Abstract
Objective
In lower-income countries rates of AIDS-defining events (ADEs) and death are high during the first year of combination antiretroviral therapy (ART). We investigated differences between foreign-born (migrant) and native-born (nonmigrant) patients initiating ART in Europe, the US and Canada, and examined rates of the most common ADEs and mortality during the first year of ART.
Design
Observational cohort study.
Methods
We studied HIV-positive adults participating in one of 12 cohorts in the Antiretroviral Therapy Cohort Collaboration (ART-CC).
Results
Of 48 854 patients, 25.6% were migrants: 16.1% from sub-Saharan Africa, 5.6% Latin America, 2.3% North Africa/Middle East, and 1.6% Asia. Incidence of ADEs during the first year of ART was 60.8 per 1000 person-years: 69.9 for migrants and 57.7 for nonmigrants [crude hazard ratio (HR) 1.18; 95% confidence interval (CI) 1.08–1.29], adjusted HR (for sex, age, CD4, HIV-1 RNA, ART regimen, prior ADE, probable route of infection and year of initiation, and stratified by cohort) 1.21 (95% CI 1.09–1.34). Rates of tuberculosis were substantially higher in migrants than nonmigrants (14.3 vs. 6.3; adjusted HR 1.94; 95% CI 1.53–2.46). In contrast, mortality was higher among nonmigrants than migrants (crude HR 0.71; 95% CI 0.61–0.84), although excess mortality was partially explained by patient characteristics at start of ART (adjusted HR 0.91; 95% CI 0.76–1.09).
Conclusions
During the first year of ART, HIV-positive migrants had higher rates of ADEs than nonmigrants. Tuberculosis was the most common ADE among migrants, highlighting the importance of screening for tuberculosis prior to ART initiation in this population.
Keywords: AIDS, antiretroviral therapy, HIV, migrant, mortality, tuberculosis
Introduction
Rates of AIDS-defining events (ADEs) and death are especially high during the first year of antiretroviral therapy (ART) among HIV-positive adults in lower-income countries [1]. Rates of disease progression among migrants from lower-income countries treated for HIV disease in higher-income nations were noted to be higher in the Netherlands [2,3], but tended to be similar to, or somewhat better than, those of nonmigrants in other populations in Europe and North America [4–7]. In a recent study of patients starting ART in Europe and followed for a median of 4 years, the Antiretroviral Therapy Cohort Collaboration (ART-CC) found that migrants had lower mortality than nonmigrants [8]. This has been attributed at least in part to a ‘healthy migrant effect,’ that a relatively high level of health is needed to migrate [9].
We studied rates and types of ADEs, and mortality, among migrants and nonmigrants during the first year of ART, among patients in Europe, the US and Canada. We examined whether differences were explained by patient characteristics at the start of ART.
Methods
The ART-CC includes 19 cohorts of HIV-positive patients starting ART in Europe, the US and Canada [8]. Our analyses included data on patients who started ART (a combination of at least two nucleoside reverse transcriptase inhibitors and boosted protease inhibitor or non-nucleoside reverse transcriptase inhibitor) at age at least 16 years between 1996 and 2009, from the 12 cohorts that routinely recorded country of birth/origin, nationality, or other related variables: The AIDS Therapy Evaluation Project, Netherlands (ATHENA); the Agence Nationale de la Recherche sur le SIDA et les hépatites virales (ANRS) CO3 Aquitaine Cohort, France; the ANRS CO4 French Hospital Database on HIV (FHDH), France; the Köln/Bonn Cohort, Germany; the Frankfurt Cohort, Germany; the Proyecto para la Informatización del Seguimiento Clínico-epidemiológico de la Infección por HIV y SIDA (PISCIS) Cohort, Spain; the Cohorte de la Red de Investigación en Sida (CoRIS), Spain; the Swiss HIV Cohort Study (SHCS), Switzerland; the HAART Observational Medical Evaluation and Research (HOMER), British Columbia, Canada; the South Alberta Clinic Cohort, Canada; Vanderbilt Comprehensive Care Clinic, US; and the University of Washington HIV Cohort, US. Patients and cohorts in whom region of origin was missing were excluded from this analysis.
Patients were classified as migrants or nonmigrants based on their region of birth, origin and/or nationality. Nonmigrants consisted of patients who were born in Europe, the US, Canada, or non-European Western countries (Australia or the Pacific). Migrant patients were classified as from sub-Saharan Africa, Latin America (Caribbean, South and Central America), North Africa/Middle East, or Asia.
Outcomes of interest were AIDS-defining events (based on 1993 Centers for Disease Control and Prevention classification criteria [10], excluding classification based only on CD4 <200 cells/µl) and all-cause mortality during the first year of ART. Patients were classified as lost to follow-up if their last visit was within the first year of ART initiation and more than a year before the study close date; patients who were lost to follow-up were censored at the date of their last study visit. Patients who were not lost to follow-up and did not experience an event during the first year were censored at 365 days. Baseline was defined as date of ART initiation. Baseline CD4 cell count and HIV-1 RNA were defined as the measurement closest to ART initiation within the 3 months prior to ART; if no such value was available (1.5 and 2.7% of CD4 and HIV-1 RNA, respectively), then the CD4/HIV-1 RNA measurement taken closest to initiation but within one month after ART was used. Patients without a baseline CD4 or HIV-1 RNA were not included in the study.
We plotted Kaplan–Meier estimates of the cumulative risk of ADE and death during the first year of ART. Estimates of the cumulative risk of ADE were similar when death was treated as a competing event (see online Supplementary Figure, http://links.lww.com/QAD/A318) [11]. Confidence intervals for incidence rates were computed using Poisson regression models. Hazard ratios for ADEs and death were estimated using Cox models. All models were stratified by cohort. Continuous covariates were included in models using restricted cubic splines (three knots for year of ART initiation, baseline age, and baseline log10-transformed HIV-1 RNA; four knots for baseline CD4 cell count). Other variables included in these analyses were sex, probable route of infection [men who have sex with men (MSM), injection drug use (IDU), heterosexual, other/unknown], clinical AIDS at ART initiation (defined by ADE not CD4 cell count), and type of initial regimen (protease inhibitor-based, NNRTI-based). All statistical analyses were performed with R statistical software (version 2.14, www.r-project.org). Analysis code is posted at http://biostat.mc.vanderbilt.edu/ Archived Analyses.
Results
Of 52 166 HIV-positive persons starting ART between 1996 and 2009 in participating cohorts in Europe, the US or Canada, region of origin was known for 48 854 (94%); patients missing region of origin were excluded from all subsequent analyses. Included patients came from eight cohorts in Europe (n = 45 715) and four cohorts in North America (n = 3139). Compared to those who were excluded, included patients were more likely to be female (30 vs. 23%), had slightly higher CD4 at ART initiation (219 vs. 198 cells/µl), were more likely to have had a prior ADE (22 vs. 15%), and less likely to have had injection drug use as the probable route of infection (12 vs. 22%).
Of the 48 854 patients included in the analysis, 12 487 (25.6%) were migrants: 7846 (16.1%) from sub-Saharan Africa, 2737 (5.6%) from Latin America, 1111 (2.3%) from North Africa/Middle East, and 793 (1.6%) from Asia. The proportion of migrants differed between the European and North American cohorts (26.3 vs. 14.0%, respectively), with migrants in European cohorts more likely to be from sub-Saharan Africa (63.5% of migrants in Europe vs. 45.1% of migrants in North America) or North Africa/Middle East (9.0 vs. 6.6%) and less likely to be from Latin America (21.7 vs. 26.9%) or Asia (5.8 vs. 21.4%) (P < 0.001).
Table 1 describes demographic characteristics and laboratory values at ART initiation among nonmigrants and migrants from different geographic areas. Migrants were more likely to be female (51 vs. 22%), younger (median age 34 vs. 38 years), more likely infected by heterosexual sex (76 vs. 33%), and slightly more immunosuppressed at baseline (median CD4 cell count 194 vs. 229 cells/µl). Median age at baseline was lowest among migrants from sub-Saharan Africa (33 years) and this group of patients had the highest proportion of females (60%). Migrants from sub-Saharan Africa had higher CD4 cell counts (median 200 cells/µl) and lower rates of ADEs prior to ART initiation (22%) than migrants from other regions. Year of ART initiation was more recent for migrants (median 2003) than nonmigrants (2001). The majority of patients initiated a regimen containing a protease inhibitor. The proportion of patients lost to follow-up during the first year of ART was 10% for nonmigrants and 11, 11, 7, and 7% for migrants from sub-Saharan Africa, Latin America, North Africa/Middle East, and Asia, respectively.
Table 1.
Characteristics of migrants and nonmigrants starting combination antiretroviral therapy (ART). Entries in table are percentage (frequency) unless otherwise shown.
| Migrants | ||||||
|---|---|---|---|---|---|---|
| Nonmigrants | Migrants | Sub-Saharan Africa |
Latin America |
North Africa/ Middle East |
Asia | |
| Number | 36367 | 12487 | 7846 | 2737 | 1111 | 793 |
| Male sex | 78% (28291) | 49% (6107) | 40% (3117) | 63% (1728) | 70% (782) | 61% (480) |
| Age (median, IQRa) | 38 (32, 45) | 34 (29, 41) | 33 (28, 40) | 36 (30, 43) | 37 (31, 45) | 34 (29, 41) |
| Probable infection route | ||||||
| Heterosexual | 33% (12062) | 76% (9541) | 87% (6857) | 58% (1580) | 64% (707) | 50% (397) |
| IDU | 15% (5535) | 2% (304) | 1% (60) | 2% (57) | 12% (138) | 6% (49) |
| MSM | 42% (15396) | 11% (1426) | 2% (158) | 31% (862) | 14% (154) | 32% (252) |
| Other/unknown | 9% (3374) | 10% (1216) | 10% (771) | 9% (238) | 10% (112) | 12% (95) |
| Year of ART initiation (median, IQR) | 2001 (‘99, ‘04) | 2003 (‘00, ’05) | 2003 (’01, ’05) | 2003 (’00, ’05) | 2002 (’99, ’05) | 2002 (’99, ’05) |
| ADEa at ART initiation | 22% (7969) | 24% (3004) | 22% (1720) | 26% (720) | 31% (341) | 28% (223) |
| CD4 count (median, IQR) | 229 (104, 360) | 194 (85, 297) | 200 (100, 298) | 186 (71, 298) | 170 (62, 290) | 170 (50, 290) |
| Log10 HIV-1 RNA (median, IQR) | 4.8 (4.1, 4.3) | 4.7 (4.0, 5.2) | 4.7 (3.9, 5.2) | 4.8 (4.2, 5.3) | 4.9 (4.2, 5.4) | 4.8 (4.3, 5.3) |
| First ART containing PIa | 65% (23724) | 62% (7723) | 64% (5002) | 56% (1528) | 64% (712) | 61% (481) |
ADE, AIDS-defining event; ART, antiretroviral therapy; IDU, injection drug use; IQR, interquartile range; MSM, men who have sex with men; PI, protease inhibitor.
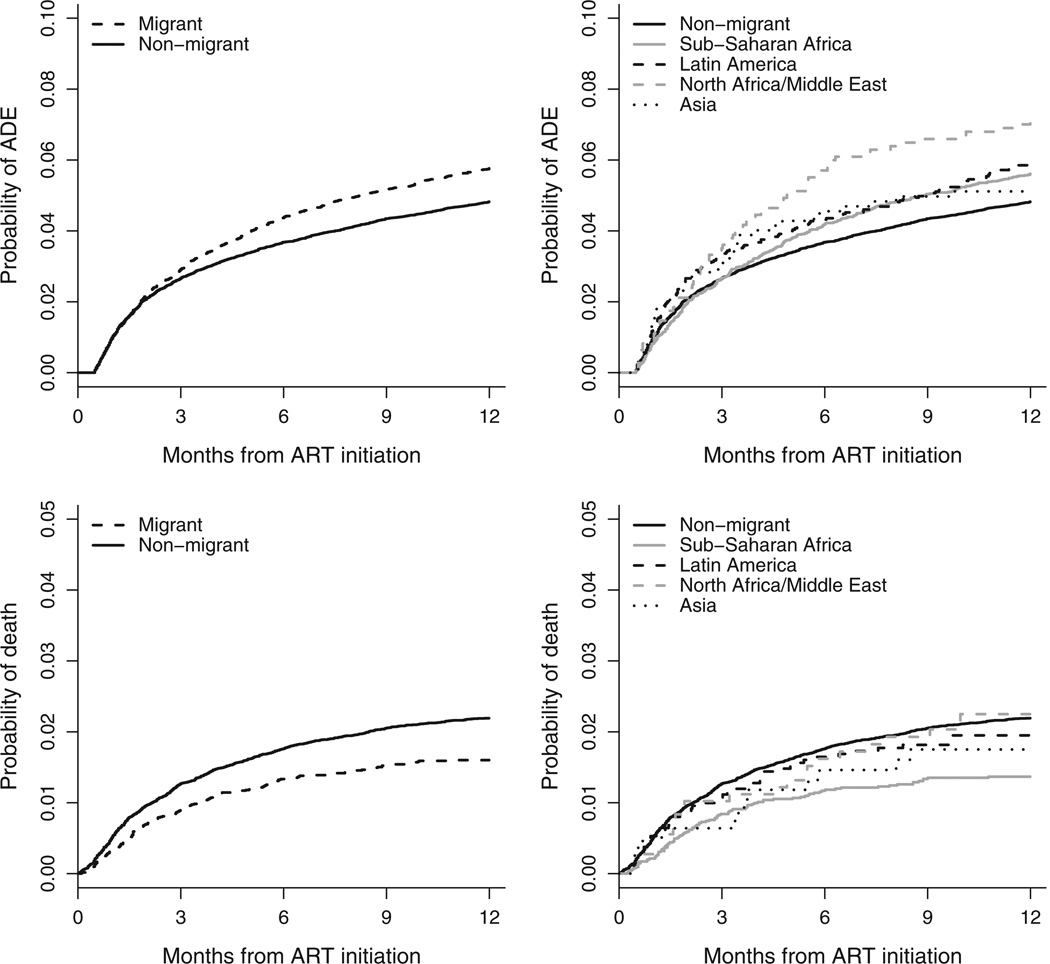
During the first year of ART, 2291 patients (4.7%) experienced a new ADE: 666 (5.3%) migrants and 1625 (4.5%) nonmigrants. The incidence of an ADE was 60.8 (95% CI 58.6–63.2) per 1000 person-years: 69.9 (95% CI 65.2–74.9) for migrants and 57.7 (95% CI 55.2–60.4) for nonmigrants. Figure 1 (upper-left panel) shows the cumulative incidence of ADEs during the first year of ART for migrants vs. nonmigrants. The crude hazard ratio for ADE during the first year of ARTwas 1.18 [95% confidence interval (CI) 1.08–1.29] for migrants compared with nonmigrants. After stratifying by cohort and adjusting for sex, age, CD4 cell count, HIV-1 RNA, type of ART, prior ADE, probable route of infection, and year of initiation, the hazard ratio was 1.21 (95% CI 1.09–1.34; P<0.001). Results were similar when limited to those whose probable route of infection was heterosexual sex (adjusted hazard ratio 1.23; 95% CI 1.08–1.40). In analyses stratified by continent (Europe and North America), there was little evidence that the ADE hazard ratio for migrants compared with nonmigrants differed between European and North American cohorts (interaction P = 0.57; adjusted hazard ratio 1.22; 95% CI 1.10–1.35 in Europe and 1.05; 0.65– 1.71 in North America).
Fig. 1. Incidence of AIDS defining events (upper panels) and death (lower panels) by region of origin (nonmigrants vs. migrantsin left panels; migrants separated into specific region of origin in right panels).
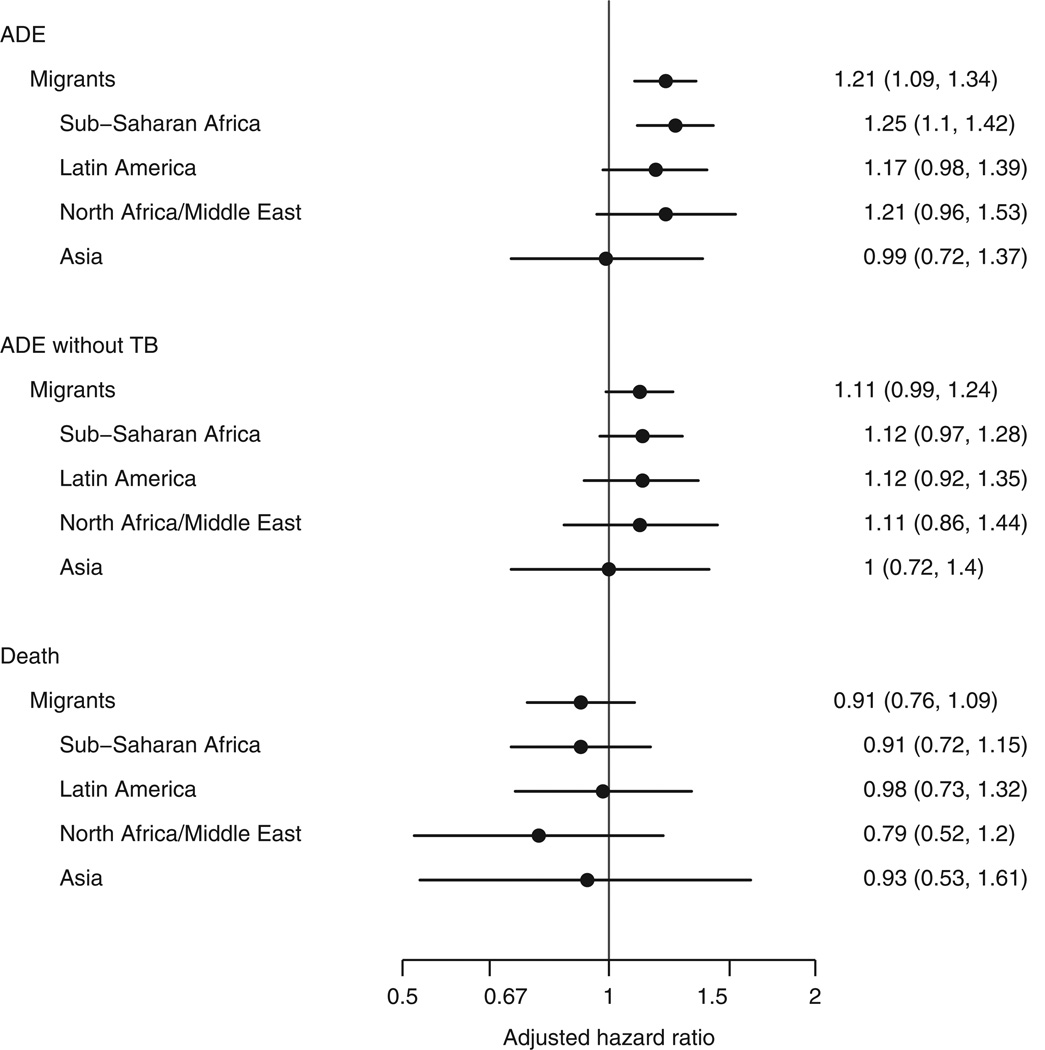
Figure 1 (lower-left panel) shows the cumulative incidence of all-cause mortality. Consistent with earlier work [8], mortality during the first year of ART was lower among migrants than nonmigrants (hazard ratio 0.71; 95% CI 0.61–0.84). The lower mortality was explained in part by patient characteristics at start of ART (adjusted hazard ratio 0.91; 95% CI 0.76–1.09; P = 0.32), particularly the younger age of migrants. The association between age and mortality was strong (hazard ratio 1.52 for 40 vs. 30 years; 95% CI 1.30–1.75; P < 0.001). When analyses only adjusted for age, the migrant vs. nonmigrant hazard ratio was 0.88 (95% CI 0.75–1.04); no other covariate had a similar impact. Results were similar when limited to those whose probable route of infection was heterosexual. Of the 805 patients who died during the first year of ART with a known cause of death (missing for 111 patients), 57.8% (465) died of an AIDS-related cause of death: 63.9% for migrants vs. 56.3% for nonmigrants. The incidence of any ADE during the first year of ART was 67.3 per 1000 person-years for migrants from sub-Saharan Africa (95% CI 61.6–73.6), and 71.8 (95% CI 62.0–83.1), 86.2 (95% CI 70.1–106.0), and 64.8 (95% CI 48.8–86.0) per 1000-person-years for migrants from Latin America, North Africa/Middle East, and Asia, respectively. Figure 1 (upper-right panel) shows the cumulative probability of ADEs during the first year of ART by region of origin. Figure 2 presents adjusted hazard ratios for ADEs comparing migrants from specific regions of origin to nonmigrants. After accounting for patient characteristics at ART initiation, the risk of ADE tended to be higher for migrants from all regions except for Asia. Figure 1 (lower-right panel) shows the cumulative probability of mortality for migrants from specific regions. Adjusted hazard ratios for death were similar for migrants from the different regions (Fig. 2).
Fig. 2. Hazard ratios for ADE, ADE not including TB, and death in the first year of ART initiation by region of origin.
The reference group is nonmigrants. All models are adjusted for sex, age, baseline CD4, baseline HIV-1 RNA, type of initial ART, prior ADE, probable route of infection, and year of ART initiation. ADE, AIDS-defining event; ART, antiretroviral therapy; TB, tuberculosis.
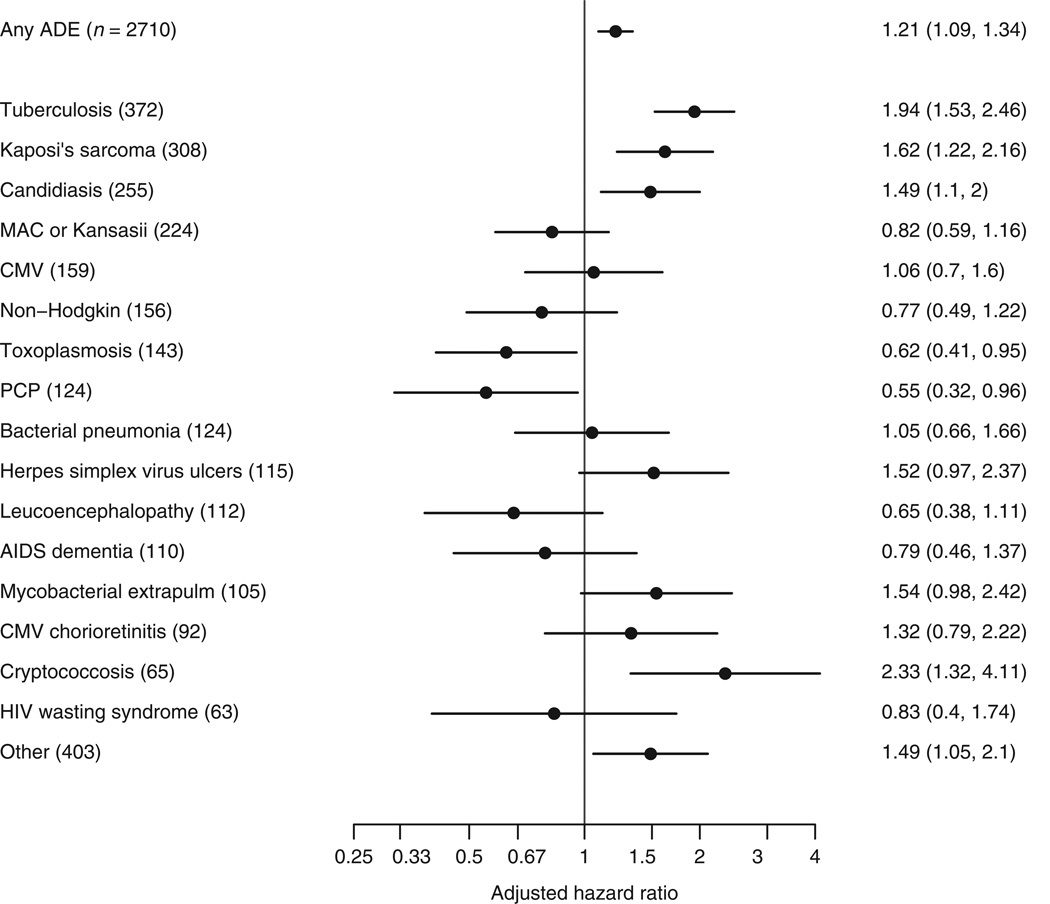
Numbers of specific ADEs and adjusted hazard ratios comparing migrants with nonmigrants are given in Fig. 3; incidence of all ADEs during the first year for migrants and nonmigrants is given in the online supplement, http://links.lww.com/QAD/A318. The most common ADEs were tuberculosis, candidiasis, Kaposi’s sarcoma, and Mycobacterium avium complex (MAC)/M. kansasii (incidence per 1000 person-years of 14.3, 8.1, 7.3, and 5.0, respectively in migrants and 6.3, 4.9, 6.8, and 5.0 in nonmigrants). Rates of tuberculosis (adjusted hazard ratio 1.94; 95% CI 1.53–2.46) and cryptococcosis (2.33; 1.32–4.11) during the first year of ART were markedly higher in migrants than nonmigrants. The incidence of tuberculosis was particularly high for migrant patients from sub-Saharan Africa, North Africa/Middle East, and Latin America (15.8, 16.3, and 12.1 per 1000 person-years, respectively); the incidence of tuberculosis for migrants from Asia was lower (5.4). After excluding tuberculosis, rates of ADE remained higher for migrants than nonmigrants (adjusted hazard ratio 1.11; 95% CI 0.99–1.24; P = 0.08; Fig. 2). Rates of Kaposi’s sarcoma (1.62; 1.22–2.16) and candidiasis (1.49; 1.10–2.00) were also higher in migrants than nonmigrants, whereas migrants experienced lower rates of toxoplasmosis (0.62; 0.41–0.95) and pneumocystis pneumonia (PCP) (0.55; CI 0.32–0.96) than nonmigrants.
Fig. 3. Hazard ratios for specific ADE in the first year of ART initiation comparing migrants with nonmigrants (reference group).
All models are adjusted for sex, age, baseline CD4, baseline HIV-1 RNA, type of initial ART, prior ADE, probable route of infection, and year of ART initiation. ADE, AIDS-defining event; ART, antiretroviral therapy.
Discussion
In this large study of persons starting ART in sites in Europe, the US, and Canada, we found higher rates of ADEs during the first year of ART, particularly of tuberculosis, among migrants than nonmigrants. These differences were most marked among migrants from sub-Saharan Africa. Rates of mortality during the first year were lower for migrants, although this was explained in part by patient age at the start of ART. Differences between migrants and nonmigrants varied between type of ADE: for example, rates of Kaposi’s sarcoma, candidiasis, and cryptococcosis were higher in migrants than nonmigrants, but rates of toxoplasmosis and PCP were lower.
Tuberculosis was the most common ADE among migrants during the first year of ART. The incidence of tuberculosis was approximately 16 cases per 1000 person-years for migrants from sub-Saharan Africa and North Africa/Middle East, and 12 per 1000 person-years for migrants from Latin America. In contrast, rates of tuberculosis were lower (between five and six cases per 1000 person-years) for migrants from Asia or nonmigrants. High rates of tuberculosis among migrants from Africa and Latin America have been reported previously and are not unexpected [14,15]. Incidence of tuberculosis among HIV-positive persons in Africa and Latin America, even those on ART, is high [16,17]. For example, a study of HIV-positive persons in South Africa found an incidence of tuberculosis of 35 cases per 1000 person-years during the first year of ART [18]. Although our observed incidence of TB after ART initiation in Europe/North America is certainly lower than that reported in sub-Saharan Africa, our results nonetheless highlight the importance of screening for tuberculosis prior to ART initiation among such patients, in addition to screening for latent infection at entry into care [19]. Current guidelines do not mention screening for tuberculosis prior to ART initiation [20–22]. Although rates of tuberculosis during the first year of ART were especially high for migrants, they were still much higher than the general population for nonmigrants, which may warrant screening in both groups [19]. These results also raise issues regarding the choice of initial regimen among migrants, given known interactions between antituberculosis medications and protease inhibitors [23–26].
Although tuberculosis drove the excess risk of ADE among migrants, rates of ADEs during the first year of ART remained elevated among migrants even when tuberculosis events were excluded. After accounting for patient characteristics, rates of cryptococcosis, Kaposi’s sarcoma and candidiasis were also higher for migrants than nonmigrants, as were several other less-frequently observed ADEs (herpes simplex virus ulcers or pneumotitis/esophagitis, extrapulmonary mycobacteria, and extrapulmonary cryptococcosis, isosporiasis diarrhea, and extrapulmonary histoplasmosis). In contrast, toxoplasmosis, PCP, and perhaps leucoencephalopathy and non-Hodgkin lymphoma appeared more prevalent among nonmigrants. Notably, many of the ADEs occurring more frequent in migrants than nonmigrants are those ADEs associated with a lower subsequent rate of mortality [27].
Others have compared rates of ADE and mortality between migrants and nonmigrants, albeit with significantly fewer patients. A study in Canada noted that immigrants presented with lower CD4 cell counts and different comorbidities, but found no statistical difference in clinical outcomes [4]. A Swiss cohort study comparing 2-year outcomes between migrants and nonmigrants from 1997 to 2001 found similar rates of ART uptake, progression to AIDS, and survival between migrants and nonmigrants [6]. In another study from the Swiss cohort, treatment-naive patients from sub-Saharan Africa had slower CD4 cell decline [5]. In a recent study conducted in Europe, Canada, and Australia, the time from seroconversion to AIDS and death was similar between migrants from sub-Saharan Africa and native-born patients [7]. In the Netherlands, patients starting ART from sub-Saharan Africa and Surinam/Netherlands Antilles tended to have similar increases in CD4 cell counts after ART initiation but were less likely to achieve virologic suppression and more likely to progress to AIDS (notably tuberculosis) or death [2,3, 12]. A study from Spain did not show differences in opportunistic infections and death between migrants and nonmigrants [13].
In an earlier study, the ART-CC noted lower mortality rates among treated HIV-positive migrants than nonmigrants [8]. That analysis did not consider ADEs and differed from the current analysis in that it included all available follow-up time, not just the first year after ART initiation [8]. In future work, we plan to investigate rates of ADEs after the first year of ART.
Strengths of this study include its large size and the geographical diversity of the patients and clinics included. Because of the large number of patients, we were able to accurately estimate the incidence of specific ADEs during the first year of ART, both in migrants and nonmigrants. We were also able to study specific regions of origin, and examine differences between patients migrating from different regions. We do not have data on length of time since migration or place of HIV-infection. Therefore, although we presume that many of the migrants in this study were infected in their region of origin, we are unable to verify this for the majority of patients. Similarly, we do not have data on virus clade. Rates of disease progression have been seen to differ according to HIV-1 subtype among patients living in Africa [28,29] which could explain some of these results. We have only considered patients who started ART; it is possible that migrants have a more difficult time engaging in care than nonmigrants, and therefore that those migrants who do start ART represent a selected group whose outcomes are expected to be better than those not on ART.
In conclusion, in this large study of HIV-positive patients initiating ART in multiple sites throughout Europe, the US, and Canada, patients who had migrated from Africa, Latin America, or the Middle East tended to have higher rates of ADEs during the first year of ART, particularly tuberculosis, and lower rates of death. These discordant results probably reflect the higher rates of opportunistic infections in developing country settings as well as a ‘healthy migrant’ effect. These findings could influence screening for specific ADEs prior to ART initiation in high-income countries.
Supplementary Material
Acknowledgements
Funding: The ART-CC is supported by UK Medical Research Council grants G0700820 and MR/J002380/1. Sources of funding of individual cohorts include the National Institutes of Health (grant numbers P30 AI54999 and P30 AI027757 for Vanderbilt and the University of Washington HIV Cohort), Pfizer and BMS (Vanderbilt), Agence Nationale de Recherche contre le SIDA (ANRS), the Institut National de la Santé et de la Recherche Médicale (INSERM), the French, Italian, Spanish and Swiss Ministries of Health, The Swiss HIV Cohort Study, supported by the Swiss National Science Foundation, the Stichting HIV Monitoring, the European Commission, the British Columbia and Alberta Governments, the Michael Smith Foundation for Health Research, the Canadian Institutes of Health Research, the VHA Office of Research and Development and unrestricted grants from Glaxo-SmithKline, Roche and Boehringer-Ingelheim. Supported in part by the ‘Spanish Network for AIDS Research’ (RIS; ISCIII-RETIC RD06/006).
Appendix
Writing committee
Bryan E. Shepherd, Cathy A. Jenkins, Deidra D. Parrish, Tracy R. Glass, Angela Cescon, Angels Masabeu, Genevieve Chene, Frank deWolf, Heidi M. Crane, Inma Jarrin, John Gill, Julia del Amo, Sophie Abgrall, Pavel Khaykin, Clara Lehmann, Suzanne M. Ingle, Margaret T. May, Jonathan A.C. Sterne, Timothy R. Sterling
| Vanderbilt: | Bryan E. Shepherd | <bryan.shepherd@vanderbilt.edu> |
| Cathy A. Jenkins | <cathy.a.jenkins@vanderbilt.edu> | |
| Deidra D. Parrish | <d.parrish@vanderbilt.edu> | |
| Timothy R. Sterling | <timothy.sterling@vanderbilt.edu> | |
| SHCS: | Tracy R. Glass | <Tracy.Glass@unibas.ch> |
| HOMER: | Angela Cescon | <acescon@cfenet.ubc.ca> |
| PISCIS: | Angels Masabeu | <amasabeu@ssibe.cat> |
| Aquitaine: | Genevieve Chene | <genevieve.chene@isped.u-bordeaux2.fr> |
| Athena: | Franf De Wolf | <f.dewolf@amc.uva.nl> |
| Washington: | Heidi M. Crane | <hcrane@U.WASHINGTON.EDU> |
| CoRIS: | Inma Jarrin | <ijarrin@isciii.es> |
| Southern Alberta: | John Gill | <John.Gill@albertahealthservices.ca> |
| CoRIS: | Julia del Amo | <jdamo@isciii.es> |
| FHDH: | Sophie Abgrall | <sophie.abgrall@AVC.APHP.FR> |
| Frankfurt: | Pavel Khaykin | <pavel.khaykin@hivcenter.de> |
| CBC: | Clara Lehmann | <clara.lehmann@uni-koeln.de> |
| Bristol: | Suzanne M. Ingle | <S.Ingle@bristol.ac.uk> |
| Margaret May | <m.t.may@bristol.ac.uk> | |
| Jonathan Sterne | <Jonathan.Sterne@bristol.ac.uk> |
Steering group
Hans-Reinhard Brodt (Frankfurt), Jordi Casabona (PISCIS), Matthias Cavassini(SHCS), Geneviève Chêne (Aquitaine), Dominique Costagliola (FHDH), François Dabis (Aquitaine), Antonella D’Arminio Monforte (ICONA), Julia del Amo (CoRIS-MD), Frank de Wolf (ATHENA), Gerd Fätkenheuer (Koln/Bonn), John Gill (South Alberta Clinic), Jodie Guest (HAVACS), David Hans-Ulrich Haerry (EATG), Robert Hogg (HOMER), Amy Justice (VACS), Amanda Mocroft (EuroSIDA), Mari Kitahata (Washington), Fiona Lampe (Royal Free), Peter Reiss (ATHENA), Michael Saag (Alabama), Tim Sterling (Vanderbilt-Meharry), Matthew Williams (UK-CAB), Robert Zangerle (Austria)
Coordinating team
Jonathan Sterne and Margaret May (Principal Investigators), Suzanne Ingle (statistician)
ART-CC Cohorts
The AIDS Therapy Evaluation Project, Netherlands (ATHENA); the Agence Nationale de la Recherche sur le SIDA et les hépatites virales (ANRS) CO3 Aquitaine Cohort, France; the ANRS CO4 French Hospital Database on HIV (FHDH), France; the Köln/Bonn Cohort, Germany; the Frankfurt Cohort, Germany; the Proyecto para la Informatización del Seguimiento Clínico-epidemiológico de la Infección por HIV y SIDA (PISCIS) Cohort, Spain; the Cohorte de la Red de Investigación en Sida (CoRIS), Spain; the Swiss HIV Cohort Study (SHCS), Switzerland; the Italian Cohort of Antiretroviral-Naïve Patients (ICONA), Italy; the Royal Free Hospital Cohort, United Kingdon; the Multicenter Study Group on EuroSIDA; the Österreichische HIV-Kohortenstudie (OEHIVKOS), Austria; the HAART Observational Medical Evaluation and Research (HOMER), British Columbia, Canada; the South Alberta Clinic Cohort, Canada; the Vanderbilt Comprehensive Care Clinic, US; the University of Washington HIV Cohort, US; the 1917 Clinic Cohort, University of Alabama, Birmingham, US; the Veterans Aging Cohort Study (VACS), US; the HIV Atlanta Veterans Affairs Cohort Study (HAVACS), US.
Footnotes
Conflicts of interest
There are no conflicts of interest.
References
- 1.Braitstein P, Brinkhof MW, Dabis F, Schechter M, Boulle A, Miotti P, et al. Mortality of HIV-1-infected patients in the first year of antiretroviral therapy: comparison between low-income and high-income countries. Lancet. 2006;367:817–824. doi: 10.1016/S0140-6736(06)68337-2. [DOI] [PubMed] [Google Scholar]
- 2.Nellen JF, Wit FW, De Wolf F, Jurriaans S, Lange JM, Prins JM. Virologic and immunologic response to highly active antiretroviral therapy in indigenous and nonindigenous HIV-1-infected patients in the Netherlands. J Acquir Immune Defic Syndr. 2004;36:943–950. doi: 10.1097/00126334-200408010-00008. [DOI] [PubMed] [Google Scholar]
- 3.Kesselring AM, Gras L, Wit FW, Smit C, Geerlings SE, Mulder JW, et al. Immune restoration and onset of new AIDS-defining events with combination antiretroviral therapy in HIV type-1-infected immigrants in the Netherlands. Antivir Ther. 2010;15:871–879. doi: 10.3851/IMP1638. [DOI] [PubMed] [Google Scholar]
- 4.Krentz H, Gill MJ. The five-year impact of an evolving global epidemic, changing migration patterns, and policy changes in a regional Canadian HIV population. Health Policy. 2009;90:296–302. doi: 10.1016/j.healthpol.2008.09.016. [DOI] [PubMed] [Google Scholar]
- 5.Muler V, von Wyl V, Yerly S, Boni J, Klimkait T, Burgisser P, et al. Swiss HIV Cohort Study. African descent is associated with slower CD4 cell count decline in treatment-naïve patients of the Swiss HIV Cohort Study. AIDS. 2009;23:1269–1276. doi: 10.1097/QAD.0b013e32832d4096. [DOI] [PubMed] [Google Scholar]
- 6.Staehelin C, Rickenbach M, Low N, Egger M, Ledergerber B, Hirschel B, et al. Swiss HIV Cohort Study. Migrants from Sub-Saharan Africa in the Swiss HIV Cohort Study: access to antiretroviral therapy, disease progression and survival. AIDS. 2003;17:2237–2244. doi: 10.1097/00002030-200310170-00012. [DOI] [PubMed] [Google Scholar]
- 7.Jarrin I, Pantazis N, Gill MJ, Geskus R, Perez-Hoyos S, Meyer L, et al. CASCADE Collaboration in EuroCoord. Uptake of combination antiretroviral therapy (cART) and HIV disease progression according to geographical origin in seroconverters in Europe, Canada, and Australia. Clin Infect Dis. 2012;54:111–118. doi: 10.1093/cid/cir814. [DOI] [PubMed] [Google Scholar]
- 8.Del Amo J, Jarrin I, Gill J, May M, Dabis F, Crane H, et al. Differences in mortality and causes of death by geographical origin and ethnicity in HIV-positive patients on Antiretroviral Therapy in Canada, Europe and United States. Clin Infect Dis. 2013 doi: 10.1093/cid/cit111. [Epub ahead of print] [DOI] [PubMed] [Google Scholar]
- 9.Razum O, Zeeb H, Rohrmann S. The ‘healthy migrant effect’: not merely a fallacy of inaccurate denominator figures. Int J Epidemiol. 2000;29:191–192. doi: 10.1093/ije/29.1.191. [DOI] [PubMed] [Google Scholar]
- 10.Centers for Disease Control. Revised classification system for HIV infection and expanded surveillance case definition for AIDS among adolescents and adults. MMWR Recomm Rep. 1992;41(RR-17):1–19. [PubMed] [Google Scholar]
- 11.Putter H, Fiocco M, Geskus RB. Tutorial in biostatistics: competing risks and multistate models. Stat Med. 2007;26:2389–2430. doi: 10.1002/sim.2712. [DOI] [PubMed] [Google Scholar]
- 12.van den Berg JB, Hak E, Vervoort SC, Hoepelman IM, Boucher CA, Schuurman R, et al. Increased risk of early virological failure in non-European HIV-1-infected patients in a Dutch cohort on highly active antiretroviral therapy. HIV Med. 2005;6:299–306. doi: 10.1111/j.1468-1293.2005.00304.x. [DOI] [PubMed] [Google Scholar]
- 13.Perez Molina JA, Rillo MM, Suarez-Lozano I, Casado Osorio JL, Cobo RT, Gonzalez PR, et al. Do HIV-infected immigrants initiating HAART have poorer treatment-related outcomes than autochthonous patients in Spain? Results of the GESIDA 5808 Study. Curr HIV Res. 2010;8:521–530. doi: 10.2174/157016210793499178. [DOI] [PubMed] [Google Scholar]
- 14.Grant AD, Bansi L, Ainsworth J, Anderson J, Delpech V, Easterbrook P, et al. Tuberculosis among people with HIV infection in the United Kingdom: opportunities for prevention? AIDS. 2009;23:2507–2515. doi: 10.1097/QAD.0b013e3283320dfd. [DOI] [PubMed] [Google Scholar]
- 15.Abgrall S, Del Giudice P, Melica G, Costagliola D FHDHANRS C04. HIV-associated tuberculosis and immigration in a high-income country: incidence trends and risk factors in recent years. AIDS. 2010;24:763–771. doi: 10.1097/QAD.0b013e3283366747. [DOI] [PubMed] [Google Scholar]
- 16.De Cock KM, Soro B, Coulibaly IM, Lucas SB. Tuberculosis and HIV infection in sub-Saharan Africa. JAMA. 1992;268:1581–1587. doi: 10.1001/jama.268.12.1581. [DOI] [PubMed] [Google Scholar]
- 17.Corbett EL, Watt CJ, Walker N, Maher D, Williams BG, Raviglione MC, Dye C. The growing burden of tuberculosis: global trends and interactions with the HIV epidemic. Arch Internal Med. 2003;163:1009–1021. doi: 10.1001/archinte.163.9.1009. [DOI] [PubMed] [Google Scholar]
- 18.Lawn SD, Badri M, Wood R. Tuberculosis among HIV-infected patients receiving HAART: long term incidence and risk factors in a South African cohort. AIDS. 2005;19:2109–2116. doi: 10.1097/01.aids.0000194808.20035.c1. [DOI] [PubMed] [Google Scholar]
- 19.Sterling TR, Lau B, Zhang J, Freeman A, Bosch RJ, Brooks JT, et al. Risk factors for tuberculosis after highly active antiretroviral therapy initiation in the United States and Canada: implications for tuberculosis screening. J Infect Dis. 2011;204:893–991. doi: 10.1093/infdis/jir421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Kaplan JE, Benson C, Holmes KH, Brooks JT, Pau A, Masur H, et al. Guidelines for prevention and treatment of opportunistic infections in HIV-infected adults and adolescents: recommendations from CDC, the National Institutes of Health, and the HIV Medicine Association of the Infectious Diseases Society of America. Morb Mortal Wkly Rep. 2009;58:1–206. [PubMed] [Google Scholar]
- 21. [last updated March, 27, 2012]; http://www.aidsinfo.nih.gov/guidelines/html/1/adult-and-adolescent-treatment-guidelines/0/
- 22.European AIDS Clinical Society Guidelines, version 6.0: October 2011. http://www.europeanaidsclinicalsociety.org. [Google Scholar]
- 23.Acosta EP, Kendall MA, Gerber JG, Alston-Smith B, Koletar SL, Zolopa AR, et al. Effect of concomitantly administered rifampin on the pharmacokinetics and safety of atazanavir administered twice daily. Antimicrob Agents Chemother. 2007;51:3104–3110. doi: 10.1128/AAC.00341-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Mallolas J, Sarasa M, Nomdedeu M, Soriano A, Lopez-Pua Y, Blanco JL, et al. Pharmacokinetic interaction between rifampicin and ritonavir-boosted atazanavir in HIV-infected patients. HIV Med. 2007;8:131–134. doi: 10.1111/j.1468-1293.2007.00442.x. [DOI] [PubMed] [Google Scholar]
- 25.Haas DW, Koletar SL, Laughlin L, Kendall MA, Suckow C, Gerber JG, et al. Hepatotoxicity and gastrointestinal intolerance when healthy volunteers taking rifampin add twice-daily atazanavir and ritonavir. J Acquir Immune Defic Syndr. 2009;50:290–293. doi: 10.1097/QAI.0b013e318189a7df. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.L’homme RF, Nijland HM, Gras L, Aarnoutse RE, van Crevel R, Boeree M, et al. Clinical experience with the combined use of lopinavir/ritonavir and rifampicin. AIDS. 2009;23:863–865. doi: 10.1097/QAD.0b013e328329148e. [DOI] [PubMed] [Google Scholar]
- 27.Mocroft A, Sterne JA, Egger M, May M, Grabar S, Furrer H, et al. Variable impact on mortality of AIDS-defining events diagnosed during combination antiretroviral therapy: not all AIDS-defining conditions are created equal. Clin Infect Dis. 2009;48:1138–1151. doi: 10.1086/597468. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Vasan A, Renjifo B, Hertzmark E, Chaplin B, Msamanga G, Essex M, et al. Different rates of disease progression of HIV type 1 infection in Tanzania based on infecting subtype. Clin Infect Dis. 2006;42:843–852. doi: 10.1086/499952. [DOI] [PubMed] [Google Scholar]
- 29.Kiwanuka N, Laeyendecker O, Robb M, Kigozi G, Arroyo M, McCutchan F, et al. Effect of human immunodeficiency virus type 1 (HIV-1) subtype on disease progression in persons from Rakai Uganda, with incident HIV-1 infection. J Infect Dis. 2008;197:707–713. doi: 10.1086/527416. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.