PA, Schmidt AW, Zorn SH, Schulz DW, Lebel LA, McLean S, Guanowsky V, Howard HR, Lowe JA, III, Heym J. Ziprasidone (CP-88,059): A New Antipsychotic with Combined Dopamine and Serotonin Receptor Antagonist Activity. J Pharmacol Exp Ther. 1995;275:101–13. [PubMed] [Google Scholar]
- Sikazwe DMN, Lyles-Eggleston MD, Li S, Ablordeppey SY. The acute EPS of Haloperidol may be unrelated to its metabolic transformation to BCPP+ Bioorg Med Chem Lett. 2003;13:3779–3782. doi: 10.1016/j.bmcl.2003.07.015. [DOI] [PubMed] [Google Scholar]
- Wadenberg M-L. Serotonergic mechanisms in neuroleptic-induced catalepsy in the rat. Neuroscience and Biobehav Rev. 1996;29:325–39. doi: 10.1016/0149-7634(95)00057-7. [DOI] [PubMed] [Google Scholar]
- Zhao C, Ming L. Sedation and disruption of maternal motivation underlie the disruptive effects of antipsychotic treatment on rat maternal behavior. Pharmacol Biochem Behav. 2009;92:147–56. doi: 10.1016/j.pbb.2008.11.006. [DOI] [PMC free article] [PubMed] [Google Scholar]