Abstract
CHGB is the major matrix protein in human catecholamine storage vesicles. CHGB genetic variation alters catecholamine secretion and blood pressure. Here effective Chgb protein under-expression was achieved by siRNA in PC12 cells, resulting in ~48% fewer secretory granules on EM, diminished capacity for catecholamine uptake (by ~79%), and a ~73% decline in stores available for nicotinic cholinergic-stimulated secretion. In vivo, loss of Chgb in knockout mice resulted in a ~35% decline in chromaffin granule abundance and ~44% decline in granule diameter, accompanied by unregulated catecholamine release into plasma. Over-expression of CHGB was achieved by transduction of a CHGB-expressing lentivirus, resulting in ~127% elevation in CHGB protein, with ~122% greater abundance of secretory granules, but only ~14% increased uptake of catecholamines, and no effect on nicotinic-triggered secretion. Human CHGB protein and its proteolytic fragments inhibited nicotinic-stimulated catecholamine release by ~72%. One conserved-region CHGB peptide inhibited nicotinic-triggered secretion by up to ~41%, with partial blockade of cationic signal transduction. We conclude that bi-directional quantitative derangements in CHGB abundance result in profound changes in vesicular storage and release of catecholamines. When processed and released extra-cellularly, CHGB proteolytic fragments exert a feedback effect to inhibit catecholamine secretion, especially during nicotinic cholinergic stimulation.
Keywords: Chromogranin B, hypertension, adrenal, chromaffin, catecholamines
INTRODUCTION
The sympathetic branch of the autonomic system exerts minute-to-minute control over the circulation. Sympathoadrenal catecholamine secretion is exocytotic (all-or-none), releasing not just catecholamines but also the proteins with which catecholamines are co-stored. The chromogranins/secretogranins comprise a family of acidic, soluble proteins that are widely stored in secretory granules with hormones, transmitters, and neuropeptides throughout the endocrine and nervous systems (1,2). Chromogranin B (CHGB), first described in the 1980s, (3,4) seems to be the quantitatively most abundant matrix protein in the core of human catecholamine storage vesicles (5,6). Expression studies in chromaffin cells suggest that CHGB plays a functional role in secretory vesicle biogenesis (7).
CHGB may have both extra-cellular and intra-cellular roles in the neuroendocrine system. Extracellular roles for CHGB are dependent on its proteolytic processing within secretory granules (8) to form smaller peptides; such peptides may have a role in the neuroendocrine/sympathoadrenal stress response to systemic infection (2), and CHGB fragments may also play an autocrine inhibitory role on the release of co-stored hormones, such as insulin (9). Within chromaffin cells and sympathetic axons,CHGB functions in sorting and trafficking of peptide hormone and neuropeptide precursors to secretory granules (10), perhaps as triggers to secretory granulogenesis (7).
Hypertension is a complex trait in which deranged autonomic control of the circulation may be an early etiological culprit (11). Expression of CHGB may mark the action of QTLs (including the CHGB locus itself) influencing exocytotic sympathoadrenal activity (12,13). CHGB is over-expressed in rodent models of genetic (14,15) as well as acquired (16) hypertension, thus suggesting augmented sympathoadrenal activity in the pathogenesis of these syndromes. Therefore CHGB gives rise to early, pathogenic “intermediate phenotypes” (17) for exploration of sympathoadrenal activity in human essential hypertension. Indeed, we reported that two common CHGB promoter SNPs, A-296C (rs236140) and A-261T (rs236141), are strongly associated with hypertension in the population (18,19), while in CHGB knockout (CHGB[−/−]) mice, we observed substantial elevations in both SBP and DBP (18). In this report, we have studied how CHGB quantitative variation (both over- and under-expression) affects not only secretory granulogenesis, but also catecholamine uptake, storage, and exocytotic release. We also describe the effects of novel CHGB fragments upon catecholamine release and its nicotinic cholinergic signal transduction pathway.
METHODS
Chromaffin cell biology
Rat pheochromocytoma (PC12) cells were cultured in a medium containing DMEM with high glucose (cat#: 11965, Invitrogen), 10% horse serum, 5% fetal bovine serum (FBS) and 1x penicillin/streptomycin/glutamine. The cells were cultured in an incubator with 6% CO2 at 37°C, and cell passage number (since initiation of the line) was between 10 and 25 in these experiments.
Secretion of catecholamines from chromaffin cells
Norepinephrine secretion from PC12 cells was assayed as described previously (20). After washing, cells treated with nicotine (60 µM; for nicotinic cholergic stimulation) or KCl (55 mM; for membrane depolarization) in physiological secretion medium. Each experiment was repeated three times.
Transmission electron microscopy (EM)
TEM on cells/tissues was conducted as previously described (21).
Silencing CHGB expression by small interfering RNA (siRNA) in PC12 cells
We used a rat CHGB siRNA siGENOME “smart pool” (Cat# M-099320-00, Dharmacon). One day before transfection, PC12 cells were grown on poly-l-lysine-coated 12-well plates and transfected for 48 h with 30, 15 or 7.5 nmol/well siRNA using the Transfectin reagent (BioRad). Silencing of CHGB expression was evaluated by immunoblotting at the protein level and real time PCR at the mRNA level. A control siRNA (Cat#: D-001210-01-05, Dharmacon) pre-designed to minimize off-target effects was used as a transfection negative control. The effect of reduced expression of CHGB on dense-core secretory granule abundance was examined by transmission electron microscopy (EM). The anti-CHGB antibody (C-19; catalog #:sc-1489, Santa Cruz Biotechnology) was a goat polyclonal IgG (provided at 200 µg/ml, and used at 1:3000) for detecting CHGB of rat or human origin.
CHGB ablation in vivo: Knockout mice, transmission EM, and catecholamine release
Mouse studies were conducted with the approval of the UCSD Animal Subjects Committee, and the ARRIVE guidelines were followed. CHGB knockout mice were generated from mouse embryonic stem cells by homologous recombination, as previously described (18,22). The effect of CHGB knockout on dense-core secretory granule abundance was examined by transmission electron microscopy (EM). Plasma catecholamines were investigated by high performance liquid chromatography coupled to an electrochemical detection (HPLC-ECD) system.
Over-expression of CHGB in PC12 cells by transduction of a CHGB lentivirus
Human cDNA clone hCHGB-pCMV6XL5 (Cat#: SC119004, Origene, Rockville, MD) was used as template. The 5′ primer, 5’- agcCCCGGGatgcagccaacgctgcttct -3’, includes a SmaI site (capitalized), while the 3′ primer, 5′- caaGTCGACtcagcccctttggctgaatt -3’, includes a SalI site (capitalized). The 2034 bp PCR product was digested with SmaI and SalI (New England Biolab), purified (Cat#: 28074, Qiagen), and ligated into corresponding site of SIN18-hPGK-tdTomato-T2A vector to generate SIN18-hPGK-tdTomato-T2A-hCHGB lentiviral vector plasmid (Supplementary Figure 1). The SIN18-hPGK-tdTomato is used as control lentiviral vector.
The standard operation procedure of lentivirus production is as described (23). Briefly, three plasmids (the lentiviral vector plasmid, the packaging plasmid pCMVDR8.74, and the vesicular stomatitis virus (VSV) glycoprotein expression plasmid pMD.G) are mixed in a ratio of 3:2:1 for transient transfection into HEK-293T cells using the calcium phosphate method. Un-concentrated viral supernatant is collected every 24 hours up to 4 days post-transfection, and a commercially available serum-free ultraculture medium (Bio-Whitttaker #12-725F) with 1 mM L-glutamine, 50 U/mL penicillin, and 50 mg/mL streptomycin is used to refeed the transfected plate. The pooled viral supernatant is filtered through 0.22 µm pores, followed by concentration and purification by 20% sucrose gradient centrifugation at 21,000 rpm for 2 hours. The viral lysate is resuspended in 1x HBSS (Hank's Balanced Salt Solution), followed by flow cytometry titration in 293T cells.
For lentiviral transduction, concentrated viral supernatant was added into PC12 cells at a MOI of 0.5, and incubated at 37 °C overnight. Cells were subjected to culture, experiment, or analysis 72 hours post-transduction.
Flow cytometry
PC12 cells grown on 100-mm plates and infected with the lenti-hPGK-tdTomato-T2A-hCHGB or lenti-hPGK-tdTomato-T2A control viruses, were suspended in Accutase detachment solution (Innovative Cell Technologies) at a concentration of 2 × 106 cells/ml. Cells (7 × 106/sample) were analyzed for tdTomato fluorescence using a MoFlo cell sorter (DakoCytomation). Fluorescence-activated cell sorting (FACS) achieved the isolation of lentivirus-infected cells. An aliquot of each cell sample was processed for immunocytochemistry (anti-HA monoclonal antibody) to evaluate the expression of CHGB, and the remaining cells were processed for characterization by biochemistries and imaging by TEM.
Prokaryotic expression and purification of CHGB: pET-hCHGB-6-His
Human CHGB cDNA encoding the mature protein (minus the 20-residue signal peptide) was excised by PCR from the eukaryotic expression plasmid hCHGB-pCMV6XL5 (Cat#: SC119004, Origene, Rockville, MD) as template. The 5′ primer, 5’-gtcCATATGatgccagtggataacaggaa-3’ is preceded by a NdeI site (capitalized) and the 3′ primer, 5′-tgaGTCGACgcccctttggctgaatttct-3’, is preceded by a SalI site (capitalized). The PCR product was digested with NdeI and SalI (New England Biolab) and gel-purified (Cat#: 28074, Qiagen). The resulting fragment of 1974 base pairs was directionally cloned into the expression vector, pET-21a-d (Novagen, Madison, WI). The final construct was designated to encode the mature (i.e., minus signal peptide) human CHGB (657 residues) with addition of the residues VDKLAAALEHHHHHH at the carboxy-terminus (thus a total of 682 residues for the final recombinant protein). The nucleotide sequences of the PCR products and the junction regions between the insert and the vector were verified from both strands by direct dideoxy-capillary sequencing.
BL21(DE3)pLysS (Novagen, Madison, WI) competent E.coli were transformed with the pET-(hCHGB-6-His) plasmid. Cells were induced with IPTG (isopropyl β-D-thiogalactopyranoside; 1 mM), and grown in LB broth containing ampicillin (50 µg/ml) at 37°C until an A600 of ~0.8 was reached. The recombinant protein was purified as described previously using its 6-His affinity tag (24). Protein concentration was determined by Bio-Rad protein assay reagent (Bio-Rad Laboratories, Hercules, CA). Aliquotted protein was stored at −70°C until further use.
Endoproteolytic cleavage of CHGB
Plasmin is serine protease found in chromaffin cells that can process CHGA to its bio-active catestatin fragment (25–27). In order liberate a “catestatin-like” peptide from CHGB, we thus turned to plasmin as a protease. Purified human recombinant CHGB protein (10 µmol/reaction) was incubated with human plasmin (EC_3.4.21.7, 2 µmol/reaction; Calbiochem catalog #527624; specific activity 10 U/mg protein) in digestion buffer (10 mM Tris-HCl, pH 8.0; 0.15 M NaCl) at 37°C for 30 min. The reaction was terminated by the addition of aprotinin (2.5 µM). For secretion assay, the cells were treated with nicotine (60 µM) in secretion medium, either alone or in combination with recombinant CHGB (3.0 µM) full-length, or pre-digested by plasmin. The digestion reaction (1 hr) contained CHGB (10 µM) and plasmin (2 µM) in a reaction volume of 120 µl of 10 mM Tris-Cl, pH 8.0 and NaCl 150 mM. After digestion, peptides were purified (and intact plasmin removed) by C18 cartridge (Waters, Milford, MA), lyophilized, and suspended in water.
LC-MS/MS
In one experiment, plasmin-digested human CHGB (without added aprotinin) was subjected to LC-MS/MS, for identification of cleavage sites. Plasmin-digested peptides were analyzed by HPLC coupled to tandem mass spectroscopy (LC-MS/MS) using nanospray ionization, in a Triple TOF 5600 hybrid mass spectrometer (AB-SCIEX) interfaced with nano-scale reversed-phase HPLC (Tempo) using a 10 cm-100 micron ID glass capillary packed with 5-µm C-18 ZorbaxTM beads (Agilent Technologies, Santa Clara, CA). Peptides were eluted from the C18 column into the mass spectrometer using a linear gradient (5–60%) of ACN (acetonitrile) at a flow rate of 250 µl/min for 1hr. The buffers used to create the ACN gradient were: Buffer A (2% ACN, 0.2% formic acid, and 0.005% TFA, in H2O) and Buffer B (0.2% formic acid, and 0.005% TFA in ACN). MS/MS data were acquired as follows: MS-1 data were obtained for 250 ms at m/z of 400 to 1250 Da, while MS-2 data spanned m/z of 50 to 2,000 Da. The independent data acquisition (IDA) parameters were: MS-1 TOF 250 milliseconds, followed by 50 MS-2 events of 25 milliseconds each, and IDA criteria of over 200 counts (threshold) and charge state +2 to +4, with a 4 second exclusion. Finally, collected data were analyzed by MASCOT® (Matrix Sciences) and Protein Pilot 4.0 (AB-SCIEX) for peptide sequence identification, with reference to the human CHGB amino acid sequence as template (UniProt P05060, NCBI NP_001810).
CHGB peptide synthesis
Five peptides were synthesized by solid-phase F-moc chemistry, with molecular mass verified by electrospray mass spectrometry (Mimotopes; Minneapolis, MN). CHGB regions were selected for a preponderance of sequence features bearing similarity to the catecholamine release-inhibitory CHGA fragment catestatin (human CHGA[352–372]) (28,29): basic isoelectric point (pI), basic amino acids (Arg, Lys; including dibasic or tribasic sites), hydrophobicity or aromaticity (Leu, Ile, Val, Phe, Tyr, His); amphiphilicity (alternating hydrophobic and cationic residue side chains); and relative abundance of the product after cleavage out of the CHGB precursor in chromaffin granules in vivo (30–32). The synthetic sequences were: hCHGB[60–67], KFEVRLLR; hCHGB[259–272], TRPRHHHGRSRPDR; hCHGB[337–354], LEWERYRGRGSEEYRAPR; hCHGB[389–419], RGLEPGKGRHHRGRGGEPRAYFMSDTREEKR; and hCHGB[480–497], RFQDKQYSSHHTAEKRKR.
Nicotinic cholinergic signal transduction: 45Ca2+ uptake
PC12 cells were seeded onto poly-L-lysine–coated six-well polystyrene culture dishes 2 days before study. Cells were rinsed with 1 ml of secretion buffer (150 mM NaCl, 5 mM KCl, 2 mM CaCl2, 10 mM Hepes, pH 7.4) every 15 minutes for 1 hour at 37°C. Then 45Ca2+ was added (2 µCi/ml, 14.95 mCi/mg, DuPont-NEN)) to Ca2+-free buffer (secretion buffer without 2 mM CaCl2), and drugs were to the labeled buffer at 37°C. The cells were incubated with labeled buffer under different conditions for 5 minutes at room temperature. Calcium uptake was stopped simultaneously in all six wells by inversion of the plate, so all wells were decanted together, followed by prompt addition of ice-cold release buffer containing 2 mL of 1 mmol/L LaCl3 for termination of further uptake of extracellular labeled calcium. The culture dishes were then rinsed twice with ice-cold release buffer. One ml of cell lysis buffer was added to cells in each well and collected for liquid scintillation counting. The data were expressed as counts per minute per well.
Statistical Analyses
Results are expressed as mean ± 1 SEM (standard error of the mean). The reported “n” refers to the number of replications. Data were evaluated by Student t test or ANOVA, followed by post hoc tests, in Excel (Microsoft, Bellevue, WA) or SPSS-17 (Chicago, Ill). P values <0.05 were considered statistically significant.
RESULTS
Silencing CHGB expression in chromaffin cells by siRNA
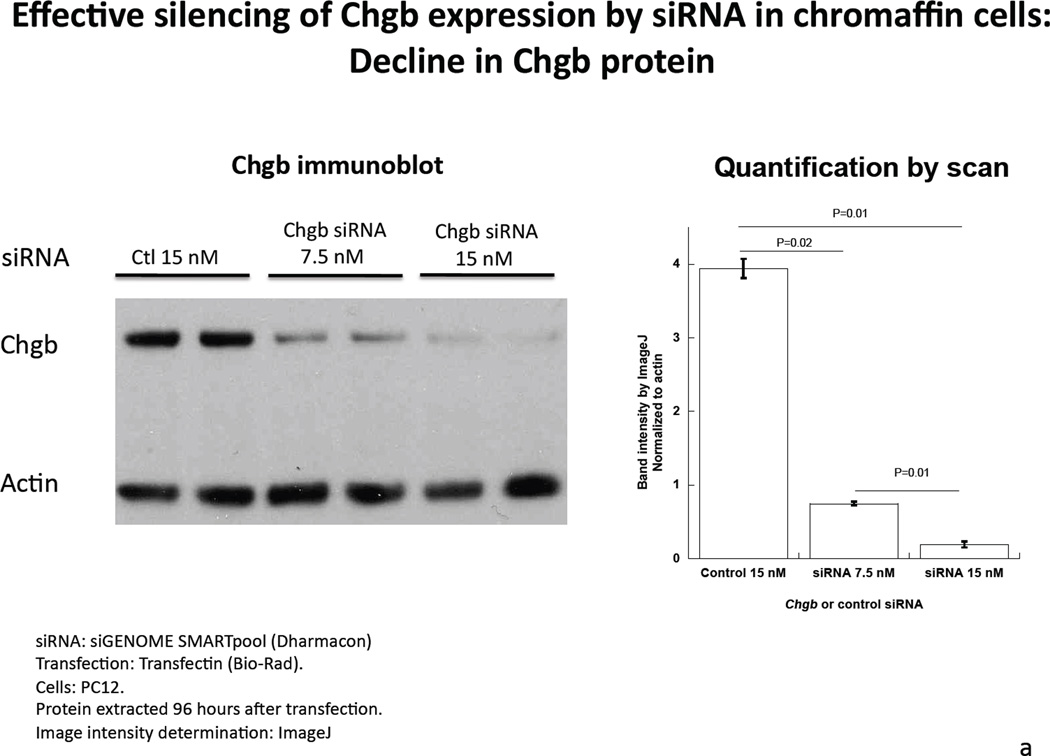
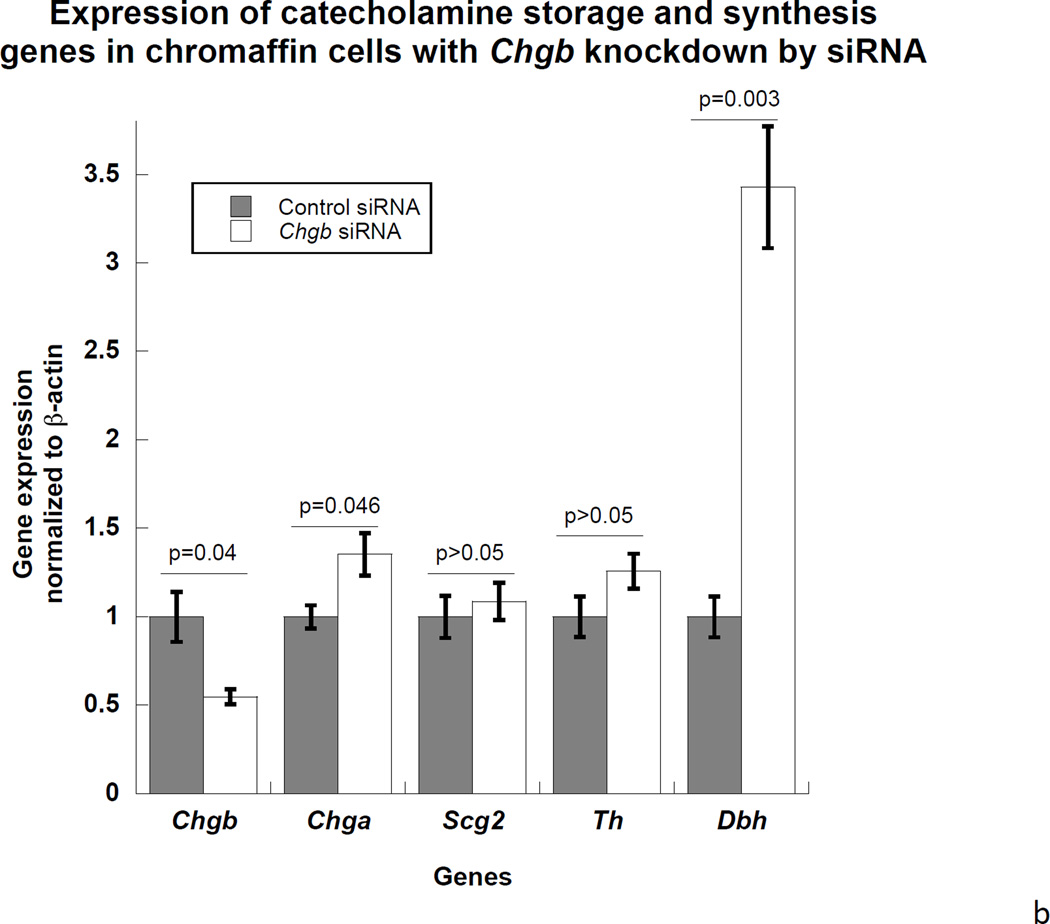
Introduction of rat CHGB siRNA into PC12 dose-dependently decreased CHGB protein expression on immunoblot. CHGB siRNA knocked down CHGB protein by 81% (p=0.02) at 7.5 nM and >95% at 15 nM (p=0.01, Figure 1a). In the catecholamine storage/synthesis pathway, after CHGB siRNA treatment real-time qPCR indicated that CHGB mRNA declined by 46% (p<0.05), while these mRNAs increased: Chga (by 35% p<0.05); and Dbh (by 243%, p=0.003). There were no changes in mRNAs encoding Scg2 or Th (Figure 1b). The increase in Dbh transcription suggests negative feedback control by cellular catecholamine content, but the mechanism of such an effect is not established.
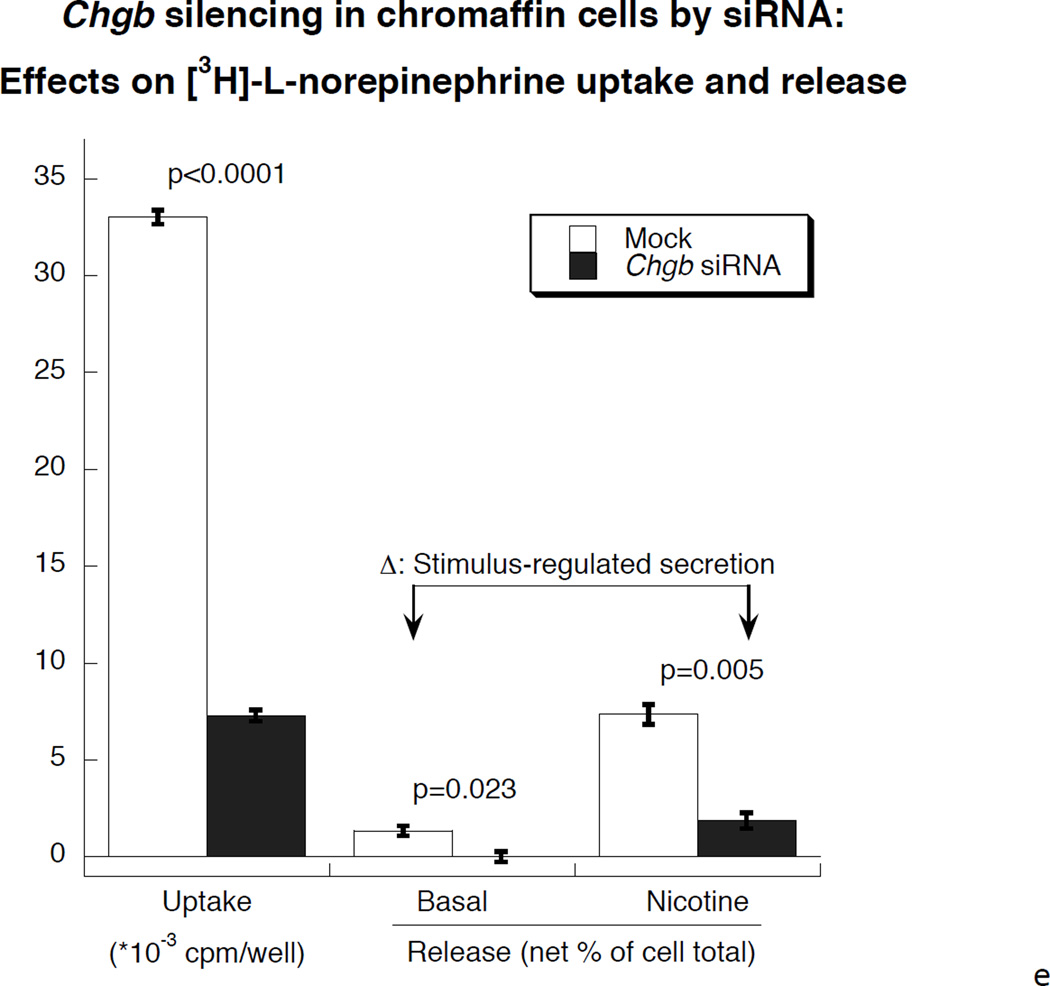
Figure 1. Under-expression of CHGB by siRNA in chromaffin cells.
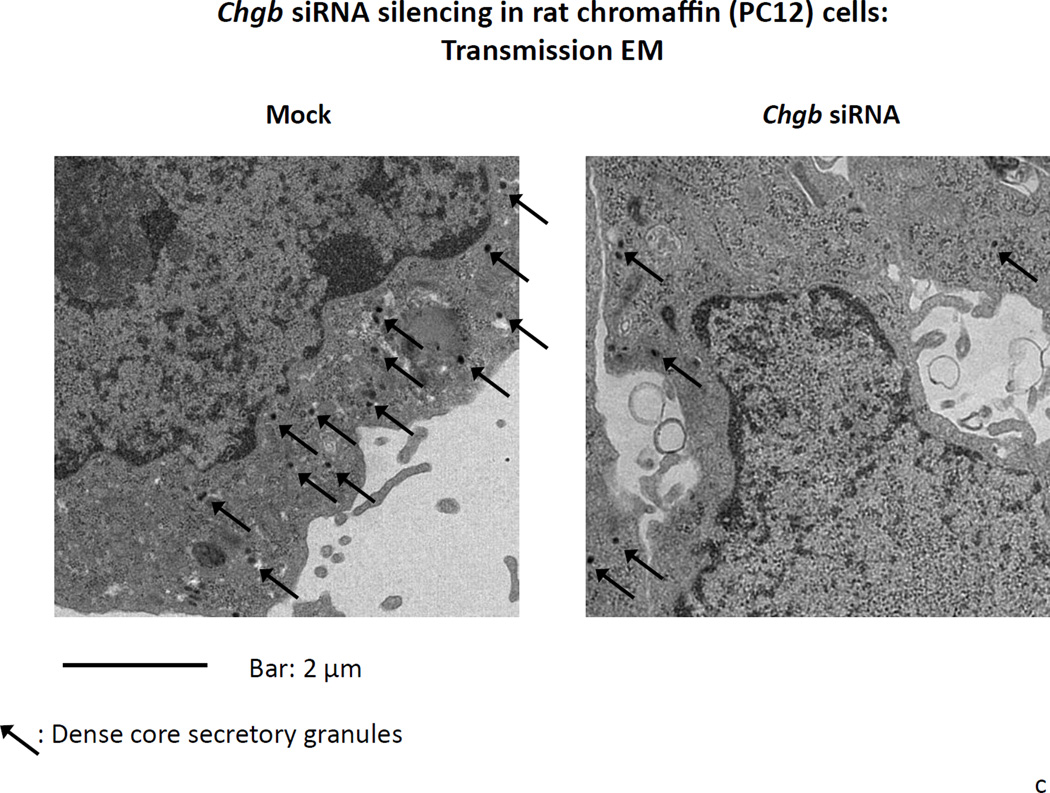
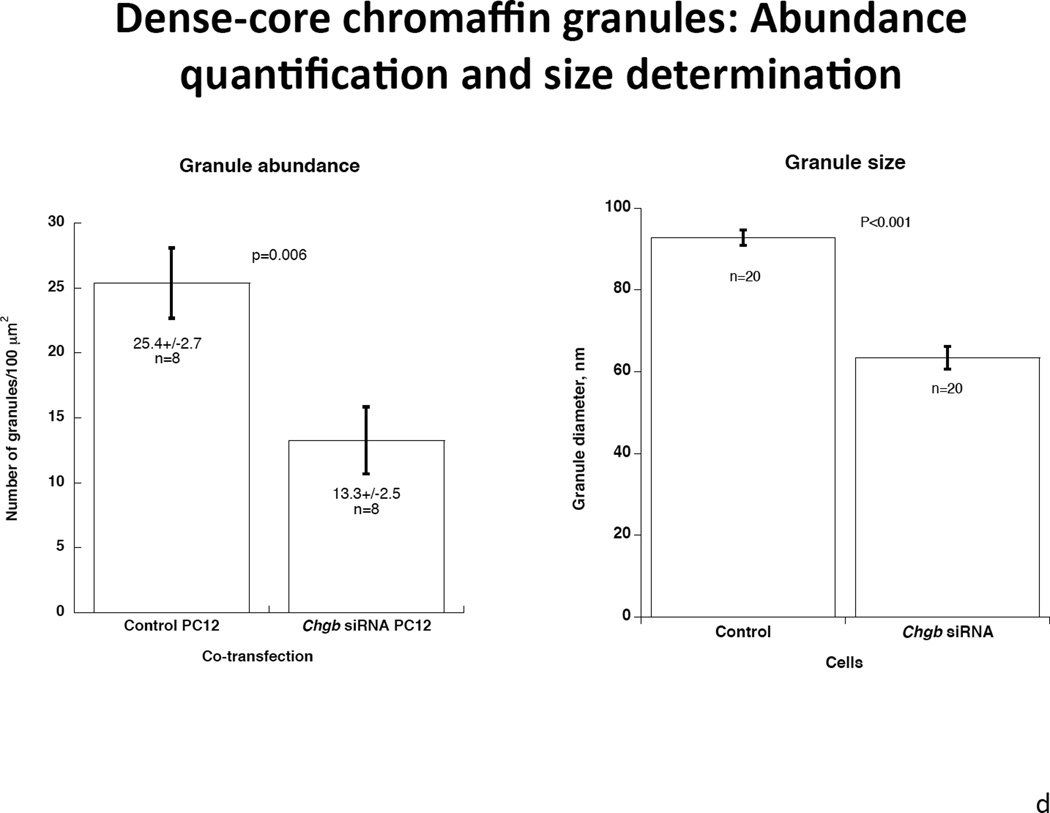
1a. Left: Immunoblot of CHGB protein in siRNA-transfected PC12 cells. The rat CHGB siRNA siGENOME “smart pool” was transfected into PC12 cells and silencing of CHGB expression was evaluated by immunoblotting at the target protein level. ~95% knockdown of CHGB expression was achieved by 15 nM siRNA. Right: Quantification of CHGB in the blot. Band intensity was scanned and scored by ImageJ. 1b. Expression of catecholamine pathway (storage, synthesis) genes in CHGB siRNA-silenced PC12 cells. The rat CHGB siRNA siGENOME “smart pool” was transfected at 15 nM into PC12 cells and transcript expression was scored by real-time qPCR, and normalized to the signal for β-actin. Each condition was repeated four times. During knockdown, the CHGB transcript was under-expressed, while Chga and Dbh were over-expressed, but Scg2 and Th were unchanged. 1c. Visualization of dense-core granules in CHGB siRNA-silenced PC12 cells by transmission EM. PC12 cells were transfected with the rat CHGB siRNA siGENOME “smart pool” at 15 nM and visualized by Zeiss EM10B electron microscope. CHGB siRNA-silenced PC12 cells display fewer dense-core granules than control (mock-transfected) cells. Arrowheads: Dense core secretory granules. Bar: 2 µm. 1d. Quantification of dense-core chromaffin granule abundance after CHGB silencing by siRNA. The data come from figure 1C. Left, granule number, n=8 cells were counted for each condition. Right, granule diameter, n=20 granules were measured for each condition. 1e. Effect of CHGB silencing on exogenous catecholamine uptake and secretagogue-triggered release. PC12 cells were labeled with [3H]-L-norepinephrine and treated with nicotine at 60 µM.The supernant and cell lysates were collected to measure radioactivity. The uptake was expressed as counts in cell lysate. The percent (%) secretion was expressed as [amount released/(amount released + amount in cell lysate)] × 100. Each condition was repeated three times. CHGB-silenced PC12 cells displayed diminished uptake of exogenous catecholamine, as well as reduced release catecholamine when stimulated by nicotine.
Effect of CHGB siRNA knockdown on catecholamine storage, uptake, or secretion in chromaffin cells
After CHGB siRNA, transmission electron micrographs indicated a ~48% less of dense core granule abundance in CHGB siRNA treated PC12 cells compared to control cells (Figure 1c). There were 25.4±2.7 granules per 100 µm2) in control PC12 cells and 13.3±2.5 granules per 100 µm2 in CHGB siRNA PC12 cells (p=0.006, Figure 1d left). Average granule diameter was 63.4±2.75 nm after CHGB siRNA, versus 92.8±1.87 nm in control cells, thus declining by ~32% during knockdown (p<0.001, Figure 1d right). Uptake of exogenous catecholamines in CHGB siRNA-silenced cells declined by ~78% compared to control (p<0.0001, Figure 1e). Secretion of catecholamines during nicotinic cholinergic stimulation also declined by ~75% (p=0.005, Figure 1e). Basal secretion of catecholamine was also diminished in CHGB siRNA-silenced cells (p=0.023, Figure 1e).
CHGB targeted ablation in vivo: CHGB knockout mice
Morphology (transmission EM of the adrenal medulla)
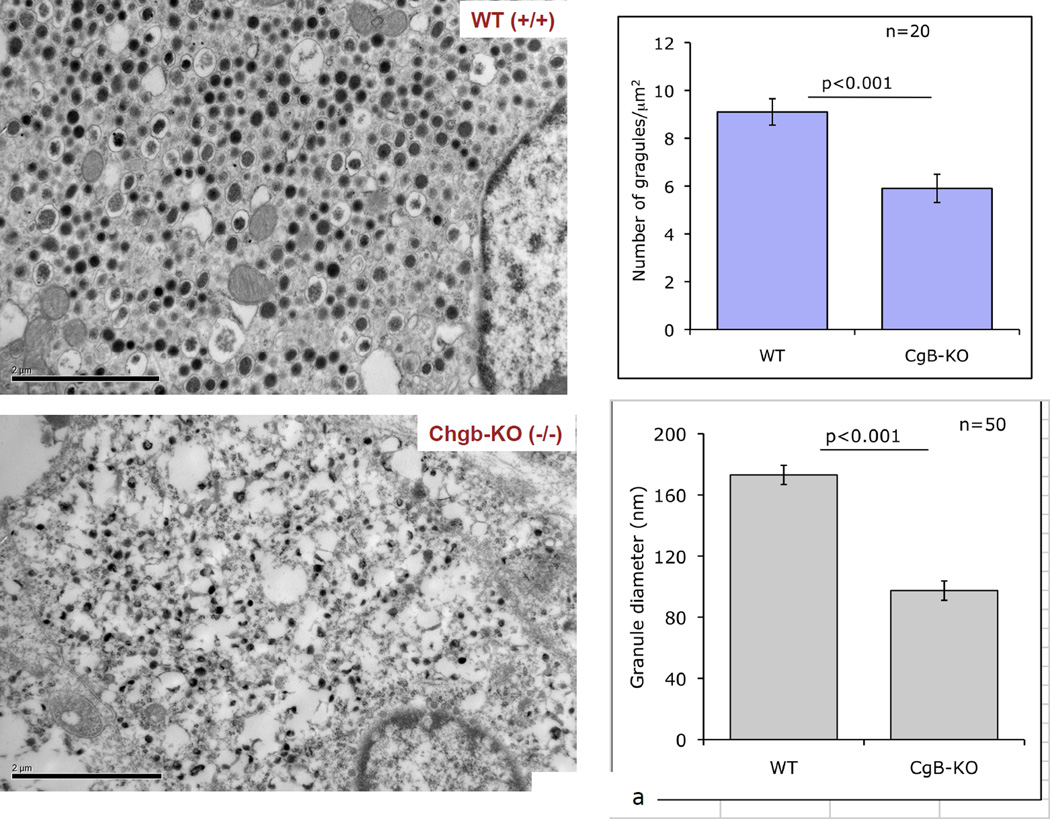
Transmission EM indicated that dense core granules in the adrenal medulla of CHGB(−/−) mice displayed both loss of abundance and decline in size. In CHGB knockout mice, the number of granules was 9.1±0.55 per µm2 in CHGB(+/+) mice versus 5.9±0.59 per in CHGB(−/−) mice, thus declining by ~35% (p<0.001, Figure 2a). The average diameter of dense core granules was 173.1±6.3 nm in CHGB(+/+) mice, declining by ~44% to 97.4±3.6 in CHGB(−/−) mice (p<0.001, Figure 2a).
Figure 2. Effects of CHGB targeted ablation in vivo: The CHGB ablation (−/−) mouse.
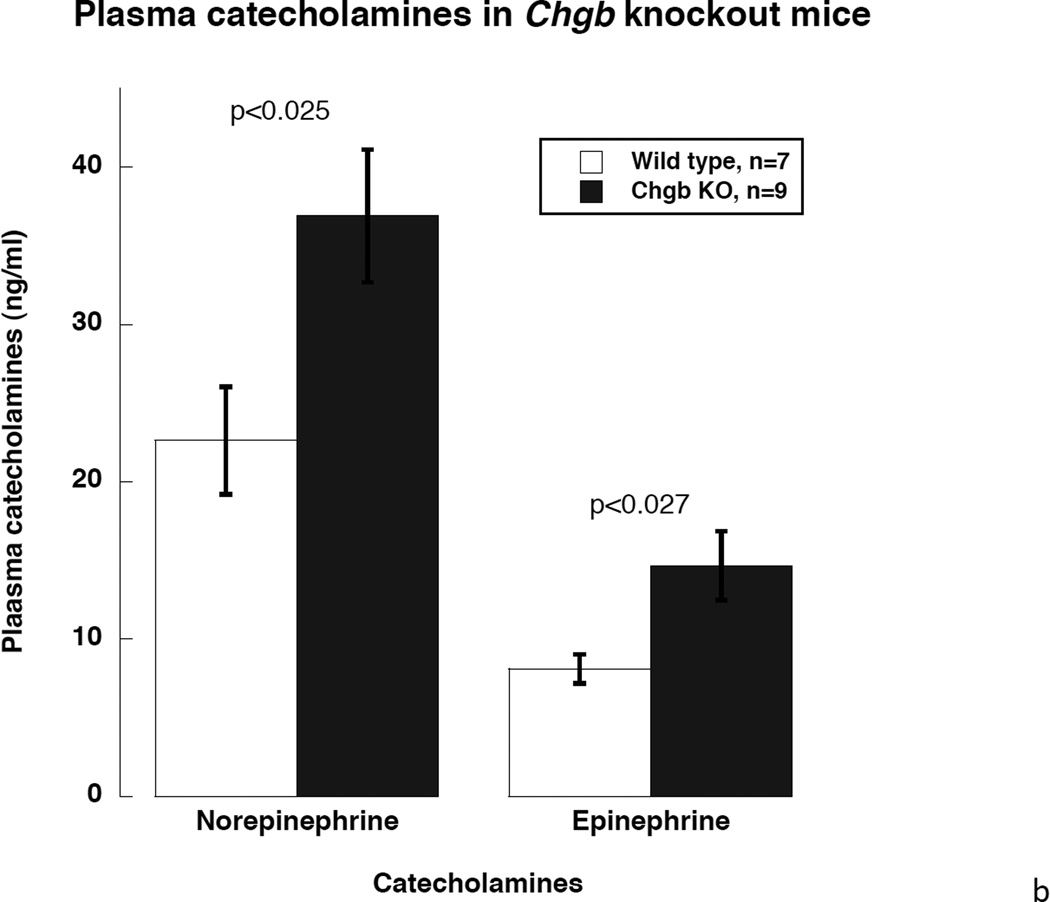
2a. Left: Morphology of the adrenal medulla by transmission EM. Visualization of dense-core granules in CHGB (+/+; top) versus CHGB (−/−; bottom) mice. Bars: 2 µm. Right: Quantification and diameter determination of dense-core chromaffin granule abundance in CHGB (+/+) versus CHGB (−/−). Both the number (n=20 cells were counted for each condition) and size of granules (n=20 granules were measured for each condition) declined in CHGB (−/−) mice. 2b. Catecholamine secretion during CHGB ablation in vivo. Mouse plasma sample were collected and catecholamines were measured by HPLC-ECD system. Both norepinephrine and epinephrine in the circulation increased after CHGB ablation. (n=7 for wild type and n=9 for Chgb knockout)
Catecholamine secretion in vivo
Here we found that plasma concentratios of both norepinephrine and epinephrine were significantly higher in CHGB knockout mice, with norepinephrine increasing from 22.6±3.4 to 36.9±4.2 ng/ml in CHGB(−/−) mice, and epinephrine from 8.1±0.9 to 14.7±2.2 ng/ml: increments of ~1.6-fold for norepinephrine and ~1.7-fold for epinephrine (each p<0.05, Figure 2b).
Over-expression of CHGB in chromaffin cells by lentivirus transduction
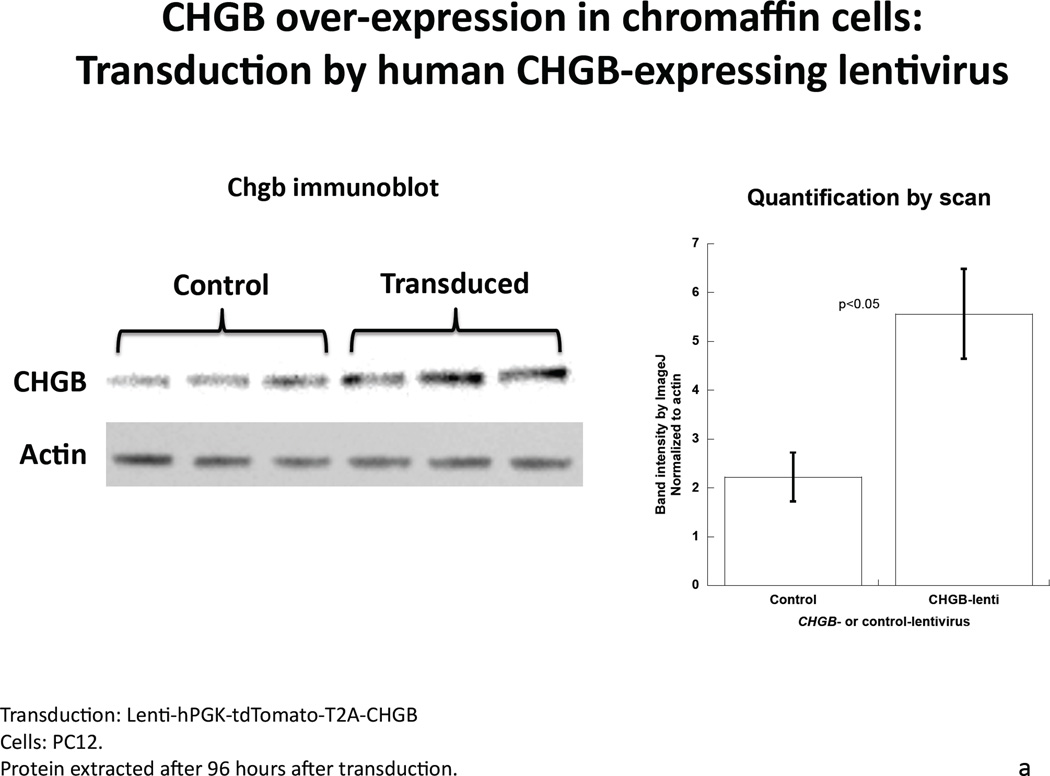
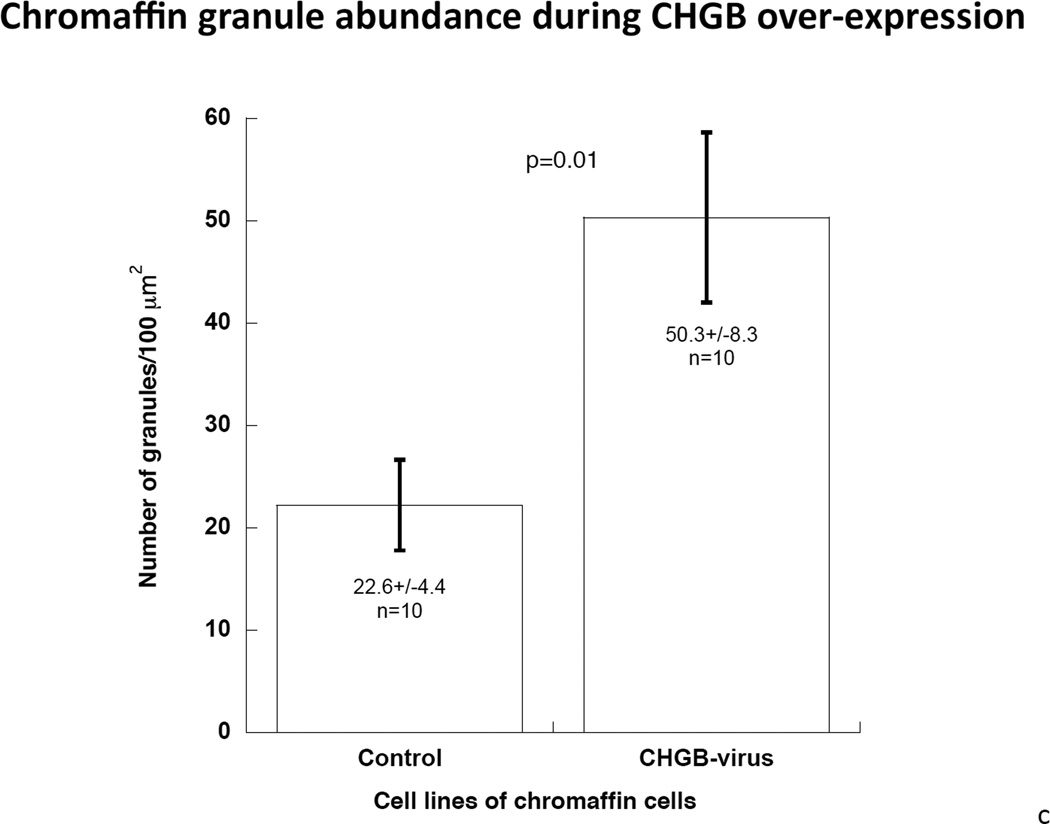
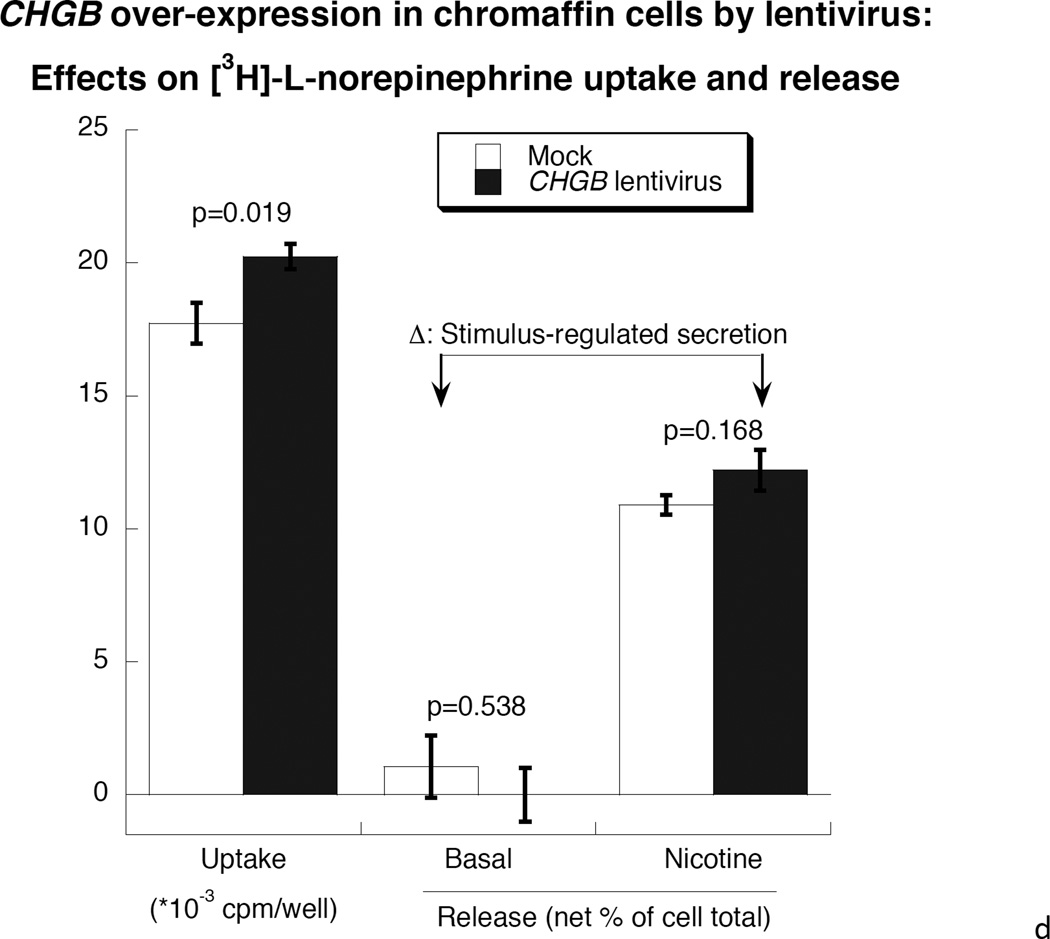
After transduction by CHGB-lentivirus, over-expressing PC12 cells were selected by flow cytometry based on the tdTomato photoprotein red fluorescence (Supplemental Figure 2). Transduction caused an increase of CHGB protein by ~127% compared to control cells (Figure 3a), while the abundance of dense core granules increased from 22.2±4.4 per high-power field (2.0 × 10−10 m2) in control cells to 50.3±8.3 in virus-infected cells (P=0.01, Figure 3b and 3c). The diameter of granules was unchanged, at 92.8±1.87 nm in control cells versus 90.9±2.98 nm in CHGB over-expressing cells. Over-expression of CHGB caused increased exogenous catecholamine uptake by ~14% (p=0.019), but did not alter nicotinic cholinergic stimulation of catecholamine release (p>0.05, Figure 3d).
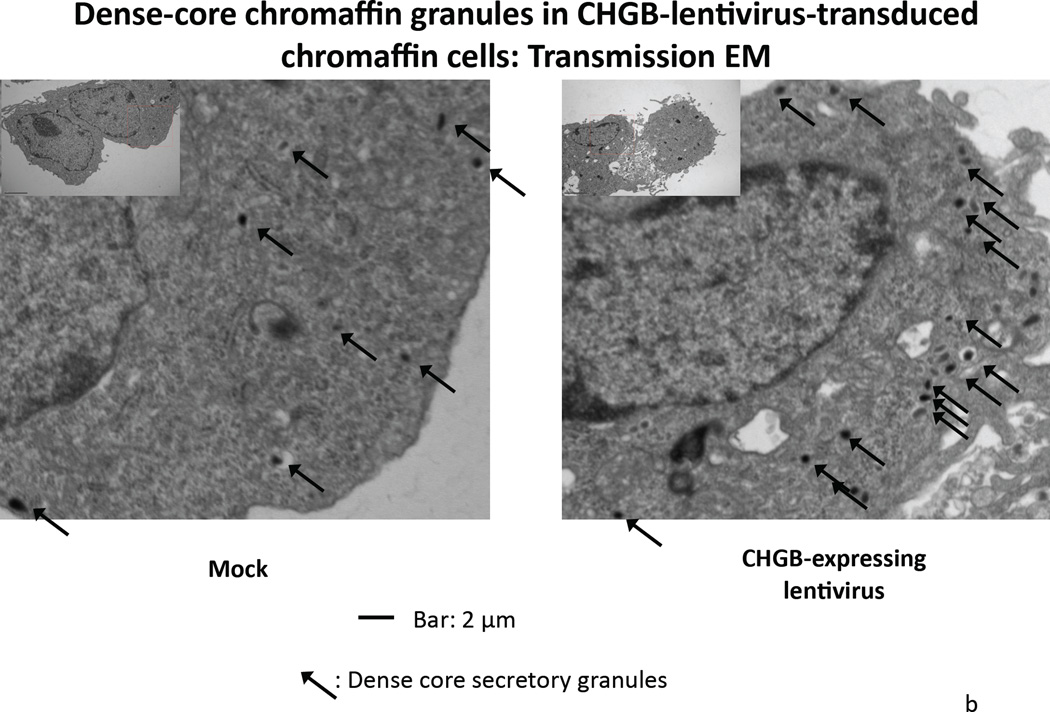
Figure 3. Over-expression of CHGB by lentiviral transduction in chromaffin cells.
3a. Left: Immunoblot of CHGB in human CHGB-lentivirus transduced PC12 cells. Right: Quantification of CHGB by scan. After 48 hours of transduction, CHGB was quantified by immunoblot with goat polyclonal IgG (sc-1489, Santa Cruz Biotechnology). Actin was used as a housekeeping probe. A ~150% increase of CHGB protein was achieved by this transduction. Band intensity was scored by ImageJ. 3b. Visualization of dense-core granules in CHGB-lentivirus-transduced PC12 cells, by transmission EM. The main/larger image magnifications are 1/12 of the originals (insets shown in upper left corners). The magnification of the original image (inset) was 10,000x. The scale bar in the original (inset) images is 2 µm. Arrowheads: Dense core secretory granules. 3c. Quantification of dense-core chromaffin granule abundance in hCHGB-lentivirus-transducted PC12 cells. The hCHGB transducted PC12 cells display more granules than control PC12 cells (p=0.01, n=10 cells were counted for each condition). Granule diameter was similar in both cell lines, at 92.8±1.87 nm in control cells and 90.9±2.98 nm in CHGB over-expressing cells. 3d. Effect of CHGB over-expression on catecholamine uptake and release in PC12 cells. Each condition was repeated three times. After the transduction of CHGB-lentivirus, the uptake of exogenous catecholamine increased by ~14%, but nicotine-stimulated secretion did not change.
Extra-cellular effects of CHGB and its proteolytic fragments: Catecholamine release from chromaffin cells
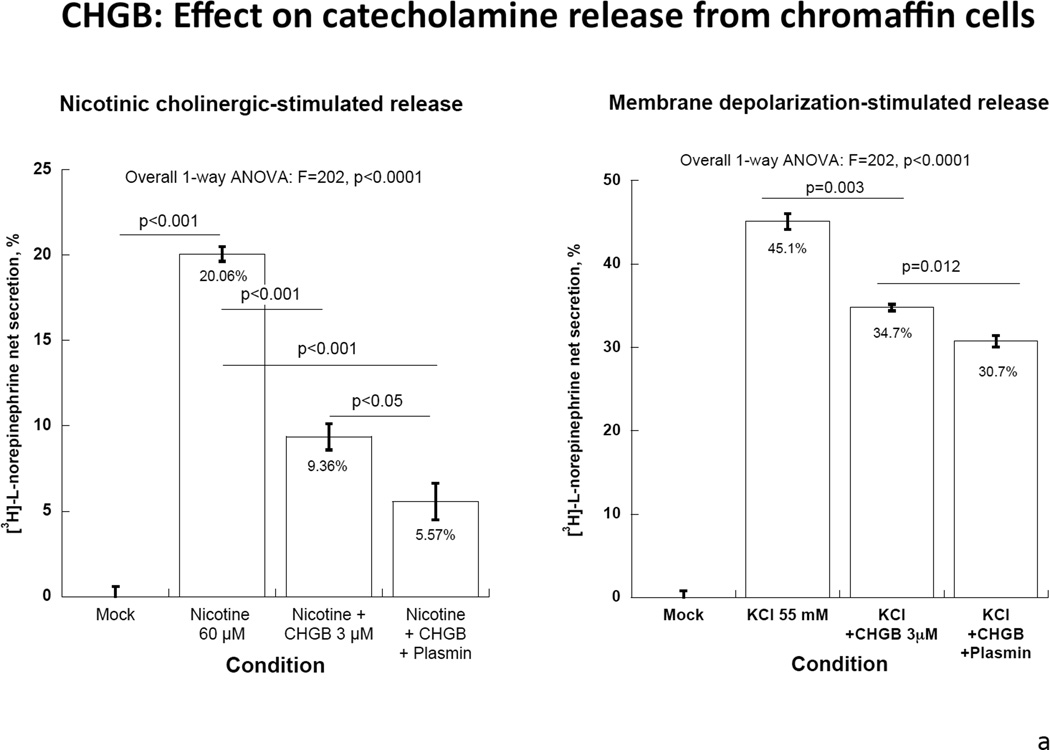
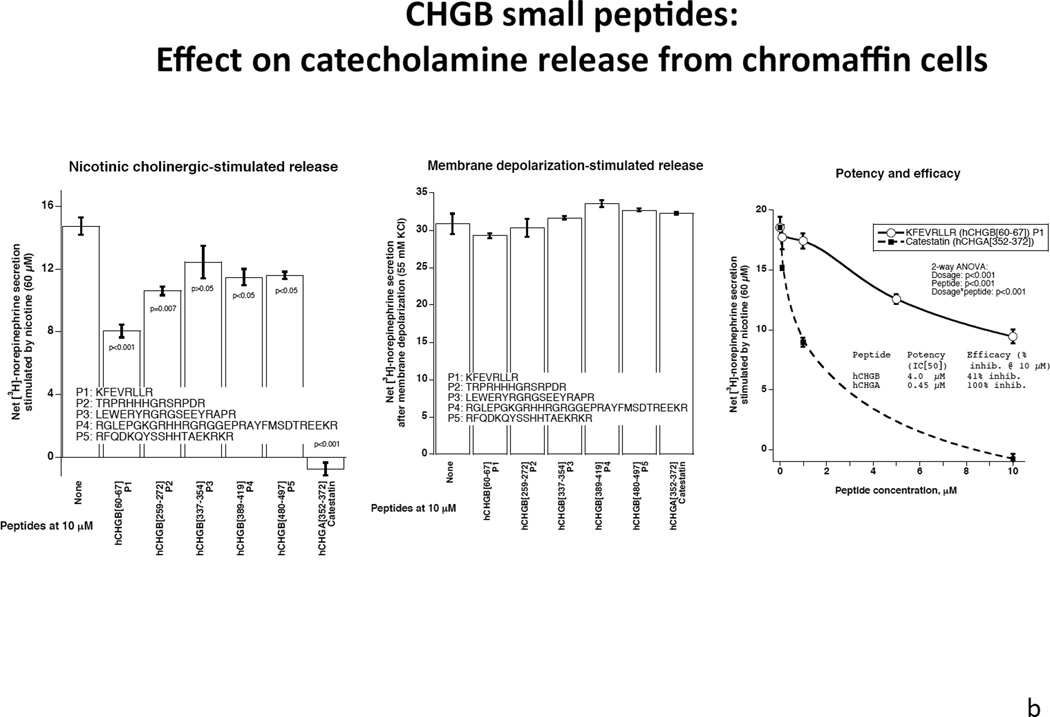
Both intact CHGB itself and its plasmin-digested fragments inhibited nicotinic cholinergic-stimulated catecholamine release, with ~53% reduction by CHGB itself and ~72% reduction by its fragments (p<0.01, Figure 4A, left). By contrast, CHGB or its fragments only moderately inhibited membrane depolarization-stimulated catecholamine release: ~23% reduction by CHGB and ~32% by its proteolytic fragments (p<0.01, Figure 4A, right).
Figure 4. Extracellular CHGB effects on catecholamine release.
Each experiment was repeated 3 times.
4a: Human CHGB protein or its proteolytically-digested fragments on nicotinic cholinergic versus membrane depolarization (KCl) -stimulated catecholamine release. CHGB protein or its plasmin-digested fragments inhibited predominantly nicotine-stimulated catecholamine release. 4b: Left: Synthetic CHGB peptides on nicotinic cholinergic stimulated catecholamine release. Catestatin (human CHGA[352–372]) served as a positive inhibitory control. Peptide human CHGB[60–67] displayed the greatest inhibitory effect among these peptides. P value is displayed for each peptide compared with negative control. Middle: Synthesized Human CHGB peptides on membrane depolarization (KCl)- stimulated catecholamine release. Catestatin (human CHGA[352–372]) served as a positive inhibitory control. No inhibitory effect was observed for these peptides (p>0.05 for each peptide).
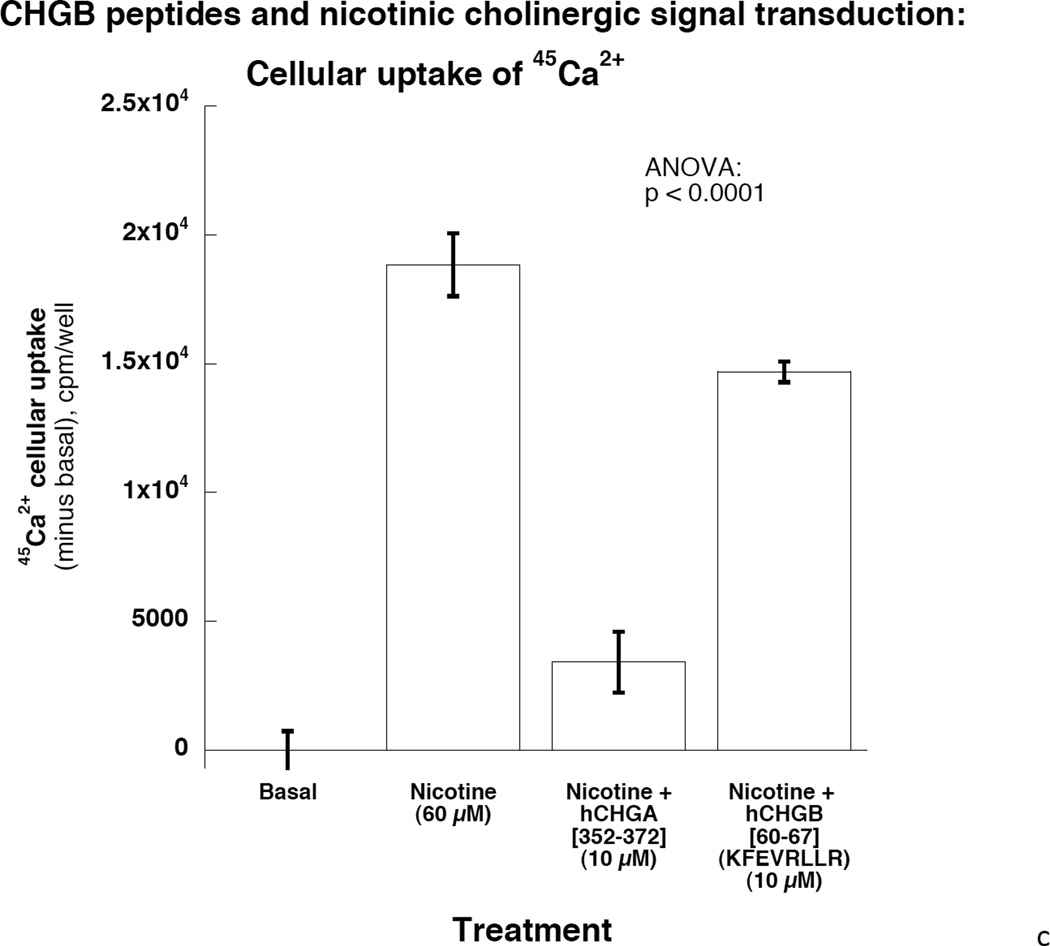
Right: Potency and efficacy of CHGB[60–67] on nicotinic cholinergic-stimulated catechalmine release. Catestatin (human CHGA[352–372]) served as a positive control. Potency and efficacy of CHGB[60–67] are displayed. 4c: CHGB peptide effect on nicotinic cholinergic cationic (Ca2+) signal transduction in chromaffin cells. PC12 cells were treated with 45Ca2+ at 2 µCi/ml and incubated under different conditions including basal, nicotine alone at 60 µM, and nicotine with hCHGA or hCHGB peptides. Catestatin (human CHGA[352–372]) served as a positive control inhibitor. Human CHGB[60–67] moderately (by ~22%) inhibited nicotine-triggered uptake of 45Ca.
Screening CHGB peptides for inhibition of nicotinic cholinergic secretion
The 5 synthetic CHGB peptides displayed a spectrum of inhibitory effects on nicotinic cholinergic secretion, with the shortest fragment CHGB[60–67] (KFEVRLLR) exerting the most prominent effect, reducing nicotinic stimulation’s effect by ~41%. As a positive control, human catestatin (CHGA[352–372]) completely abrogated nicotine’s effect (Figure 4b, left). By contrast, synthetic CHGB peptides had little or no effect on catecholamine release when stimulated by membrane depolarization (Figure 4b, middle), suggesting an element of nicotinic pathway specificity for the secretory effect.
Potency and efficacy of CHGB peptide CHGB[60–67] (KFEVRLLR) to inhibit nicotinic cholinergic-stimulated catecholamine secretion, as well as its cationic signal transduction in chromaffin cells
The inhibitory effect of CHGB[60–67] on nicotinic cholinergic secretion was dose-dependent in PC12 cells. The potency (IC[50]) was 4 µM for CHGB[60–67], versus 0.45 µM for catestatin, while the efficacy (maximal effect, at 10 µM peptide) was ~49% inhibiton for CHGB[60–67] versus ~100% for catestatin (Figure 4b, right). Nicotine stimulated uptake of 45Ca2+, an effect strongly (~82%) inhibited by catestatin, and moderately (~22%) inhibited by CHGB[60–67] (p<0.0001, Figure 4c). The inhibitory peptide sequence, CHGB[60–67] (K60FEVRLLR67), was 100% (i.e., perfectly) conserved across human (P05060), rat (O35314), and mouse (P16014) CHGB protein sequences (UniProt identifiers <http://www.uniprot.org>). This oligopeptide region is located within an amphiphilic domain (similar to the CHGA fragment catestatin) (33), and more specifically within an amphiphilic helical (or coiled-coil) patch, as previously described for both CHGA and CHGB (34).
Endoproteolytic cleavage of CHGB in the region of the active secretory peptide
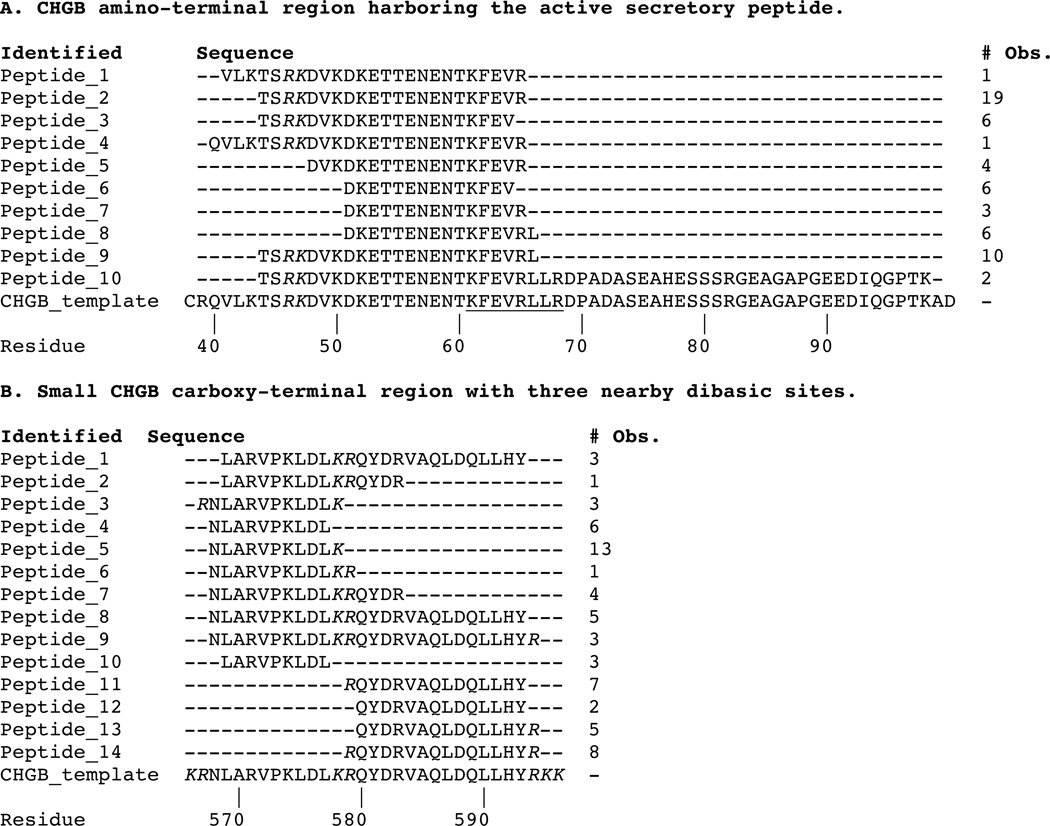
We analyzed plasmin-liberated human CHGB peptides by LC-MS/MS, to position the cleavage sites for this chromaffin cell endoproteolytic system. In the region of the active peptide (Table 1A), extensive cleavage was noted, primarily at monobasic sites (i.e., R or K), though also at dibasic site R46K47. As a control to explore endoprotease cleavage targets elsewhere in CHGB, we also evaluated a region (CHGB565–596) containing three dibasic sites – K566R567, K578R579, and R595K596K597 – that bound two putative peptides for liberation (CHGB568–577 and CHGB580–594); each of these three dibasic sites was utilized by plasmin, and the expected peptides were formed (Table 1B).
Table 1. Tandem MS on human CHGB after endoproteolytic cleavage by plasmin.
Alignments by Clustal Omega (1.2.0).
Legend: Italics = paired basic residues; Underlined = Active peptide tested; # Obs = Number of times that peptide was observed in spectra.
 |
DISCUSSION
Overview
The chromogranin/secretogranin acidic proteins are mainly located within neuroendocrine secretory vesicles such as chromaffin granules and large dense-core vesicles (LDCVs) of monoaminergic neurons (1,2). In these organelles, chromogranins constitute the main protein component of the intravesicular matrix. An important property of chromogranins is their ability to bind solutes, thereby allowing LDCVs to accumulate large amounts of catecholamines while maintaining their osmotic stability(35,36), an effect essential for the maintenance of neurosecretory activity. Several functions have been proposed for chromogranin B (CHGB), including granule biogenesis and sorting (7,10), Ca2+ sequestration and release (37), and a source of bioactive peptides (38,39). Alterations in CHGB expression accompany several human diseases of disordered neurotransmitter storage or release, such as hypertension (18,19,40), schizophrenia (41,42), and Alzheimer disease (43). While previous studies of CHGB abundance suggested alterations in storage granule abundance, quantitative studies of physiological processes underpinning catecholamine synthesis, uptake, storage, and secretagogue-triggered release have been lacking.
CHGB under- and over-expression in catecholaminergic cells, as well as in vivo
In PC12 cells, si-RNA silencing successfully diminished CHGB protein expression, with consequent reduction of chromaffin granule abundance, uptake of exogenous catecholamines, and secretagogue-stimulated catecholamine release (Figure 1e). Conversely, expression viral transduction successfully increased CHGB expression, with an increase in chromaffin granule abundance, but only minor changes in cellular ability to uptake catecholamines or respond to secretory stimulation (Figure 3d). Thus, the range of CHGB concentrations between physiological and zero have profound consequences for catecholamine metabolism, while elevation of CHGB beyond the physiological range does influence granule morphogenesis, but seems to have fewer physiological effects, suggesting a sufficient or saturating CHGB concentration under basal circumstances.
Both we (Figure 1b) and Diaz-Vera et al (22) found changes in expression of other chromaffin cell mRNAs during diminution of CHGB expression, especially over-expression of Chga mRNA. We speculate that such an increase may have dampened the already impressive consequences of CHGB depletion (Figure 1e). Diaz-Vera et al (22) noted diminished adrenal medullary storage of epinephrine and norepinephrine, consistent with our finding of decreased catecholamine uptake capacity (Figure 1e).
In vivo (targeted ablation of the mouse CHGB locus), we found similar changes in granule morphology (Figure 2a), with apparently unregulated catecholamine release (Figure 2b), which may underlie the substantially elevated BP observed in the CHGB(−/−) mouse (18). Of note, we previously observed similar catecholamine release and BP effects in the Chga knockout mouse (28).
Using an expressed frog CHGA (fCHGA) cDNA and a non-chromaffin cell line (COS-7), Montero-Hadjadje, et al demonstrated that CHGA has the intrinsic capacity to induce formation of mobile secretory granules and to promote the sorting and release of peptide hormones (44). Our finding of a role for CHGB on the biogenesis of dense core granules (in chromaffin and PC12 cells) indicate that these two homologous proteins exert similar functions.
CHGB peptide regions on catecholamine release
The CHGA peptide catestatin (human CHGA[352–372]) inhibits the physiological (nicotinic cholinergic) pathway of catecholamine release in chromaffin cells (28,29) and polymorphisms in the human catestatin region are associated with risk of hypertension (45). Here we observed a similar (though less potent) inhibitory effect on nicotinic stimulated secretion for CHGB and its proteolytic fragments (Figure 4a). We then synthesized several peptides that potentially mimic the effects of catestatin, and found that such peptides can inhibit nicotinic cholinergic-stimulated catecholamine release (Figure 4b left) and cationic signal transduction (Figure 4c) in chromaffin cells, albeit at lower potency and efficacy than catestatin. The complete lack of effect of CHGB synthetic peptides on catecholamine secretion triggered by membrane depolarization (Figure 4b center) reinforces the specificity of such peptides for the nicotinic cholinergic pathway.
The active human CHGB region is completely conserved across mammalian species (human, rat, mouse), and the target region is extensively cleaved by the chromaffin cell plasmin endoproteolytic system (Table 1A), though we have not yet positioned the precise boundaries of the active peptide as formed in vivo; as a control, the same system exerts the expected cleavage pattern on multiple dibasic sites in the human CHGB substrate (Table 1B). A more extensive synthetic peptide scan across the entire CHGB protein (or its highly conserved regions) could better establish the active region(s) of the protein.
Conclusions and perspectives
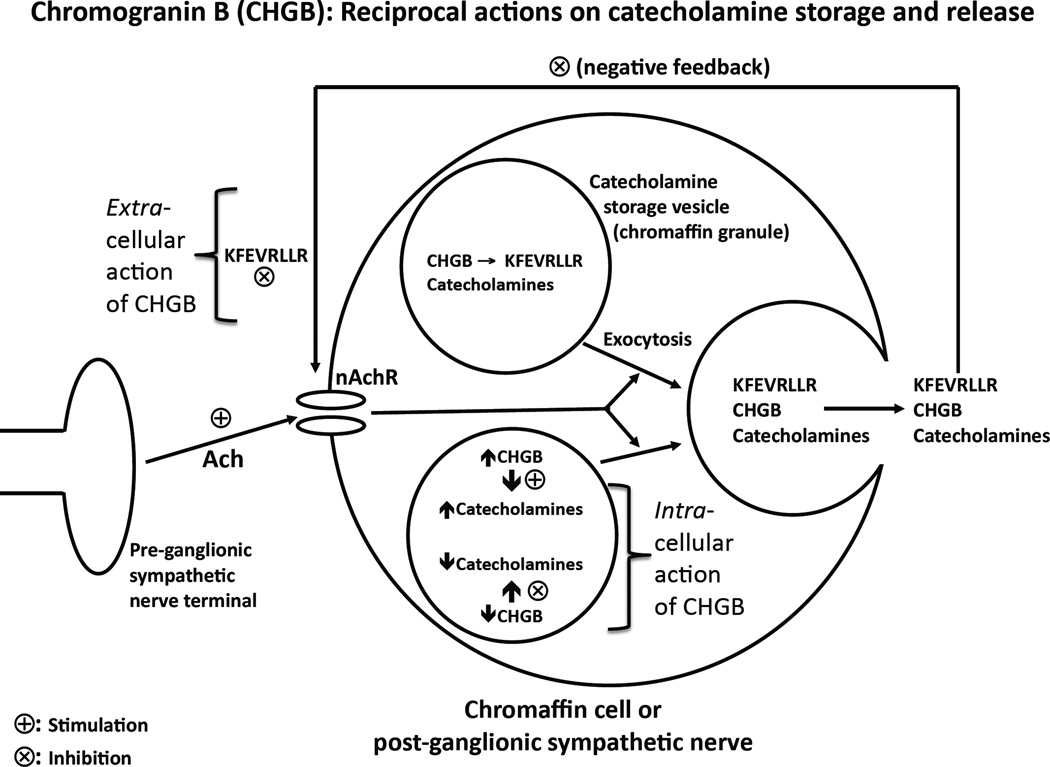
Within chromaffin cells, CHGB participates in assembly of catecholamine secretory vesicles (dense-core granules), and governs the secretory capacity of these granules under the nicotinic stimulation. After proteolytic cleavage and release into the extracellular space, CHGB (and particularly its peptide hCHGB[60–67]) seems to exert a negative feedback effect to inhibit the physiological secretory response to acetylcholine. The autocrine effects of released/processed CHGB on secretion (Figure 5) would be most likely to occur on chromaffin cells, whose chromaffin granules (or LDV [large dense core vesicles]) typically contain both chromogranins and catecholamines, though even sympathetic nerve termini which contain substantial numbers of SDVs (small dense core vesicles, without chromogranins) also harbor some chromogranin-bearing LDVs (46).
Figure 5. Schema: Reciprocal actions of CHGB on catecholamine storage (stimulation) and release (inhibition).
The figure synthesizes the consequences of our CHGB results for these physiological processes. Within chromaffin cells, CHGB participates in assembly of catecholamine secretory vesicles (dense-core granules), and governs the secretory capacity of these granules under the nicotinic stimulation. After proteolytic cleavage and release into the extracellular space, CHGB (and particularly its peptide hCHGB[60–67]) seems to exert a negative feedback effect to inhibit the secretory response to acetylcholine.
Derangements of CHGB expression may thus lead to impaired catecholamine storage, with consequently elevated constitutive catecholamine release, eventuating in systemic hypertension. Our data provide direct bi-directional evidence implicating CHGB in vesicular storage of catecholamines, and also novel evidence implicating released CHGB in control of exocytosis. Thus CHGB emerges as a multi-functional protein/peptide, with both intra-cellular and extracellular activities (Figure 5), whose novel properties may contribute to a spectrum of catecholaminergic disease states.
Supplementary Material
ACKNOWLEDGEMENTS
Support: National Institutes of Health, Department of Veterans Affairs.
Glossary of abbreviations
- BP
Blood pressure
- CHGA
Chromogranin A
- CHGB
Chromogranin B (human: CHGB; rodent: CHGB)
- cPPT
central polypurine tract
- DBP
Diastolic blood pressure
- HBSS
Hank's Balanced Salt Solution
- hCHGB
Human CHGB
- HR
Heart rate
- hPGK
Human phosphoglycerate kinase
- HPLC-ECD
high performance liquid chromatography coupled to an electrochemical detection
- IPTG
Isopropylβ-D-1-thiogalactopyranoside
- KO
Knock-out
- LC-MS/MS
C-18 reverse-phase Liquid Chromatography, followed by tandem Mass Spectroscopy
- LDCV
large dense-core vesicle
- LTR
Long terminal repeat
- M.O.I.
Multiplicity of infection
- PCR
Polymerase chain reaction
- PC12
Rat pheochromocytoma cell line
- QTL
Quantitative Trait Locus
- siRNA
Small interfering RNA
- SBP
Systolic blood pressure
- T2A
2A peptide, self-cleavage
- TEM
Transmission electron microscopy
- UTR
Untranslated region
- VSV
Vesicular stomatitis virus
- WPRE
Woodchuck hepatitis virus Posttranscriptional Regulatory Element
Footnotes
Conflicts of interest: None to declare.
REFERENCES
- 1.Huttner WB, Gerdes HH, Rosa P. The granin (chromogranin/secretogranin) family. Trends Biochem Sci. 1991;16:27–30. doi: 10.1016/0968-0004(91)90012-k. [DOI] [PubMed] [Google Scholar]
- 2.Taupenot L, Harper KL, O'Connor DT. The chromogranin-secretogranin family. N Engl J Med. 2003;348:1134–1149. doi: 10.1056/NEJMra021405. [DOI] [PubMed] [Google Scholar]
- 3.Rosa P, Hille A, Lee RW, Zanini A, De Camilli P, Huttner WB. Secretogranins I and II: two tyrosine-sulfated secretory proteins common to a variety of cells secreting peptides by the regulated pathway. J Cell Biol. 1985;101:1999–2011. doi: 10.1083/jcb.101.5.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Falkensammer G, Fischer-Colbrie R, Winkler H. Biogenesis of chromaffin granules: incorporation of sulfate into chromogranin B and into a proteoglycan. J Neurochem. 1985;45:1475–1480. doi: 10.1111/j.1471-4159.1985.tb07215.x. [DOI] [PubMed] [Google Scholar]
- 5.O'Connor DT, Frigon RP, Sokoloff RL. Human chromogranin A. Purification and characterization from catecholamine storage vesicles of human pheochromocytoma. Hypertension. 1984;6:2–12. doi: 10.1161/01.hyp.6.1.2. [DOI] [PubMed] [Google Scholar]
- 6.Schober M, Fischer-Colbrie R, Schmid KW, Bussolati G, O'Connor DT, Winkler H. Comparison of chromogranins A, B, and secretogranin II in human adrenal medulla and pheochromocytoma. Lab Invest. 1987;57:385–391. [PubMed] [Google Scholar]
- 7.Huh YH, Jeon SH, Yoo SH. Chromogranin B-induced secretory granule biogenesis: comparison with the similar role of chromogranin A. J Biol Chem. 2003;278:40581–40589. doi: 10.1074/jbc.M304942200. [DOI] [PubMed] [Google Scholar]
- 8.Gill BM, Barbosa JA, Dinh TQ, Garrod S, O'Connor DT. Chromogranin B: isolation from pheochromocytoma, N-terminal sequence, tissue distribution and secretory vesicle processing. Regul Pept. 1991;33:223–235. doi: 10.1016/0167-0115(91)90216-4. [DOI] [PubMed] [Google Scholar]
- 9.Karlsson E, Stridsberg M, Sandler S. Chromogranin-B regulation of IAPP and insulin secretion. Regul Pept. 2000;87:33–39. doi: 10.1016/s0167-0115(99)00105-6. [DOI] [PubMed] [Google Scholar]
- 10.Natori S, Huttner WB. Chromogranin B (secretogranin I) promotes sorting to the regulated secretory pathway of processing intermediates derived from a peptide hormone precursor. Proc Natl Acad Sci U S A. 1996;93:4431–4436. doi: 10.1073/pnas.93.9.4431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Binder A. A review of the genetics of essential hypertension. Curr Opin Cardiol. 2007;22:176–184. doi: 10.1097/HCO.0b013e3280d357f9. [DOI] [PubMed] [Google Scholar]
- 12.Greenwood TA, Cadman PE, Stridsberg M, et al. Genome-wide linkage analysis of chromogranin B expression in the CEPH pedigrees: implications for exocytotic sympathochromaffin secretion in humans. Physiol Genomics. 2004;18:119–127. doi: 10.1152/physiolgenomics.00104.2003. [DOI] [PubMed] [Google Scholar]
- 13.Greenwood TA, Rao F, Stridsberg M, et al. Pleiotropic effects of novel trans-acting loci influencing human sympathochromaffin secretion. Physiol Genomics. 2006;25:470–479. doi: 10.1152/physiolgenomics.00295.2005. [DOI] [PubMed] [Google Scholar]
- 14.O'Connor DT, Takiyyuddin MA, Printz MP, et al. Catecholamine storage vesicle protein expression in genetic hypertension. Blood Press. 1999;8:285–295. doi: 10.1080/080370599439508. [DOI] [PubMed] [Google Scholar]
- 15.Schober M, Howe PR, Sperk G, Fischer-Colbrie R, Winkler H. An increased pool of secretory hormones and peptides in adrenal medulla of stroke-prone spontaneously hypertensive rats. Hypertension. 1989;13:469–474. doi: 10.1161/01.hyp.13.5.469. [DOI] [PubMed] [Google Scholar]
- 16.Takiyyuddin MA, De Nicola L, Gabbai FB, et al. Catecholamine secretory vesicles. Augmented chromogranins and amines in secondary hypertension. Hypertension. 1993;21:674–679. doi: 10.1161/01.hyp.21.5.674. [DOI] [PubMed] [Google Scholar]
- 17.Lillie EO, O'Connor DT. Early phenotypic changes in hypertension: a role for the autonomic nervous system and heredity. Hypertension. 2006;47:331–333. doi: 10.1161/01.HYP.0000203980.44717.aa. [DOI] [PubMed] [Google Scholar]
- 18.Zhang K, Rao F, Rana BK, et al. Autonomic function in hypertension: Role of genetic variation at the catecholamine storage vesicle protein chromogranin B (CHGB) Circ Cardiovasc Genet. 2009;2:46–56. doi: 10.1161/CIRCGENETICS.108.785659. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Zhang K, Rao F, Wang L, et al. Common functional genetic variants in catecholamine storage vesicle protein promoter motifs interact to trigger systemic hypertension. J Am Coll Cardiol. 2010;55:1463–1475. doi: 10.1016/j.jacc.2009.11.064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Biswas N, Vaingankar SM, Mahata M, et al. Proteolytic cleavage of human chromogranin a containing naturally occurring catestatin variants: differential processing at catestatin region by plasmin. Endocrinology. 2008;149:749–757. doi: 10.1210/en.2007-0838. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Courel M, Rodemer C, Nguyen ST, et al. Secretory granule biogenesis in sympathoadrenal cells: identification of a granulogenic determinant in the secretory prohormone chromogranin A. J Biol Chem. 2006;281:38038–38051. doi: 10.1074/jbc.M604037200. [DOI] [PubMed] [Google Scholar]
- 22.Diaz-Vera J, Morales YG, Hernandez-Fernaud JR, et al. Chromogranin B gene ablation reduces the catecholamine cargo and decelerates exocytosis in chromaffin secretory vesicles. J Neurosci. 2010;30:950–957. doi: 10.1523/JNEUROSCI.2894-09.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Tiscornia G, Singer O, Verma IM. Production and purification of lentiviral vectors. Nat Protoc. 2006;1:241–245. doi: 10.1038/nprot.2006.37. [DOI] [PubMed] [Google Scholar]
- 24.Taylor CV, Taupenot L, Mahata SK, et al. Formation of the catecholamine release-inhibitory peptide catestatin from chromogranin A. Determination of proteolytic cleavage sites in hormone storage granules. J Biol Chem. 2000;275:22905–22915. doi: 10.1074/jbc.M001232200. [DOI] [PubMed] [Google Scholar]
- 25.Jiang Q, Taupenot L, Mahata SK, et al. Proteolytic cleavage of chromogranin A (CgA) by plasmin. Selective liberation of a specific bioactive CgA fragment that regulates catecholamine release. J Biol Chem. 2001;276:25022–25029. doi: 10.1074/jbc.M101545200. [DOI] [PubMed] [Google Scholar]
- 26.Parmer RJ, Mahata M, Gong Y, et al. Processing of chromogranin A by plasmin provides a novel mechanism for regulating catecholamine secretion. J Clin Invest. 2000;106:907–915. doi: 10.1172/JCI7394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Conlon JM, Hamberger B, Grimelius L. Isolation of peptides arising from the specific posttranslational processing of chromogranin A and chromogranin B from human pheochromocytoma tissue. Peptides. 1992;13:639–644. doi: 10.1016/0196-9781(92)90167-2. [DOI] [PubMed] [Google Scholar]
- 28.Mahapatra NR, O'Connor DT, Vaingankar SM, et al. Hypertension from targeted ablation of chromogranin A can be rescued by the human ortholog. J Clin Invest. 2005;115:1942–1952. doi: 10.1172/JCI24354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Mahata SK, Mahata M, Parmer RJ, O'Connor DT. Desensitization of catecholamine release. The novel catecholamine release-inhibitory peptide catestatin (chromogranin a344–364) acts at the receptor to prevent nicotinic cholinergic tolerance. J Biol Chem. 1999;274:2920–2928. doi: 10.1074/jbc.274.5.2920. [DOI] [PubMed] [Google Scholar]
- 30.Wegrzyn JL, Bark SJ, Funkelstein L, et al. Proteomics of dense core secretory vesicles reveal distinct protein categories for secretion of neuroeffectors for cell-cell communication. J Proteome Res. 2010;9:5002–5024. doi: 10.1021/pr1003104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Gupta N, Bark SJ, Lu WD, et al. Mass spectrometry-based neuropeptidomics of secretory vesicles from human adrenal medullary pheochromocytoma reveals novel peptide products of prohormone processing. J Proteome Res. 2010;9:5065–5075. doi: 10.1021/pr100358b. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Hook V, Bark S, Gupta N, et al. Neuropeptidomic components generated by proteomic functions in secretory vesicles for cell-cell communication. AAPS J. 2010;12:635–645. doi: 10.1208/s12248-010-9223-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Kennedy BP, Mahata SK, O'Connor DT, Ziegler MG. Mechanism of cardiovascular actions of the chromogranin A fragment catestatin in vivo. Peptides. 1998;19:1241–1248. doi: 10.1016/s0196-9781(98)00086-2. [DOI] [PubMed] [Google Scholar]
- 34.Mosley CA, Taupenot L, Biswas N, et al. Biogenesis of the secretory granule: chromogranin A coiled-coil structure results in unusual physical properties and suggests a mechanism for granule core condensation. Biochemistry. 2007;46:10999–11012. doi: 10.1021/bi700704r. [DOI] [PubMed] [Google Scholar]
- 35.Videen JS, Mezger MS, Chang YM, O'Connor DT. Calcium and catecholamine interactions with adrenal chromogranins. Comparison of driving forces in binding and aggregation. J Biol Chem. 1992;267:3066–3073. [PubMed] [Google Scholar]
- 36.Helle KB, Reed RK, Pihl KE, Serck-Hanssen G. Osmotic properties of the chromogranins and relation to osmotic pressure in catecholamine storage granules. Acta Physiol Scand. 1985;123:21–33. doi: 10.1111/j.1748-1716.1985.tb07556.x. [DOI] [PubMed] [Google Scholar]
- 37.Yoo SH, Jeon CJ. Inositol 1,4,5-trisphosphate receptor/Ca2+ channel modulatory role of chromogranin A. a Ca2+ storage protein of secretory granules. J Biol Chem. 2000;275:15067–15073. doi: 10.1074/jbc.M909391199. [DOI] [PubMed] [Google Scholar]
- 38.Strub JM, Garcia-Sablone P, Lonning K, et al. Processing of chromogranin B in bovine adrenal medulla. Identification of secretolytin, the endogenous C-terminal fragment of residues 614–626 with antibacterial activity. Eur J Biochem. 1995;229:356–368. doi: 10.1111/j.1432-1033.1995.tb20476.x. [DOI] [PubMed] [Google Scholar]
- 39.Winkler H, Laslop A, Leitner B, Weiss C. The secretory cocktail of adrenergic large dense-core vesicles: the functional role of the chromogranins. Adv Pharmacol. 1998;42:257–259. doi: 10.1016/s1054-3589(08)60742-5. [DOI] [PubMed] [Google Scholar]
- 40.Zhang K, Chen Y, Wen G, et al. Catecholamine storage vesicles: role of core protein genetic polymorphisms in hypertension. Curr Hypertens Rep. 2011;13:36–45. doi: 10.1007/s11906-010-0170-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Landen M, Grenfeldt B, Davidsson P, et al. Reduction of chromogranin A and B but not C in the cerebrospinal fluid in subjects with schizophrenia. Eur Neuropsychopharmacol. 1999;9:311–315. doi: 10.1016/s0924-977x(98)00042-x. [DOI] [PubMed] [Google Scholar]
- 42.Marksteiner J, Lechner T, Kaufmann WA, et al. Distribution of chromogranin B-like immunoreactivity in the human hippocampus and its changes in Alzheimer's disease. Acta Neuropathol (Berl) 2000;100:205–212. doi: 10.1007/s004010000239. [DOI] [PubMed] [Google Scholar]
- 43.Marksteiner J, Kaufmann WA, Gurka P, Humpel C. Synaptic proteins in Alzheimer's disease. J Mol Neurosci. 2002;18:53–63. doi: 10.1385/JMN:18:1-2:53. [DOI] [PubMed] [Google Scholar]
- 44.Montero-Hadjadje M, Elias S, Chevalier L, et al. Chromogranin A promotes peptide hormone sorting to mobile granules in constitutively and regulated secreting cells: role of conserved N- and C-terminal peptides. J Biol Chem. 2009;284:12420–12431. doi: 10.1074/jbc.M805607200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Rao F, Wen G, Gayen JR, et al. Catecholamine release-inhibitory peptide catestatin (chromogranin A(352–372)): naturally occurring amino acid variant Gly364Ser causes profound changes in human autonomic activity and alters risk for hypertension. Circulation. 2007;115:2271–2281. doi: 10.1161/CIRCULATIONAHA.106.628859. [DOI] [PubMed] [Google Scholar]
- 46.O'Connor DT, Klein RL, Thureson-Klein AK, Barbosa JA. Chromogranin A: localization and stoichiometry in large dense core catecholamine storage vesicles from sympathetic nerve. Brain Res. 1991;567:188–196. doi: 10.1016/0006-8993(91)90795-w. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.